Abstract
Animal models suggest that Bax and Bak play an essential role in the implementation of apoptosis and as a result can hinder tumorigenesis. We analyzed the expression of these proteins in 50 human glioblastoma multiforme (GBM) tumors. We found that all the tumors expressed Bak, while three did not express Bax. In vitro, Bax-deficient GBM (BdGBM) exhibited an important resistance to various apoptogenic stimuli (e.g., UV, staurosporine, and doxorubicin) compared to the Bax-expressing GBM (BeGBM). Using an antisense strategy, we generated Bak− BeGBM and Bak− BdGBM, which enabled us to show that the remaining sensitivity of the BdGBM to apoptosis was due to the overexpression of Bak. Bax/Bak single or double deficiency had no influence on either the clonogenicity or the growth of tumors in Swiss nude mice. Of note, Bak− BeGBM cells were resistant to apoptosis induced by caspase 8 (C8) but not to that induced by granzyme B (GrB). Cells lacking both Bax and Bak (i.e., Bak− BdGBM) were completely resistant to all stimuli including the microinjection of C8 and GrB. We show that GrB-cleaved Bid and C8-cleaved Bid differ in size and utilize preferentially Bax and Bak, respectively, to promote cytochrome c release from mitochondria. Our results suggest that Bax deficiency is compensated by an increase of the expression of Bak in GBM and show, for the first time in human cancer, that the double Bax and Bak deficiency severely impairs the apoptotic program.
Evasion of apoptosis is one of the cardinal points acquired by a cancerous cell to facilitate tumorigenesis (15, 17, 21, 24). The implementation of the apoptotic program is under the control of a family of multidomain proteins with Bcl-2 as its founder (1, 26). The Bcl-2 family can be divided in two classes with antagonistic properties: antiapoptotic (Bcl-2, Bcl-XL, and Mcl-1, etc.) and proapoptotic (Bax, Bak, and Bok, etc.). A third family of proapoptotic proteins present a homology with Bcl-2 limited to its BH3 domain (1, 4). Proteins of this “BH3-only” group mediate apoptosis in response to specific stimuli (1, 4). Knockout studies in mice have shown that the presence of at least one of the two proapoptotic multidomain proteins, Bax and Bak, is necessary for the execution of the apoptotic program (7, 44, 48). In the absence of these proteins, the apoptotic program is severely impaired downstream of BH3 protein activation (7, 44, 48). Several works have emphasized the role of Bax in both cancer progression and/or resistance to chemo- or radiotherapy-induced apoptosis in human and in animal models (11, 12, 20, 27, 28, 31, 33, 34, 38, 45, 46, 47). The loss of the expression of Bax in a subset of human colon cancer with the microsatellite mutator phenotype (MMP) confers a selective advantage to cancer cell growth in vivo and in vitro (33, 20, 47). Conversely, it has recently been shown that the expression of an apoptogenic variant of Bax (Bax Ψ) was associated with a longer survival of glioblastoma multiforme (GBM) patients (6), one of the most dreadful types of tumors (18). Bax Ψ was found to be expressed in 25% of the patients examined while very few tumors lacked the expression of Bax (6). We have thus examined the sensitivity to apoptosis of the latter tumors with a particular emphasis on immune-induced apoptosis as the elimination of malignant cells by the immune system appears to be critical for the prevention of tumor development and/or expansion (35). The immune system induced apoptosis in these cells by two main mechanisms: the death receptor and cytotoxic pathways (19, 35). Both pathways induce apoptosis mainly through the proteolytic activation of the BH3-only proapoptotic protein Bid (29, 30) directly in the case of the cytotoxic pathway through granzyme B (GrB) and indirectly through caspase 8 (C8) in the death receptor pathway (19, 35).
In vitro, Bax-deficient GBM (BdGBM) derived from resected tumors were more resistant to chemo- or UV-induced apoptosis than control tumors (Bax-expressing GBM [BeGBM]). Using an antisense strategy, we show that Bak accounted for the remaining sensitivity to apoptosis and that double deficient cells Bax−/Bak− GBM were completely resistant to all death stimuli. We also report that Bax− or Bak− cells are equally sensitive to GrB but that the absence of Bak impaired the response of GBM cells to C8.
In this work, we show that in GBM, GrB and C8 induced different proteolytic truncations of Bid (p13-tBid and p15-tBid, respectively) which exhibited different affinities towards Bax and Bak. We thus postulate that these specific cross talks between Bax or Bak and the truncated forms of Bid generated by the cytotoxic pathway (GrB) or the death receptor pathway (C8) could allow the maintenance of immune-induced cell death in tumors.
MATERIALS AND METHODS
Reagents.
Unless otherwise stated, biochemical reagents used in this study were obtained from Sigma (St. Quentin Fallavier, France). Cell culture reagents were obtained from Invitrogen (Cergy Pontoise, France).
Patients.
GBM tumors were obtained from patients operated on at the Clinique de Neurochirurgie at the Hospital of Nantes over the years 1997 to 2000 and were classified according to the World Health Organization classification as astrocytoma grade 4 (23). Patient material as well as records (diagnosis, age, sex, and date of death) were used with confidentiality according to French laws and the recommendations of the French National Committee of Ethics.
Cell culture and proliferation.
Immediately after resection, the tumors were washed and stored in Ringer's solution at 4°C. Within 2 to 4 h, the tumors were washed with cold phosphate-buffered saline (PBS) prior to mechanical dissociation using a Medimachine (Dako, Trappes, France) and 50 μM Medicon (Dako) according to the manufacturer's instructions. Cells were plated at a density of 5,000 cells/ml and cultured in RPMI 1640 supplemented with 10% fetal calf serum (FCS), penicillin (100 U/ml), streptomycin (100 μg/ml), and 2 mM glutamine for 3 days and then transferred in RPMI 1640-10% FCS supplemented with 1% G5, penicillin (100 U/ml), streptomycin (100 μg/ml) and 2 mM glutamine. Proliferation was estimated using a methylthiazoletetrazolium assay (Promega) according to the manufacturer's instruction. The cells were passaged 10 times before being used in experiments in order to avoid contamination with nontumoral cells. To inhibit Bak expression in GBM, cells were transfected with two consecutive electroporations with 10 μg of plasmid encoding the anti-full-length sequence of human Bak as described by Eguchi et al. (10). The bulk of cells were then selected for 20 days with G418 (250 μg/ml) and used as such to avoid clonal bias.
Immunoblots, RNA purification and RT-PCR.
Tumors and primary cultures were homogenized vol/vol in RIPA buffer (PBS containing 1% NP-40, 0.5% Na-deoxycholate, 0.1% sodium dodecyl sulfate [SDS], 1 nM Na-vanadate, and complete inhibitor cocktail) and analyzed for protein expression by standard immunoblotting methods. RNA purification and reverse transcription (RT)-PCR were also performed as previously described (6). For RT-PCR analyses, 40 ng of cDNA was amplified in a final volume of 50 μl using 1 U of Taq polymerase (Invitrogen). Thirty amplification cycles consisting of 95°C for 30 s, 55°C for 30 s, and 72°C for 30 s were performed. The number of cycles was within the linear phase of amplification and amplified products were analyzed in a 1.5% agarose-Tris-borate-EDTA gel. The bands were quantified using the IPLab Gel Program. The DNA methylation pattern of the CpG islands surrounding the TATA box of the Bax gene was investigated by methylation-specific PCR according to the protocol described by Herman et al. (16). Control, methylated (normal control) and ummethylated (positive control) DNAs were obtained from Intergen (Oxford, United Kingdom). In vitro demethylation treatment of BeGBM and BdGBM cells was performed by the addition of 15 mM 5-aza-2′-deoxycytidine (5aza2dC) for 18 days.
