Abstract
Programmed capillary regression is essential for development, but little is known about the mechanism behind this phenomenon. In this study, we characterized the molecular determinants of capillary regression utilizing the pupillary membrane (PM) in the newborn rat's eye. We observed in the 1-day-culture system that apoptotic endothelial cells decrease in number with the addition of a natural antagonist, Noggin, strongly suggesting the involvement of the bone morphogenetic protein (BMP) family in PM regression. In addition, the lens-conditioned medium (Lens-CM) induced apoptosis of HUVE cells and inhibited endothelial tubulogenesis, which were completely blocked by both Noggin and the BMP4-specific neutralizing antibody. Activation of BMP4 pathway in endothelial cells was confirmed by both the up-regulation of Msx genes correlated with apoptosis and the translocation of Smad1 into the nucleus. We showed a transient expression of BMP4 in Lens-CM by immunoprecipitation assay. Furthermore, the transcorneal injection of BMP4 in rats enhanced the apoptosis of PMs, while that of Noggin attenuated it. These results indicate that BMP4 pathways play pivotal roles in capillary regression in a paracrine manner between lens and PMs.
The development of the vasculature, which serves as a nutrient and waste pipeline, is a fundamental requirement for organ development and differentiation during embryogenesis. The plasticity of the vasculature is evident during ontogeny and is maintained under physiological conditions. Microvascular remodeling, including the growth of new vessels and regression of others, is a complex process that involves endothelial cell growth and death, and plays a major role in the early development of the vascular system (12, 29, 40). Indeed, recent studies indicate that the balance between pro-apoptotic and antiapoptotic signals determines the vasculature: in addition to endothelial cell proliferation, endothelial cell apoptosis is tightly associated with the vascular regression. To date, several hypothetical mechanisms of vascular regression have been proposed, based on findings on the change of blood flow distribution, vascular obstruction and physical vascular stretching. Hence, it is important to reveal the molecular mechanisms underlying vascular regression, although the complicated processes of vascular remodeling remain to be elucidated.
In the mammalian eye, hyaloid vessels and the pupillary membrane (PM), a temporary capillary network in the anterior chamber of the lens and iris diaphragm, nourish the immature lens, retina, and vitreous body during morphogenesis. In rodents, the PM regresses during the 2nd week after birth presumably as an adaptation to allow efficient light transmission to the retina (29). This phenomenon is one example of the regression of a capillary network in a developmentally programmed manner. Here we characterized the molecular determinants of capillary network regression utilizing newborn rat PMs as a model system.
Bone morphogenetic proteins (BMPs), members of a rapidly expanding subclass of the transforming growth factor β (TGF-β) superfamily, are involved in the proliferation and differentiation of many different tissues and organs (19, 20, 36). Several lines of evidence suggest that the activity of BMPs is associated with developmentally regulated apoptosis (5, 10). In particular, BMP4 mediates apoptosis in the prospective neural crest cells (15), in the dorsal portion of the chick optic cup during morphogenesis of the eye (44) and in the interdigital space of the developing limbs in birds (11, 32, 48). Furthermore, BMP4 has crucial roles for optic development, especially for the lens induction process in mice (9). Recently, a group of proteins, the Smads, have been identified as important transducers of the TGF-β signal in a variety of species. BMP4 stimulates the phosphorylation and translocation to the nucleus of Smad1, where it regulates the transcription of target genes such as homeobox genes encoding Msx1 and Msx2 (30, 46). Localization and expression data suggest that Msx1 and Msx2 are the downstream effectors of the BMP4 signaling pathway in various developing systems, including mouse toothbuds, chick hindbrain, and spinal cord (15, 45). In addition, expression of BMP4 led to an expansion of Msx1/2 preceding apoptosis (8, 34). Therefore, it seems that BMP4 and Msx genes are generally involved in morphogenesis, cell differentiation, and also induction of apoptosis (5, 8, 15, 48).
In this work, we describe a reciprocal interaction between the lens and PMs important for triggering the regression of capillary endothelial cells in PMs. The conditioned medium of the lens (obtained from the PM regression phase) induced both apoptosis of endothelial cells and regression of tubules, which was completely suppressed by Noggin, a factor known to inhibit the functions of BMP2, -4, and -7 by binding directly to BMPs (4). Indeed, a significant amount of BMP4 was transiently secreted from the lens. The transcorneal injection of BMP4 in vivo further confirmed the importance of BMP4 in promoting apoptosis in PMs. Overall, the results presented here provide strong evidence that BMP4 secreted from the lens contributes to apoptosis in a paracrine fashion during capillary regression in PMs.
MATERIALS AND METHODS
Materials.
The purified recombinant mouse Noggin/fc Chimera, recombinant human BMP2, BMP4, and BMP7 and recombinant human vascular endothelial growth factor (VEGF) were obtained from R&D Systems (Minneapolis, Minn.). Antibodies used are as follows. Anti-human BMP2 antibody and anti-human BMP4 antibody (the BMP4-specific neutralizing antibody) were purchased from R&D Systems. The mature human and rat forms of BMP4 are 98.3% identical at the amino acid level. Anti-BMP4 (N-16) antibody (sc-6896) and anti-BMP7 (N-19) antibody (sc-6899) were from Santa Cruz Biotechnology, Inc. (Santa Cruz, Calif.), and anti-human MADR1/Smad1 antibody was from Upstate Biotechnology, Inc. (Lake Placid, N.Y.). Anti-phospho Smad1/5/8 antibody and anti-cleaved caspase-3 antibody were from Cell Signaling (Beverly, Mass.), and horseradish peroxidase (HRP)-conjugated anti-mouse immunoglobulin G (IgG) antibody and HRP-conjugated anti-rabbit IgG antibody were from Amersham Pharmacia (Piscataway, N.J.).
Animals.
Male F344/N Slc rats were obtained from SLC Inc. (Hamamatsu, Japan). Ages of rats are noted as postnatal days (e.g., day 8).
Cell culture.
Primary cultures of human umbilical vein endothelial (HUVE) cells were purchased from Morinaga (Yokohama, Japan) and maintained in a complete medium containing 5% (vol/vol) fetal bovine serum (FBS) and growth factors (Nissui, Tokyo, Japan) according to the manufacturer's instructions.
One-day-organ culture.
The technique for dissection of the rat PM was performed essentially as described by Lang et al. (29). The tissue was transferred onto the center of a Millipore Millicell-CM culture insert (Millipore Corp., Bedford, Mass.) containing M199 (Nissui) plus 5% (vol/vol) FBS and maintained at 37°C in 5% CO2 for 24 h.
Conditioned medium.
Organs were rinsed with phosphate-buffered saline (PBS), and cultured on a Millipore Millicell-CM culture insert (Millipore Corp.) for 2 days at 37°C in 5% CO2 with M199 medium alone. The incubation medium was collected and centrifuged (15,000 × g) for 20 min at 4°C, filtered through a 0.22-μm-pore-size filter, and frozen at −80°C. For each experiment, aliquots of CM were diluted 1:2.
Reverse transcription PCR.
Total RNA from various rat tissues or HUVE cells was converted to cDNA with an M-MLV reverse transcriptase (Life Technologies, Inc., Gaithersburg, Md.) and random hexamers according to the manufacturer's instructions. Aliquots of the cDNAs were used for PCR amplification with specific primers. The PCR products were separated on 2% agarose gels and stained with ethidium bromide.
