Abstract
d-Hydantoinase (d-HYD) is an industrial enzyme that is widely used in the production of d-amino acids which are precursors for semisynthesis of antibiotics, peptides, and pesticides. This report describes the crystal structure of d-hydantoinase from Burkholderia pickettii (HYDBp) at a 2.7-Å resolution. The structure of HYDBp consists of a core (α/β)8 triose phosphate isomerase barrel fold and a β-sheet domain, and the catalytic active site consists of two metal ions and six highly conserved amino acid residues. Although HYDBp shares only moderate sequence similarity with d-HYDs from Thermus sp. (HYDTsp) and Bacillus stearothermophilus (HYDBst), whose structures have recently been solved, the overall structure and the structure of the catalytic active site are strikingly similar. Nevertheless, the amino acids that compose the substrate-binding site are less conserved and have different properties, which might dictate the substrate specificity. Structural comparison has revealed insights into the molecular basis of the differential thermostability of d-HYDs. The more thermostable HYDTsp contains more aromatic residues in the interior of the structure than HYDBp and HYDBst. Changes of large aromatic residues in HYDTsp to smaller residues in HYDBp or HYDBst decrease the hydrophobicity and create cavities inside the structure. HYDTsp has more salt bridges and hydrogen-bonding interactions and less oxidation susceptible Met and Cys residues on the protein surface than HYDBp and HYDBst. Besides, HYDTsp also contains more rigid Pro residues. These factors are likely to make major contributions to the varying thermostability of these enzymes. This information could be exploited in helping to engineer more thermostable mesophilic enzymes.
Dihydropyrimidinases (EC 3.5.2.2) are a family of enzymes that catalyze the reversible hydrolytic ring opening of the amide bond in five- or six-membered cyclic diamides (54, 62). In humans, they are responsible for the hydrolysis of dihydropyrimidines at the second step of reductive catabolism of pyrimidines. The homologous enzymes in microorganisms are hydantoinases (HYDs) which catalyze the hydrolysis of 5-substituted hydantoins to the enantiomerically pure N-carbamyl amino acids. The latter can be converted chemically or enzymatically to the corresponding optically pure amino acids (50, 54, 64). In the biotechnology industry, HYD is widely used in the production of d-amino acids, including d-p-hydroxyphenylglycine, which are the precursors for semisynthesis of antibiotics, peptide hormones, pyrethroids, and pesticides (3, 49, 53, 54). Depending on their stereoselectivities and specificities on substrates, HYDs are often classified as d-, l-, or non-enantio specific (47, 67).
One major consideration in the application of enzymes for biocatalysis is thermostability. Screening of various thermophiles has successfully discovered several thermostable d-HYDs from Thermus sp. CBS30380 and Lu1220 (28), and Bacillus stearothermophilus SD-1 (38) and GH-2 (51). Substantial efforts have been made to study the specificity and thermostability of HYDs from different microorganisms (29-31, 43, 44, 47, 51). Directed evolution techniques have been used to generate enzymes with new properties or enhanced stereochemical specificity and thermostability (29, 43). Structural studies of HYDs have been carried out to understand the structure and function of these enzymes. Recently, crystal structures of d-HYDs from both Thermus sp. (HYDTsp) (2) and B. stearothermophilus (HYDBst) (13) and of l-HYD from Arthrobacter aurescens (HYDAau) (1) have been solved. In addition, structures of several other amidohydrolases have been reported in the past years, including structures of the urease α-subunit from Klebsiella aerogenes (23) and Bacillus pasteurii (5, 6), dihydroorotase from Escherichia coli (58), and phosphotriesterase from Pseudomonas diminuta (60). Analyses of amino acid sequences of various amidohydrolases show that HYD, dihydropyrimidinase, dihydroorotase, urease, phosphotriesterase, and adenosine deaminase belong to a distantly related superfamily (22, 42). Though these enzymes share less similarity in amino acid sequence (always less than 30%), their tertiary structures adopt the same TIM (triose phosphate isomerase) barrel fold (1, 2, 5, 6, 13, 23, 58, 60).
In this paper, we report the crystal structure of d-HYD from Burkholderia pickettii (HYDBp) at a 2.7-Å resolution. In contrast with other d-HYDs of known structure which are all from thermophiles, HYDBp is from a mesophile and its thermostability is relatively low. Comparison of this structure with those of HYDTsp and HYDBst has provided some insights into the molecular basis of the varying thermostability of these enzymes. This information could provide a rationale for generating new mesophilic enzymes with improved thermostability.
MATERIALS AND METHODS
Expression and purification of HYDBp.
