Abstract
Previously we showed that recombinant adenoviral helper-dependent (HD) vectors result in long-term transgene expression levels in vivo which slowly declined by 95% over a period of 1 year. In this study, we further establish that this was not predominantly immune mediated. To determine if cell turnover was responsible for the loss of transgene expression, we induced rapid hepatocyte cell cycling in mouse liver, by performing a surgical two-thirds partial hepatectomy. We observed a 55 and 65% reduction in transgene expression levels and a 50 and 71% loss of vector genomes for the HD vector and the first-generation adenoviral vector. In sharp contrast, in nonviral, episomal plasmid DNA-injected mice, transgene expression levels and DNA copy numbers decreased by 95 and 99%, respectively. These findings suggest that cell division alone was not the primary reason for the slow decrease in transgene expression levels and that recombinant adenoviral vectors have a more robust mechanism for maintaining persistence during cell cycling. Several potential mechanisms are proposed.
Helper-dependent (HD) adenoviral vectors were demonstrated to result in long-term expression levels of therapeutic genes in vivo (7, 15, 23, 24, 30, 32). The in vivo performance of adenoviral vectors was shown to be dependent on the DNA sequences placed in the adenoviral vector (14, 29), the transgene expression cassette (10, 14, 34), the mouse strain (3, 20, 33), the immediate early inflammatory response, and cell-mediated and humoral immune responses (7, 18, 32, 38). The persistence of adenoviral vector genomes and transgene expression levels still remains a major obstacle for first-generation (fg), multidefective, and HD vectors. Little is known about the mechanism which is responsible for the maintenance of the vector DNA. We recently showed, for both HD gene deletion adenoviral vectors and fg vectors delivered into the livers of mice, a concordant 95% falloff in transgene expression and vector genome copy number over a period of a year (7). The absence of a detectable humoral immune response against the transgene product and similar findings with immunodeficient mice strongly suggest that an immune response was not responsible for the falloff in gene expression. This phenomenon does not appear to be unique to rodents, since Morral et al. (23) showed a 92% decline in gene expression in nonhuman primates infused with an HD adenoviral vector over a period of 2 years. The progressive deletion of the adenoviral genome does not seem to be strongly influenced by the antiviral immune response, because it occurs in both immunocompromised (4, 20, 33) and immunocompetent (13) mice for first-generation, multidefective, and helper-dependent adenoviral vectors. This is further supported by the study of Wadsworth et al. (36), who boosted the antiadenoviral CTL response in liver-transduced mice and found no enhanced loss in transgene expression.
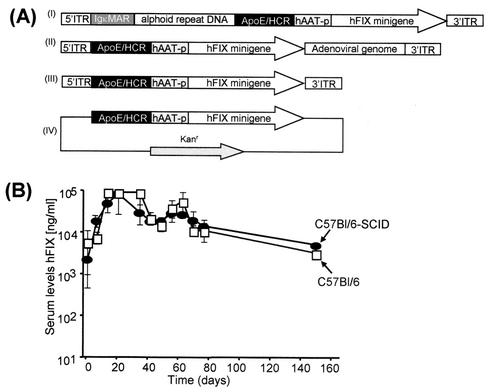
To further establish that the loss of gene expression in fg and gene deletion adenoviral vector-treated mice was not the result of an antigen-specific immune response directed against the transduced cells or transgene product, C57BL/6 mice and C57BL/6-SCID mice (Jackson Laboratory) (n = 5 per group) were injected with 8 × 108 transducing units of the HD vector AdFTC/hFIX (Fig. 1A, I). The production of the HD vector AdFTC/hFIX was performed as previously described (7, 11). The final adenoviral vector preparations were diluted in phosphate-buffered saline (PBS), and a total volume of 200 μl was injected into the tail vein. We found that the time courses of transgene expression were similar for C57BL/6 and C57BL/6-SCID mice (Fig. 1B). The highest hFIX expression levels were observed at 2 weeks postinjection (up to 80,000 ng/ml). The expression levels remained stable for the next months, followed by a slow decline in hFIX concentrations in serum for both C57BL/6 and C57BL/6-SCID mice. Taken together, these findings further support that a cell-mediated immune response is not the major factor responsible for the slow decline in hFIX expression levels and the drop of genome copy numbers over time. The progressive clearance mechanism of adenoviral vector genomes may be independent of B cells, T cells, and NK cells and suggests that other factors might influence genome persistence.
FIG. 1.