Clonogenicity and growth in Swiss nude mice.
For the clonogenicity assay, 400 BdgBM, BeGBM, or Bak antisense-transfected BdGBM and BeGBM cells were plated into six-well plates (3 ml per well) in semisolid medium containing 0.3% agar and allowed to adhere overnight. After 21 days, colonies were fixed and stained with 0.5% crystal violet. Only colonies with diameters of ≥2 mm were counted.
For in vivo growth experiments, six Swiss nude mice received subcutaneous injections in the flank with 106 BdGBM, BeGBM, or Bak antisense-transfected BdGBM and BeGBM cells. Tumoral progression was monitored by measuring, every 4 days over a period of 30 days, the size of the tumors formed.
Induction of apoptosis in vitro and confocal analyses.
Apoptosis was induced in semiconfluent cells (50 to 70%) BdGBM or BeGBM cells by the addition of staurosporine (STS), doxorubicin (DOXO), or UVB treatment as indicated. Cell death was evaluated 24 h later, as it corresponded in all cases to the maximum loss of cell viability. Apoptosis was quantified by measuring the release of lactate dehydrogenase (LDH) in the culture medium as previously described (5, 6). For immunohistochemical detection of Bax, Bak, and GFAP, the cells were cultured for 7 days before analysis by laser confocal microscopy as previously described (6).
Microinjection.
Microinjections were performed as described by Juin et al. (22). Briefly, cells were seeded on glass coverslips the day prior to microinjection using sterile microcapillaries (femtotips II; Eppendorf) mounted on an automated microinjection system (Eppendorf). Recombinant proteins were dissolved in PBS together with dextran (70 kDa)-conjugated lysine-fixable Oregon green (0.5% final concentration; Molecular Probes) as a coinjection marker. Typically, 100 to 150 cells were injected using identical pressures (150 hPa) and times (0.1 s).
Cell-free assay and preparation of p13- and p15-tBid.
Mitochondrial isolation and incubation with purified or in vitro-translated Bid, Bax, or Bak as well as protein binding and cytochrome c (CYT-C) release assays were performed as described previously (5).
The coding region of Bid was subcloned into pTrcHis2-TOPO plasmid (Invitrogen). The His-tagged proteins were expressed in bacteria and purified according to the manufacturer's instructions. In vitro, 100 μg of full-length Bid (fl-Bid)-His was incubated with 300 U of IETDase activity GrB (Alexis Biochemicals) or C8 (Calbiochem) at 37°C for 3 h to generate the p13- and p15-tBid fragments. The digestion product was purified on Ni-Sepharose prior to microinjection and SDS-polyacrylamide gel electrophoresis (SDS-PAGE) analysis.
RESULTS
Bax deficiency in primary cultures of GBM.
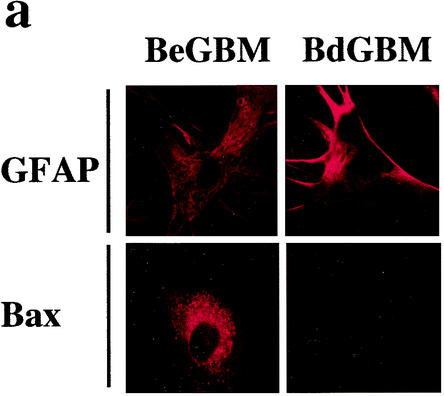
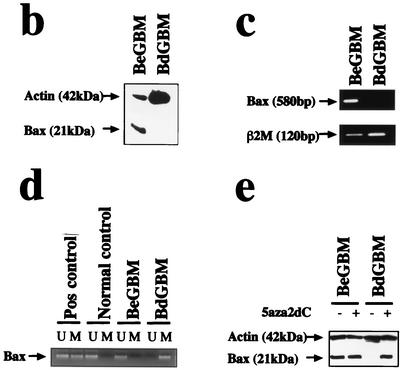
We found, in a series of 50 patients with GBM, that only three tumors lacked the expression of Bax (6). No difference in age, sex, and Karnofski index was found between the latter patients and patients with tumors expressing Bax α (data not shown). We obtained primary cultures from 2 tumors lacking the expression of Bax (BdGBM 38 and 44) and from 2 tumors expressing Bax α (BeGBM 12 and 24). The persistence of the lack of expression of Bax in BdGBM was confirmed by immunostaining (Fig. 1a) and by immunoblotting (Fig. 1b). Bax could be found under the same conditions in BeGBM cells (Fig. 1a and b). As show in Fig. 1c, no mRNA encoding Bax could be found in the BdGBM cells. We have recently observed that Bax expression in GBM was partially controlled by methylation of the gene (6). Methylation of the promoter regions of CpG-rich sites in genes is the major mechanism for the silencing of many genes in tumors (3). We thus compared the methylation pattern of the Bax gene in BeGBM and in BdGBM cells using a methylation-specific PCR method (16). As shown in Fig. 1d, hypermethylation of the 5′ untranslated region of the Bax gene around the TATA box was observed in BdGBM but not in BeGBM cells and thus could be responsible for the lack of transcription of the Bax mRNAs in BdGBM cells. To confirm this, BdGBM cells were treated with the methylation inhibitor 5aza2dC as described by Soengas et al. (36). As show in Fig. 1e, the 5aza2dC treatment was sufficient to restore the expression of Bax in BdGBM, confirming the existence of an epigenetic silencing of Bax in the latter tumors.
FIG. 1.
Absence of expression of Bax in a subset of GBM. (a) Confocal microscopy analysis of in vitro Bax expression in BdGBM and in BeGBM cells using the GFAP as glial marker and as a control of the level of protein expression. (b) Immunoblot analysis of Bax confirming the absence of the protein in BdGBM cells in vitro. Representative immunoblots were done with 50 μg of BeGBM and 150 μg of BdGBM loaded on SDS-PAGE. (c) mRNA encoding Bax was examined by RT-PCR using the SMART system as described in Materials and Methods using β2-macroglobulin as a control as described in reference 6. (d) Methylation specific PCR analysis of the Bax promoter region in BeGBM and in BdGBM cells. PCR was performed on genomic DNA purified from BdGBM or BeGBM cells. The DNA was untreated (U) or modified (M) by bisulfite sodium as originally described by Herman et al. (16). Pos control, ummethylated DNA; Normal control, methylated DNA. A gel representative of results from three independent experiments performed on each cell line is illustrated. (e) 5aza2dc treatment of GBM cells restored the expression of Bax in BdGBM cells as shown by immunoblotting with anti-Bax antibodies using actin as an internal control (50 μg of protein was loaded in each lane).
Roles of Bax and Bak in the response of GBM cells to chemo- and irradiation-induced apoptosis.
