Abstract
The protein kinase Bcr is a negative regulator of cell proliferation and oncogenic transformation. We identified Bcr as a ligand for the PDZ domain of the cell junction and Ras-interacting protein AF-6. The Bcr kinase phosphorylates AF-6, which subsequently allows efficient binding of Bcr to AF-6, showing that the Bcr kinase is a regulator of the PDZ domain-ligand interaction. Bcr and AF-6 colocalize in epithelial cells at the plasma membrane. In addition, Bcr, AF-6, and Ras form a trimeric complex. Bcr increases the affinity of AF-6 to Ras, and a mutant of AF-6 that lacks a specific phosphorylation site for Bcr shows a reduced binding to Ras. Wild-type Bcr, but not Bcr mutants defective in binding to AF-6, interferes with the Ras-dependent stimulation of the Raf/MEK/ERK pathway. Since AF-6 binds to Bcr via its PDZ domain and to Ras via its Ras-binding domain, we propose that AF-6 functions as a scaffold-like protein that links Bcr and Ras to cellular junctions. We suggest that this trimeric complex is involved in downregulation of Ras-mediated signaling at sites of cell-cell contact to maintain cells in a nonproliferating state.
Ras proteins are small GTP-binding proteins mediating signal transduction pathways from plasma membrane receptors to the nucleus. They are involved in the control of cell proliferation, differentiation, and apoptosis. A direct downstream effector of activated Ras is the serine/threonine kinase Raf (29). Raf phosphorylates and activates the kinase MEK, which in turn stimulates the kinase ERK (17). Another Ras-dependent pathway signals through phosphatidylinositol 3-kinase and Akt. Recently, a cross-talk between these two Ras-dependent pathways was described which can lead to either cell proliferation or differentiation (36, 50). A further putative Ras effector is the AF-6 protein (19).
The human AF-6 gene has been described first as a fusion partner of the ALL-1 gene in a subset of acute lymphoblastic leukemia caused by a chromosomal translocation t(6;11) (32). The N-terminal part of AF-6 contains two Ras-binding domains (31), a forkhead-associated domain (14), and a class V myosin homology region, DIL (30). Furthermore, a PDZ (PSD-95, Dlg, ZO-1) domain and a proline-rich region are located at the C-terminal part of AF-6 (Fig. 1A). PDZ domains are 80 to 90 amino acids long and are characterized by a hydrophobic pocket with a conserved motif, G-L-G-F (37). This pocket interacts with the C-terminal residues of ligands, which can be divided into two major classes depending on the corresponding PDZ domains (40). Class I PDZ domains mainly interact with ligands containing the motif S/T-X-Φ, where Φ is a hydrophobic residue, such as valine, leucine, or isoleucine, and X is an unspecified residue. Class II PDZ domains prefer a C-terminal Φ-X-Φ sequence. Other ligands can be divided into minor classes (12). PDZ domain-containing proteins promote clustering of receptors or other proteins preferentially at cellular junctions (8, 11). AF-6 contains one PDZ domain and interacts via this domain with proteins such as the junctional adhesion molecule JAM, the poliovirus receptor-related protein PRR2/nectin, and several members of the Eph receptor family of receptor tyrosine kinases (4, 6, 13, 42). In addition, AF-6 binds in a PDZ domain-independent manner to cytoplasmic proteins, such as the small GTPases Ras and Rap1, the deubiquitinating enzyme FAM, the tight junction protein ZO-1, the vinculin-binding protein ponsin, and profilin, a modulator of actin polymerization (1, 25, 43, 48). AF-6 colocalizes with tight junctions and adhesion junctions and is involved in connecting the junctional complexes with the cortical actin cytoskeleton. The importance of AF-6 during development has been demonstrated with AF-6-deficient mice, which died 10 days postcoitum due to defects at cell-cell junctions and had reduced cell polarity of neuroepithelial cells (49). The Drosophila melanogaster homologue of AF-6, Canoe, is also targeted to junctional complexes in embryonic epithelia. Loss-of-function mutants of Canoe lead to failure in the dorsal closure of embryonic epidermis, demonstrating an essential function in Drosophila morphogenesis (41).
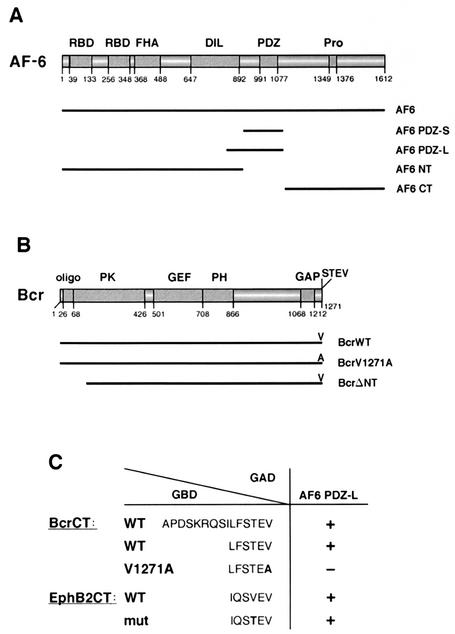
FIG. 1.
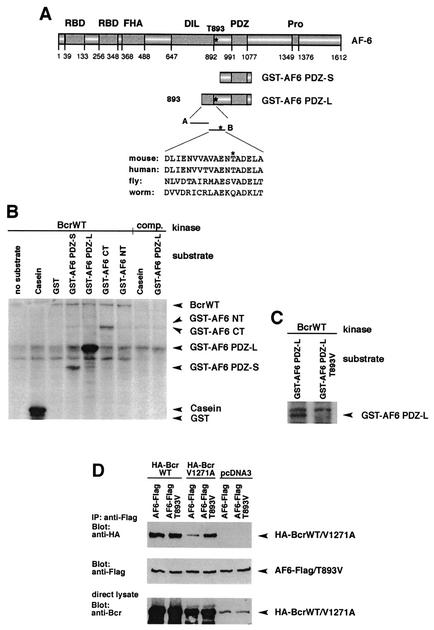
Bcr is a ligand of the PDZ domain of AF-6. (A) Scheme of the domain structure of AF-6. Numbers indicate the amino acid positions. The human AF-6 contains two Ras-binding domains (RBD), a forkhead-associated (FHA) domain, a class V myosin homology region named the DIL domain, a PDZ domain, and a proline-rich region (Pro). Constructs used in this study express full-length AF-6 (AF6), the N terminus of AF-6 corresponding to residues 1 to 914 (AF6 NT), the PDZ domain comprising residues 915 to 1129 (AF6 PDZ-S), and the C terminus of AF-6 containing residues 1130 to 1612 (AF6 CT). AF6 PDZ-L is expressed by a mouse AF-6 cDNA clone coding for the residues 867 to 1145 of mouse AF-6 that correspond to amino acids 850 to 1129 of human AF-6 (4). (B) Scheme of the domain structure of Bcr. Numbers indicate the amino acid positions. Bcr contains an oligomerization sequence (oligo), a serine/threonine protein kinase (PK) domain, a GEF function, a pleckstrin homology (PH) domain, and a GAP domain. At the extreme C terminus, Bcr contains a PDZ domain-binding motif (STEV). Bcr constructs used express full-length Bcr (BcrWT), a mutant in which the C-terminal valine is replaced by alanine (BcrV1271A), and a kinase-defective N-terminal deletion mutant that codes for residues 168 to 1271 (BcrΔNT). Notice that the mutant BcrΔNT still contains the PDZ domain-binding motif. (C) C-terminal sequences of Bcr and EphB2 were tested for their ability to bind to the AF-6 PDZ domain (AF6 PDZ-L) in a Gal4-dependent yeast two-hybrid assay (5). The wild-type sequences contain the C-terminal 15 or 6 residues of Bcr (BcrCT) or EphB2 (EphB2CT) as indicated. The construct BcrCT V1271A contains the C-terminal residue alanine instead of valine, which destroys the putative PDZ domain-binding motif. The construct EphB2CTmut comprises threonine instead of valine at position −2, where the last residue indicates position 0. This construct resembles the C terminus of Bcr and has been shown to bind to the AF-6 PDZ domain (13). All C-terminal sequences were expressed as fusions with the Gal4 DNA-binding domain (GBD), whereas the PDZ domain of AF-6 (AF6 PDZ-L) was expressed as a fusion to the Gal4 activation domain (GAD). Interaction between the C-terminal sequences and the PDZ domain of AF-6 was detected by a β-galactosidase activity assay; interaction is indicated by a plus, no interaction is indicated by a minus (38).
