Abstract
Recombinant adeno-associated virus type 5 (rAAV-5) is known to efficiently transduce airway epithelia via apical infection. In contrast, rAAV-2 has been shown to be inherently ineffective at transducing airway epithelia from the apical surface. However, tripeptide proteasome inhibitors (such as LLnL) can dramatically enhance rAAV-2 transduction from the apical surface of human polarized airway epithelia by modulating the intracellular trafficking and processing of the virus. To further investigate potential differences between rAAV-2 and rAAV-5 that might explain their altered ability to transduce airway epithelia from the apical membrane, we examined the functional involvement of the ubiquitin/proteasome pathway and rate-limiting aspects of second-strand synthesis for these two rAAV serotypes. To this end, we conducted studies to compare the extent to which LLnL alters transduction efficiencies with both rAAV-2 and rAAV-2/5 by using luciferase and enhanced green fluorescent protein (EGFP) reporter vectors. Our results demonstrate that the coadministration of LLnL at the time of viral infection significantly enhanced transduction of both rAAV-2/5 and rAAV-2 from the apical surface of airway epithelia. Although rAAV-2/5 was slightly more effective at transducing epithelia from the apical membrane, rAAV-2 transduction was superior to that of rAAV-2/5 in the presence of proteasome inhibitors. Interestingly, the basolateral membrane entry pathways for both serotypes were not significantly affected by the addition of LLnL, which suggests that apical and basolateral infectious pathways possess distinctive intracellular processing pathways for both rAAV-2 and rAAV-5. Studies comparing the transduction of short self-complementary (scAAV) to full-length conventional AAV EGFP vectors suggested that second-strand synthesis of rAAV genomes was not rate limiting for either serotype or altered by proteasome inhibitors following apical infection of polarized airway epithelia. These findings suggest that both rAAV-2 and rAAV-5 share similar intracellular viral processing barriers that involve the ubiquitin/proteasome system, but do not appear to involve second-strand synthesis.
Recombinant adeno-associated virus (rAAV) is one of the most promising vectors for gene delivery to airway epithelia for the gene therapy of cystic fibrosis (8). The most commonly studied AAV vector for gene transfer is derived from the type 2 serotype (rAAV-2). However, rAAV-2 has been shown to be inherently ineffective at transducing airway epithelia from the apical surface (3-5, 9). This limitation has hindered clinical applications of this serotype in human gene therapy trials for cystic fibrosis (1). Traditional hypotheses have suggested that the lack of apical receptors or coreceptors for AAV-2 may be the cause of its inefficient transduction (3, 4, 14). Later studies, which evaluated cellular uptake of rAAV-2 and the ability of proteasome inhibitors to enhance rAAV-2 transduction (i.e., transgene expression) from the apical membrane, modified earlier hypotheses to suggest that intracellular barriers for AAV-2 transduction might exist and act as the major rate-limiting factor for AAV transduction (5). Recent advances in the development of new AAV serotype vectors have demonstrated that both rAAV-5 and rAAV-6 are capable of efficiently infecting airway epithelia from the apical membrane (2, 9, 12). For example, one study demonstrated that rAAV-5 was more effective than rAAV-2 at transducing airway epithelia through the apical membrane at low titers of infection (14).
We hypothesized that the increased efficiency of rAAV-5 transduction from the apical membrane of airway epithelia may be due to a lack of proteasome barrier involvement in AAV-5, as compared to AAV-2, transduction. Previous work evaluating the effect of the ubiquitin/proteasome pathway on rAAV-2/5 pseudotyped virus (AAV-2 genome with type 5 capsids) in cell lines indeed suggests that ubiquitination of the AAV-5 capsid is similar to that observed with rAAV-2 (13). However, the effect of proteasome inhibitors on rAAV-5 transduction in polarized human airway models has yet to be evaluated. To this end, the present study has attempted to more clearly define the functional involvement of the ubiquitin/proteasome system in rAAV-2 and rAAV-2/5 transduction of airway epithelia following apical and basolateral infection. Furthermore, rate-limiting aspects of second-strand genome conversion, as well as the effect of proteasome inhibitors on this process, were also evaluated for the two serotypes by using self-complementary rAAV (scAAV) vectors.
