Abstract
Retroviruses must gain access to the host cell nucleus for subsequent replication and viral propagation. Human immunodeficiency virus type 1 (HIV-1) and other primate lentiviruses are distinguished from the gammaretroviruses by their ability to infect nondividing cells such as macrophages, an important viral reservoir in vivo. Rather than requiring nuclear membrane breakdown during cell division, the HIV-1 preintegration complex (PIC) enters the nucleus by traversing the central aqueous channel of the limiting nuclear pore complex. The HIV-1 PIC contains three nucleophilic proteins, matrix, integrase, and Vpr, all of which have been implicated in nuclear targeting. The mechanism by which Vpr can display such nucleophilic properties and yet also be available for incorporation into virions assembling at the plasma membrane is unresolved. We recently characterized Vpr as a nucleocytoplasmic shuttling protein that contains two novel nuclear import signals and an exportin-1-dependent nuclear export signal (NES). We now demonstrate that mutation of this NES impairs the incorporation of Vpr into newly formed virions. Furthermore, we find that the Vpr NES is required for efficient HIV replication in tissue macrophages present in human spleens and tonsils. These findings underscore how the nucleocytoplasmic shuttling of Vpr not only contributes to nuclear import of the HIV-1 PIC but also enables Vpr to be present in the cytoplasm for incorporation into virions, leading to enhancement of viral spread within nondividing tissue macrophages.
Human immunodeficiency virus type 1 (HIV-1) and other primate lentiviruses are able to infect nondividing cells, notably terminally differentiated macrophages (41), an important viral reservoir within the infected host (31, 34, 53). This biological feature distinguishes the lentiviruses from the oncoretroviruses (or gammaretroviruses), in which cell division associated with nuclear membrane dissolution is required for infection (33, 42, 62). HIV-1 is also able to infect resting, nondividing T cells in lymphoid tissues (13). These nondividing T cells may contribute to the establishment of protected reservoirs in the host, undermining attempts to eradicate virus-producing cells in the long term (5, 6, 15, 56, 77).
After entry by fusion and uncoating, the viral reverse transcriptase complex traverses the cytoplasm while reverse transcribing the two strands of RNA into DNA (20), forming the viral preintegration complex (PIC). The nuclear envelope forms a barrier that the PIC must negotiate. The nuclear envelope is studded with nuclear pore complexes (NPCs) that form a conduit with a central aqueous channel mediating bidirectional transport of many macromolecules. The NPC corresponds to a 125-MDa structure comprising 50 to 100 polypeptides. Many of these proteins are members of the nucleoporin family characterized by FG repeats (46). During active transport, the central aqueous channel accommodates protein complexes as large as 25 nm in diameter. However, the HIV-1 PIC exhibits a Stokes diameter of 56 nm and represents one of the largest known cargoes successfully transported across the NPC (48). How HIV-1 performs this feat of “molecular gymnastics” remains unknown (66).
All retroviruses contain three major open reading frames, including gag (generates the viral core after intravirion processing of the p55gag precursor polypeptide), pol (encodes the reverse transcriptase, integrase, and protease enzymes), and env (directs the production of the transmembrane and surface glycoproteins). In addition, the primate lentiviruses contain genes for regulatory (rev and tat) and accessory (vpr, vpx, vpu, vif, and nef) proteins. Viral protein R (Vpr) is highly conserved in vivo (47, 65) and serves many functions in the viral life cycle yet is frequently lost during in vitro propagation of the virus (25, 80), highlighting an experimental limitation of such in vitro culture systems.
Vpr induces G2 cell cycle arrest in proliferating human cells when it is overexpressed (1, 25, 28, 60) or in the context of infection with a recombinant vector (37). This effect correlates with the production of herniations in the nuclear envelope (10). Arrest in the G2 phase of the cell cycle enhances viral replication, in part by increasing the activity of the long terminal repeat (25). Other studies suggest that the prolonged G2 arrest induced by Vpr promotes apoptosis of the infected cell, perhaps leading to increased virion release and enhanced viral burden (57, 70-72, 78). Although it has been suggested that Vpr inhibits apoptosis early and promotes apoptosis late during the course of HIV-1 infection (9), we have not observed a consistent effect of Vpr on T-cell depletion in infected lymphoid histocultures (14).
Structural studies performed with full-length, synthetic Vpr indicate that this 96-amino-acid, 14-kDa protein contains a helix-turn-helix domain between residues 17 and 50 and an α-helical stretch between residues 53 and 78 (30). The carboxy-terminal region of Vpr corresponds to an arginine-rich segment that can influence the stability and, potentially, the structure of the entire protein (79). In a previous study, we defined two unique nuclear targeting signals within Vpr: one residing within the arginine-rich carboxy-terminal segment and a second that depends on highly conserved leucines present in the two α-helical regions (35, 64). The distal leucine-rich helix also contains a nuclear export signal (NES) (64). This NES utilizes the chromosome maintenance region 1 protein (CRM1) (17, 52), which binds to the leucine-rich NES directly and mediates export through the NPC in a leptomycin B-sensitive manner (51, 76). However, the biological significance of the nucleocytoplasmic shuttling properties of Vpr remains unknown.
Vpr is predominantly found in the nuclei of HIV-1-infected cells (43), probably reflecting the strength of its two nuclear targeting signals (64). This nucleophilic property of Vpr, coupled with its presence in the viral PIC, led to the observation that Vpr facilitates more efficient HIV-1 replication in nondividing monocyte-derived macrophages (4, 8, 22, 29). In vitro assays further supported a direct role for Vpr in PIC import (58, 59). However, more recent studies have questioned the role of Vpr in the nuclear uptake of the HIV-1 PIC (2) and have also shown that Vpr is not required for HIV infection of nondividing T cells (14). Further, it is unclear how this nucleophilic protein is incorporated into virions, which are assembled in the cytoplasm at or near the plasma membrane.
In this study, we investigated whether the nuclear export function of Vpr contributes to virion incorporation and whether virion Vpr contributes to viral replication in tissue macrophages. For these studies, we have employed HIV molecular clones containing a mutation (L67A) in the distal helix of Vpr that selectively compromises the nuclear export phenotype of Vpr while maintaining its nuclear import and G2 cell cycle-arresting functions in HIV-1-infected peripheral blood mononuclear cells (PBMCs). We have evaluated the growth of wild-type and mutant viruses in both the T-cell and macrophage compartments of lymphoid histocultures produced with human tonsillar or splenic tissue. HIV infection in the ex vivo lymphoid histoculture system is likely to more closely approximate the conditions encountered in vivo than does infection of mitogen-stimulated PBMCs (23, 24). This tissue-based system is composed of a mixture of HIV-1 targets, including lymphocytes, macrophages, and the supporting cellular network. It requires no addition of cytokines or activating or differentiating agents like those used in more homogeneous primary cellular systems.
MATERIALS AND METHODS
Plasmids.
