Abstract
Nova is a neuron-specific RNA binding protein targeted in patients with the autoimmune disorder paraneoplastic opsoclonus-myoclonus ataxia, which is characterized by failure of inhibition of brainstem and spinal motor systems. Here, we have biochemically confirmed the observation that splicing regulation of the inhibitory GABAA receptor γ2 (GABAARγ2) subunit pre-mRNA exon E9 is disrupted in mice lacking Nova-1. To elucidate the mechanism by which Nova-1 regulates GABAARγ2 alternative splicing, we systematically screened minigenes derived from the GABAARγ2 and human β-globin genes for their ability to support Nova-dependent splicing in transient transfection assays. These studies demonstrate that Nova-1 acts directly on GABAARγ2 pre-mRNA to regulate E9 splicing and identify an intronic region that is necessary and sufficient for Nova-dependent enhancement of exon inclusion, which we term the NISE (Nova-dependent intronic splicing enhancer) element. The NISE element (located 80 nucleotides upstream of the splice acceptor site of the downstream exon E10) is composed of repeats of the sequence YCAY, consistent with previous studies of the mechanism by which Nova binds RNA. Mutation of these repeats abolishes binding of Nova-1 to the RNA in vitro and Nova-dependent splicing regulation in vivo. These data provide a molecular basis for understanding Nova regulation of GABAARγ2 alternative splicing and suggest that general dysregulation of Nova's splicing enhancer function may underlie the neurologic defects seen in Nova's absence.
The regulation of eukaryotic gene expression involves an ordered sequence of events within various subcellular compartments. RNA binding proteins (RBPs) play a critical role in this process, acting at multiple levels to regulate and diversify gene expression, and such functions are likely to be of particular importance to neurons. RBPs regulate processes such as alternative splicing, which is an important mechanism for generating complexity in specialized cells, particularly in neurons. For example, all of the major neurotransmitter receptors contain subunits that are alternatively spliced, and these variants can alter receptor localization, ligand binding, signal transduction, and electrophysiological properties (13, 20, 44, 51). Moreover, RBPs and aberrant splicing of mRNAs encoding proteins critical for proper functioning of neurons have been associated with a number of neurological diseases (reviewed in reference 19). To understand the role RBPs play in regulating gene expression in neurons, it would be necessary to identify their complement of RNA targets and to understand how they are recognized amidst the complexity of the cellular environment.
Regulation of alternative splicing occurs through recognition of cis-acting sequences in pre-mRNA by trans-acting factors. cis-acting repressor elements that mediate the exclusion of the neuron-specific exons in nonneuronal tissues have been identified through studies of neuron-specific splicing in transcripts such as c-src, the GABAA receptor γ2 (GABAARγ2) subunit, the N-methyl-d-aspartate receptor NR1 subunit, and clathrin light-chain B mRNAs (3, 9, 10, 60, 63, 64). These repressor sequences are mostly pyrimidine rich and, when present in trans as RNA competitors in vitro, stimulate neuronal splicing patterns (10, 64). A number of generally expressed trans-acting factors, including hnRNP H, hnRNP F, KSRP, and PTB (3, 10, 12, 46), have been found to bind such sequences and are believed to play important roles in regulating alternative splicing.
In addition to the contribution of generally expressed factors, it has long been suspected that tissue-specific factors regulate alternative splicing in the brain (1). Study of the paraneoplastic neurologic disorders (PNDs) has resulted in the identification of Nova-1 (7), a neuron-specific protein involved in splicing regulation (32). PNDs are rare immune-mediated disorders associated with ectopic expression of neuron-specific proteins in tumors (reviewed in references 15 and 48). Patients harbor characteristic high titers of antibodies specific for these antigens in their sera, and immune targeting of these antigens in the central nervous system (CNS) is thought to result in the neuronal death and neurological symptoms seen in PNDs. Nova proteins are the target antigens in paraneoplastic opsoclonus-myoclonus ataxia, a PND characterized by loss of inhibitory control of motor neurons in the spinal cord and brainstem.
The Nova proteins are RBPs containing 3 KH-type (for hnRNP K homology) RNA binding domains. Widely expressed KH domain-containing proteins have been shown to play roles in a number of aspects of RNA processing, including mRNA stability and translational control (hnRNP K and hnRNP E1/E2) (35, 49), subcellular localization of RNAs to specific sites in the cell cytoplasm (ZBP-1 and Vera) (17, 55), and RNA splicing (KSRP [46] and SF1 [2] in mammals, PSI [57] in Drosophila, and MER-1 [21] in Saccharomyces cerevisiae). Nova-1 is expressed exclusively in neurons of the CNS, including the brainstem and spinal motor neurons targeted in paraneoplastic opsoclonus-myoclonus ataxia. Nova-1 null mice display a severe phenotype. Although they appear normal at birth, they are markedly smaller than their wild-type littermates at as little as 3 to 4 days postbirth and display symptoms of motor impairment such as motor weakness and an action-induced tremor (32).
Nova proteins act as sequence-specific RNA binding proteins. A consensus binding sequence determined by in vitro RNA selection consists of a stem-loop RNA containing (UCAUY)3 in the loop region (6). This binding is sequence specific, as a number of discrete nucleotide substitutions, such as mutagenesis of this core to (UCUUY)3, abolish RNA binding. Studies using the third Nova KH domain in isolation identified high-affinity targets containing a single UCAY element, again within a hairpin loop context (33), and allowed solution of the X-ray structure of a Nova KH3/RNA cocrystal. The crystal structure reveals that the RNA stem-loop presents the UCAY sequence to a “molecular vise” formed by a cleft between the variable and invariant protein loops of the Nova KH3 domain (42). In particular, the CA dinucleotide forms contacts with the protein to create pseudo-Watson-Crick base pairs and predicts that these residues are absolutely necessary for binding to occur. The structure agrees precisely with Nova KH3 domain-RNA binding studies in which RNA mutations still conforming to the consensus HYCAY (where H denotes A, C, or U and Y denotes C or U) are tolerated (33).
These studies led to the identification of GlyRα2 inhibitory neurotransmitter receptor subunit pre-mRNA as a Nova target RNA. Nova binds this transcript 80 nucleotides (nt) upstream of an alternatively spliced exon, E3A, which is spliced in a mutually exclusive manner with a downstream exon, E3B. These exons encode part of the extracellular domain of the receptor and thus may be involved in ligand binding (39). Nova protein in mouse brain lysates coimmunoprecipitates with GlyRα2 pre-mRNA (6) and can be UV cross-linked to an RNA encoding a region of the GlyRα2 intronic sequence (32). Cotransfection of GlyRα2 minigene constructs with a Nova-1 expression plasmid demonstrated that Nova-1 is able to act directly on GlyRα2 pre-mRNA to enhance the inclusion of GlyRα2 E3A and that this action is dependent on the integrity of the intronic (UCAU)3 Nova binding site (32).
Analysis of GlyRα2 alternative splicing in vivo provided genetic support for these conclusions, as Nova-1 null mice showed a decrease in utilization of GlyRα2 E3A relative to wild-type littermates (32). This defect in alternative splicing regulation was restricted to regions that normally express Nova-1 and was specific. Regulation of a number of alternatively spliced RNAs was unaffected in Nova-1 null mice, with one notable exception: alternative splicing of exon 9 in the inhibitory neurotransmitter receptor subunit pre-mRNA, GABAARγ2, was disrupted in Nova-1 null mice in a dose-dependent manner, and these defects were again restricted to regions which normally express Nova-1.
Alternative splicing of GABAARγ2 cassette exon E9 generates two mRNA isoforms, γ2L (long [E9 included]) and γ2S (short), which show differential spatial and temporal expression characteristics (24, 27, 59). Inclusion of E9 adds 8 amino acids to an intracellular loop of the protein which contain a consensus site for phosphorylation by protein kinase C (37, 61). Phosphorylation of this site has been shown to modulate GABAAR function (38). Mice engineered to express only the short form of the GABAARγ2 subunit display a higher level of anxiety than control mice (30) and increased sensitivity to benzodiazepines (53). Studies aimed at trying to understand the mechanism behind regulation of GABAARγ2 splicing have shown that nucleotides within the exon and the adjacent splice sites are essential for regulation in a neuronal versus a nonneuronal cell line (63). PTB has been shown to inhibit γ2L splicing, leading to the hypothesis that splicing is regulated by a mechanism involving derepression of splicing at this site in neurons of the CNS (3, 64). However, it is unknown whether these observations are relevant to γ2L splicing in neurons and/or whether they involve Nova protein.
To understand how Nova acts to regulate GABAARγ2 splicing, we have determined the sequence requirements for Nova-1 regulation of E9 inclusion. We find that previously mapped elements important for the regulation of splicing in tissue culture are not involved in Nova regulation of γ2L. Remarkably, a broad and unbiased screen for regulatory elements over 3.7 kb of GABAARγ2 pre-mRNA revealed that a single intronic YCAY repeat element is necessary and sufficient to mediate Nova-dependent regulation. These studies define Nova as a neuron-specific splicing regulator that functions through action on a newly identified intronic splicing enhancer which lies far downstream of the regulated exon.
MATERIALS AND METHODS
RNA preparation, RNase protection assays, and RT-PCR.