DNA sequences used for vector production and in vivo performance of HD vectors in immunodeficient mice. (A) Recombinant adenoviral and nonviral vector genomes used in this study. All vector genomes contained the identical hFIX expression cassette in which the hFIX minigene including a portion of the first hFIX gene intron and the 3′ untranslated region (3′UTR) was driven by the human alpha-1-antitrypsin promoter (hAAT), the liver-specific hepatocyte control region (HCR), and the apolipoprotein enhancer (ApoE). (I) DNA sequences used for production of the HD vector AdFTC/hFIX. A 16.2-kb of alphoid repeat DNA fragment from human chromosome 17 was flanked by a 4.2-kb fragment containing the left terminus of adenovirus type 5 (nt 1 to 452), two copies of the immunoglobulin κMAR (IgκMAR) and by a 7.2-kb fragment containing the hepatocyte control region, a multiple cloning site, the hFIX expression cassette, and the right terminus of adenovirus type 5 (nt 35796 to 35935). (II) The first-generation adenoviral vector fgAd/hFIX contained the hFIX expression cassette and was deleted for the adenoviral early genes E1 and E3. (III) Linear DNA construct L-ITR/hFIXmg, in which the hFIX expression cassette was flanked by the adenoviral 5′ and 3′ ITR. For high-pressure tail vein injection of a linear DNA fragment in which the hFIX expression cassette was flanked by the adenoviral ITRs, the plasmid pL-ITR/hFIXmg was digested with PacI, which cuts twice, to release the plasmid backbone. (IV) Circular DNA construct pHM5/hFIXmg, in which our most robust hFIX expression cassette was placed in the plasmid pHM5. (B) The duration of transgene expression levels from an HD adenoviral vector shows the same time courses in C57/BL/6 and C57BL/6-SCID mice. For comparison, 8 × 109 transducing units of the HD vector AdFTC/hFIX (see Fig. 2A) were injected via the tail vein into C57/BL/6 (•) and C57BL/6-SCID (□) mice. Serum samples were collected by retro-orbital bleeding, and hFIX concentrations in serum were monitored weekly by enzyme-linked immunosorbent assay as described earlier (7).
To further analyze the mechanism involved in adenoviral genome persistence, we evaluated the loss of vector in the liver of mice during cell cycling to determine if hepatocyte turnover was responsible for the slow decline in transgene expression levels and loss of vector genomes in vivo. Cell cycling of hepatocytes was induced by performing a surgical two-thirds partial hepatectomy after vector delivery as previously described (28). The principle of the experiment is that after hepatectomy, almost every hepatocyte will undergo one or two cell doublings until the original liver mass is reconstituted. During cell cycling, episomal vectors that are not directly attached to the chromosomal DNA or contain separate centromeres/telomeres will not be maintained during cell division. This is consistent with our previously established studies that demonstrated a 95% reduction in episomal plasmid or recombinant adeno-associated virus (AAV)-mediated transgene expression and vector DNA, while integrating vectors maintained gene expression during and after liver regeneration (6, 22, 25). It is important to note that because we removed approximately two-thirds of the liver, at a minimum, 66% of the vector DNA would be removed at the time of surgery.
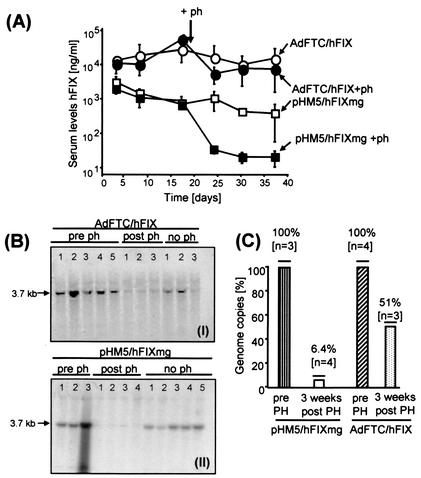
We compared the persistence of the HD vector genome AdFTC/hFIX (n = 8) (Fig. 1A, I) with the nonviral plasmid vector pHM5/hFIXmg (8) (n = 8) (Fig. 1A, IV). To deliver nonviral DNA into mouse liver, a high-pressure tail vein injection was performed as previously described (19, 37). Briefly, 20 μg of the respective DNA diluted in 2 ml of phosphate-buffered saline was injected into the tail vein of C57BL/6 mice in 5 to 7 s. Two weeks after hepatic infusion of 109 transducing units of the HD adenoviral vector AdFTC/hFIX or 20 μg of the circular plasmid pHM5/hFIXmg in mice, a surgical two-thirds partial hepatectomy was performed. Before partial hepatectomy, hFIX levels in serum from the HD vector AdFTC/hFIX and pHM5/hFIXmg were stable at about 40,000 and 2,000 ng/ml, respectively (Fig. 2A). Three weeks post-partial hepatectomy, hFIX expression levels dropped by about 56% (18,000 ng/ml) for HD vector AdFTC/hFIX-treated animals and 96% (80 ng/ml) for circular plasmid pHM5/hFIXmg-treated animals (Fig. 2A). Mice injected with either AdFTC/hFIX or pHM5/hFIXmg that did not receive a surgical two-thirds partial hepatectomy maintained stable hFIX expression throughout the experiment.