The gene of HYDBp was cloned from genomic DNA of B. pickettii by Southern blotting. The plasmid pXZPH2, which contains the HYDBp gene, was constructed as previously described (66). The B. pickettii strain used in this study has been deposited with the China General Microbiological Culture Collection Center (entry no. 0520.1). One milliliter of an overnight preculture of E. coli BL21(DE3)/pXZPH2 in Luria broth supplemented with 100 μg of kanamycin/ml was inoculated into 100 ml of the same medium and grown to an optical density at 600 nm of 0.6. After a 3-h induction with 1 mM isopropyl-β-d-thiogalactopyranoside (IPTG) at 28°C, the cells were harvested by centrifugation at 2,000 × g for 7 min and resuspended in 30 ml of Tris-HCl buffer (20 mM Tris-HCl [pH 8.0], 10 mM imidazole, and 500 mM NaCl) and stored at −20°C for further use. The frozen cells were thawed on ice and then passed three times through an ice-cold French pressure cell (SLM-Aminco) operating at an internal pressure of 1,000 lb/in2. Cell debris was precipitated by centrifugation at 22,000 × g for 30 min. The supernatant solution was subjected to purification.
HYDBp was purified by affinity chromatography with an Ni-nitrilotriacetic acid agrose column (Qiagen). The extract was first loaded onto the column and washed with 25 ml of binding buffer (20 mM Tris-HCl [pH 8.0], 5 mM imidazole, and 500 mM NaCl). It was then washed with 25 ml of washing buffer (20 mM Tris-HCl [pH 8.0], 60 mM imidazole, and 500 mM NaCl) to elute any nonspecific binding protein. The tightly bound HYDBp was finally eluted with elution buffer (20 mM Tris-HCl [pH 8.0], 1 M imidazole, and 500 mM NaCl). All steps were carried out at 4°C to minimize proteolysis of the target protein. The results of sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) of the protein samples before and after purification are shown in Fig. 1A. The final purification factor is 22, and the total yield of HYDBp activity of the purified enzyme is 16% in relation to the crude extract (Table 1).
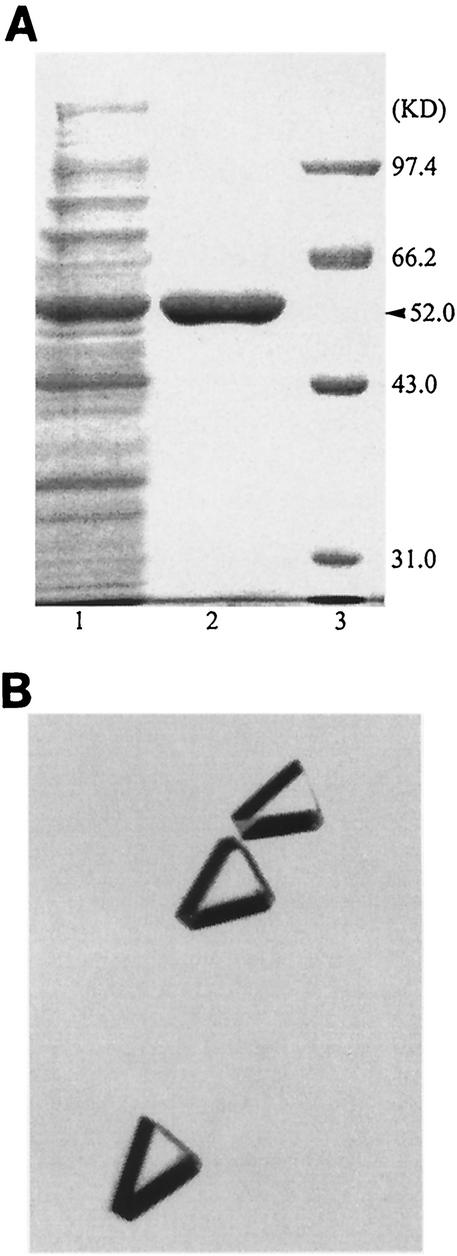
FIG. 1.
(A) SDS-PAGE of HYDBp protein samples with Coomassie blue staining before and after purification. Lane 1, the cell extract of E. coli BL21(DE3)/pXZPH2; lane 2, purified HYDBp; lane 3, molecular mass standards. (B) Crystals of HYDBp used for structure determination in this study.
TABLE 1.
Summary of purification of d-HYD from E. coli BL21(DE3)/pXZPH2
| Purification step | Sample vol (ml) | Total protein (mg) | Total activity (U) | Sp act (U/mg) | Purifi- cation factor | Activity yield (%) |
|---|---|---|---|---|---|---|
| Cell extract | 25.0 | 600 | 30.8 | 0.05 | 1.0 | 100 |
| Ni-NTAa affinity | 4.0 | 4.4 | 5.0 | 1.13 | 22.6 | 16.6 |
NTA, nitrilotriacetic acid.