MATERIALS AND METHODS
rAAV vectors and experimental conditions.
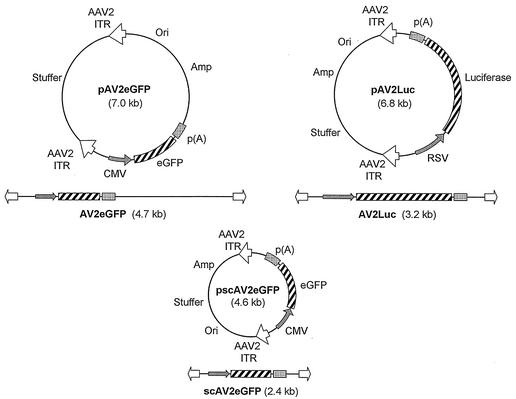
Stocks of recombinant virus for AV2Luc, AV2eGFP, scAV2eGFP, AV2/5Luc, AV2/5eGFP, and scAV2/5eGFP were generated with the previously described proviral plasmids (Fig. 1) and methods (13). All genomes harbored AAV-2 inverted terminal repeats (ITRs) from pSub201 (11), and vectors with the AV2/5 nomenclature were pseudotyped with AAV-5 capsid as previously described (13). Luciferase vectors (AV2Luc and AV2/5Luc) utilized the Rous sarcoma virus (RSV) promoter and were 3.7 kb in length. Two types of enhanced green fluorescent protein (EGFP)-based vectors (self-complementary and full-length) harbored an identical EGFP expression cassette driven by the cytomegalovirus (CMV) immediate-early promoter. The self-complementary vectors had viral genomes 2.3 kb in length and were called “scAV2eGFP and scAV2/5eGFP.” The conventional full-length vectors, termed “AV2eGFP” and “AV2/5eGFP,” had viral genomes 4.7 kb in length. Primary human airway epithelial cells were obtained from the bronchi of lungs that were removed from organ donation or from surgical polypectomies. Epithelial cells were collected by enzymatic digestion and were grown at an air-liquid interface for more than 3 weeks until electrical resistance was greater than 300 Ω (4, 6). For infection studies, rAAV (multiplicity of infection [MOI] = 10,000 particles per cell) was added to either the apical or basolateral side of the epithelia in the presence or absence of 40 μM LLnL (N-acetyl-l-leucyl-l-leucyl-norleucine; Calbiochem, San Diego, Calif.). Viruses and chemicals were removed after 24 h of infection, and epithelia were evaluated for gene expression at two time points ranging from 5 to 18 days postinfection. Transepithelial electrical resistance was determined at the time of analysis to confirm that the integrity of the epithelial layer was intact for each sample. For studies in HeLa cells, full-length and self-complementary vectors were evaluated at an MOI of 1,000 particles per cell.
FIG. 1.
rAAV vectors. Proviral plasmids used to generate the rAAV vectors are shown for pAV2Luc, pAV2eGFP, and pscAV2eGFP. The viral genomic structure is given below each proviral plasmid.
Luciferase activity assays.
Luciferase activity in infected polarized human airway epithelia was measured with a Promega luciferase assay kit. At 5 and 14 days postinfection, polarized airway cells were lysed with 200 μl of lysis buffer and subjected to microcentrifugation at 10,000 × g for 60 s. The supernatant was then incubated with the luciferase substrate in a model TD-20/20 luminometer (Turner Designs Instruments, Sunnyvale, Calif.) per the manufacturer's instructions, and luciferase activity was determined at a sensitivity of 47%.
Image acquisition and analysis of GFP expression.
