Abstract
The cellular immunogenicity of formulated plasmid DNA and replication-defective human adenovirus serotype 5 (Ad5) vaccine vectors expressing a codon-optimized human immunodeficiency virus type 1 gag gene was examined in baboons. The Ad5 vaccine was capable of inducing consistently strong, long-lived CD8+-biased T-cell responses and in vitro cytotoxic activities. The DNA vaccine-elicited immune responses were weaker than those elicited by the Ad5 vaccine and highly variable; formulation with chemical adjuvants led to moderate increases in the levels of Gag-specific T cells. Increasing the DNA-primed responses with booster doses of either Ad5 or modified vaccinia virus Ankara vaccines suggests a difference in the relative levels of cytotoxic and helper responses. The implications of these results are discussed.
Virus-specific cellular immunity, particularly that associated with CD3+ CD8+ cytotoxic T lymphocytes (CTLs) (6, 15, 23-25, 27), has been increasingly recognized to have an important role in controlling persistent human immunodeficiency virus type 1 (HIV-1) infection. Recently, we reported on the efficacy of using CRL1005 adjuvant-formulated DNA and recombinant adenovirus type 5 (Ad5) vectors that express simian immunodeficiency virus SIVmac239 gag to protect rhesus macaques (Macaca mulatta) against disease progression from a pathogenic infection with simian-human immunodeficiency virus SHIV89.6P (25). In developing a vaccine against HIV-1, it is important to evaluate the cellular immunogenicity of equivalent vectors expressing HIV-1 gag in nonhuman primates. The most commonly used rhesus macaques weigh considerably less (3 to 12 kg) than human adults. In contrast, adult baboons (genus Papio) are nearly half the size of adult humans. They can be infected with several simian-human immunodeficiency virus strains (1, 14) and have been shown to develop AIDS-like disease symptoms when infected with HIV-2 (5). They thus provide a suitable alternative immunological model that may closely approximate vaccine-induced immune responses in humans. Prior to this work, vaccine testing in baboons (4, 9, 13, 17, 26) had largely involved candidates aimed at generating virus-specific neutralizing antibodies. Hence, techniques for quantitative analyses of antigen-specific T-cell responses in this primate system were limited.
In this study, we evaluated the cellular immune responses induced in adult baboons by using several vector approaches for treatment of HIV-1. A DNA vaccine was constructed by inserting a synthetic gene for HIV-1 CAM1 gag, which used human-biased codons (16), into the V1Jns plasmid under the control of the human cytomegalovirus-human intron A promoter and bovine growth hormone terminator (8, 25). The Ad5 vaccine construct is a prototypic vector containing an E1 deletion replaced with the expression cassette for the same codon-optimized gag gene. Methods for constructing and characterizing such vectors have been described earlier (25). The modified vaccinia virus Ankara (MVA) vector was constructed by inserting the same gag gene into the PmeI site of the pLW22 shuttle vector (20). gag was placed under the control of a synthetic early-late vaccinia virus promoter and was inserted into the site of deletion II in the MVA genome by homologous recombination. The generation of the vaccine in chicken embryo fibroblasts and the characterization of the recombinant MVA virus have been described elsewhere (20).
Groups of three baboons (Papio cynocephalus; 25 to 34 kg each) were given priming immunizations with (i) 5 mg of V1Jns-HIV-1 gag (the DNA vaccine) in phosphate-buffered saline, (ii) 5 mg of the DNA vaccine formulated with 700 μg of aluminum phosphate (HCI Biosector, Frederikssund, Denmark), (iii) 5 mg of the DNA vaccine formulated with 7.5 mg of the adjuvant CRL1005 (CytRx Corp., Atlanta, Ga.) and 0.75 mM benzyalkonium chloride (Spectrum, Gardena, Calif.), or (iv) 1011 viral particles (VP) of replication-incompetent Ad5-HIV-1 gag (the Ad5 vaccine). Each vaccine was prepared in a 1-ml volume and injected at a single site in the deltoid muscle of an anesthetized animal by using a tuberculin syringe. In addition to intramuscular (i.m.) immunization, Ad5-HIV-1 gag was also injected subcutaneously at four proximal sites (250 μl per site) above the deltoid muscle area. Table 1 details the priming and booster immunizations that each baboon received. All animal care and treatment were performed in accordance with standards approved by the Institutional Animal Care and Use Committee.
TABLE 1.
