Abstract
The antiapoptotic protein PED/PEA-15 features an Akt phosphorylation motif upstream from Ser116. In vitro, recombinant PED/PEA-15 was phosphorylated by Akt with a stoichiometry close to 1. Based on Western blotting with specific phospho-Ser116 PED/PEA-15 antibodies, Akt phosphorylation of PED/PEA-15 occurred mainly at Ser116. In addition, a mutant of PED/PEA-15 featuring the substitution of Ser116→Gly (PEDS116→G) showed 10-fold-decreased phosphorylation by Akt. In intact 293 cells, Akt also induced phosphorylation of PED/PEA-15 at Ser116. Based on pull-down and coprecipitation assays, PED/PEA-15 specifically bound Akt, independently of Akt activity. Serum activation of Akt as well as BAD phosphorylation by Akt showed no difference in 293 cells transfected with PED/PEA-15 and in untransfected cells (which express no endogenous PED/PEA-15). However, the antiapoptotic action of PED/PEA-15 was almost twofold reduced in PEDS116→G compared to that in PED/PEA-15WT cells. PED/PEA-15 stability closely paralleled Akt activation by serum in 293 cells. In these cells, the nonphosphorylatable PEDS116→G mutant exhibited a degradation rate threefold greater than that observed with wild-type PED/PEA-15. In the U373MG glioma cells, blocking Akt also reduced PED/PEA-15 levels and induced sensitivity to tumor necrosis factor-related apoptosis-inducing ligand apoptosis. Thus, phosphorylation by Akt regulates the antiapoptotic function of PED/PEA-15 at least in part by controlling the stability of PED/PEA-15. In part, Akt survival signaling may be mediated by PED/PEA-15.
PED/PEA-15 is a recently identified cytosolic protein featuring ubiquitous expression (8, 6). PED/PEA-15 has been shown to exert antiapoptotic action through distinct mechanisms. First, PED/PEA-15 inhibits formation of the death-inducing signaling complex (DISC) and caspase 3 activation by different apoptotic cytokines including FASL, tumor necrosis factor alpha, and tumor necrosis factor-related apoptosis-inducing ligand (TRAIL) (7, 16, 22). At least in part, this action is accomplished through the death-effector-domain of PED/PEA-15 upon PED/PEA-15 recruitment to the DISC (7, 16, 22). Secondly, PED/PEA-15 inhibits the induction of different stress-activated protein kinases (SAPKs) triggered by growth factor deprivation, hydrogen peroxide, and anisomycin (5). This action of PED/PEA-15 is exerted by the blocking of an upstream event in the SAPK activation cascade (5) and requires the interaction of PED with ERK1/2 (14, 17).
PED/PEA-15 is phosphorylated at Ser116 by calcium-calmodulin kinase II (CaMKII) (2) facilitating further phosphorylation by protein kinase C (PKC) at Ser104 (23). Thus, PED/PEA-15 is present in the cell in the unphosphorylated (N), singly phosphorylated (Pa), and doubly phosphorylated (Pb) forms. Previous studies have shown that only the Pb form of PED/PEA-15 can be recruited to the DISC and inhibit TRAIL apoptotic signaling (31). In addition, the antiapoptotic action of PED/PEA-15 requires PKC activity (7, 16, 22), indicating that, in the cell, PED/PEA-15 function is regulated by phosphorylation. However, whether kinases different from PKC and CaMKII may trigger survival or antiapoptotic signals by regulating PED/PEA-15 function is currently unknown.
Akt/PKB is a serine/threonine kinase that plays a major role in transducing proliferative and survival signals intracellularly (15, 21, 25). Akt/PKB has been demonstrated to phosphorylate a number of proteins involved in apoptotic signaling cascades, including the BCL2 family member BAD (12), the protease caspase 9 (4), the Forkhead transcription factor FRLH (3), and p21CipWAF1 (24). Phosphorylation of these proteins prevents apoptosis through several different mechanisms. For instance, unphosphorylated BAD induces cell death by forming heterodimers with BCL-XL and generating BAX homodimers (12). Upon activation of Akt/PKB, phosphorylated BAD promotes cell survival by binding the 14-3-3 protein, which prevents BAD association to BCL-XL (12). At variance, in the case of p21CipWAF1, phosphorylation by Akt/PKB results in increased stability, promoting cell survival (24).
The finding that PED/PEA-15 possesses a low-stringency Akt/PKB phosphorylation consensus led us to analyze the possibility that PED/PEA-15 may also represent a relevant Akt/PKB substrate and that PED/PEA-15 phosphorylation by this kinase may regulate the antiapoptotic function of PED/PEA-15. In the present work we demonstrate that Akt, in addition to CaMKII, phosphorylates PED at Ser116 in vitro and in vivo, regulating PED/PEA-15 function on cellular apoptosis. In part, Akt survival signaling may be mediated by PED/PEA-15.
MATERIALS AND METHODS
Materials.
Media, sera, and antibiotics for cell culture and the Lipofectamine reagent were purchased from Invitrogen Ltd. (Paisley, United Kingdom). Rabbit polyclonal Akt antibodies were from Santa Cruz Biotechnology (Santa Cruz, Calif.), and phosphokinase antibodies were from New England Biolabs Inc. (Beverly, Mass.). PED/PEA-15 antibodies have been previously reported (6). The HA-Akt, HA-AktmΔ4-129, and HA-AktK179M plasmids were donated by G. L. Condorelli (La Sapienza University of Rome) and have been previously reported (13), while recombinant Akt (rAkt) was purchased from Upstate Biotechnology Inc. (Lake Placid, N.Y.). Antisera against phospho-Serine116 PED/PEA-15 (pSer116 PED Ab) were prepared in rabbits by PRIMM (Milan, Italy) by using the PED/PEA-15 KLH-conjugated phosphopeptide NH2-CKDIIRQP(Sp)EEEIIKLAP-COOH. The specificity of this antiserum is shown in Fig. 2 in the present paper. The S70 (HIFEISRRPDLL), S104 (LTRIPSAKKYKD), and S116 (IIRQPSEEIIK) PED/PEA-15 dodecapeptides were also synthesized by PRIMM. Sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) reagents were purchased from Bio-Rad (Richmond, Va.). Western blotting and ECL reagents and radiochemicals were from Amersham (Arlington Heights, Ill.). All other reagents were from Sigma (St. Louis, Mo.).
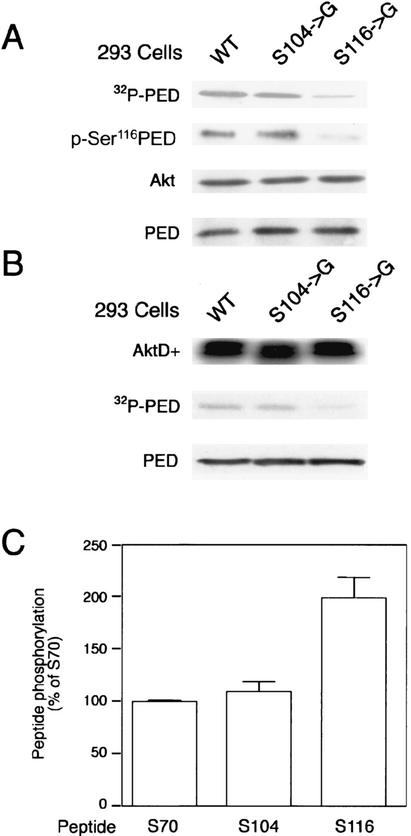
FIG. 2.