Primer pairs were designed as follows: rat glyceraldehyde-3-phosphate dehydrogenase (GAPDH) (5′-AGTTCAACGGCACAGTCAAG-3′, 5′-TCAGCTCTGGGATGACCTTG-3′); human GAPDH (5′-TCACCATCTTCCAGGAGCGAGA-3′, 5′-GGGTGTCGCTGTTGAAGTCAGA-3′); rat VEGF (5′-GCACCCACGACAGAAGGGGA-3′, 5′-TCACCGCCTTGGCTTGTCACAT-3′); rat flk1 (5′-CAGAAAAGGAGATGCCCGAC-3′, 5′-TCCAGAGTTTTCAGCTCTTC-3′); rat flt1 (5′-GACTTCTTCTGTCTCAACAAGGAT-3′, 5′-TTGAAGCAGGTCACCGAGCTTCTC-3′); rat sflt1 (5′-GGTTAGCACATTGGTGGTGGC-3′, 5′-TGTGTGGTACAATCATTCCTCC-3′); rat Ang1 (5′-GAAAATTATACTCAGTGGCTGGAAA-3′, 5′-TTCTAGGATTTTATGCTCTAATAAA-3′); rat Ang2 (5′-AAAGAGTACAAAGAGGGCTTCGGGA-3′, 5′-GTAGTACCACTTGATACCGTTGAAC-3′); rat Tie1 (5′-ACGAGCACCACCGTCCGTGG-3′, 5′-CTCCAGCACAGGGTAACTCA-3′); rat Tie2 (5′-TGACGTGAAGATCAAGAATG-3′, 5′-TTGGCCTTCCTGTTTAGGGC-3′); rat ALK6 (5′-CATTTGGCGCTGAGCTATGAC-3′, 5′-GACATCCAGAGGTGACAACAG-3′); Smad5 (5′-CAACACAGCCTTCTGGTTCA-3′, 5′-TTGACAACAAACCCAAGCAG-3′); rat Smad6 (5′-CCGGGCGGCTGCGTGCTGGTGCC-3′, 5′-GGTTGAGGGAGTAGTGACGAGG-3′); rat Smad7 (5′-ATGTTCAGGACCAAACGATC-3′, 5′-GACCCGGGCCCCCGCGGGCG-3′); Msx1 (5′-CCCCGCTTCTCCCCGCC-3′, 5′-GCGGCCATCTTCAGCTTCTCCAG-3′); Msx2 (5′-ATGAGCCCTACCACCTGCACC-3′, 5′-TTAGGACAGGTGGTACATGCC-3′). Primers for rat Bcl-X, rat Smad1, rat BMP2, rat BMP4, rat BMP7, rat ALK3, and rat BMPR II were synthesized according to the sequences previously reported (6, 16, 42).
Immunohistochemistry.
Cells were fixed with 3.7% paraformaldehyde in PBS, permeabilized with 0.1% (vol/vol) Triton X-100, and incubated with the specific antibodies overnight at 4°C. For the anti-Smad1 antibody, specimens were incubated with a biotinylated goat anti-rabbit IgG (Vector Laboratories, Inc.) followed by an HRP-conjugated streptavidin (Dakopatts, Glostrup, Denmark), and visualized by incubation with peroxide substrate, 3-amino-9-ethyl carbazol (AEC) solution (Vector Laboratories, Inc.). For the anti-cleaved caspase-3 antibody and the anti-phospho Smad1/5/8 antibody, a rhodamine- or a fluorescein isothiocyanate-conjugated goat anti-rabbit IgG (ICN Pharmaceuticals, Inc., Aurora, Ohio) was used as secondary antibody.
Assessment of apoptosis.
Apoptotic cells were detected using a terminal deoxynucleotidyltransferase-mediated dUTP-biotin nick end labeling (TUNEL) kit (In situ cell death detection kit [fluorescein], Roche Diagnostics Co.) according to the manufacturer's instructions. To assess the nuclear morphology of the cells, the samples were stained with Hoechst 33258 (Wako, Tokyo, Japan). The specimens were then observed under a fluorescence microscope.
Cell fractionation.
Cells were rinsed with ice-cold PBS, suspended in ice-cold buffer A (20 mM HEPES-NaOH [pH 7.3], 10 mM MgCl2, 1 mM EDTA, 1 mM EGTA, 10 mM NaF, 1 mM Na3VO4, 25 mM β-glycerophosphate, aprotinin [20 μg/ml], leupeptin [10 μg/ml]) and homogenized with a Dounce homogenizer. After centrifugation (100 × g) for 10 min at 4°C, the supernatant was further centrifuged (100,000 × g) for 60 min at 4°C to obtain the cytoplasmic fraction. The pellet from the first centrifugation was washed with buffer A and resuspended in buffer A containing 1% (vol/vol) Triton X-100. After centrifugation (100,000 × g) for 30 min at 4°C, the supernatant was referred to as the nuclear fraction.
Immunoprecipitation and Immunoblotting.
Cells were dissolved in IP buffer (20 mM Tris-HCl [pH 7.5], 250 mM NaCl, 3 mM EDTA, 3 mM EGTA, 0.5% [vol/vol] Nonidet P-40, 1 mM phenylmethylsulfonyl fluoride, 0.1 mM Na3VO4, 3 mM β-glycerophosphate, leupeptin [10 μg/ml]), and the supernatant after centrifugation (15,000 × g) for 10 min at 4°C was used as a cell lysate. The anti-BMP4 antibody (R&D Systems) was conjugated to protein A-Sepharose (Amersham Pharmacia). The lysates were mixed gently with the antibody/protein A-Sepharose complex for 16 h at 4°C, and the precipitates were washed four times with IP buffer. The precipitated proteins were subsequently separated by SDS-PAGE, and transferred onto a polyvinylidene difluoride membrane (Immobilon; Millipore Corp.). The membrane was stained with the specific antibodies, followed by visualization with enhanced chemiluminescence detection reagents (Amersham Pharmacia).
Angiogenesis by HUVE cells.
An angiogenesis assay kit obtained from Kurabo (Osaka, Japan) was used according to the manufacturer's instructions with minor modifications. After 12 days, the cells were fixed at room temperature with 3.7% paraformaldehyde in PBS and permeabilized with 0.1% (vol/vol) Triton X-100 for 5 min. The cells were incubated with the anti-human CD31 antibody for 1 h at 37°C, and further with an alkaline phosphatase-conjugated goat anti-mouse IgG antibody. Visualization was achieved with 5-bromo-4-chloro-3-indolyl phosphate-nitroblue tetrazolium. Tubules were analyzed under a bright-field microscope and the total length in a fixed area (millimeters per square millimeter) of 20 randomly chosen fields per well was calculated.
To assess the apoptotic HUVE cells in tubules, the cells were double-stained with the anti-human CD31 antibody and Hoechst 33258, and both CD31+ cells and CD31+ apoptotic cells were counted in 25 different fields. The percentage of apoptotic HUVE cells was expressed as a ratio of the number of CD31+ apoptotic cells to the total number of CD31+ cells.
Transcorneal injection.
The transcorneal injection was performed essentially as described by Diez-Roux et al. (7) with modifications. The reagents injected were dissolved in PBS with 0.1% (wt/vol) bovine serum albumin (BSA). Since only one eye of each animal was injected reagents, the contralateral eye was used as an internal control. For control injections, 0.1% BSA solution in PBS was used.
RESULTS
Apoptosis of endothelial cells during rat PM regression via a VEGF-independent mechanism.
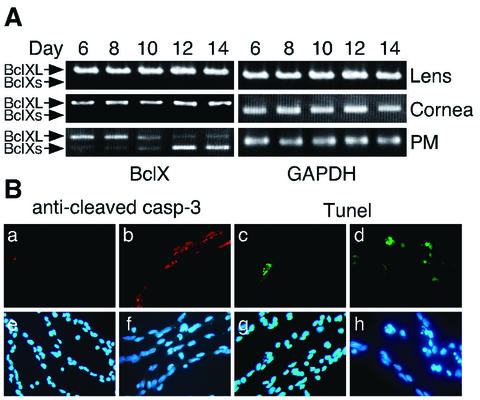
To investigate the mechanism of capillary regression, we dissected and carried out a RT-PCR analysis of rat PMs daily. Bcl-X is known to play an important role in the regulation of developmental and tissue-specific programmed cell death (2, 14), and two different Bcl-X mRNA species, antiapoptotic Bcl-XL (long form) and proapoptotic Bcl-Xs (short form), have been identified. Therefore, we assessed the relative expression of Bcl-Xs over Bcl-XL in rat PMs from postnatal days 6 to 14 by competitive RT-PCR (Fig. 1A). The expression level of Bcl-Xs increased from day 8 and reached a peak around day 14, when the capillary network in the PM had mostly disappeared. These results are consistent with the previous observations that the PM normally starts to regress between postnatal days 5 and 7 in the rats (7). On the other hand, the Bcl-Xs form was not observed either in the lens or cornea at any stages examined.
