Abstract
The regulation of telomerase reverse transcriptase (TERT) plays an important role in the proliferative capacity and survival of cells. Here, we report that exogenously as well as endogenously induced oxidative stress leads to translocation of endogenous as well as overexpressed human TERT from the nucleus into the cytosol. TERT is transported through the nuclear pores in a leptomycin-sensitive and Ran GTPase-dependent process. H2O2-induced nuclear export of TERT is preceded by TERT tyrosine phosphorylation at position 707 and prevented by the Src kinase family inhibitor PP1. Oxidative stress-induced nuclear export of TERT depends on association with the Ran GTPase. In contrast, mutation of tyrosine 707 inhibits phosphorylation induced by oxidative stress and prevents association with Ran and nuclear export of TERT. Moreover, inhibition of tyrosine phosphorylation at 707 increases the antiapoptotic capacity of TERT. Taken together, depletion of nuclear TERT by tyrosine phosphorylation-dependent nuclear export of TERT is a novel mechanism for regulation of TERT localization, which reduces the antiapoptotic activity of TERT.
Telomeres are the physical ends of the chromosomes. They maintain chromosome stability, genetic integrity, and cell viability in a variety of different species (2, 10). Telomeres can also function as a mitotic clock, since telomeres are progressively shortened during each cell division. Telomere shortening forces human primary cells to stop dividing when a critical minimum telomere length is reached (8, 16). The enzyme telomerase, a ribonucleoprotein, counteracts the shortening of telomeres. Telomerase contains a catalytical subunit, the telomerase reverse transcriptase (TERT), which uses a small integral RNA component as a template for the synthesis of the dGT-rich strand of telomeres (20, 21, 42). Introduction of the active subunit of the telomerase, TERT, into human cells extended both their life span and their telomeres to lengths typical of young cells (4, 9, 63). In addition to the well-known function of TERT to counteract telomere shortening, TERT has been implicated in promotion of cell survival (45, 50). Suppression of telomerase enzyme activity promotes apoptosis of neuronal cells, germ cells, and thymocytes, whereas overexpression of TERT prevents apoptosis by interfering with a premitochondrial step in the cell death cascade (27, 38, 61, 65). Likewise, forced expression of TERT prevents apoptosis of cardiac myocytes (45).
The regulation of TERT involves transcriptional and posttranscriptional mechanisms. Sequence analysis has revealed that the human TERT promoter contains binding sites for several transcription factors (35). There is also growing evidence for posttranscriptional regulation of TERT. Thus, telomerase enzyme activity can be posttranscriptionally regulated by the kinases c-Abl, protein kinase C, ERK1/2, and Akt (5, 27, 30, 31, 33, 35, 38, 54, 61, 65). Moreover, binding proteins, like heat shock proteins (HSPs), seem to be required for telomerase enzyme activity (29). Recently, the translocation of TERT has been described as a third mechanism for posttranscriptional regulation (36, 53). Thereby, TERT was shown to be imported from the cytoplasm into the nucleus in T cells and smooth muscle cells upon stimulation with growth factors (36, 41). Another study demonstrated that 14-3-3 signaling proteins bind to TERT and thereby prevent nuclear export (53). Moreover, a recent study demonstrated that active human telomerase has a regulated intranuclear localization that is dependent on the cell cycle state, transformation, and DNA damage (60).
Generally, the exchange of small molecules and macromolecules in and out of the nucleus proceeds through nuclear pore complexes (51). Nuclear pores allow passage in essentially two modes: passive diffusion and facilitated translocation (43). Since passive diffusion is inefficient as the diffusing objects approach a size limit of 40 kDa (46), larger macromolecules cross the nuclear pore in a complex consisting of transport receptors—exportins for nuclear export and importins for nuclear import—and the small GTPase Ran (43). The gradient in Ran-GTP concentration across the nuclear envelope thereby is crucial for the directionality of the transport (43).
Reactive oxygen species (ROS) have been implicated in aging, apoptosis, and numerous diseases (6, 12, 22, 59). Upon production of high levels of ROS from exogenous or endogenous sources, the redox balance is perturbed and cells are shifted into a state of oxidative stress, which subsequently leads to modifications of intracellular proteins and lipids and to direct DNA damage (47). When the stress is severe, survival of the cell is dependent on the repair or replacement of damaged molecules, which can result in induction of apoptosis in severely damaged cells.
Since the enzyme TERT seems to be involved in processes of aging and apoptosis, we wanted to disclose a potential link between ROS and human TERT (hTERT), by investigating the regulation of hTERT by oxidative stress. Here, we demonstrate that oxidative stress induces the depletion of hTERT from the nucleus via export through the nuclear pores. Nuclear export is initiated by ROS-induced phosphorylation of tyrosine 707 within hTERT by the Src kinase family. Interference with the Src kinase family-dependent tyrosine phosphorylation of hTERT inhibits depletion of nuclear hTERT and thereby potentiates the antiapoptotic capacity of hTERT.
MATERIALS AND METHODS
cDNA cloning and plasmids.
The hTERT construct was kindly donated by R. A. Weinberg (40). hTERT was subcloned into the pcDNA3.1myc-his vector (hTERTwt) or the pShooter vector, which targets TERT to the nucleus (hnucTERT) (Invitrogen). The nuclear pShooter mammalian expression vector incorporates signal sequences into hTERT to direct TERT to the nuclear location. hTERTY707F was introduced into the hTERT wild type (hTERTwt) by site-directed mutagenesis (Stratagene).
The Ran wild type (Ran wt) was cloned out of cDNA by using sense (5′-GCGAATTCATGGCTGCGCAGGGAGAG) and antisense (5′-GCGGATCCGAACAGGTCATCCTCATCCGGGAGAGC) primers incorporating EcoRI and BamHI restriction sites. The amplified DNA was restriction digested with EcoRI and BamHI and subcloned into pcDNA3.1myc-his vector. Sequencing confirmed the correct orientation and the entire coding region. Ran Q69L, Ran G19V, and RanT24N mutants were introduced into Ran wt by site-directed mutagenesis (Stratagene).
Cell culture and transfection.
293 cells were maintained in Dulbecco's modified Eagle's medium containing 10% fetal calf serum. 293 cells were transfected with Lipofectamine/Plus according to the manufacturer's protocol (Gibco BRL) as previously described (24) with a transfection efficiency of 90% ± 4%.
Telomerase enzyme activity measurement.
Telomerase enzyme activity was measured with a commercially available PCR-based assay according to the manufacturer's protocol (Roche) as previously described (58).
In brief, after PCR amplification, PCR products were used for detection of telomerase enzyme activity by either (i) enzyme-linked immunosorbent assay (ELISA) or (ii) telomerase-mediated DNA laddering. (i) For ELISA, The PCR products are immobilized via the biotin-labeled TS primers (provided with the assay) to a streptavidin-coated microtiter plate. The linearity of the assay was ensured by the positive controls provided by the company, and as negative controls, H2O was used in the presence of the biotinylated primers. (ii) For the telomerase-mediated DNA laddering, PCR products were resolved on a 12% nondenaturing polyacrylamide gel. After transfer to positively charged nylone membranes, the 6-bp telomerase-specific ladder was detected with streptavidin-horseradish peroxidase and the enhanced chemiluminescence system (Amersham).
Separation of nuclear and cytosolic fractions.
Nuclear and cytosolic fractions were separated with a commercially available kit according to the manufacturer's protocol (Pierce). In brief, cells were scraped off the dish and centrifuged at 800 × g for 5 min at 4°C. The resulting pellet was resolved in cytosolic extraction reagent I (CERI buffer; provided by Pierce) and incubated for 10 min at 4°C. After addition of CERII buffer (provided by Pierce) and further incubation for 1 min at 4°C, samples were centrifuged for 5 min at 16,000 × g for 5 min at 4°C. The resulting supernatant contained the cytosolic fraction. The resulting pellet was resuspended in nuclear extraction reagent (NER) buffer and incubated for 60 min at 4°C. After centrifugation for 15 min at 16,000 × g at 4°C, the resulting supernatant was obtained as the nuclear fraction. The purity of the nuclear and cytosolic fractions was ensured by immunoblotting with topoisomerase 1 (nuclear) and HSP70 (cytosolic).