Identification of the in vitro Akt phosphorylation site of PED/PEA-15. (A) 293 cells expressing the wild-type (WT), the Ser104→Gly or the Ser116→Gly PED/PEA-15 cDNAs were solubilized and precipitated with PED/PEA-15 antibodies. PED/PEA-15 precipitates were phosphorylated with AktWT as outlined in the legend to Fig. 1, and samples were subjected to SDS-PAGE and autoradiography. For control, aliquots of the phosphorylation mixtures were also blotted with Akt, pSer116 PED/PEA-15, or PED/PEA-15 antibodies as indicated. (B) Constitutively active AktD+ (recombinant; 0.5 μg) was incubated with PED/PEA-15 antibody precipitates from 293PEDWT, PEDS104->G, and 293S116→G cells. Phosphorylation reactions were performed as outlined above, and samples were subjected to SDS-PAGE and autoradiography. To ensure equal amounts of Akt and PED/PEA-15 in each assay, aliquots of the samples were also blotted with Akt or PED/PEA-15 antibodies. The autoradiographs shown are representative of three (A) and four (B) independent experiments. (C) Synthetic dodecapeptides matching the sequence of PED/PEA-15 surrounding Ser70, Ser104, or Ser116 (PEP70, PEP104, or PEP116, respectively) were incubated with recombinant AktD+ (0.5 μg). Phosphorylation reactions were initiated by addition of 10 μCi of [γ-32P]ATP and prolonged for 30 min. Peptide phosphorylation was then quantitated as described in Materials and Methods. Each bar represents the mean ± standard deviation of five independent experiments. Based on t test analysis, the difference in phosphorylation of the PEP116 and those of the other peptides was significant at P values of <0.002.
Plasmid preparation, cell culture, and transfection and apoptosis assays.
The PEDS116→G and PEDS104→G mutant cDNAs were prepared by using a pcDNA3PED/PEA-15 cDNA and the site-directed mutagenesis kit by Stratagene (Heidelberg, Germany) according to the kit manufacturer's instructions. Sequences were confirmed by the Sanger method with the T7 sequencing kit (Pharmacia-LKB, Milan, Italy). Stable expression of the mutant cDNAs in 293 cells was achieved as reported in reference 5. The 293 cells expressing the wild-type PED/PEA-15 cDNA have been described in reference 16. The cells were cultured in Dulbecco's modified Eagle's medium (DMEM) supplemented with 10% fetal calf serum, 100 IU of penicillin/ml, 100 IU of streptomycin/ml, and 2% l-glutamine in a humidified CO2 incubator as reported in reference 5. Transient transfection of wild-type or mutant Akt cDNAs in these cells was accomplished by using the Lipofectamine method according to the manufacturer's instructions. Briefly, the cells were cultured in 60-mm-diameter dishes and incubated for 24 h in serum-free DMEM supplemented with 3 μg of cDNA and 15 μl of Lipofectamine reagent. An equal volume of DMEM supplemented with 20% fetal calf serum was then added for 5 h, followed by replacement with DMEM supplemented with 10% serum for 24 h before the assays. The U373MG human glioma cells were generously provided by C. Hao (University of Alberta, Edmonton, Canada). These cells were cultured and transfected as described in reference 16.
Apoptosis was quantitated by using the Apoptosis ELISA Plus kit (Roche Diagnostics GmbH, Mannheim, Germany) according to the manufacturer's instructions.
Western blot analysis, PED/PEA-15 phosphorylation, and determination of Akt activity.
For Western blotting, the cells were solubilized in lysis buffer (50 mM HEPES [pH 7.5], 150 mM NaCl, 4 mM EDTA, 10 mM Na4PO7, 2 mM Na3VO4, 100 mM NaF, 10% glycerol, 1% Triton X-100, 1 mM phenylmethylsulfonyl fluoride, 100 mg of aprotinin/ml, 1 mM leupeptin) for 60 min at 4°C. Cell lysates were clarified at 5,000 × g for 15 min. Solubilized proteins were then separated by SDS-PAGE and transferred onto 0.45-μm-pore-size Immobilon-P membranes (Millipore, Bedford, Mass.). Upon incubation with the primary and secondary antibodies, immunoreactive bands were detected by ECL according to the manufacturer's instructions.
For studying phosphorylation of PED/PEA-15 in intact cells, 293 cells were transiently transfected with the different Akt constructs as indicated. After 24 h, the cells were rinsed with 150 mM NaCl, incubated in phosphate- and serum-free culture medium for 16 h at 37°C, and then further incubated for 8 h in this same medium supplemented with 200 μCi of [32P]orthophosphate/ml. Insulin (final concentration, 100 nM) was then added, and the cells were rapidly rinsed with ice-cold saline followed by solubilization with 0.5 ml of lysis buffer per dish for 1 h at 4°C. Lysates were centrifuged at 5,000 × g for 20 min, and solubilized proteins were precipitated with PED/PEA-15 antibodies, separated by SDS-PAGE, and revealed by autoradiography. In these assays, insulin action on PED phosphorylation was dose dependent, with half-maximal effect at 3 nM and maximal effect at 100 nM.
Akt activity was assayed in vitro as previously reported (11). Briefly, 293 cells expressing either the wild-type or the mutant PED/PEA-15 cDNAs were solubilized in lysis buffer and lysates were clarified by centrifugation at 5,000 × g for 20 min. Two hundred micrograms of the lysates were immunoprecipitated with Myc antibodies. The precipitates (or 2 μg of recombinant PED) were incubated in a kinase reaction mixture containing 20 mM HEPES [pH 7.2], 10 mM MgCl2, 10 mM MnCl2, 1 mM dithiothreitol, 5 mM ATP, 0.2 mM EGTA, 1 mM protein kinase inhibitor, 10 μCi of [γ-32P]ATP, and 0.4 μg of rAkt or Akt or CaMKII immunoprecipitates, as indicated. Alternatively, rAkt was incubated in the kinase reaction mixture in the presence of 1 mM concentration of the S70, S104, or S116 PED/PEA-15 dodecapeptides. Phosphorylation reactions were prolonged for 10 min, stopped by cooling on ice, and spotted on phosphocellulose disk papers. Disks were washed with 1% H3PO4, and disk-bound radioactivity was quantified by liquid scintillation counting. Alternatively, phosphorylated proteins were separated by SDS-PAGE and analyzed by autoradiography. The stoichiometry of PED phosphorylation by Akt and CaMKII was determined by allowing the reactions to proceed for 1 h at 30°C in the presence of excess kinase to saturate the reaction mixture. The number of moles of phosphate transferred per mole of recombinant PED was calculated based on a [γ-32P]ATP standard curve.
PED/PEA-15-Akt interaction.
To investigate the interaction of PED/PEA-15 with Akt, PED/PEA-15-glutathione S-transferase (GST) fusion protein was generated. To this end, wild-type PED/PEA-15 cDNA was amplified by using the following two sets of primers: PED/PEA-15 5′EcoRI (5′-CCGGAATTCATGGTTGAGTACGGGACCCTC-3′) and PED/PEA-15 3′SalI (5′-GTCGACTCAGGCCTTCTTCGGTGGGGGGACC-3′). PED/PEA-15 cDNA was then cloned in the pGEX 4T1plasmid, and the sequence was confirmed by using the T7 sequencing kit. Lysates from Akt-transfected cells (500 μg) or 0.4 μg of recombinant Akt were incubated in the presence of 50 μl of Sepharose-bound GST-PED/PEA-15 (approximately 2 μg) for 2 h at 4°C. Beads were washed four times with TNT buffer (0.5% NP-40, 25 mM TRIS [pH 7.5], 30 mM MgCl2, 40 mM NaCl, 1 mM dithiothreitol) and then resuspended in Laemmli buffer followed by boiling for 4 min and centrifugation at 25,000 × g for 3 min. Supernatants were analyzed by SDS-PAGE and blotting with Akt antibodies.