FIG. 1.
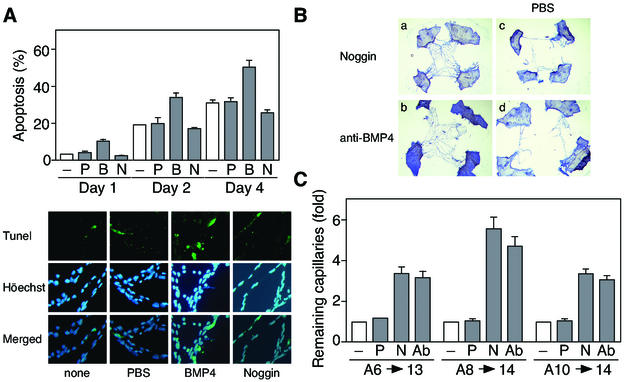
Time course of the rat PM regression. (A) The relative amounts of Bcl-XL (upper bands) and Bcl-Xs (lower bands) mRNAs at different stages in the lens, cornea and PM as determined by competitive RT-PCR (See Materials and methods). The PCR products obtained using Bcl-X-specific primers were separated on 2% agarose gels and stained with ethidium bromide. The results of GAPDH amplification confirm both the quality and quantity of RNA analyzed. (B) Apoptosis, detected by immmunostaining with the anti-cleaved caspase 3 antibody (a and b) or TUNEL (c and d) in a PM from day 8 (a, c, e, and g) or day 12 (b, d, f, and h) rats. The same specimens were counterstained with Hoechst 33258 (e to h). In both experiments, the results indicated are representative of several experiments.
In addition, using TUNEL and immunohistochemical analysis with the anti-cleaved caspase 3 antibody, positive staining in PMs was frequently observed from day 8 (Fig. 1B). Thus, we confirmed that the PM capillary endothelial cells gradually disappeared via the process of apoptosis.
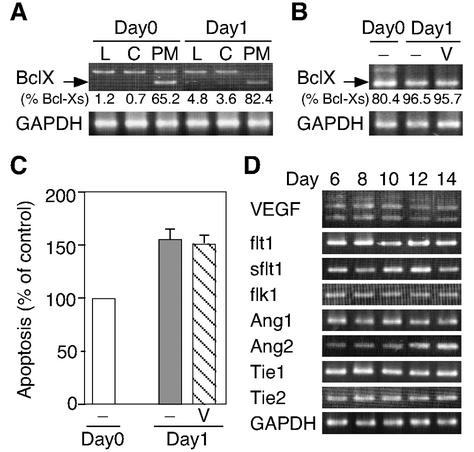
Meesson et al. (35) proposed that VEGF supplied by blood flow is necessary for the survival of endothelial cells in PMs. To examine this possibility, we established a 1-day-organ-culture system. In this system, the expression pattern of Bcl-Xs (the ratio of Bcl-Xs to total Bcl-X) revealed that apoptosis occurred specifically in the PM, not in the lens or cornea, similar to the case in vivo (Fig. 2A). To investigate whether VEGF was necessary for endothelial cell survival, PMs were explanted with or without VEGF in the 1-day-culture system. To our surprise, as shown in Fig. 2B and C, VEGF failed to suppress the apoptosis in the PMs. Furthermore, the expression levels of VEGF and its receptors involved in angiogenesis showed no marked change throughout the regression phase. The transcript of sflt1, a physiological negative regulator of VEGF, was only slightly increased (Fig. 2D). In contrast, angiopoietin 2 (Ang2) was up-regulated as shown in a previous report (33). Therefore, some signal factor(s) other than VEGF may regulate this apoptotic process.
FIG. 2.
VEGF fails to inhibit apoptosis in the PMs of day 8 rats in a 1-day-culture system. (A) Apoptosis is specific to the PM in 1-day culture, detected by RT-PCR analysis of the Bcl-XL and Bcl-Xs expression profile. Amplifications were performed with Bcl-X-specific primers on templates from the lens (L), cornea (C) and PM of day 8 rats (Day0) or their 1-day culture (Day1) as described for Fig. 1A. The data shown are representative of several experiments. An arrow indicates the bands due to BclXs mRNAs and the upper bands are due to Bcl-XL. The ratio of Bcl-Xs/total Bcl-X (%) was calculated by densitometric analysis. (B) Effect of VEGF on apoptosis of PM cells in the 1-day culture system. PMs of day 8 rats were cultured with (V) or without (−) VEGF (50 ng/ml) for 24 h at 37°C. RT-PCR analysis was performed as described for panel A. The results are representative of several experiments. An arrow indicates the bands due to BclXs mRNAs. The ratio of Bcl-Xs/total Bcl-X (percent) was calculated by densitometric analysis. (C) Quantification of the apoptotic index in PMs under the conditions indicated. Relative values compared with the apoptotic index for Day0 (100%) are expressed as the mean ± standard error (n = 5). (D) Developmental expression of various mRNAs involved in angiogenesis. RT-PCR analysis was performed as described for Fig. 1A. Representative data from several independent experiments are shown.
Lens-CM contains apoptotic factors for the rat PM.
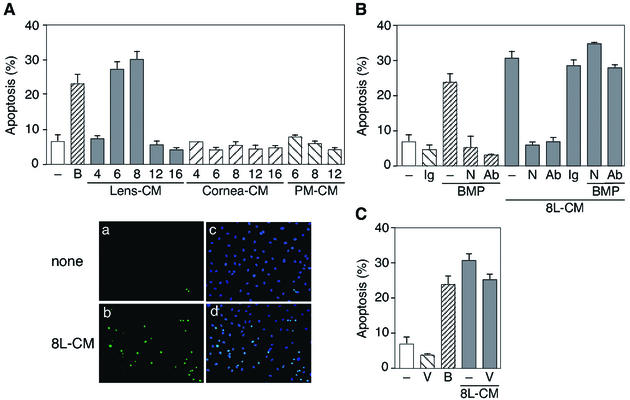
During vertebrate development, apoptosis can result from changes in the extracellular environment (43). It is well known that members of the TGF-β superfamily, especially BMPs, can serve as morphogens, playing fundamental roles governing morphogenetic processes (17, 38, 39). Since morphogens (e.g., BMP4) are secreted molecules, we focused on the role of the lens interacting with PMs in the process of PM regression. To test the hypothesis that the capillary apoptosis in the PM is induced by death factors secreted from the lens, we examined whether the lens-conditioned medium (Lens-CM) induces the apoptosis of HUVE cells. Figure 3A shows that only Lens-CM, especially in day 6 and day 8 rats, significantly increased the levels of apoptosis over control cultures. Recombinant BMP4 gave a similar result. In addition, Lens-CM was found to induce the apoptosis of other endothelial cells, but not of fibroblast cells (data not shown). To address the possible role of BMPs during PM regression, we investigated the effect of Noggin on this apoptosis. Noggin and the neutralizing antibody to BMP4 strongly inhibited the Lens-CM-mediated apoptosis of HUVE cells, which was reversed by adding an excess of recombinant BMP4 (Fig. 3B), suggesting that BMPs (especially BMP4) are good candidate molecules for Lens-CM-induced apoptosis. Consistent with the idea that VEGF-independent signaling is active in PMs, VEGF failed to rescue Lens-CM-induced apoptosis (Fig. 3C).