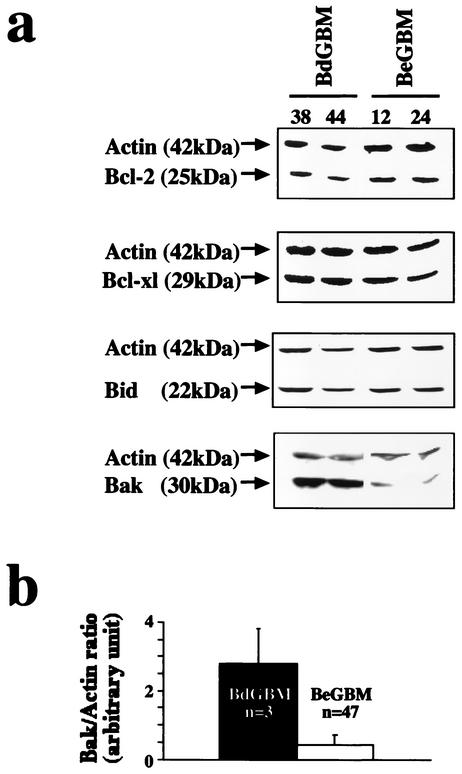
We examined by immunoblotting the expression of several apoptotic-related proteins in the BdGBM and BeGBM cell lines. As shown in Fig. 2a, little or no difference in the expression of Bcl-2 or Bcl-XL, two antiapoptotic proteins, was observed between BeGBM and BdGBM cells. A similar situation was found for the expression of the BH3-only proapoptotic Bid which was not significantly different in BeGBM and BdGBM cells (P = 0.19). Conversely, we found that the expression of the multidomain proapoptotic protein Bak was up-regulated in BdGBM both in vitro (Fig. 2a) and in vivo (i.e., in the resected tumors: P = 0.045) (Fig. 2b).
FIG. 2.
Bak is overexpressed in BdGBM. (a) Immunoblot analyses of Bcl-2, Bcl-XL and Bid expression in BeGBM and in BdGBM cells. Immunoblotting with a Bak antibody revealed that this protein was overexpressed in BdGBM cells; 50 μg of protein was loaded in each lane and actin was used as a control. (b) The ratio between Bak and actin in the resected tumors was calculated from immunoblots and represented as a histogram.
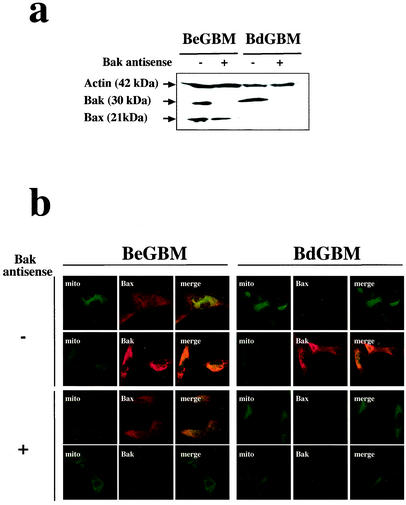
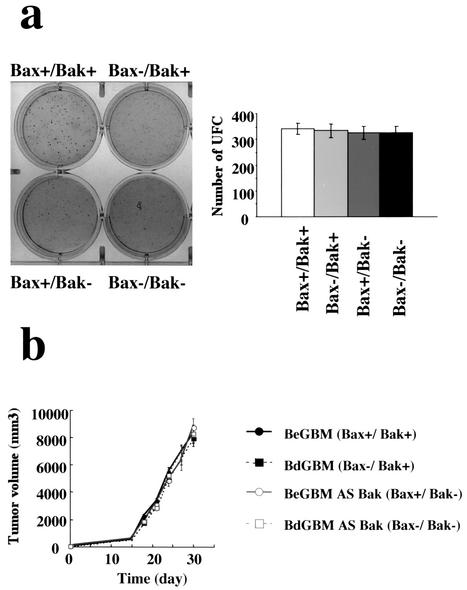
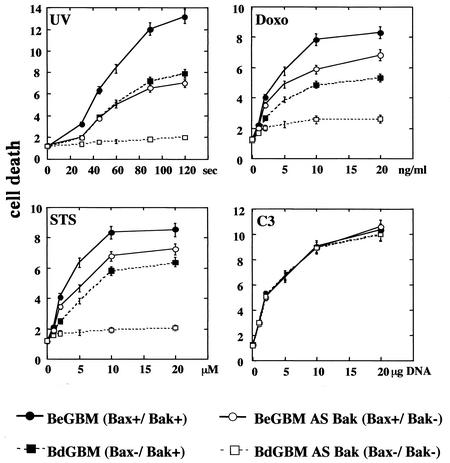
To evaluate the importance of the overexpression of Bak in BdGBM, we generated Bax−/Bak− cells from BdGBM cells and Bax+/Bak− cells from BeGBM cells using an antisense strategy (see Materials and Methods). Immunoblotting (Fig. 3a) and laser confocal microscopy (Fig. 3b) were used to confirm the suppression of Bak expression in all antisense-transfected BdGBM and BeGBM cells. Deficiency in Bax expression has been reported to confer a growth selective advantage in human cancer cells (33). However, in our case, we did not observe any influence of single Bax− or Bak− and double Bax−/Bak− deficiency on the clonogenicity (Fig. 4a) and tumoral growth in the Swiss nude mice (Fig. 4b). We analyzed the response of these cell lines to UVB- or drug-induced (i.e., STS and DOXO) apoptosis. As shown in Fig. 5, BeGBM cells were more resistant to UV-induced apoptosis than the BdGBM. The fact that this resistance in BdGBM could be overcome by the expression of a Bax transgene or by a 5aza2dC treatment (data not shown) indicated that it was a consequence of the lack of expression of Bax. In BeGBM cells, the Bak antisense transfection decreased but did not abolish the resistance to UVB-induced apoptosis, while in BdGBM cells, this treatment led to a complete resistance to UVB (Fig. 5). Similar results were obtained with drug-induced apoptosis (i.e., DOXO and STS) (Fig. 5). Of note, in all cases the sensitivity to apoptosis of the Bak− cells was always lower than that of their Bax− counterparts (Fig. 5). A very likely explanation is that, in the latter case, the overexpression of Bak compensated for Bax loss while the Bak antisense transfection treatment, because it decreased Bax expression (Fig. 3), induced a greater resistance to apoptosis than that produced by the sole absence of Bak. The expression of a transgene encoding C3 induced massive apoptosis independently of Bax or Bak expressions, suggesting that the postmitochondrial apoptotic apparatus was fully functional in all cell lines (Fig. 5). These results suggest that, as observed in the double deficient mice (44), Bax and Bak play overlapping roles in irradiation- or drug-induced apoptosis in human tumoral cells and the cells lacking both Bax and Bak are completely resistant to multiple apoptotic stimuli.
FIG. 3.
Bak overexpression in BdGBM cells can be inhibited by full-length Bak antisense transfection. The inhibition of Bak expression by the full-length Bak antisense in the cell lines was analyzed by immunoblotting using actin and Bax as controls (a) and by confocal microscopy with mitochondria labeled with Mitotrackter green (mito) as a control (b).
FIG. 4.
Single or double Bax and/or Bak deficiency influenced neither GBM cell clonogenicity nor their growth in the nude mice. (a) The clonogenicity assays were performed as described in Materials and Methods; histograms were drawn from the results obtained from six independent experiments. Data are given as numbers of UFC (colony-forming units) ± standard deviations. (b) Tumoral growth in Swiss nude mice (data shown were obtained from six different mice for each tumor). Standard deviations are shown. Bax+/Bak+ = BeGBM, Bax−/Bak+ = BdGBM, Bax+/Bak− = antisense Bak-transfected BeGBM cells, and Bax−/Bak− = antisense Bak-transfected BdGBM cells.
FIG. 5.