RESULTS
In vitro phosphorylation of PED/PEA-15 by Akt.
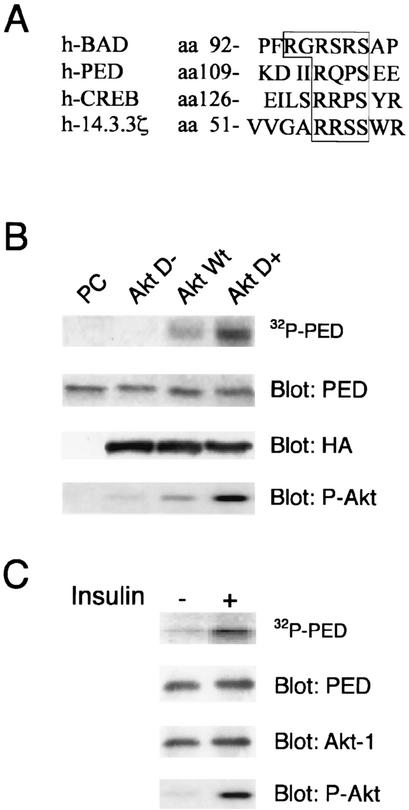
PED/PEA-15 sequence analysis revealed the presence of a low-stringency Akt phosphorylation motif including the Ser116 residue of PED/PEA-15 (Fig. 1A). To verify the hypothesis that PED/PEA-15 is an Akt substrate, we first expressed the hemagglutinin (HA)-tagged wild-type Akt (AktWT), the constitutively active HA-AktmΔ4-129 (AktD+), and the inactive HA-AktK179 M mutant cDNAs (AktD-) in 293 human embryonic kidney cells, because these cells express no detectable levels of endogenous PED/PEA-15. HA antibody-precipitated Akt from these cells was then tested for its ability to phosphorylate His-tagged recombinant PED/PEA-15 in vitro. SDS-PAGE analysis of the phosphorylation mixtures revealed that wild-type Akt induced PED/PEA-15 phosphorylation (Fig. 1B). The constitutively active Akt induced fourfold-greater phosphorylation of PED/PEA-15 than did wild-type Akt. In contrast, precipitated inactive Akt or HA precipitates from cells transfected with the empty vector (PC) did not. Blotting with PED/PEA-15 and HA antibodies revealed that these variations were not due to differences in the amounts of either PED/PEA-15 or Akt in the assays. In addition, blotting with specific antibodies toward the key Akt Serine activation site (serine473; P-Akt) showed that the different phosphorylation levels of PED/PEA-15 closely paralleled the Akt activation state in each assay. Endogenous Akt precipitated from 293 cells upon stimulation with 100 nM insulin also induced a fivefold increase in PED/PEA-15 phosphorylation over the level observed with unstimulated cells (Fig. 1C). Again, blotting with PED/PEA-15, Akt-1, and phospho-Akt antibodies indicated that the changes in PED/PEA-15 phosphorylation reflected differences in the activation state of Akt rather than different amounts of Akt or PED/PEA-15 in the assays. PED was indeed phosphorylated by Akt with a stoichiometry of 0.82 mol of phosphate/mol of recombinant PED, a value close to that observed in the case of CaMKII phosphorylation (0.87 mol of phosphate/mol of PED; the difference is not statistically significant).
FIG. 1.
In vitro phosphorylation of PED/PEA-15 by Akt (A) Partial amino acid sequence alignment of serine phosphorylation sites in Akt substrates. hBAD (29); hCREB (18, 30); h14.3.3.ζ (19, 27); hPED/PEA-15 (2). Sequences have been aligned to maximize the homology in the region upstream from the Ser116 of PED/PEA-15. The single-letter amino acid code is used. (B) The HA-tagged wild-type Akt (AktWT), constitutively active HA-AktmΔ4-129 (AktD+), inactive HA-AktK179 M (AktD-) mutant cDNAs, or the PcDNA3 empty vector (PC) was transfected in 293 cells. Cells maintained in the presence of 10% serum were solubilized, and cell lysates were precipitated with HA antibodies. Precipitated Akt was incubated with 2 μg of His-tagged recombinant PED/PEA-15 in the presence of 10 μCi of [γ-32P]ATP. The phosphorylation reaction was terminated with Laemmli buffer, and samples were subjected to SDS-PAGE and autoradiography. For control, the gels were also blotted with PED/PEA-15, HA, and Akt phosphoserine473 antibodies (P-Akt), as indicated. (C) 293 cells were stimulated with 100 nM insulin for 10 min and solubilized as described in Materials and Methods, and lysates were immunoprecipitated with Akt1 antibodies. Precipitated Akt was incubated with recombinant PED/PEA-15, and PED/PEA-15 phosphorylation was assayed as described above. For control, the gels were blotted with PED/PEA-15, Akt-1, and P-Akt antibodies. The autoradiographs shown are representative of three (B) and four (C) independent experiments.
Identification of PED/PEA-15 phosphorylation site by Akt.
To prove that the Ser116 of PED/PEA-15 is phosphorylated by Akt, we generated a cDNA mutant of PED/PEA-15 featuring the Ser116→Gly substitution. We then stably expressed the PED/PEA-15 Ser116→Gly cDNA and the wild-type PED/PEA-15 cDNA in 293 cells (293S116→G and 293PEDWT cells; three independent clones of cells expressing the mutant and four expressing the wild-type PED were selected and characterized in detail). For control, three independent clones of cells expressing a second mutant cDNA of PED/PEA-15, featuring the Ser104→ Gly substitution, were also selected and used (PEDS104→G cells; the Ser104→Gly mutation abolishes PED/PEA-15 phosphorylation by PKC [23]). Precipitates of these cells with PED/PEA-15 antibodies were phosphorylated by using HA precipitates from insulin-exposed cells expressing wild-type HA-Akt. In these assays, the Ser116→G mutant of PED/PEA-15 showed a fourfold-decreased phosphorylation by Akt compared to that of wild-type PED/PEA-15 (Fig. 2A). Phosphorylation of the Ser104→Gly mutant showed no significant difference from that of wild-type PED/PEA-15. In addition, blotting with antisera raised against a specific phosphoSerine116 PED/PEA-15 peptide showed a 15-fold-decreased phosphorylation of the Ser116→G compared to that of the Ser104→G PED mutant or the wild-type PED. Also, incubation of recombinant active Akt with same amounts of PED/PEA-15 precipitated from cells expressing either the wild-type or the S116→G mutant PED/PEA-15 cDNAs showed 10-fold-decreased phosphorylation of the PED/PEA-15 mutant compared to that of the wild-type (Fig. 2B). In this same assay, however, the active Akt phosphorylated the Ser104→Gly mutant of PED/PEA-15 at a level similar to that observed with wild-type PED/PEA-15. In vitro, the active Akt also phosphorylated twofold more effectively a synthetic dodecapeptide featuring the sequence of PED/PEA-15 surrounding Ser116 (S116 peptide) than a PED/PEA-15 dodecapeptide including Ser70 (S70 peptide; Fig. 2C). An additional PED/PEA-15 dodecapeptide including Ser104 (S104 peptide) was phosphorylated to a level similar to that of the S70 peptide. Thus, in vitro, Akt phosphorylates PED/PEA-15 on Ser116.
In vivo phosphorylation of PED by Akt.