Immunoprecipitation and immunoblotting.
Lysates (500 μg) were immunoprecipitated with 5 μg of Ran antibody or myc antibody overnight at 4°C. After incubation with A/G Plus agarose (Santa Cruz) for 2 h at 4°C, the resulting beads were washed, subjected to sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) sample buffer, and dissolved by SDS-PAGE.
Immunoblotting was performed with antibodies directed against TERT (1:200, overnight, 4°C; Calbiochem); Ran, myc, and topoisomerase 1 (2 h, 1:250; all Santa Cruz); tubulin (2 h, 1:500; Neomarkers); HSP70 and phosphotyrosine clone 4G10 (overnight, 4°C, 1:500; Upstate Biotechnologies); and actin (2 h, 1:2,000; Sigma). Antibodies were detected by the Amersham ECL enhanced chemiluminescence system.
Immunostaining.
For immunostaining, cells were fixed in 4% paraformaldehyde and permeabilized with 0.3% Triton X-100 and 3% bovine serum albumin in phosphate-buffered saline (PBS).
For coimmunostaining, cells were first incubated with an antibody against myc (mouse, 1:50; overnight, 4°C; Santa Cruz), and Texas red-conjugated Fab fragment antimouse antibody was used as secondary antibody (1:250, 1 h; Jackson ImmunoResearch, Inc.). Since the antitubulin antibody is from the same host species as the myc antibody (mouse), the cells were then incubated with an excess of unconjugated Fab fragment antimouse antibody to block the first secondary antibody step. Afterwards, cells were incubated with tubulin antibody (mouse, 1:50, 2 h; Neomarkers) and with fluorescein isothiocyanate (FITC)-conjugated Fab fragment antimouse antibody (1:150, 1 h, room temperature; Jackson ImmunoResearch, Inc.). Nuclei were counterstained with 0.2 μg of DAPI (4′,6′-diamidino-2-phenylindole) per ml.
For confocal microscopy, cells were incubated with tubulin antibody (mouse, 1:50, 2 h; Neomarkers) and with FITC-conjugated Fab fragment antimouse antibody (1:150, 1 h, room temperature; Jackson ImmunoResearch, Inc.). Afterwards, cells were incubated with an antibody against myc directly conjugated with tetramethyl rhodamine isothiocyanate (sc-40; 1:100, 2 h; Santa Cruz). When indicated, nuclei were incubated with TO-PRO-3 iodide (1:4,000, 5 min; Molecular Probes). Cells were visualized by confocal microscopy (Zeiss LSM 510 META; magnification, 1:63 oil).
To quantify the cells with predominantly nuclear TERT-myc staining, 300 cells per staining were counted by three independent observers.
Negative controls were performed as follows. Texas red-conjugated Fab fragment antimouse antibody was used, and coimmunostaining with tubulin was performed as described above. Cells were visualized by fluorescence microscopy (Zeiss Axiovert 100; magnification, 1:63).
Detection of oxidative stress.
Cells were incubated with 20 μM 2′,7′ dichlorodihydrofluorescein diacetate (H2DCF-DA) or 5 μM dihydroethidium (DHE) for 30 min (Molecular Probes). Cells were trypsinized for 2 min, the reaction was stopped with media, and cells were washed with PBS. Fluorescence intensity was measured by fluorescence-activated cell sorter (FACS) analysis.
Apoptosis.
Apoptosis was determined by FACS analysis with annexin V-phycoerythrin (PE) binding and 7-amino-actinomycin (7AAD)-FITC staining (Pharmingen). Apoptotic cells were defined as annexin V positive and 7AAD negative. In brief, cells were trypsinized in the dish and pelleted. After being washed twice with annexin binding buffer (10 mM HEPES, 140 mM NaCl, 2.5 mM CaCl2 [pH 7.4]), cell pellets were resuspended in 50 μl of annexin binding buffer and incubated with 2.5 ng (each) of annexin V-PE and 7AAD-FITC per ml for 20 min. The reaction was terminated by addition of 250 μl of annexin binding buffer and then analyzed by FACS.
RESULTS
Exogenous oxidative stress induces translocation of hTERT.
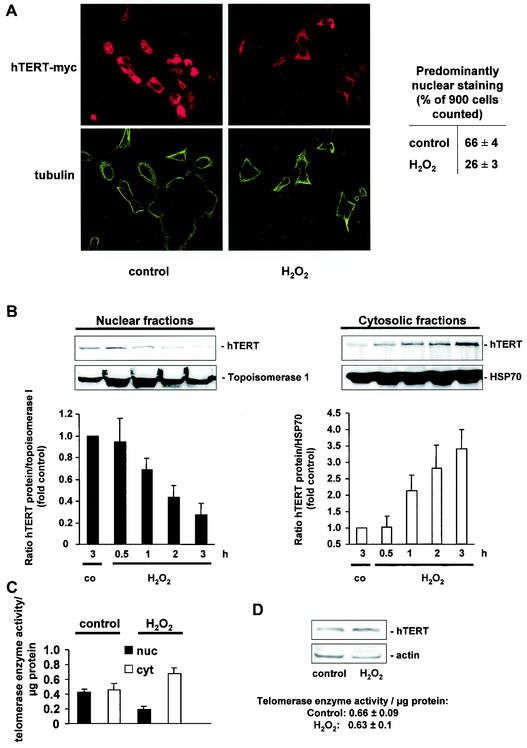
To investigate the effects of oxidative stress on hTERT localization, cells were incubated with 500 μM H2O2 for 3 h. H2O2 treatment resulted in a profound time-dependent change from predominantly nuclear to a predominantly cytoplasmic localization of myc-tagged hTERT protein (Fig. 1A). Likewise, biochemical separation of the nuclear and cytoplasmic fractions revealed that H2O2 time dependently reduced the endogenous hTERT protein in the nuclear fraction associated with a concomitant increase in cytosolic hTERT protein (Fig. 1B). Moreover, telomerase activity in the nuclear and cytosolic fractions was significantly altered after H2O2 incubation (Fig. 1C). Control experiments demonstrated that H2O2 treatment did not affect the overall expression of the hTERT protein or telomerase enzyme activity (Fig. 1D).
FIG. 1.
Exogenous oxidative stress induces translocation of hTERT. (A) A representative immunostaining using confocal microscopy is shown for 293 cells overexpressing myc-tagged hTERTwt with or without treatment with 500 μM H2O2 for 3 h. (Upper panels) hTERT-myc staining (red). (Lower panels) Tubulin staining to visualize the cell structure (green). Results from five different transfected dishes are shown. Cells with predominantly nuclear hTERT staining were counted by three independent investigators. The quantification is shown on the right side. (B) 293 cells were incubated with H2O2 (500 μM) for the indicated times, and nuclear and cytosolic fractions were separated. Immunoblotting was performed with an antibody against hTERT, and equal loading was confirmed either with topoisomerase 1 (nuclear fractions) or HSP90 (cytosolic fractions) (n = 4). Blots were scanned and semiquantitatively analyzed (lower panels). co, control. (C) Telomerase enzyme activity was measured in nuclear (nuc) and cytosolic (cyt) fractions of cells treated with 500 μM H2O2 for 3 h (n = 3 to 4). (D) 293 cells were incubated with H2O2 for 3 h, and hTERT protein levels were measured in whole-cell lysates (n = 3). A representative Western blot is shown. Equal loading was confirmed with actin. Enzymatic activity of the samples is shown in the lower part of panel D.
Endogenous oxidative stress induces the translocation of hTERT.