To evaluate the longitudinal kinetics of gene expression in the same samples, we performed quantitative morphometry for GFP expression. Fluorescent images were acquired at days 5 and 18 postinfection. A Hamamatsu ORCA high-sensitivity, high-speed digital camera and Improvision OpenLab V3.05 software were used for fluorescent imaging. The image acquisition parameters were set to be exactly the same for the time course comparisons, and a blank culture filter well was used as the background for normalization. Ten images were randomly captured in the plane of focus for each experimental sample. The acquired images were then analyzed by NIH Image V1.62. The average fluorescent intensity of expressing cells and the area of GFP expression were used as indices for quantification of GFP expression.
Southern blot analysis of rAAV vector genomes.
Viral DNAs were prepared from the purified scAAV and full-length rAAV stocks by proteinase K digestion followed by phenol and phenol-chloroform extraction. Structure analysis of the viral genomes was performed in 0.9% alkaline agarose gels (50 mM NaOH, 1 mM EDTA) followed by Southern blotting as previously described (10). Hirt DNAs from rAAV-infected cells were also prepared and analyzed as previously described (13) in order to evaluate differences in gene conversion between full-length and self-complementary vectors. Southern blots were probed with a 32P-labeled EGFP probe.
Statistical analysis.
Data are presented as mean values ± standard errors. For the comparisons of two means, statistical significance was evaluated with an unpaired Student's t test. For multiple comparisons, one-way analysis of variance (ANOVA) followed by the Student-Newman-Keuls multiple comparison test was used. Differences were considered significant at P < 0.05.
RESULTS AND DISCUSSION
Polarity of infection with rAAV-2 and rAAV-2/5.
rAAVs have demonstrated great promise as vectors for gene delivery to the airway and the treatment of cystic fibrosis. However, studies evaluating the rAAV-2 vectors that are currently used in clinical trials have suggested that transduction from the apical membrane of polarized human airway epithelia with this serotype is extremely inefficient and is 200-fold less efficient than that following basolateral membrane infection (1, 5). This difference in the polarity of transduction with rAAV-2 appears not to be caused by major differences in the binding or endocytosis of virus at apical or basolateral membranes, but rather appears to involve differential susceptibility to the ubiquitin/proteasome system. Small tripeptide proteasome inhibitors have been shown to selectively augment long-term transduction efficiencies with rAAV-2 from the apical membrane, but not from the basolateral membrane (5). A recent report demonstrating the increased efficiency of transduction in polarized human airway epithelia using rAAV-5 (14) prompted us to evaluate whether transduction with this serotype was also affected by proteasome inhibitors.
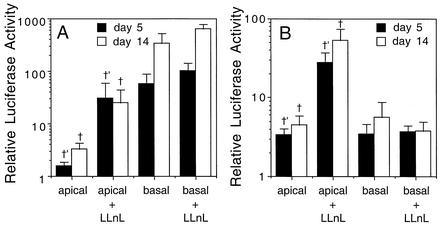
We had originally hypothesized that the increased efficiency of rAAV-5 transduction from the apical membrane of airway epithelium may be due to a lack of ubiquitin or proteasome involvement in AAV-5 transduction, as opposed to AAV-2 transduction. The rationale for this hypothesis stemmed from the fact that AAV-5 appears to infect epithelia through 2,3-linked sialic acid binding receptors (12), while the apical membrane entry pathway in airway epithelia for AAV-2 has yet to be identified (4, 5). These differences in receptor entry pathways suggested the potential for differences in intracellular trafficking for the two serotypes of rAAV. In contrast to the previous study, which evaluated rAAV-2 and rAAV-5 transduction efficiencies from the apical membrane (14), our comparisons using luciferase reporter vectors demonstrated only marginally (two- to threefold) better AV2/5Luc transduction from the apical membrane, as compared to AV2Luc, at 5 days postinfection (Fig. 2). At 14 days postinfection, both serotypes led to similar levels of transduction from the apical membrane. One potential explanation for the disparity between these observations, which used an identical airway model system, is the fact that the MOIs of infection used in the present study were 10-fold greater than those previously evaluated by Zabner and colleagues (14). Hence, the AAV-2 receptor may have a lower binding affinity than AAV-5 receptors. Interestingly, transduction with AV2Luc was far more efficient (∼100-fold) from the basolateral membrane than from basolateral or apical infection with AV2/5Luc (Fig. 2). Despite the 100-fold-greater transduction of rAAV-2 from the basolateral membrane compared to that from the apical membrane, rAAV-2/5 demonstrated no polarity preference in transduction with equivalent levels of transgene expression following either basolateral or apical infection. These findings demonstrate that the polarity of airway epithelia differentially affects transduction with these two serotypes of AAV.