We also examined the phosphorylation of PED/PEA-15 by Akt in intact 293PEDWT cells upon metabolic labeling with [32P]orthophosphate. Incubation of 293PEDWT cells with 100 nM insulin for 10 min stimulated PED/PEA-15 phosphorylation twofold above the basal level (Fig. 3A). Overexpression of similar levels of wild-type or active Akt cDNAs also increased phosphorylation of PED/PEA-15 1.8- and 3.2-fold, respectively. The same results were obtained with two further independent clones of 293PEDWT cells (not shown), indicating that, at least in part, insulin may induce PED/PEA-15 phosphorylation through Akt activation. Consistent with the role of Akt in insulin- and serum-stimulated phosphorylation of PED, preincubation of 293 cells with the phosphatidylinositol (PI) 3-kinase blockers Wortmannin or LY294002 inhibited insulin- as well as serum-stimulated phosphorylation of PED/PEA-15 by >70% (Fig. 3B). These decreases in phosphorylation of PED were accompanied by a decrease of similar size in phosphorylation of the key site of BAD by Akt.
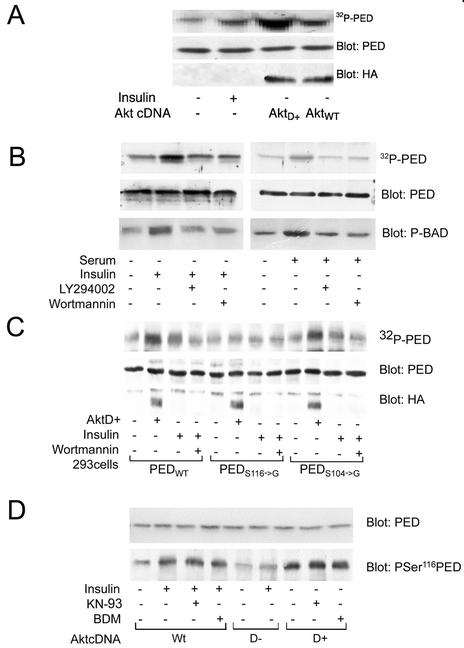
FIG. 3.
PED/PEA-15 phosphorylation by Akt in intact cells. (A) The HA-tagged wild-type and constitutively active Akt (AktWT and AktD+, respectively) were transiently transfected in 293PEDWT cells. The cells were then labeled with [32P]orthophosphate and, when indicated, exposed to 100 nM insulin for 10 min. Cells were lysed and precipitated with PED/PEA-15 antibodies, and precipitated proteins were analyzed by SDS-PAGE and autoradiography. For control, aliquots of the samples were also blotted with PED/PEA-15 or HA antibodies. (B) 293PEDWT cells were labeled with [32P]orthophosphate, preincubated with 50 nM Wortmannin or 110 μM LY294002 for 30 min, and stimulated with either 100 nM insulin (left panel) or 10% serum (right panel) for a further 10 min. The cells were solubilized and immunoprecipitated with PED/PEA-15 antibodies, and precipitates were subjected to SDS-PAGE and autoradiography. For control, the gels were subsequently blotted with either PED/PEA-15 or phosphoBAD antibodies. (C) 293PEDWT, 293S116→G, and 293S104→G cells were transiently transfected with the active AktD+ cDNA (HA tagged) and labeled with [32P]orthophosphate. The cells were then incubated with 50 nM Wortmannin and further stimulated with insulin as indicated. Cell lysates were precipitated with PED/PEA-15 antibodies and analyzed by SDS-PAGE and autoradiography. For control, aliquots of the cell lysates were also blotted with PED/PEA-15 or HA antibodies. (D) 293PEDWT cells were transfected with the wild-type (WT), the constitutively active (D+) or inactive (D−) Akt, incubated with 50 μM KN-93 or 100 nM BDM, and then stimulated with 100 μM insulin as indicated. Cell lysates were blotted with pSer116 PED/PEA-15 antibodies and, for control, reblotted with PED/PEA-15 antibodies. Filters were revealed by ECL and autoradiography. The autoradiographs shown are representative of three (A, B, and D) and four (C) independent experiments.
Since Akt phosphorylates PED/PEA-15 at Ser116 in vitro, we asked whether Ser116 is also phosphorylated in intact cells. To answer this question, we compared PED/PEA-15 phosphorylation in the 293PEDWT, the 293S116→G, and the 293S104→G cells upon expression of the constitutively active HA-Akt or insulin stimulation. As shown in Fig. 3C, PED/PEA-15 phosphorylation by active Akt was evident in the 293PEDWT and 293S104→G (3.2-fold increase) but not the 293S116→G cells. Insulin also increased PED/PEA-15 phosphorylation twofold and 40% in the 293PEDWT and the 293S104→G cells, respectively, but elicited only a slight 20% increase in the 293PEDS116→G cells. In addition, Wortmannin preincubation of the cells almost completely inhibited insulin-stimulated phosphorylations of wild-type PED/PEA-15 and of the Ser104→Gly mutant but showed no effect on that of the Ser116→Gly mutant, indicating that the latter phosphorylation is independent of Akt. Blotting with phospho-Ser116 PED antibodies revealed that wild-type PED underwent phosphorylation in cells transfected with wild-type Akt (upon insulin exposure) or with the active, but not the inactive, Akt (Fig. 3D). Importantly, insulin-induced phosphorylation of PED at Ser116 was unaffected by cell preincubation with KN-93 and bisindolylmaleimide (BDM), which inhibit CaMKII and PKC, respectively. It appears therefore that Ser116 represents the major Akt phoshorylation site of PED/PEA-15 in intact cells as well as in vitro.
Action of Akt on PED function in 293 cells.
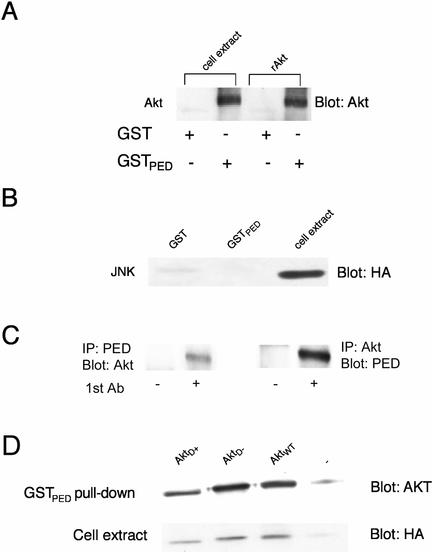
To further investigate the functional significance of Akt-PED/PEA-15 interaction, we first analyzed whether Akt directly binds PED/PEA-15. To this end, we incubated PED/PEA-15-GST fusion protein with either recombinant active Akt or lysates from cells expressing the constitutively active cDNA of Akt. Bound proteins were then pulled down and blotted with Akt antibodies. As shown in Fig. 4A, PED/PEA-15-GST bound both recombinant and transfected Akt. No Akt binding was evident upon incubation with GST alone. PED/PEA-15-GST did not bind to HA precipitates from cells expressing an HA-JNK-1 cDNA either, indicating specificity of the PED/PEA-15-Akt interaction (Fig. 4B). Based on overlay binding studies, PED/PEA-15 bound Akt and ERK1 with similar affinities (data not shown). Importantly, precipitation of 293PEDWT lysates with PED/PEA-15 antibodies followed by blotting with Akt antibodies, or vice versa, also revealed coprecipitation of PED/PEA-15 with Akt, indicating that Akt-PED/PEA-15 binding may occur in intact cells as well as in vitro (Fig. 4C). PED/PEA-15-GST incubation of extracts from cells expressing constitutively active, kinase-defective, and wild-type HA-Akt cDNAs followed by Akt blotting revealed that the mutant, the wild-type, and the endogenous Akt equally bound to PED/PEA-15 (Fig. 4D). Direct blotting of the cell extract with HA antibodies showed similar levels of transfected Akt in the cells. It appeared therefore that the formation of the PED/PEA-15-Akt complex is independent of Akt activity.