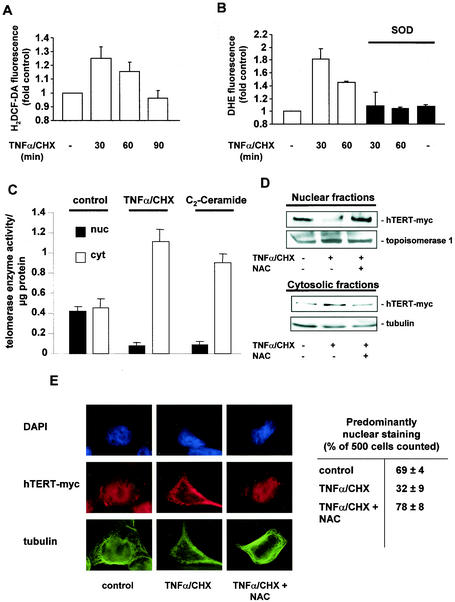
Next, we investigated whether endogenous formation of ROS is sufficient to induce translocation of hTERT. Tumor necrosis factor alpha combined with cycloheximide (TNF-α/CHX) is known to induce endogenous oxidative stress (14, 26, 62). Indeed, incubation with TNF-α/CHX time dependently increased formation of endogenous ROS, with maximum levels after 30 min as determined by FACS analysis with H2DCF-DA or DHE (Fig. 2A and B). The increase in endogenous ROS formation was blocked by polyethylene glycol-superoxide dismutase (PEG-SOD) (Fig. 2B). The resulting induction of endogenous ROS was accompanied by a translocation of telomerase enzyme activity from the nucleus into the cytosol (Fig. 2C). C2-ceramide, another stimulus known to induce endogenous oxidative stress with a maximum at 6 h in neuronal PC12 cells (14), also profoundly altered the localization of telomerase enzyme activity after 6 h of treatment (Fig. 2C).
FIG.2.
Endogenous oxidative stress induces nuclear export of hTERT. (A) 293 cells were incubated for the indicated times with 100 ng of TNF-α per ml and 10 μg of CHX per ml (TNF-α/CHX). Production of endogenous ROS was measured with H2DCF-DA by FACS analysis (n = 3). (B) After preincubation with PEG-SOD for 12 h, 293 cells were incubated for the indicated times with TNF-α/CHX (n = 3). Production of endogenous ROS was measured with DHE by FACS analysis. (C) Telomerase enzyme activity was measured in nuclear (nuc) and cytosolic (cyt) fractions of cells treated with 10 μM C2-ceramide for 6 h or TNF-α/CHX for 1 h (n = 3 to 4). (D) After preincubation with 10 mM NAC for 1 h, 293 cells overexpressing myc-tagged, full-length hTERT (hTERT-myc) were incubated with TNF-α/CHX for 1 h, and nuclear and cytosolic fractions were separated. Immunoblotting was performed with an antibody against myc, and equal loading was confirmed either with topoisomerase 1 or tubulin. (n = 3). (E) A representative immunostaining is shown for 293 cells overexpressing myc-tagged hTERTwt either untreated or treated with TNF-α/CHX for 1 h and 10 mM NAC as indicated. (Upper panels) Nuclear staining with DAPI (blue). (Middle panels) hTERT-myc staining (red). (Lower panels) Tubulin staining (green). Results are shown from five different transfected dishes. Cells with predominantly nuclear hTERT staining were counted by three independent investigators. The quantification is shown on the right side.
To further demonstrate that endogenous and overexpressed hTERT (hTERT-myc) showed similar changes in localization after ROS treatment, we overexpressed hTERT into 293 cells. Cells were then incubated with TNF-α/CHX for 30 min in the presence or absence of the antioxidant N-acetylcysteine (NAC). The localization of overexpressed hTERT was also altered from the nucleus into the cytoplasm after TNF-α/CHX treatment, as demonstrated by Western blot analysis of cytoplasmic and nuclear fractions (Fig. 2D) and immunostaining (Fig. 2E). NAC inhibited TNF-α/CHX-induced changes in cellular hTERT localization (Fig. 2D and E).
Nuclear export of hTERT occurs via the nuclear pores.
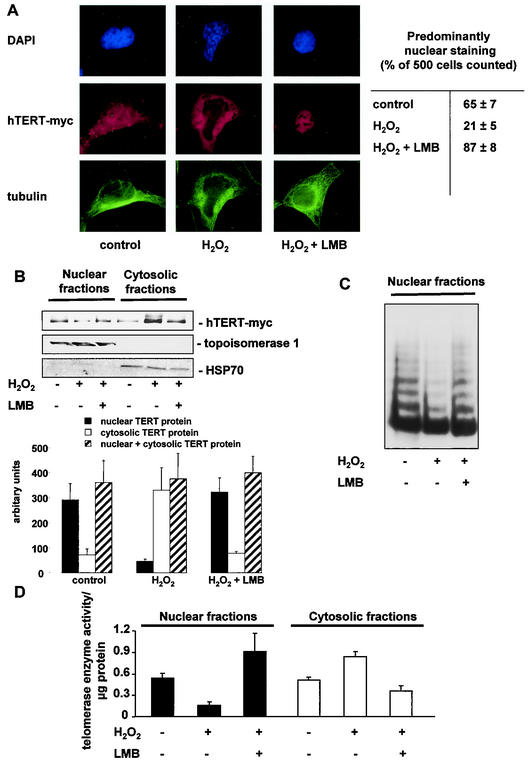
Having demonstrated that exogenous and endogenous oxidative stress causes changes in the localization of endogenous as well as overexpressed hTERT, we wanted to get insights into the underlying mechanism. Recently, it has been shown that the nuclear export receptor CRM1 can bind to TERT (53). This receptor in concert with the GTPase Ran can bind large cargo molecules and transport them through the nuclear pores into the cytosol. The best-characterized inhibitor for CRM1-Ran-dependent transport is leptomycin B (31a). Therefore, we investigated whether leptomycin B inhibits oxidative stress-induced hTERT translocation. Indeed, leptomycin B inhibited H2O2-induced hTERT export into the cytosol, as shown by immunofluorescence microscopy and immunoblotting after biochemical separation of the nuclear and cytosolic fractions (Fig. 3 A and B). Moreover, H2O2-induced translocation of telomerase enzyme activity from the nuclei into the cytosol was prevented by leptomycin B treatment (Fig. 3C and D).
FIG. 3.
Nuclear export of hTERT occurs through the nuclear pores. (A) A representative immunostaining is shown for 293 cells overexpressing full-length, myc-tagged hTERT. Cells were incubated with 500 μM H2O2 for 3 h or were preincubated with 10 ng of leptomycin B (LMB) per ml for 30 min followed by 500 μM H2O2 for 3 h. (Upper panels) Nuclear staining with DAPI (blue). (Middle panels) hTERT-myc staining (red). (Lower panels) Tubulin staining (green). Results are shown from five different transfected dishes. Cells with predominantly nuclear hTERT staining were counted by three independent investigators. The quantification is shown on the right side. (B) 293 cells overexpressing full-length, myc-tagged hTERT (hTERT-myc) were preincubated with 10 ng of LMB per ml for 30 min and incubated with H2O2 for 3 h. Immunoblotting of the nuclear and cytosolic fractions was performed with an antibody against myc (upper panel), topoisomerase 1 as a loading control for nuclear fractions (middle panel), and HSP70 for cytosolic fractions (lower panels) (n = 4). The bar graphs below show the densitometric analysis (n = 4). (C) Telomerase enzyme activity was measured in nuclear and cytosolic fractions of cells in the presence or absence of 500 μM H2O2 or 500 μM H2O2 and 10 ng of LMB per ml. A representative telomeric repeat amplification product assay is shown. (D) Telomerase enzyme activity was measured in nuclear and cytosolic fractions of cells in the presence or absence of 500 μM H2O2 or 500 μM H2O2 plus 10 ng of LMB per ml (n = 3).