Double Bax/Bak deficiency abolished sensitivities toward apoptosis in GBM. Bax+/Bak+ (BeGBM), Bax−/Bak+ (BdGBM), Bax+/Bak− (antisense Bak-transfected BeGBM cells) and Bax−/Bak− (antisense Bak-transfected BdGBM) cells were treated with UV irradiation (10 s to 120 s) and increasing concentrations of DOXO (up to 20 ng/ml) or STS (up to 20 μM). Cells were transfected with increasing concentrations of plasmid encoding human C3 (up to 20 μg of plasmid) or an empty plasmid (20 μg) used as a control. It should be noted that no significant cell death was recorded in mock-transfected cells after selection. Cell death was quantified after 24 h using the release of LDH (measured as described in Materials and Methods) in the cell culture medium. Representative results from one of 3 independent experiments are illustrated for each treatment.
Role of Bax and Bak in apoptosis induced by GrB or C8.
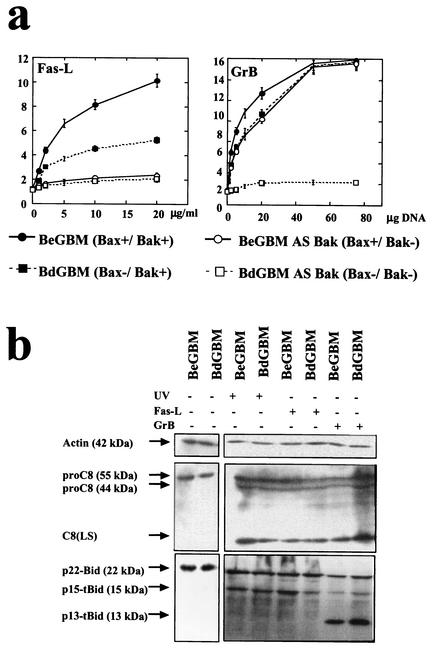
The link between dysregulation of apoptosis and progression of cancer can, at least partially, be associated with tumor resistance to immune surveillance (35). It has been recently shown that the main effectors of the immune-induced cell death are the death receptor pathway mainly through the apex caspase C8 and the cytotoxic pathway through the perforin/GrB system (35, 40). We thus examined the resistance to apoptosis induced by a death receptor (i.e., FasL) and by the cytotoxic pathway (GrB). As illustrated in Fig. 6a, BdGBM cells were still sensitive to FasL; however, the inhibition of the expression of Bak completely inhibited the apoptosis induced by this death ligand. Conversely, BeGBM and BdGBM cells were equally sensitive to GrB-induced apoptosis but not the Bax−/Bak− cells (Fig. 6a). These results confirm that the presence of Bax and Bak is required for the immune-induced apoptosis and show that in GBM, Bak is the main effector of the death receptor-induced apoptosis. As immune-induced apoptosis occurred mainly through the truncation of Bid by C8 (death receptor pathway) or by GrB (2, 32, 37), we examined the cleavage of fl-Bid as well as the activation of proC8 in BdGBM and BeGBM treated with UVB or Fas ligand (FasL) (20 μg/ml) or transfected with a GrB transgene. In all cases, the induction of apoptosis in BdGBM and BeGBM cells induced a similar activation of C8 (Fig. 6b). The cleavage of fl-Bid was significantly affected by the nature of the death inducer: UV irradiation or FasL treatment induced a cleavage of fl-Bid into a p15-tBid while GrB produced a distinct cleavage to yield two fragments, a major p13-tBid and a minor p15-tBid (Fig. 6b), as indeed previously reported (14, 43). Interestingly, treatments with FasL and GrB yielded similar patterns of C8 and truncated Bid in BeGBM and BdGBM. The latter results indicated that the different sensitivities toward these stimuli occurred downstream of these “activation steps” in these cells.
FIG. 6.
Sensitivity of single or double Bax/Bak-deficient GBM cells toward immune-induced apoptosis. (a) Bax+/Bak+ (BeGBM), Bax−/Bak+ (BdGBM), Bax+/Bak− (antisense Bak-treated BeGBM cells) and Bax−/Bak− (antisense Bak-treated BdGBM) cells were treated with increasing concentrations of FasL (from 0 to 20 μg/ml) or were transfected with increasing concentrations of plasmid (up to 200 μg of plasmid) encoding GrB. Cell death was quantified after 24 h of treatment using the release of LDH as an assay. Representative results from one of three independent experiments are illustrated for each treatment. (b) BeGBM or BeGBM cells treated with UVB irradiation (60 s) or FasL (10 μg/ml) or transfected with a plasmid encoding GrB (10 μg) were harvested 24 h after the start of the treatment. Cell extracts (50 μg) were analyzed for pro-caspase 8 (proC8) cleavage into the large subunit of caspase 8 (C8 LS) as a marker. The processing of fl-Bid (p22Bid) into p15-tBid or p13-tBid was analyzed in the same extracts using a 12 to 20% gradient SDS-PAGE.
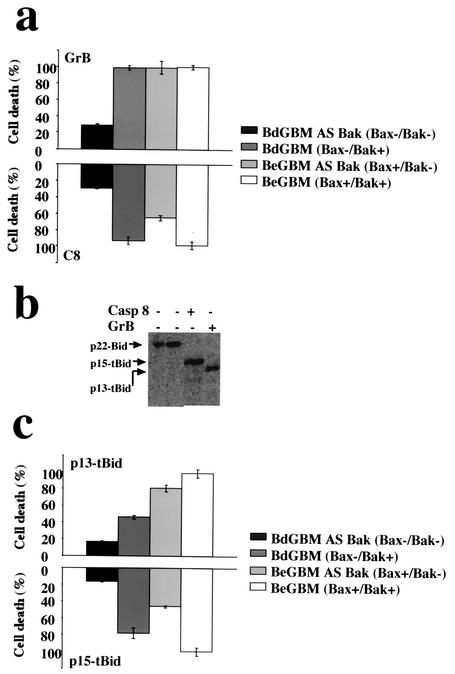
Since Bax or Bak deficiency resulted in different levels of resistance toward FasL- and GrB-induced apoptosis, we examined the effect of microinjection of C8 and GrB on Bax- and/or Bak-deficient GBM cells. Pure GrB and C8, at concentrations which represented IETDase activities similar to that measured in FasL-treated cells (data not shown), were microinjected into the GBM cell lines. Cell death was monitored for up to 3 h, at which stage most of the BeGBM cells microinjected with either C8 or GrB displayed typical morphological features of apoptosis (data not shown). As illustrated in Fig. 7a, with the notable exception of the Bax−/Bak− GBM, all cells were equally sensitive to the presence of GrB. Conversely, the loss of Bak appeared to drastically influence the apoptotic response to the microinjection of C8 (Fig. 7a). Next, we asked if the distinct cleavage patterns of Bid generated by the C8 or GrB were responsible for the differential role of Bak or Bax in the sensitization toward these enzymes. One hundred micrograms of fl-Bid was incubated with GrB or C8 and the cleavage products were analyzed by SDS-PAGE and immunoblotting. As shown in Fig. 7b, C8 produced a single Bid fragment with an apparent molecular mass of 15 kDa (p15-tBid) while GrB generated a truncated form of 13 kDa (p13-tBid). This result suggested that the p15-tBid observed in GrB-transfected cells (Fig. 6b) was due to the GrB-induced C8 activity. BdGBM or BeGBM cells with or without Bak were microinjected with purified in vitro-generated p13-tBid or p15-tBid. The microinjection of p13-tBid or p15-tBid into these double deficient cells induced little apoptosis while Bax+/Bak+ cells (BeGBM) were equally and highly sensitive to both truncated forms of Bid (Fig. 7c). Bax− cells (BdGBM) were resistant to p13-tBid-induced apoptosis but still sensitive to p15-tBid (Fig. 7c) while Bak− cells were sensitive to p13-tBid but resistant to p15-tBid (Fig. 7c). These results were consistent with results obtained with C8 and indicated that Bak plays a major role in the response to a C8/p15-tBid. On the other hand, the sensitivity toward GrB, observed in single deficient cells, could result from a GrB toxicity independent of its cleavage of fl-Bid or from its ability to generate both the p15 and p13 truncated forms of tBid (Fig. 6b).