Using a modified guanidine-acid phenol protocol (11), total RNA was extracted from mouse tissues. RNA from tissue culture cells was purified using the RNeasy Mini kit system (Qiagen). RNase protection assays were performed using the RPA III kit (Ambion) according to the manufacturer's instructions and 5 μg of total RNA isolated from mouse spinal cords. Hybridization was performed at 56°C, and digests were performed with RNase T1. Using T7 RNA polymerase from gel-purified PCR products generated with primers gaba-rpa-F (5′-GAAATTAATACGACTCACTATAGGGAGTTGAATGGTTGCTGATCTGGGACG), gaba-rpa-R (5′-TCCCGCTCGTCGTCTGGTATGGCACCCTGCATTATTTTGTC), cla-rpa-F (5′-GAAATTAATACGACTCACTATAGGGAGGGGGTCTCCTCCTTGGATTCT), and cla-rpa-R (5′-TCCCGCTCGTCGTCTGACCGAACAGGAGTGGCGGGAG) or from plasmid pTRI-β-Actin-Mouse antisense control template (Ambion), antisense probes were in vitro transcribed. Probes were labeled by incorporation of [α-32P]UTP, and specific activities were adjusted by incorporation of unlabeled UTP to give protected fragments of approximately equal intensities for each message. Reverse transcription-PCR (RT-PCR) was performed as previously described (Jensen et al. [32]). PCR primers to human β-globin were E1F (5′-CTGAGGAGAAGTCTGCCGTTACTG) and E3R (5′-CAGCACACAGACCAGCACGTTG).
Plasmid constructs.
The GABAARγ2 minigene containing full-length introns was generated by long-range PCR using primers γ2 GABA F and γ2 GABA R (32) and a subclone derived from mouse BAC 608F8 (Research Genetics). The product was cloned into pGem-T-Easy (Promega) and transferred to pcDNA3 (Invitrogen) via EcoRI. Mutations of minigene constructs were made with a QuikChange site-directed mutagenesis kit (Stratagene) and specifically designed primers (Operon).
Using plasmid sp64-HβΔ6 (a kind gift from A. R. Krainer) as the template, the β-globin minigene was generated by PCR using primers glo-KpnF (5′-ATGGTACCTGACTCCTGAGGAGAAG) and glo-Rc (5′-CCACTTTCTGATAGGCAGCC) and cloned into pcDNA3 (Invitrogen) via KpnI and EcoRI. Exon 2 was shortened by PCR. Chimeric minigene constructs were generated by PCR.
cDNAs encoding T7-tagged Nova-1 including alternative exon H and T7-tagged hnRNP E1 were cloned into pCI-neo (Promega). All DNA constructs generated by PCR or site-directed mutagenesis were sequenced in their entirety. Plasmid DNA for transfection into cell lines was prepared by a modified cesium chloride method (56) and dialyzed with 1× Tris-EDTA buffer.
Cell transfection.
Using Fugene6 (Roche) as described by the manufacturer, N2A and 293T cells (American Type Culture Collection) were transfected with 2.25 μg of total DNA in a mixture composed of 0.25 μg of the appropriate minigene and variable amounts of pNova-1 and empty pcIneo vector. After 40 h, the cells were washed with 1× phosphate-buffered saline, collected by scraping, and halved for RNA extraction and protein extraction. Western blotting was performed using horseradish peroxidase-conjugated anti-T7 tag monoclonal antibody (Novagen) and detected by chemiluminescence (NEN).
Fusion protein synthesis.
Full-length recombinant T7/His-tagged Nova-1 fusion protein was produced and purified as previously described (41). Full-length Nova-1 cDNA including alternative exon H was cloned into pET21a (Novagen) and transformed into BL21-competent cells, followed by standard IPTG (isopropyl-β-d-thiogalactopyranoside) induction and purification by nickel chelation chromatography.
Nitrocellulose filter binding assays and boundary mapping.
Labeled RNA (100 to 200 fmol) was incubated with the indicated concentrations of protein. Protein-RNA mixes were incubated in a total volume of 50 μl of 1× BB (200 mM KOAc, 10 mM TrisOAc [pH 7.7], 1 mM MgOAc, 10 mM dithiothreitol, 1 mg of heparin/ml) for 15 min at room temperature. Binding solutions were passed through nitrocellulose filters (0.45-μm pore size; Millipore) and washed with 5 ml of 1× BB without dithiothreitol or heparin. Data were plotted as the percentage of total bound RNA versus the log of the protein concentration, and Kds were determined with Kaleidograph software (Synergy Software). SB2 RNA was synthesized as previously described (6). GABA wild-type and mutant RNAs were transcribed using T7 RNA polymerase and gel-purified PCR templates. Glo RNA was transcribed using T7 RNA polymerase directly from minigene pGlo2Δ digested with BbsI to yield a 175-nt RNA. Boundary mapping was performed essentially as described previously (14), with binding conditions identical to those used for nitrocellulose filter binding assays.
RESULTS
Splicing defects in Nova-1 null mice.
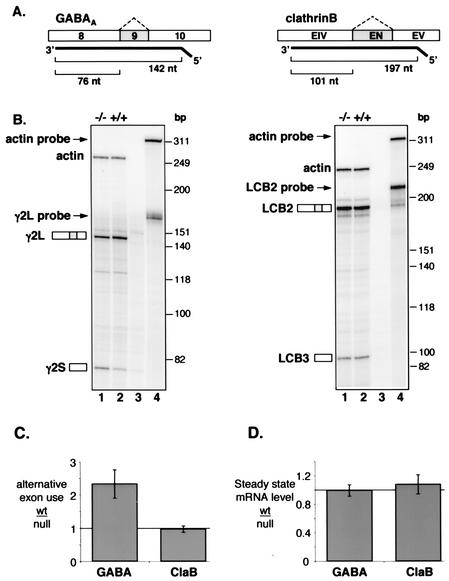
To confirm and extend the previous analysis of Nova-1 null mice by Jensen et al. (32), RNase protection assays were performed to assess the relative abundance of GABAARγ2-spliced mRNAs. RNA isolated from the spinal cord of Nova-1 null mice displayed a decrease of roughly 2.5-fold in the ratio of E9-inclusive (γ2L) to E9-exclusive (γ2S) splice forms of GABAARγ2 (Fig. 1 B and C). Moreover, no difference was detected in the total abundance of GABAARγ2 mRNA when compared to that of actin (Fig. 1D), indicating that the decrease in γ2L is accompanied by a complementary increase in the γ2S spliced form. As a control for the specificity of Nova action, we examined the alternative splicing and steady-state levels of clathrin light chain B (ClaB), which were found to be unchanged in the Nova-1 null mice, as expected (Fig. 1B to D). These results demonstrate specific, Nova-dependent alterations in alternative splicing, but not stability, of the γ2L isoform of GABAARγ2 pre-mRNA in the CNS of mice.
FIG. 1.
Changes in alternative splicing but not steady-state levels of GABAARγ2 transcripts in Nova-1 null mice. (A) Schematic representation of body-labeled antisense RNA probes used for RNase protection assays and the resulting product sizes used for quantitation. The cassette exons shown in grey are alternatively spliced, producing γ2L (contains exon 9) and γ2S (lacks exon 9) forms of the GABAARγ2 mRNA and LCB2 (contains exon EN) and LCB3 (lacks exon EN) forms of the ClaB mRNA (60). An antisense RNA probe specific for β-actin mRNA was also made. (B) Spinal cord RNA isolated from P9 (GABAARγ2 analysis) and P11 (ClaB) Nova-1 null mice (−/−) and their wild-type (+/+) littermates was analyzed by an RNase protection assay. Lanes 3, yeast RNA controls; lanes 4, undigested probes. (C and D) Quantitation of the data presented in panel B together with additional RNA samples (n = 5 littermate pairs). Products were quantitated by phosphorimager and corrected for the expected number of 32P-labeled uridines. (C) The ratio of long to short alternatively spliced products measured in control animals (wt) relative to the respective ratio for the corresponding Nova-1 null littermate. (D) For steady-state comparisons, the total of both alternatively spliced products was normalized to the amount of actin message detected in the same lane and compared between control animals (wt) and the corresponding Nova-1 null littermate. The solid bars represent the average changes in wild-type-null exon usage (C) or mRNA level (D); error bars represent standard deviations.
Nova regulates GABAARγ2 alternative splicing in cell lines.