A hemagglutinin (HA) epitope was introduced at the amino terminus of NL4-3 Vpr to form HA-Vpr as previously described (65). For subcellular localization studies, we used a green fluorescence protein (GFP)-pyruvate kinase (PK)-Vpr chimera (64). This GFP-PK-Vpr fusion protein or relevant Vpr mutants allowed subcellular localization by fluorescence microscopy. Since the backbone is larger (≈90 kDa) than the passive diffusion size of the NPC (≈60 kDa), its import and export occur by active transport (52). The nuclear localization sequence (NLS)-GFP-PK-Vpr construct has been characterized (64), and an L67A mutation was derived by cloning the NLS (PKKKRKV) from the simian virus 40 (SV40) large T antigen at the amino terminus of the GFP-PK-VprL67A chimera (64). HIV-1 infection experiments were performed using HIV-1107, composed of an NL4-3 viral backbone modified to contain the V1 to V3 loop from the envelope of the chemokine receptor 5 (CCR5)-dependent primary isolate, Ba-L (73). This modification enables the 107 virus to infect macrophages and CCR5+ CD4+ T cells. The 107 virus containing the VprL67A point mutation was constructed by using paired PCR primers overlapping the EcoRI site and a downstream NheI site located distal to the end of the V3 loop. This amplicon was cloned directly into the digested wild-type viral construct and sequenced to verify that only this single point mutation had been introduced. The construction of the HIV-1 NL4-3 ΔVpr strain has been described previously (14).
Cell cultures, transfections, and microscopic analysis.
Expression vector DNA was transfected into HeLa or 293T cells with calcium phosphate. Cells were cultured in Dulbecco modified Eagle medium (GIBCO BRL, Gaithersburg, Md.) supplemented with 10% fetal bovine serum, 2 mM l-glutamine, penicillin G (100 U/ml), and streptomycin (100 μg/ml). All plasmids were transfected with either 4 μg of DNA per well of six-well plates or 3 μg in experiments incorporating 1 μg of an expression vector encoding the red fluorescent protein (RFP) (pDsRed1-N1) (Clontech, Palo Alto, Calif.). Cells plated on coverslips for microscopic analyses were washed with phosphate-buffered saline, fixed for 10 min in 1% paraformaldehyde, and rinsed in water. The coverslips were then inverted and mounted on glass slides using Gel Mount (Biomeda Corp., Foster City, Calif.). Nuclei were visualized with Hoechst 33342 stain (10 μg/ml; Molecular Probes, Eugene, Oreg.) added to the paraformaldehyde. Cells were analyzed with a Nikon TE 300 Quantum fluorescence microscope and a Hamamatsu Orca II charge-coupled device camera.
Heterokaryon analyses.
Heterokaryons were generated as described previously (64, 69). Briefly, transfected 293T cells were cultured for 24 h, washed, trypsinized, and replated overnight at a 1:10 ratio with excess untransfected HeLa cells to achieve a total cell concentration of 1.5 × 106 per well. The cells were exposed to cycloheximide (25 μg/ml) for 1 h to prevent de novo protein synthesis and then subjected to membrane fusion by the addition of 50% polyethylene glycol for 3 min. The cells were washed with phosphate-buffered saline (PBS) and incubated for 1 h with cycloheximide. The pDsRed1-N1 vector expressing RFP was also included in the transfections. RFP localizes in the nucleus and cytoplasm of the donor cell and diffuses into the entire cytoplasm of the newly formed heterokaryon, thus delineating its boundaries. However, while RFP is present in the donor nucleus of the heterokaryon, it does not enter the newly fused nuclei (recipient nuclei) during this 2-h procedure. Thus, it is possible to discern readily whether the test protein linked to the GFP shuttles from the donor nucleus (red) to the recipient nuclei (unstained) within the heterokaryon.
Western blot and coimmunoprecipitation analyses.
Coimmunoprecipitation experiments were performed by cotransfecting the HA-Vpr vector with Pr55ΔMA-GFP (11), which contains the p6 domain that mediates Vpr binding. Pr55ΔMA-GFP lacks the RNA retention signal in MA that inhibits RNA export and subsequent expression (63). An equivalent number of 293T cells (600,000 cells) were transfected and harvested 48 h later in 500 μl of lysis buffer (50 mM HEPES [pH 7.9], 250 mM NaCl, 0.5% NP-40 detergent, 0.5 mM EDTA supplemented with protease inhibitor [Roche] [1 tablet/ml], 100 μM phenylmethylsulfonyl fluoride). Lysates were aliquoted for direct analysis or incubated with monoclonal mouse antibody HA.11 immobilized on Sepharose Fast Flow beads (Covance) for 15 h at 4°C and then washed three times with lysis buffer. The beads were boiled for 5 min in loading buffer to dissociate any bound proteins before analysis by sodium dodecyl sulfate-polyacrylamide gel electrophoresis. Western blotting was performed with monoclonal anti-HA (Boehringer Manheim), polyclonal anti-GFP (Clontech), monoclonal anti-p24gag (NEN) or polyclonal rabbit anti-Vpr (35) antiserum diluted 1:2,000.
PBMC isolation and infection.
HIV-seronegative blood was obtained by leukopheresis, and mononuclear cells were isolated on Histopaque-1077, washed with PBS, and activated with phytohemagglutinin (Sigma, St. Louis, Mo.) at 50 μg/ml. After 24 h, the cells were washed and cultured in RPMI supplemented with interleukin-2 (10 U/ml; Roche). Cultures were infected with HIV-1 by resuspending 107 cells and 200 ng of viral stocks in 1 ml of medium for 2 h before washing them and culturing them at 106 cells/ml.
Cell cycle analyses.
Cell cycle experiments were performed with pEGFP expression vector (Clontech) cotransfected into 293T cells at a 1:7 ratio with the indicated HA control or HA-Vpr expression vector. After culture for 24 to 48 h, the cells were trypsinized and fixed for 30 min in 2% formaldehyde. The cells were next washed with PBS and treated with 1 mg of RNase A per ml plus 0.01 mM To-Pro-3 iodide (Molecular Probes) in PBS for 30 min. Cellular DNA content in the transfected (GFP+) and untransfected (GFP−) cells was assessed with a FACScan flow cytometer. DNA profiles were analyzed with FlowJo software (Treestar). PBMCs, after 5 days of infection, were processed similarly except that fixation and permeabilization were performed with a solution of 1% paraformaldehyde, 1 mg of human immunoglobulin G per ml, and 0.1% Tween 20 in fluorescence-activated cell sorter buffer (PBS with 2% fetal calf serum). Lymphocytes were analyzed by first gating on live, cycling cells as determined by cell size (forward scatter) and granularity (side scatter) and by DNA content with mock-infected controls. This cycling gate was then interrogated identically between the different infections for intracellular Gag polyprotein expression with the KC57 monoclonal anti-p24 antibody (1:50 dilution) (Coulter).
Preparation of viral stocks.
Molecular clones of HIV-1 proviruses were transfected into 293T cells as described above, and the culture supernatants were collected after 48 h. These viral preparations were centrifuged for 10 min at 5,000 rpm in a Beckman GH 3.7 rotor before being aliquoted and frozen. The p24gag concentration was determined by a standard enzyme-linked immunosorbent assay (NEN), and the 50% tissue culture infective dose (TCID50) was determined by limiting dilution using pooled, phytohemagglutinin-activated PBMC cultures as targets (23, 55). Virus (300 ng of p24gag) was collected by ultracentrifugation at 40,000 × g for 90 min to assess virion incorporation of Vpr.
Culture and infection of human lymphoid tissues ex vivo.