The human Bcr (breakpoint cluster region) is a multidomain protein with several enzymatic functions. Bcr contains a serine/threonine protein kinase domain, PK (26), a guanine nucleotide exchange factor (GEF) function, and a GTPase-activating protein (GAP) domain (Fig. 1B). The GEF and GAP domains can modulate the activity of Rho-type GTPases (2, 46). Furthermore, Bcr contains an oligomerization domain at its N terminus, a pleckstrin homology domain, which may be involved in protein-protein interaction or binding of acidic phospholipids, and a C-terminal sequence (S-T-E-V) that is a ligand for a PDZ domain as shown in this study.
Bcr is a negative regulator of the small GTPase Rac. This has been demonstrated with Bcr-deficient mice, where the loss of Bcr upregulates the production of a reactive oxygen species by the NADPH-oxidase complex through Rac (45). Simultaneous disruption of Bcr and the closely related protein Abr leads to specific abnormalities in postnatal cerebellar development that correlates with the loss of Bcr and Abr RacGAP activity (16). In addition, Bcr reduces via its GAP function the Rac1-dependent activation of the protein kinase Pak1, an activator of the JNK pathway (24).
Bcr is well known as part of the chimeric fusion protein Bcr-Abl that arises by translocation of Bcr from chromosome 9 to 22, t(9;22). This translocation is named Philadelphia chromosome, and it is found in 95% of patients with chronic myelogenous leukemia (7). The Bcr-Abl fusion protein carries a constitutively active, highly upregulated tyrosine kinase activity compared to that of the kinase activity of c-Abl in normal cells. Bcr and Abl downregulate each other's kinase activity. On the one hand, Abl and Bcr-Abl phosphorylate tyrosine residues in the kinase domain of the Bcr protein and in the Bcr part of Bcr-Abl, thereby downregulating the serine/threonine kinase activity of Bcr and Bcr-Abl (22, 23). On the other hand, the N terminus of Bcr negatively regulates the tyrosine kinase activity of Abl and Bcr-Abl and thereby reduces their transformation abilities (47).
Except for the autophosphorylation of Bcr, only one substrate has been described, the adaptor protein 14-3-3 (34). Furthermore, Bcr has been shown to bind to Raf through 14-3-3, but the function of this complex is unclear (3).
Here we demonstrate the colocalization of Bcr with AF-6 in epithelial cells at the plasma membrane. Bcr phosphorylates AF-6, which allows efficient binding of the C terminus of Bcr to the PDZ domain of AF-6. We mapped the major Bcr-dependent phosphorylation site on AF-6 to Thr 893. This residue is involved in the regulation of the interaction between Bcr and AF-6. Bcr increases binding of AF-6 to Ras, and an exchange of Thr 893 to Val reduces this interaction. In addition, Bcr interferes with the Ras-dependent stimulation of the Raf/MEK/ERK signal transduction pathway. We suggest that AF-6 is a scaffold-like protein that links Bcr and Ras to a single complex. This trimeric complex could be important for downregulation of Ras-mediated signaling at cell-cell junctions to maintain cells in a nonproliferating state.
MATERIALS AND METHODS
Antibodies.
Immunoprecipitation and immunoblotting of Bcr and AF-6 proteins were performed with rabbit polyclonal anti-Bcr C20 and anti-Bcr N20 antibody (Santa Cruz Biotechnology) for Bcr and mouse monoclonal antibody (MAb) clone 35 (Transduction Laboratories) for AF-6, respectively. Ras was immunoprecipitated with rat MAb clone Y13-238 (Calbiochem) and was blotted with mouse MAb Ras clone 18 (Transduction Laboratories). ERK2 was detected with anti-ERK C14 antibody (Santa Cruz Biotechnology), and activated forms of ERK1 and ERK2 were detected with anti-phospho p44/p42 mitogen-activated protein kinase (Cell Signaling Technology, Inc.). The hemagglutinin (HA)- and Flag-tagged proteins were analyzed by using mouse MAb 12CA5 (Roche Diagnostics) for HA and mouse MAb M2 (Sigma) for Flag. Expression of glutathione-S-transferase (GST) fusion proteins was controlled by immunoblotting with anti-GST antibody (Amersham Pharmacia Biotech).
Plasmids.
The full-length bcr cDNA (44) was subcloned into the expression vector pcDNA3 (Invitrogen), resulting in a plasmid expressing wild-type Bcr (BcrWT) (Fig. 1B). The mutant BcrV1271A, in which the C-terminal residue valine was replaced by alanine, was prepared by in vitro site-directed mutagenesis (QuikChange; Stratagene). BcrΔNT is an N-terminal deletion mutant which expresses the residues 168 to 1271 of Bcr. In addition, an HA-tagged Bcr (HA-BcrWT) was constructed by insertion of an oligonucleotide which codes for the HA tag in frame 5′ to the coding sequence of Bcr.
The cytomegalovirus-driven vectors coding for the full-length human AF6 (AF6-Flag) and the PDZ domain of murine AF-6 (Flag-AF6 PDZ-L) were described elsewhere (4) (Fig. 1A). The AF-6 mutants AF6-T893D-Flag and AF6-T893V were prepared by in vitro site-directed mutagenesis. Flag-AF6 PDZ-S expresses the PDZ domain of human AF-6, residues 915 to 1129. In addition, sequences of the human af-6 cDNA coding for the N terminus (AF6 NT; residues 1 to 914), the PDZ domain (AF6 PDZ-S), and the C terminus (AF6 CT; residues 1130 to 1612) were cloned into the vector pGEX-6P2 (Amersham Pharmacia Biotech). pGEX-AF6 PDZ-L encodes the PDZ domain of murine AF-6 (38). The plasmids encoding HA-ERK2 and RasV12 were described elsewhere (51). DNA sequencing and synthesis of oligonucleotides used for cloning were performed by Microsynth GmbH (Balgach, Switzerland).
Preparation of GST fusion proteins.
GST fusion proteins were expressed in Escherichia coli BL21plus (Stratagene) in Luria-Bertani medium, supplemented with 100 μg of ampicillin/ml, 100 μg of chloramphenicol/ml, and 20 μg of tetracycline/ml. At an optical density at 600 nm of 0.6 to 0.8 the expression of the fusion proteins was induced with isopropyl-β-d-thiogalactopyranoside (final concentration, 0.5 mM) for 6 h at 28°C. Cells from a 200-ml culture were harvested and resuspended in 10 ml of a solution containing GST buffer, phosphate-buffered saline (PBS) supplemented with 1 mM phenylmethylsulfonyl fluoride, 1.4 μg of Trasylol/ml, and 1 mM benzamidine. Cells were lysed by three freeze/thaw cycles and subsequent sonification. The lysate was then centrifuged for 15 min at 25,000 × g at 4°C. GST fusion proteins were recovered from the supernatant by incubation with 200 μl of glutathione (GSH)-agarose beads (Sigma) in GST buffer supplemented with 0.1% Triton X-100. For pull-down assays GSH-agarose beads carrying 5 μg of GST fusion protein per reaction were used. For in vitro kinase assays GST fusion proteins were eluted from the GSH-agarose beads by incubation with 5 mM glutathione. One microgram of eluted GST fusion protein was used as substrate for an in vitro kinase assay.
Cell transfection.
Human embryonic kidney 293 (HEK293) cells were cultured at 37°C and 5% CO2 in Dulbecco's modified Eagle medium containing 10% fetal calf serum, 100 U of penicillin/ml, and 100 μg of streptomycin/ml. Transfections were performed based on standard calcium-phosphate methods.
Briefly, cells were cultured to about 50% confluency. Four hours prior to transfection medium was renewed. For transfection 10 to 25 μg of plasmid DNA (per 10-cm-diameter dish) was diluted into 500 μl of 2× CaCl2 buffer (250 mM); the amount of plasmid DNA for transfection was kept constant by addition of empty vector pcDNA if necessary. After adding 500 μl of 2× BBS [50 mM N,N-bis(2-hydroxyethyl)-2-aminoethanesulfonic acid (BES; pH 6.91), 280 mM NaCl, 1.5 mM Na2HPO4] the samples were vortexed for 1 min and were incubated for 20 min at room temperature. The mixture was then added dropwise to the cells. Cells were incubated at 37°C and 3% CO2 for 16 to 18 h. The medium was replaced with fresh medium, and cells were incubated for 18 to 36 h at 37°C and 5% CO2 prior to lysis. For transfection of cells plated on 6-cm-diameter dishes, 4 to 10 μg of plasmid DNA and 200 μl of each 2× CaCl2 buffer and 2× BBS were used.
Immunoprecipitations and immunoblots.