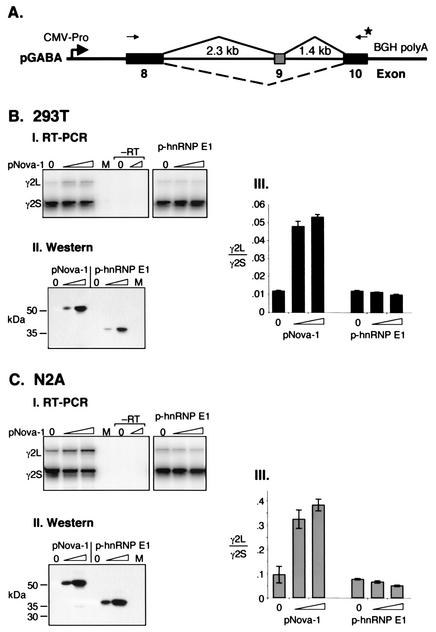
To assess the sequence requirements necessary for Nova-1 action on GABAARγ2 alternative splicing, a minigene construct was generated which contains the entire mouse genomic region surrounding the alternatively spliced exon, E9, extending into the adjacent exons, E8 and E10 (pGABA; Fig. 2A). This construct was transiently transfected into N2A or 293T cells in the presence or absence of pNova-1 plasmid encoding full-length Nova-1 protein, and spliced products were analyzed by semi-quantitative RT-PCR. Consistent with the analysis of GABAARγ2L splicing in Nova-1 null mice, cotransfection of pNova-1 caused a dose-dependent increase in E9 inclusion, resulting in an up to fivefold increase in the ratio of E9-inclusive (γ2L) to E9-exclusive (γ2S) splice forms in 293T cells (Fig. 2B). Similar results were seen with a mouse neuroblastoma cell line, N2A, for which a fourfold increase in exon 9 utilization was observed (Fig. 2C). No products were detected in control cells (Fig. 2BI and CI, lanes M), indicating that spliced products were derived from pGABA. Notably, the baseline level of E9 inclusion without the addition of exogenous Nova-1 was approximately 10-fold higher in N2A cells than in 293T cells, perhaps reflecting the fact that N2A cells express endogenous Nova proteins whereas 293T cells do not (data not shown).
FIG. 2.
GABAARγ2L splicing is enhanced specifically by Nova-1 in heterologous cell lines. (A) Schematic representation of the GABAARγ2 (pGABA) minigene containing the full mouse intronic regions surrounding and including exon 9 plus shortened exons 8 and 10 constructed for transient transfection assays. The primers used for RT-PCR analysis are depicted as arrows; the star denotes the 32P-labeled primer. Figure is not to scale. (B and C) RNA was isolated from 293T (B) or N2A (C) cells transiently transfected with pGABA and increasing amounts of pNova-1 (0, 0.5, or 2.0 μg) or p-hnRNP E1 (0, 0.05, or 0.2 μg) mammalian expression plasmid and analyzed by RT-PCR. Mock transfections were performed using no plasmid DNA (lanes M). (Panels I) RT-PCRs were performed in duplicate using controls without RT, representatives of which are shown (−RT). (Panels II) Western blot using anti-T7-tagged antibody showing titration of T7-tagged Nova-1 and hnRNP E1 protein levels after transfection. (Panels III) Quantitation of the data presented in panels I plus two additional independent transfections (n = 3). The bars represent the ratios of γ2L/γ2S spliced products ± standard deviations.
In contrast to the action of Nova-1, addition of exogenous hnRNP E1 caused a modest decrease in the utilization of GABAARγ2 E9 (Fig. 2B and C). Western blotting and immunofluorescence microscopy confirmed that using the plasmid concentrations indicated, comparable levels of transfected Nova-1 and hnRNP E1 were expressed and both proteins were localized to the nucleus (Fig. 2BII and CII and data not shown). Since hnRNP E1 and Nova contain three KH-type RNA binding domains whose sequences and spacings are similar, these results are consistent with the hypothesis that sequence-specific interactions between Nova-1 and GABAARγ2 RNA are necessary for splicing regulation.
Defining the region of GABAARγ2 pre-mRNA necessary for Nova-1 regulation.
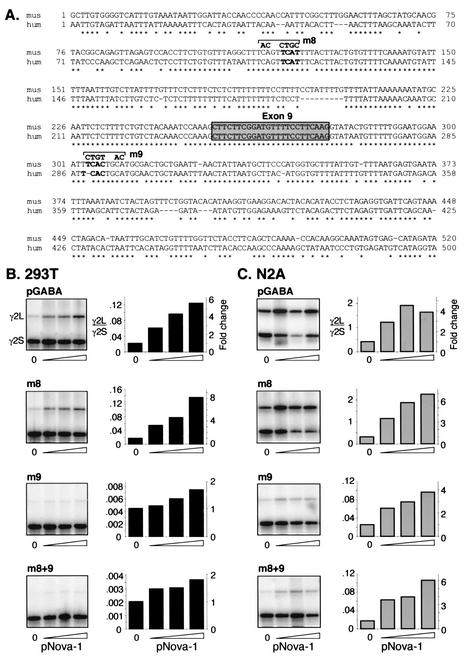
Genomic sequences surrounding GABAARγ2 E9 from humans and mice were examined to identify putative conserved Nova-1 binding sites. Within approximately 250 nt on either side of E9, only two YCAY elements conserved between humans and mice were found, one upstream of E9 in intron 8 and one downstream in intron 9. These elements plus an adjacent CA dinucleotide were mutated (Fig. 3), and the resulting pGABA mutants were assessed for Nova-1-dependent splicing. No changes in E9 splicing were seen upon mutation of intron 8 in 293T or N2A cells (Fig. 3A and Fig. 3B, panel m8). In contrast, a 5- or 15-fold decrease in E9 splicing was seen upon mutation of intron 9 in 293T cells and N2A cells, respectively, reducing γ2L to barely detectable levels in the absence of pNova-1. Despite this, cotransfection of pNova-1 led to an increase in E9 inclusion in both cell lines (Fig. 3B and C, panels m9), although quantitation of these effects in 293T was difficult due to very low abundance of the γ2L species. A construct harboring both mutations (Fig. 3B and C, panel m8+9) behaved in a manner identical to that of the intron 9 mutation, indicating that the two mutated sites do not act synergistically. We conclude that neither the intron 8 or 9 mutation disrupts the major site of Nova-1 action. Although we have not further explored the effect of the intron 9 mutation, its action on basal E9 splicing might be due to disruption of a TGCATG element, a common component of downstream intronic enhancer elements. Various numbers of repeats of this sequence have been shown to be important for alternative splicing of fibronectin (31), fibroblast growth factor receptor 2 (16, 43), src (47), calcitonin/CGRP (28), nonmuscle myosin II heavy-chain B (34), and possibly AMP deaminase (22).
FIG. 3.
Mutations within the adjacent intronic regions do not interfere with Nova's ability to regulate GABAARγ2 exon 9 alternative splicing. (A) Sequence comparisons of intronic regions surrounding GABAARγ2 exon 9. Genomic sequences surrounding and encompassing GABAARγ2 E9 (gray box) from human (hum) and mouse (mus) were aligned by ClustalW alignment (MacVector). Conserved YCAY elements are shown in boldface characters. m8 and m9 represent mutations made in the mouse minigene within introns 8 and 9, respectively. (B and C) The mutations depicted in panel A were made in pGABA, either individually or in combination, and the resulting minigenes were cotransfected with increasing amounts of pNova-1 (0, 0.5, 1.0, or 2.0 μg) into 293T (B) or N2A (C) cells. Spliced products were analyzed as described for Fig. 2. The graphs represent quantitation of the autoradiograph data shown in the left column of each panel; the left y axes indicate the ratios of γ2L/γ2S spliced products, and the right y axes indicate the severalfold changes in these ratios upon addition of pNova-1. Titration of transfected pNova-1 expression was monitored by Western blotting (data not shown). Similar results were obtained in at least two independent transfection experiments.
To identify sites capable of mediating Nova-dependent GABAARγ2 E9 inclusion, we used exonuclease III to generate pGABA deletion constructs harboring introns of differing lengths. These experiments were used as an initial means to screen the 3.7 kb of intronic sequence for potential Nova-dependent elements, bearing in mind that introns are known to harbor widely scattered elements that might affect splicing (43). Screening of over 20 constructs in transfected N2A cells demonstrated that most of introns 8 and 9 can be deleted without eliminating Nova-dependent splicing regulation (data not shown). Using this deletion data, we generated a truncated GABAARγ2 minigene, pGABAΔ, derived from the shortest intron 8 and intron 9 deletions that retained Nova-responsive E9 splicing. pGABAΔ recapitulated the Nova-dependent inclusion of E9 seen with the full-length construct, showing a 6- to 14-fold increase in E9 utilization upon the addition of Nova-1 in N2A and 293T cells, respectively, and a lower level of basal E9 inclusion in 293T cells compared to that in N2A cells (Fig. 4A). Taken together, these data indicate that the size of introns 8 and 9, and most of their sequence content, is not critical for Nova-dependent regulation of GABAARγ2 E9 splicing.
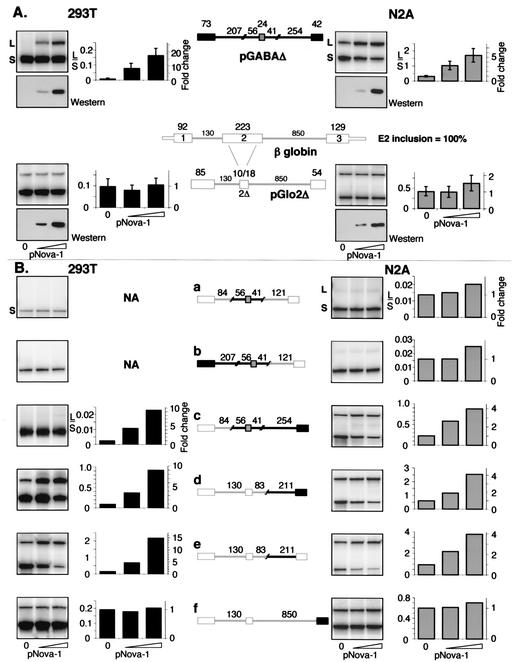
FIG. 4.