The ex vivo lymphoid histoculture system was used to more closely approximate HIV infection events occurring in vivo. This system supports viral infection and replication in the absence of exogenous cytokine stimulation and provides the diverse array of cells present in normal lymphoid tissues (23, 26, 55). Noninflammatory spleen or tonsil tissue (obtained from the National Disease Research Interchange) was sectioned into 2- to 3-mm blocks and cultured as described previously (23). Six blocks per well were inoculated with HIV-1 by dropwise addition of 50 TCID50. At the indicated times, the medium was collected from the wells to monitor the replication kinetics or the tissue was mechanically disrupted and subjected to flow cytometric analysis as described above for PBMCs. In addition, cells derived from lymphoid tissue were immunostained with various antibodies, including anti-CD3, anti-CD4, anti-CD14, and anti-CD68. Replicates represent data collected from three separate wells.
RESULTS
Segregation of the Vpr NES from the overlapping nuclear import signal.
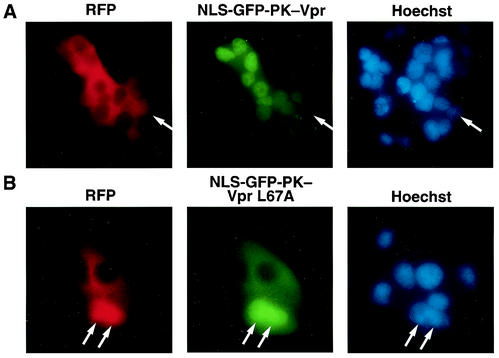
We previously mapped an NES to the leucine-rich domain (LQQLL) of the distal helix spanning amino acids 64 to 68 of Vpr (64). Our mutational analysis further revealed that the L67A analogue was effectively imported into the nucleus but that L64A and L68A Vpr analogues were not. We next investigated the nuclear export properties of the L67A mutant. Wild-type and L67A Vpr were fused at the carboxy terminus of the NLS-GFP-PK chimera, and heterokaryon shuttling studies were performed (Fig. 1). The fusion protein containing wild-type Vpr effectively relocalized into the acceptor nuclei in the heterokaryons, indicating effective shuttling. However, a chimera containing the VprL67A mutant failed to exit the donor nucleus. GFP-PK-VprL67A localizes to the nucleus after addition of the export inhibitor leptomycin B (64) but appears predominantly in the cytoplasm at baseline, indicating that some degree of the nuclear export function is retained (data not shown). However, when measured against the import strength of the SV40 NLS, as shown in these experiments, the nuclear export properties of the L67A Vpr mutant were significantly compromised.
FIG. 1.
Identification of a Vpr mutation that segregates nuclear import from nuclear export. The SV40 NLS was cloned into the GFP-PK-Vpr fusion protein to direct this predominantly cytoplasmic shuttling protein into the nucleus. Cells were fused with polyethylene glycol in the presence of cycloheximide (to prevent de novo production of Vpr in the cytoplasm) to examine whether the fusion protein, although on average appearing nuclear, was actually shuttling as indicated by exit from the donor nucleus and accumulation in the recipient nucleus. RFP was included to demarcate the boundaries of the heterokaryon and to mark the transfected (donor) nucleus red (arrows; also shown in the Hoechst staining panel) but not the recipient nuclei (devoid of RFP) introduced by cell fusion. Note that the NLS-GFP-PK-Vpr was able to exit the donor nucleus and accumulate in the recipient nuclei (A) while the L67A mutant was defective for such shuttling (B).
The VprL67A export mutant causes G2 cell cycle arrest.
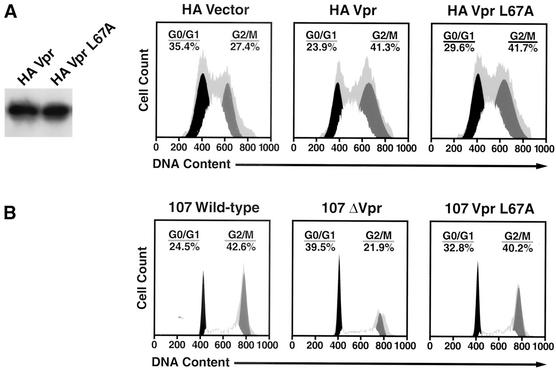
To assess whether the L67A mutant interferes with the G2-arresting properties of Vpr, an HA-VprL67A expression vector was prepared and introduced into 293T cells. Western blotting revealed that wild-type Vpr and the VprL67A mutant were expressed at comparable levels (Fig. 2A). Cell cycle analysis showed that the levels of G2 cell cycle arrest were similar in cells transfected with wild-type and L67A export mutant Vpr. To verify that this mutant causes cell cycle arrest at levels achieved during HIV-1 infection of primary cells, we generated proviral molecular clones containing the single VprL67A point mutation or lacking Vpr altogether. Stocks of wild-type 107, 107L67A, and 107ΔVpr generated after transfection of 293T cells were equally infectious as determined by TCID50/p24gag content (data not shown). PBMCs were infected with these viruses, and the cell cycle profiles were assessed in the p24gag-positive lymphocytes (those productively infected and making the p55gag precursor of p24gag) and p24gag-negative lymphocytes by analyzing their DNA contents (Fig. 2B). The 107- and 107VprL67A-infected lymphocytes displayed similar increases in the percentage of cells with a 4N complement of DNA compared with 107ΔVpr-infected lymphocytes, indicating retention of G2-arresting properties by the L67A mutant in these primary cells.
FIG. 2.
The VprL67A mutant induces cell cycle arrest. Cells were analyzed for DNA content, and cell cycle profiles were determined with FlowJo software using the Watson Pragmatic model. Primary data are represented by the gray background. (A) Both wild-type Vpr and the L67A-Vpr export mutant were expressed at comparable levels (left panel) in 293T cells and induced a similar accumulation of cells at the G2/M interface, characterized by a 4N complement of DNA. (B) The Vpr L67A export mutant was introduced as a single amino acid change in the HIV-1 CCR5-dependent 107 proviral backbone. PBMCs were infected for 5 days and stained to assess intracellular p24 production, and the infected lymphocytes (anti-p24gag+) were analyzed for DNA content. Note the significant accumulation of cells paused or arrested at the G2/M phase of the cell cycle for lymphocytes infected with the wild-type and VprL67A mutant viruses, compared with the control 107ΔVpr virus.
Compromise of Vpr export results in decreased incorporation of Vpr into virions.
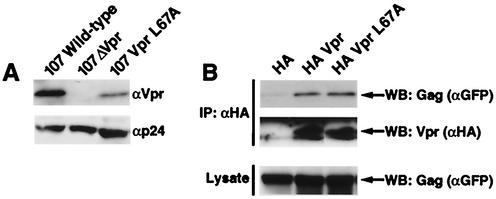
One possible function for Vpr nuclear export is to ensure that there are adequate amounts of this protein in the cytoplasmic compartment to allow its incorporation into new virions. Since 107, 107VprL67A, and 107ΔVpr were equally infectious in PBMC cultures, similar amounts of viral particles, as measured by p24gag, were pelleted and examined for virion-associated Vpr (Fig. 3A). Markedly less (<3%) VprL67A than wild-type Vpr was associated with virions. However, these findings do not exclude the possibility that the L67A mutation interfered with the binding of Vpr to the p6 component of the p55gag precursor and, as a result, was not effectively recruited to the virion. Although previous mapping studies indicated that the p6-binding domain of Vpr is located in the helix-turn-helix domain at the amino terminus (44, 45), we performed immunoprecipitation experiments to directly test whether the L67A mutant binds as well as p6 wild-type Vpr does (Fig. 3B). These studies revealed indistinguishable levels of binding of VprL67A and wild-type Vpr to Gag as measured with the Pr55ΔMA-GFP fusion protein. Immunoprecipitation and immunoblotting confirmed that similar amounts of HA-tagged Vpr and Gag proteins were expressed under each of these conditions.