Cells were washed with ice-cold PBS and were harvested in lysis buffer (50 mM Tris-HCl [pH 7.5], 100 mM NaCl, 0.2 mM EDTA, 1 mM dithiothreitol, 0.5% Triton X-100 supplemented with 1.4 μg of Trasylol/ml, 1 mM phenylmethylsulfonyl fluoride, 1 mM benzamidine, 10 μM leupeptin, 1 mM Na3VO4, 20 mM β-glycerol-phosphate, 25 mM NaF, 1 μg of pepstatin/ml). Cells were lysed on ice and then were centrifuged for 10 min at 16,000 × g and 4°C. Supernatants were incubated with antibodies for 2 h, and then protein G-Sepharose was added for 1 h. The immunocomplexes were collected by centrifugation for 1 min at 16,000 × g and were washed three times with 500 μl of lysis buffer. The washed immunoprecipitates were taken up in sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) sample buffer (2% SDS, 10% glycerine, 62.5 mM Tris-HCl [pH 6.8], 0.1% bromphenol blue) and were boiled for 5 min at 95°C. Equal amounts of lysates were separated by SDS-PAGE with 10% (for Bcr and AF-6) and 12.5% (for Ras) gels. Proteins were transferred onto nitrocellulose membranes. Membranes were blocked with Blotto buffer (50 mM Tris-HCl [pH 7.5], 150 mM NaCl, 0.1% Triton X-100, and 5% [wt/vol] dried low-fat milk powder) and were probed with the first antibodies (dilution, 1:1,000) before being incubated with goat anti-mouse or goat anti-rabbit secondary antibody coupled to horseradish peroxidase (dilution, 1:10,000). Immunoreactive proteins were visualized by enhanced chemiluminescence (ECL; Amersham Pharmacia Biotech).
Immunofluorescence microscopy.
Madin-Darby canine kidney cells (MDCK; American Type Culture Collection) were cultured on a coverglass, and immunofluorescence microscopy was performed as described previously (4). Briefly, cells were fixed with 3% paraformaldehyde in PBS and were permeabilized with 0.5% Triton X-100 in PBS. Samples were incubated with primary antibodies followed by treatment with either rhodamine-conjugated anti-rabbit immunoglobulin G (IgG) or fluorescein-conjugated anti-mouse IgG antibodies. Samples were embedded in Mowiol (Hoechst) and were examined with a confocal laser scanning microscope (model TCS4D; Leica). Images were further processed and merged with the Imaris software (Bitplane).
In vitro protein kinase assays.
In vitro kinase assays were performed as described previously (33). Immunocomplexes of Bcr expressed in HEK293 cells were washed three times with lysis buffer and twice with kinase buffer (20 mM HEPES [pH 7.5], 50 mM NaCl, 25 mM MnCl2, 0.1 mM Na3VO4, 10 mM β-glycerol-phosphate). Immunocomplexes were resuspended in 40 μl of kinase buffer containing 1 μg of affinity-purified recombinant GST fusion protein as substrate. Reactions were started by adding a mixture of 4 μl of 2 μM ATP and 0.5 μl of [γ-32P]ATP (5 μCi; Redivue; Amersham Pharmacia Biotech). After incubation at 30°C for 30 min the reactions were stopped by adding 15 μl of 4× SDS-PAGE sample buffer. Samples were boiled for 5 min, separated by SDS-PAGE on 10% gels, and transferred onto nitrocellulose membrane. Radioactively labeled proteins were visualized by autoradiography, and unlabeled proteins were visualized by immunoblotting with the appropriate antibody as indicated.
RESULTS
Bcr interacts with AF-6 via its PDZ domain.
The extreme C terminus of Bcr contains the amino acid sequence S-T-E-V that is typical for a PDZ ligand. The putative PDZ domain was unknown. We suspected that AF-6 contained this PDZ domain, since it binds to exactly the same C-terminal residues of a known ligand—a mutant of the EphB2 receptor (13) (Fig. 1C). We confirmed the interaction between Bcr and AF-6 in a yeast two-hybrid assay. Peptides corresponding to the C-terminal 15 or even 6 residues of Bcr were sufficient to target the Gal4 DNA-binding domain to the AF-6 PDZ domain fused to the Gal4 activation domain (Fig. 1C). In contrast, a peptide of Bcr V1271A in which the highly specific hydrophobic C-terminal residue, valine, was replaced by alanine was no longer able to bind to the AF-6 PDZ domain.
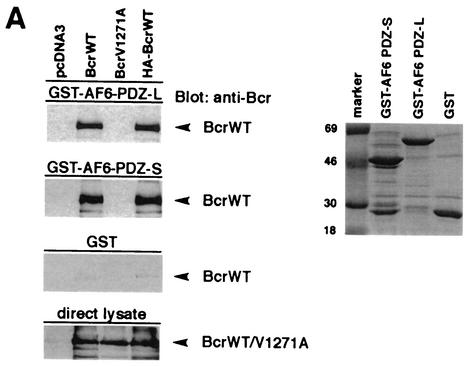
To further confirm the interaction between Bcr and AF-6 in mammalian cells, we performed pull-down assays by using the PDZ domain of AF-6 fused to the GST. GST-AF6 PDZ that contains residues 915 to 1129 was designated the short (PDZ-S) domain, whereas a longer version that carries an additional 65 residues at the N terminus was designated the large (PDZ-L) PDZ domain (Fig. 1A). These two GST-PDZ fusion proteins and the GST protein alone as control were incubated with lysates of HEK293 cells overexpressing BcrWT and an HA-tagged version (HA-BcrWT). Furthermore, a Bcr C-terminal mutant, BcrV1271A, was used which no longer functions as a PDZ ligand. As expected, BcrWT but not the mutant BcrV1271A bound to the PDZ domains of GST-AF6 PDZ-S and GST-AF6 PDZ-L (Fig. 2A, left panels). Controls performed with the empty vector pcDNA3 or with GST protein alone did not bind. Expression of Bcr proteins in HEK293 and the amount of GST proteins used for the pull-down assays were monitored by immunoblotting with anti-Bcr antibody and by protein staining with Coomassie brilliant blue, respectively (Fig. 2A, lower left panel and the right panel). Thus, the C terminus of Bcr is a ligand of AF-6 in mammalian cells.
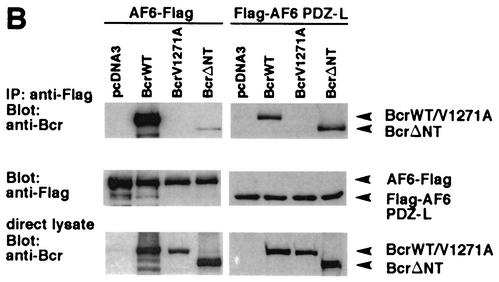
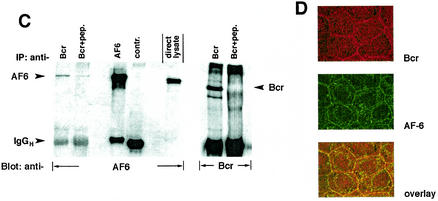
FIG.2.
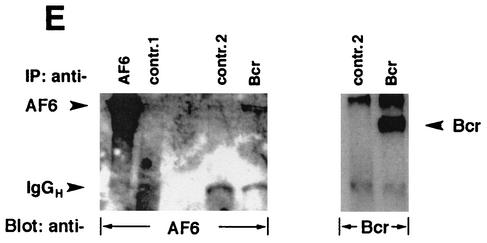
Bcr binds to AF-6 in a PDZ domain-dependent manner in vitro and in vivo. (A) HEK293 cells transfected with plasmids coding for BcrWT, BcrV1271A, and HA-BcrWT or empty vector (pcDNA3) were lysed and incubated with GST-AF6 PDZ-L, GST-AF6 PDZ-S, or GST immobilized on GSH-agarose beads as indicated. Proteins bound to GST fusion proteins were fractionated by SDS-PAGE and were immunoblotted with anti-Bcr antibody. As control for the expression of Bcr proteins, direct lysates of 293 cells were immunoblotted with anti-Bcr antibody. The amount of the GST fusion proteins was controlled by Coomassie brilliant blue staining of the SDS-polyacrylamide gel (right panel). (B) HEK293 cells were cotransfected with plasmids coding for AF6-Flag or Flag-AF6 PDZ-L together with Bcr plasmids as indicated. Cell lysates were incubated with anti-Flag antibody (IP). Coprecipitation of Bcr proteins was analyzed by immunoblotting (Blot) with anti-Bcr antibody. Efficiency of immunoprecipitation of Flag-tagged AF6 proteins with anti-Flag antibody and the amount of Bcr protein in direct lysates was monitored by immunoblotting with anti-Flag and anti-Bcr antibody, respectively. (C) Coprecipitation of endogenous AF-6 and Bcr in MDCK cells. The lysate of MDCK cells was incubated with anti-Bcr antibody in the absence or presence of Bcr peptide (+pep.) followed by immunoblotting with anti-AF-6 antibody (lanes 1 and 2). Expression of endogenous AF-6 and Bcr was verified by immunoprecipitation with anti-AF6 and anti-Bcr antibody, respectively, and immunoblotting with the same antibody (lanes 3 and 6) and by analysis of direct lysate with anti-AF-6 antibody (lane 5). A control mouse MAb (anti-cdc2/p34) (lane 4) and the Bcr peptide used as antigen (lane 7) proved the specificity of the antibody reaction. (D) Endogenous Bcr and AF-6 colocalize in MDCK cells. Confluent MDCK cells were double stained with rabbit polyclonal antibody against anti-Bcr and mouse MAb against anti-AF-6. As secondary antibodies rhodamine-conjugated anti-rabbit IgGs and fluorescein-conjugated anti-mouse IgGs were used. (E) Coprecipitation of endogenous AF-6 and Bcr in mouse brain extract. The lysate was treated as indicated and described in (C). Contr. 1, mouse monoclonal anti-cdc2/p34 antibody; Contr. 2, rabbit polyclonal anti-cdk2 antibody.