The intronic sequence upstream of GABAARγ2 E10 is sufficient for regulation of alternative splicing by Nova-1. (A) A truncated GABAARγ2 minigene (pGABAΔ) was generated by fusing intron deletion constructs (data not shown). A β-globin minigene (pGloΔ2) was derived from the human gene, with the central region of exon 2 deleted such that exon 2Δ retains 10 bp 5′ and 18 bp 3′ of the deletion site. The sizes (in base pairs) of the exonic and intronic sequences contained in the minigenes are noted above the schematics. Minigenes were cotransfected into 293T or N2A cells with increasing amounts of pNova-1 (0, 0.5, or 2.0 μg), and spliced products were measured by RT-PCR and phosphorimage analysis as described for Fig. 2. Titration of transfected pNova-1 expression was monitored by Western blotting using α-T7-tagged antibody (lower panel of each pair of panels). Quantitation of the data plus that of additional experiments (n = 4 for pGABAΔ, n = 5 for pGlo2Δ) is shown at the right side of each pair of panels. The bars represent L/S exon use ratios ± the standard deviations. (B) Chimeric constructs were made by ligating portions of the GABAARγ2 (black lines and filled boxes) and β-globin (gray lines and empty boxes) constructs shown in panel A. Minigenes were cotransfected into 293T or N2A cells with increasing amounts of pNova-1 (0, 0.5, or 2.0 μg), and spliced products were measured by RT-PCR by using primers specific for the outside exons and phosphorimage analysis as described for Fig. 2. The graphs represent quantitation of the autoradiograph data shown in the left column of each panel; the left y axes indicate the ratios of L/S spliced products, and the right y axes indicate the severalfold changes in these ratios upon addition of pNova-1. NA, not applicable (no long form detected). Titration of transfected pNova-1 expression was monitored by Western blotting (data not shown). Similar results were obtained in at least two independent transfection experiments.
To further delineate Nova-responsive elements, a heterologous minigene was constructed using the human β-globin gene as a backbone into which elements from pGABAΔ can be inserted (Fig. 4A). Since exon size can directly influence splicing efficiency (4, 18), the central region of β-globin exon 2 was deleted to give a central exon size of 28 nt (pGlo2Δ) (Fig. 4A), making it comparable in size to GABAARγ2 E9 (24 nt). pGlo2Δ showed a level of alternative exon inclusion (29% inclusion of exon E2Δ) similar to that seen with pGABAΔ (24% inclusion of exon E9) in N2A cells, although the exon was less well utilized in 293T cells (9% inclusion of exon E2Δ). However, pGlo2Δ was not regulated upon the addition of pNova-1 in either cell line (Fig. 4A).
A chimeric construct in which 121 nt (surrounding and including E9) were inserted into pGlo2Δ was not able to mediate Nova-dependent splicing (Fig. 4Ba). In addition, inclusion of additional GABAARγ2 sequence derived from E8 and its downstream intron was not sufficient to regain Nova-dependent alternative splicing of E9 (Fig. 4Bb). However, inclusion of the region derived from the constitutively spliced exon E10 and its upstream intron did restore Nova-dependent splicing regulation, indicating that this region is necessary for Nova's effect (Fig. 4Bc). Moreover, the 211-nt sequence upstream of exon E10 was itself able to mediate Nova-dependent regulation of GABAARγ2 alternative splicing when placed into the heterologous pGlo2Δ minigene, and E10 by itself did not contribute to the effect (Fig. 4Bd to f). These results indicate that the 211-nt intronic element immediately upstream of E10 in GABAARγ2 pre-mRNA is sufficient to mediate Nova-dependent regulation of alternative splicing (Fig. 4B).
Defining a Nova-dependent intronic splicing enhancer (NISE).
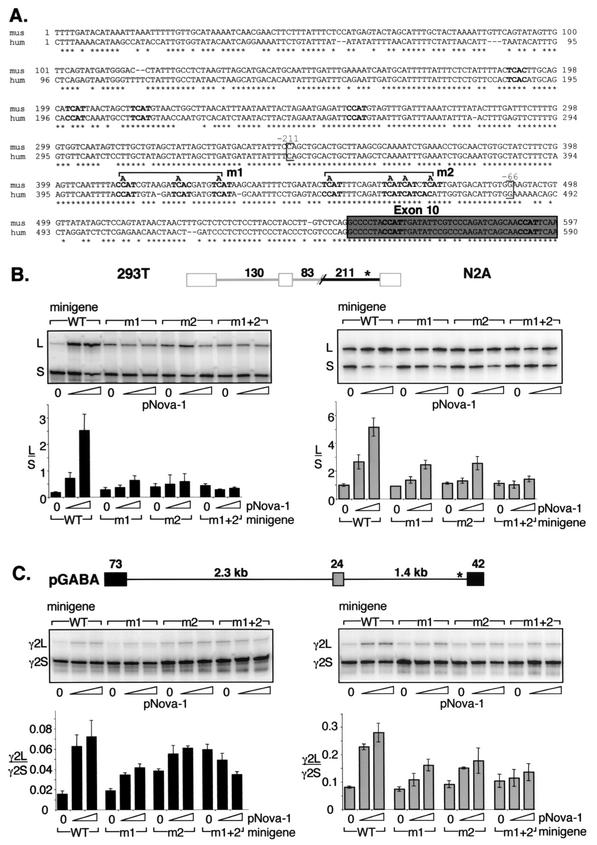
This 211-nt intronic element contains seven YCAY repeats (Fig. 5A) and is highly conserved between mouse and human sequences. Mutations were made in the first three (Fig. 5B, lanes m1), last four (lanes m2), or all seven (lanes m1+2) YCAY elements, mutating each to YAAY, a sequence which does not bind to Nova (6, 32, 42). In 293T cells, these mutations increased the baseline level of E9 inclusion in the absence of cotransfected pNova-1 up to 2.4-fold, possibly due to inadvertent generation or disruption of a binding site for another protein factor. Nonetheless, mutation of the first three or last four YCAY elements markedly reduced the effect of Nova in 293T cells from a 14.5-fold effect to a 2.5-fold (Fig. 5B, lanes m1) or 1.8-fold (lanes m2) effect on splicing, respectively, and mutation of all seven repeats abolished the effect of Nova on alternative splicing (lanes m1+2).
FIG.5.
Mutagenesis of the YCAY repeats close to E10 abolishes Nova-dependent regulation of GABAARγ2 minigene alternative splicing. (A) Genomic sequences upstream of GABAARγ2 exon 10 (gray box) from mouse (mus) and human (hum) were aligned by ClustalW alignment (MacVector). Conserved YCAY elements are shown in boldface characters. Brackets indicate the region corresponding to the in vitro transcribed RNA used as described for Fig. 6B and are numbered relative to exon 10. m1 and m2 represent mutations of three and four YCAY repeats to YAAY, respectively. (B) Chimeric minigenes containing the mutations depicted in panel A were cotransfected into 293T or N2A cells with increasing amounts of pNova-1 (0, 0.5, or 2.0 μg), and spliced products were measured by RT-PCR and phosphorimage analysis as described for Fig. 2. The transfected pNova-1 expression was monitored by Western blotting (data not shown). Quantitation of the data is shown below representative autoradiographs and is presented as L/S exon use ratios ± standard deviations for three independent transfections. WT, wild type. (C) GABAARγ2 minigenes containing the mutations depicted in panel A were cotransfected into 293T or N2A cells with increasing amounts of pNova-1 (0, 0.5, or 2.0 μg), and spliced products were measured by RT-PCR and phosphorimage analysis as described for Fig. 2. The transfected pNova-1 expression was monitored by Western blotting (data not shown). Quantitation of the data is shown below representative autoradiographs and is presented as γ2L/γ2S exon use ratios ± standard deviations for three independent transfections. WT, wild type.
In N2A cells, which express endogenous Nova proteins, mutation of the Nova binding site would be expected to decrease the baseline level of E9 inclusion in the absence of cotransfected pNova-1. However, no change was seen, likely due to a competing increase in E9 inclusion identical to that observed in 293T cells. Nonetheless, the mutations showed identical behavior with respect to Nova-dependent exon inclusion in N2A cells (Fig. 5B), and taken together, these data demonstrate that YCAY repeats within a conserved 211-nt intronic element are absolutely necessary for Nova-dependent regulation of GABAARγ2 alternative splicing in the context of the chimeric minigene. Since these repeats are both necessary and sufficient to mediate Nova-dependent enhancement of exon inclusion, we refer to this region as a NISE element.
To confirm the role of the NISE element in mediating Nova action in the context of the native GABAARγ2 pre-mRNA, identical YCAY-to-YAAY mutations were made in pGABA, which harbors the intact GABAARγ2 genomic sequence between E8 and E10 (Fig. 5C). Cotransfection of these plasmids with pNova-1 into 293T or N2A cells yielded results similar to those seen with the chimeric pGlo2Δ constructs. In N2A cells, mutation of the first three or last four YCAY repeats reduced Nova-dependent regulation from a 3.5- to a 2.2- or 1.9-fold effect, respectively (Fig. 5C). Mutation of all seven YCAY repeats (Fig. 5C, lanes m1+2) markedly diminished the effect of Nova-1 to a 1.3-fold change, a change comparable to the background effect of Nova on pGlo2Δ in N2A cells (compare Fig. 4A).