FIG. 4.
In vitro interaction of PED/PEA-15 and Akt. (A) Lysates from 293PEDWT cells expressing the AktD+ mutant (500 μg of cell protein) and recombinant AktD+ (rAkt; 0.4 μg) were incubated with agarose-bound GST-PED/PEA-15 or, for control, agarose-bound GST, as indicated. Pulled-down complexes were then analyzed by SDS-PAGE followed by Western blotting with Akt antibodies. Filters were revealed by ECL and autoradiography. (B) In parallel experiments, 293PEDWT cells were transiently transfected with HA-tagged JNK-1 cDNA. Cell lysates (500 μg of protein) were incubated with agarose-bound GST-PED/PEA-15 or GST, as indicated, pulled-down, and analyzed by blotting with HA antibodies and autoradiography. (C) 293PEDWT cells were solubilized, and cell lysates were immunoprecipitated with either PED/PEA-15 or preimmune antisera followed by blotting with Akt antibodies (left panel). Alternatively, the lysates were precipitated with Akt or nonimmune antibodies followed by blotting with PED/PEA-15 antibodies. Filters were revealed by ECL and autoradiography. (D) 293PEDWT cells were transiently transfected with the HA-tagged active AktD+, inactiveAktD−, or AktWT cDNAs as indicated. Cell lysates were analyzed by GST-PED/PEA-15 pull-down assays as outlined above. For control, aliquots of the cell extracts were also blotted with HA antibodies followed by ECL and autoradiography. The autoradiographs shown are representative of four (A) and three (B, C, and D) independent experiments.
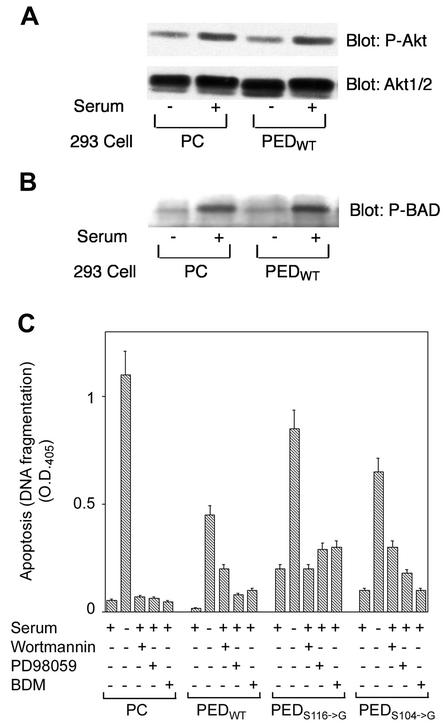
PED exerts a broad antiapoptotic action (5, 7, 16, 22). The finding that PED/PEA-15 binds and is phosphorylated by Akt led us to hypothesize that the antiapoptotic action of PED/PEA-15 may be contributed by activation of Akt signaling, or it may be regulated by phosphorylation by Akt, or both. To answer these questions, we compared the serum activation of Akt in the 293PEDWT cells with that in cells transfected with the empty plasmid (293PC). As shown in Fig. 5A, there was no difference between the phosphorylation levels of the key Akt serine activation sites in these two cell types. Also, BAD phosphorylation by Akt featured no difference in the 293PEDWT and the 293PC cells (Fig. 5B), indicating that Akt activity is not affected by cellular levels of PED/PEA-15. We therefore investigated whether phosphorylation by Akt regulates the antiapoptotic function of PED/PEA-15. To this end, we compared the apoptosis induced by growth factor deprivation in untransfected 293 cells with the apoptosis induced in cells expressing comparable levels of wild-type or mutant PED/PEA-15. Consistent with previous findings (6), the expression of wild-type PED/PEA-15 resulted in a >2-fold decrease in apoptosis following serum deprivation compared to that observed for the 293 cells transfected with the empty vector (293PC cells; Fig. 5C). But this protective effect was >60% less evident in cells expressing the nonphosphorylatable S116→G mutant of PED/PEA-15. In comparison, substitution of Ser104→Gly reduced PED/PEA-15 antiapoptotic function by only 35%. The levels of ERK activation by serum in cells expressing wild-type PED/PEA-15, in cells expressing mutant PED/PEA-15, and in untransfected 293 cells were not different (data not shown).
FIG. 5.
Effects of phosphorylation by Akt on the antiapoptotic action of PED/PEA-15. (A) 293PEDWT and 293PC control cells were deprived of serum overnight, followed by 10% serum stimulation as indicated. The cells were solubilized, and cell lysates were subjected to SDS-PAGE followed by immunoblotting with Akt phosphoserine473 antibodies (P-Akt). For control, aliquots of the lysates were also blotted with Akt1/2 antibodies. (B) Further aliquots of the cell lysates were blotted with phospho-BAD (P-BAD). Filters were revealed by ECL and autoradiography. The autoradiographs shown are representative of three (A) and four (B) independent experiments. (C) 293PC, 293PEDWT, 293S116→G, and 293S104→G cells were incubated in the presence or the absence of serum for 48 h. Cells were further incubated with 50 nM Wortmannin, 50 μM PD98059, or 100 nM BDM, as indicated. Apoptosis was quantitated by the ELISA Plus detection kit as described in Materials and Methods. Bars represent the means ± standard deviations from four duplicate experiments. Based on t test analysis, the difference in apoptosis following serum deprivation between the 293S116→G and 293PEDWT cells is significant at P values of <0.01. O.D.405, optical density at 405 nm.
293PEDWT cells featured very low levels of apoptosis when maintained in the presence of serum. However, exposure of these cells to Wortmannin and BDM increased apoptosis nine- and fivefold, respectively. According to previous observations in 293 cells (5), cell treatment with the MEK inhibitor PD98059 also induced an increase in apoptosis of almost fourfold. The effects of Wortmannin, PD98059, and BDM were more pronounced than in untransfected cells (P < 0.001 by t test analysis), indicating an important role of PED/PEA-15 in their elicitation. The 293S116→G and 293S104→G cells exhibited, respectively, nine- and fivefold-higher basal rates of apoptosis compared to the 293PEDWT cells (P < 0.001). Wortmannin had no further effect on apoptosis in the 293S116→G cells, whereas it increased the apoptosis in the 293S104→G cells threefold. In contrast, BDM slightly increased apoptosis in the 293S116→G cells (40%; P < 0.05) but had no effect at all in those expressing the Ser104→G mutant. PD98059 increased apoptosis by 40% and twofold, respectively, in the 293S116→G and the 293S104→G cells. Thus, Akt as well as PKC phosphorylations exerted an important role in regulating PED function.
Akt action on PED stability in 293 cells.
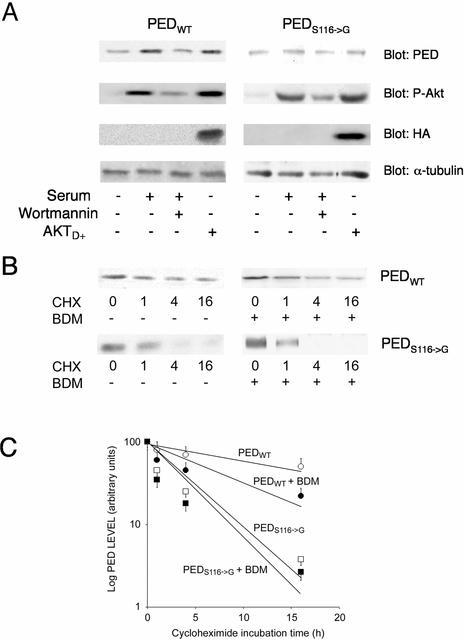
To elucidate the significance of phosphorylation by Akt for PED/PEA-15 function, we tested the possibility that Akt phosphorylation affects the half-life of PED/PEA-15 in the cells. We first compared the expression levels of wild-type PED/PEA-15 in cells maintained in the presence and in the absence of serum, when phosphorylation of the key Akt activation site is maximal and undetectable, respectively. As shown in Fig. 6A, PED/PEA-15 levels were fivefold higher in the cells exposed to serum than in the starved cells. Also, the effect of serum on the expression level of PED/PEA-15 was reduced threefold by Wortmannin, in parallel with the effect of serum on Akt phosphorylation. Very similar results were obtained in the presence of 100 nM insulin instead of serum (data not shown). More compelling, in the serum-starved cells, expression of the constitutively active Akt mutant resulted in PED/PEA-15 expression levels comparable to those measured in cells maintained in the presence of serum. In addition, both the effect of serum and that of the active Akt on PED/PEA-15 levels were absent in the 293S116→G cells, demonstrating the role of Akt phosphorylation in determining the intracellular levels of PED/PEA-15.