FIG. 2.
The gutless adenoviral vector AdFTC/hFIX results in persistent transgene expression levels during the cell cycle in vivo. (A) Comparison of hFIX expression levels derived from episomal circular nonviral DNA and gutless adenoviral vector genomes. transducing units (109) of gutless adenoviral vector AdFTC/hFIX (•, ○) and 20 μg of the nonviral vector pHM5/hFIXmg (▪, □) were transduced into mouse liver. To induce the liver cell cycle, 2 weeks postinjection a surgical two-thirds partial hepatectomy was performed [•, ▪]. The hFIX expression levels were monitored before and up to 3 weeks post-partial hepatectomy by serum hFIX ELISA. Means ± standard deviation are shown. (B) Southern blot analyses and quantification of adenoviral (I) and nonviral (II) vector DNA in mouse liver. (C) The relative loss of vector genomes before and after partial hepatectomy was determined by phosphoimager analyses.
To establish if the posthepatectomy decline in gene expression was due to a loss of vector genomes, we determined the relative amount of recombinant vector DNA pre- and post-partial hepatectomy by performing Southern blot analyses (Fig. 2B). Liver genomic DNA was extracted as previously described (17). Twenty micrograms of genomic liver DNA pre- and post-partial hepatectomy was digested with HindIII a restriction enzyme nuclease which releases 3.74 kb from the input DNA. The genomic liver DNA was separated by gel electrophoresis, transferred to a nitrocellulose membrane, and hybridized with a [α-32]dCTP-labeled cDNA hFIX probe (1.6-kb HindIII/EcoRI fragment from pAAVCM2), using a random priming kit (Stratagene). We found that the adenoviral vector genomes remain more stable than a circular plasmid DNA (nonviral) vector. Three weeks post-partial hepatectomy, the amount of nonviral vector DNA and adenoviral vector DNA dropped by approximately 94 and 49%, respectively (Fig. 2C). The loss of vector DNA was concordant with the falloff in transgene expression. Taken together, our results presented in this study are in contrast to the common understanding of episomal adenoviral vector genomes because this data and previous studies all suggest that the genomes are slowly lost over time. While adenovirus-mediated gene expression and genomes slowly declined by about 95% over a period of a year, AAV-mediated hepatic gene expression and vector genomes do not extensively decline, even though most of the vector genomes remain episomal (25). Taken together, recombinant adenoviral vectors are not lost during hepatocyte cell division, suggesting that they become uniquely associated with chromosomal DNA during the cell cycle.
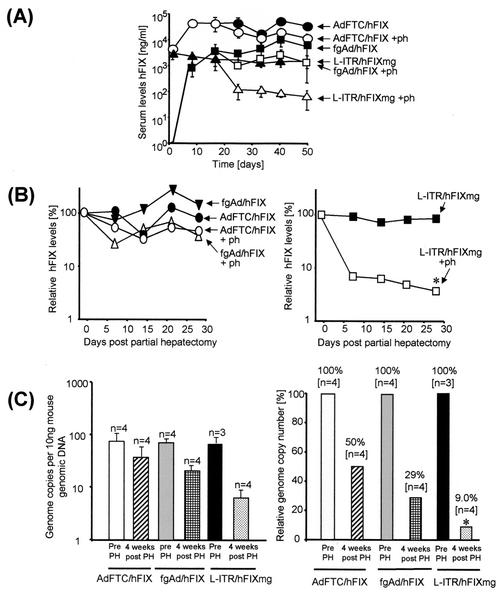
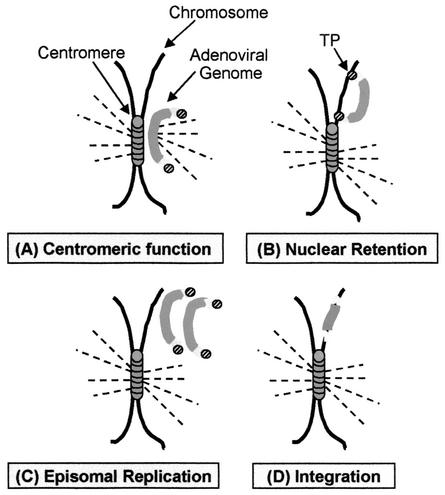
To address whether the persistence of adenoviral vector genomes was similar for other types of adenoviral vectors and/or if the linear terminal ends of the adenoviral DNA alone were sufficient for persistence, we performed a further study which compared the HD vector, AdFTC/hFIX, the first-generation adenoviral vector fgAd/hFIX (Fig. 1A, II), and a linear nonviral DNA fragment with the identical expression cassette flanked by the adenoviral inverted terminal repeats (ITR) (L-ITR/hFIXmg) (Fig. 1A, III). To generate L-ITR/hFIXmg, the vector pBITRpTP (unpublished data) was digested with ClaI and a SpeI linker was added. The hFIX expression cassette from pBS-ApoEHCR-hAATp-FIX-Int-3′UTR-pA (21) was ligated into the SpeI site of pITRSpeI, resulting in pL-ITR/hFIXmg. We infused 8 × 108 transducing particles of the adenoviral vector fgAdFTC/hFIX (n = 