In 293T cells, mutation of the YCAY repeats altered the baseline level of E9 inclusion up to 3.9-fold; however, the magnitude of the Nova effect was markedly reduced from a 4.7-fold to a 2.2-fold (Fig. 5B, lanes m1) or 1.6-fold (lanes m2) effect. Mutation of all seven YCAY repeats (Fig. 5B, lanes m1+2) resulted in a paradoxical 1.7-fold decrease in the inclusion of E9 upon the addition of Nova-1 in 293T cells. Similar baseline and paradoxical Nova-dependent effects were also seen upon mutation of YCAY repeats to YAAY in GlyRα2 minigenes (32). While not fully understood, the aberrant effect of Nova might reflect a dominant-negative action of the protein under these conditions. Taken together, these results indicate that the YCAY repeats within the NISE are necessary for the regulation of GABAARγ2 E9 alternative splicing by Nova-1 in the context of the wild-type pre-mRNA.
In vitro analysis of Nova binding to GABAARγ2 RNA.
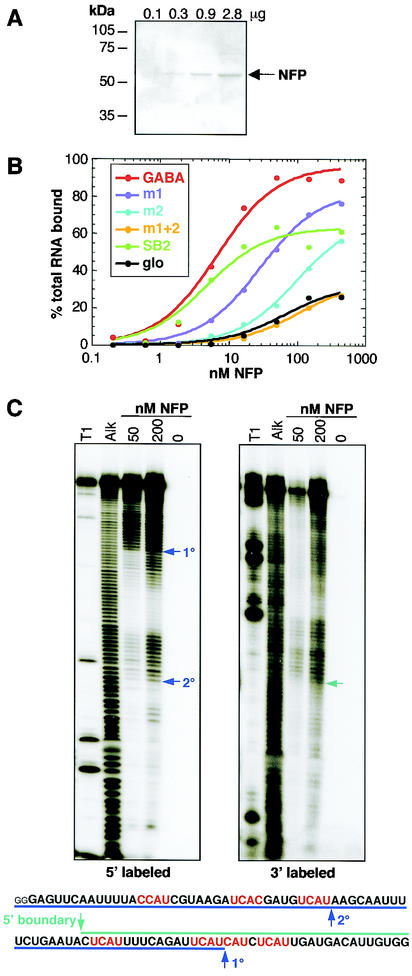
To assess whether Nova protein directly interacts with the NISE element defined above, we performed nitrocellulose filter binding assays with purified RNA and Nova-1 protein. Purified full-length Nova-1 fusion protein (NFP) (Fig. 6A) is capable of binding a 159-nt RNA encompassing the NISE element (Fig. 5A) with high affinity (Kd, 7 nM) similar to the affinity with which Nova binds to RNA selection winners (SB2; Kd, 5 nM) (6) (Fig. 6B). Mutation of the first three YCAY repeats to YAAY results in a modest decrease of approximately 4-fold in NFP binding affinity (Kd, 29 nM), while mutation of the last four reduces binding affinity by >14-fold (Kd, 100 nM). Mutation of all seven repeats reduced the binding affinity >100-fold to a level similar to that of NFP binding to an irrelevant β-globin RNA of similar length (Fig. 6B). These results are consistent with the conclusion that Nova binds directly to the NISE element in vivo to mediate GABAARγ2 E9 alternative splicing.
FIG. 6.
Nova-1 binds with high affinity to GABAARγ2 RNA in vitro. (A) His-tagged NFP was expressed in Escherichia coli and purified over a nickel column. Aliquots of increasing concentrations were analyzed by sodium dodecyl sulfate-PAGE followed by staining with Coomassie stain. (B) Nitrocellulose filter binding assays were performed using the NFP shown in panel A and six RNAs transcribed in vitro. GABA, RNA corresponding to the region of GABAARγ2 intron 8 highlighted in Fig. 5, with the addition of the sequences GGGAG at the 5′ end and CUAGCAAA at the 3′ end derived from the PCR primers used to amplify the template prior to in vitro transcription; m1 and m2, mutations of three and four YCAY repeats to YAAY in GABA, respectively; SB2, RNA obtained by RNA selection with Nova-1 (6); glo, RNA derived from human β-globin which spans regions of exon 1 and intron 1 and contains no YCAY elements. (C) Mapping the boundaries of Nova-1 binding to GABAARγ2 intron 9 RNA. RNA was labeled with 32P at either the 5′ or 3′ end and subjected to mild alkaline hydrolysis (Alk). The RNA was then incubated with NFP at a final concentration of 50 or 200 nM, and protein:RNA complexes werecaptured by filtration through nitrocellulose filters. Bound RNAs were eluted and analyzed by denaturing PAGE. RNase T1 digestion (T1) was used to generate size standards. Boundaries are highlighted by arrows and indicated on the transcribed RNA sequence shown at the bottom of each panel. 1°, primary boundary; 2°, secondary boundary. Small letters represent sequences derived from primers.
To map the RNA sequence necessary for high-affinity Nova-1 binding, the 5′ and 3′ boundaries of the smallest RNA that retains high affinity to Nova-1 were determined (Fig. 6C). In vitro-transcribed RNA was labeled with 32P at either the 5′ or 3′ end and subjected to mild alkaline hydrolysis. This RNA was then incubated with NFP, and bound RNAs were captured by filtration through nitrocellulose filters, eluted, and analyzed by denaturing polyacrylamide gel electrophoresis (PAGE). The 5′ boundary mapped just upstream of the fourth YCAY tetranucleotide such that four YCAY repeats were contained within the smallest bound RNA. The position of the boundary at 1 nt upstream of a YCAY sequence agrees with structural data indicating that this nucleotide makes stacking interactions with the Nova KH domain in such a way as to contribute to binding affinity (42). We detected two 3′ boundaries; the predominant boundary (Fig. 6C) occurred immediately downstream of the fifth YCAY repeat. A secondary boundary (Fig. 6C) immediately downstream of the third YCAY repeat was apparent at a high protein concentration (200 nM), suggesting that NFP binds these RNAs with lower affinity. The results suggest that a minimum of four or five YCAY repeats are necessary for high-affinity binding of the RNA to Nova-1.
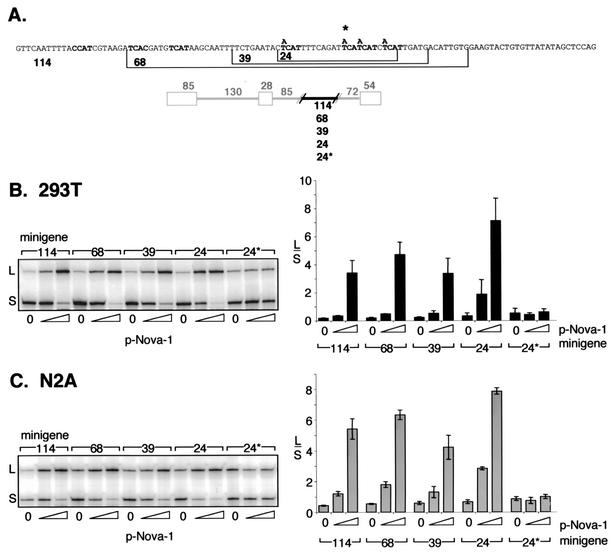
To correlate the in vitro binding studies with the function of Nova-1 on the regulation of alternative splicing, additional β-globin-based minigenes harboring GABAARγ2 intronic elements of 114, 68, 39, or 24 nt from intron 9 were assayed by transfection into 293T and N2A cells and were all found to retain Nova-dependent splicing capacity (Fig. 7). Reduction of the element to only 24 nt containing a total of four YCAY repeats was sufficient to mediate Nova-dependent alternative splicing. This contrasts with the relatively small effect of Nova on pGABA retaining only these four repeats (Fig. 5). However, two UCAU repeats separated by a single nucleotide are present in the β-globin pre-mRNA at 21 nt upstream of the site at which GABAARγ2 sequences were inserted (data not shown), which might have contributed to Nova binding and splicing regulation. Mutation of the four GABAARγ2-derived repeats (Fig. 7) abolished Nova-1's effect. Taken together with the in vitro binding studies, these results are consistent with the conclusion that Nova-1 binding to a small set of YCAY intronic repeats downstream of the regulated exon is necessary and sufficient for regulation of alternative splicing and imply that the number of YCAY repeats correlates with Nova binding affinity and the magnitude of splicing regulation.
FIG. 7.
A 24-nt intronic sequence is sufficient for regulation of alternative splicing by Nova-1 in a heterologous context. (A) DNA sequences derived from GABAARγ2 intron 9 contained within the chimeric minigenes used in panels B and C. Numbers specify the lengths (in base pairs) of the GABAARγ2 intronic regions cloned into the β-globin minigene. The star denotes the presence of four C-to-A mutations within the 24-nt GABAARγ2 sequence. (B and C) The chimeric minigenes depicted in panel A were cotransfected into 293T (B) or N2A (C) cells with increasing amounts (0, 0.5, or 2.0 μg) of pNova-1, and spliced products were measured by RT-PCR and phosphorimage analysis as described for Fig. 2. The titration of transfected pNova-1 expression was monitored by Western blotting (data not shown). Representative autoradiographs are shown at the left side of each panel; graphs summarize data from three independent transfections. The bars represent the ratios of L/S spliced products ± standard deviations.