FIG. 3.
The Vpr L67A nuclear export mutant is less efficiently incorporated into newly formed virions yet retains the ability to bind to Gag. (A) Virions were collected by centrifugation and analyzed by sodium dodecyl sulfate-polyacrylamide gel electrophoresis. Levels of p24 Gag are shown in the lower panel, and levels of intravirion Vpr are shown in the upper panel. Note the substantial decrease (>97%) in the amount of VprL67A associated with the virion compared with wild-type Vpr (top row). (B) HA-tagged Vpr constructs were cotransfected with p55ΔMAGFP. This vector encodes a large segment of Gag, including the p6 domain, but does not require Rev for its expression. Immunoprecipitation (IP, top two rows) of exogenously expressed HA fusion proteins and subsequent immunoblotting (WB) were used to evaluate the interaction of wild-type Vpr and the Vpr L67A analogue with Gag. Expression levels of the Vpr proteins and the Gag fusion protein are shown in the lower two panels. Note the equivalent interaction of VprL67A and wild-type Vpr with the Gag fusion protein (top panel).
Vpr export is required for efficient infection of macrophages present in human lymphoid tissues.
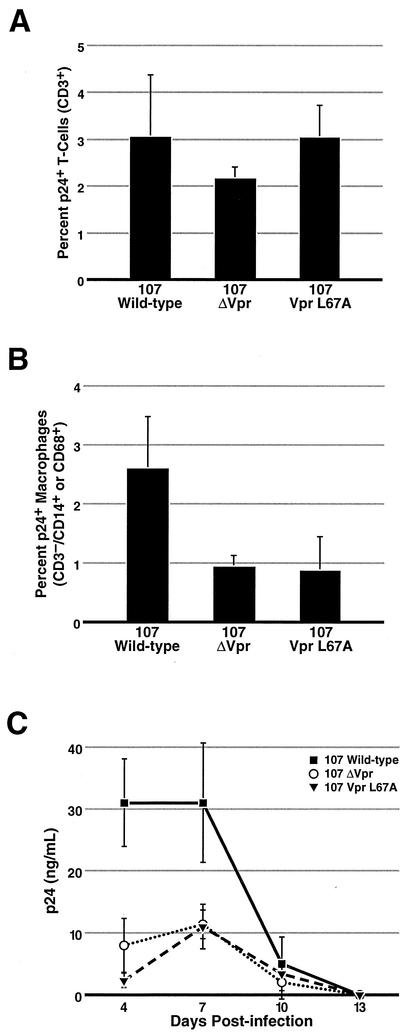
Spleen and tonsil tissue offer an important tool to study the replication of HIV-1 in what appears to be a more physiologically relevant system. As is true for activated PBMC infection, we observed no dependence on Vpr for replication of CXCR4-dependent viruses (14). Consistent with this observation, examination of the number of infected T cells by intracellular anti-p24gag immunostaining showed no dependence on Vpr for CCR5-dependent viral infection of CD4+ T lymphocytes (Fig. 4A). However, when the number of HIV-producing macrophages was assessed, a 60% reduction was consistently observed with the CCR5-dependent virus containing the Vpr export mutant (L67A) compared to wild-type virus. Viruses lacking Vpr displayed an equivalent decrease. Since both nuclear import and G2 cell cycle arrest are intact with VprL67A, these results suggest that nuclear export of Vpr is important for viral spread to macrophages present in lymphoid tissues. Because nuclear export appears to be important for incorporation of Vpr into virions, these data imply that Vpr is required during the initial phase of infection of target macrophages in these tissues. Of note, although macrophages represent only about 1% of the lymphoid tissue, we observed a 67% reduction in the amount of p24gag present in the media of these lymphoid histocultures after infection with virus containing the VprL67A export mutant or lacking Vpr altogether. These findings support the notion that macrophages are an important reservoir that disproportionately contribute to viral burden in these lymphoid tissues (14).
FIG. 4.
An HIV-1 molecular clone containing the Vpr L67A export mutant can infect T cells efficiently but is as defective as ΔVpr HIV-1 for infection of tissue macrophages. Lymphoid histocultures prepared from tonsil and spleen (represented here) were infected with 50 TCID50 per block of tissue with the indicated CCR5-dependent 107 virus. After 7 to 10 days, the blocks were mechanically dispersed and examined by fluorescence-activated cell sorting using the indicated cell surface markers and intracellular anti p24gag staining. There were six blocks per well, and error bars represent the standard deviation for three wells. (A) There was no difference in the number of infected T cells (CD3+ lymphocytes) in the presence or absence of Vpr. (B) A 60% reduction in the number of infected macrophages (CD3− CD14+ and/or CD68+) was observed for the ΔVpr and the VprL67A mutants compared with viruses expressing wild-type Vpr. (C) Supernatants of these cultures were colleted over the course of the experiment and analyzed for p24gag levels by enzyme-linked immunosorbent assay. There was a 67% decrease in the amount of secreted p24gag in the cultures infected with the ΔVpr or the VprL67A virus compared with the wild-type virus. These results were reproduced in more than four independent experiments with human spleen and tonsil tissue.
DISCUSSION
This study shows that the nuclear export property of the nucleocytoplasmic shuttling protein Vpr is required for efficient incorporation of Vpr into virions, which is required for efficient HIV-1 replication in tissue macrophages. We studied a Vpr mutant (L67A) that is compromised in its nuclear export property but retains wild-type nuclear import and causes G2 cell cycle arrest. Using this mutant in the context of a molecular HIV clone to infect lymphoid histocultures derived from human tonsil or spleen tissue, we found that the limited incorporation of the Vpr L67A mutant into virions reduced the number of infected tissue macrophages by 60%. A similar reduction was obtained with viruses lacking the entire Vpr gene. In contrast, the L67A Vpr export mutant had no effect on levels of HIV infection of T cells within the same lymphoid cultures. Nevertheless, we observed a 67% reduction in viral production in these tissues, even though only 1% of the available cellular targets for infection are macrophages. These findings support the notion that both virion-associated Vpr and macrophage targets of viral replication are key factors in the overall HIV-1 load generated in these lymphoid tissues. Both HIV replication in macrophages and PIC import depend upon Vpr. This is further supported by the observation that the HIV-2/simian immunodeficiency virus SM Vpx protein aids in PIC import into the cell nucleus (16) and also enhances infections of macrophages in lymphoid tissues (14). These data also add support to the proposal that infected macrophages significantly contribute to the viral load of HIV-1-infected patients (14, 26, 31, 34, 53).