8) or AdFTC/hFIX (n = 8) or 20 μg of L-ITR/hFIXmg into the livers of C57B/6 mice. Two weeks postinjection, after the transgene expression levels were stabilized, a two-thirds partial hepatectomy was performed. We found that the transgene expression levels for both the gene deleted and the fg adenoviral vector were equally persistent. One day pre-partial hepatectomy, the serum hFIX concentrations were 40,000 ng/ml for AdFTC/hFIX and 3,700 ng/ml for fgAd/hFIX, and 4 weeks post-partial hepatectomy, the serum hFIX concentrations dropped to 18,000 and 1,300 ng/ml, respectively (Fig. 3A). This was in sharp contrast to the hFIX expression levels derived from the nonviral vector L-ITR/hFIXmg, which dropped from 1,700 ng/ml pre-partial hepatectomy to less than 100 ng/ml after hepatectomy. For comparison, the relative hFIX expression levels pre- and post-partial hepatectomy are summarized in Fig. 3B. The relative hFIX expression dropped 55% for AdFTC/hFIX, 65% for fgAd/hFIX (Fig. 3B, left panel), and 95% for L-ITR/hFIXmg (Fig. 3B, right panel) (P ≤ 0.05, analysis of variance (ANOVA) analysis with a post-hoc Fisher test). The dramatic loss of serum hFIX concentrations after hepatic infusion of linear nonviral DNA in mice that received a two-thirds partial hepatectomy was similar to what we observed in a previous study (6). In concordance with these findings, we observed a dramatic clearance of the nonviral vector genomes after partial hepatectomy, which was significantly less for both the first-generation and HD adenoviral vectors (Fig. 3C, left panel). The total vector genome copy numbers per 10 ng of liver genomic DNA pre- and post-partial hepatectomy for the HD vector AdFTC, the fg adenoviral vector fgAd/hFIX, and the nonviral vector L-ITR/hFIXmg were determined by a quantitative real-time PCR assay. For this purpose the SYBR Green PCR Master Mix (catalog no. 4309155; PE Biosystems) in combination with the GeneAmp5700 sequence detection system was used. The PCR product was located in the hFIX cDNA [nucleotides (nt) 5,032 to 5,800 from pBS-ApoEHCR(s)-hAATp-hFIXmg-bpA], and for the amplification, a hFIX forward primer (5′AAGATGCCAAACCAGGTCAATT3′) and a hFIX reverse primer (5′GATAGAGCCTCCACAGAATGCA3′) were used. The results are summarized in Fig. 3C (right panel) and expressed as the relative genome copy number. There was a 50 and 71% decline for adenoviral vector AdFTC/hFIX-treated animals and fgAd/hFIX-treated animals, respectively, and in concordance with the hFIX expression levels, we observed a 91% loss of nonviral vector genomes (P ≤ 0.05, ANOVA analysis with a post-hoc Fisher test). For the control mice that were not subjected to a surgical two-thirds partial hepatectomy, stable hFIX concentrations in serum over the duration of the experiment were obtained (Fig. 3A). In summary, this study implicates that episomal adenoviral vector genomes have a more robust nuclear retention and that most of the vector DNA remaining in the liver after partial hepatectomy, unlike that observed with AAV or naked plasmid DNA, remained in mouse liver after regeneration. Several factors which influence genome stability may be responsible for adenoviral vector persistence during hepatocyte turnover. As schematically shown in Fig. 4, we present possible models that would mechanistically explain these results. The models include vector DNA which (i) has centromeric functions (Fig. 4A), (ii), contains nuclear retention signals (Fig. 4B), (iii) replicates as an episome (Fig. 4C), or (iv) integrates into the host genome (Fig. 4D) during the cell cycle.