DISCUSSION
The observation that the human genome contains a smaller number of genes than expected has enhanced the appreciation of alternative splicing as a mechanism for generating proteomic complexity. Comparisons of transcribed sequence databases with genomic data suggest that alternative splicing is particularly abundant in the brain (62), and understanding alternative splicing regulation in the brain is of profound importance (19, 25). While the mechanisms by which tissue-specific regulated alternative splicing decisions are controlled are not fully understood, an overriding theme appears to be the balance between positive and negative signals in pre-mRNA, which interact with constitutive or tissue-specific protein factors to promote or inhibit exon usage.
Nova-1 is a neuron-specific RBP important for neuronal survival and regulation of alternative splicing in mammals (32). We have previously used in vitro methods to identify RNA binding targets for Nova and in turn used those targets to drive toward identification of an in vivo function for Nova binding to YCAY elements in an intron of GlyRα2 pre-mRNA to regulate exon inclusion. However, the generality of this phenomenon was not previously known. The fortuitous identification of GABAARγ2 as a second transcript whose alternative splicing was affected by Nova allowed us to prospectively examine a mechanism regarding which we had no prior understanding.
A number of factors have been previously reported to influence splicing of GABAARγ2 E9, including the small exon size, a weak 5′ splice site, sequence elements within and upstream of E9 (63), and the RBP PTB (3). The finding that Nova-1 null mice showed a decrease in the level of inclusion of E9 (Fig. 1) (32) indicated that Nova-1 also plays a role in regulation of GABAARγ2 alternative splicing but did not address whether Nova acts directly on GABAARγ2 pre-mRNA, and if so, whether Nova binds to an element resembling the YCAY sequences selected in vitro. Here we show that GABAARγ2 E9 inclusion is regulated by Nova action on a distal downstream intronic YCAY repeat element. These results provide an important verification of Nova function as a sequence-specific splicing factor, underscore the significance of YCAY repeats as Nova targets in vivo, and provide insight into the mechanisms of tissue-specific splicing regulation.
The NISE element.
Several lines of evidence demonstrate that Nova regulates GABAARγ2 E9 inclusion by a direct action on clustered YCAY intronic repeats. First, the NISE element contains seven YCAY repeats, which are known to be the preferred RNA sequence for Nova KH domain binding (6, 33, 42), and these are conserved between mice and humans in GABAARγ2 (Fig. 5A). Second, mutation of these YCAY repeats to YAAY completely abolished Nova's effect on splicing (Fig. 5B and C), indicating that these sequences are necessary for Nova-dependent splicing regulation. Third, Nova binds with high affinity to the wild-type but not the mutated sequence corresponding to this region (Fig. 6B). Boundary mapping experiments indicated that this binding abruptly drops when the sequence contains fewer than four UCAY repeats (Fig. 6C). Consistent with this result, we found that a 24-nt element spanning the last four YCAY repeats is sufficient to mediate Nova-dependent E9 inclusion (Fig. 7). These findings do not entirely exclude the possibility that other sequences within the GABAARγ2 pre-mRNA contribute to Nova-dependent splicing of E9; however, our data indicate that any such additional Nova regulatory regions would play a minor regulatory role and would be dependent on the integrity of the NISE element.
The requirement for a number of YCAY elements for high-affinity Nova-1 binding suggests that alternative splicing regulation of GABAARγ2 might be mediated through the binding of multiple KH domains to multiple YCAY repeats within the RNA. A similar mechanism has been reported for Vg1 mRNA localization mediated by VgRBP/Vera. Binding to the Vg1 mRNA requires two KH didomains, and these KH didomains bind RNA cooperatively (23). It is possible that a single Nova molecule can bind up to three YCAY elements within an RNA. Moreover, Nova proteins can multimerize (41, 42, 52), suggesting that several Nova protein molecules cooperatively bind an RNA target. Biophysical evidence suggests that both of these mechanisms can apply to the formation of Nova-RNA complexes (54). Either mechanism can effectively increase the affinity of binding to an RNA target and help to explain the requirement for several YCAY elements in the GABAARγ2 pre-mRNA for regulation of alternative splicing by Nova.
A general principle of RBP action can be seen in this data. RNA targets of high specificity yet low complexity can be recognized in vivo via clustering of the target elements. This can be seen in several examples of RNA translational control and localization mediated by RBP-target RNA interactions. These include the action of hnRNPs K and E1 on LOX mRNA translation in erythrocytes which requires clustered differentiation control elements (49, 50), the action of bruno on oskar mRNA translation in Drosophila which requires clustered bruno response elements (36), and the action of Vg1RBP/Vera on Vg1 and VegT mRNA localization in Xenopus which requires clustered VM1 elements (5, 40). Our finding that clustered YCAY repeats are necessary and sufficient to mediate the action of Nova helps confirm this principle and extend it to alternative splicing.
Mechanism of Nova action to regulate alternative splicing.
The mechanism by which Nova-1 enhances E9 inclusion is unclear. In some instances of alternative splicing control, enhancement of splicing is mediated through inhibition of a negative signal. For example, PTB acts on CT/CGRP through inhibition of a competing pseudoexon (45). PTB has also been shown to inhibit inclusion of GABAARγ2 E9, and nucleotides adjacent to and within E9 are important for this function (3, 64). This observation led to the proposal that inclusion of E9 in neurons of the CNS is regulated by a mechanism involving derepression of splicing by PTB. The finding that GABAARγ2L exon utilization is dysregulated in Nova-1 null mice suggested that Nova is a candidate for mediation of this derepression (32), perhaps through competitive binding to the pre-mRNA. Our findings that the NISE element lies further than 1 kb downstream of E9 and that a small (as short as 24 nt) sequence element corresponding to the region of Nova-1 binding is sufficient to mediate Nova-dependent enhancement of an upstream exon in a heterologous system argue against this hypothesis of Nova action on GABAARγ2L exon utilization.
Alternatively, enhancement can be achieved by positive action such as recruitment of essential splicing factors to an otherwise poorly utilized splice site, as has been proposed for KSRP enhancement of src N1 inclusion (46), or the action of the downstream enhancer of (NMHC)-B N30 inclusion (26). Conceptually, Nova-dependent enhancement of GABAARγ2L exon utilization is likely to involve facilitation of splicing of an intron adjacent to E9. It is unclear whether this facilitation involves the intron upstream [in similarity to (NMHC)-B N30] or downstream (in similarity to KSRP/src N1) of E9. However, comparison with Nova's action on GlyRα2 splicing (and the simplest biochemical hypothesis) would predict that Nova enhances splicing of the intron in which it resides. In vitro splicing assays utilizing simplified RNA substrates similar to those performed by Guo and Kawamoto (26) would be helpful in assessing this possibility.
The extremely close positioning of the Nova binding site at only 80 nt upstream of the splice acceptor signals of exon 10 is intriguing. The majority of splicing regulatory sequences that have been characterized lie within or close to the exon that they regulate (58), including those reported to affect GABAARγ2 E9. Although our data confirm that mutations within the region surrounding E9 cause a dramatic decrease in the basal level of E9 inclusion, Nova acts at a distance—the NISE element is approximately 1,250 nt downstream of E9. One possibility is that Nova recruits factors that interact with and help assemble the basal splicing machinery in a manner analogous to the distant action of transcriptional enhancer elements. Transcriptional enhancers recruit DNA binding proteins which, in turn, help to recruit the basal transcription machinery to the promoter, which often lies many kilobases away, through protein-protein interactions (reviewed in reference 29). In this context, we hypothesize that Nova binding to the NISE recruits proteins that directly or indirectly interact with either the upstream 5′ splice site and/or the downstream 3′ splice site. Yeast two-hybrid results have indicated that Nova can bind to spliceosomal elements present at both the 3′ and 5′ splice sites, namely, U1-70K and U2AF65, as well as some SR proteins, including SRp20 (G. Stefani and R. Darnell, unpublished data). Through interactions such as these, Nova may serve a bridging function between the nearby 3′ splice site and the next upstream 5′ splice site.
Another possibility for the mechanism of Nova action is that Nova slows down the kinetics of spliceosome assembly at E10 by interfering with binding or assembly of essential factors at this site. This might then enhance splicing to E9 by allowing more time for assembly to take place at an otherwise weakly recognized site and thus increase the chances of recognition of this exon. A similar kinetic mechanism has been proposed to explain the influence of transcription on alternative splicing through differences in RNA polymerase II processivity rates (reviewed in reference 8). However, it is difficult to reconcile this hypothesis with what is known about Nova regulation of GlyRα2 splicing. Nova enhances GlyRα2 E3A inclusion through binding to an element which lies at the same distance upstream of E3A that the NISE element lies upstream of GABAARγ2 E10 (32). If a similar kinetic mechanism involving interference of spliceosome assembly at the adjacent 3′ splice site were involved in regulation of GlyRα2 splicing, Nova binding would be expected to repress E3A inclusion.
The identification of the NISE element in GABAARγ2 pre-mRNA offers several advantages over the study of GlyRα2 for the further elucidation of the biochemical mechanism of Nova-dependent alternative splicing regulation. First, alternative splicing of GABAARγ2 involves the skipping or inclusion of a single cassette exon rather than the more complex scenario of mutual exclusion of the two alternative exons E3A and E3B in GlyRα2. Second, this study demonstrated that the NISE element is necessary for Nova-dependent regulation of E9 splicing in the context of the full-length introns; this has not been shown for GlyRα2, in part because the Nova binding site lies within an intron approximately 35 kb in length, necessitating the use of truncated introns in the minigenes used to assay GlyRα2 splicing. Finally, we have extended previous analyses by demonstrating that the NISE element is sufficient to mediate Nova-dependent enhancement of exon inclusion in a heterologous context, enabling us to conclude that the exact nature of the surrounding splice sites and other regulatory elements within the pre-mRNA is not critical for Nova's action. Furthermore, this simplified substrate is useful in in vitro splicing assays (Stefani and Darnell, unpublished), providing a potentially powerful tool to help determine the biochemical mechanism by which Nova regulates alternative splicing.