FIG. 3.
Lens-CM induces apoptosis of HUVE cells. (A) HUVE cells were cultured in M199 containing 5% FBS with various organ-CM at different stages (days after birth) or BMP4 (50 ng/ml). After 24 h of culture, TUNEL-positive cells were counted. Data are shown as the mean percent apoptosis ± standard error from several independent experiments (upper panel). Representative fields of HUVE cells cultured in M199 with (b and d) or without (a and c) Lens-CM from day 8 rats (8L-CM), stained with TUNEL reagents (a and b) or Hoechst 33258 (c and d) are shown (lower panels). (B) Effect of Noggin and the BMP4-specific neutralizing antibody on Lens-CM-induced apoptosis of HUVE cells. Cells were cultured with or without Noggin (250 ng/ml), anti-BMP4 antibody (250 ng/ml) or BMP4 (50 ng/ml) under the conditions indicated for 24 h at 37°C. Excess amounts of BMP4 (500 ng/ml) were added when cultured with 8L-CM as indicated. Data are expressed as in panel A. (C) Effect of VEGF on Lens-CM-induced apoptosis of HUVE cells. Cells were cultured with or without VEGF (50 ng/ml) or BMP4 (50 ng/ml) for 24 h at 37°C under the conditions indicated. Data are expressed as in panel A. Abbreviations: Ab, anti-BMP4 antibody; B, BMP4; Ig, normal mouse IgG; N, Noggin; V, VEGF.
Lens-CM inhibits tubular formation in coculture system.
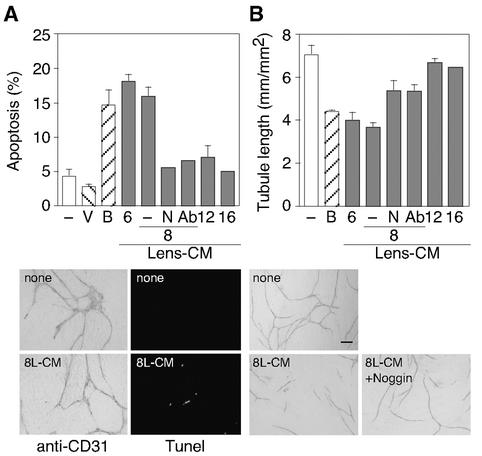
Next, the apoptotic effect of Lens-CM on the regression of tubules was assessed. We used a coculture system of HUVE cells and fibroblasts as a model of angiogenesis in vivo, which appeared more similar to the environment in vivo than that used in current in vitro assays, such as the three-dimensional collagen gel culture or the matrigel assay.
As shown in Fig. 4A, both Lens-CM (during the regression phase) and BMP4 increased the apoptotic response of HUVE cells in tubules. Simultaneously, the Lens-CM or BMP4 significantly reduced tubular formation by HUVE cells (Fig. 4B). These proapoptotic and inhibitory effects of Lens-CM were completely inhibited by the treatment with Noggin or the neutralizing antibody to BMP4.
FIG. 4.
Lens-CM inhibits tubular formation by HUVE cells. HUVE cells were cocultured with human fibroblasts for 10 days, and subjected to incubation with or without VEGF (50 ng/ml), BMP4 (50 ng/ml), Noggin (250 ng/ml) or the BMP4-specific neutralizing antibody (250 ng/ml) under the conditions indicated for 2 days. (A) Quantification of apoptotic HUVE cells by counting pyknotic nuclei. Data are shown as the mean percent apoptosis ± standard error from several independent experiments (upper panel). Representative apoptotic tubules, immunostained with the anti-CD31 antibody or TUNEL are shown (lower panels). (B) Measurements of tubule length. The results are shown as the mean ± standard error (n = 4) (upper panel). Representative fields stained with the anti-CD31 antibody are shown (lower panels). Abbreviations: Ab, anti-BMP4 antibody; B, BMP4; N, Noggin; V, VEGF. Scale bar: 100 μm.
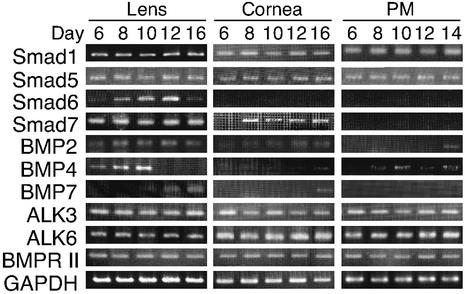
If BMPs had local apoptosis-inducing activity, then the components of its specific transduction pathway should be expressed. Thus, we examined the expression profile of BMP family ligands, receptors and transducers during PM regression by RT-PCR (Fig. 5). Interestingly, the expression of BMP4 in the lens was transiently up-regulated at the beginning of PM regression. On the other hand, inhibitory-Smads, Smad6 and -7, which function as inhibitors of BMP-Smad signaling (18, 21, 22, 23, 37) were strongly expressed in the lens, but not in the PMs. These results suggest an important role for BMP4 secreted from the lens in inducing PM apoptosis in a paracrine manner.
FIG. 5.
Developmental changes in BMP4 mRNA levels of rat lens. RT-PCR analysis of ligands, receptors and transducers of BMP family signals was performed at various stages of organs. Representative data are shown from several experiments performed as described for Fig. 1A.
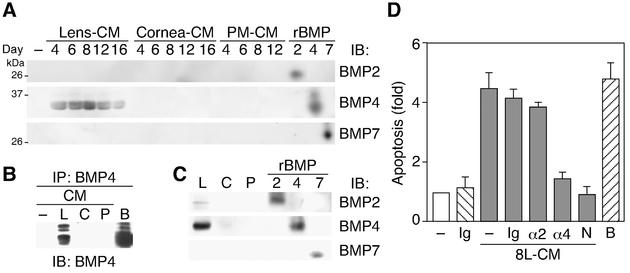
Lens, but not cornea or PM, transiently secretes BMP4.
To clarify whether Lens-CM actually contains BMP family ligands, an immunoblotting analysis using specific antibodies was performed. As shown in Fig. 6A, specific BMP4 bands were evident in Lens-CM during the regression phase, but not in Cornea-CM or in PM-CM. These results were confirmed by immunoprecipitation experiments (Fig. 6B). On the other hand, neither BMP2 nor BMP7 could be detected at any stage in organ-CMs (Fig. 6A). In addition, BMP4 was expressed dominantly in the lens itself, compared with the cornea or PMs (Fig. 6C). Moreover, the apoptosis-inducing activity of Lens-CM on HUVE cells was significantly decreased by immunodepletion of a factor from Lens-CM with the anti-BMP4 antibody (Fig. 6D). Immunodepletion with either the anti-BMP2 antibody or normal mouse IgG did not show clear suppressive effects.
FIG. 6.
Identification of BMP4 as a factor required for Lens-CM-induced apoptosis of HUVE cells. (A and B) Developmental enhancement of BMP4 secretion from lens. Organ-CM (A) or protein immunoprecipitated by the anti-BMP4 antibody from organ-CM of day 8 rats (B) was stained with the specific antibodies. (C) Expression profile of BMP4 in organs. Lens, cornea and PMs were lysed and subjected to immunoblotting analysis using the specific antibodies. Results (A to C) are representative ones of at least three independent experiments. Anti-BMP4 antibody used for immunoblotting (A and C) and immunoprecipitation (B) was obtained from R&D Systems. In (B), anti-BMP4 antibody from Santa Cruz was used for immunoblotting. (D) Inhibitory effect of neutralizing antibody to BMP4 on Lens-CM-induced apoptosis. HUVE cells were cultured in M199 containing 5% FBS with or without Lens-CM from day 8 rats (8L-CM). Before use, the 8L-CM was immunodepleted with or without (−) the normal mouse IgG (Ig), the anti-BMP2 antibody (α2) and the anti-BMP4 antibody (α4). After 24 h of culture, TUNEL-positive cells were counted. HUVE cells cultured with Lens-CM and Noggin (250 ng/ml, N) were used as a positive control. Relative apoptotic cell numbers are shown as the mean ± standard error from several independent experiments. Abbreviations: B, BMP4; C, cornea; L, lens; N, Noggin; P, PM.