FIG. 7.
Different forms of truncated Bid generated by C8 and GrB affect differently the single or double deficient GBM cells. (a) GBM cells with distinct Bax and Bak phenotypes were microinjected with GrB and C8 concentrations exhibiting similar IETDase activities (5 U) and mixed with Oregon green dextran as a microinjection marker. Cell death was assessed morphologically by fluorescent microscopy as described by Juin et al. (22). (b) Specific cleavage of fl-Bid (4 fmol) into p15-tBid by C8 (300 U of IETDase) and into p13-tBid by GrB (300 U of IETDase) in a cell-free assay. (c) Similar amounts of p13-tBid or p15-tBid (0.1 ng/μl) were microinjected, and cell death was quantified as in panel a.
p13-tBid and p15-tBid interact differently with Bax and Bak.
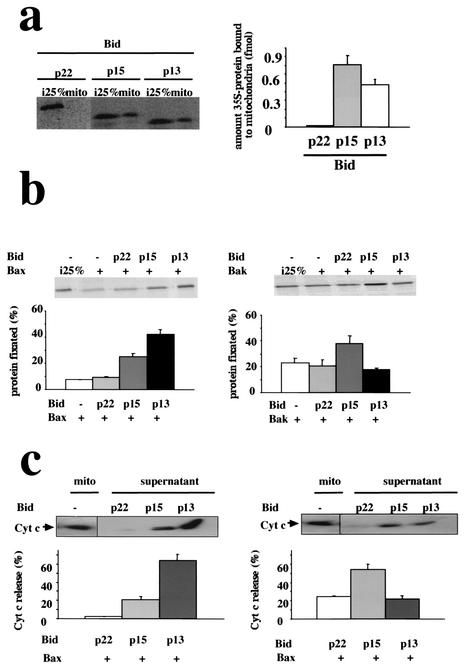
The latter results suggested that the truncated forms of Bid could be involved in separate pathways which would implicate either Bax or Bak. To address this question, we used a cell-free system to analyze the association of p15-tBid and p13-tBid with mitochondria as well as their functional interactions with Bak or Bax. As illustrated in Fig. 8a, p13-tBid and p15-tBid associated readily with mitochondria in a cell-free system as previously described by Wei et al. (43). On the other hand, no or little association of fl-Bid with mitochondria was observed under our conditions. It has been shown that the interaction of Bax with tBid triggered its association with the mitochondrial outer membrane (9, 13, 25). In the cell-free system, we observed that the binding of 4 fmol of Bax to mitochondria was unaffected by the coincubation with equimolar concentrations of fl-Bid but was significantly augmented by a similar concentration of p13-tBid or p15-tBid (Fig. 8b). Interestingly, the addition of p13-tBid induced a greater association of Bax with mitochondria than that of p15-tBid (P = 0.0001) (Fig. 8b), indicating that p13-tBid was more efficient than the p15-tBid in the activation of Bax. Conversely, the insertion of Bak into mitochondria, which occurred spontaneously (41), was enhanced by the addition of p15-tBid but not by that of p13-tBid (P = 0.0048) (Fig. 8c). tBid has been shown to cooperate with Bax to promote the liberation of CYT-C from mitochondria (9, 13, 43). In the cell-free assay, the presence of fl-Bid did not provoke a Bax- or Bak-dependent release of CYT-C (Fig. 8d and e). In the presence of Bax, p13-tBid induced the rapid release of CYT-C from mitochondria, while p15-tBid triggered a more moderate release of CYT-C (Fig. 8d). Conversely, in the presence of Bak, p15-tBid induced a rapid and massive release of CYT-C while the presence of p13-tBid had little effect on it (Fig. 8e). Of note, neither p13-tBid nor p15-tBid induced the release of CYT-C in the absence of exogenous Bax and Bak (data not shown). Taken together, our results suggested that p13-tBid had a higher impact on Bax- than on Bak-induced CYT-C release and that a reverse trend was observed with p15-tBid.
FIG. 8.
Preferential cooperation of p15-tBid with Bak and p13-tBid with Bax in a cell-free assay. (a) 35S-fl-Bid was digested by C8 or GrB as described in Fig. 7, and 4 fmol of 35S-C8-cleaved fl-Bid (p15-tBid) or 35S-GrB-cleaved Bid (p13-tBid) was incubated for 30 min at 37°C with 50 μg of mitochondria before centrifugation for 10 min at 10,000 × g at 4°C. The binding of 35S-Bid to mitochondria was analyzed by SDS-PAGE and autoradiography of the mitochondrial pellet. One femtomole (25% of the initial input of 35S-labeled protein [i.e., 25%]), was added to the mitochondrial binding assay mix as a loading control. The influence of the different forms of Bid on Bax (b) and Bak (c) association in the cell-free system was assessed by the association of 35S-Bax and 35S-Bak with mitochondria after incubation with equimolar concentrations of cold Bid (4 fmol). Mitochondrial pellets were analyzed after SDS-PAGE and autoradiography as described for panel a. p13-tBid and p15-tBid involvement in Bax- (d) or Bak- (e) induced CYT-C release from mitochondria. After binding of Bax and Bak to mitochondria in the absence or the presence of different forms of Bid, mitochondria were pelleted for 10 min at 10,000 × g at 4°C and the presence of CYT-C in the supernatant was analyzed by immunoblotting. Data (± standard deviations) were obtained from at least 3 independent experiments.
DISCUSSION
The importance of Bax in tumor progression has been revealed in numerous studies of human tumors as well as in animal models (11, 12, 20, 27, 28, 31, 33, 38, 45, 47). However, based on the putative fundamental role of the loss of Bax in tumor progression and aggressivity (20, 28, 31, 38), a surprisingly low number of GBM tumors lacked its expression (i.e., 6%). In addition, the mean survival of these patients was not significantly different from that of the 30 Bax α-positive tumor patients (10.8 ± 3 months versus 11.2 ± 5 months; P = 0.654), while the 17 patients expressing the gain of function variant Bax Ψ had a significant increase in their survival (19 ± 3 months, P = 0.019) (6). Thus, the loss of Bax appears to have less impact on tumor growth than the increase in its proapoptotic properties. Consistent with these results, but contrary to recent results in mice (8), the inactivation of Bax and/or Bak facilitated neither the clonogenicity nor the tumor growth (Fig. 4). In GBM, Bax deficiency was linked to the methylation of the gene (Fig. 1), a mechanism which has been associated with the silencing of tumor suppressors in many cancers (3). The absence of Bax in GBM cells decreased but did not abolish the apoptotic response to various cell death inducers (Fig. 5). However, contrary to a previous report by Zhang et al. (47), the persistence of an apoptotic response was not due to a change in the level of antiapoptotic proteins such as Bcl-2 or Bcl-XL but rather to the compensatory increase in the expression of Bak, a proapoptotic member of the Bcl-2 family (Fig. 2). The latter point was highlighted using the antisense strategy described by Eguchi et al. (10) to generate Bak-deficient BeGBM (Bax+/Bak−) or BdGBM (Bax−/Bak−) cells (Fig. 3). The double deficient GBM cells were highly resistant to various apoptotic stimuli (Fig. 5) and thus behaved like the equivalent cells derived from the double knockout of Bax and Bak in mice (7, 8, 44). The overexpression of Bak in BdGBM cells did not fully compensate for Bax deficiency in UVB- or chemo-induced cell death (Fig. 5). The latter point suggested that either Bax fulfilled a more fundamental role in the control of apoptosis in GBM than Bak or that Bax and Bak had distinct specificities toward cell death stimuli.