FIG. 3.
Relative transgene expression levels in partially hepatectomized mice after delivery of AdFTC/hFIX, fgAd/hFIX, and L-ITR/hFIXmg into mouse liver. Transducing units (8 × 108) of AdFTC/hFIX (n = 8) and 109 transducing units of the fg adenoviral vector fgAd/hFIX (n = 8) were injected into the tail vein of 8-week-old C57BL/6 mice, and 20 μg of the linear DNA construct L-ITR/hFIXmg (n = 8) was injected via high-pressure tail vein injection. Two weeks postinjection, some mice were subjected to a two-thirds surgical partial hepatectomy (○, □, ▵), and the hFIX expression levels were monitored by hFIX ELISA. The livers of control mice which did not receive a partial hepatectomy were infused at the same time with the adenoviral vectors AdFTC/hFIX and fgAd/hFIX and linear DNA L-ITR/hFIX (•, ▪, ▴). (A) Comparison of the serum hFIX concentration in all groups as determined by enzyme-linked immunosorbent assay. The relative hFIX expression levels are shown as percentages relative to their levels at the time of partial hepatectomy. (B) The left panel demonstrates the relative hFIX expression levels for the adenoviral vectors AdFTC/hFIX (○, •) and fgAd/hFIX (□, ▪), and the right panel shows the relative hFIX levels for the linear nonviral vector L-ITR/hFIXmg (▵, ▴). (C) Relative loss of adenoviral and nonviral DNA molecules after a surgical two-thirds partial hepatectomy. The total vector genome copy number per 10 ng of liver genomic DNA pre- and post-partial hepatectomy for the HD vector AdFTC, the fg adenoviral vector fgAd/hFIX, and the nonviral vector L-ITR/hFIXmg was determined by a quantitative real-time PCR assay. The left panel shows the total genome copy number per 10 ng of genomic DNA, and the right panel summarizes the relative number of vector DNA molecules pre- and post-partial hepatectomy. The asterisk indicates that there was a significant difference in the drop of hFIX expression levels or genome copy numbers compared to the other groups (P ≤ 0.05, ANOVA with a post-hoc Fisher's test).
FIG. 4.
Potential mechanisms which may cause episomal persistence of adenoviral vector genomes during cell cycling in vivo are shown schematically. Four different mechanisms are proposed. (A) The adenoviral vector genome functions as a centromere. During cell division the spindle apparatus binds to the adenoviral vector genome, and it will be maintained in one of the daughter cells. (B) The adenoviral vector genome has a stronger nuclear retention mechanism than nonviral DNA. Adenoviral DNA molecules are tightly attached to the host DNA chromatin via the adenoviral terminal protein; thus, they will be maintained during cell division. (C) The episomal adenoviral genome replicates. (D) Integration of the adenoviral genome into the host genome occurs during the cell cycle in vivo.
In a previous study we found that for fg adenoviral vectors with a deletion of E1, adenoviral genome replication was not the major factor responsible for vector stability in quiescent cells in vitro and in vivo (26). We believe that these findings make replication of adenoviral vector genomes as a potential mechanism for persistence during cell cycling highly unlikely. Various studies demonstrated that the integration efficiency of adenoviral vector genomes occurs only at a very low efficiency (12). All of the evidence together implies that centromeric functions and/or nuclear retention signals in the adenoviral vector genome and not episomal replication or integration are responsible for vector persistence of adenoviral DNA molecules during cell cycle in vivo. Many eukaryotic viruses are maintained as extrachromosomal DNA molecules. There is strong evidence that, e.g., Epstein-Barr virus and bovine papillomavirus are attached to the chromosome (5, 35) to ensure proper segregation of the viral DNA and nuclear retention during cell division. A similar mechanism might be responsible for persistence of adenoviral vector genomes during the cell cycle in mouse hepatocytes.