To reduce the salt concentration and maintain the stability of the protein, stepwise buffer exchanges by dialysis with Centricon 30 (Amicon) were carried out to find a proper buffer solution. The eluted protein fractions were first dialyzed with a buffer containing 20 mM Tris-HCl (pH 8.0), 100 mM imidazole, and 500 mM NaCl; then with a buffer consisting of 20 mM Tris-HCl (pH 8.0), 100 mM imidazole, and 100 mM NaCl; and finally with a storage buffer (20 mM Tris-HCl [pH 8.0], 50 mM imidazole, and 100 mM NaCl). The purified protein solution was concentrated to 15 mg/ml by ultrafiltration (Centricon 30) and then stored in the storage buffer for biochemical studies and crystallization experiments. A low concentration of imidazole was found to be essential to keep HYDBp stable in the storage buffer.
Enzymatic activity assay of HYDBp.
The thermostability of HYDBp was determined by measuring the residual enzymatic activity of HYDBp after the enzyme was incubated at various temperatures in the 40 to 70°C range for 30 min. For the activity assay, a fixed amount of the enzyme was first incubated at a certain temperature for 30 min and then incubated with 0.1% d,l-hydroxyphenylhydantoin in 100 mM phosphate buffer (pH 8.0) at 50°C for 30 min in a total volume of 300 μl. Two hundred fifty microliters of the sample was removed from the reaction system and mixed with 550 μl of dimethylaminobenzaldehyde solution (5% wt/vol) in 6 M HCl. The mixture was centrifuged, and its optical density was measured at 438 nm. The concentration of N-carbamyl-d-hydroxyphenylglycine was determined from a calibration plot. One unit of HYD activity was defined as the amount of enzyme required to produce 1 μmol of N-carbamyl-d-hydroxyphenylglycine from d,l-hydroxyphenylhydantoin per min under the specified conditions.
Crystallization and data collection.
Crystallization was performed by using the hanging-drop variant of the vapor diffusion method with equal volumes of the protein solution and the reservoir solution. The reservoir solution contains 16% polyethylene glycol 6000, 0.1 M Bicine, and 0.005 M CdCl2 (pH 8.5). Crystals of HYDBp grew to a size of 0.2 by 0.2 by 0.1 mm3 after 2 to 3 days at 4°C with the shape of a trigonal prism (Fig. 1B).
X-ray diffraction data were collected from a crystal that was mounted with a nylon loop and flash cooled in a cold gaseous stream of N2 (−180°C). The cryoprotectant contains 18% (wt/vol) glycerol and the reservoir solution. A total of 244 oscillation images with 0.5° of oscillation were collected by using an in-house Rigaku R-AXIS IV++ and CuK α radiation (wavelength of 1.5418 Å) focused with a confocal mirror. The diffraction data were recorded on dual image plates and processed and scaled together with CrystalClear (52). Crystals of HYDBp diffracted X rays to a resolution better than 2.7 Å. Analysis of the diffraction data indicated that crystals of HYDBp belong to monoclinic space group P21 with unit cell dimensions of a (68.7 Å), b (170.5 Å), c (87.3 Å), and β (95.2°). The final diffraction data set contained 53,883 unique reflections [I/σ (I)>0] with an Rmerge(I) of 0.095 and a completeness of 98.5% to a 2.7-Å resolution. The diffraction data statistics are summarized in Table 2. One asymmetric unit contains four HYDBp molecules, corresponding to a Matthews coefficient (VM) of 2.5 Å3/Da (40) and a solvent content of 49.3%.
TABLE 2.