FIG. 2.
LLnL augments rAAV-2 and rAAV-2/5 transduction from the apical membrane of polarized airway epithelia. The effect of proteasome inhibitor coadministration on transduction of AV2Luc (A) and AV2/5Luc (B) was evaluated following apical and basolateral infection of human polarized airway epithelia at an MOI of 10,000 particles per cell in the presence and absence of LLnL (40 μM). Luciferase activity was measured at 5 and 14 days postinfection. Values represent the mean (± standard error) relative luciferase activity for three independent tissue samples. For each tissue sample, two to three transwells were evaluated (n = 6 to 9 total). Significant differences in gene expression following LLnL treatment were only seen following apical infection (P < 0.01 using the Student's t test) for the comparisons marked † and †′.
Proteasome inhibitors augment rAAV-2 and rAAV-2/5 transduction from the apical membrane.
With the notion that polarity differentially influences rAAV-2 and rAAV-2/5 transduction in airway epithelia, we reasoned that the degree of ubiquitin or proteasome involvement might also differ between these two serotypes. To test this hypothesis, we directly compared the effect of the proteasome inhibitor LLnL on rAAV transduction using these two serotypes. Studies using luciferase reporter vectors demonstrated 30-fold (at 5 days postinfection) and 10-fold (at 14 days post-infection) augmentations in apical AV2Luc transduction when LLnL was coadministered at the time of viral infection (Fig. 2). In contrast to our original hypothesis, AV2/5Luc apical transduction was also augmented by LLnL treatment (8- to 11-fold) at both 5- and 14-day time points (Fig. 2). These findings suggest that the ubiquitin/proteasome system is a common feature that affects transduction with both serotypes from the apical membrane. Support for a unifying hypothesis of ubiquitin and proteasome involvement in rAAV transduction with both type 2 and type 5 serotypes has recently been reported (13). Yan and colleagues have demonstrated that both AAV-2 and AAV-5 capsids are receptive to ubiquitination in HeLa cells. Interestingly, at the same time points, treatment of LLnL did not significantly influence transduction with either AV2Luc or AV2/5Luc following basolateral infection. These findings suggest some aspect of epithelial polarity directs the manner in which the ubiquitin/proteasome pathway influences transduction with both serotypes.
Proteasome inhibitors similarly augment transduction of self-complementary and full-length rAAV vectors.