Since the fg adenoviral vector and the HD vector share similar features during induced cell cycle in vivo, it will be of great interest to analyze which elements in the two vectors have similar functions with regard to maintenance of vector genomes during cell division. For example, it was demonstrated that elements in the fg adenoviral vector might function as matrix attachment regions (31), which would be one common element which exists in both the fg and the HD vector. The HD adenoviral vector genome used in this study contains immunoglobulin κMAR (27) as a matrix attachment region and a centromeric region of alphoid repeat DNA from human chromosome 17 (16) as an origin of replication. It was shown that if a mammalian origin of replication and matrix attachment regions are placed in a single nonviral vector, this combination is sufficient to provide episomal persistence during cell division in vitro (2). Another similarity between fg and HD vectors is that TP is covalently bound to the ITRs, which was shown to stabilize the adenoviral vector genome. TP is the proteolytical cleavage product of its precursor preterminal protein (pTP). It is known that pTP is involved in adenoviral vector replication by forming a heterodimer with the viral DNA polymerase, and there is strong evidence that the adenoviral vector genome is associated via pTP with the nuclear matrix, generating a replication complex (1, 9, 31). Moreover, it was demonstrated that pTP prolongs vector persistence in vivo (17). A valid model must take into account the more rapid rate of vector loss using adenovirus-based vectors than with other episomal vectors, such as some plasmid vectors and AAV, during the normal aging process, versus the more rapid loss of nonadenoviral episomal genomes during acute and robust hepatocellular regeneration. The terminal protein complexes may be directly bound the host DNA chromatin, as indicated in Fig. 4B, or indirectly via the nuclear matrix (1, 9, 31), allowing the vector genomes to be retained during regeneration, but the protein may have a finite half-life in vivo that over time, when lost, destabilizes the adenoviral genome, resulting in a slow decline in vector genomes over time. At this time we favor such a mechanism, but further studies will be required to definitively establish this model. New insights into the persistence of episomal vector DNA may be useful for the development of improved episomal nonviral vectors. Taken together, this work provides new insight into adenoviral biology as an episomal system and shows that HD adenoviral vectors are a powerful tool for gene therapy approaches.
Acknowledgments
We thank Leonard Meuse for technical assistance.
A.E. is a recipient of the Judith Graham Pool postdoctoral fellowship by the National Hemophilia Foundation. This work was supported by NIH grant DK49022.
REFERENCES
- 1.Angeletti, P. C., and J. A. Engler. 1998. Adenovirus preterminal protein binds to the CAD enzyme at active sites of viral DNA replication on the nuclear matrix. J. Virol. 72:2896-2904. [DOI] [PMC free article] [PubMed]
- 2.Baiker, A., C. Maercker, C. Piechaczek, S. B. Schmidt, J. Bode, C. Benham, and H. J. Lipps. 2000. Mitotic stability of an episomal vector containing a human scaffold/matrix-attached region is provided by association with nuclear matrix. Nat. Cell Biol. 2:182-184. [DOI] [PubMed] [Google Scholar]
- 3.Barr, D., J. Tubb, D. Ferguson, A. Scaria, A. Lieber, C. Wilson, J. Perkins, and M. A. Kay. 1995. Strain related variations in adenovirally mediated transgene expression from mouse hepatocytes in vivo: comparisons between immunocompetent and immunodeficient inbred strains. Gene Ther. 2:151-155. [PubMed] [Google Scholar]
- 4.Brough, D. E., C. Hsu, V. A. Kulesa, G. M. Lee, L. J. Cantolupo, A. Lizonova, and I. Kovesdi. 1997. Activation of transgene expression by early region 4 is responsible for a high level of persistent transgene expression from adenovirus vectors in vivo. J. Virol. 71:9206-9213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Calos, M. P. 1998. Stability without a centromere. Proc. Natl. Acad. Sci. USA 95:4084-4085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chen, Z. Y., S. R. Yant, C. Y. He, L. Meuse, S. Shen, and M. A. Kay. 2001. Linear DNAs concatemerize in vivo and result in sustained transgene expression in mouse liver. Mol. Ther. 3:403-410. [DOI] [PubMed] [Google Scholar]