Conclusions.
Beginning with the in vivo finding that GABAARγ2L exon utilization is perturbed in Nova-1 null mice and working backwards to biochemical function, we have found an intronic Nova binding element—the NISE—that is necessary and sufficient to mediate Nova-dependent splicing regulation. This is the first demonstration that a small RNA element is both necessary and sufficient to mediate the action of a neuron-specific mammalian splicing activator. The NISE element was identified using a systematic approach to screen for Nova-dependent regulatory elements. The finding that the NISE element also conforms to the consensus Nova binding sequences predicted in vitro further validates the usefulness of RNA selection as a means to identify biologically relevant targets and leads to the prediction that it might be possible to identify additional Nova targets through bioinformatic screenings. The work presented here, completing a cycle of experiments in vitro to in vivo and back, validates our identification of Nova binding sites as targets for Nova-dependent regulation and furthers our understanding of how Nova acts as a regulator of alternative splicing in neurons.
Acknowledgments
We thank A. R. Krainer for the sp64-HβΔ6 plasmid from which the β-globin constructs were derived. We are grateful to all members of the laboratory, especially K. B. Jensen and J. C. Darnell, for advice and helpful discussions and for critical reading of the manuscript.
B.K.D. was supported in part by a B. Duke Glenn Fellowship. This research was supported by the Howard Hughes Medical Institute (B.K.D. and R.B.D.), the Burroughs Wellcome Fund, and NIH R01 NS40955 and NS34389 (R.B.D.).
REFERENCES
- 1.Amara, S. G., V. Jonas, M. G. Rosenfeld, E. S. Ong, and R. M. Evans. 1982. Alternative RNA processing in calcitonin gene expression generates mRNAs encoding different polypeptide products. Nature 298:240-244. [DOI] [PubMed] [Google Scholar]
- 2.Arning, S., P. Gruter, G. Bilbe, and A. Kramer. 1996. Mammalian splicing factor SF1 is encoded by variant cDNAs and binds to RNA. RNA 2:794-810. [PMC free article] [PubMed] [Google Scholar]
- 3.Ashiya, M., and P. J. Grabowski. 1997. A neuron-specific splicing switch mediated by an array of pre-mRNA repressor sites: evidence of a regulatory role for the polypyrimidine tract binding protein and a brain-specific PTB counterpart. RNA 3:996-1015. [PMC free article] [PubMed] [Google Scholar]
- 4.Berget, S. M. 1995. Exon recognition in vertebrate splicing. J. Biol. Chem. 270:2411-2414. [DOI] [PubMed] [Google Scholar]
- 5.Bubunenko, M., T. L. Kress, U. D. Vempati, K. L. Mowry, and M. L. King. 2002. A consensus RNA signal that directs germ layer determinants to the vegetal cortex of Xenopus oocytes. Dev. Biol. 248:82-92. [DOI] [PubMed] [Google Scholar]
- 6.Buckanovich, R. J., and R. B. Darnell. 1997. The neuronal RNA binding protein Nova-1 recognizes specific RNA targets in vitro and in vivo. Mol. Cell. Biol. 17:3194-3201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Buckanovich, R. J., J. B. Posner, and R. B. Darnell. 1993. Nova, the paraneoplastic Ri antigen, is homologous to an RNA-binding protein and is specifically expressed in the developing motor system. Neuron 11:657-672. [DOI] [PubMed] [Google Scholar]
- 8.Caceres, J. F., and A. R. Kornblihtt. 2002. Alternative splicing: multiple control mechanisms and involvement in human disease. Trends Genet. 18:186-193. [DOI] [PubMed] [Google Scholar]
- 9.Chan, R. C., and D. L. Black. 1995. Conserved intron elements repress splicing of a neuron-specific c-src exon in vitro. Mol. Cell. Biol. 15:6377-6385. (Erratum, 17:2970, 1997.) [DOI] [PMC free article] [PubMed]
- 10.Chan, R. C., and D. L. Black. 1997. The polypyrimidine tract binding protein binds upstream of neural cell-specific c-src exon N1 to repress the splicing of the intron downstream. Mol. Cell. Biol. 17:4667-4676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chomczynski, P., and N. Sacchi. 1987. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal. Biochem. 162:156-159. [DOI] [PubMed] [Google Scholar]
- 12.Chou, M.-Y., N. Rooke, C. W. Turck, and D. L. Black. 1999. hnRNP H is a component of a splicing enhancer complex that activates a c-src alternative exon in neuronal cells. Mol. Cell. Biol. 19:69-77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Conn, P. J., and J. P. Pin. 1997. Pharmacology and functions of metabotropic glutamate receptors. Annu Rev. Pharmacol. Toxicol. 37:205-237. [DOI] [PubMed] [Google Scholar]
- 14.Darnell, J. C., K. B. Jensen, P. Jin, V. Brown, S. T. Warren, and R. B. Darnell. 2001. Fragile X mental retardation protein targets G quartet mRNAs important for neuronal function. Cell 107:489-499. [DOI] [PubMed] [Google Scholar]
- 15.Darnell, R. B. 1996. Onconeural antigens and the paraneoplastic neurologic disorders: at the intersection of cancer, immunity, and the brain. Proc. Natl. Acad. Sci. USA 93:4529-4536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Del Gatto, F., A. Plet, M. C. Gesnel, C. Fort, and R. Breathnach. 1997. Multiple interdependent sequence elements control splicing of a fibroblast growth factor receptor 2 alternative exon. Mol. Cell. Biol. 17:5106-5116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Deshler, J. O., M. I. Highett, T. Abramson, and B. J. Schnapp. 1998. A highly conserved RNA-binding protein for cytoplasmic mRNA localization in vertebrates. Curr. Biol. 8:489-496. [DOI] [PubMed] [Google Scholar]
- 18.Dominski, Z., and R. Kole. 1991. Selection of splice sites in pre-mRNAs with short internal exons. Mol. Cell. Biol. 11:6075-6083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dredge, B. K., A. D. Polydorides, and R. B. Darnell. 2001. The splice of life: alternative splicing and neurological disease. Nat. Rev. Neurosci. 2:43-50. [DOI] [PubMed] [Google Scholar]
- 20.Ehlers, M. D., W. G. Tingley, and R. L. Huganir. 1995. Regulated subcellular distribution of the NR1 subunit of the NMDA receptor. Science 269:1734-1737. [DOI] [PubMed] [Google Scholar]
- 21.Engebrecht, J. A., K. Voelkel-Meiman, and G. S. Roeder. 1991. Meiosis-specific RNA splicing in yeast. Cell 66:1257-1268. [DOI] [PubMed] [Google Scholar]
- 22.Genetta, T., H. Morisaki, T. Morisaki, and E. W. Holmes. 2001. A novel bipartite intronic splicing enhancer promotes the inclusion of a mini-exon in the AMP deaminase 1 gene. J. Biol. Chem. 276:25589-25597. [DOI] [PubMed] [Google Scholar]
- 23.Git, A., and N. Standart. 2002. The KH domains of Xenopus Vg1RBP mediate RNA binding and self-association. RNA 8:1319-1333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Glencorse, T. A., A. N. Bateson, and M. G. Darlison. 1992. Differential localization of two alternatively spliced GABAA receptor γ2-subunit mRNAs in the chick brain. Eur. J. Neurosci. 4:271-277. [DOI] [PubMed] [Google Scholar]
- 25.Grabowski, P. J., and D. L. Black. 2001. Alternative RNA splicing in the nervous system. Prog. Neurobiol. 65:289-308. [DOI] [PubMed] [Google Scholar]
- 26.Guo, N., and S. Kawamoto. 2000. An intronic downstream enhancer promotes 3′ splice site usage of a neural cell-specific exon. J. Biol. Chem. 275:33641-33649. [DOI] [PubMed] [Google Scholar]
- 27.Gutierrez, A., Z. U. Khan, and A. L. De Blas. 1994. Immunocytochemical localization of gamma 2 short and gamma 2 long subunits of the GABAA receptor in the rat brain. J. Neurosci. 14:7168-7179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hedjran, F., J. M. Yeakley, G. S. Huh, R. O. Hynes, and M. G. Rosenfeld. 1997. Control of alternative pre-mRNA splicing by distributed pentameric repeats. Proc. Natl. Acad. Sci. USA 94:12343-12347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hertel, K. J., K. W. Lynch, and T. Maniatis. 1997. Common themes in the function of transcription and splicing enhancers. Curr. Opin. Cell Biol. 9:350-357. [DOI] [PubMed] [Google Scholar]