Proteasome inhibitors may act to augment rAAV transduction at several levels, including: (i) intracellular trafficking to the nucleus, (ii) viral uncoating, and/or (iii) viral genome conversion or second-strand synthesis. Under the premise that the ubiquitin/proteasome pathway helps to facilitate intracellular processing of rAAV, we reasoned that genome conversion of rAAV vectors is unaltered by the application of proteasome inhibitors. However, since rAAV intracellular trafficking to the nucleus is increased by proteasome inhibition in polarized airway epithelia (5), some aspects of enhanced genome conversion are difficult to directly address. For example, if LLnL enhances gene conversion events by altering the activity of nuclear DNA synthesis enzymes or the receptiveness of the viral genome to gene conversion processes, the outcome would be indistinguishable from an increase in viral genome accumulation in the nucleus in the absence of enhancement of gene conversion. Although earlier studies have confirmed that LLnL enhances nuclear trafficking of rAAV in polarized airway epithelia using in situ localization of 35S-labeled rAAV-2 (5), we could not exclude the possibility that LLnL may also affect functions involved in second-strand synthesis, since both scenarios could result in increased gene expression. In this study, we sought to use an alternative genetic approach to address whether LLnL influences gene conversion events by comparing full-length and self-complementary EGFP-based AAV vectors. Conventional AAV vectors that package a full-length 4.7-kb single-stranded genome require host cell synthesis of the complementary second strand for transgene expression. However, when the viral genome is half the length of the wild type, the rAAV can package double-stranded Rf monomers to give so-called “self-complementary” vectors (10). Expression of scAAV vectors is characterized by more immediate and higher levels of gene expression (10), since they bypass the requirement for gene conversion that has been recognized as a rate-limiting step in rAAV transduction for many cell types (7). Using this type of vector, we reasoned that gene expression endpoints could then be capable of preferentially evaluating intracellular processes that affect rAAV transduction while minimizing influences responsible for gene conversion. Furthermore, by comparing both full-length and self-complementary vectors in the presence or absence of proteasome inhibitor, the extent to which proteasome inhibition enhances intracellular processing and/or nuclear gene conversion involved in rAAV transduction could be inferred.
Studies using self-complementary rAAV-2 and rAAV-2/5 vectors require the use of an alternative reporter gene that is smaller than luciferase. To this end, we developed a set of full-length (AV2eGFP and AV2/5eGFP) and self-complementary (scAV2eGFP and scAV2/5eGFP) vectors that were either 4.7 or 2.3 kb in length, respectively. In contrast to luciferase studies, the evaluation of EGFP provides several advantages. First, it has the ability to evaluate the kinetics of gene expression longitudinally over time using the same samples, a factor that is critical for evaluating scAAV vectors that have an altered kinetics of transduction. Second, it also provides the advantage of being able to address both changes in fluorescent intensity and in the number of cells expressing EGFP. To this end, we developed a quantitative morphometric approach to evaluate several indices of EGFP expression, including fluorescent intensity, cell number, and area of EGFP expression. To minimize the subjective judgment for image acquisition, fluorescent fields were randomly taken for each well, and more than 36 images were analyzed for each experimental group. The relationship between fluorescent area and positive cell number counts was linear for all samples tested (data not shown). We have chosen to present only the intensity and area determination as a basis for comparison between different treatment conditions (Fig. 3 and 4).
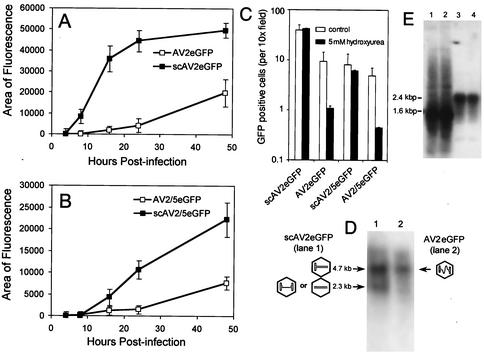
FIG. 3.