- 7.Ehrhardt, A., and M. A. Kay. 2002. A new adenoviral helper-dependent vector results in long-term therapeutic levels of human coagulation factor IX at low doses in vivo. Blood 99:3923-3930. [DOI] [PubMed] [Google Scholar]
- 8.Ehrhardt, A., D. P. Peng, H. Xu, L. Meuse, and M. A. Kay. 2003. Optimization of cis-acting elements for gene expression from nonviral vectors in vivo. Hum. Gene Ther. 14:215-225. [DOI] [PubMed] [Google Scholar]
- 9.Fredman, J. N., and J. A. Engler. 1993. Adenovirus precursor to terminal protein interacts with the nuclear matrix in vivo and in vitro. J. Virol. 67:3384-3395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Grave, L., D. Dreyer, A. Dieterle, P. Leroy, A. I. Michou, C. Doderer, A. Pavirani, M. Lusky, and M. Mehtali. 2000. Differential influence of the E4 adenoviral genes on viral and cellular promoters. J. Gene Med. 2:433-443. [DOI] [PubMed] [Google Scholar]
- 11.Hartigan-O'Connor, D., C. Barjot, R. Crawford, and J. S. Chamberlain. 2002. Efficient rescue of gutted adenovirus genomes allows rapid production of concentrated stocks without negative selection. Hum. Gene Ther. 13:519-531. [DOI] [PubMed] [Google Scholar]
- 12.Harui, A., S. Suzuki, S. Kochanek, and K. Mitani. 1999. Frequency and stability of chromosomal integration of adenovirus vectors. J. Virol. 73:6141-6146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Harvey, B. G., S. Worgall, S. Ely, P. L. Leopold, and R. G. Crystal. 1999. Cellular immune responses of healthy individuals to intradermal administration of an E1-E3- adenovirus gene transfer vector. Hum. Gene Ther. 10:2823-2837. [DOI] [PubMed] [Google Scholar]
- 14.Kay, M. A., C. N. Landen, S. R. Rothenberg, L. A. Taylor, F. Leland, S. Wiehle, B. Fang, D. Bellinger, M. Finegold, A. R. Thompson, et al. 1994. In vivo hepatic gene therapy: complete albeit transient correction of factor IX deficiency in hemophilia B dogs. Proc. Natl. Acad. Sci. USA 91:2353-2357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kim, I. H., A. Jozkowicz, P. A. Piedra, K. Oka, and L. Chan. 2001. Lifetime correction of genetic deficiency in mice with a single injection of helper-dependent adenoviral vector. Proc. Natl. Acad. Sci. USA 98:13282-13287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Krysan, P. J., S. B. Haase, and M. P. Calos. 1989. Isolation of human sequences that replicate autonomously in human cells. Mol. Cell. Biol. 9:1026-1033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lieber, A., C. Y. He, and M. A. Kay. 1997. Adenoviral preterminal protein stabilizes mini-adenoviral genomes in vitro and in vivo. Nat. Biotechnol. 15:1383-1387. [DOI] [PubMed] [Google Scholar]
- 18.Lieber, A., C. Y. He, L. Meuse, D. Schowalter, I. Kirillova, B. Winther, and M. A. Kay. 1997. The role of Kupffer cell activation and viral gene expression in early liver toxicity after infusion of recombinant adenovirus vectors. J. Virol. 71:8798-8807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Liu, F., Y. Song, and D. Liu. 1999. Hydrodynamics-based transfection in animals by systemic administration of plasmid DNA. Gene Ther. 6:1258-1266. [DOI] [PubMed] [Google Scholar]
- 20.Lusky, M., M. Christ, K. Rittner, A. Dieterle, D. Dreyer, B. Mourot, H. Schultz, F. Stoeckel, A. Pavirani, and M. Mehtali. 1998. In vitro and in vivo biology of recombinant adenovirus vectors with E1, E1/E2A, or E1/E4 deleted. J. Virol. 72:2022-2032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Miao, C. H., K. Ohashi, G. A. Patijn, L. Meuse, X. Ye, A. R. Thompson, and M. A. Kay. 2000. Inclusion of the hepatic locus control region, an intron, and untranslated region increases and stabilizes hepatic factor IX gene expression in vivo but not in vitro. Mol. Ther. 1:522-532. [DOI] [PubMed] [Google Scholar]
- 22.Miao, C. H., A. R. Thompson, K. Loeb, and X. Ye. 2001. Long-term and therapeutic-level hepatic gene expression of human factor IX after naked plasmid transfer in vivo. Mol. Ther. 3:947-957. [DOI] [PubMed] [Google Scholar]