Summary of diffraction data and structure refinement
| Statistica (unit) | Result |
|---|---|
| No. of crystals | 1 |
| No. of images | 244 |
| Temp (°C) | −180 |
| Space group | P21 |
| Cell parameter(s) | |
| a, b, c (Å) | 68.7, 170.5, 87.3 |
| β (°) | 95.2 |
| Resolution range (Å) | 15.0-2.70 |
| No. of observed reflections | 139,372 |
| No. of unique reflections (I/σ > 0) | 53,883 |
| Completeness (%) (2.78-2.70 Å bin) | 98.5 (96.8) |
| Rmergeb (%) (2.78-2.70 Å bin) | 9.5 (23.7) |
| Resolution range (Å) | 15.0-2.70 |
| No. of reflections | |
| (Fo ≥ σ [Fo]) (2.78-2.70 Å bin) | 53,756 (4,281) |
| Free R set (2.78-2.70 Å bin) | 2,681 (221) |
| Completeness (%) (2.78-2.70 Å bin) | 98.3 (96.6) |
| R factorc (%) (2.78-2.70 Å bin) | 22.0 (30.3) |
| Free R factor (%) (2.78-2.70 Å bin) | 23.9 (32.4) |
| No. of residues | 457 |
| No. of: | |
| Protein atoms | 3,513 |
| Zn atoms | 2 |
| Water molecules | 126 |
| Average B factor (Å2) of: | 28.8 |
| Protein main chain atoms | 28.0 |
| Protein side chain atoms | 29.8 |
| Zn atoms | 21.9 |
| Water molecules | 26.8 |
| RMS bond lengths (Å) | 0.007 |
| RMS bond angles (°) | 1.4 |
| RMS dihedral angles (°) | 23.3 |
| RMS improper angles (°) | 0.86 |
| Ramachandran plot (%) | |
| Most favored regions | 86.9 |
| Allowed regions | 12.1 |
| Generously allowed | 1.0 |
The first group contains statistics for diffraction data, and the second group contains statistics for refinement and model.
Rmerge = Σ||Io| − 〈I〉|/Σ〈I〉.
R factor = Σ||Fo| − |Fc||/Σ|Fo|.
Structure determination and refinement.
The structure of HYDBp was solved by using the molecular replacement (MR) method as implemented in the program of CNS (8). Initial attempts with the α-subunit of urease from K. aerogenes (Protein Data Bank [PDB] entry 2KAU) (23) and dihydroorotase from E. coli (PDB entry 1J79) (58) as the starting search models were unsuccessful. With the availability of two d-HYD structures from Thermus sp. (PDB entries 1GKP and 1GKQ) in the PDB, the crystal structure of HYDTsp (PDB entry 1GKQ) was used as the template model for the MR search. HYDBp is homologous to HYDTsp with 39% sequence identity and 61% sequence similarity (Table 3). The starting model was modified from the coordinates of HYDTsp in such a way that the identical residues between HYDTsp and HYDBp were unchanged, whereas the variant residues were mutated to alanines. Since both gel filtration and dynamic light scattering results suggested that HYDBp exists as a dimer in solution (see Results and Discussion), a dimer, instead of monomer or tetramer, was used as the searching model. In retrospect, the dimer model indeed yielded better and clearer MR solutions than the tetramer model because the tetrameric arrangement differs slightly in different HYD structures. The self-rotation function search demonstrated the presence of twofold noncrystallographic symmetry (NCS), suggesting that there is a tetramer per asymmetric unit. Cross rotation function searches with data between a 15- and 4-Å resolution yielded two pairs of equally outstanding solutions (3 σ higher than the next peak). Each pair of peaks differed only slightly from the other. Subsequent translation function searches also produced two pairs of outstanding solutions (2.5 σ higher than the next peak), each corresponding to one dimer. Each pair of solutions has the same rotation but a different translation along the unique b-axis. Cross-validation showed that with one dimer being fixed, the second translation function search gave only the alternate solution. The initial phases computed from the MR solution yielded an R factor of 44.7% and a free R factor of 45.1%. Rigid-body refinement with each monomer of the model as a rigid-body group did not change the R factor and free R factor much (both decreased by 0.1%). After 200 cycles of conjugate-gradient energy minimization with CNS, the R factor and free R factor dropped to 34.4 and 42.3%, respectively. A composite omit map computed with the phases from the MR solution showed that the structure model fits the electron density very well and most of the omitted side chains had well-ordered electron density. This further confirmed the correctness of the MR solution.
TABLE 3.
Comparison of HYDBp with other known HYD structures
| HYD structure | % Sequence identity (similarity) to:
|
RMSD (Å) (Cα atoms) of:
|
Total monomer surface area (Å2)a | Buried accessible surfaces (Å2)a of:
|
|||||
|---|---|---|---|---|---|---|---|---|---|
| HYDBp | HYDBst | HYDTsp | HYDAau | Monomer | Tetramer | A/B | A/C | ||
| HYDBp (1NFG) | 51 (69) | 39 (61) | 28 (53) | 16,622 | 2,414 | 2,132 | |||
| HYDBst (1K1D) | 51 (69) | 40 (63) | 30 (51) | 1.01 (452) | 2.02 (1,500) | 16,978 | 2,167 | 1,959 | |
| HYDTsp (1GKP/1GKQ) | 39 (61) | 40 (63) | 27 (46) | 1.15 (447)/1.08 (446) | 2.20 (1,622)/1.68 (1,751) | 16,461/16,396 | 2,472/2,552 | 1,748/1,728 | |
| HYDAau (1GKR) | 28 (53) | 30 (51) | 27 (46) | 1.62 (418) | 2.46 (890) | 16,656 | 1,334 | 1,734 | |
Surface areas were computed by using the program AREAIMOL (34), with a probe radius of 1.4 Å and a point density of 50 points/Å2.