Translocation of hTERT is dependent on GTPase Ran.
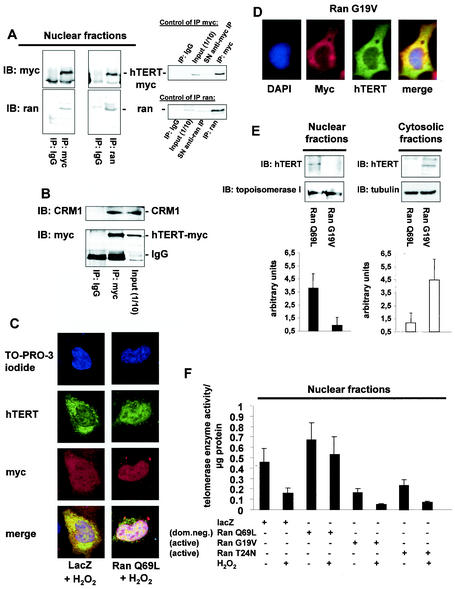
Next, we investigated the involvement of GTPase Ran and CRM1 in hTERT translocation in hTERT-overexpressing 293 cells. Coimmunoprecipitation studies using antibodies against either myc or Ran revealed that hTERT indeed associates with Ran (Fig. 4A) and CRM1 (Fig. 4B). The causal involvement of Ran in mediating the nuclear export of hTERT was investigated by overexpressing a dominant-negative GTPase-deficient mutant (Ran Q69L), which cannot hydrolyze GTP and was shown to inhibit export of proteins from the nucleus (32, 34, 52). Overexpression of Ran Q69L inhibited H2O2-induced export of hTERT, as assayed by immunofluorescence and telomerase enzyme activity measurements (Fig. 4C, E, and F). In contrast, expression of the active Ran mutants (Ran G19V and Ran T24N) induced nuclear export of hTERT already under basal conditions (Fig. 4D to F) (data not shown). These data indicate that the translocation of hTERT induced by oxidative stress from the nucleus into the cytosol is dependent on the GTPase Ran.
FIG. 4.
Nuclear export of hTERT is dependent on the GTPase Ran. (A) Lysates from 293 cells overexpressing myc-tagged, full-length hTERT were immunoprecipitated (IP) with an anti-myc antibody (left panels), an anti-Ran antibody, or an anti-mouse immunoglobulin G (IgG) antibody as indicated. Immunoblot (IB) analysis was performed against anti-Ran (lower panels) or anti-myc (upper panels) antibody. The right panels show the quality of the immunoprecipitations using an anti-myc or anti-Ran antibody. The input lane represents 1/10 of the cell lysate used for immunoprecipitation. SN, supernatant of immunoprecipitate. (B) Lysates from 293 cells overexpressing myc-tagged hTERTwt were immunoprecipitated with an antimouse IgG or an anti-myc antibody as indicated, and immunoblot analysis was performed with an anti-CRM-1 antibody (upper panel). Membranes were stripped and reprobed with an anti-myc antibody (lower panel). The input lane represents 1/10 of the cell lysate used for immunoprecipitation. (C) A representative immunostaining is shown from cells overexpressing myc-tagged LacZ or myc-tagged Ran Q69L (dominant negative) incubated with H2O2 for 3 h. Cells were stained with TO-PRO-3 iodide to visualize the nuclei (blue, upper panel) and anti-TERT antibody followed by a goat anti-rabbit FITC-conjugated antibody (green, second upper panel) and anti-myc antibody (red, second lower panel). Cells were visualized by confocal microscopy. (D) A representative immunostaining is shown from cells overexpressing myc-tagged Ran G19V (active). Cells were stained with DAPI to visualize the nuclei (blue, left panel) and anti-TERT antibody followed by a biotin-labeled secondary antibody and streptavidin-FITC (green, second left panel) and anti-myc antibody (red, second right panel). (E) Nuclear and cytosolic lysates of cells overexpressing Ran Q69L or Ran G19V were dissolved by SDS-PAGE. Immunoblotting was performed with an antibody against hTERT (upper panels), and equal loading was confirmed either with topoisomerase 1 (nuclear fractions) or tubulin (cytosolic fractions) (n = 4) (middle panels). Densitometric analysis of four independent experiments is shown (lower panels). (F) Telomerase enzyme activity was measured in nuclear lysates of cells overexpressing LacZ, Ran Q69L, Ran G19V, or Ran T24N (n = 4) incubated in the presence or absence of H2O2 for 3 h.
Nuclear export of hTERT is dependent on tyrosine phosphorylation by the Src kinase family at tyrosine 707.
Next, we investigated the signaling induced by oxidative stress, which triggers the nuclear export of hTERT. It is known that ROS induce rapid activation of the Src kinase family in different cell types (23, 56). Therefore, we first investigated hTERT tyrosine phosphorylation. Treatment with 500 μM H2O2 resulted in a time-dependent increase in hTERT tyrosine phosphorylation, with a maximum achieved after 15 min of incubation, which returned to baseline after 1 h (Fig. 5A and B) (data not shown). Of note, hTERT tyrosine phosphorylation (maximum of 15 min) preceded the translocation of hTERT, which appeared after 1 h (Fig. 1B and 3B, respectively). Since members of the Src kinase family were found to be localized in the perinuclear region and in the nucleus (49, 55, 57), we tested whether the Src kinase family is involved in hTERT tyrosine phosphorylation. Therefore, we used the specific Src kinase inhibitor PP1 (25). PP1 significantly inhibited H2O2-induced nuclear hTERT tyrosine phosphorylation (Fig. 5A and B). PP1 also inhibited the nuclear export of hTERT, detected by immunostaining and immunoblotting (Fig. 5C) (data not shown), as well as the reduction in nuclear telomerase enzyme activity induced by H2O2 after 3 h (Fig. 5D).
FIG. 5.
Src kinase family-dependent tyrosine phosphorylation plays a role in nuclear export of hTERT. (A) 293 cells overexpressing myc-tagged hTERTwt were incubated with 500 μM H2O2 in the presence or absence of 10 μM specific Src kinase family inhibitor PP1 for 15 min. Lysates of nuclear fractions were immunoprecipitated (IP) with an anti-myc antibody, and immunoblot (IB) analysis was performed with an antiphosphotyrosine antibody (upper panel). Immunoprecipitations were controlled by immunoblotting with anti-myc antibody (lower panel). A representative immunoblot is shown. The right panel shows the quality of the immunoprecipitation with an anti-myc antibody. The input lane represents 1/10 of cell lysate used for immunoprecipitation. SN, supernatant of immunoprecipitate. (B) Densitometric analysis of three independent experiments is shown. (C) 293 cells overexpressing myc-tagged hTERTwt were incubated with 500 μM H2O2 in the presence or absence of 10 μM specific Src kinase family inhibitor PP1 for 2 h. A representative immunostaining is shown. (Upper panels) Nuclear staining with DAPI (blue). (Middle panels) hTERT-myc staining (red). (Lower panels) Tubulin staining (green). Results are shown from five different transfected dishes. Cells with predominantly nuclear hTERT staining were counted by three independent investigators. The quantification is shown on the right side. (D) Telomerase enzyme activity was measured in nuclear fractions of cells treated with 500 μM H2O2 or 500 μM H2O2 and 10 μM PP1 for the indicated times. Densitometric analysis is shown (n = 3).