- 23.Morral, N., W. O'Neal, K. Rice, M. Leland, J. Kaplan, P. A. Piedra, H. Zhou, R. J. Parks, R. Velji, E. Aguilar-Cordova, S. Wadsworth, F. L. Graham, S. Kochanek, K. D. Carey, and A. L. Beaudet. 1999. Administration of helper-dependent adenoviral vectors and sequential delivery of different vector serotype for long-term liver-directed gene transfer in baboons. Proc. Natl. Acad. Sci. USA 96:12816-12821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Morsy, M. A., M. Gu, S. Motzel, J. Zhao, J. Lin, Q. Su, H. Allen, L. Franlin, R. J. Parks, F. L. Graham, S. Kochanek, A. J. Bett, and C. T. Caskey. 1998. An adenoviral vector deleted for all viral coding sequences results in enhanced safety and extended expression of a leptin transgene. Proc. Natl. Acad. Sci. USA 95:7866-7871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nakai, H., S. R. Yant, T. A. Storm, S. Fuess, L. Meuse, and M. A. Kay. 2001. Extrachromosomal recombinant adeno-associated virus vector genomes are primarily responsible for stable liver transduction in vivo. J. Virol. 75:6969-6976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nelson, J. E., and M. A. Kay. 1997. Persistence of recombinant adenovirus in vivo is not dependent on vector DNA replication. J. Virol. 71:8902-8907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Okada, S., K. Tsutsui, S. Seki, and T. Shohmori. 1996. Subdomain structure of the matrix attachment region located within the mouse immunoglobulin kappa gene intron. Biochem. Biophys. Res. Commun. 222:472-477. [DOI] [PubMed] [Google Scholar]
- 28.Park, F., K. Ohashi, W. Chiu, L. Naldini, and M. A. Kay. 2000. Efficient lentiviral transduction of liver requires cell cycling in vivo. Nat. Genet. 24:49-52. [DOI] [PubMed] [Google Scholar]
- 29.Parks, R. J., J. L. Bramson, Y. Wan, C. L. Addison, and F. L. Graham. 1999. Effects of stuffer DNA on transgene expression from helper-dependent adenovirus vectors. J. Virol. 73:8027-8034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Reddy, P. S., K. Sakhuja, S. Ganesh, L. Yang, D. Kayda, T. Brann, S. Pattison, D. Golightly, N. Idamakanti, A. Pinkstaff, M. Kaloss, C. Barjot, J. S. Chamberlain, M. Kaleko, and S. Connelly. 2002. Sustained human factor VIII expression in hemophilia A mice following systemic delivery of a gutless adenoviral vector. Mol. Ther. 5:63-73. [DOI] [PubMed] [Google Scholar]
- 31.Schaak, J., P. Schedl, and T. Shenk. 1990. Transcription of adenovirus and HeLa cell genes in the presence of drugs that inhibit topoisomerase I and II function. Nucleic Acids Res. 18:1499-1508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Schiedner, G., N. Morral, R. J. Parks, Y. Wu, S. C. Koopmans, C. Langston, F. L. Graham, A. L. Beaudet, and S. Kochanek. 1998. Genomic DNA transfer with a high-capacity adenovirus vector results in improved in vivo gene expression and decreased toxicity. Nat. Genet. 18:180-183. [DOI] [PubMed] [Google Scholar]
- 33.Schowalter, D. B., C. L. Himeda, B. L. Winther, C. B. Wilson, and M. A. Kay. 1999. Implication of interfering antibody formation and apoptosis as two different mechanisms leading to variable duration of adenovirus-mediated transgene expression in immune-competent mice. J. Virol. 73:4755-4766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Van Linthout, S., D. Collen, and B. De Geest. 2002. Effect of promoters and enhancers on expression, transgene DNA persistence, and hepatotoxicity after adenoviral gene transfer of human apolipoprotein a-I. Hum. Gene Ther. 13:829-840. [DOI] [PubMed] [Google Scholar]
- 35.Voitenleitner, C., and M. Botchan. 2002. E1 protein of bovine papillomavirus type 1 interferes with E2 protein-mediated tethering of the viral DNA to mitotic chromosomes. J. Virol. 76:3440-3451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wadsworth, S. C., H. Zhou, A. E. Smith, and J. M. Kaplan. 1997. Adenovirus vector-infected cells can escape adenovirus antigen-specific cytotoxic T-lymphocyte killing in vivo. J. Virol. 71:5189-5196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zhang, G., V. Budker, and J. A. Wolff. 1999. High levels of foreign gene expression in hepatocytes after tail vein injections of naked plasmid DNA. Hum. Gene Ther. 10:1735-1737. [DOI] [PubMed] [Google Scholar]
- 38.Zhang, Y., N. Chirmule, G. P. Gao, R. Qian, M. Croyle, B. Joshi, J. Tazelaar, and J. M. Wilson. 2001. Acute cytokine response to systemic adenoviral vectors in mice is mediated by dendritic cells and macrophages. Mol. Ther. 3:697-707. [DOI] [PubMed] [Google Scholar]