- 30.Homanics, G. E., N. L. Harrison, J. J. Quinlan, M. D. Krasowski, C. E. Rick, A. L. de Blas, A. K. Mehta, F. Kist, R. M. Mihalek, J. J. Aul, and L. L. Firestone. 1999. Normal electrophysiological and behavioral responses to ethanol in mice lacking the long splice variant of the γ2 subunit of the γ-aminobutyrate type A receptor. Neuropharmacology 38:253-265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Huh, G. S., and R. O. Hynes. 1994. Regulation of alternative pre-mRNA splicing by a novel repeated hexanucleotide element. Genes Dev. 8:1561-1574. [DOI] [PubMed] [Google Scholar]
- 32.Jensen, K. B., B. K. Dredge, G. Stefani, R. Zhong, R. J. Buckanovich, H. J. Okano, Y. Y. Yang, and R. B. Darnell. 2000. Nova-1 regulates neuron-specific alternative splicing and is essential for neuronal viability. Neuron 25:359-371. [DOI] [PubMed] [Google Scholar]
- 33.Jensen, K. B., K. Musunuru, H. A. Lewis, S. K. Burley, and R. B. Darnell. 2000. The tetranucleotide UCAY directs the specific recognition of RNA by the Nova K-homology 3 domain. Proc. Natl. Acad. Sci. USA 97:5740-5745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kawamoto, S. 1996. Neuron-specific alternative splicing of nonmuscle myosin II heavy chain-B pre-mRNA requires a cis-acting intron sequence. J. Biol. Chem. 271:17613-17616. [PubMed] [Google Scholar]
- 35.Kiledjian, M., X. Wang, and S. A. Liebhaber. 1995. Identification of two KH domain proteins in the alpha-globin mRNP stability complex. EMBO J. 14:4357-4364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kim-Ha, J., K. Kerr, and P. M. Macdonald. 1995. Translational regulation of oskar mRNA by bruno, an ovarian RNA-binding protein, is essential. Cell 81:403-412. [DOI] [PubMed] [Google Scholar]
- 37.Kofuji, P., J. B. Wang, S. J. Moss, R. L. Huganir, and D. R. Burt. 1991. Generation of two forms of the gamma-aminobutyric acidA receptor gamma 2-subunit in mice by alternative splicing. J. Neurochem. 56:713-715. [DOI] [PubMed] [Google Scholar]
- 38.Krishek, B. J., X. Xie, C. Blackstone, R. L. Huganir, S. J. Moss, and T. G. Smart. 1994. Regulation of GABAA receptor function by protein kinase C phosphorylation. Neuron 12:1081-1095. [DOI] [PubMed] [Google Scholar]
- 39.Kuhse, J., A. Kuryatov, Y. Maulet, M. L. Malosio, V. Schmieden, and H. Betz. 1991. Alternative splicing generates two isoforms of the alpha 2 subunit of the inhibitory glycine receptor. FEBS Lett. 283:73-77. [DOI] [PubMed] [Google Scholar]
- 40.Kwon, S., T. Abramson, T. P. Munro, C. M. John, M. Kohrmann, and B. J. Schnapp. 2002. UUCAC- and vera-dependent localization of VegT RNA in Xenopus oocytes. Curr. Biol. 12:558-564. [DOI] [PubMed] [Google Scholar]
- 41.Lewis, H. A., H. Chen, C. Edo, R. J. Buckanovich, Y. Y. Yang, K. Musunuru, R. Zhong, R. B. Darnell, and S. K. Burley. 1999. Crystal structures of Nova-1 and Nova-2 K-homology RNA-binding domains. Structure 7:191-203. [DOI] [PubMed] [Google Scholar]
- 42.Lewis, H. A., K. Musunuru, K. B. Jensen, C. Edo, H. Chen, R. B. Darnell, and S. K. Burley. 2000. Sequence-specific RNA binding by a Nova KH domain: implications for paraneoplastic disease and the fragile X syndrome. Cell 100:323-332. [DOI] [PubMed] [Google Scholar]
- 43.Lim, L. P., and P. A. Sharp. 1998. Alternative splicing of the fibronectin EIIIB exon depends on specific TGCATG repeats. Mol. Cell. Biol. 18:3900-3906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lin, Z., S. Haus, J. Edgerton, and D. Lipscombe. 1997. Identification of functionally distinct isoforms of the N-type Ca2+ channel in rat sympathetic ganglia and brain. Neuron 18:153-166. [DOI] [PubMed] [Google Scholar]
- 45.Lou, H., D. M. Helfman, R. F. Gagel, and S. M. Berget. 1999. Polypyrimidine tract-binding protein positively regulates inclusion of an alternative 3′-terminal exon. Mol. Cell. Biol. 19:78-85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Min, H., C. W. Turck, J. M. Nikolic, and D. L. Black. 1997. A new regulatory protein, KSRP, mediates exon inclusion through an intronic splicing enhancer. Genes Dev. 11:1023-1036. [DOI] [PubMed] [Google Scholar]
- 47.Modafferi, E. F., and D. L. Black. 1997. A complex intronic splicing enhancer from the c-src pre-mRNA activates inclusion of a heterologous exon. Mol. Cell. Biol. 17:6537-6545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Musunuru, K., and R. B. Darnell. 2001. Paraneoplastic neurologic disease antigens: RNA-binding proteins and signaling proteins in neuronal degeneration. Annu. Rev. Neurosci. 24:239-262. [DOI] [PubMed] [Google Scholar]
- 49.Ostareck, D. H., A. Ostareck-Lederer, M. Wilm, B. J. Thiele, M. Mann, and M. W. Hentze. 1997. mRNA silencing in erythroid differentiation: hnRNP K and hnRNP E1 regulate 15-lipoxygenase translation from the 3′ end. Cell 89:597-606. [DOI] [PubMed] [Google Scholar]
- 50.Ostareck-Lederer, A., D. H. Ostareck, N. Standart, and B. J. Thiele. 1994. Translation of 15-lipoxygenase mRNA is inhibited by a protein that binds to a repeated sequence in the 3′ untranslated region. EMBO J. 13:1476-1481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Picetti, R., A. Saiardi, T. Abdel Samad, Y. Bozzi, J. H. Baik, and E. Borrelli. 1997. Dopamine D2 receptors in signal transduction and behavior. Crit. Rev. Neurobiol. 11:121-142. [DOI] [PubMed] [Google Scholar]
- 52.Polydorides, A. D., H. J. Okano, Y. Y. Yang, G. Stefani, and R. B. Darnell. 2000. A brain-enriched polypyrimidine tract-binding protein antagonizes the ability of Nova to regulate neuron-specific alternative splicing. Proc. Natl. Acad. Sci. USA 97:6350-6355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Quinlan, J. J., L. L. Firestone, and G. E. Homanics. 2000. Mice lacking the long splice variant of the gamma 2 subunit of the GABA(A) receptor are more sensitive to benzodiazepines. Pharmacol. Biochem. Behav. 66:371-374. [DOI] [PubMed] [Google Scholar]
- 54.Ramos, A., D. Hollingworth, S. A. Major, S. Adinolfi, G. Kelly, F. W. Muskett, and A. Pastore. 2002. Role of dimerization in KH/RNA complexes: the example of Nova KH3. Biochemistry 41:4193-4201. [DOI] [PubMed] [Google Scholar]
- 55.Ross, A. F., Y. Oleynikov, E. H. Kislauskis, K. L. Taneja, and R. H. Singer. 1997. Characterization of a β-actin mRNA zipcode-binding protein. Mol. Cell. Biol. 17:2158-2165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Sambrook, J., E. F. Fritsch, and T. Maniatis. 1989. Molecular cloning: a laboratory manual, 2nd ed. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, N.Y.
- 57.Siebel, C. W., A. Admon, and D. C. Rio. 1995. Soma-specific expression and cloning of PSI, a negative regulator of P element pre-mRNA splicing. Genes Dev. 9:269-283. [DOI] [PubMed] [Google Scholar]
- 58.Smith, C. W., and J. Valcarcel. 2000. Alternative pre-mRNA splicing: the logic of combinatorial control. Trends Biochem. Sci. 25:381-388. [DOI] [PubMed] [Google Scholar]
- 59.Wang, J. B., and D. R. Burt. 1991. Differential expression of two forms of GABAA receptor gamma 2-subunit in mice. Brain Res. Bull. 27:731-735. [DOI] [PubMed] [Google Scholar]
- 60.Wang, Z., and P. J. Grabowski. 1996. Cell- and stage-specific splicing events resolved in specialized neurons of the rat cerebellum. RNA 2:1241-1253. [PMC free article] [PubMed] [Google Scholar]
- 61.Whiting, P., R. M. McKernan, and L. L. Iversen. 1990. Another mechanism for creating diversity in gamma-aminobutyrate type A receptors: RNA splicing directs expression of two forms of gamma 2 phosphorylation site. Proc. Natl. Acad. Sci. USA 87:9966-9970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Xu, Q., B. Modrek, and C. Lee. 2002. Genome-wide detection of tissue-specific alternative splicing in the human transcriptome. Nucleic Acids Res. 30:3754-3766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Zhang, L., M. Ashiya, T. G. Sherman, and P. J. Grabowski. 1996. Essential nucleotides direct neuron-specific splicing of gamma 2 pre-mRNA. RNA 2:682-698. [PMC free article] [PubMed] [Google Scholar]
- 64.Zhang, L., W. Liu, and P. J. Grabowski. 1999. Coordinate repression of a trio of neuron-specific splicing events by the splicing regulator PTB. RNA 5:117-130. [DOI] [PMC free article] [PubMed] [Google Scholar]