The ability of tumors to evade apoptotic death is one of the hallmarks of cancer defined by Hanahan and Weinberg (15), and the immune system appears to be one of the main effectors of cell death in tumors (35). The immune system exerts its antitumoral surveillance mainly through cell death induced by cytotoxic T lymphocytes and NK cells (19, 35). Cytotoxic T lymphocytes and NK cells use different effectors to mediate apoptosis in target cells: the death receptor mechanism with the FasL/Fas receptor system as model and the perforin/GrB cytotoxic pathway (35, 40). The ligation of the death ligands to their receptors, such as that of FasL to Fas receptor, initiates cell death by the activation of the intracellular initiator caspase C8 which in turn can induce apoptosis either through the direct activation of C3 in type I cells or by using mitochondria as an obligatory amplifier of the death signal in type II cells (19). Cytolytic granules function through the serine protease GrB which activates apoptosis mainly by the mitochondrial pathway (2, 32, 37, 39, 42). We show here that Bax deficiency decreases the sensitivity of GBM to FasL but not to C3 and that this sensitivity is completely abolished by the suppression of Bak expression both in BeGBM and BdGBM cells (Fig. 6 and 7) These results suggest that GBM are type II cells and that Bak plays a fundamental role in C8-induced apoptosis as previously suggested by Wang et al. (42). Of note, no difference in the sensitivity toward GrB was observed under the same conditions in the double deficient cells as little or no signs of apoptosis were observed (Fig. 6 and 7).
Recent experiments have shown that both Bax and Bak and thus the mitochondrial pathway are coupled to the Fas pathway/C8 through the BH3-only proapoptotic protein Bid (9, 13, 14, 29, 30, 46). No significant difference in the expression of Bid was observed between BeGBM and BdGBM cells (Fig. 2) or in cells transfected with Bak antisense (data not shown). Treatment of the BeGBM or BdGBM cells with UVB irradiation, FasL or GrB leads to a similar activation of C8 but generates different forms of truncated Bid (Fig. 6). GrB has been shown to cleave Bid at Asp75 and C8 at Asp59 (14). This result is consistent with the pattern of generation of the p13-tBid and the p15-tBid, observed in BeGBM and BdGBM cells, by treatments which utilize one or both enzymes (Fig. 6). Of note, the activation of the GrB pathway in GBM cells induces mainly p13-tBid with a minor p15-tBid while in an acellular assay pure GrB produces only the p13-tBid (Fig. 6). The latter results are likely to be due to a direct activation of C8 by GrB, which led to the production of p15-tBid and as such could explain the discrepancies between the microinjection of GrB and that of p13-tBid observed in Fig. 7. The microinjection of different forms of tBid showed that p13-tBid induced cell death more efficiently in the presence of Bax than in that of Bak while p15-tBid seems to synergize better with Bak than with Bax (Fig. 7). Using a cell-free system, we found that p13-tBid and p15-tBid displayed similar affinities for mitochondria, while fl-Bid did not associate with the organelle (Fig. 8). The presence of p13-tBid enhanced the association of Bax to mitochondria as well as the Bax-dependent release of CYT-C from the organelle (Fig. 8.). On the other hand, Bak association with mitochondria was stimulated by the addition of p15-tBid and unaffected by that of p13-tBid (Fig. 8). However, the Bak-dependent release of CYT-C from mitochondria was more affected by the presence of p15-tBid than by that of p13-tBid (Fig. 8). Taken together, our results suggest the existence of a preferential cross talk between p13-tBid and Bax which is preferentially used in the GrB pathway whereas p15-tBid appeared to cooperate better with Bak than with Bax and to be used in the cell death receptor/C8 pathway (see Fig. 9).
FIG. 9.
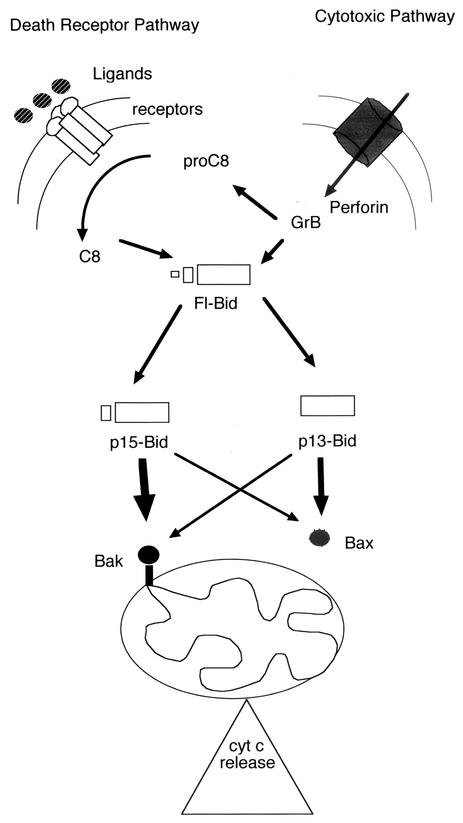
Schematic representation of the cross talks between Bak and the death receptor pathway and Bax and the cytotoxic pathway. The immune system can trigger cell death in target GBM cells by the death receptor pathway (i.e., interaction of FasL with its plasma membrane receptor Fas) or by the cytotoxic pathway (i.e., the translocation of GrB throughout the target plasma membrane mediated by the perforin proteins). The activation of C8 is achieved by activation of the death receptor pathway or by GrB (cf. Fig. 6b). In the latter case, fl-Bid is cleaved into two fragments: p13-tBid directly by GrB and p15-tBid by the GrB-activated C8 (Fig. 6b). Hence, GrB generates both products of cleavage of fl-Bid and C8 generates only p15-tBid (Fig. 7a). p13-tBid has a preferential interaction with Bax and triggers its association with mitochondria while p15 appears to preferentially interact with Bak to induce the release of CYT-C from mitochondria and the subsequent activation of the execution phase of apoptosis (Fig. 7c and 8).
In conclusion, we show in this work that the loss of Bax which occurs in a minority of GBM is accompanied by the overexpression of Bak. Of note, Leblanc et al. (27) have observed this type of regulation in Bax-deficient cells derived from human colon cancer which were treated with subapoptotic amounts of DNA-damaging drugs. BdGBM were untreated at the time of the resection but it is tempting to speculate that a similar process occurs in BdGBM as they would carry more naturally occurring DNA damages because of the absence of Bax. In these tumors, the overexpression of Bak would act as a safeguard against tumor expansion as it allows a certain sensitivity of the malignant cells to apoptosis. This could ensure the elimination of totally genetically aberrant tumoral cells which in turn could interfere with tumoral growth. Alternatively, the BdGBM could arise from tumors which primitively expressed high levels of both Bax and Bak which would have been selected against the high level of expression of Bax. In any case, the preservation of the sensitivity of GBM to death ligand- and/or to GrB-induced apoptosis could provide important insights into the development of new therapies for GBM.