Vpr is incorporated into the HIV-1 PIC and is thought to facilitate its nuclear import in cooperation with the matrix (MA) (3, 22, 27, 75) and integrase (2, 21) proteins as well as the central DNA flap (81); however, the actual involvement of integrase and the central DNA flap has recently been questioned (12). The specific role of MA in PIC import has also been questioned (19, 50, 61) but may be partially explained by recognition that p55gag is a nucleocytoplasmic shuttling protein (11). Finally, even the role of Vpr during PIC import has been controversial. Vpr facilitates replication in monocyte-derived macrophages, but only when virus is added at a low multiplicity of infection (21). Impaired viral DNA import requires mutations in both Vpr and MA, suggesting at least some redundancy in their functions (29). Others have concluded that Vpr does not contribute to HIV infection of monocyte-derived macrophages at all independently of MA (27) or even with the loss of both these nuclear import signals (40). However, in the more biologically relevant context of lymphoid tissue, Vpr clearly facilitated replication in tissue macrophages (14), and in contrast to other studies (32), its presence in the virion is required for this effect. It is clear that Vpr does not facilitate replication in artificially arrested T cells (2, 8, 27). Further, in our previous work utilizing the CXCR-4-dependent HIV strain NL4-3, we demonstrated that Vpr does not facilitate HIV infection of resting T cells infected in ex vivo lymphoid histocultures (14). Vpr also does not contribute to overall viral production when macrophages are excluded from infection, as seen with NL4-3 infection of lymphoid tissues (14). These findings suggest that Vpr exerts its effects in a cell type-restricted manner.
Jenkins et al. have confirmed the nucleocytoplasmic shuttling properties of Vpr (36). Curiously, in this study, Vpr export was insensitive to leptomycin B even though mutations in the predicted leucine-rich, CRM-1-binding domain abrogate the phenotype (36, 64). Our prior studies demonstrated that leptomycin B impairs nuclear export of Vpr (64). Jenkins et al. also suggested that nuclear export of Vpr was not required for virion incorporation when virions were produced in the presence of cotransfected plasmids encoding a Vpr export mutant. While Vpr is specifically incorporated into the virion through interaction with the p6 portion of the p55gag precursor protein (7, 39, 54, 79), the stoichiometry, once thought to be 1:1 with p55gag in the virion, is now estimated to be as low as 1:7 with capsid (49) or even as low as 14 to 18 Vpr molecules per virion (67). More importantly, the quantity of Vpr in the virion can be greatly enhanced (up to ca. 40-fold) by cotransfecting cells with Vpr and proviral DNA expression plasmids (67, 68). Such overexpression experiments involving Vpr must therefore be interpreted with caution. Specifically, the levels of Vpr achieved in the study by Jenkins et al. (36) may have led to increased cytoplasmic concentrations of Vpr that masked the key function of the NES within Vpr. In our experiments, the VprL67A mutant was cloned into the viral genome and thus was expressed at levels characteristic of wild-type HIV-1 infection. Under these conditions, the Vpr NES is key for full incorporation of Vpr into virions.
The absence of Vpr does not completely prevent the infection of tissue macrophages. Vpr enhances such infections either by acting synergistically with the other import factors or by providing a redundant signal for more efficient import of the PIC across the NPC. It has been suggested that Vpr acts like an importin-β homologue through its direct binding to nucleoporins within the NPC, although this appears to be context dependent (18, 58, 74). While MA and integrase utilize the importin-α/importin-β-dependent pathway of nuclear import, Vpr possesses noncanonical NLSs and is not imported exclusively through these classical mechanisms (21, 22, 35, 38, 64). Thus HIV-1 may have adapted a novel strategy to bypass cellular defense mechanisms targeted at excluding viruses from the nucleus. Importantly, Vpr does not facilitate the infection of resting, nondividing T cells in the same tissue context where macrophage infection is enhanced (13, 14). Therefore, cell-specific factors must dictate whether Vpr is required for or is active in PIC import. In view of the unexpectedly large contribution of infected macrophages to the viral burden in lymphatic tissues, interrupting macrophage-dependent growth by compromising Vpr action in vivo could lead to a sharp decline in the viral burden in infected patients.
Acknowledgments
We thank Robin Givens for assistance in the preparation of the manuscript; John Carroll, Jack Hull, Stephen Gonzales, and Chris Goodfellow for assistance with graphics; and Stephen Ordway and Gary Howard for editorial assistance.
M.P.S. was supported by NIH/NIAID grant K08-AI01866. L.A.E. was supported by the Biomedical Sciences Graduate Program (BMS) and the National Institutes of Health (NIH) Medical Scientist Training Program (MSTP) at University of California, San Francisco (UCSF). This work was supported by NIH grant R01-AI45234 (W.C.G.) and the UCSF-GIVI Center for AIDS Research (NIH P30-MH59037) (W.C.G.) and in part by NIH grant R01-AI43695 (M.A.G.), the UCSF-California AIDS Research Center (CC99-SF-001) (M.A.G. and W.C.G.), and the J. David Gladstone Institutes (M.A.G. and W.C.G.).
REFERENCES
- 1.Bartz, S. R., M. E. Rogel, and M. Emerman. 1996. Human immunodeficiency virus type 1 cell cycle control: Vpr is cytostatic and mediates G2 accumulation by a mechanism which differs from DNA damage checkpoint control. J. Virol. 70:2324-2331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bouyac-Bertoia, M., J. D. Dvorin, R. A. Fouchier, Y. Jenkins, B. E. Meyer, L. I. Wu, M. Emerman, and M. H. Malim. 2001. HIV-1 infection requires a functional integrase NLS. Mol. Cell 7:1025-1035. [DOI] [PubMed] [Google Scholar]
- 3.Bukrinsky, M. I., S. Haggerty, M. P. Dempsey, N. Sharova, A. Adzhubel, L. Spitz, P. Lewis, D. Goldfarb, M. Emerman, and M. Stevenson. 1993. A nuclear localization signal within HIV-1 matrix protein that governs infection of non-dividing cells. Nature 365:666-669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bukrinsky, M. I., N. Sharova, M. P. Dempsey, T. L. Stanwick, A. G. Bukrinskaya, S. Haggerty, and M. Stevenson. 1992. Active nuclear import of human immunodeficiency virus type 1 preintegration complexes. Proc. Natl. Acad. Sci. USA 89:6580-6584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chun, T. W., R. T. Davey, Jr., M. Ostrowski, J. Shawn Justement, D. Engel, J. I. Mullins, and A. S. Fauci. 2000. Relationship between pre-existing viral reservoirs and the re-emergence of plasma viremia after discontinuation of highly active anti-retroviral therapy. Nat. Med. 6:757-761. [DOI] [PubMed] [Google Scholar]