Structure refinement was carried out by using the conjugate-gradient energy minimization, torsion-constrained molecular dynamics-simulated annealing, group B factor refinement, and individual B factor refinement protocols in CNS. A bulk solvent correction was applied in all refinements. After the first round of structure refinement, fourfold NCS was derived from the superposition of four monomers of the structure model. From the second round of refinement, strict fourfold NCS constraints were imposed throughout the refinement procedure and map calculations. This increased the data-to-parameter ratio by a factor of 4, corresponding to about 12 observations per parameter refined. Model building was facilitated by using the graphics program O (26) and guided by SIGMAA-weighted 2mFo-DFc and mFo-DFc maps and composite omit maps. The free R factor was calculated in all stages of model building and structure refinement to monitor the progress. Since relaxation of strict fourfold NCS constraints to NCS restraints did not improve the R factor and free R factor significantly (decreased only 0.1 and 0.3%, respectively), strict NCS constraints were applied throughout the refinement process. Electron density corresponding to two metal ions at the catalytic active site was present in the initial difference Fourier maps. Though the Cd2+ ion was present in the crystallization solution, refinement with Zn2+ gave better R factors and more-reasonable B factors than with Cd2+. Therefore, the metal ions are tentatively assigned as Zn2+ ions in this structure. Also, at the active site, there was a strong positive electron density in the difference Fourier maps near Lys148, suggesting a carboxylation of this residue. After Lys148 was replaced with the carboxylated lysine (Kcx), the positive difference peak disappeared. Water molecules were not included in the model until the R factor decreased to approximately 27% after a few cycles of model building and refinement. Electron density peaks in difference Fourier maps at a height of at least 2.5 σ were assigned as water molecules if they had reasonable geometry in relation to hydrogen bond donors or acceptors from amino acids or other water molecules.
Structure refinement of the final atomic model converged to an R factor of 22.0% and a free R factor of 23.9% to a 2.7-Å resolution. The final model contains residues 1 to 457, two zinc ions, and 126 water molecules. All atoms were refined with restrained individual isotropic temperature factors and full occupancy. The stereochemistry of the protein model was analyzed with the programs PROCHECK (33) and CNS (8). The root-mean-squared deviations (RMSDs) of bond lengths and angles for the final model are 0.007 Å and 1.4°, respectively. Analysis of the protein main chain conformation shows 99.0% of amino acid residues in the most favored or allowed regions of the Ramachandran plot; no amino acid residues are located in the disallowed region. The mean error in atomic positions was estimated by the Luzzati method (39) to be 0.34 Å. Secondary structure assignments were made by using the DSSP program (27). A summary of the diffraction data and structure refinement statistics is given in Table 2. Figure 2 shows a representative SIGMAA-weighted 2mFo-DFc map at a 2.7-Å resolution at the catalytic active site.
FIG. 2.
A representative SIGMAA-weighted 2mFo-DFc map (1σ contour level) at a 2.7-Å resolution in the region of the catalytic active site. The final coordinates of the structure are shown with a ball-and-stick model.
PDB accession code.
The coordinates and structure factors of the d-HYD from B. pickettii have been deposited with the Research Collaboratory for Structural Bioinformatics Protein Data Bank (PDB) (entry 1NFG) for immediate release.
RESULTS AND DISCUSSION
Characterization of HYDBp.
HYDBp consists of 457 amino acids. Both reducing and nonreducing SDS-PAGE analyses show a single band at 52 kDa. Mass spectrometric analyses yield a molecular mass of 52,253 Da and confirm the homogeneity of the protein sample. Although most HYDs can form either dimers or tetramers in solution and require an oligomeric structure for activity and thermostability (36, 37, 48), all HYDs with known structures form tetramers in crystal structures (1, 2, 13). Gel filtration analysis of HYDBp indicated a molecular mass of approximately 105 kDa. Dynamic light scattering analyses showed that HYDBp is homogeneously dispersed in solution (at a protein concentration of 1 mg/ml) and has a molecular mass of 110 kDa. These results suggest that HYDBp exists as a homodimer in solution.