We extended this study by asking whether full-length protein of AF-6 was able to bind to Bcr. For that purpose, HEK293 cells were cotransfected with plasmids expressing Flag-tagged full-length AF6 (AF6-Flag) together with a plasmid encoding BcrWT. Again, the mutant BcrV1271A was used together with another Bcr mutant, BcrΔNT, which lacks the N-terminal 167 residues, including the ATP-binding site. This deletion abrogates the kinase activity of Bcr (data not shown) while the C-terminal sequence is unaffected (Fig. 1B). The AF-6 protein was immunoprecipitated with anti-Flag antibody and was immunoblotted with anti-Bcr antibody (Fig. 2B, upper left panel). BcrWT, but not the mutant BcrV1271A, coprecipitated with the full-length AF6-Flag protein. Also, binding of AF-6 to the mutant BcrΔNT was very weak. Full-length AF-6 behaves differently than the isolated PDZ domain of AF-6 (Flag-AF6 PDZ-L) that bound to BcrWT as well as to the mutant BcrΔNT (Fig. 2B, uppermost right panel). Again, as expected BcrV1271A did not bind to the isolated PDZ domain of AF-6. Control experiments show the expression of Flag-tagged AF-6 protein and Bcr (Fig. 2B, middle and lower panels).
These studies demonstrate that Bcr binds to the full-length AF-6 protein via its PDZ domain only if the Bcr kinase is intact. This suggests that phosphorylation induces a conformational change of AF-6, which makes its PDZ domain accessible for efficient binding to Bcr (see below).
A convincing proof of the biological relevance of the interaction between Bcr and AF-6 is the interaction of the endogenous proteins. To test this, Bcr and AF-6 were analyzed in epithelial MDCK cells where both proteins are well expressed (Fig. 2C, lanes 3 and 6). Indeed, AF-6 coprecipitated with Bcr (Fig. 2C, lane 1). Competition of the Bcr antibody by its antigen reduced precipitation of AF-6 supporting the specificity of the interaction (Fig. 2C, lane 2).
Furthermore, we analyzed the localization of endogenous AF-6 and Bcr in MDCK cells. Double staining of confluent cells with antibodies directed against Bcr and AF-6 demonstrated colocalization of both proteins at the plasma membrane, as shown in the overlay (Fig. 2D). Rabbit antibody against the HA peptide and mouse antibody against the Flag peptide were used as negative controls (data not shown).
In addition, we tested mouse brain extract for the presence of Bcr/AF-6 complexes. In this case also we were able to show the interaction between Bcr and AF-6 (Fig. 2E).
All in all, these experiments demonstrate that Bcr and AF-6 can form a stable complex in cells, with or without overexpression, and in brain tissue.
Bcr phosphorylates AF-6.
The results of our experiments demonstrate that the interaction between Bcr and full-length AF-6 protein depends on a functional kinase domain of Bcr (Fig. 2B), since deletion of the kinase domain of Bcr abrogates its binding to AF-6. This suggests that AF-6 is a substrate for Bcr. To test whether Bcr phosphorylates AF-6, in vitro kinase assays were performed. Immunopurified Bcr served as the kinase source, and purified GST-AF6 proteins served as the substrates. Four different GST-AF6 fusion proteins were prepared: GST-AF6 NT, GST-AF6 PDZ-S, GST-AF6 PDZ-L, and GST-AF6 CT (Fig. 1A and Fig. 3A). The full-length GST-AF6 protein could not be used because the protein was not stable in E. coli (data not shown). The kinase reaction resulted in strong phosphorylation of GST-AF6 PDZ-L by Bcr. In contrast, the proteins GST-AF6 PDZ-S and GST-AF6 CT were phosphorylated only weakly, and the protein GST-AF6 NT was not phosphorylated at all (Fig. 3B). The intensity of phosphorylation of GST-AF6 PDZ-L was equal to that of casein, a known substrate of Bcr in vitro. The specificity of the Bcr kinase activity was demonstrated by competition of the anti-Bcr antibody with the corresponding peptide antigen. In this control, neither GST-AF6 PDZ-L nor casein was phosphorylated by Bcr. Since GST-AF6 PDZ-L was a much better substrate than GST-AF6 PDZ-S, the major phosphorylation site for the Bcr kinase should be located in the region of 65 residues unique to GST-AF6 PDZ-L. Therefore, two peptides representing this region were tested as substrates for Bcr (Fig. 3A). Bcr phosphorylates the peptide comprising residues 881 to 898 (peptide B) but not the peptide comprising residues 851 to 885 (peptide A) (data not shown). Peptide B contains one potential phosphorylation site, Thr 893, which is conserved in AF-6 of human and mouse (Fig. 3A). A single amino acid was introduced into GST-AF6 PDZ-L that replaces threonine with valine (T893V). This protein was no longer a substrate for the Bcr kinase, indicating that Thr 893 of AF-6 is a phosphorylation site for Bcr in vitro (Fig. 3C). In all reactions the amount of Bcr proteins and GST fusion proteins used in the kinase assays were controlled by immunoblotting with anti-Bcr antibody and anti-GST antibody, respectively (data not shown).
FIG. 3.
Bcr phosphorylates AF-6 at threonine 893. (A) Schematic representation of AF-6 and its constructs used for the in vitro kinase assay. Peptides A and B represent the sequence unique to GST-AF6 PDZ-L. The sequence of peptide B containing T893 is depicted. This sequence is highly conserved among human and mouse AF-6 but is less conserved in the AF-6 homologs of fly and worm that lack the putative phosphorylation site at the position corresponding to T893. (B) Bcr phosphorylates AF-6. Immunopurified BcrWT prepared from HEK293 cells overexpressing BcrWT was used as kinase. The following GST proteins served as substrates: GST fused to AF6 PDZ-S, AF6 PDZ-L, AF6 CT, and AF6 NT. The specificity of the Bcr kinase activity was checked by using casein and GST alone as positive and negative controls, respectively, and by competition (comp.) of the anti-Bcr antibody with its peptide antigen. The amount of Bcr and of GST fusion proteins was verified by immunoblotting with anti-Bcr and anti-GST antibody (data not shown). (C) Bcr phosphorylates AF-6 at Thr 893. In vitro kinase assays were performed as described for panel B. As substrate, GST-AF6 PDZ-L and GST-AF6 PDZ-L T893V were tested. (D) Thr 893 of AF-6 regulates binding to BcrV1271A. HEK293 cells were transfected with Bcr and AF-6 constructs as indicated. Lysates were incubated with anti-Flag antibody, and coprecipitation of HA-Bcr proteins was analyzed by immunoblotting with anti-HA antibody. Expression of AF-6 and Bcr proteins was controlled by immunoblotting the direct lysate with the appropriate antibody. WT, wild type.
Since Bcr phosphorylates AF-6 at Thr 893, we asked whether the mutant AF6-T893V, which lacks a phosphorylation site at residue 893, differs in binding to Bcr. Coexpression of BcrWT with AF-6 or the mutant AF6-T893V resulted in a similar amount of complex formation between Bcr and AF-6 (Fig. 3D). Interestingly, BcrV1271A, which contains a defective PDZ domain-binding motif, distinguished between AF-6 and AF6-T893V in that BcrV1271A showed only a residual binding to AF-6, whereas binding to AF6-T893V was much stronger (Fig. 3D). This indicates that phosphorylation of Thr 893 can negatively regulate the binding of BcrV1271A to AF-6.
In summary, these results demonstrate that Bcr phosphorylates AF-6 and that one specific phosphorylation site is Thr 893, which is located in the center of AF-6 in a linker region close to the PDZ domain of AF-6. In addition, phosphorylation of AF-6 at Thr 893 seems to regulate the interaction between Bcr and AF-6 under certain conditions.
Bcr forms a trimeric complex with AF-6 and Ras.