Analysis of full-length and self-complementary EGFP-expressing AAV vectors in HeLa cells. In an effort to characterize the previously described differences in the expression patterns of full-length and self-complementary rAAV vectors, analysis was performed following infection of HeLa cells at an MOI of 1,000 particles per cell. (A) Quantification of relative EGFP-expressing area for AV2eGFP and scAV2eGFP vectors. (B) Quantification of relative EGFP-expressing area for AV2/5eGFP and scAV2/5eGFP vectors. The values represent the mean (± standard error) for three independent infections. For each experimental sample, 10 random fields were captured for quantification by NIH Image. NIH Image quantification of relative intensity demonstrated a direct correlation with relative mean area calculations (data now shown). (C) Quantification of EGFP expression for conventional full-length vectors and self-complementary vectors in the presence of the DNA synthesis inhibitor HU. HeLa cells at 30% confluence were treated with 5 mM HU 24 h prior to infection with AV2eGFP, AV2/5eGFP, scAV2eGFP, or scAV2/5eGFP vectors at an MOI of 1,000 particles per cell. The GFP-positive cells were then quantified at 24 h postinfection. The values represent the mean (± standard error) GFP-positive cells per ×10 field for three independent infections (10 random fields were quantified for each sample). (D) Evaluation of scAV2eGFP (lane 1) and AV2eGFP (lane 2) viral DNA harvested from purified virus on NaOH agarose gels. Southern blot analysis of the NaOH gel was performed with an EGFP probe. Schematic representations of predicted viral genome structure and molecular size (kilobases) are given adjacent to the Southern blot. (E) Southern blot analysis of Hirt DNA harvested from HeLa cells infected with AV2eGFP (lanes 1 and 2) and scAV2eGFP (lanes 3 and 4) at 24 h postinfection. Southern blots were probed with a 32P-labeled EGFP DNA probe. The 1.6-kb hybridizing bands from AV2eGFP-infected cells are single stranded. The 2.4-kb hybridizing bands from scAV2eGFP-infected cells are the size of double-stranded genomes.
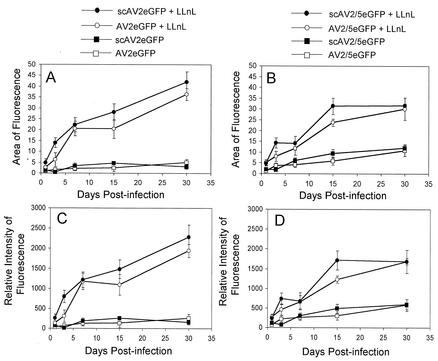
FIG. 4.
Quantification of EGFP expression following apical infection of polarized human airway epithelia with self-complementary and full-length EGFP vectors. Both the relative mean area of fluorescence (A and B) and the mean fluorescent intensity (C and D) were evaluated following transduction with AV2eGFP and scAV2eGFP (A and C) or AV2/2eGFP and scAV2/5eGFP (C and D) vectors in the presence or absence of LLnL (40 μM) at an MOI of 10,000 particles per cell. Fluorescent images were acquired on 1, 3, 7, 15, and 30 days postinfection. The background was adjusted to the level of uninfected transwells and held constant for analysis of each group. The values represent the mean (± standard error) for three independent tissue samples. For each tissue sample, three transwells were evaluated by imaging 10 random fields in each sample at the various time points (n = 9 total transwells for each experimental point). Statistical analyses of expression patterns for the various vector and LLnL treatment groups were performed by ANOVA followed by the Student-Newman-Keuls multiple comparison test. No significant differences were noted between AVeGFP and scAVeGFP vector groups for both serotypes in the presence or absence of LLnL. However, statistical differences (P < 0.05) were seen when comparing AVeGFP (−LLnL) and AVeGFP (+LLnL) or scAVeGFP (−LLnL) and scAVeGFP (+LLnL) for both serotypes.