Immunization schedule and vaccines useda
| Animals | Priming immunization | Booster at wk 24b |
|---|---|---|
| 11017, 11361, 14232 | 1011 VP of Ad5 at wk 0 and 4 (i.m.) | 1011 VP of Ad5 (i.m.) |
| 10041, 11039, 11096 | 1011 VP of Ad5 at wk 0 and 4 (s.c.) | 1011 VP of Ad5 (s.c.) |
| 10432, 8698, 10875 | None | 108 VP of Ad5 (i.m.) |
| 9895, 11251, 14811 | DNA at wk 0, 4, and 8 (i.m.) | 108 VP of Ad5 (i.m.) |
| 8988, 9783, 10843 | DNA-alum at wk 0, 4, and 8 (i.m.) | 108 VP of Ad5 (i.m.), 109 PFU of MVA (i.m.), —c |
| 10544, 11247, 11876 | DNA-CRL1005 at wk 0, 4, and 8 (i.m.) | 109 PFU of MVA (i.m.), 109 PFU of MVA (i.m.), 108 VP of Ad5 (i.m.) |
| 11029 (naïve) | None | None |
s.c., subcutaneously; Ad5, Ad5-HIV-1 gag; MVA, MVA-HIV-1 gag; DNA, 5 mg of V1Jns-HIV-1 gag; alum, 700 μg of aluminum phosphate; CRL1005, 7.5 mg of CRL1005 with 0.75 mM benzyalkonium chloride.
For the group in which a different booster vaccine is given to each animal, the order of booster vaccines listed corresponds to that of animals listed in the first column.
This animal died of an unrelated cause after week 12.
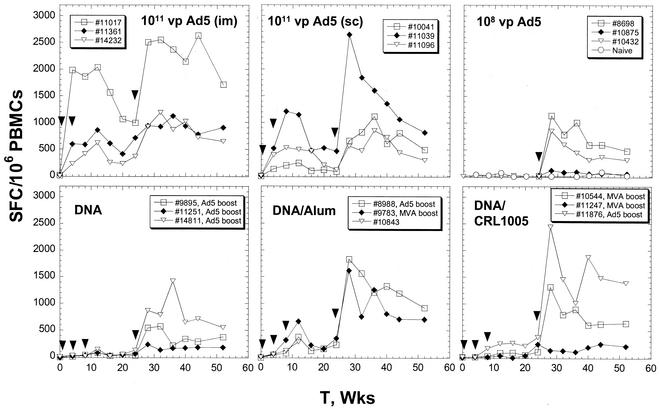
The levels of Gag-specific T cells (Fig. 1) from peripheral blood mononuclear cell (PBMC) preparations were quantified by the enzyme-linked immunospot (ELISPOT) assay by using the same reagent sets described elsewhere (2). All antigen-specific stimulation reactions were achieved by using a pool of 20-amino-acid (aa) peptides that overlapped by 10 aa and that encompassed the entire Gag product sequence (SynPep, Dublin, Calif.). The length of each peptide is inclusive to allow detection of both CD4+- and CD8+-T-cell determinants. Priming immunizations with 1011 VP of the Ad5-HIV-1 gag vector induced in all six vaccinees high levels of Gag-specific T cells which peaked at levels ranging from 530 to 2,050 spot-forming T cells (SFC)/106 PBMCs at weeks 8 to 12 (Fig. 1). The magnitude of these responses was independent of the route of administration. Immunization of the Ad5 vaccinees with a third dose of the virus resulted in improved levels of circulating Gag-specific T cells (1.3- to 4-fold and 2- to 10-fold higher than the prebooster peak and set point levels, respectively). The anti-Ad5 neutralizing antibody titers were determined by testing serially diluted serum samples for the ability to block the expression of human secreted alkaline phosphatase from cells infected with an Ad5-human secreted alkaline phosphatase vector; the titers for the Ad5 vaccinees ranged from 139 to 4,000 at week 24. Animals 11017 and 11039 showed the lowest neutralizing antibody titers (139 and 201, respectively) and the largest absolute increases in T-cell responses. The fact that the immune responses were increased despite high levels of vector-specific neutralizing immunity suggests that only a small amount of the Ad5 vaccine was necessary to effect a significant expansion of the existing T-cell pool.
FIG. 1.