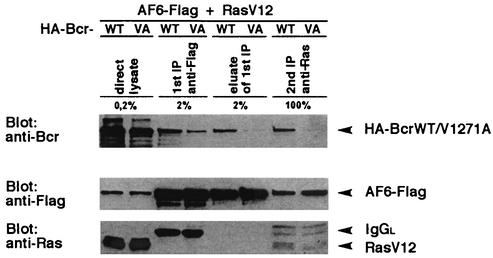
AF-6 has been described as a potential Ras effector protein that interacts via its Ras-binding domain with the activated form of Ras (19). As we have shown that AF-6 binds to Bcr, the question arose whether AF-6 binds to both proteins at the same time and could form a trimeric complex. To analyze this possibility, BcrWT, AF-6, and the activated Ras mutant, RasV12, were coexpressed in HEK293 cells, and a sequential precipitation was carried out. First, the cell lysate was incubated with anti-Flag antibody to precipitate AF6-Flag and AF-6-associated proteins. After elution of AF6-Flag from the antibody by competition with the Flag peptide, the eluate was immunoprecipitated in a second step with anti-Ras antibody. In a trimeric complex, AF-6, Ras, and Bcr should be detectable in the first and second immunocomplex. This is clearly shown in the second immunocomplex with anti-Ras antibody (Fig. 4). In the first immunocomplex with anti-Flag antibody, where only a fiftieth was loaded on the gel, Bcr but not Ras is detectable. This indicates that more Bcr is complexed to AF-6 than RasV12, suggesting that in addition to trimers, dimers between AF-6 and Bcr also are present in this first immunocomplex. Furthermore, Bcr can oligomerize and therefore could be overrepresented in the AF-6 complex compared to RasV12. As control the mutant BcrV1271A was coexpressed with AF-6 and Ras and showed a reduced binding to AF-6 and no coprecipitation with Ras. These results indicate that AF-6 is a bifunctional molecule that binds Bcr via its PDZ domain and active Ras via its Ras-binding domain, located at the C-terminal and N-terminal part of AF-6, respectively.
FIG. 4.
Bcr, AF-6, and Ras form a trimeric complex. HEK293 cells were cotransfected with BcrWT or BcrV1271A, respectively, together with RasV12 and AF6-Flag. The expression of the proteins was confirmed directly by immunoblot (direct lysate). The first immunoprecipitation (1st IP) was performed with anti-Flag antibody. The Flag complex was eluted from the antibody by incubation with Flag peptide (eluate of 1st IP) and was reprecipitated with anti-Ras antibody (2nd IP). With the exception of the second immunoprecipitation, only small aliquots of the total sample were loaded on the gel as indicated. Bcr, AF-6, and Ras were detected by immunoblotting with appropriate antibodies. IgGL, Ig light chain.
Bcr increases the formation of the complex of AF-6 with Ras.
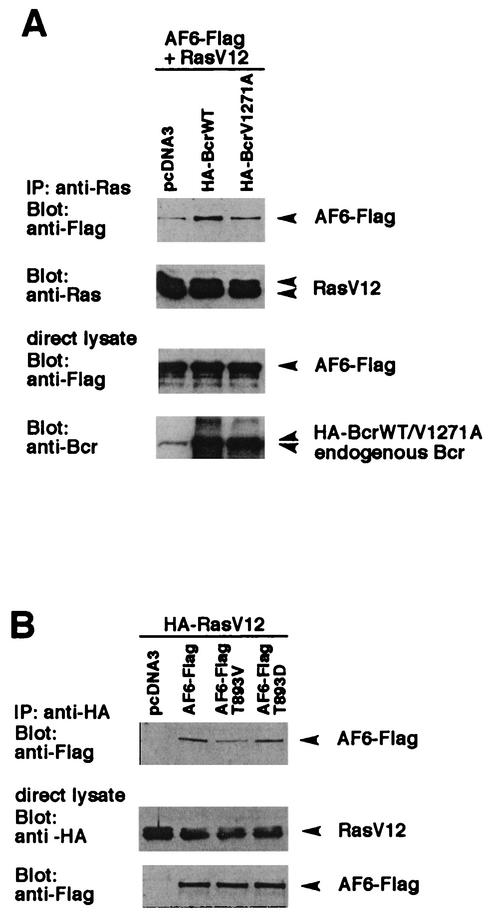
The experiments described above demonstrate that AF-6 can connect Bcr and activated Ras. Since Bcr phosphorylates AF-6 and thereby regulates their interaction, the influence on Ras was of interest. In order to test this, a lysate of HEK293 cells, overexpressing AF6-Flag and the activated mutant RasV12 together with BcrWT or the mutant BcrV1271A, were immunoprecipitated with anti-Ras antibody. The analysis of Ras complexes by immunoblotting with anti-Flag antibody revealed that overexpression of BcrWT but not of BcrV1271A led to an increase in the amount of AF-6 bound to active Ras (Fig. 5A). The expression of AF-6, Bcr, and Ras was controlled by immunoblotting the cell lysates with the appropriate antibodies. This experiment showed that Bcr increased the binding of AF-6 to activated Ras in a reaction that requires binding of its C terminus to the PDZ domain of AF-6.
FIG. 5.
Bcr modulates the formation of the AF-6/Ras complex. (A) Coexpression of BcrWT increases the complex formation between AF-6 and RasV12. HEK293 cells were transfected with BcrWT or BcrV1271A, respectively, together with RasV12 and AF6-Flag. Cell lysates were incubated with anti-Ras antibody, and coprecipitation of AF6-Flag was detected by immunoblotting with anti-Flag antibody. The amount of precipitated RasV12 and the expression of Bcr and AF-6 were checked by immunoblotting with the appropriate antibodies as indicated. (B) Prevention of phosphorylation of AF-6 by Bcr reduces binding of AF-6 to Ras. HA-RasV12 was coexpressed with AF6-Flag or AF-6 mutant proteins, AF6 T893V and AF6 T893D, that carry a single amino acid exchange at position 893 from Thr to Val or Asp, respectively. Cell lysates were incubated with anti-HA antibody for immunoprecipitation of HA-RasV12, and coprecipitation of AF6-Flag proteins was detected by immunoblotting with anti-Flag antibody. Expression of HA-RasV12 and AF6-Flag proteins was monitored by immunoblotting with the appropriate antibodies as indicated. IP, immunoprecipitation.
Since Bcr can increase the binding of AF-6 to Ras, the role of its kinase activity was tested by using the AF6-T893V mutant, which cannot be phosphorylated by Bcr. Another mutant, AF6-T893D, that should mimic the phosphorylation by a negatively charged residue was used for comparison. As can be seen in Fig. 5B, the mutant AF6-T893V showed reduced binding to Ras compared to that of wild-type AF-6. The amount of mutant AF6-T893D protein bound to Ras is not significantly increased compared to that of wild-type AF-6. This could be explained if the phosphorylation of the threonine residue is not sufficiently mimicked by the replacement through a negatively charged residue. Such a phenomenon has been described for other proteins (35).
In summary, the experiments demonstrate that phosphorylation of AF-6 by Bcr is important to regulate the interaction between AF-6 and Ras. In addition, Thr 893 of AF-6 seems to play an essential role in this process.
Bcr downregulates Ras-mediated ERK activation.
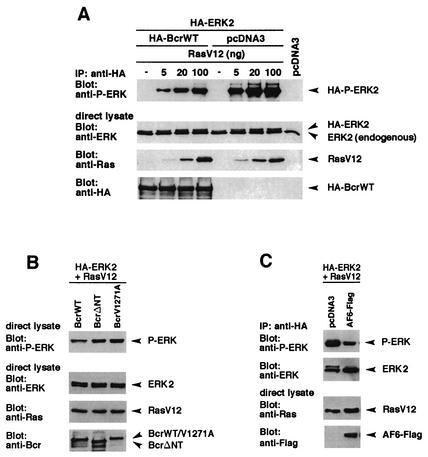
It has been shown previously that the two Ras-interacting proteins, AF-6 and Raf-1, can compete with each other for binding to the Ras effector domain in vitro (15). Furthermore, overexpression of the Ras-binding domain of AF-6 downregulates the stimulation of the fos promoter, which depended on the Ras/Raf/MEK/ERK pathway (48). Since Bcr can form a trimeric complex with AF-6 and Ras, we tested whether Bcr could be responsible for the downregulation of the Ras/MEK/ERK pathway. Therefore, BcrWT, ERK2, and increasing amounts of the activated Ras mutant RasV12 were coexpressed in HEK293 cells. HA-ERK2 was immunoprecipitated with anti-HA antibody and was immunoblotted with anti-phosphospecific ERK antibody that only detected the phosphorylated activated form of ERK2. BcrWT interferes with Ras-induced ERK stimulation, as can be seen by comparison with cells transfected with the control plasmid (Fig. 6A, upper panel). Controls show the expression of BcrWT, RasV12, and ERK (Fig. 6A, lower panels). Thus, Bcr negatively regulates Ras-mediated signaling.
FIG. 6.