FIG. 6.
Akt action on PED/PEA-15 stability in 293 cells. (A) 293PEDWT, and 293S116→G cells were transiently transfected with the active HA-AktD+ mutant as indicated. The cells were maintained in the absence or the presence of serum for 16 h. During the last 3 h of incubation, Wortmannin was added at a final concentration of 50 nM, as indicated. The cells were then solubilized and sequentially blotted with PED/PEA-15, P-Akt, HA, and α-tubulin antibodies. Filters were revealed by ECL and autoradiography. The autoradiograph shown is representative of three independent experiments. (B) Alternatively, 293PEDWT and 293S116→G cells were deprived of serum for 14 h and then further incubated with 40 μg of cycloheximide/ml for the indicated times in the absence or the presence of 100 nM BDM. Cells were then solubilized, and 100 μg of cell proteins were subjected to SDS-PAGE followed by blotting with PED/PEA-15 antibodies, ECL, and autoradiography. (C) Autoradiographs were analyzed by laser densitometry. Each data point is the mean ± standard deviation of four independent experiments, one of which is shown in panel B.
Next, we directly examined whether Akt phosphorylation may increase the stability of PED/PEA-15. We incubated 293PEDWT and 293S116→G cells with the protein synthesis inhibitor cycloheximide. We then compared the degradation rates of PED/PEA-15 in the two cell types. As shown in Fig. 6B, in the 293PEDWT cells, almost 50% of the initial levels of PED/PEA-15 were still present after 6 h of incubation with cycloheximide. The disappearance of PED/PEA-15 was much faster in the 293S116→G cells, since in these cells 50% of PED/PEA-15 levels became undetectable within only 1 h. Again, the difference in PED stability in the 293PEDWT and the 293S116→G cells was confirmed in two other clones of each cell line (data not shown). Earlier reports showed that PKC phosphorylation at Ser104 depends on previous phosphorylation at Ser116 (23). Thus, phosphoserine104 might also contribute to PED stability. Accordingly, wild-type PED stability also decreased when PKC activity was blocked by BDM, although less evidently than with the Ser116→Gly mutant. Hence, upon treatment with BDM, 50% of the initial levels of wild-type PED were present after 3 h of incubation with cycloheximide. Consistent with the permissive role of Ser116 phosphorylation on that of Ser104 (23), treatment with BDM caused only a slight further increase in the degradation rate of PEDS116→G.
Action of Akt on PED stability and function in human glioma cells.
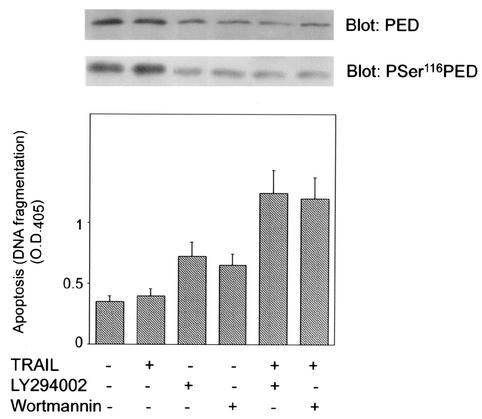
To further address the significance of PED phosphorylation by Akt, we have blocked Akt activity in the U373MG human glioma cell line. These cells feature high levels of PED determining resistance to the apoptotic cytokine TRAIL (16, 31). Similar to what was observed with the 293 cells, in the U373MG glioma cells preincubation with either Wortmannin or LY294002 caused an 80% increase in apoptosis (P < 0.001; Fig. 7). This induction of apoptosis was accompanied by a >3-fold decrease in PED phosphorylation at Ser116 and in the cellular levels of PED (Fig. 7, top). Interestingly, both treatment with Wortmannin and treatment with LY294002 rescued sensitivity to TRAIL apoptosis. Wortmannin and LY294002 also reduced Akt activity as well as PED recruitment to TRAIL-induced DISC by >80% (data not shown), supporting the major role of this kinase in controlling the cellular function of PED in the glioma as well as in other cells.
FIG. 7.
Akt action on PED function in U373MG cells. U373MG cells were preincubated with 100 μM LY294002 or 50 nM Wortmannin for 3 h and then exposed to 300 ng of TRAIL/ml, as indicated. Apoptosis was quantitated by evaluating the level of DNA fragmentation by using the ELISA Plus detection kit as described in Materials and Methods. Some of the cells were also solubilized and Western blotted with either PED/PEA-15 or pSer116 PED/PEA-15 antibodies (top). Bars represent the means ± standard deviations of duplicate determinations from three independent experiments.
DISCUSSION
PED/PEA-15 is a recently identified protein featuring a broad antiapoptotic function (5, 7, 16, 22). PED/PEA-15 inhibits the apoptotic signal of FasL, tumor necrosis factor alpha, and TRAIL (7, 16, 22) and also blocks apoptosis following the activation of several SAPKs (6). Previous work in our own as well as in other laboratories has shown that phosphorylation by PKC plays an important role in enabling the antiapoptotic function of PED/PEA-15 (7, 16, 22). In the present paper we demonstrate that PED/PEA-15 phosphorylation by Akt also regulates PED/PEA-15 control of cell apoptosis.
We have shown that Akt phosphorylates PED/PEA-15 in vitro as well as in intact cells. PED/PEA-15 sequence analysis revealed a low-stringency Akt phosphorylation site, Ser116. Hence, in vitro, Akt phosphorylates the wild-type PED/PEA-15 but not a mutant PED/PEA-15 featuring the Ser116→Gly substitution. Akt also phosphorylates twofold more effectively a synthetic peptide featuring the sequence of PED/PEA-15 surrounding Ser116 than two other equally sized PED/PEA-15 peptides including either Ser104 or Ser70. In addition, active Akt does not phosphorylate the PEDS116→G mutant when expressed in 293 cells, whereas it does phosphorylate wild-type PED/PEA-15. Finally, Western blotting studies with a specific phospho-Ser116 PED/PEA-15 antiserum identified Ser116 as the major Akt phosphorylation site of PED/PEA-15 in vivo and in vitro. Akt substrate serines are usually embedded in RXRXXS consensus sequences (1). In contrast, the 5-amino-acid region of PED/PEA-15 upstream from Ser116 exhibits only a single Arg residue. The same structural feature has been reported for the Akt Ser substrates of CREB (10) and of the 14-3-3ζ scaffold protein (27). In the case of PED/PEA-15, we showed that PED/PEA-15 directly binds to Akt. The binding is independent of Akt activity, as it effectively occurs to both constitutively active and inactive Akt mutants as well as to wild-type Akt. In addition, PED/PEA-15 binds Akt with an affinity similar to that exhibited for ERK1, a known PED/PEA-15 ligand (14, 17) (data not shown). It is possible, therefore, that in the cell PED/PEA-15 recruits Akt and that this facilitates PED/PEA-15 phosphorylation by the kinase.