Levels of Gag-specific T cells in baboons immunized with DNA and viral vaccine vectors as a function of time (T). For the ELISPOT assay, 2 × 105 to 4 × 105 PBMCs were plated on each well and the wells were incubated in the absence or presence of peptides at a 4-μg/ml concentration per peptide. The plotted values are differences in the numbers of IFN-γ SFC per 106 PBMCs for a given sample mixed with the Gag peptide pool and the blank control. The average blank control count was about 18 SFC/106 PBMCs with a standard deviation of 28 SFC/106 PBMCs. Arrowheads indicate the times of immunization with the vectors described in each panel. The DNA-, DNA-aluminum phosphate (Alum)-, and DNA-CRL1005-vaccinated animals received booster doses of either 108 VP of Ad5 or 109 PFU of MVA-HIV-1 gag vector at week 24.
Baboons immunized with the DNA vaccine alone developed low levels of antigen-specific T cells following the priming immunizations (<138 SFC/106 PBMCs) (Fig. 1). The responses (293 to 705 SFC per 106 PBMCs at week 12) were improved by combining the DNA vaccine with aluminum phosphate. One animal in the DNA-aluminum phosphate-vaccinated group (animal 10843) died due to an unrelated cause during the course of the priming regimen. In the cohort vaccinated with DNA formulated with CRL1005 adjuvant, the levels of circulating Gag-specific T cells were not significantly higher than those in the DNA-alone control group in this animal model system. The animals that received DNA-alone vaccines were given a low (108 VP) booster dose of Ad5-HIV-1 gag at week 24. The low Ad5-HIV-1 gag booster dose is designed to simulate a case in which the subject has a preexisting Ad5-specific immunity capable of 99.9% neutralization of a 1011-VP dose. Each of three baboons in a control group was inoculated i.m. with a single dose of 108 VP of the Ad5 vaccine (booster control group) at the same time that the other baboons received the booster immunization. The levels of Gag-specific T cells in the DNA-alone vaccinees following the booster dose increased to 200 to 1,500 SFC/106 cells. These levels were comparable to those in the booster control group, suggesting that the DNA immunizations did not significantly prime for HIV-1 T-cell responses.
Several studies have shown that optimal HIV-1-specific T-cell responses are obtained by administering boosters of recombinant poxvirus vaccines such as the MVA vaccine to animals receiving DNA priming immunizations (3, 11). We decided that the remaining five DNA-adjuvant vaccinees were best utilized by comparing the booster effects of the Ad5 vaccine with those of the MVA vaccine (Table 1). Gag-specific responses in all DNA-adjuvant vaccinees were augmented by immunization with either viral booster at week 24 (Fig. 1). The two animals receiving DNA-adjuvant priming and Ad5 booster immunizations (animals 8988 and 11876) developed peak T-cell responses in the range of 1,800 to 2,500 SFC/106 cells, which were markedly higher than those of the booster control group animals. Animals that received the MVA booster likewise showed high postbooster T-cell levels (200 to 1,500 SFC/106 cells), but these levels were slightly lower than those observed in animals from the same DNA-adjuvant groups that were given the Ad5 booster. A control cohort for the MVA booster was not included in this study. However, immunization of rhesus macaques with the same MVA-HIV-1 gag vector resulted in consistently weak Gag-specific T-cell responses (<150 SFC/106 PBMCs) (7).
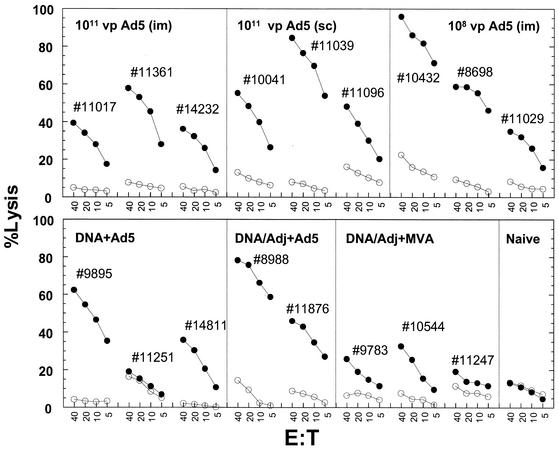
Cytotoxic functions of the vaccine-induced T-cell responses (Fig. 2) were examined by in vitro killing of autologous B-lymphoid cell lines (BLCLs) pulsed with the 20-aa peptide pool by using established protocols (10). At week 36, all animals (with the exception of animal 11251) that received at least a single injection of the Ad5 vaccine exhibited consistently strong CTL activity (>40% at an effector-to-target cell ratio of 40:1). Prior to the booster doses, none of the DNA vaccinees showed any Gag-specific cytotoxic activity above that of the unpulsed BLCL controls (data not shown). All three animals that received MVA boosters at week 24 exhibited much weaker CTL killing efficiencies than those of the DNA vaccinees that received Ad5 boosters (Fig. 2).