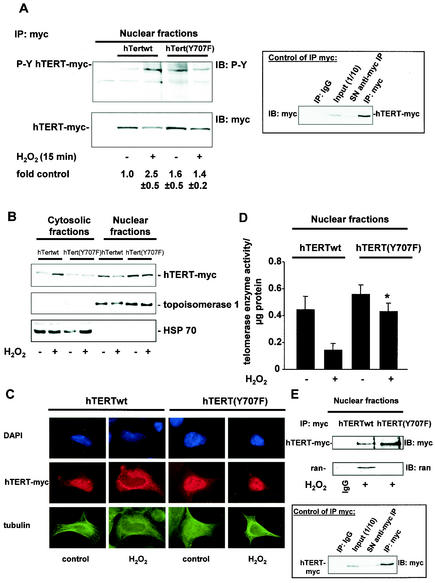
hTERT exhibits a putative Src kinase phosphorylation site at tyrosine 707. BLAST analysis revealed that this putative Src kinase phosphorylation site is conserved between Tetrahymena thermophila, Xenopus laevis, mice, and humans. Therefore, we created a hTERT mutant replacing tyrosine 707 with nonphosphorylatable phenylalanine: hTERT(Y707F). In contrast to hTERTwt, incubation with H2O2 did not induce tyrosine phosphorylation of hTERT(Y707F) (Fig. 6A). Moreover, H2O2-stimulated nuclear export of TERT(Y707F) was inhibited (Fig. 6B and C), and H2O2-induced reduction of nuclear telomerase enzyme activity was prevented in cells overexpressing hTERT(Y707F) (Fig. 6D). Since hTERT(Y707F) is not exported from the nucleus, we investigated the association of hTERT(Y707F) with Ran to determine whether tyrosine phosphorylation of 707 is the signal for complex formation. Indeed, immunoprecipitation studies revealed that, in contrast to hTERTwt, hTERT(Y707F) did not associate with Ran (Fig. 6E). Taken together, these data demonstrate that tyrosine phosphorylation of hTERT at tyrosine 707 signals oxidative stress-induced nuclear export of hTERT.
FIG. 6.
Phosphorylation at tyrosine 707 signals nuclear export of hTERT. (A) 293 cells overexpressing myc-tagged hTERTwt or hTERT(Y707F) were incubated with 500 μM H2O2 for 15 min. Lysates of nuclear fractions were immunoprecipitated (IP) with an anti-myc antibody, and immunoblot (IB) analysis was performed with an antiphosphotyrosine antibody (PY [upper panel]). Immunoprecipitations were controlled by immunoblotting with anti-myc antibody (lower panel). A representative immunoblot is shown. The right panel shows the quality of the immunoprecipitation using an anti-myc antibody. The input lane represents 1/10 of cell lysate used for immunoprecipitation. SN, supernatant of immunoprecipitate. (B) 293 cells overexpressing myc-tagged hTERTwt or hTERT(Y707F) were incubated with H2O2 for 2 h. Immunoblotting of the nuclear and cytosolic fractions was performed with an antibody against myc (upper panel), topoisomerase 1 (middle panel), and HSP70 (lower panel). Representative immunoblots are shown (n = 5). (C) 293 cells overexpressing myc-tagged hTERTwt or hTERT(Y707F) were incubated with 500 μM H2O2 for 2 h. A representative immunostaining is shown. (Upper panels) Nuclear staining with DAPI (blue). (Middle panels) hTERT-myc staining (red). (Lower panels) Tubulin staining (green). (D) 293 cells overexpressing myc-tagged hTERTwt or hTERT(Y707F) were incubated with H2O2 for 2 h, and telomerase enzyme activity in the nuclear fractions was measured. Densitometric analysis is shown (n = 3). *, P < 0.05 versus hTERTwt plus H2O2. (E) Lysates from 293 cells overexpressing myc-tagged hTERTwt or hTERT(Y707F) were incubated with H2O2 for 2 h. Cell lysates were immunoprecipitated with an anti-myc antibody, and immunoblot analysis was performed with an anti-Ran antibody (middle panel) and an anti-myc antibody (upper panel). The lower panel shows the quality of the immunoprecipitation using an anti-myc antibody. The input lane represents 1/10 of the cell lysate used for immunoprecipitation.
Nuclear localization of hTERT enhances its antiapoptotic function.
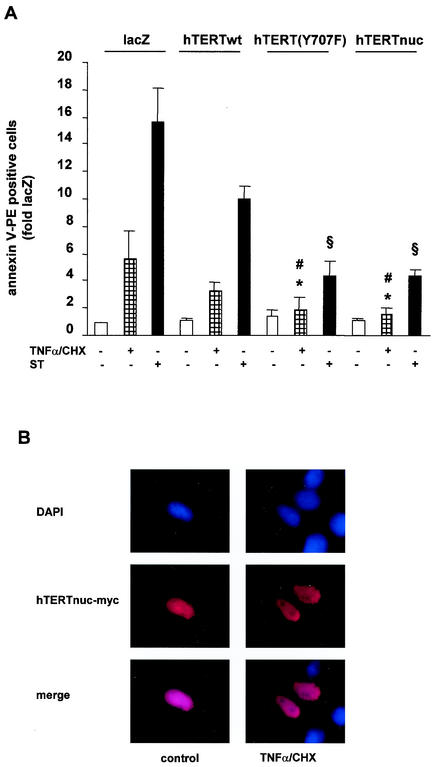
High levels of ROS are known to induce apoptosis in various cell types (44, 56). Different studies demonstrated that TERT exerts an antiapoptotic effect (28, 61). Therefore, we investigated whether nuclear localization of hTERT may enhance the antiapoptotic capacity of hTERT. Indeed, overexpression of hTERT(Y707F), which is resistant to ROS-induced nuclear export, revealed more pronounced antiapoptotic capacity than hTERTwt (Fig. 7A). To further prove the concept that nuclear localization of hTERT is important for its antiapoptotic capacity, we subcloned hTERTwt into a nuclear shooter vector, which contains three nuclear targeting sequences at the C terminus and leads to forced expression of the protein in the nucleus (13, 48). Overexpression of the nuclear targeted hTERT (hTERTnuc) also exerted an enhanced apoptosis-suppressive effect comparable to that of hTERT(Y707F) (Fig. 7A), demonstrating that nuclear localization of hTERT importantly contributes to the antiapoptotic function of hTERT. Control experiments revealed that hTERTnuc localized exclusively in the nucleus, even under treatment with apoptotic stimuli (Fig. 7B). Moreover, the enhanced antiapoptotic effect of hTERTnuc and hTERT(Y707F) was not due to increased telomerase enzyme activity, because hTERTwt, hTERTnuc, and hTERT(Y707F) revealed similar telomerase enzyme activity in whole-cell homogenates (data not shown).
FIG. 7.
Nuclear localization of hTERT enhances its antiapoptotic function. (A) 293 cells were transfected with LacZ, hTERTwt, hTERT(Y707F), or hTERTnuc and incubated with 10 μM staurosporine (ST) or 100 ng of TNF-α per ml and 10 μg of CHX per ml (TNF-α/CHX) for 3 h. Apoptosis was measured with annexin V by FACS analysis. *, P < 0.01 versus LacZ plus TNF-α/CHX; #, P < 0.05 versus hTERTwt plus TNF-α/CHX; §, P < 0.05 versus hTERTwt plus staurosporine (n = 4 to 6). (B) 293 cells were transfected with hTERTnuc and then incubated with TNF-α and cycloheximide for 3 h. A representative immunostaining is shown (n = 5). (Upper panels) Staining of nuclei with DAPI (blue). (Middle panels) hTERTnuc-myc staining (red). The lower panels show the merge.
DISCUSSION
The data from the present study demonstrate that exogenous and endogenous ROS stimulate hTERT translocation from the nucleus into the cytoplasm via the nuclear pores in a CRM1/Ran-dependent manner. Under basal conditions, 30% of the cell population showed cytosolic staining of hTERT. A reasonable explanation for this phenomenon could be that cells are in different stages of the cell cycle under basal conditions. Indeed, cell cycle stages are known to influence nucleocytoplasmic transport (37). Further studies are needed to determine the influence of cell cycle stages on hTERT translocation.