- 6.Chun, T. W., L. Stuyver, S. B. Mizell, L. A. Ehler, J. A. Mican, M. Baseler, A. L. Lloyd, M. A. Nowak, and A. S. Fauci. 1997. Presence of an inducible HIV-1 latent reservoir during highly active antiretroviral therapy. Proc. Natl. Acad. Sci. USA 94:13193-13197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cohen, E. A., G. Dehni, J. G. Sodroski, and W. A. Haseltine. 1990. Human immunodeficiency virus vpr product is a virion-associated regulatory protein. J. Virol. 64:3097-3099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Connor, R. I., B. K. Chen, S. Choe, and N. R. Landau. 1995. Vpr is required for efficient replication of human immunodeficiency virus type-1 in mononuclear phagocytes. Virology 206:935-944. [DOI] [PubMed] [Google Scholar]
- 9.Conti, L., P. Matarrese, B. Varano, M. C. Gauzzi, A. Sato, W. Malorni, F. Belardelli, and S. Gessani. 2000. Dual role of the HIV-1 vpr protein in the modulation of the apoptotic response of T cells. J. Immunol. 165:3293-3300. [DOI] [PubMed] [Google Scholar]
- 10.de Noronha, C. M., M. P. Sherman, H. W. Lin, M. V. Cavrois, R. D. Moir, R. D. Goldman, and W. C. Greene. 2001. Dynamic disruptions in nuclear envelope architecture and integrity induced by HIV-1 Vpr. Science 294:1105-1108. [DOI] [PubMed] [Google Scholar]
- 11.Dupont, S., N. Sharova, C. DeHoratius, C. M. Virbasius, X. Zhu, A. G. Bukrinskaya, M. Stevenson, and M. R. Green. 1999. A novel nuclear export activity in HIV-1 matrix protein required for viral replication. Nature 402:681-685. [DOI] [PubMed] [Google Scholar]
- 12.Dvorin, J. D., P. Bell, G. G. Maul, M. Yamashita, M. Emerman, and M. H. Malim. 2002. Reassessment of the roles of integrase and the central DNA flap in human immunodeficiency virus type 1 nuclear import. J. Virol. 76:12087-12096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Eckstein, D. A., M. L. Penn, Y. D. Korin, D. D. Scripture-Adams, J. A. Zack, J. F. Kreisberg, M. Roederer, M. P. Sherman, P. S. Chin, and M. A. Goldsmith. 2001. HIV-1 actively replicates in naive CD4(+) T cells residing within human lymphoid tissues. Immunity 15:671-682. [DOI] [PubMed] [Google Scholar]
- 14.Eckstein, D. A., M. P. Sherman, M. L. Penn, P. S. Chin, C. M. De Noronha, W. C. Greene, and M. A. Goldsmith. 2001. HIV-1 Vpr enhances viral burden by facilitating infection of tissue macrophages but not nondividing CD4(+)T cells. J. Exp. Med. 194:1407-1419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Finzi, D., M. Hermankova, T. Pierson, L. M. Carruth, C. Buck, R. E. Chaisson, T. C. Quinn, K. Chadwick, J. Margolick, R. Brookmeyer, J. Gallant, M. Markowitz, D. D. Ho, D. D. Richman, and R. F. Siliciano. 1997. Identification of a reservoir for HIV-1 in patients on highly active antiretroviral therapy. Science 278:1295-1300. [DOI] [PubMed] [Google Scholar]
- 16.Fletcher, T. M., III, B. Brichacek, N. Sharova, M. A. Newman, G. Stivahtis, P. M. Sharp, M. Emerman, B. H. Hahn, and M. Stevenson. 1996. Nuclear import and cell cycle arrest functions of the HIV-1 Vpr protein are encoded by two separate genes in HIV-2/SIV(SM). EMBO J. 15:6155-6165. [PMC free article] [PubMed] [Google Scholar]
- 17.Fornerod, M., M. Ohno, M. Yoshida, and I. W. Mattaj. 1997. CRM1 is an export receptor for leucine-rich nuclear export signals. Cell 90:1051-1060. [DOI] [PubMed] [Google Scholar]
- 18.Fouchier, R. A., B. E. Meyer, J. H. Simon, U. Fischer, A. V. Albright, F. Gonzalez-Scarano, and M. H. Malim. 1998. Interaction of the human immunodeficiency virus type 1 Vpr protein with the nuclear pore complex. J. Virol. 72:6004-6013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fouchier, R. A., B. E. Meyer, J. H. Simon, U. Fischer, and M. H. Malim. 1997. HIV-1 infection of non-dividing cells: evidence that the amino-terminal basic region of the viral matrix protein is important for Gag processing but not for post-entry nuclear import. EMBO J. 16:4531-4539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Frankel, A. D., and J. A. Young. 1998. HIV-1: fifteen proteins and an RNA. Annu. Rev. Biochem. 67:1-25. [DOI] [PubMed] [Google Scholar]
- 21.Gallay, P., T. Hope, D. Chin, and D. Trono. 1997. HIV-1 infection of nondividing cells through the recognition of integrase by the importin/karyopherin pathway. Proc. Natl. Acad. Sci. USA 94:9825-9830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gallay, P., V. Stitt, C. Mundy, M. Oettinger, and D. Trono. 1996. Role of the karyopherin pathway in human immunodeficiency virus type 1 nuclear import. J. Virol. 70:1027-1032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Glushakova, S., B. Baibakov, L. B. Margolis, and J. Zimmerberg. 1995. Infection of human tonsil histocultures: a model for HIV pathogenesis. Nat. Med. 1:1320-1322. [DOI] [PubMed] [Google Scholar]
- 24.Glushakova, S., B. Baibakov, J. Zimmerberg, and L. B. Margolis. 1997. Experimental HIV infection of human lymphoid tissue: correlation of CD4+ T cell depletion and virus syncytium-inducing/non-syncytium-inducing phenotype in histocultures inoculated with laboratory strains and patient isolates of HIV type 1. AIDS Res. Hum. Retroviruses 13:461-471. [DOI] [PubMed] [Google Scholar]
- 25.Goh, W. C., M. E. Rogel, C. M. Kinsey, S. F. Michael, P. N. Fultz, M. A. Nowak, B. H. Hahn, and M. Emerman. 1998. HIV-1 Vpr increases viral expression by manipulation of the cell cycle: a mechanism for selection of Vpr in vivo. Nat. Med. 4:65-71. [DOI] [PubMed] [Google Scholar]
- 26.Grivel, J. C., M. L. Penn, D. A. Eckstein, B. Schramm, R. F. Speck, N. W. Abbey, B. Herndier, L. Margolis, and M. A. Goldsmith. 2000. Human immunodeficiency virus type 1 coreceptor preferences determine target T-cell depletion and cellular tropism in human lymphoid tissue. J. Virol. 74:5347-5351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Haffar, O. K., S. Popov, L. Dubrovsky, I. Agostini, H. Tang, T. Pushkarsky, S. G. Nadler, and M. Bukrinsky. 2000. Two nuclear localization signals in the HIV-1 matrix protein regulate nuclear import of the HIV-1 pre-integration complex. J. Mol. Biol. 299:359-368. [DOI] [PubMed] [Google Scholar]
- 28.He, J., S. Choe, R. Walker, P. Di Marzio, D. O. Morgan, and N. R. Landau. 1995. Human immunodeficiency virus type 1 viral protein R (Vpr) arrests cells in the G2 phase of the cell cycle by inhibiting p34cdc2 activity. J. Virol. 69:6705-6711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Heinzinger, N. K., M. I. Bukinsky, S. A. Haggerty, A. M. Ragland, V. Kewalramani, M. A. Lee, H. E. Gendelman, L. Ratner, M. Stevenson, and M. Emerman. 1994. The Vpr protein of human immunodeficiency virus type 1 influences nuclear localization of viral nucleic acids in nondividing host cells. Proc. Natl. Acad. Sci. USA 91:7311-7315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Henklein, P., K. Bruns, M. P. Sherman, U. Tessmer, K. Licha, J. Kopp, C. M. de Noronha, W. C. Greene, V. Wray, and U. Schubert. 2000. Functional and structural characterization of synthetic HIV-1 Vpr that transduces cells, localizes to the nucleus, and induces G2 cell cycle arrest. J. Biol. Chem. 275:32016-32026. [DOI] [PubMed] [Google Scholar]