FIG. 2.
51Cr release assay of CTL killing by baboon effector cells of autologous target BLCLs that either were unpulsed (open circles) or were pulsed with a pool of 20-mer peptides derived from HIV-1 Gag (closed circles). Effector cells were prepared from PBMCs that were collected at week 36 (12 weeks after the booster dose) and were restimulated with a vaccinia virus expressing HIV-1 gag for 2 weeks. The percentages of specific lysis were recorded at effector-to-target cell ratios (E:T) ranging from 40 to 5. In all cases, the spontaneous lysis did not exceed 15% of the maximum 51Cr release values. Adj, adjuvant; sc, subcutaneous.
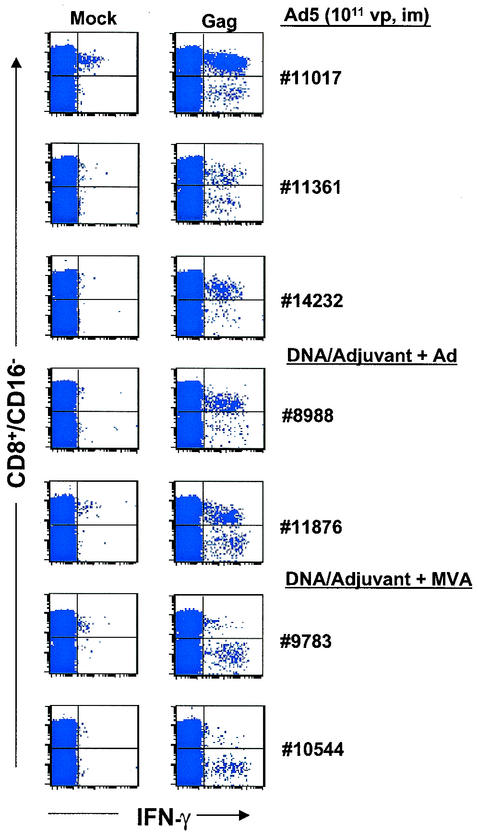
The intracellular cytokine staining method (ICS) has been widely utilized to distinguish the relative contributions of CD4+ and CD8+ cells to the overall T-cell responses (18). We have identified suitable commercial antibodies for staining markers (CD3, CD16, CD4, CD8, and gamma interferon [IFN-γ]) on baboon PBMCs. Anti-CD3 or anti-CD16 monoclonal antibodies can be used interchangeably in order to exclude cytokine-producing CD8+ NK cells (CD3− CD16+) (19). At week 28, all six Ad5-HIV-1 gag vaccinees showed detectable levels of effector CD8+ T cells mixed with lower amounts of CD4+ T cells (range of anti-Gag CD8+:CD4+ cell ratios, 1.4:1 to 10:1) (Table 2; Fig. 3) following overnight restimulation with the 20-aa peptide pool. Prior to any viral boost, all DNA vaccinees exhibited largely CD4+ antigen-specific IFN-γ-producing cells (<0.14% of CD16− T cells) (data not shown).
TABLE 2.
Percentages of CD16− T lymphocytes that are Gag- specific CD8+ or CD4+ cells in vaccinated animalsa
| Vaccine (route) | Animal | % CD16− T cells that wereb:
|
||
|---|---|---|---|---|
| Priming | Booster | CD4+ | CD8+ | |
| Ad5 | Ad5 | 11017 | 0.17 | 1.84 |
| 11361 | 0.10 | 0.25 | ||
| 14232 | 0.06 | 0.52 | ||
| Ad5 (s.c.) | Ad5 (s.c.) | 10041 | 0.10 | 0.14 |
| 11039 | 0.09 | 0.73 | ||
| 11096 | 0.06 | 0.18 | ||
| None | Ad5 | 8698 | 0.01 | 0.51 |
| 10875 | 0.02 | 0.06 | ||
| 10432 | 0.04 | 0.20 | ||
| DNA | Ad5 | 9895 | 0.07 | 0.03 |
| 11251 | 0.05 | 0.02 | ||
| 14811 | 0.05 | 0.38 | ||
| DNA-alum | Ad5 | 8988 | 0.07 | 0.56 |
| DNA-CRL1005 | Ad5 | 11876 | 0.26 | 0.67 |
| DNA-alum | MVA | 9783 | 0.35 | 0.02 |
| DNA-CRL1005 | MVA | 10544 | 0.23 | 0.04 |
| 11247 | 0.02 | 0.01 | ||
Data are based on IFN-γ ICS analyses of PBMCs collected at week 28 (4 weeks postbooster). s.c., subcutaneously; alum, aluminum phosphatase.