Previous studies indicated that TERT localization is a highly regulated process. Thus, TERT is translocated from cytoplasm to the nucleus after activation of T lymphocytes (36). Likewise, stimulation of smooth muscle cells with growth factors increased nuclear TERT (41). These studies suggest that proproliferative stimuli induce an increase in nuclear telomerase enzyme activity to maintain telomere length and subsequently the proliferative capacity of the cells. In contrast, the present study demonstrates that ROS induce the export of hTERT. The question remains how ROS initiate complex formation and export. Several previous studies—mainly with yeast—have shown involvement of oxidative stress in nuclear transport via the Ran GTPase. However, these studies reported that ROS induced the shuttling of proteins from the cytoplasm to the nucleus (11). One of the identified targets in mammals that is shuttled from the cytoplasm into the nucleus upon ROS formation is mitogen-activated protein kinase 1/2 (MAPK1/2) (1). We could also document that the MAPK1/2 is reduced in the cytoplasmic fraction in response to oxidative stress under conditions in which hTERT is exported from the nucleus into the cytoplasm (data not shown). Thus, nuclear export of hTERT induced by ROS appears to be mediated by a rather specific mechanism instead of a general effect of ROS on the export machinery.
We demonstrate that the nuclear export of hTERT is regulated by a Ran GTPase-dependent process via the export receptor CRM1. This is evidenced by coimmunoprecipitation of hTERT with Ran and CRM1. These data are in accordance with recent findings by Seimiya et al., who showed that different TERT mutants can be exported from the nucleus (53). Moreover, pharmacological inhibition of the CRM1-binding capacity to its export cargos with leptomycin B or overexpression of a dominant-negative Ran construct prevented ROS-induced nuclear export of hTERT. In contrast to recent findings, we were unable to detect any coimmunoprecipitation of hTERT with 14-3-3 proteins (data not shown), which led us speculate that nuclear export of hTERT induced by oxidative stress may involve a different mechanism. Moreover, it has to be noted that the association of TERT with 14-3-3 proteins seems to be independent of the phosphorylation status of TERT, which also differs from the study presented here (53).
We further identified tyrosine phosphorylation of hTERT as the signal required for nuclear export by a Ran GTPase-dependent process. Oxidative stress is well known to regulate signaling pathways by tyrosine phosphorylation (23, 56). Our data demonstrate that the putative Src phosphorylation site at position Y707 of hTERT is phosphorylated after H2O2 stimulation and that Y707 phosphorylation is required for hTERT-Ran binding. Thus, Src kinase-dependent phosphorylation of TERT mediates TERT binding to Ran in response to oxidative stress to facilitate nuclear export via the export receptor CRM1. In contrast, inhibition of the tyrosine kinase c-Abl, which was previously shown to phosphorylate and inactivate TERT (31), did not prevent H2O2-induced tyrosine phosphorylation of hTERT (data not shown). Interestingly, tyrosine phosphorylation has been described as a signal for nucleocytoplasmic shuttling for different targets, including transcription factors STAT5 and STAT1 (39, 64) and now for hTERT as reported here. Moreover, we detected localization of src in the nucleus, which further underscored the concept that hTERT tyrosine phosphorylation takes place in the nucleus in a Src kinase family-dependent manner (data not shown). Thus, tyrosine phosphorylation in concert with nuclear localization signals and nuclear export signals makes an important contribution to the cellular localization of proteins.
The activity of TERT is not limited to the extension of telomere ends but also appears to be involved in regulation of apoptosis (3, 7, 15, 27, 38, 45, 61). The molecular mechanism by which TERT regulates apoptosis is not yet clear. It has recently been shown that overexpression of hTERT into fibroblasts prevents oxidative stress-induced apoptosis, but did not inhibit oxidative stress-induced replicative senescence (19). Therefore, it is tempting to speculate that hTERT is involved in regulation of apoptosis independent of the preservation of telomere length. This assumption is supported by data from Gonzalez-Suarez, who demonstrated that in keratinocyte-targeted telomerase-transgenic mice, the epidermis is highly responsive to phorbol ester and wound healing is increased, although telomere length is not changed (18). These data suggest that telomerase actively promotes proliferation in cells independent of telomere length. Moreover, these telomerase-transgenic mice showed an increased incidence of spontaneous cancer compared to their wild-type littermates, despite the fact that both wild-type and transgenic mice have very long telomeres (17). In line with these findings, the antiapoptotic capacity of hTERT shown in this study was detected within a few hours after transfection of the hTERT constructs. Thus, hTERT seems to exert its antiapoptotic activity in the nucleus, presumably due to illegitimate healing of DNA breaks (19) and independent of direct telomere elongation (3).
Taken together, our data disclose a mechanism by which exogenous H2O2 as well as endogenous oxidative stress can induce dramatic changes in hTERT localization. Prevention of nuclear export of hTERT by using a nonphosphorylatable Y707 construct or a nuclear targeting vector significantly enhanced the antiapoptotic activity of TERT against ROS-dependent apoptosis induction. Thus, depletion of nuclear hTERT by ROS may amplify cellular sensitivity to apoptosis, irrespective of the effects on telomere length reduction and cellular senescence.
Acknowledgments
We thank Susanne Ficus and Christine Goy for expert technical assistance.
This work was supported by the Deutsche Forschungsgemeinschaft (Ha 2868/2-1 and SFB 553 Project C21B6). Jörg Hoffmann was supported by a fellowship from the Boehringer Ingelheim Fond.
REFERENCES
- 1.Adachi, M., M. Fukuda, and E. Nishida. 1999. Two co-existing mechanisms for nuclear import of MAP kinase: passive diffusion of a monomer and active transport of a dimer. EMBO J. 18:5347-5358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Blackburn, E. H. 2000. Telomere states and cell fates. Nature 408:53-56. [DOI] [PubMed] [Google Scholar]
- 3.Blasco, M. A. 2002. Telomerase beyond telomeres. Nat. Rev. Cancer 2:627-633. [DOI] [PubMed] [Google Scholar]
- 4.Bodnar, A. G., M. Ouellette, M. Frolkis, S. E. Holt, C. P. Chiu, G. B. Morin, C. B. Harley, J. W. Shay, S. Lichtsteiner, and W. E. Wright. 1998. Extension of life-span by introduction of telomerase into normal human cells. Science 279:349-352. [DOI] [PubMed] [Google Scholar]
- 5.Breitschopf, K., A. M. Zeiher, and S. Dimmeler. 2001. Proatherosclerotic factors induce telomerase inactivation in endothelial cells through an Akt-dependent mechanism. FEBS Lett. 493:21-25. [DOI] [PubMed] [Google Scholar]