- 31.Hockett, R. D., J. M. Kilby, C. A. Derdeyn, M. S. Saag, M. Sillers, K. Squires, S. Chiz, M. A. Nowak, G. M. Shaw, and R. P. Bucy. 1999. Constant mean viral copy number per infected cell in tissues regardless of high, low, or undetectable plasma HIV RNA. J. Exp. Med. 189:1545-1554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hrimech, M., X. J. Yao, F. Bachand, N. Rougeau, and E. A. Cohen. 1999. Human immunodeficiency virus type 1 (HIV-1) Vpr functions as an immediate-early protein during HIV-1 infection. J. Virol. 73:4101-4109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Humphries, E. H., and H. M. Temin. 1974. Requirement for cell division for initiation of transcription of Rous sarcoma virus RNA. J. Virol. 14:531-546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Igarashi, T., C. R. Brown, Y. Endo, A. Buckler-White, R. Plishka, N. Bischofberger, V. Hirsch, and M. A. Martin. 2001. Macrophages are the principal reservoir and sustain high virus loads in rhesus macaques after the depletion of CD4+ T cells by a highly pathogenic simian immunodeficiency virus/HIV type 1 chimera (SHIV): implications for HIV-1 infections of humans. Proc. Natl. Acad. Sci. USA 98:658-663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Jenkins, Y., M. McEntee, K. Weis, and W. C. Greene. 1998. Characterization of HIV-1 vpr nuclear import: Analysis of signals and pathways. J. Cell Biol. 143:875-885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Jenkins, Y., P. V. Sanchez, B. E. Meyer, and M. H. Malim. 2001. Nuclear export of human immunodeficiency virus type 1 Vpr is not required for virion packaging. J. Virol. 75:8348-8352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Jowett, J. B., V. Planelles, B. Poon, N. P. Shah, M. L. Chen, and I. S. Chen. 1995. The human immunodeficiency virus type 1 Vpr gene arrests infected T cells in the G2 + M phase of the cell cycle. J. Virol. 69:6304-6313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Karni, O., A. Friedler, N. Zakai, C. Gilon, and A. Loyter. 1998. A peptide derived from the N-terminal region of HIV-1 Vpr promotes nuclear import in permeabilized cells: elucidation of the NLS region of the Vpr. FEBS Lett. 429:421-425. [DOI] [PubMed] [Google Scholar]
- 39.Kondo, E., F. Mammano, E. A. Cohen, and H. G. Gottlinger. 1995. The p6gag domain of human immunodeficiency virus type 1 is sufficient for the incorporation of Vpr into heterologous viral particles. J. Virol. 69:2759-2764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kootstra, N. A., and H. Schuitemaker. 1999. Phenotype of HIV-1 lacking a functional nuclear localization signal in matrix protein of gag and Vpr is comparable to wild-type HIV-1 in primary macrophages. Virology 253:170-180. [DOI] [PubMed] [Google Scholar]
- 41.Lewis, P., M. Hensel, and M. Emerman. 1992. Human immunodeficiency virus infection of cells arrested in the cell cycle. EMBO J. 11:3053-3058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lewis, P. F., and M. Emerman. 1994. Passage through mitosis is required for oncoretroviruses but not for the human immunodeficiency virus. J. Virol. 68:510-516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lu, Y. L., P. Spearman, and L. Ratner. 1993. Human immunodeficiency virus type 1 viral protein R localization in infected cells and virions. J. Virol. 67:6542-6550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Mahalingam, S., V. Ayyavoo, M. Patel, T. Kieber-Emmons, and D. B. Weiner. 1997. Nuclear import, virion incorporation, and cell cycle arrest/differentiation are mediated by distinct functional domains of human immunodeficiency virus type 1 Vpr. J. Virol. 71:6339-6347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Mahalingam, S., S. A. Khan, M. A. Jabbar, C. E. Monken, R. G. Collman, and A. Srinivasan. 1995. Identification of residues in the N-terminal acidic domain of HIV-1 Vpr essential for virion incorporation. Virology 207:297-302. [DOI] [PubMed] [Google Scholar]
- 46.Mattaj, I. W., and L. Englmeier. 1998. Nucleocytoplasmic transport: the soluble phase. Annu. Rev. Biochem. 67:265-306. [DOI] [PubMed] [Google Scholar]
- 47.Meyers, G., S. Wain-Hobson, L. E. Henderson, B. T. Korber, K.-T. Jeang, and G. N. Pavlakis (ed.). 1994. Human retroviruses and AIDS. Los Alamos National Laboratory, Los Alamos, N.M.
- 48.Miller, M. D., C. M. Farnet, and F. D. Bushman. 1997. Human immunodeficiency virus type 1 preintegration complexes: studies of organization and composition. J. Virol. 71:5382-5390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Muller, B., U. Tessmer, U. Schubert, and H. G. Krausslich. 2000. Human immunodeficiency virus type 1 Vpr protein is incorporated into the virion in significantly smaller amounts than gag and is phosphorylated in infected cells. J. Virol. 74:9727-9731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Nie, Z., D. Bergeron, R. A. Subbramanian, X. J. Yao, F. Checroune, N. Rougeau, and E. A. Cohen. 1998. The putative alpha helix 2 of human immunodeficiency virus type 1 Vpr contains a determinant which is responsible for the nuclear translocation of proviral DNA in growth-arrested cells. J. Virol. 72:4104-4115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Nishi, K., M. Yoshida, D. Fujiwara, M. Nishikawa, S. Horinouchi, and T. Beppu. 1994. Leptomycin B targets a regulatory cascade of crm1, a fission yeast nuclear protein, involved in control of higher order chromosome structure and gene expression. J. Biol. Chem. 269:6320-6324. [PubMed] [Google Scholar]
- 52.Ossareh-Nazari, B., F. Bachelerie, and C. Dargemont. 1997. Evidence for a role of CRM1 in signal-mediated nuclear protein export. Science 278:141-144. [DOI] [PubMed] [Google Scholar]
- 53.Pantaleo, G., C. Graziosi, L. Butini, P. A. Pizzo, S. M. Schnittman, D. P. Kotler, and A. S. Fauci. 1991. Lymphoid organs function as major reservoirs for human immunodeficiency virus. Proc. Natl. Acad. Sci. USA 88:9838-9842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Paxton, W., R. I. Connor, and N. R. Landau. 1993. Incorporation of Vpr into human immunodeficiency virus type 1 virions: requirement for the p6 region of gag and mutational analysis. J. Virol. 67:7229-7237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Penn, M. L., J. C. Grivel, B. Schramm, M. A. Goldsmith, and L. Margolis. 1999. CXCR4 utilization is sufficient to trigger CD4+ T cell depletion in HIV-1-infected human lymphoid tissue. Proc. Natl. Acad. Sci. USA 96:663-668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Pierson, T., J. McArthur, and R. F. Siliciano. 2000. Reservoirs for HIV-1: mechanisms for viral persistence in the presence of antiviral immune responses and antiretroviral therapy. Annu. Rev. Immunol. 18:665-708. [DOI] [PubMed] [Google Scholar]