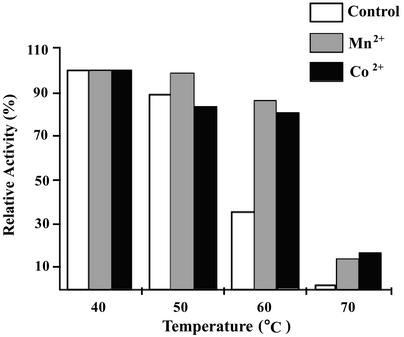
The enzymatic activity of HYDBp was assayed by measurement of N-carbamyl-d-hydroxyphenylglycine produced from d,l-hydroxyphenylhydantoin. Effects of various metal ions on the enzymatic activity were examined. The results indicate that HYDBp is a metal-dependent HYD. Though the enzymatic activity of HYDBp can be stimulated by various metal ions, including Co2+, Mn2+, Zn2+, Fe2+, and Ni2+, the most effective metal is Co2+ (Z. Xu, J. Hong, and Y. Yang, unpublished data). Variation of the enzymatic activity of HYDBp with pH and temperature either in the absence or in the presence of Co2+ or Mn2+ shows that optimal catalysis occurs at pH 8.0 and 50°C (Z. Xu et al., unpublished). Therefore, to examine the thermostability of HYDBp, the residual activity of HYDBp was measured at pH 8.0 and 50°C following incubation of the enzyme at temperatures of 40, 50, 60, and 70°C for 30 min (Fig. 3). In the absence of metal ions, HYDBp retains 89 and 35% of the enzymatic activity after incubation at 50 and 60°C, respectively (the residual enzymatic activity after incubation at 40°C is taken as 100%). When incubated at temperatures above 70°C, the activity of HYDBp is completely lost. In the presence of Co2+ or Mn2+, the enzymatic activities of HYDBp after incubation at 50°C are comparable to those without metal ions. However, HYDBp maintains more than 80 and 15% of its enzymatic activity after incubation with either Co2+ or Mn2+ at 60 or 70°C, respectively. These results indicate that HYDBp is fairly thermostable and the presence of metal ions enhances the thermostability of HYDBp.
FIG. 3.
Enzymatic stability of HYDBp at different temperatures. Control, incubated in the absence of any metal ions; Mn2+, incubated in the presence of Mn2+ (1 mM); Co2+, incubated in the presence of Co2+ (1 mM).
Overall structure of HYDBp.
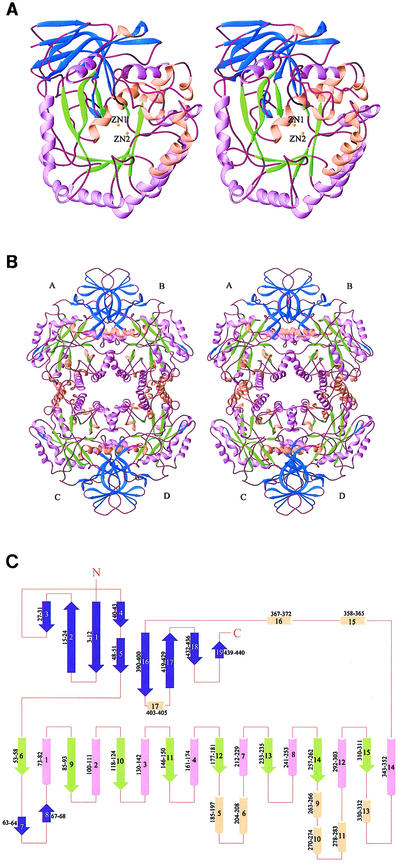
The overall structure and topology of HYDBp are illustrated in Fig. 4. Similar to other HYD structures (1, 2, 13), the HYDBp monomer consists of two domains. The large domain has a classic (α/β)8 TIM barrel fold containing strands β6 and β9 to β15, and helices α1 to α4, α7 to α8, α12, and α14, ranging from residues 53 to 355. The TIM barrel is elliptically distorted and is extended by some loops, six short α-helices (α5-α6, α9-α11, and α13), and a two-strand β-sheet (β7-β8). The longest β-strand (β9) has nine amino acid residues, and the shortest (β15) contains only two amino acid residues. The sizes of the barrel α-helices also vary between 10 (α1) and 18 (α7) residues. A β-sheet domain which comprises both the N and C termini is located on top of the central (α/β)8 barrel. This β-sheet domain consists of nine β-strands, five of them from the N terminus and the other four from the C terminus. Strands β1 and β4, and β1, β5, and β16 are parallel, whereas all other strands are antiparallel (Fig. 4C). These β-strands form three β-sheets. Four long β-strands (β1, β2, β16, and β17) have sharp bends in the middle and each participates in two β-sheets. The first β-sheet consists of the N-terminal portion of β1 (residues 3 to 6), the C-terminal portion of β2 (residues 21 to 24), β3 (residues 27 to 31), and β4 (residues 40 to 43). This β-sheet is continued in the adjacent monomer related by twofold NCS to form an eight-strand β-sheet. The second β-sheet is composed of the C-terminal portion of β1 (residues 8 to 12), the N-terminal portion of β2 (residues 15 to 19), β5 (residues 48 to 51), the N-terminal portion of β16 (residues 390 to 395), the C-terminal portion of β17 (residues 423 to 429), β18 (residues 432 to 436), and β19 (residues 439 to 440). These two β-sheets form a bilayer sandwich. The third β-sheet, which flanks one side of the TIM barrel, is formed by the C-terminal portion of β16 (residues 397 to 400) and the N-terminal portion of β17 (residues 419 to 421). At the C terminus there are three short α-helices (α15 to α17) which separate the TIM barrel and the β-sheet domain. The C terminus is located at the opposite side of the hydrophobic cleft which hosts the catalytic active site (see below). The N terminus is also far away from the active site. Consistent with biochemical data, both termini appear not to be essential for the catalytic activity of the enzyme (31).