Mock-subtracted values.
FIG. 3.
Intracellular staining for IFN-γ production by PBMCs collected from selected baboons at week 28. The animals received multiple high doses of Ad5-HIV-1 gag or DNA-adjuvant priming immunizations followed by either an MVA or an Ad5 (Ad) booster. PBMCs were treated overnight with brefeldin, anti-human CD28 (anti-hCD28, clone L293; Becton Dickinson, Franklin Lakes, N.J.), and anti-hCD49d (clone L25; Becton Dickinson) with or without antigen stimulation. They were then stained with anti-hCD16-Cy-Cychrome (clone 3G8; BD-PharMingen), anti-hCD4-allophycocyanin (anti-hCD4-APC, clone SK3; Becton Dickinson), anti-hCD8-APC (clone SK1; Becton Dickinson), and fluorescein isothiocyanate-anti-hIFN-γ (clone 1D1K; MABTECH, Nacka, Sweden). The plots illustrate IFN-γ staining of CD16− CD8+ and CD16− CD8− cells incubated in the absence (left panels) or presence (right panels) of the 20-aa Gag peptides. In every case, the numbers of CD16− CD8− IFN-γ+ T cells normalized to 106 lymphocytes matched those of CD16− CD4+ IFN-γ+ cells, suggesting that in the plots shown, the events in the CD8− population represented helper responses.
The types of responses in the adjuvant-formulated-DNA vaccinees after booster vaccinations with either the Ad5 or the MVA vector were also compared. Two of the three animals that received MVA-HIV-1 gag boosters exhibited responses with a stronger CD4+ component than a CD8+ component (CD4+:CD8+ cell ratios of 6:1 and 10:1 for animals 9783 and 10544, respectively) (Fig. 3); in the third animal, the overall response was much weaker, which was consistent with the ELISPOT assay results. It should be noted that the CD4+ and CD8+ distributions were similar when either a 15-mer peptide pool (with 11-aa overlaps) or a 20-mer peptide pool was used for stimulation (data not shown). In contrast, the two animals that received the Ad5 booster dose developed CD8+-biased T-cell responses (antigen-specific CD8+:CD4+ ratios of 2:1 and 8:1, respectively). This trend was likewise observed in rhesus macaques immunized with a heterologous DNA-CRL1005-Ad5 priming-booster regimen (7).
HIV-1-specific cellular responses in all vaccinees were assessed at week 52, or about 7 months after the final immunization. All animals maintained levels of Gag-specific effector T cells to within 30 to 80% of the postbooster peak levels. The antigen-specific T-cell counts were consistently higher than those at the set point (week 24) after the priming series, suggesting an immunological benefit of the booster treatment.
The successful development of an HIV-1 vaccine for either prophylactic or therapeutic application will require gene delivery systems that are potent and consistent in their ability to elicit effective cellular immune responses. Herein, we provide evidence that a nonreplicating human adenovirus vector is capable of inducing consistently strong and stable HIV-1-specific cytotoxic T-cell responses in a large nonhuman primate system. First, 10 out of the 11 baboons that received immunizations of the Ad5-HIV-1 gag vaccine one or more times during the study produced high levels of Gag-specific T cells (0.01 to 0.25% of lymphocytes) that were sustained for at least several months. Second, the majority of the Gag-specific cellular immune responses consistently originated from CD8+ T cells, a finding which is in agreement with the observed high efficiencies of in vitro killing by vaccinee PBMCs of peptide-pulsed autologous cell lines. Third, the virus is effective with even a single low-dose immunization of 108 VP.
One limitation of viral vaccine vectors such as Ad5 or MVA involves the negative effect of preexisting vector-specific immunity on the induced immunity against the vaccine antigen. This limitation can be circumvented by priming with a nonimmunogenic vector system such as DNA and then following that with a heterologous viral booster (7, 28). Here we provide evidence suggesting that an effective priming vaccine using adjuvant-formulated DNA combined with an Ad5 booster can result in a T-cell immune response that approaches those observed with multiple high doses of an Ad5-based vaccine.