Preliminary analysis of transduction, using both full-length and self-complementary EGFP vectors in HeLa cells, confirmed that both scAV2eGFP and scAV2/5eGFP demonstrated a faster onset of EGFP expression and overall higher transduction than the full-length viruses, AV2eGFP and AV2/5eGFP (Fig. 3A and B). The functional analysis of these two types of viral vectors was also performed in HeLa cells pretreated with hydroxyurea (HU), a DNA synthesis inhibitor, for 24 h prior to infection. As shown in Fig. 3C, pretreatment with HU did not affect transgene expression from the self-complementary vectors scAV2eGFP and scAV2/5eGFP. In contrast, AV2eGFP and AV2/5eGFP transduction was inhibited 10-fold by the same concentration of HU. These experiments support earlier reports that scAAV vector transduction is not rate limited by second-strand synthesis (10). Evaluation of vector genomes from purified scAAV and full-length AAV viruses also confirmed that approximately 75% of packaged genomes from scAAV vectors were indeed Rf monomers (Fig. 3D). The remaining shorter genomes in the scAAV vector preparations may represent either noncovalently linked double-stranded packaged genomes or single-stranded packaged genomes. Furthermore, analysis of Hirt DNA from HeLa cells that were infected with the two vector types demonstrated that at early times postinfection, self-complementary vectors had a significantly higher percentage of double-stranded genomes than full-length vectors (Fig. 3E). The high percentage of double-stranded genomes in Hirt DNA from scAAV vector-infected cells suggests that the residual 2.3-kb genomes seen on NaOH gels of scAAV vectors (Fig. 3D, lane 1) may primarily originate from double-stranded noncovalently linked genomes copackaged into a single virion as opposed to single-stranded packaged genomes.
To evaluate whether second-strand synthesis was a major rate-limiting step in the transduction of polarized airway epithelia cells from the apical membrane, we performed experiments evaluating the kinetics of EGFP expression between scAV2eGFP, scAV2/5eGFP, AV2eGFP, and AV2/5eGFP vectors. Results from these experiments (Fig. 4) demonstrated no significant alteration in the onset or longevity of EGFP expression for either AAV-2 or AAV-2/5 vector sets. Similarly, no differences in the expression profile of self-complementary or full-length vectors were seen for either serotype in the presence of LLnL. These findings demonstrate that second-strand synthesis is not the rate-limiting step that prevents efficient transduction from the apical membrane. Furthermore, the fact that both self-complementary and full-length vectors were similarly augmented by proteasome inhibitor suggests that aspects of viral transduction that occur prior to second-strand synthesis most likely limit transduction from the apical membrane for both rAAV-5 and rAAV-2 serotypes.
In summary, the results from the present study demonstrate that proteasome inhibitors can augment transduction of both rAAV-2 and rAAV-5 in airway epithelia following apical membrane infection. The influence of proteasome inhibitors on transduction with these two serotypes appears to involve intracellular viral processing and may include the rate of movement to the nucleus and/or viral uncoating. Studies with self-complementary vectors have appeared to rule out second-strand synthesis as a rate-limiting step in this transduction process. The fact that proteasome inhibitor had no significant effect on basolateral transduction of either rAAV-2 or rAAV-5 also supports the notion that second strand synthesis is not rate limiting in airway epithelia. These studies suggest that the proteasome inhibitor LLnL does not significantly alter the level or activity of host enzymes involved in AAV single-stranded genome conversion. Further investigation into the mechanism by which proteasome inhibitors selectively augment rAAV transduction from the apical membrane of airway epithelia may lead to more effective strategies to improve transduction to the airway for both rAAV-5 and rAAV-2.
Acknowledgments
This work was supported by NIH RO1 HL58340 and Targeted Genetics Corporation.
We gratefully acknowledge P. Karp and J. Zabner of the Gene Therapy Center (P30 DK54759) Cells and Tissue Core and the University of Iowa DERC (NIDDK) for tissue culture media supplies. We also thank Kevin Wyne and Jude Gustafson for proofreading the manuscript.