Lens-CM mediates BMP4 signaling.
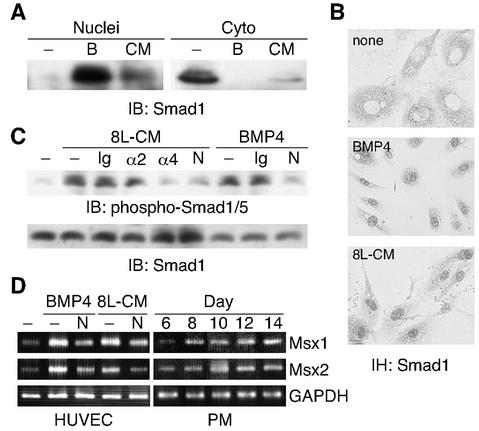
Next, we examined whether the BMP4-Smad1/5 (Smad1 and/or Smad5) pathway is involved in the Lens-CM-mediated apoptosis. The propagation of BMP4 signaling downstream is achieved by the activation of receptor type-I kinase, the phosphorylation of Smad1 and the translocation of the phosphorylated Smad1 into the nucleus (28). Furthermore, BMP4-mediated apoptosis appears to be transcriptionally regulated by the activity of the homeobox genes encoding Msx1 and Msx2 (8, 34). The results demonstrated that the treatment of HUVE cells with Lens-CM was associated with decreases in cytoplasmic Smad1 and concomitant increases in nuclear Smad1 (Fig. 7A). As a control, the nuclear and cytoplasmic fractions were also subjected to immunoblotting with antibodies specific to nuclei or cytoplasm to ensure the purity of the preparation (data not shown).
FIG. 7.
BMP4 signaling in HUVE cells. (A) Lens-CM promotes the translocation of Smad1 to the nucleus. HUVE cells were stimulated with Lens-CM from day 8 rats (CM) or BMP4 (50 ng/ml, B) for 2 h. Cells were harvested, fractionated into nuclear (Nuclei) and cytoplasmic (Cyto) fractions, and subjected to immunoblotting analysis using the anti-Smad1 antibody. (B) Immunohistochemistry using the anti-Smad1 antibody of HUVE cells, stimulated as described for panel A. (C) Phosphorylation of Smad1/5 in HUVE cells. HUVE cells were pretreated with or without Noggin (250 ng/ml, N) and stimulated with Lens-CM from day 8 rats (8L-CM) or BMP4 (50 ng/ml). Before use, the 8L-CM was immunodepleted with or without (−) the normal mouse IgG (Ig), the anti-BMP2 antibody (α2) and the anti-BMP4 antibody (α4), for 2 h. Cells were harvested and subjected to immunoblotting analysis using the anti-phospho Smad1/5/8 antibody. (D) Lens-CM induces the expression of Msx1 and Msx2 mRNAs. HUVE cells were cultured with or without Noggin (250 ng/ml) under the conditions indicated. After 30 h of culture, cells were harvested and subjected to RT-PCR analysis with specific primers as described for Fig. 1A. Developmental changes in rat PMs were also examined. Results in the figure are representative of several experiments.
To confirm the subcellular redistribution of Smad1 in HUVE cells, we carried out an immunohistochemical analysis. Staining with the anti-Smad1 antibody clearly showed that the use of Lens-CM led to a nuclear accumulation of Smad1 (Fig. 7B). Similar results were obtained from BMP4-treated cells (Fig. 7A and B). In addition, the phosphorylation of Smad1/5 in HUVE cells induced by Lens-CM was strongly suppressed by an immunodepletion treatment of Lens-CM with the anti-BMP4 antibody but not with anti-BMP2 antibody (Fig. 7C). Moreover, we assessed whether Lens-CM could induce the expression of putative Smad1 target genes, Msx1 and Msx2. In support of this idea, Fig. 7D (left panels) showed that both Lens-CM and BMP4 increased Msx1 and Msx2 mRNA levels. Noggin significantly inhibited the up-regulation of both target genes to control levels. Given these observations, it is likely that BMP4 plays a major role in the Lens-CM-induced apoptosis of HUVE cells.
BMP4 induces phosphorylation of Smad1/5 and promotes apoptosis in the PM in vivo.
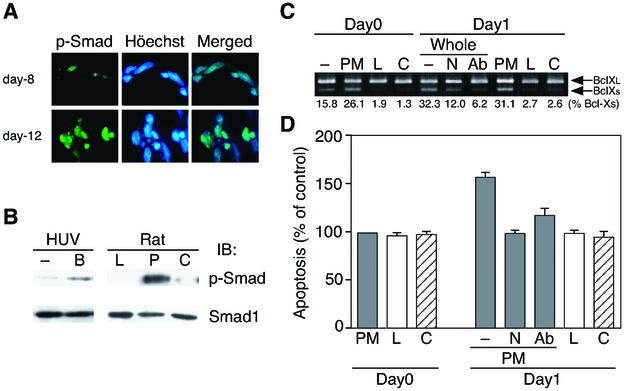
To test whether BMP4 signaling is really active in the PMs throughout developmental capillary regression, we examined the phosphorylation of Smad1/5 in rat PMs by immunohistochemical and immunoblotting analyses. Interestingly, phosphorylated Smad1/5 was clearly expressed in endothelial cells in PMs (Fig. 8A). As shown in Fig. 8B, Smad1/5 was strongly phosphorylated in endothelial cells from the day 8 rat PMs. As a control, BMP4 also phosphorylated Smad1/5 in HUVE cells (Fig. 8B, left panel). Moreover, the expression of Msx1 and Msx2 were up-regulated throughout the regression phase in the PMs (Fig. 7D, right panels).
FIG. 8.
BMP4 signal is active in the PMs. (A) Immunohistochemical analysis of PMs from day 8 or day 12 rats using the anti-phospho Smad1/5/8 antibody. The same specimens were counterstained with Hoechst 33258. (B) Phosphorylation of Smad1/5 in PMs from day 8 rats. Lens (L), PM (P), and cornea (C) harvested from rats were subjected to immunoblotting analysis using the anti-phospho Smad1/5/8 antibody. HUVE cells, stimulated with (B) or without (−) BMP4 (50 ng/ml) for 2 h, were used as a control. (C) Effect of Noggin and the BMP4-specific neutralizing antibody on the apoptosis of PMs in the 1-day-whole-culture system. The orbits of day 8 rats were cultured with medium alone (−), Noggin (250 ng/ml, N) or the anti-BMP4 antibody (250 ng/ml, Ab) for 24 h at 37°C (Day1). RT-PCR analysis for the whole orbits (−, N, and Ab), PM, lens (L) and cornea (C) was performed as described in Fig. 1A. In addition, whole orbits were separated into parts, the PM, lens and cornea. Data are representative of several experiments. The ratio of Bcl-Xs/total Bcl-X (%) was calculated by densitometric analysis. (D) Quantification of the apoptotic index in the PMs from day 8 rats under the same conditions as described above in the 1-day-culture system. Data are expressed as in Fig. 2C.
We next examined the BMP4-induced paracrine regulation of the PM apoptosis in an orbit 1-day-whole-culture system. As shown in Fig. 8C and D, both Noggin and the BMP4-specific neutralizing antibody repressed the up-regulation of Bcl-Xs (lower bands) and reversed the extent of apoptosis to that of day 0, specifically in the PMs, not in the lens or cornea.