- 57.Poon, B., K. Grovit-Ferbas, S. A. Stewart, and I. S. Y. Chen. 1998. Cell cycle arrest by Vpr in HIV-1 virions and insensitivity to antiretroviral agents. Science 281:266-269. [DOI] [PubMed] [Google Scholar]
- 58.Popov, S., M. Rexach, L. Ratner, G. Blobel, and M. Bukrinsky. 1998. Viral protein R regulates docking of the HIV-1 preintegration complex to the nuclear pore complex. J. Biol. Chem. 273:13347-13352. [DOI] [PubMed] [Google Scholar]
- 59.Popov, S., M. Rexach, G. Zybarth, N. Reiling, M. A. Lee, L. Ratner, C. M. Lane, M. S. Moore, G. Blobel, and M. Bukrinsky. 1998. Viral protein R regulates nuclear import of the HIV-1 pre-integration complex. EMBO J. 17:909-917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Re, F., D. Braaten, E. K. Franke, and J. Luban. 1995. Human immunodeficiency virus type 1 Vpr arrests the cell cycle in G2 by inhibiting the activation of p34cdc2-cyclin B. J. Virol. 69:6859-6864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Reil, H., A. A. Bukovsky, H. R. Gelderblom, and H. G. Gottlinger. 1998. Efficient HIV-1 replication can occur in the absence of the viral matrix protein. EMBO J. 17:2699-2708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Roe, T., T. C. Reynolds, G. Yu, and P. O. Brown. 1993. Integration of murine leukemia virus DNA depends on mitosis. EMBO J. 12:2099-2108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Schwartz, S., B. K. Felber, and G. N. Pavlakis. 1992. Distinct RNA sequences in the gag region of human immunodeficiency virus type 1 decrease RNA stability and inhibit expression in the absence of Rev protein. J. Virol. 66:150-159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Sherman, M. P., C. M. de Noronha, M. I. Heusch, S. Greene, and W. C. Greene. 2001. Nucleocytoplasmic shuttling by human immunodeficiency virus type 1 Vpr. J. Virol. 75:1522-1532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Sherman, M. P., C. M. de Noronha, D. Pearce, and W. C. Greene. 2000. Human immunodeficiency virus type 1 Vpr contains two leucine-rich helices that mediate glucocorticoid receptor coactivation independently of its effects on G2 cell cycle arrest. J. Virol. 74:8159-8165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Sherman, M. P., and W. C. Greene. 2002. Slipping through the door: HIV entry into the nucleus. Microbes Infect. 4:67-73. [DOI] [PubMed] [Google Scholar]
- 67.Singh, S. P., D. Lai, M. Cartas, D. Serio, R. Murali, V. S. Kalyanaraman, and A. Srinivasan. 2000. Epitope-tagging approach to determine the stoichiometry of the structural and nonstructural proteins in the virus particles: amount of vpr in relation to gag in HIV-1. Virology 268:364-371. [DOI] [PubMed] [Google Scholar]
- 68.Singh, S. P., P. Tungaturthi, M. Cartas, B. Tomkowicz, T. A. Rizvi, S. A. Khan, V. S. Kalyanaraman, and A. Srinivasan. 2001. Virion-associated HIV-1 Vpr: variable amount in virus particles derived from cells upon virus infection or proviral DNA transfection. Virology 283:78-83. [DOI] [PubMed] [Google Scholar]
- 69.Stauber, R. H., and G. N. Pavlakis. 1998. Intracellular trafficking and interactions of the HIV-1 Tat protein. Virology 252:126-136. [DOI] [PubMed] [Google Scholar]
- 70.Stewart, S. A., B. Poon, J. B. Jowett, and I. S. Chen. 1997. Human immunodeficiency virus type 1 Vpr induces apoptosis following cell cycle arrest. J. Virol. 71:5579-5592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Stewart, S. A., B. Poon, J. B. Jowett, Y. Xie, and I. S. Chen. 1999. Lentiviral delivery of HIV-1 Vpr protein induces apoptosis in transformed cells. Proc. Natl. Acad. Sci. USA 96:12039-12043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Stewart, S. A., B. Poon, J. Y. Song, and I. S. Chen. 2000. Human immunodeficiency virus type 1 vpr induces apoptosis through caspase activation. J. Virol. 74:3105-3111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Toohey, K., K. Wehrly, J. Nishio, S. Perryman, and B. Chesebro. 1995. Human immunodeficiency virus envelope V1 and V2 regions influence replication efficiency in macrophages by affecting virus spread. Virology 213:70-79. [DOI] [PubMed] [Google Scholar]
- 74.Vodicka, M. A., D. M. Koepp, P. A. Silver, and M. Emerman. 1998. HIV-1 Vpr interacts with the nuclear transport pathway to promote macrophage infection. Genes Dev. 12:175-185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.von Schwedler, U., R. S. Kornbluth, and D. Trono. 1994. The nuclear localization signal of the matrix protein of human immunodeficiency virus type 1 allows the establishment of infection in macrophages and quiescent T lymphocytes. Proc. Natl. Acad. Sci. USA 91:6992-6996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Wolff, B., J. J. Sanglier, and Y. Wang. 1997. Leptomycin B is an inhibitor of nuclear export: inhibition of nucleo-cytoplasmic translocation of the human immunodeficiency virus type 1 (HIV-1) Rev protein and Rev-dependent mRNA. Chem. Biol. 4:139-147. [DOI] [PubMed] [Google Scholar]
- 77.Wong, J. K., M. Hezareh, H. F. Gunthard, D. V. Havlir, C. C. Ignacio, C. A. Spina, and D. D. Richman. 1997. Recovery of replication-competent HIV despite prolonged suppression of plasma viremia. Science 278:1291-1295. [DOI] [PubMed] [Google Scholar]
- 78.Yao, X. J., A. J. Mouland, R. A. Subbramanian, J. Forget, N. Rougeau, D. Bergeron, and E. A. Cohen. 1998. Vpr stimulates viral expression and induces cell killing in human immunodeficiency virus type 1-infected dividing Jurkat T cells. J. Virol. 72:4686-4693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Yao, X. J., R. A. Subbramanian, N. Rougeau, F. Boisvert, D. Bergeron, and E. A. Cohen. 1995. Mutagenic analysis of human immunodeficiency virus type 1 Vpr: role of a predicted N-terminal alpha-helical structure in Vpr nuclear localization and virion incorporation. J. Virol. 69:7032-7044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Yedavalli, V. R., C. Chappey, and N. Ahmad. 1998. Maintenance of an intact human immunodeficiency virus type 1 vpr gene following mother-to-infant transmission. J. Virol. 72:6937-6943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Zennou, V., C. Petit, D. Guetard, U. Nerhbass, L. Montagnier, and P. Charneau. 2000. HIV-1 genome nuclear import is mediated by a central DNA flap. Cell 101:173-185. [DOI] [PubMed] [Google Scholar]