Bcr interferes with Ras-mediated ERK2 activation in a PDZ ligand-dependent manner. (A) HEK293 cells plated on 6-cm-diameter dishes were cotransfected with plasmids coding for HA-BcrWT or empty vector (4 μg) together with HA-ERK2 (1 μg) and increasing amounts of RasV12 as indicated. Cell lysates were incubated with anti-HA antibody, and the activity of immunoprecipitated HA-ERK2 was determined by immunoblotting with anti-phospho-ERK2 (anti-P-ERK) antibody. Expression of HA-BcrWT, HA-ERK2, and RasV12 was verified by immunoblotting with the appropriate antibodies as indicated. (B) HEK293 cells, which expressed BcrWT, BcrΔNT, or BcrV1271A together with HA-ERK2 and RasV12 (20 ng) were analyzed for the amount of phosphorylated, activated ERK2 as described for panel A. Expression of proteins was controlled by immunoblotting with the appropriate antibodies as indicated. (C) AF-6 can reduce Ras-mediated ERK2 activation. AF-6 and control vector were transfected in HEK293 cells together with RasV12 and HA-ERK and were analyzed as described for panel A. IP, immunoprecipitation.
To test whether this effect of Bcr on Ras signaling depends on the C terminus of Bcr, which acts as ligand to the PDZ domain, BcrWT and BcrV1271A were compared for their effect on ERK activation. BcrWT and BcrV1271A were cotransfected in HEK293 cells together with activated RasV12. Overexpression of BcrWT resulted in a significant reduction of ERK stimulation compared to that of cells expressing BcrV1271A (Fig. 6B, upper panel). In addition, the kinase-defective mutant BcrΔNT did not lead to the reduction of the Ras-dependent ERK activation, indicating a role of the Bcr kinase activity on this inhibitory effect. The expression of the proteins was controlled by immunoblotting with the appropriate antibodies (Fig. 6B, lower panels).
These data imply that the effect of Bcr on Ras signaling depends on a PDZ domain-containing protein. AF-6 binds to Bcr and is well expressed in 293 cells (data not shown). Therefore, we tested whether AF-6 can interfere with Ras-dependent ERK activation. Indeed, overexpression of AF-6 also reduces ERK activation (Fig. 6C).
Taken together, these data demonstrate that an active Bcr kinase downregulates the Ras-dependent stimulation of ERK. This effect of Bcr depends on a functional kinase domain of Bcr and its C terminus, which is a ligand for the PDZ domain of AF-6.
DISCUSSION
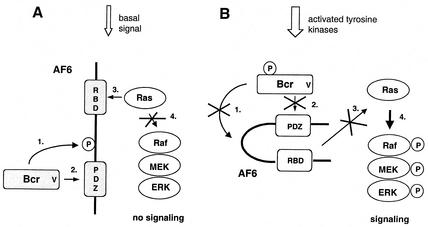
Here we demonstrate a novel function of Bcr as a negative regulator of Ras signaling. To achieve this effect, the Bcr kinase phosphorylates AF-6, binds to its PDZ domain, and allows AF-6 to interact with Ras and keep Ras away from downstream signaling (the proposed model is depicted in Fig. 7). This mechanism may be responsible for maintaining the cell in a nonproliferative state where the Bcr kinase is constitutively active.
FIG. 7.
Proposed model depicting the effect of Bcr on Ras-dependent stimulation of ERK via AF-6. (A) In quiescent cells the constitutively active Bcr phosphorylates AF-6 (step 1), which leads to the interaction of the PDZ domain of AF-6 with the PDZ-binding motif of Bcr (step 2). This interaction increases the affinity of AF-6 for Ras via the Ras binding domain (RBD) (step 3) and prevents binding of Raf to Ras (step 4). Under these conditions the protein kinase cascade composed of Raf, MEK, and ERK is not activated. (B) Phosphorylation of Bcr on tyrosine residues inactivates its protein kinase activity. Therefore, Bcr cannot phosphorylate (step 1) and cannot bind to AF-6 (step 2). Thus, AF-6 does not compete with Raf for Ras (step 3) and does not interfere with the Ras-dependent activation of the protein kinase cascade (step 4). P represents phosphorylation of proteins.
We recognized by sequence analysis that the C terminus of Bcr contains a motif typical for ligands of PDZ domains, S-T-E-V, and indeed, the C terminus of Bcr binds to the PDZ domain of AF-6. Several other ligands are known to bind to the PDZ domain of AF-6, such as cell adhesion molecules and signal transducers. AF-6 colocalizes with these binding partners at sites of cell-cell contact. We show that AF-6 colocalizes with Bcr at the plasma membrane in confluent epithelial cells. Also, in primary tissue, such as brain tissue, Bcr and AF-6 are expressed in similar regions (4, 10). Moreover, both proteins can be coprecipitated from mouse brain lysate (Fig. 2E). These data suggest a biological role for the interaction between Bcr and AF-6 at the cellular membrane.
We show that Bcr phosphorylates AF-6. Since no homolog of Bcr exists in invertebrates, Bcr-dependent phosphorylation and regulation of AF-6 must be specific for vertebrates. The importance of phosphorylation for the interaction between Bcr and AF-6 was demonstrated here by comparison of the BcrWT and the kinase-defective mutant BcrΔNT and showed that the PDZ domain of full-length AF-6 is accessible only for efficient binding after phosphorylation. We mapped the major phosphorylation site of Bcr on AF-6 to Thr 893, a site that is conserved in AF-6 of human and mouse but not in AF-6 homologues of fly and worm (Fig. 3A). This supports the notion that this Bcr-dependent phosphorylation of AF-6 is specific for vertebrates. AF-6 and AF6-T893V did not differ in their ability to bind to BcrWT. This indicates that in addition to phosphorylation of AF-6 at Thr 893 there has to be a further phosphorylation event which regulates phosphorylation-dependent binding of AF-6 to BcrWT. However, the mutant BcrV1271A, which cannot bind to the PDZ domain of AF-6, reveals differences. In the case of AF-6, which can be phosphorylated at Thr 893, the interaction is reduced. In the case of AF6-T893V, which can no longer be phosphorylated at this site, the interaction to BcrV1271A is much stronger. Thus, under certain conditions phosphorylation of AF-6 at Thr 893 may play a regulatory role, e.g., by affecting the specificity of the binding to the PDZ domain or to another binding site.
The phosphorylation site Thr 893 is located in a linker-like central part of AF-6 and is close to the PDZ domain. Phosphorylation in the proximity of a PDZ domain for regulation of ligand binding is a novel phenomenon. In other cases, the binding between ligand and PDZ domain is also regulated by phosphorylation of the ligand. This is shown for the S/T-X-Φ motif interacting with class I PDZ domains. Phosphorylation of S/T in this motif regulates the binding affinity of, e.g., the E6 protein of the human papilloma virus to the PDZ protein hDlg (18). This demonstrates the relevance of phosphorylation for the interaction of PDZ domains with their ligands.
Bcr is not the only kinase that phosphorylates AF-6, thereby regulating binding of its ligand to the PDZ domain. Receptor tyrosine kinases of the Eph receptor family have been shown to phosphorylate AF-6 (13). Bcr and Eph receptors have in common that they are both negative regulators of proliferation. The Bcr kinase has a function in quiescent cells where it is constitutively active. Eph receptors, when activated by ephrins, lead to inhibition of proliferation and induction of differentiation (28). In both cases AF-6 is involved in maintenance of a nonproliferative state. We propose this in a model (Fig. 7A).
AF-6 has previously been shown to compete with Raf for binding to active Ras in vitro (15). In addition, overexpression of the Ras-binding domain of AF-6 inhibits Ras/Raf-dependent c-fos promoter stimulation (48). Here we show that overexpression of the full-length AF-6 protein can interfere with Ras-dependent signaling and inhibits activation of ERK2. This could be the result of a residual low-affinity binding of overexpressed AF-6 independently of its phosphorylation state. We show that BcrWT but not the mutant BcrV1271A, which is defective in binding to AF-6, reduced Ras-dependent activation of ERK2. We attribute this to direct binding of BcrWT to endogenous AF-6 under conditions where AF-6 is phosphorylated and the Ras-binding site is accessible. This requires the kinase function of Bcr and its ability to bind to the PDZ domain of AF-6. The Bcr kinase is active in nonproliferating cells and inhibits low basal signal activity, which we mimic here by low expression of active Ras (Fig. 6 and 7A). However, when the Bcr kinase activity is downregulated by tyrosine phosphorylation, Bcr is not able to reduce Ras-dependent signaling (20, 22) (Fig. 7B). In the developing system of the Drosophila eye, the AF-6 homolog Canoe also modulates the Ras activity (27), although it does so by other regulators, since a homolog of human Bcr does not exist in Drosophila. Additional signaling mechanisms may have evolved to control the more complex organization in vertebrates.