Comparison of the immune responses of the adjuvant-formulated-DNA vaccinees given an Ad5 or an MVA booster is suggestive of differences in polarization in the phenotype of the Gag-specific T cells. The Ad5 booster was particularly potent in eliciting a CD8+-biased T-cell response. In contrast, none of the three animals that received the MVA vector produced a CD8+-biased Gag-specific immunity. It should be noted that the numbers of animals tested were limited and that these findings should be reinforced with studies of other nonhuman primate systems as well as of other poxviruses (12, 21). It has been suggested that potent CD8+- and CD4+-T-cell responses are both critical in controlling an HIV infection (22, 29), yet the relative magnitude and the intrinsic quality of both types of responses remain unclear. It would appear that such immunological parameters can be controlled by the nature of the vaccine modality. Ultimately, the potential of these vaccine approaches can be assessed only with clinical trials involving healthy and HIV-1-infected individuals.
Acknowledgments
We thank Bernard Moss and Vanessa Hirsch (National Institutes of Health) for providing the MVA vectors.
REFERENCES
- 1.Allan, J. S., P. Ray, S. Broussard, E. Whitehead, G. Hubbard, T. Butler, K. Brasky, P. Luciw, C. Cheng-Mayer, J. A. Levy, K. Steimer, J. Li, J. Sodroski, and M. Garcia-Moll. 1995. Infection of baboons with simian/human immunodeficiency viruses. J. Acquir. Immune Defic. Syndr. Hum. Retrovirol. 15:429-441. [PubMed] [Google Scholar]
- 2.Altfeld, M. A., A. Trocha, R. L. Eldridge, E. S. Rosenberg, M. N. Phillips, M. M. Addo, R. P. Sekaly, S. A. Kalmas, S. A. Burchett, K. McIntosh, B. D. Walker, and P. J. R. Goulder. 2000. Identification of dominant optimal HLA-B60- and HLA-B61-restricted cytotoxic T-lymphocyte (CTL) epitopes: rapid characterization of CTL responses by enzyme-linked immunospot assay. J. Virol. 74:8541-8549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Amara, R. R., F. Villinger, J. D. Altman, S. L. Lydy, S. P. O'Neil, S. I. Staprans, D. C. Montefiori, Y. Xu, J. G. Herndon, L. S. Wyatt, M. A. Candido, N. L. Kozyr, P. L. Earl, J. M. Smith, H. L. Ma, B. D. Grimm, M. L. Hulsey, J. Miller, H. M. McClure, J. M. McNicholl, B. Moss, and H. L. Robinson. 2001. Control of a mucosal challenge and prevention of AIDS by a multiprotein DNA/MVA vaccine. Science 292:69-74. [DOI] [PubMed] [Google Scholar]
- 4.Anderson, K. P., C. Lucas, C. V. Hanson, H. F. Londe, A. Izu, T. Gregory, A. Ammann, P. W. Berman, and J. W. Eichberg. 1989. Effect of dose and immunization schedule on immune response of baboons to recombinant glycoprotein 120 of HIV-1. J. Infect. Dis. 160:960-969. [DOI] [PubMed] [Google Scholar]
- 5.Barnett, S. W., K. K. Murthy, B. G. Herndier, and J. A. Levy. 1994. An AIDS-like condition induced in baboons by HIV-2. Science 266:642-646. [DOI] [PubMed] [Google Scholar]
- 6.Borrow, P., H. Lewicki, B. H. Hahn, G. M. Shaw, and M. B. A. Oldstone. 1994. Virus-specific CD8+ cytotoxic T-lymphocyte activity associated with control of viremia in primary human immunodeficiency virus type 1 infection. J. Virol. 68:6103-6110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Casimiro, D. R., L. Chen, T.-M. Fu, R. K. Evans, M. J. Caulfield, M.-E. Davies, A. Tang, M. Chen, L. Huang, V. Harris, D. C. Freed, K. A. Wilson, S. Dubey, D. Zhu, D. Nawrocki, H. Mach, R. Troutman, L. Isopi, D. Williams, W. Hurni, Z. Xu, J. G. Smith, S. Wang, X. Liu, L. Guan, R. Long, W. Trigona, G. J. Heidecker, H. C. Perry, N. Persaud, T. J. Toner, Q. Su, X. Liang, R. Youil, M. Chastain, A. J. Bett, D. B. Volkin, E. A. Emini, and J. W. Shiver. 2003. Comparative immunogenicity in rhesus monkeys of DNA plasmid, recombinant vaccinia virus, and replication-defective adenovirus vectors expressing a human immunodeficiency virus type 1 gag gene. J. Virol. 77:6305-6313. [DOI] [PMC free article] [PubMed]