Acknowledgments
We thank the staff of the department of Neurosurgery (Y. Lajat) and of the Laboratoire d'Anatomo-Cytopathology B, Hospital of Nantes (Paulette Cuillère) for providing us with tumor samples and advice. We thank I. Theodorou (Inserm U543) for the gift of human GrB cDNA.
P.-F.C. is a recipient of a fellowship from the Ligue Contre le Cancer du Doubs et du Pays de Montbeliard. This work was supported by grants from INSERM and La Ligue Contre le Cancer.
REFERENCES
- 1.Adams, J. M., and S. Cory. 2002. The Bcl-2 family: regulators of the cellular life-or-death switch. Nat. Rev. Cancer 2:647-656. [DOI] [PubMed] [Google Scholar]
- 2.Barry, M., J. A. Heibein, M. J. Pinkoski, S. F. Lee, R. W. Moyer, D. R. Green, and R. C. Bleackley. 2000. Granzyme B short-circuits the need for caspase 8 activity during granule-mediated cytotoxic T-lymphocyte killing by directly cleaving Bid. Mol. Cell. Biol. 20:3781-3794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bayling, S. B., M. Esteller, M. R. Rountree, K. E. Bachman, and J. G. Herman. 2001. Aberrant patterns of DNA methylation, chromatin formation and gene expression in cancer. Hum. Mol. Genet. 10:687-692. [DOI] [PubMed] [Google Scholar]
- 4.Bouillet, P., and A. Strasser. 2002. BH3-only proteins—evolutionarily conserved proapoptotic Bcl-2 family members essential for initiating programmed cell death. J. Cell Sci. 115:1567-1574. [DOI] [PubMed] [Google Scholar]
- 5.Cartron, P. F., C. Moreau, L. Oliver, E. Mayat, K. Meflah, and F. M. Vallette. 2002. Involvement of the N-terminus of Bax in its intracellular localization and function. FEBS Lett. 512:95-100. [DOI] [PubMed] [Google Scholar]
- 6.Cartron, P. F., L. Oliver, S. Martin, C. Moreau, M. T. LeCabellec, P. Jezequel, K. Meflah, and F. M. Vallette. 2002. The expression of a new variant of the pro-apoptotic molecule Bax, Bax ψ, is correlated with an increased survival of glioblastoma multiforme patients. Hum. Mol. Genet. 11:675-687. [DOI] [PubMed] [Google Scholar]
- 7.Cheng, E. H., M. C. Wei, S. Weiler, R. A. Flavell, T. W. Mak, T. Lindsten, and S. J. Korsmeyer. 2001. BCL-2, BCL-X(L) sequester BH3 domain-only molecules preventing BAX- and BAK-mediated mitochondrial apoptosis. Mol. Cell 8:705-711. [DOI] [PubMed] [Google Scholar]
- 8.Degenhardt, K., G. Chen, T. Lindstein, and E. White. 2002. BAX and BAK mediate p53-independent suppression of tumorigenesis. Cancer Cell 2:193-203. [DOI] [PubMed] [Google Scholar]
- 9.Desagher, S., A. Osen-Sand, A. Nichols, R. Eskes, S. Montessuit, S. Lauper, K. Maundrell, B. Antonsson, and J. C. Martinou. 1999. Bid-induced conformational change of Bax is responsible for mitochondrial cytochrome c release during apoptosis. J. Cell Biol. 144:891-901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Eguchi, H., K. Suga, H. Saji, M. Toi, and S. L. Nakachi. 2000. Different expression patterns of Bcl-2 family genes in breast cancer by estrogen receptor status with special reference to pro-apoptotic Bak gene. Cell Death Differ. 7:439-446. [DOI] [PubMed] [Google Scholar]
- 11.Eischen, C. M., M. F. Roussel, S. J. Korsmeyer, and J. L. Cleveland. 2001. Bax loss impairs Myc-induced apoptosis and circumvents the selection of p53 mutation during Myc-mediated lymphomagenesis. Mol. Cell. Biol. 21:7653-7662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Eischen, C. M., J. E. Rehg, S. J. Korsmeyer, and J. L. Cleveland. 2002. Loss of Bax alters tumor numbers in ARF-deficient mice. Cancer Res. 62:2184-2191. [PubMed] [Google Scholar]
- 13.Eskes, R., S. Desagher, B. Antonsson, and J. C. Martinou. 2000. Bid induces the oligomerization and insertion of Bax into the outer mitochondrial membrane. Mol. Cell. Biol. 20:929-935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gross, A., X. M. Yin, K. Wang, M. C. Wei, J. Jockel, C. Milliman, H. Erdjument-Bromage, P. Tempst, and S. J. Korsmeyer. 1999. Caspase- cleaved Bid targets mitochondria and is required for cytochrome c release while BCL-XL prevents this release but not tumor necrosis factor-R1/Fas death. J. Biol. Chem. 274:1156-1163. [DOI] [PubMed] [Google Scholar]
- 15.Hanahan, D., and R. A. Weinberg. 2000. The hallmarks of cancer. Cell 100:57-70. [DOI] [PubMed] [Google Scholar]
- 16.Herman, J. G., J. R. Graff, S. Myohanen, B. D. Nelkin, and S. B. Baylin. 1996. Methylation-specific PCR: a novel PCR assay for the methylation status of CpG islands. Proc. Natl. Acad. Sci. USA 93:9821-9826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hickman, J. A. 2002. Apoptosis and tumourigenesis. Curr. Opin. Genet. Dev. 12:67-72. [DOI] [PubMed] [Google Scholar]
- 18.Holland, E. C. 2000. Glioblastoma multiforme: the terminator. Proc. Natl. Acad. Sci. USA 97:6242-6244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Igney, F. H., and P. H. Krammer. 2002. Death and anti-death: tumor resistance to apoptosis. Nat. Rev. Cancer 2:277-288. [DOI] [PubMed] [Google Scholar]
- 20.Ionov, Y., H. Yamamoto, S. Krajewski, J. C. Reed, and M. Perucho. 2000. Mutational inactivation of the pro-apoptotic gene BAX confers selective advantage during tumor clonal evolution. Proc. Natl. Acad. Sci. USA 97:10872-10877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Johnstone, R. W., A. A. Ruefli, and S. W. Lowe. 2002. Apoptosis: a link between cancer genetics and chemotherapy. Cell 108:153-164. [DOI] [PubMed] [Google Scholar]
- 22.Juin, P., A. Hunt, T. Littlewood, B. Griffiths, L. Brown-Swigart, S. Korsmever, and G. Evan. 2002. c-Myc functionally cooperates with Bax to induce apoptosis. Mol. Cell. Biol. 22:6158-6169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kleihues, P., P. C. Burger, and B. W. Scheithauer. 1993. The new W.H.O. classification of brain tumours. Brain Pathol. 3:255-268. [DOI] [PubMed] [Google Scholar]
- 24.Korsmeyer, S. J. 1999. BCL-2 gene family and the regulation of programmed cell death. Cancer Res. 59(Suppl.):1693s-1700s. [PubMed]