- 6.Chandra, J., A. Samali, and S. Orrenius. 2000. Triggering and modulation of apoptosis by oxidative stress. Free Radic. Biol. Med. 29:323-333. [DOI] [PubMed] [Google Scholar]
- 7.Chin, L., S. E. Artandi, Q. Shen, A. Tam, S. L. Lee, G. J. Gottlieb, C. W. Greider, and R. A. DePinho. 1999. p53 deficiency rescues the adverse effects of telomere loss and cooperates with telomere dysfunction to accelerate carcinogenesis. Cell 97:527-538. [DOI] [PubMed] [Google Scholar]
- 8.Colgin, L. M., and R. R. Reddel. 1999. Telomere maintenance mechanisms and cellular immortalization. Curr. Opin. Genet. Dev. 9:97-103. [DOI] [PubMed] [Google Scholar]
- 9.Counter, C. M., W. C. Hahn, W. Wei, S. D. Caddle, R. L. Beijersbergen, P. M. Lansdorp, J. M. Sedivy, and R. A. Weinberg. 1998. Dissociation among in vitro telomerase activity, telomere maintenance, and cellular senescence. Proc. Natl. Acad. Sci. USA 95:14723-14728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.de Lange, T. 1998. Telomeres. Nature 392:753-754. [DOI] [PubMed] [Google Scholar]
- 11.Ferrigno, P., F. Posas, D. Koepp, H. Saito, and P. A. Silver. 1998. Regulated nucleo/cytoplasmic exchange of HOG1 MAPK requires the importin beta homologs NMD5 and XPO1. EMBO J. 17:5606-5614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Finkel, T., and N. J. Holbrook. 2000. Oxidants, oxidative stress and the biology of aging. Nature 408:239-247. [DOI] [PubMed] [Google Scholar]
- 13.Fischer-Fantuzzi, L., and C. Vesco. 1988. Cell-dependent efficiency of reiterated nuclear signals in a mutant simian virus 40 oncoprotein targeted to the nucleus. Mol. Cell. Biol. 8:5495-5503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.France-Lanord, V., B. Brugg, P. P. Michel, Y. Agid, and M. Ruberg. 1997. Mitochondrial free radical signal in ceramide-dependent apoptosis: a putative mechanism for neuronal death in Parkinson's disease. J. Neurochem. 69:1612-1621. [DOI] [PubMed] [Google Scholar]
- 15.Fu, W., J. G. Begley, M. W. Killen, and M. P. Mattson. 1999. Anti-apoptotic role of telomerase in pheochromocytoma cells. J. Biol. Chem. 274:7264-7271. [DOI] [PubMed] [Google Scholar]
- 16.Furumoto, K., E. Inoue, N. Nagao, E. Hiyama, and N. Miwa. 1998. Age-dependent telomere shortening is slowed down by enrichment of intracellular vitamin C via suppression of oxidative stress. Life Sci. 63:935-948. [DOI] [PubMed] [Google Scholar]
- 17.González-Suárez, E., J. M. Flores, and M. A. Blasco. 2002. Cooperation between p53 mutation and high telomerase transgenic expression in spontaneous cancer development. Mol. Cell. Biol. 22:7291-7301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gonzalez-Suarez, E., E. Samper, A. Ramirez, J. M. Flores, J. Martin-Caballero, J. L. Jorcano, and M. A. Blasco. 2001. Increased epidermal tumors and increased skin wound healing in transgenic mice overexpressing the catalytic subunit of telomerase, mTERT, in basal keratinocytes. EMBO J. 20:2619-2630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gorbunova, V., A. Seluanov, and O. Pereira-Smith. 2002. Expression of hTERT does not prevent stress-induced senescence in normal human fibroblasts, but protects the cells from stress-induced apoptosis and necrosis. J. Biol. Chem. 277:38540-38549. [DOI] [PubMed] [Google Scholar]
- 20.Greider, C. W., and E. H. Blackburn. 1985. Identification of a specific telomere terminal transferase activity in Tetrahymena extracts. Cell 43:405-413. [DOI] [PubMed] [Google Scholar]
- 21.Greider, C. W., and E. H. Blackburn. 1989. A telomeric sequence in the RNA of Tetrahymena telomerase required for telomere repeat synthesis. Nature 337:331-337. [DOI] [PubMed] [Google Scholar]
- 22.Griendling, K. K., and D. G. Harrison. 1999. Dual role of reactive oxygen species in vascular growth. Circ. Res. 85:562-563. [DOI] [PubMed] [Google Scholar]
- 23.Griendling, K. K., D. Sorescu, B. Lassegue, and M. Ushio-Fukai. 2000. Modulation of protein kinase activity and gene expression by reactive oxygen species and their role in vascular physiology and pathophysiology. Arterioscler. Thromb. Vasc. Biol. 20:2175-2183. [DOI] [PubMed] [Google Scholar]
- 24.Haendeler, J., M. Ishida, L. Hunyady, and B. C. Berk. 2000. The third cytoplasmic loop of the angiotensin II type 1 receptor exerts differential effects on extracellular signal-regulated kinase (ERK1/ERK2) and apoptosis via Ras- and Rap1-dependent pathways. Circ. Res. 86:729-736. [DOI] [PubMed] [Google Scholar]
- 25.Hanke, J. H., J. P. Gardner, R. L. Dow, P. S. Changelian, W. H. Brissette, E. J. Weringer, B. A. Pollok, and P. A. Connelly. 1996. Discovery of a novel, potent, and Src family-selective tyrosine kinase inhibitor. Study of Lck- and FynT-dependent T cell activation. J. Biol. Chem. 271:695-701. [DOI] [PubMed] [Google Scholar]
- 26.Heller, R. A., and M. Kronke. 1994. Tumor necrosis factor receptor-mediated signaling pathways. J. Cell Biol. 126:5-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hemann, M. T., K. L. Rudolph, M. A. Strong, R. A. DePinho, L. Chin, and C. W. Greider. 2001. Telomere dysfunction triggers developmentally regulated germ cell apoptosis. Mol. Biol. Cell 12:2023-2030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hemann, M. T., M. A. Strong, L. Y. Hao, and C. W. Greider. 2001. The shortest telomere, not average telomere length, is critical for cell viability and chromosome stability. Cell 107:67-77. [DOI] [PubMed] [Google Scholar]
- 29.Holt, S. E., D. L. Aisner, J. Baur, V. M. Tesmer, M. Dy, M. Ouellette, J. B. Trager, G. B. Morin, D. O. Toft, J. W. Shay, W. E. Wright, and M. A. White. 1999. Functional requirement of p23 and Hsp90 in telomerase complex. Genes Dev. 13:817-826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kang, S. S., T. Kwon, D. Y. Kwon, and S. I. Do. 1999. Akt protein kinase enhances human telomerase activity through phosphorylation of telomerase reverse transcriptase subunit. J. Biol. Chem. 274:13085-13090. [DOI] [PubMed] [Google Scholar]
- 31.Kharbanda, S., V. Kumar, S. Dhar, P. Pandey, C. Chen, P. Majumder, Z. M. Yuan, Y. Whang, W. Strauss, T. K. Pandita, D. Weaver, and D. Kufe. 2000. Regulation of the hTERT telomerase catalytic subunit by the c-Abl tyrosine kinase. Curr. Biol. 10:568-575. [DOI] [PubMed] [Google Scholar]
- 31a.Kudo, N., N. Matsumori, H. Taoka, D. Fujiwara, E. P. Schreiner, B. Wolff, M. Yoshida, and S. Horinouchi. 1999. Leptomycin B inactivates CRM/exportin 1 by covalent modification at a cysteine residue in the central conserved region. Proc. Natl. Acad. Sci. USA 96:9112-9117. [DOI] [PMC free article] [PubMed]
- 32.Lee, S.-H., and M. Hannink. 2001. The N-terminal nuclear export sequence of IkBalpha is required for RanGTP-dependent binding to CRM-1. J. Biol. Chem. 276:23599-23606. [DOI] [PubMed] [Google Scholar]
- 33.Li, H., L. Zhao, Z. Yang, J. W. Funder, and J. P. Liu. 1998. Telomerase is controlled by protein kinase Calpha in human breast cancer cells. J. Biol. Chem. 273:33436-33442. [DOI] [PubMed] [Google Scholar]