- 8.Caulfield, M. J., S. Wang, J. G. Smith, T. W. Tobery, X. Liu, M.-E. Davies, D. R. Casimiro, T.-M. Fu, A. Simon, R. K. Evans, E. A. Emini, and J. Shiver. 2002. Sustained peptide-specific gamma interferon T-cell response in rhesus macaques immunized with human immunodeficiency virus gag DNA vaccines. J. Virol. 76:10038-10043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cleland, J. L., A. Lim, A. Daugherty, L. Barron, N. Desjardin, E. T. Duenas, D. J. Eastman, J. C. Vennari, T. Wrin, P. Berman, K. K. Murthy, and M. F. Powell. 1998. Development of a single-shot subunit vaccine for HIV-1. 5. Programmable in vivo autoboost and long lasting neutralizing response. J. Pharm. Sci. 87:1489-1495. [DOI] [PubMed] [Google Scholar]
- 10.Fu, T.-M., D. C. Freed, W. L. Trigona, L. Guan, L. Zhu, R. Long, N. V. Persaud, K. Manson, S. Dubey, and J. W. Shiver. 2001. Evaluation of cytotoxic T-lymphocyte responses in human and nonhuman primate subjects infected with human immunodeficiency virus type 1 or simian/human immunodeficiency virus. J. Virol. 75:73-82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hanke, T., R. V. Samuel, T. J. Blanchard, V. C. Neumann, T. M. Allen, J. E. Boyson, S. A. Sharpe, N. Cook, G. L. Smith, D. I. Watkins, M. P. Cranage, and A. J. McMichael. 1999. Effective induction of simian immunodeficiency virus-specific cytotoxic T lymphocytes in macaques by using a multiepitope gene and DNA prime-modified vaccinia virus Ankara boost vaccination regimen. J. Virol. 73:7524-7532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hel, Z., J. Nacsa, W. P. Tsai, A. Thornton, L. Giuliani, J. Tartaglia, and G. Franchini. 2002. Equivalent immunogenicity of the highly attenuated poxvirus-based ALVAC-SIV and NYVAC-SIV vaccine candidates in SlVmac251-infected macaques. Virology 304:125-134. [DOI] [PubMed] [Google Scholar]
- 13.Irwin, M. J., L. S. Laube, V. Lee, M. Austin, S. Chada, C.-G. Anderson, K. Townsend, D. J. Jolly, and J. F. Warner. 1994. Direct injection of a recombinant retroviral vector induces human immunodeficiency virus-specific immune responses in mice and nonhuman primates. J. Virol. 68:5036-5044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Klinger, J. M., S. Himathongkham, H. Legg, P. A. Luciw, and S. W. Barnett. 1998. Infection of baboons with a simian immunodeficiency virus/HIV-1 chimeric virus constructed with an HIV-1 Thai subtype E envelope. AIDS 12:849-857. [DOI] [PubMed] [Google Scholar]
- 15.Koup, R. A., J. T. Safrit, Y. Cao, C. A. Andrews, G. McLeod, W. Borkowsky, C. Farthing, and D. D. Ho. 1994. Temporal association of cellular immune responses with the initial control of viremia in primary human immunodeficiency virus type 1 syndrome. J. Virol. 68:4650-4655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lathe, R. 1985. Synthetic oligonucleotide probes deduced from amino acid sequence data. Theoretical and practical considerations. J. Mol. Biol. 183:1-12. [DOI] [PubMed] [Google Scholar]
- 17.Locher, C. P., S. A. Witt, B. G. Herndier, K. Tenner-Racz, P. Racz, and J. A. Levy. 2001. Baboons as an animal model for human immunodeficiency virus pathogenesis and vaccine development. Immunol. Rev. 183:127-140. [DOI] [PubMed] [Google Scholar]
- 18.Maino, V. C., and L. J. Picker. 1998. Identification of functional subsets by flow cytometry: intracellular detection of cytokine expression. Cytometry 34:207-215. [DOI] [PubMed] [Google Scholar]