Ras is not the only GTPase that interacts with AF-6. Other GTPases, such as Rap1A, Rit, and Rin, also bind to the Ras-binding domain of AF-6 (1, 21, 39). Rap1A and AF-6 colocalize at the plasma membrane of epithelial cells, and overexpression of Rap1 maintains their epithelial morphology (1). In contrast, high expression of active Ras gives rise to disruption of cell-cell contacts. Thus, AF-6 might regulate different cellular responses, such as cell adhesion, differentiation, or morphogenesis, through interaction with Ras or Rap1A (52). Whether Bcr also modulates a functional interaction between Rap1A and AF-6 is under investigation.
We identified a trimeric complex between Bcr, AF-6, and Ras. Alternatively, Bcr can also be linked to the Ras pathway through the adaptor protein 14-3-3, yet the biological significance has not been clearly shown. Indeed, Bcr phosphorylates 14-3-3 and also interacts via 14-3-3 with the Ras effector Raf (3, 34). However, this interaction does not depend on a PDZ domain-containing protein. Thus, there are two substrates of Bcr, AF-6 and 14-3-3, both of which might regulate the Ras/Raf/MEK/ERK pathway, one affecting Ras and the other one affecting Raf. Here we demonstrate the function of Bcr as a negative regulator of Ras, which acts via AF-6 in a PDZ domain-dependent manner.
Bcr contains additional functional domains besides its kinase domain, e.g., the GAP domain, which regulates Rho/Rac-dependent signaling. Rho-dependent signaling is involved in organization of the actin cytoskeleton at sites of cell-cell contact (9). AF-6 also associates with the cytoskeleton through binding to profilin, which is a regulator for actin polymerization and participates in cortical actin assembly (1). Thus, the Bcr/AF-6 complex could play an important role in regulating signaling pathways at sites of cellular junctions, but it may as well be important for the structural organization of the cell.
Acknowledgments
We thank Gunnar Schuetz for help in the initial phase of this project and Martin Schwemmle for preparation of the mouse brain extracts. We also thank Jochen Heinrich, Algirdas Ziogas, Jovan Pavlovic, and Beatrice Pilger for helpful discussion and critical reading of the manuscript.
This work was supported by the Swiss National Science Foundation, the Swiss Cancer League, the Krebsliga des Kantons Zürich, and the BMBF.
REFERENCES
- 1.Boettner, B., E. Govek, J. Cross, and L. van Aelst. 2000. The junctional multidomain protein AF-6 is a binding partner of the Rap1A GTPase and associates with the actin cytoskeletal regulator profilin. Proc. Natl. Acad. Sci. USA 97:9064-9069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Boguski, M. S., and F. McCormick. 1993. Proteins regulating Ras and its relatives. Nature 366:643-654. [DOI] [PubMed] [Google Scholar]
- 3.Braselmann, S., and F. McCormick. 1995. Bcr and Raf form a complex in vivo via 14-3-3 proteins. EMBO J. 14:4839-4848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Buchert, M., S. Schneider, V. Meskenaite, M. T. Adams, E. Canaani, T. Baechi, K. Moelling, and C. M. Hovens. 1999. The junction-associated protein AF-6 interacts and clusters with specific Eph receptor tyrosine kinases at specialized sites of cell-cell contact in the brain. J. Cell Biol. 144:361-371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Durfee, T., K. Becherer, P. L. Chen, S. H. Yeh, Y. Yang, A. E. Kilburn, W. H. Lee, and S. J. Elledge. 1993. The retinoblastoma protein associates with the protein phosphatase type 1 catalytic subunit. Genes Dev. 7:555-569. [DOI] [PubMed] [Google Scholar]
- 6.Ebnet, K., C. U. Schulz, M. K. Meyer zu Brickwedde, G. G. Pendl, and D. Vestweber. 2000. Junctional adhesion molecule interacts with the PDZ-domain-containing proteins AF-6 and ZO-1. J. Biol. Chem. 275:27979-27988. [DOI] [PubMed] [Google Scholar]
- 7.Faderl, S., M. Talpaz, Z. Estrov, S. O'Brien, R. Kurzrock, and H. M. Kantarjian. 1999. The biology of chronic myeloid leukemia. N. Engl. J. Med. 341:164-172. [DOI] [PubMed] [Google Scholar]
- 8.Fanning, A. S., and J. M. Anderson. 1999. PDZ domains: fundamental building blocks in the organization of protein complexes at the plasma membrane. J. Clin. Investig. 103:767-772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fanning, A. S., L. L. Mitic, and J. M. Anderson. 1999. Transmembrane proteins in the tight junction barrier. J. Am. Soc. Nephrol. 10:1337-1345. [DOI] [PubMed] [Google Scholar]
- 10.Fioretos, T., J. W. Voncken, T. Z. Baram, F. Kamme, J. Groffen, and N. Heisterkamp. 1995. Regional localization and developmental expression of the bcr gene in rodent brain. Cell. Mol. Biol. Res. 41:97-102. [PMC free article] [PubMed] [Google Scholar]
- 11.Garner, C. C., J. Nash, and R. L. Huganir. 2000. PDZ domains in synapse assembly and signalling. Trends Cell Biol. 10:274-280. [DOI] [PubMed] [Google Scholar]
- 12.Harris, B. Z., and L. Lim. 2001. Mechanism and role of PDZ domains in signalling complex assembly. J. Cell Sci. 114:3219-3231. [DOI] [PubMed] [Google Scholar]
- 13.Hock, B., B. Böhme, T. Karn, K. Kaibuchi, T. Yamamoto, U. Holtrich, S. Holland, T. Pawson, H. Rübsamen-Waigmann, and K. Strebhardt. 1998. PDZ-domain-mediated interaction of the Eph-related receptor tyrosine kinase EphB3 and the ras-binding protein AF-6 depends on the kinase activity of the receptor. Proc. Natl. Acad. Sci. USA 95:9779-9784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hofmann, K., and P. Bucher. 1995. The FHA domain: a putative nuclear signalling domain found in protein kinases and transcription factors. Trends Biochem. Sci. 20:347-349. [DOI] [PubMed] [Google Scholar]
- 15.Hu, C. D., K. Kariya, G. Kotani, M. Shirouzu, S. Yokoyama, and T. Kataoka. 1997. Coassociation of Rap1A and Ha-Ras with Raf-1 N-terminal region interferes with Ras-dependent activation of Raf-1. J. Biol. Chem. 272:11702-11705. [DOI] [PubMed] [Google Scholar]
- 16.Kaartinen, V., I. Gonzalez-Gomez, J. W. Voncken, L. Haataja, E. Faure, A. Nagy, J. Groffen, and N. Heisterkamp. 2001. Abnormal function of astroglia lacking Abr and Bcr RacGAPs. Development 128:4217-4227. [DOI] [PubMed] [Google Scholar]
- 17.Kolch, W. 2000. Meaningful relationships: the regulation of the Ras/Raf/Mek/Erk pathway by protein interactions. Biochem. J. 351:289-305. [PMC free article] [PubMed] [Google Scholar]
- 18.Kuhne, C., D. Gardiol, C. Guarnaccia, H. Amenitsch, and L. Banks. 2000. Differential regulation of human papillomavirus E6 by protein kinase A: conditional degradation of human discs large protein by oncogenic E6. Oncogene 19:5884-5891. [DOI] [PubMed] [Google Scholar]
- 19.Kuriyama, M., N. Harada, S. Kuroda, T. Yamamoto, M. Nakafuku, A. Iwamatsu, D. Yamamoto, R. Prasad, C. Croce, E. Canaani, and K. Kaibuchi. 1996. Identification of AF-6 and Canoe as putative targets for Ras. J. Biol. Chem. 271:607-610. [DOI] [PubMed] [Google Scholar]
- 20.Li, J., and T. E. Smithgall. 1996. Coexpression with Bcr induces activation of Fes tyrosine kinase and phosphorylation of specific N-terminal Bcr tyrosine residues. J. Biol. Chem. 271:32930-32936. [DOI] [PubMed] [Google Scholar]