REFERENCES
- 1.Aitken, M. L., R. B. Moss, D. A. Waltz, M. E. Dovey, M. R. Tonelli, S. C. McNamara, R. L. Gibson, B. W. Ramsey, B. J. Carter, and T. C. Reynolds. 2001. A phase I study of aerosolized administration of tgAAVCF to cystic fibrosis subjects with mild lung disease. Hum. Gene Ther. 12:1907-1916. [DOI] [PubMed] [Google Scholar]
- 2.Auricchio, A., E. O'Connor, D. Weiner, G. P. Gao, M. Hildinger, L. Wang, R. Calcedo, and J. M. Wilson. 2002. Noninvasive gene transfer to the lung for systemic delivery of therapeutic proteins. J. Clin. Investig. 110:499-504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bals, R., W. Xiao, N. Sang, D. J. Weiner, R. L. Meegalla, and J. M. Wilson. 1999. Transduction of well-differentiated airway epithelium by recombinant adeno-associated virus is limited by vector entry. J. Virol. 73:6085-6088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Duan, D., Y. Yue, Z. Yan, P. B. McCray, and J. F. Engelhardt. 1998. Polarity influences the efficiency of recombinant adeno-associated virus infection in differentiated airway epithelia. Hum. Gene Ther. 9:2761-2776. [DOI] [PubMed] [Google Scholar]
- 5.Duan, D., Y. Yue, Z. Yan, J. Yang, and J. F. Engelhardt. 2000. Endosomal processing limits gene transfer to polarized airway epithelia by adeno-associated virus. J. Clin. Investig. 105:1573-1587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Duan, D., Y. Zhang, and J. F. Engelhardt. 1998. Gene delivery to the airway, p. 13.9.1-13.9.34. In N. C. Dracopoli, J. L. Haines, B. R. Korf, D. T. Moir, C. C. Morton, C. E. Seidman, J. G. Seidman, and D. R. Smith (ed.), Current protocols in human genetics. John Wiley & Sons, Inc., New York, N.Y.
- 7.Ferrari, F. K., T. Samulski, T. Shenk, and R. J. Samulski. 1996. Second-strand synthesis is a rate-limiting step for efficient transduction by recombinant adeno-associated virus vectors. J. Virol. 70:3227-3234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Flotte, T. R., and B. L. Laube. 2001. Gene therapy in cystic fibrosis. Chest 120:124S-131S. [DOI] [PubMed]
- 9.Halbert, C. L., J. M. Allen, and A. D. Miller. 2001. Adeno-associated virus type 6 (AAV6) vectors mediate efficient transduction of airway epithelial cells in mouse lungs compared to that of AAV2 vectors. J. Virol. 75:6615-6624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.McCarty, D. M., P. E. Monahan, and R. J. Samulski. 2001. Self-complementary recombinant adeno-associated virus (scAAV) vectors promote efficient transduction independently of DNA synthesis. Gene Ther. 8:1248-1254. [DOI] [PubMed] [Google Scholar]
- 11.Samulski, R. J., L.-S. Chang, and T. Shenk. 1987. A recombinant plasmid from which an infectious adeno-associated virus genome can be excised in vitro and its use to study viral replication. J. Virol. 61:3096-3101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Walters, R. W., S. M. Yi, S. Keshavjee, K. E. Brown, M. J. Welsh, J. A. Chiorini, and J. Zabner. 2001. Binding of adeno-associated virus type 5 to 2,3-linked sialic acid is required for gene transfer. J. Biol. Chem. 276:20610-20616. [DOI] [PubMed] [Google Scholar]
- 13.Yan, Z., R. Zak, G. W. Luxton, T. C. Ritchie, U. Bantel-Schaal, and J. F. Engelhardt. 2002. Ubiquitination of both adeno-associated virus type 2 and 5 capsid proteins affects the transduction efficiency of recombinant vectors. J. Virol. 76:2043-2053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zabner, J., M. Seiler, R. Walters, R. M. Kotin, W. Fulgeras, B. L. Davidson, and J. A. Chiorini. 2000. Adeno-associated virus type 5 (AAV5) but not AAV2 binds to the apical surfaces of airway epithelia and facilitates gene transfer. J. Virol. 74:3852-3858. [DOI] [PMC free article] [PubMed] [Google Scholar]