FIG. 4.
Structure of HYDBp. (A) Ribbon diagram (10) showing the overall structure of the HYDBp monomer viewed from the catalytic active site. (B) Tetramer of HYDBp showing the intersubunit interfaces. (C) Secondary structural topology of HYDBp. α-Helices are shown as cylinders; β-strands are shown as arrows. The amino acid residue ranges of the secondary structural elements are illustrated. The β-strands of the small β-sheet domain are shown in blue; the β-strands of the TIM barrel are shown in green; α-helices of the TIM barrel are shown in lavender; and the other α-helices are shown in pink. The two zinc ions at the catalytic site are illustrated as yellow spheres.
Subunit interfaces of HYDBp.
Although HYDBp appears to exist as a homodimer in solution, it forms a tetramer in the crystal structure, similar to all other HYDs with known structures (1, 2, 13). The tetramer of HYDBp possesses 222-point group symmetry and has a dimension of 124 by 87 by 83 Å; the monomer has a dimension of 76 by 52 by 47 Å. The tetramer core is made up of α-helices of the TIM barrel (Fig. 4B). There are two different types of subunit interfaces in the tetramer. The large interface is formed between monomers A and B and monomers C and D and is dominated by an extended eight-strand β-sheet with four strands from each subunit as well as coiled-coil interactions between three α-helices of the central (α/β)8 barrel (α8 and α12) and its extension (α16). The small interface is formed between monomers A and C and monomers B and D and is dominated by interactions with four α-helices (α4, α5, α7, and α8) from each subunit. The first interface is tighter and buries 2,414 Å2 of solvent-accessible surface from each monomer; the second is less compact and buries 2,132 Å2 of solvent-accessible surface from each monomer. The extents of both interfaces are in the range expected for protein-protein interactions (25). There are no direct contacts between monomers A and D and monomers B and C. The interface contacts involve both hydrophilic (including hydrogen bonding and electrostatic) interactions and hydrophobic interactions. Since both the gel filtration and dynamic light scattering data suggest that the stable form of HYDBp is a dimer in solution, it is not clear yet whether the tetrameric form observed in the crystal structure is of any biological significance or is merely an artifact of the high protein concentration in the crystallization condition.
Biochemical data have shown that the enzymatic activities of HYDs require metal ions as cofactors (54). In addition to their participation at the catalytic active site (see discussion later), it has been suggested that in HYDAau, metal ions might also be involved in the intersubunit interactions and the removal of metal ions dissociates the oligomeric form to the monomer (45, 46). However, in all crystal structures of HYDs reported so far, including HYDAau, no metal ion has been observed at the intersubunit interfaces. There is no metal ion detected at the intersubunit interface in this structure either.
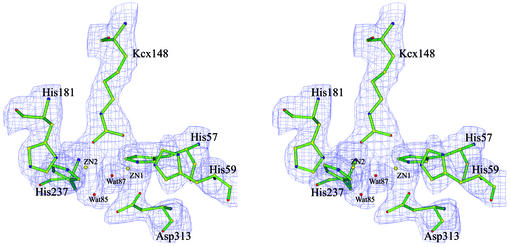
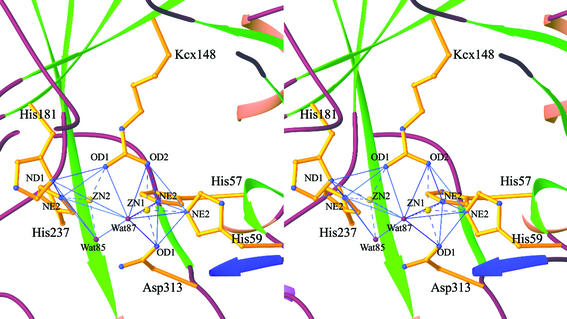
Structure of the active site.