- 21.Linnemann, T., M. Geyer, B. K. Jaitner, C. Block, H. R. Kalbitzer, A. Wittinghofer, and C. Herrmann. 1999. Thermodynamic and kinetic characterization of the interaction between the Ras binding domain of AF6 and members of the Ras subfamily. J. Biol. Chem. 274:13556-13562. [DOI] [PubMed] [Google Scholar]
- 22.Liu, J., Y. Wu, G. Z. Ma, D. Lu, L. Haataja, N. Heisterkamp, J. Groffen, and R. B. Arlinghaus. 1996. Inhibition of Bcr serine kinase by tyrosine phosphorylation. Mol. Cell. Biol. 16:998-1005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lu, D., J. Liu, M. Campbell, J. Q. Guo, N. Heisterkamp, J. Groffen, E. Canaani, and R. Arlinghaus. 1993. Tyrosine phosphorylation of P160 Bcr by P210 Bcr-Abl. Blood 82:1257-1263. [PubMed] [Google Scholar]
- 24.Lu, W., and B. J. Mayer. 1999. Mechanism of activation of Pak1 kinase by membrane localization. Oncogene 18:797-806. [DOI] [PubMed] [Google Scholar]
- 25.Mandai, K., H. Nakanishi, A. Satoh, K. Takahashi, K. Satoh, H. Nishioka, A. Mizoguchi, and Y. Takai. 1999. Ponsin/SH3P12: an l-afadin- and vinculin-binding protein localized at cell-cell and cell-matrix adherens junctions. J. Cell Biol. 144:1001-1017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Maru, Y., and O. N. Witte. 1991. The bcr gene encodes a novel serine/threonine kinase activity within a single exon. Cell 67:459-468. [DOI] [PubMed] [Google Scholar]
- 27.Matsuo, T., K. Takahashi, S. Kondo, K. Kaibuchi, and D. Yamamoto. 1997. Regulation of cone cell formation by Canoe and Ras in the developing Drosophila eye. Development 124:2671-2680. [DOI] [PubMed] [Google Scholar]
- 28.Miao, H., B. R. Wei, D. M. Peehl, Q. Li, T. Alexandrou, J. R. Schelling, J. S. Rhim, J. R. Sedor, E. Burnett, and B. Wang. 2001. Activation of EphA receptor tyrosine kinase inhibits the Ras/MAPK pathway. Nat. Cell Biol. 3:527-530. [DOI] [PubMed] [Google Scholar]
- 29.Moelling, K., B. Heimann, P. Beimling, U. R. Rapp, and T. Sander. 1984. Serine- and threonine-specific protein kinase activities of purified gag-mil and gag-raf proteins. Nature 312:558-561. [DOI] [PubMed] [Google Scholar]
- 30.Ponting, C. P. 1995. AF-6/cno: neither a kinesin nor a myosin, but a bit of both. Trends Biochem. Sci. 20:265-266. [DOI] [PubMed] [Google Scholar]
- 31.Ponting, C. P., and D. R. Benjamin. 1996. A novel family of Ras-binding domains. Trends Biochem. Sci. 21:422-425. [DOI] [PubMed] [Google Scholar]
- 32.Prasad, R., Y. Gu, H. Alder, T. Nakamura, O. Canaani, H. Saito, K. Huebner, R. P. Gale, P. C. Nowell, K. Kuriyama, et al. 1993. Cloning of the ALL-1 fusion partner, the af-6 gene, involved in acute myeloid leukemias with the t(6;11) chromosome translocation. Cancer Res. 53:5624-5628. [PubMed] [Google Scholar]
- 33.Radziwill, G., M. Niehof, C. Rommel, and K. Moelling. 1995. Direct interaction and N-terminal phosphorylation of c-Jun by c-Mil/Raf. Proc. Natl. Acad. Sci. USA 92:1421-1425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Reuther, G. W., H. Fu, L. D. Cripe, R. J. Collier, and A. M. Pendergast. 1994. Association of the protein kinases c-Bcr and Bcr-Abl with proteins of the 14-3-3 family. Science 266:129-133. [DOI] [PubMed] [Google Scholar]
- 35.Rommel, C., G. Radziwill, K. Moelling, and E. Hafen. 1997. Negative regulation of Raf activity by binding of 14-3-3 to the amino terminus of Raf in vivo. Mech. Dev. 64:95-104. [DOI] [PubMed] [Google Scholar]
- 36.Rommel, C., B. A. Clarke, S. Zimmermann, L. Nunez, R. Rossman, K. Reid, K. Moelling, G. D. Yancopoulos, and D. J. Glass. 1999. Differentiation stage-specific inhibition of the Raf-Mek-Erk pathway by Akt. Science 286:1738-1741. [DOI] [PubMed] [Google Scholar]
- 37.Saras, J., and C. H. Heldin. 1996. PDZ domains bind carboxy-terminal sequences of target proteins. Trends Biochem. Sci. 21:455-458. [DOI] [PubMed] [Google Scholar]
- 38.Schneider, S., M. Buchert, O. Georgiev, B. Catimel, M. Halford, S. A. Stacker, T. Baechi, K. Moelling, and C. M. Hovens. 1999. Mutagenesis and Selection of PDZ domains that bind new protein targets. Nat. Biotechnol. 17:170-175. [DOI] [PubMed] [Google Scholar]
- 39.Shao, H., K. Kadano-Okuda, B. S. Finlin, and D. A. Andres. 1999. Biochemical characterization of the Ras-related GTPases Rit and Rin. Arch. Biochem. Biophys. 371:207-219. [DOI] [PubMed] [Google Scholar]
- 40.Songyang, Z., A. S. Fanning, C. Fu, S. Xu, S. M. Marfatia, A. H. Chishti, A. Crompton, A. C. Chan, J. M. Anderson, and L. C. Cantley. 1997. Recognition of unique carboxyl-terminal motifs by distinct PDZ domains. Science 275:73-77. [DOI] [PubMed] [Google Scholar]
- 41.Takahashi, K., T. Matsuo, T. Katsube, R. Ueda, and Y. Daisuke. 1998. Direct binding between two PDZ domain proteins Canoe and ZO-1 and their roles in regulation of the jun N-terminal kinase pathway in Drosophila morphogenesis. Mech. Dev. 78:97-111. [DOI] [PubMed] [Google Scholar]
- 42.Takahashi, K., H. Nakanishi, M. Miyahara, K. Mandai, K. Satoh, A. Satoh, H. Nishioka, J. Aoki, A. Nomoto, A. Mizoguchi, and Y. Takai. 1999. Nectin/Prr: an immunoglobulin-like cell adhesion molecule recruited to cadherin-based adherens junctions through interaction with Afadin, a PDZ domain-containing protein. J. Cell Biol. 145:539-549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Taya, S., T. Yamamoto, K. Kano, Y. Kawano, A. Iwamatsu, T. Tsuchiya, K. Tanaka, M. Kanai-Azuma, S. A. Wood, J. S. Mattick, and K. Kaibuchi. 1998. The Ras target AF-6 is a substrate of the fam deubiquitinating enzyme. J. Cell Biol. 142:1053-1062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Timmons, M. S., and O. N. Witte. 1989. Structural characterization of the bcr gene product. Oncogene 4:559-567. [PubMed] [Google Scholar]
- 45.Voncken, J. W., H. van Schaick, V. Kaartinen, K. Deemer, T. Coates, B. Landing, P. Pattengale, O. Dorseuil, G. M. Bokoch, J. Groffen, and N. Heisterkamp. 1995. Increased neutrophil respiratory burst in bcr-null mutants. Cell 80:719-728. [DOI] [PubMed] [Google Scholar]
- 46.Wittinghofer, A., K. Scheffzek, and M. R. Ahmadian. 1997. The interaction of Ras with GTPase-activating proteins. FEBS Lett. 410:63-67. [DOI] [PubMed] [Google Scholar]
- 47.Wu, Y., G. Ma, D. Lu, F. Lin, H. Xu, J. Liu, and R. B. Arlinghaus. 1999. Bcr: a negative regulator of the Bcr-Abl oncoprotein. Oncogene 18:4416-4424. [DOI] [PubMed] [Google Scholar]
- 48.Yamamoto, T., N. Harada, Y. Kawano, S. Taya, and K. Kaibuchi. 1999. In vivo interaction of AF-6 with activated Ras and ZO-1. Biochem. Biophys. Res. Commun. 259:103-107. [DOI] [PubMed] [Google Scholar]
- 49.Zhadanov, A. B., D. W. Provance, Jr., C. A. Speer, J. D. Coffin, D. Goss, J. A. Blixt, C. M. Reichert, and J. A. Mercer. 1999. Absence of the tight junctional protein AF-6 disrupts epithelial cell-cell junctions and cell polarity during mouse development. Curr. Biol. 9:880-888. [DOI] [PubMed] [Google Scholar]
- 50.Zimmermann, S., and K. Moelling. 1999. Phosphorylation and regulation of Raf by Akt (protein kinase B). Science 286:1741-1744. [DOI] [PubMed] [Google Scholar]
- 51.Ziogas, A., I. C. Lorenz, K. Moelling, and G. Radziwill. 1998. Mitotic Raf-1 is stimulated independently of Ras and is active in the cytoplasm. J. Biol. Chem. 273:24108-24114. [DOI] [PubMed] [Google Scholar]
- 52.Zwartkruis, F. J., and J. L. Bos. 1999. Ras and Rap1: two highly related small, GTPases with distinct function. Exp. Cell Res. 253:157-165. [DOI] [PubMed] [Google Scholar]