Finally, to investigate whether BMP4 derived from the lens actually promotes in vivo the apoptosis of PMs in a paracrine manner, BMP4 was injected transcorneally into day 8 rats. Twenty-four hours after injection, PM regression was examined (day 1). As shown in Fig. 9A, BMP4 significantly promoted apoptosis compared with controls (PBS injected). In contrast, injections of Noggin revealed that the frequency of apoptosis was significantly reduced compared with controls. Importantly, prolonged treatment with BMP4 and Noggin (2 or 4 days after injections) further enhanced these effects. BMP4 strongly increased the number of apoptotic endothelial cells, whereas Noggin prevented regression to a large degree (Fig. 9A). Moreover, when Noggin or the BMP4-specific neutralizing antibody was injected at days 6, 8, and 10, the number of remaining capillary cells was increased approximately four to six times compared with the controls (PBS injection) and untreated PMs (Fig. 9B and C). Therefore, BMP4 secreted from the lens may promote apoptosis in the PM through the induction of Smad1/5 as a paracrine factor.
FIG. 9.
BMP4 promotes apoptosis in the PMs. (A) Effect of BMP4 and Noggin on apoptosis in PMs in vivo. Transcorneal injections of PBS (P), BMP4 (200 ng, B) and Noggin (500 ng, N) were performed with day 8 rats. After 1, 2, or 4 days, PMs were dissected and TUNEL-positive cells were counted. Data are shown as the mean percent apoptosis ± standard error (n = 30) (upper panel). Representative fields of PMs stained with TUNEL are shown (lower panels). (B) Morphological change of pupillary membranes at day 14 rats after injections of reagents at day 10. (a and b) Persistent PMs at day 14 after injection of Noggin (500 ng) (a) or the BMP4-specific neutralizing antibody (1 μg) (b). (c and d) The contralateral PMs injected with PBS from the animals in panels a and b. (C) Quantification of the remaining capillary cells in PMs. Noggin (500 ng, N), the BMP4-specific neutralizing antibody (1 μg, Ab) and PBS (P) injections were performed at days 6, 8, and 10, and this was followed by dissection at day 13 or 14 as indicated. The remaining capillary cells were analyzed by NIH image and expressed relative values to untreated PMs (−). Data are shown as the mean ± standard error (n = 50 to 60).
Overall, the results obtained in this study indicate that BMP4-Smad1/5 signaling is activated in PMs throughout the developmental capillary regression in a paracrine fashion (Fig. 10).
FIG. 10.
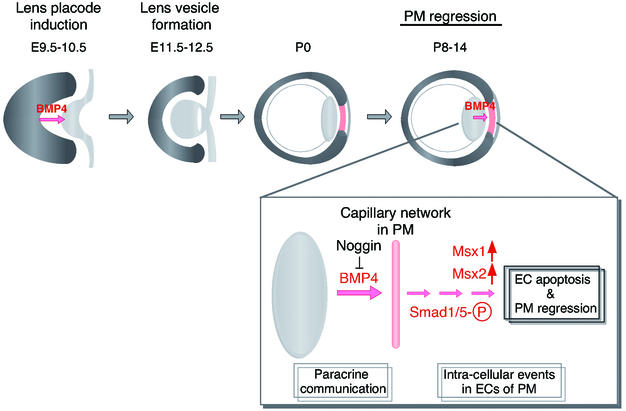
BMP4 contributes to the development of the eye (a schematic model). At approximately embryonic day 9.5 to 10.5, the optic vesicle contacts with the surface ectoderm to induce lens placode. BMP4, one of the factors releasing by optic vesicle, is first detectable in the anterior region of the optic cup, and its activity is necessary for the lens placode induction during early stages of eye morphogenesis. Coordinated invagination of the lens placode and optic vesicle in the region of contact results in the positioning of the lens vesicle in the center of the bilayered optic cup (embryonic day 11.5 to 12.5). Reciprocal interactions between the lens vesicle and optic cup are required for continued growth and morphogenesis of the developing eye. During the later stages of ocular development, PMs nourishes the immature lens. Then, matured lens secretes BMP4, and this BMP4 activity is responsible for the apoptotic cell death in PMs (P8 to P14) to lead its regression as shown in this study. Although the phosphorylation of Smad1/5 was detected, it is not clear whether Smad1 and/or Smad5 is the mediator of apoptosis in PM.
DISCUSSION
Apoptosis plays a major role in the morphogenesis and remodeling of organs according to internal and external environmental changes during development. Here, we found that BMP4 secreted from the lens is involved in endothelial apoptosis, especially capillary regression in the PM.
There is evidence to suggest that cell fate is determined by interactions within the morphogenetic field over the course of organogenesis. Lens formation is the result of a reciprocal inductive interaction between the optic vesicle and the surface ectoderm. Several BMP family members have been reported to be expressed throughout the development of the eye in mice (9, 24, 26, 31, 44). In mice, BMP4 has critical roles during the lens induction process. In addition, it is known that PMs, which nourish the immature lens, disappear during the later stages of ocular development. To our surprise, BMP4 plays two important roles in the development of the lens: one in the process of lens induction during embryogenesis and the other in the capillary network regression of PMs secreted from the lens epithelial cells as shown in this study (Fig. 10).
Recently, Meeson et al. (35) proposed that a lack of blood flow results in synchronous apoptosis along the capillary segment of PM, due to the shortage of VEGF supplied by blood. A simple physical block of plasma flow is suggested to cause the apoptosis of endothelial cells in the circulation of corpus luteum in the ovary (1). A decrease in hemodynamic force was also shown to trigger apoptosis in endothelial cells (25). The expression of a number of genes involved in thrombosis, homeostasis and inflammation is regulated by vascular shear stress (41). Thus, the decrease in hemodynamic force and the cessation of blood flow may also play some role in vascular regression in vivo. However, contrary to the previous report, VEGF failed to repress apoptosis in the PMs in our 1-day-culture system (Fig. 2C). Even without blood flow in the capillaries under this system, Noggin strongly inhibited apoptosis in the PMs (Fig. 8C and D). Therefore, we consider that the BMP4 secreted from the lens during the day 6 to 10 after birth is the major initiator for PM regression, and the decrease in the level of VEGF contributes in a minor way or to a later phase of endothelial cell apoptosis.
Diez-Roux et al. (7) reported that macrophages play an important role in the PM regression. Macrophages are well known to participate in the late stage of apoptosis, such as recognition of apoptosis-induced cells and phagocytosis of those cells. Thus, we suggest that the expression of BMP4 shown in this study initiates the first step of apoptosis in PM regression, then macrophages are recruited and involved in the second stage of this process to remove the died capillary endothelial cells.
Given the expression pattern of the BMP4 gene and related genes in the lens, cornea, and PM (Fig. 5), the question arises why PMs are sensitive to BMP4-induced apoptosis, but lens and cornea are not. Several explanations are possible. (i) The endothelial cells in PMs are more unstable than the epithelial cells in lens and cornea, thus, a difference of cell type determines the sensitivity to BMP4-induced apoptosis. (ii) The mRNAs of the BMP4 receptors (ALK3, ALK6, and BMPR II) are expressed in lens, cornea and PM (Fig. 5). However, the lens and cornea but not the PM strongly express the transcripts of Smad6 and -7, inhibitory Smads against TGF-β signals. Thus, Smad6 and -7 may efficiently block an autocrine-type BMP4-induced apoptosis in the lens and a paracrine-type apoptosis in the cornea. (iii) BMP4 receptors function in the PMs to transduce apoptotic signals, but these receptors might be less functional or inactive in lens and cornea due to a rapid protein turnover or due to an association with an unidentified inhibitor(s).