- 25.Korsmeyer, S. J., M. C. Wei, M. Saito, S. Weiler, K. J. Oh, and P. H. Schlesinger. 2000. Pro-apoptotic cascade activates BID, which oligomerizes BAK or BAX into pores that result in the release of cytochrome c. Cell Death Differ. 7:1166-1173. [DOI] [PubMed] [Google Scholar]
- 26.Kroemer, G., and J. C. Reed. 2000. Mitochondrial control of cell death. Nat. Med. 6:513-519. [DOI] [PubMed] [Google Scholar]
- 27.LeBlanc, H., D. Lawrence, E. Varfolomeev, K. Totpal, J. Morlan, P. Schow, S. Fong, R. Schwall, D. Sinicropi, and A. Ashkenazi. 2002. Tumor-cell resistance to death receptor-induced apoptosis through mutational inactivation of the proapoptotic Bcl-2 homolog Bax. Nat. Med. 8:274-281. [DOI] [PubMed] [Google Scholar]
- 28.Li, B., and Q. P. Dou. 2000. Bax degradation by the ubiquitin/proteasome-dependent pathway: involvement in tumor survival and progression. Proc. Natl. Acad. Sci. USA 97:3850-3855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Li, H., H. Zhu, C. J. Xu, and J. Yuan. 1998. Cleavage of Bid by caspase 8 mediates the mitochondrial damage in the Fas pathway of apoptosis. Cell 94:491-500. [DOI] [PubMed] [Google Scholar]
- 30.Luo, X., I. Budihardjo, H. Zou, C. Slaughter, and X. Wang. 1998. Bid, a Bcl-2 interacting protein, mediates cytochrome c release from mitochondria in response to activation of cell death receptors. Cell 94:481-490. [DOI] [PubMed] [Google Scholar]
- 31.Meijerink, J. P., E. J. Mensink, K. Wang, T. W. Sedlak, A. W. Sletjes, T. de Witte, G. Waksman, and S. J. Korsmeyer. 1998. Hematopoietic malignancies demonstrate loss-of-function mutations of BAX. Blood 91:2991-2997. [PubMed] [Google Scholar]
- 32.Pinkoski, M. J., N. J. Waterhouse, J. A. Heibein, B. B. Wolf, T. Kuwana, J. C. Goldstein, D. D. Newmeyer, R. C. Bleackley, and D. R. Green. 2001. Granzyme B-mediated apoptosis proceeds predominantly through a Bcl-2 inhibitable mitochondrial pathway. J. Biol. Chem. 276:12060-12067. [DOI] [PubMed] [Google Scholar]
- 33.Rampino, N., H. Yamamoto, Y. Ionov, Y. Li, H. Sawai, J. C. Reed, and M. Perucho. 1997. Somatic frameshift mutations in the BAX gene in colon cancers of the microsatellite mutator phenotype. Science 275:967-969. [DOI] [PubMed] [Google Scholar]
- 34.Shibata, M. A., M. L. Liu, M. C. Knudson, E. Shibata, K. Yoshidome, T. Bandey, S. J. Korsmeyer, and J. E. Green. 1999. Haploid loss of Bax leads to accelerated mammary tumor development in C3 (1)/SV40-Tag transgenic mice: reduction in protective apoptotic response at the preneoplastic stage. EMBO J. 18:2692-2701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Smyth, M. J., D. I. Godfrey, and J. A. Trapani. 2001. A fresh look at tumor immunosurveillance and immunotherapy. Nat. Immunol. 2:293-299. [DOI] [PubMed] [Google Scholar]
- 36.Soengas, M. S., P. Capodieci, D. Polsky, J. Mora, M. Esteller, X. Opitz-Araya, R. McCombie, J. G. Herman, W. L. Gerald, Y. A. Lazebnik, C. Cordon-Cardo, and S. W. Lowe. 2001. Inactivation of the apoptosis effector Apaf-1 in malignant melanoma. Nature 409:207-211. [DOI] [PubMed] [Google Scholar]
- 37.Sutton, V. R., J. E. Davis, M. Cancilla, R. W. Johnstone, A. A. Ruefli, K. Sedelis, K. A. Browne, and J. A. Trapani. 2000. Initiation of apoptosis by granzyme B requires direct cleavage of bid, but not direct granzyme B-mediated caspase activation. J. Exp. Med. 192:1403-1413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Theodorakis, P., E. Lomonosova, and E. Chinnadurai. 2002. Critical requirement of BAX for manifestation of apoptosis induced by multiple stimuli in human epithelial cancer cells. Cancer Res. 62:3373-3376. [PubMed] [Google Scholar]
- 39.Thomas, D. A., L. Scorrano, G. V. Putcha, S. J. Korsmeyer, and T. J. Ley. 2001. Granzyme B can cause mitochondrial depolarization and cell death in the absence of BID, BAX, and BAK. Proc. Natl. Acad. Sci. USA 98:14985-14990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Trapani, J. A., and M. J. Smyth. 2002. Functional significance of the perforin/granzyme cell death pathway. Nat. Rev. Immunol. 2:735-747. [DOI] [PubMed] [Google Scholar]
- 41.Tremblais, K., L. Oliver, P. Juin, M. T. LeCabellec, K. Meflah, and F. M. Vallette. 1999. The C-terminus of Bax is not a membrane addressing/anchoring signal. Biochem. Biophys. Res. Commun. 260:582-591. [DOI] [PubMed] [Google Scholar]
- 42.Wang, G. Q., E. Wieckowski, L. A. Goldstein, B. R. Gastam, A. Rabinovitz, A. Gambotto, S. Li, B. Fang, X. M. Yin, and H. Rabinowich. 2001. Resistance to granzyme B-mediated cytochrome c release in Bak-deficient cells. J. Exp. Med. 194:1325-1337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wei, M. C., T. Lindstein, V. K. Mootha, S. Weiler, A. Gross, M. Ashiya, C. B. Thompson, and S. J. Korsmeyer. 2000. tBID, a membrane-targeted death ligand, oligomerizes BAK to release cytochrome c. Genes Dev. 14:2060-2071. [PMC free article] [PubMed] [Google Scholar]
- 44.Wei, M. C., W. X. Zong, E. H. Y. Cheng, T. Lindstem, V. Panoutsakopoulou, A. J. Ross, K. A. Roth, G. R. MacGregor, G. B. Thompson, and S. J. Kormeyer. 2001. Proapoptotic BAX and BAK; a requisite gateway to mitochondrial dysfunction and death. Science 292:727-730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Yin, C., C. M. Knudson, S. J. Kormeyer, and T. Van Dyke. 1997. Bax suppresses tumorigenesis and stimulates apoptosis in vivo. Nature 385:637-640. [DOI] [PubMed] [Google Scholar]
- 46.Yin, X. M., K. Wang, A. Gross, Y. Zhao, S. Zinkel, B. Klocke, K. A. Roth, and S. J. Korsmeyer. 1999. Bid-deficient mice are resistant to Fas-induced hepatocellular apoptosis. Nature 400:886-891. [DOI] [PubMed] [Google Scholar]
- 47.Zhang, L., J. Yu, B. H. Park, K. W. Kinzler, and B. Vogelstein. 2000. Role of BAX in the apoptotic response to anticancer agents. Science 290:989-992. [DOI] [PubMed] [Google Scholar]
- 48.Zong, W. X., T. Lindstem, A. J. Ross, G. R. MacGregor, and G. B. Thompson. 2001. BH3-only proteins that bind pro-survival Bcl-2 family members fail to induce apoptosis in the absence of Bax and Bak. Genes Dev. 15:1481-1486. [DOI] [PMC free article] [PubMed] [Google Scholar]