- 19.Malyguine, A. M., S. Saadi, J. L. Platt, and J. R. Dawson. 1996. Differential expression of natural killer cell markers: human versus baboon. Transplantation 62:1319-1324. [DOI] [PubMed] [Google Scholar]
- 20.Ourmanov, I., C. R. Brown, B. Moss, M. Carroll, L. Wyatt, L. Pletneva, S. Goldstein, D. Venzon, and V. M. Hirsch. 2000. Comparative efficacy of recombinant modified vaccinia virus Ankara expressing simian immunodeficiency virus (SIV) Gag-Pol and/or Env in macaques challenged with pathogenic SIV. J. Virol. 74:2740-2751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Paoletti, E. 1996. Applications of pox virus vectors to vaccination: an update. Proc. Natl. Acad. Sci. USA 93:11349-11353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rosenberg, E. S., J. M. Billingsley, A. M. Caliendo, S. L. Boswell, P. E. Sax, S. A. Kalams, and B. D. Walker. 1997. Vigorous HIV-1-specific CD4+ T cell responses associated with control of viremia. Science 278:1447-1451. [DOI] [PubMed] [Google Scholar]
- 23.Rowland-Jones, S. L., T. Dong, L. Dorrell, G. Ogg, P. Hansasuta, P. Krausa, J. Kimani, S. Sabally, K. Ariyoshi, J. Oyugi, K. S. MacDonald, J. Bwayo, H. Whittle, F. A. Plummer, and A. J. McMichael. 1999. Broadly cross-reactive HIV-specific cytotoxic T-lymphocytes in highly-exposed persistently seronegative donors. Immunol. Lett. 66:9-14. [DOI] [PubMed] [Google Scholar]
- 24.Schmitz, J. E., M. J. Kuroda, S. Santra, V. G. Sasseville, M. A. Simon, M. A. Lifton, P. Racz, K. Tenner-Racz, M. Dalesandro, B. J. Scallon, J. Ghrayeb, M. A. Forman, D. C. Montefiori, E. P. Rieber, N. L. Letvin, and K. A. Reimann. 1999. Control of viremia in simian immunodeficiency virus infection by CD8+ lymphocytes. Science 283:857-860. [DOI] [PubMed] [Google Scholar]
- 25.Shiver, J. W., T.-M. Fu, L. Chen, D. R. Casimiro, M. Davies, R. K. Evans, Z.-Q. Zhang, A. J. Simon, W. L. Trigona, S. A. Dubey, L. Huang, V. A. Harris, R. S. Long, X. Liang, L. Handt, W. A. Schleif, L. Zhu, D. C. Freed, N. V. Persaud, L. Guan, K. S. Punt, A. Tang, M. Chen, K. A. Wilson, K. B. Collins, G. J. Heidecker, V. R. Fernandez, H. C. Perry, J. G. Joyce, K. M. Grimm, J. C. Cook, P. M. Keller, D. S. Kresock, H. Mach, R. D. Troutman, L. A. Isopi, D. M. Williams, Z. Xu, K. E. Bohannon, D. B. Volkin, D. C. Montefiori, A. Miura, G. R. Krivulka, M. A. Lifton, M. J. Kuroda, J. E. Schmitz, N. L. Letvin, M. J. Caulfield, A. J. Bett, R. Youil, D. C. Kaslow, and E. A. Emini. 2002. Replication-incompetent adenoviral vaccine vector elicits effective anti-immunodeficiency-virus immunity. Nature 415:331-335. [DOI] [PubMed] [Google Scholar]
- 26.Stephens, D. M., J. W. Eichberg, N. L. Haigwood, K. S. Steimer, D. Davis, and P. J. Lachmann. 1992. Antibodies are produced to the variable regions of the external envelope glycoprotein of human immunodeficiency virus type 1 in chimpanzees infected with the virus and baboons immunized with a candidate recombinant vaccine. J. Gen. Virol. 73:1099-1106. [DOI] [PubMed] [Google Scholar]
- 27.Wyand, M. S., K. Manson, D. C. Montefiori, J. D. Lifson, R. P. Johnson, and R. C. Desrosiers. 1999. Protection by live, attenuated simian immunodeficiency virus against heterologous challenge. J. Virol. 73:8356-8363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yang, Z., L. S. Wyatt, W. Kong, Z. Moodie, B. Moss, and G. J. Nabel. 2003. Overcoming immunity to a viral vaccine by DNA priming before vector boosting. J. Virol. 77:799-803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zajac, A. J., K. Murali-Krishna, J. N. Blattman, and R. Ahmed. 1998. Therapeutic vaccination against chronic viral infection: the importance of cooperation between CD4+ and CD8+ T cells. Curr. Opin. Immunol. 10:444-449. [DOI] [PubMed] [Google Scholar]