Ang2 is a natural antagonist for Ang1, opposing the effect of Ang1-mediated stabilization by acting as a competitive antagonist at the Tie2 receptor (33). In adult mice and humans, Ang2 is expressed only at sites of vascular remodeling. The ovarian vasculature is one of the few locations where the nonpathological development, maintenance and regression of vessels occur in the adult. A rise in the Ang2/Ang1 ratio has been shown in the corpus luteum during luteolysis. This ratio increased significantly in the late luteal phase, suggesting that overexpression of Ang2 during luteolysis could reflect the regression of capillaries (13, 33). Notably, we observed a marked shift in the ratio of Ang2 to Ang1 expression during PM regression from day 10 to 14 after birth (Fig. 2D). Considering the previous observations, these findings suggest that the up-regulation of Ang2 promotes vascular breakdown, which follows later during PM regression. This is intriguing because differences in the temporal expression of Ang2 versus Ang1 are consistent with the cyclic ovarian angiogenesis, suggesting that a balance of these angiogenic factors may also control vessel degeneration in PMs at later stages.
Moreover, we showed that the expression of proapoptotic BclXs (short band in RT-PCR) increased in association with the activation of caspase 3 according to the apoptotic process in the PMs (Fig. 1). These results are analogous to previous observations of a dramatic vascular remodeling, the closure and regression of the umbilical artery and ductus arteriosus during the perinatal period (3, 27). Therefore, the balance of Bcl-Xs and Bcl-XL is also an important determinant of these physiological apoptotic processes of endothelial cells including PM regression.
In this study, we demonstrated that the secreted molecule BMP4 plays a key role in the regression of the capillary network in the anterior chamber of the lens at later stage of development. The role of the BMP pathway in endothelial cell biology is not well understood. Decidualization is a process involving tissue swelling and vascular remodeling. Recently, BMP2, -4, and -8 were found in mouse decidua, suggesting that BMP signaling is involved in this process (47). Taken together, it is noteworthy that there is an intimate link between BMP4 signaling and endothelial apoptosis in general physiologic vascular regression and/or remodeling. Further investigation is necessary to elucidate the molecular basis of the effect of the paracrine factor, BMP4, on vascular cell apoptosis and characterize the actual role of apoptosis as a determinant of vascular structure in vivo.
Acknowledgments
This work was supported by Grants-in-Aid for Special Project Research on Cancer-Bioscience (12215024) from the Ministry of Education, Science, Sports, and Culture of Japan; for the Research Fellowships of the Japan Society for the Promotion on Science for Young Scientists; and for the program Research for the Future of the Japan Society for the Promotion of Science.
REFERENCES
- 1.Azmi, T. I., and J. D. O'Shea. 1984. Mechanism of deletion of endothelial cells during regression of the corpus luteum. Lab. Investig. 51:206-217. [PubMed] [Google Scholar]
- 2.Boise, L. H., M. Gonzalez-Garcia, C. E. Postema, L. Ding, T. Lindsten, L. A. Turka, X. Mao, G. Nunez, and C. B. Thompson. 1993. bcl-x, a bcl-2-related gene that functions as a dominant regulator of apoptotic cell death. Cell 74:597-608. [DOI] [PubMed] [Google Scholar]
- 3.Cho, A., D. W. Courtman, and B. L. Langille. 1995. Apoptosis (programmed cell death) in arteries of the neonatal lamb. Circ. Res. 76:168-175. [DOI] [PubMed] [Google Scholar]
- 4.Cho, K. W., and I. L. Blitz. 1998. BMPs, Smads and metalloproteases: extracellular and intracellular modes of negative regulation. Curr. Opin. Genet. Dev. 8:443-449. [DOI] [PubMed] [Google Scholar]
- 5.Coucouvanis, E., and G. R. Martin. 1999. BMP signaling plays a role in visceral endoderm differentiation and cavitation in the early mouse embryo. Development 126:535-546. [DOI] [PubMed] [Google Scholar]
- 6.Dick, A., W. Risau, and H. Drexler. 1998. Expression of Smad1 and Smad2 during embryogenesis suggests a role in organ development. Dev. Dyn. 211:293-305. [DOI] [PubMed] [Google Scholar]
- 7.Diez-Roux, G., and R. A. Lang. 1997. Macrophages induce apoptosis in normal cells in vivo. Development 124:3633-3638. [DOI] [PubMed] [Google Scholar]
- 8.Ferrari, D., A. C. Lichtler, Z. Z. Pan, C. N. Dealy, W. B. Upholt, and R. A. Kosher. 1998. Ectopic expression of Msx-2 in posterior limb bud mesoderm impairs limb morphogenesis while inducing BMP-4 expression, inhibiting cell proliferation, and promoting apoptosis. Dev. Biol. 197:12-24. [DOI] [PubMed] [Google Scholar]
- 9.Furuta, Y., and B. L. Hogan. 1998. BMP4 is essential for lens induction in the mouse embryo. Genes Dev. 12:3764-3775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Furuta, Y., D. W. Piston, and B. L. Hogan. 1997. Bone morphogenetic proteins (BMPs) as regulators of dorsal forebrain development. Development 124:2203-2212. [DOI] [PubMed] [Google Scholar]
- 11.Ganan, Y., D. Macias, M. Duterque-Coquillaud, M. A. Ros, and J. M. Hurle. 1996. Role of TGFβs and BMPs as signals controlling the position of the digits and the areas of interdigital cell death in the developing chick limb autopod. Development 122:2349-2357. [DOI] [PubMed] [Google Scholar]
- 12.Gobe, G., J. Browning, T. Howard, N. Hogg, C. Winterford, and R. Cross. 1997. Apoptosis occurs in endothelial cells during hypertension-induced microvascular rarefaction. J. Struct. Biol. 118:63-72. [DOI] [PubMed] [Google Scholar]
- 13.Goede, V., T. Schmidt, S. Kimmina, D. Kozian, and H. G. Augustin. 1998. Analysis of blood vessel maturation processes during cyclic ovarian angiogenesis. Lab. Investig. 78:1385-1394. [PubMed] [Google Scholar]
- 14.Gonzalez-Garcia, M., R. Perez-Ballestero, L. Ding, L. Duan, L. H. Boise, C. B. Thompson, and G. Nunez. 1994. bcl-XL is the major bcl-x mRNA form expressed during murine development and its product localizes to mitochondria. Development 120:3033-3042. [DOI] [PubMed] [Google Scholar]
- 15.Graham, A., P. Francis-West, P. Brickell, and A. Lumsden. 1994. The signalling molecule BMP4 mediates apoptosis in the rhombencephalic neural crest. Nature 372:684-686. [DOI] [PubMed] [Google Scholar]
- 16.Gross, R. E., M. F. Mehler, P. C. Mabie, Z. Zang, L. Santschi, and J. A. Kessler. 1996. Bone morphogenetic proteins promote astroglial lineage commitment by mammalian subventricular zone progenitor cells. Neuron 17:595-606. [DOI] [PubMed] [Google Scholar]
- 17.Gurdon, J. B., P. Harger, A. Mitchell, and P. Lemaire. 1994. Activin signalling and response to a morphogen gradient. Nature 371:487-492. [DOI] [PubMed] [Google Scholar]