- 34.Lindsay, M. E., J. M. Holaska, K. Welch, B. M. Paschal, and I. G. Macara. 2001. Ran-binding protein 3 is a cofactor for CRM1-mediated nuclear protein export. J. Cell. Biol. 153:1391-1402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Liu, J. P. 1999. Studies of the molecular mechanisms in the regulation of telomerase activity. FASEB J. 13:2091-2104. [DOI] [PubMed] [Google Scholar]
- 36.Liu, K., R. J. Hodes, and N.-P. Weng. 2001. Telomerase activation in human T lymphocytes does not require increase in telomerase reverse transcriptase (hTERT) protein but is associated with hTERT phosphorylation and nuclear translocation. J. Immunol. 166:4826-4830. [DOI] [PubMed] [Google Scholar]
- 37.Macara, I. G. 2001. Transport into and out of the nucleus. Microbiol. Mol. Biol. Rev. 65:570-594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mattson, M. P., and W. Klapper. 2001. Emerging roles for telomerase in neuronal development and apoptosis. J. Neurosci. Res. 63:1-9. [DOI] [PubMed] [Google Scholar]
- 39.McBride, K. M., G. Banninger, C. McDonald, and N. C. Reich. 2002. Regulated nuclear import of STAT1 transcription factor by direct binding of importin-alpha. EMBO J. 21:1754-1763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Meyerson, M., C. M. Counter, E. N. Eaton, L. W. Ellisen, P. Steiner, S. D. Caddle, L. Ziaugra, R. L. Beijersbergen, M. J. Davidoff, Q. Liu, S. Bacchetti, D. A. Haber, and R. A. Weinberg. 1997. hEST2, the putative human telomerase catalytic subunit gene, is up-regulated in tumor cells and during immortalization. Cell 90:785-795. [DOI] [PubMed] [Google Scholar]
- 41.Minamino, T., and S. Kourembanas. 2001. Mechanisms of telomerase induction during vascular smooth muscle cell proliferation. Circ. Res. 89:237-243. [DOI] [PubMed] [Google Scholar]
- 42.Nakamura, T. M., and T. R. Cech. 1998. Reversing time: origin of telomerase. Cell 92:587-590. [DOI] [PubMed] [Google Scholar]
- 43.Nakielny, S., and G. Dreyfuss. 1999. Transport of proteins and RNAs in and out the nucleus. Cell 99:677-690. [DOI] [PubMed] [Google Scholar]
- 44.Nordberg, J., and E. S. Arner. 2001. Reactive oxygen species, antioxidants, and the mammalian thioredoxin system. Free Radic. Biol. Med. 31:1287-1312. [DOI] [PubMed] [Google Scholar]
- 45.Oh, H., G. E. Taffet, K. A. Youker, M. L. Entman, P. A. Overbeek, L. H. Michael, and M. D. Schneider. 2001. Telomerase reverse transcriptase promotes cardiac muscle cell proliferation, hypertrophy, and survival. Proc. Natl. Acad. Sci. USA 98:10308-10313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Paine, P. L., L. C. Moore, and S. B. Horowitz. 1975. Nuclear envelope permeability. Nature 254:109-114. [DOI] [PubMed] [Google Scholar]
- 47.Parman, T., M. J. Wiley, and P. G. Wells. 1999. Free radical-mediated oxidative DNA damage in the mechanism of thalidomide teratogenicity. Nat. Med. 5:582-585. [DOI] [PubMed] [Google Scholar]
- 48.Persic, L., M. Righi, A. Roberts, H. R. Hoogenboom, A. Cattaneo, and A. Bradbury. 1997. Targeting vectors for intracellular immunisation. Gene 187:1-8. [DOI] [PubMed] [Google Scholar]
- 49.Rongish, B. J., and W. H. Kinsey. 2000. Transient nuclear localization of Fyn kinase during development in zebrafish. Anat. Rec. 260:115-123. [DOI] [PubMed] [Google Scholar]
- 50.Rudolph, K. L., S. Chang, M. Millard, N. Schreiber-Agus, and R. A. DePinho. 2000. Inhibition of experimental liver cirrhosis in mice by telomerase gene delivery. Science 287:1253-1258. [DOI] [PubMed] [Google Scholar]
- 51.Ryan, K. J., and S. R. Wente. 2000. The nuclear pore complex: a protein machine bridging the nucleus and cytoplasm. Curr. Opin. Cell Biol. 12:361-371. [DOI] [PubMed] [Google Scholar]
- 52.Sachdev, S., S. Bagchi, D. D. Zhang, A. C. Mings, and M. Hannink. 2000. Nuclear import of IkBα is accomplished by a Ran-independent transport pathway. Mol. Cell. Biol. 20:1571-1582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Seimiya, H., H. Sawada, Y. Muramatsu, M. Shimizu, K. Ohko, K. Yamane, and T. Tsuruo. 2000. Involvement of 14-3-3 proteins in nuclear localization of telomerase. EMBO J. 19:2652-2661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Seimiya, H., M. Tanji, T. Oh-Hara, A. Tomida, I. Naasani, and T. Tsuruo. 1999. Hypoxia up-regulates telomerase activity via mitogen-activated protein kinase signaling in human solid tumor cells. Biochem. Biophys. Res. Commun. 260:365-370. [DOI] [PubMed] [Google Scholar]
- 55.Sloan-Lancaster, J., W. Zhang, J. Presley, B. L. Williams, R. T. Abraham, J. Lippincott-Schwartz, and L. E. Samelson. 1997. Regulation of ZAP-70 intracellular localization: visualization with the green fluorescent protein. J. Exp. Med. 186:1713-1724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Suzuki, Y. J., H. J. Forman, and A. Sevanian. 1997. Oxidants as stimulators of signal transduction. Free Radic. Biol. Med. 22:269-285. [DOI] [PubMed] [Google Scholar]
- 57.Uetz, P., S. Fumagalli, D. James, and R. Zeller. 1996. Molecular interaction between limb deformity proteins (formins) and Src family kinases. J. Biol. Chem. 271:33525-33530. [DOI] [PubMed] [Google Scholar]
- 58.Vasa, M., K. Breitschopf, A. M. Zeiher, and S. Dimmeler. 2000. Nitric oxide activates telomerase and delays endothelial cell senescence. Circ. Res. 87:540-542. [DOI] [PubMed] [Google Scholar]
- 59.White, C. R., T. A. Brock, L. Y. Chang, J. Crapo, P. Briscoe, D. Ku, W. A. Bradley, S. H. Gianturco, J. Gore, B. A. Freeman et al. 1994. Superoxide and peroxynitrite in atherosclerosis. Proc. Natl. Acad. Sci. USA 91:1044-1048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Wong, J. M., L. Kusdra, and K. Collins. 2002. Subnuclear shuttling of human telomerase induced by transformation and DNA damage. Nat. Cell Biol. 4:731-736. [DOI] [PubMed] [Google Scholar]
- 61.Wong, K. K., S. Chang, S. R. Weiler, S. Ganesan, J. Chaudhuri, C. Zhu, S. E. Artandi, K. L. Rudolph, G. J. Gottlieb, L. Chin, F. W. Alt, and R. A. DePinho. 2000. Telomere dysfunction impairs DNA repair and enhances sensitivity to ionizing radiation. Nat. Genet. 26:85-88. [DOI] [PubMed] [Google Scholar]
- 62.Woo, C. H., Y. W. Eom, M. H. Yoo, H. J. You, H. J. Han, W. K. Song, Y. J. Yoo, J. S. Chun, and J. H. Kim. 2000. Tumor necrosis factor-alpha generates reactive oxygen species via a cytosolic phospholipase A2-linked cascade. J. Biol. Chem. 275:32357-32362. [DOI] [PubMed] [Google Scholar]
- 63.Yang, J., E. Chang, A. M. Cherry, C. D. Bangs, Y. Oei, A. Bodnar, A. Bronstein, C. P. Chiu, and G. S. Herron. 1999. Human endothelial cell life extension by telomerase expression. J. Biol. Chem. 274:26141-26148. [DOI] [PubMed] [Google Scholar]
- 64.Zeng, R., Y. Aoki, M. Yoshida, K. Arai, and S. Watanabe. 2002. Stat5B shuttles between cytoplasm and nucleus in a cytokine-dependent and -independent manner. J. Immunol. 168:4567-4575. [DOI] [PubMed] [Google Scholar]
- 65.Zhu, H., W. Fu, and M. P. Mattson. 2000. The catalytic subunit of telomerase protects neurons against amyloid beta-peptide-induced apoptosis. J. Neurochem. 75:117-124. [DOI] [PubMed] [Google Scholar]