When expressed in 293 cells, the S116→G mutant PED/PEA-15 showed an almost twofold decrease in antiapoptotic function compared to wild-type PED/PEA-15. In 293 cells, PED inhibition of apoptosis requires PED activation of ERKs (5). However, reduced antiapoptotic function of the Ser116→G mutant did not appear to depend on changes in its interaction with ERKs, as ERK activation levels were not different in cells expressing the mutant and in wild-type cells (data not shown). Interestingly, a pharmacological blocking of wild-type PED/PEA-15 phosphorylation at Ser116 was accompanied by a significant increase in 293 cell apoptosis, even in the presence of serum. These findings suggest that, at least in the 293 cells, PED may switch from an unphosphorylated proapoptotic form to a phosphorylated antiapoptotic form. Hence, expression of the Ser116→Gly mutant induces apoptosis, likely contributing to the reduced protection from apoptosis observed in 293PEDS116→G compared to the 293PEDWT cells. Kubes et al. reported that endothelin-activated CaMKII also phosphorylates PED/PEA-15 at Ser116 (23). Consistent with our findings, endothelin exerts antiapoptotic action in different cell types (9). It is unlikely that CaMKII serves as a downstream mediator of Akt action on PED/PEA-15 phosphorylation in the intact cell, as blocking of CaMKII does not inhibit Akt phosphorylation of PED/PEA-15 at Ser116. More likely, therefore, PED/PEA-15 represents a common target for multiple kinases transducing survival signals triggered by growth factors and cytokines, supporting an important role of PED/PEA-15 in the cellular regulation of apoptotic programs.
Earlier works indicated that PED/PEA-15 phosphorylation at Ser116 facilitates subsequent phosphorylation by PKC at Ser104. In the present report, we show that the single substitution Ser104→G of PED/PEA-15 also reduced the antiapoptotic activity of PED/PEA-15, but only by 30%. These findings indicate that impaired phosphorylation at Ser104 may account, in part, for reducing PED/PEA-15 antiapoptotic action when phosphorylation of Ser116 is disabled. Consistent with this possibility, blocking of PKC only slightly increased apoptosis in 293S116→G cells.
Despite the relevance of phosphorylation at Ser116, other factors seemed to be involved in regulating PED/PEA-15 antiapoptotic function in 293 cells. Hence, apoptosis induced by serum starvation was less in cells expressing the S116→G mutant than in cells expressing no PED/PEA-15 at all. Akt regulation of PED function is not unique to the 293 cells, however. In fact, we report that blocking of Akt in human glioma cells expressing high levels of PED/PEA-15 simultaneously decreases PED/PEA-15 phosphorylation at Ser116 and rescues sensitivity to the apoptotic cytokine TRAIL.
Akt regulates a number of cellular functions including cell survival (25). Different substrates are phosphorylated by Akt and converted into survival proteins (3, 4, 12, 24). This function, in turn, is accomplished by regulating protein-protein interaction (12), by affecting protein localization (3) or protein stability (24). In the present work, we show that Akt phosphorylation increases the stability of PED/PEA-15 in the cell. Hence, PED/PEA-15 levels are low in cells subjected to serum starvation, when Akt activity is inhibited, and in cells subjected to pharmacological blocking of the PI 3-K/Akt pathway. PED/PEA-15 cellular levels increase upon expression of a constitutively active Akt mutant. In addition, we report that the S116→G PED/PEA-15 mutant features reduced stability compared to that of wild-type PED/PEA-15. At variance with wild-type PED/PEA-15, the S116→G mutant is unaffected by Akt. Thus, at least in part, Akt regulates PED protein stability and antiapoptotic function by phosphorylating PED/PEA-15 at Ser116. PED/PEA-15 degradation is blocked by treatment of the cells with the proteasome inhibitor lactacystin, suggesting that PED/PEA-15 intracellular levels are regulated by the ubiquitin pathway (data not shown). Whether this hypothesis holds and whether PED/PEA-15 ubiquitination is, in turn, inhibited by Akt phosphorylation is currently being investigated in our laboratory.
The PED/PEA-15 gene is amplified in human breast cancer (20) as well as in other tumors and PED/PEA-15 overexpression may have a role in skin carcinogenesis (S. Santopietro and J. Portella, personal communication). Akt is also upregulated in a number of human cancers (26, 28, 29). In this work, we demonstrated that PED/PEA-15 is a substrate for Akt and obtained evidence that Akt phosphorylation and stabilization of PED/PEA-15 is a previously unrecognized mechanism involved in Akt survival signaling. Simultaneous increases in PED/PEA-15 cellular levels and Akt activity might function cooperatively in tumorigenesis and/or tumor progression in humans.
Acknowledgments
This work was supported in part by the European Community (grant QLRT-1999-00674 to F.B. [EUDG program]), grants from the Associazione Italiana per la Ricerca sul Cancro (AIRC) to F.B. and P.F., and the Ministero dell'Università e della Ricerca Scientifica. The financial support of Telethon—Italy is gratefully acknowledged.
Alessandra Trencia and Anna Perfetti contributed equally to this work.
REFERENCES
- 1.Alessi, D. R., F. B. Caudwell, M. Andjelkovic, B. A. Hemmings, and P. Cohen. 1996. Molecular basis for the substrate specificity of protein kinase B; comparison with MAPKAP kinase-1 and p70 S6 kinase. FEBS Lett. 399:333-338. [DOI] [PubMed] [Google Scholar]
- 2.Araujo, H., N. Danzinger, J. Cordier, J. Glowinski, and H. Chneiweiss. 1993. Characterization of PEA-15, a major substrate for protein kinase C in astrocytes. J. Biol. Chem. 268:5911-5920. [PubMed] [Google Scholar]
- 3.Brunet, A., A. Bonni, M. J. Zigmond, M. Z. Lin, P. Juo, L. S. Hu, M. J. Anderson, K. C. Arden, J. Blenis, and M. E. Greenberg. 1999. Akt promotes cell survival by phosphorylating and inhibiting a Forkhead transcription factor. Cell 96:857-868. [DOI] [PubMed] [Google Scholar]
- 4.Cardone, M. H., N. Roy, H. R. Stennicke, G. S. Salvesen, T. F. Franke, E. Stanbridge, S. Fricsh, and J. C. Reed. 1998. Regulation of cell death protease caspase-9 by phosphorylation. Science 282:1318-1321. [DOI] [PubMed] [Google Scholar]
- 5.Condorelli, G., A. Trencia, G. Vigliotta, A. Perfetti, U. Goglia, E. Autori, A. Cassese, A. Musti, C. Miele, S. Santopietro, P. Formisano, and F. Beguinot. 2002. Multiple members of the mitogen-activated protein kinase family are necessary for PED/PEA-15 anti-apoptotic function. J. Biol. Chem. 277:11013-11018. [DOI] [PubMed] [Google Scholar]