- 18.Hayashi, H., S. Abdollah, Y. Qiu, J. Cai, Y. Y. Xu, B. W. Grinnell, M. A. Richardson, J. N. Topper, M. A. Gimbrone, Jr., J. L. Wrana, and D. Falb. 1997. The MAD-related protein Smad7 associates with the TGFβ receptor and functions as an antagonist of TGFβ signaling. Cell 89:1165-1173. [DOI] [PubMed] [Google Scholar]
- 19.Hogan, B. L. 1996. Bone morphogenetic proteins in development. Curr. Opin. Genet. Dev. 6:432-438. [DOI] [PubMed] [Google Scholar]
- 20.Hogan, B. L. 1996. Bone morphogenetic proteins: multifunctional regulators of vertebrate development. Genes Dev. 10:1580-1594. [DOI] [PubMed] [Google Scholar]
- 21.Imamura, T., M. Takase, A. Nishihara, E. Oeda, J. Hanai, M. Kawabata, and K. Miyazono. 1997. Smad6 inhibits signalling by the TGF-β superfamily. Nature 389:622-626. [DOI] [PubMed] [Google Scholar]
- 22.Ishisaki, A., K. Yamato, S. Hashimoto, A. Nakao, K. Tamaki, K. Nonaka, P. ten Dijke, H. Sugino, and T. Nishihara. 1999. Differential inhibition of Smad6 and Smad7 on bone morphogenetic protein- and activin-mediated growth arrest and apoptosis in B cells. J. Biol. Chem. 274:13637-13642. [DOI] [PubMed] [Google Scholar]
- 23.Ishisaki, A., K. Yamato, A. Nakao, K. Nonaka, M. Ohguchi, P. ten Dijke, and T. Nishihara. 1998. Smad7 is an activin-inducible inhibitor of activin-induced growth arrest and apoptosis in mouse B cells. J. Biol. Chem. 273:24293-24296. [DOI] [PubMed] [Google Scholar]
- 24.Jena, N., C. Martin-Seisdedos, P. McCue, and C. M. Croce. 1997. BMP7 null mutation in mice: developmental defects in skeleton, kidney, and eye. Exp. Cell Res. 230:28-37. [DOI] [PubMed] [Google Scholar]
- 25.Kaiser, D., M. A. Freyberg, and P. Friedl. 1997. Lack of hemodynamic forces triggers apoptosis in vascular endothelial cells. Biochem. Biophys. Res. Commun. 231:586-590. [DOI] [PubMed] [Google Scholar]
- 26.Karsenty, G., G. Luo, C. Hofmann, and A. Bradley. 1996. BMP 7 is required for nephrogenesis, eye development, and skeletal patterning. Ann. N. Y. Acad. Sci. 785:98-107. [DOI] [PubMed] [Google Scholar]
- 27.Kim, H. S., M. Aikawa, K. Kimura, M. Kuro-o, K. Nakahara, T. Suzuki, H. Katoh, E. Okamoto, Y. Yazaki, and R. Nagai. 1993. Ductus arteriosus. Advanced differentiation of smooth muscle cells demonstrated by myosin heavy chain isoform expression in rabbits. Circulation 88:1804-1810. [DOI] [PubMed] [Google Scholar]
- 28.Kretzschmar, M., F. Liu, A. Hata, J. Doody, and J. Massague. 1997. The TGF-β family mediator Smad1 is phosphorylated directly and activated functionally by the BMP receptor kinase. Genes Dev. 11:984-995. [DOI] [PubMed] [Google Scholar]
- 29.Lang, R., M. Lustig, F. Francois, M. Sellinger, and H. Plesken. 1994. Apoptosis during macrophage-dependent ocular tissue remodelling. Development 120:3395-3403. [DOI] [PubMed] [Google Scholar]
- 30.Liu, F., A. Hata, J. C. Baker, J. Doody, J. Carcamo, R. M. Harland, and J. Massague. 1996. A human Mad protein acting as a BMP-regulated transcriptional activator. Nature 381:620-623. [DOI] [PubMed] [Google Scholar]
- 31.Luo, G., C. Hofmann, A. L. Bronckers, M. Sohocki, A. Bradley, and G. Karsenty. 1995. BMP-7 is an inducer of nephrogenesis, and is also required for eye development and skeletal patterning. Genes Dev. 9:2808-2820. [DOI] [PubMed] [Google Scholar]
- 32.Macias, D., Y. Ganan, T. K. Sampath, M. E. Piedra, M. A. Ros, and J. M. Hurle. 1997. Role of BMP-2 and OP-1 (BMP-7) in programmed cell death and skeletogenesis during chick limb development. Development 124:1109-1117. [DOI] [PubMed] [Google Scholar]
- 33.Maisonpierre, P. C., C. Suri, P. F. Jones, S. Bartunkova, S. J. Wiegand, C. Radziejewski, D. Compton, J. McClain, T. H. Aldrich, N. Papadopoulos, T. J. Daly, S. Davis, T. N. Sato, and G. D. Yancopoulos. 1997. Angiopoietin-2, a natural antagonist for Tie2 that disrupts in vivo angiogenesis. Science 277:55-60. [DOI] [PubMed] [Google Scholar]
- 34.Marazzi, G., Y. Wang, and D. Sassoon. 1997. Msx2 is a transcriptional regulator in the BMP4-mediated programmed cell death pathway. Dev. Biol. 186:127-138. [DOI] [PubMed] [Google Scholar]
- 35.Meeson, A. P., M. Argilla, K. Ko, L. Witte, and R. A. Lang. 1999. VEGF deprivation-induced apoptosis is a component of programmed capillary regression. Development 126:1407-1415. [DOI] [PubMed] [Google Scholar]
- 36.Mehler, M. F., P. C. Mabie, D. Zhang, and J. A. Kessler. 1997. Bone morphogenetic proteins in the nervous system. Trends Neurosci. 20:309-317. [DOI] [PubMed] [Google Scholar]
- 37.Nakao, A., M. Afrakhte, A. Moren, T. Nakayama, J. L. Christian, R. Heuchel, S. Itoh, M. Kawabata, N. E. Heldin, C. H. Heldin, and P. ten Dijke. 1997. Identification of Smad7, a TGFβ-inducible antagonist of TGF-β signalling. Nature 389:631-635. [DOI] [PubMed] [Google Scholar]
- 38.Nellen, D., R. Burke, G. Struhl, and K. Basler. 1996. Direct and long-range action of a DPP morphogen gradient. Cell 85:357-368. [DOI] [PubMed] [Google Scholar]
- 39.Neumann, C., and S. Cohen. 1997. Morphogens and pattern formation. Bioessays 19:721-729. [DOI] [PubMed] [Google Scholar]
- 40.Pollman, M. J., L. Naumovski, and G. H. Gibbons. 1999. Endothelial cell apoptosis in capillary network remodeling. J. Cell. Physiol. 178:359-370. [DOI] [PubMed] [Google Scholar]
- 41.Resnick, N., and M. A. Gimbrone, Jr. 1995. Hemodynamic forces are complex regulators of endothelial gene expression. FASEB J. 9:874-882. [DOI] [PubMed] [Google Scholar]
- 42.Rong, P., A. M. Bennie, W. R. Epa, and G. L. Barrett. 1999. Nerve growth factor determines survival and death of PC12 cells by regulation of the bcl-x, bax, and caspase-3 genes. J. Neurochem. 72:2294-2300. [DOI] [PubMed] [Google Scholar]
- 43.Ruoslahti, E., and J. C. Reed. 1994. Anchorage dependence, integrins, and apoptosis. Cell 77:477-478. [DOI] [PubMed] [Google Scholar]
- 44.Trousse, F., P. Esteve, and P. Bovolenta. 2001. Bmp4 mediates apoptotic cell death in the developing chick eye. J. Neurosci. 21:1292-1301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Vainio, S., I. Karavanova, A. Jowett, and I. Thesleff. 1993. Identification of BMP-4 as a signal mediating secondary induction between epithelial and mesenchymal tissues during early tooth development. Cell 75:45-58. [PubMed] [Google Scholar]
- 46.Whitman, M. 1998. Smads and early developmental signaling by the TGFβ superfamily. Genes Dev. 12:2445-2462. [DOI] [PubMed] [Google Scholar]
- 47.Ying, Y., and G. Q. Zhao. 2000. Detection of multiple bone morphogenetic protein messenger ribonucleic acids and their signal transducer, Smad1, during mouse decidualization. Biol. Reprod. 63:1781-1786. [DOI] [PubMed] [Google Scholar]
- 48.Zou, H., and L. Niswander. 1996. Requirement for BMP signaling in interdigital apoptosis and scale formation. Science 272:738-741. [DOI] [PubMed] [Google Scholar]