- 6.Condorelli, G., G. Vigliotta, C. Iavarone, M. Caruso, C. G. Tocchetti, F. Andreozzi, M. F. Tecce, A. Cafieri, P. Formisano, L. Beguinot, and F. Beguinot. 1998. PED/PEA-15 gene controls glucose transport and is overexpressed in type 2 diabetes mellitus. EMBO J. 17:3858-3865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Condorelli, G., G. Vigliotta, C. Iavarone, A. Trencia, P. Andalò, F. Oriente, C. Miele, M. Caruso, P. Formisano, and F. Beguinot. 1999. PED/PEA-15: an anti-apoptotic molecule that regulates FAS/TNFR1-induced apoptosis. Oncogene 18:4409-4415. [DOI] [PubMed] [Google Scholar]
- 8.Danzinger, N., M. Yokohama, T. Jay, J. Cordier, J. Glowinski, and H. Chneiweiss. 1995. Cellular expression, developmental regulation, and phylogenic conservation of PEA-15, the astrocytic major phosphoprotein and protein kinase C substrate. J. Neurochem. 64:1016-1025. [DOI] [PubMed] [Google Scholar]
- 9.Del Bufalo, D., V. Di Castro, A. Biroccio, M. Varmi, D. Salani, L. Rosano, D. Trisciuoglio, F. Spinella, and A. Bagnato. 2002. Endothelin-1 protects ovarian carcinoma cells against paclitaxel-induced apoptosis: requirement for Akt activation. Mol. Pharmacol. 61:524-532. [DOI] [PubMed] [Google Scholar]
- 10.Du, K., and M. Montminy. 1998. CREB is a regulatory target for the protein kinase Akt/PKB. J. Biol. Chem. 273:32377-32379. [DOI] [PubMed] [Google Scholar]
- 11.Dudek, H., S. R. Datta, T. F. Franke, M. Brinbaum, R. Yao, G. M. Cooper, R. A. Segal, D. R. Kaplan, and M. E. Greenberg. 1997. Regulation of neuronal survival by the serine-threonine protein kinase Akt. Science 275:661-665. [DOI] [PubMed] [Google Scholar]
- 12.Dudek, H., S. R. Datta, X. Tao, S. Masters, H. Fu, Y. Gotoh, and M. E. Greenberg. 1997. Akt phosphorylation of BAD couples survival signals to the cell-intrinsic death machinery. Cell 91:231-241. [DOI] [PubMed] [Google Scholar]
- 13.Eves, E. M., W. Xiong, A. Bellacosa, S. G. Kennedy, P. N. Tsichlis, M. R. Rosner, and N. Hay. 1998. Akt, a target of phosphatidylinositol 3-kinase, inhibits apoptosis in a differentiating neuronal cell line. Mol. Cell. Biol. 18:2143-2152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Formstecher, E., J. W. Ramos, M. Fauquet, D. A. Calderwood, J. C. Hsieh, B. Canton, X. T. Nguyen, J. V. Barnier, J. Camonis, M. H. Ginsberg, and H. Chneiweiss. 2001. PEA-15 mediates cytoplasmic sequestration of ERK MAP kinase. Dev. Cell 1:239-250. [DOI] [PubMed] [Google Scholar]
- 15.Franke, T. F., D. R. Kaplan, L. C. Cantly, and A. Toker. 1997. Direct regulation of the Akt proto-oncogene product by phosphatidylinositol-3,4-bisphosphate. Science 275:665-668. [DOI] [PubMed] [Google Scholar]
- 16.Hao, C., F. Beguinot, G. Condorelli, A. Trencia, H. T. Chen, E. G. Van Meir, V. W. Yong, I. F. Parney, W. H. Roa, and K. C. Petruk. 2001. Induction and intracellular regulation of tumor necrosis factor-related apoptosis-inducing ligand (TRAIL) mediated apoptosis in human malignant glioma cells. Cancer Res. 61:1-9. [PubMed] [Google Scholar]
- 17.Hill, J. M., H. Vaidyanathan, J. W. Ramos, M. H. Ginsberg, and M. H. Werner. 2002. Recognition of ERK MAP kinase by PEA-15 reveals a common docking site within the death domain and death effector domain. EMBO J. 21:6494-6504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hoeffler, J. P., T. E. Meyer, Y. Yun, J. L. Jameson, and J. F. Habener. 1988. Cyclic AMP-responsive DNA-binding protein: structure based on a cloned placental cDNA. Science 242:1430-1433. [DOI] [PubMed] [Google Scholar]
- 19.Honda, R., Y. Ohba, and H. Yasuda. 1997. 14-3-3 zeta protein binds to the carboxyl half of mouse wee1 kinase. Biochem. Biophys. Res. Commun. 230:262-265. [DOI] [PubMed] [Google Scholar]
- 20.Hwang, S., W. Kuo, J. F. Cochron, R. C. Gurman, T. Tsukamoto, G. Bandyopadhyay, K. Mambo, and C. C. Collins. 1997. Assignment of HMAT1, the human homolog of the murine mammary transforming gene (MAT1) associated with tumorigenesis, to 1q21.1, a region frequently gained in human breast cancers. Genomics 42:540-542. [DOI] [PubMed] [Google Scholar]
- 21.Khwaja, A., S. Rodriguez-Viciana Wennstrom, P. H. Warne, and J. Downward. 1997. Matrix adhesion and Ras transformation both activate a phosphoinositide 3-OH kinase and protein kinase B/Akt cellular survival pathway. EMBO J. 16:2783-2793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kitsberg, D., E. Formstecher, M. Fauquet, M. Kubes, J. Cordier, B. Canton, G. Pan, M. Rolli, J. Glowinski, and H. Chneiweiss. 1999. Knock-out of the neural death effector domain protein PEA-15 demonstrates that its expression protects astrocytes from TNFalpha-induced apoptosis. J. Neurosci. 19:8244-8251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kubes, M., J. Cordier, J. Glowinski, J. A. Girault, and H. Chneiweiss. 1998. Endothelin induces a calcium-dependent phosphorylation of PEA-15 in intact astrocytes: identification of Ser104 and Ser116 phosphorylated, respectively, by protein kinase C and calcium/calmodulin kinase II in vitro. J. Neurochem. 71:1303-1314. [DOI] [PubMed] [Google Scholar]
- 24.Li, Y., D. Dowbenko, and L. A. Lasky. 2002. AKT/PKB phosphorylation of p21Cip/WAF1 enhances protein stability of p21Cip/WAF1 and promotes cell survival. J. Biol. Chem. 277:11352-11361. [DOI] [PubMed] [Google Scholar]
- 25.Marte, B. M., and J. Downward. 1997. PKB/Akt: connecting phosphoinositide 3-kinase to cell survival and beyond. Trends Biochem. Sci. 22:355-358. [DOI] [PubMed] [Google Scholar]
- 26.Nicholson, K. M., and N. G. Anderson. 2002. The protein kinase B/Akt signalling pathway in human malignancy. Cell Signal. 14:381-395. [DOI] [PubMed] [Google Scholar]
- 27.Powell, D. W., M. J. Rane, Q. Chen, S. Singh, and K. R. McLeish. 2002. Identification of 14-3-3zeta as a protein kinase B/Akt substrate. J. Biol. Chem. 277:21639-21642. [DOI] [PubMed] [Google Scholar]
- 28.Testa, J. R., and A. Bellacosa. 2001. AKT plays a central role in tumorigenesis. Proc. Natl. Acad. Sci. USA 98:10983-10985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Vivano, I., and C. L. Sawyers. 2002. The phosphatidylinositol 3-kinase AKT pathway in human cancer. Nat. Rev. Cancer 2:489-502. [DOI] [PubMed] [Google Scholar]
- 30.Wang, H. G., U. R. Rapp, and J. C. Reed. 1996. Bcl-2 targets the protein kinase Raf-1 to mitochondria. Cell 87:629-638. [DOI] [PubMed] [Google Scholar]
- 31.Xiao, C., B. F. Yang, N. Asadi, F. Beguinot, and C. Hao. 2002. Tumor necrosis factor-related apoptosis-inducing ligand-induced death-inducing signaling complex and its modulation by c-FLIP and PED/PEA-15 in glioma cells. J. Biol. Chem. 277:25020-25025. [DOI] [PubMed] [Google Scholar]