The catalytic active site is located in a deep hydrophobic cleft which is situated at one end of the TIM barrel distant from the β-sheet domain (Fig. 4). The cleft is exposed to solvent, but not to the tetramer interfaces; so the active site is accessible to the substrate. The active site consists of six strictly conserved amino acid residues which are His57, His59, His181, His237, Asp313, and the carboxylated lysine Kcx148 (Fig. 5). These residues are also highly conserved in other amidohydrolases, including dihydroorotase and urease. The carboxylated lysine 148 participates in metal coordination, and its presence is required for the enzymatic activity of HYD. A γ-carboxylated lysine is present at the equivalent site in other HYDs with known structures as well as in the structures of ureases, phosphotriesterase, dihydroorotase, and ribulose-1,5-bisphosphate carboxylase/oxygenase (2).
FIG. 5.
Structure of the catalytic active site of HYDBp. The six strictly conserved amino acid residues are shown with side chains. The two Zn2+ ions are shown as large yellow spheres, and the two conserved water molecules are shown as small red spheres. The coordination interactions are shown as dashed lines. Both Zn ions have distorted trigonal bipyramidal geometry.
As in the structure of HYDTsp, there are two Zn2+ ions and two conserved water molecules observed at the catalytic active site. One Zn2+ (Zn1) is coordinated by His57 Nɛ2, His59 Nɛ2, Kcx148 Ox2, Asp313 Oδ1, and a water molecule (Wat87). The other Zn2+ (Zn2) is coordinated by Kcx148 Ox1, His181 Nδ1, His237 Nɛ2, and two water molecules (Wat85 and Wat87). However, the coordination geometries of the metal ions and the positions of the two water molecules in HYDBp differ from those in HYDTsp. In HYDBp, both coordinations adopt distorted trigonal bipyramidal geometry (Fig. 5). Wat87 bridges the two zinc ions and is positioned at the vertices of the two trigonal bipyramids, and Wat85 coordinates with Zn2 at the equatorial position. In HYDTsp, Zn1 has a distorted trigonal bipyramidal geometry and Zn2 has a slightly distorted square pyramidal geometry (2). The bridging water molecule takes the planar position while the other water molecule takes the vertex position of Zn1 coordination. No water molecule was observed in HYDBst structure, potentially owing to the moderate resolution (3 Å) of the structure (13). The bridging water molecule is believed to be deprotonated and act as an active nucleophile in the hydrolytic reaction (2). Whether the differences in the metal coordination geometry and/or the positions of conserved water molecules at the active site in different HYD structures have any biological significance or merely reflect the difference in the resolutions of different structures is unclear. Determination of high-resolution crystal structures of HYDs in complexes with various substrates might resolve this question. Nevertheless, the structures of urease have shown that the coordination geometry does vary depending on the bound substrates (5, 6, 23, 24).
So far there is no substrate-bound HYD structure. However, the structure of the substrate-binding site and the interactions between substrate and HYD have been modeled based on the structures of ureases and dihydroorotase (1, 2, 13). The substrate-binding site contains recognition sites for both the amide group and the exocyclic substituent of hydantoin. Structural comparison indicates that the functional recognition site in HYDBp comprises four conserved residues: Tyr153, His181, Thr286, and Asn335. These residues would form several hydrogen bonds with the amide group of hydantoin, and these interactions discriminate the ring orientation of the substrate. Though the equivalent residue of Thr286 in HYDTsp and HYDBst is serine, the change of Ser to Thr does not affect its hydrogen-bonding interaction with the substrate. The exocyclic substituent recognition site in HYDBp consists primarily of residues Thr62, Ser64, Gln93, Phe150, Tyr153, and Asn157. Except for Phe150 and Tyr153, the other residues at the equivalent positions in different HYDs are less conserved. Both HYDTsp and HYDBst have hydrophobic residues at these positions while HYDBp contains hydrophilic residues at these positions. It has been suggested that their interactions with hydantoin might control the substrate specificity and enantioselectivity (1, 2, 13). The preference for hydrophilic residues at the exocyclic substituent recognition site in HYDBp might suggest that HYDBp might favor hydantoins containing the polar substituent as a substrate. It would be interesting to see if HYDBp has a substrate specificity different from those of HYDTsp and HYDBst.
Comparison of HYD structures from different species.
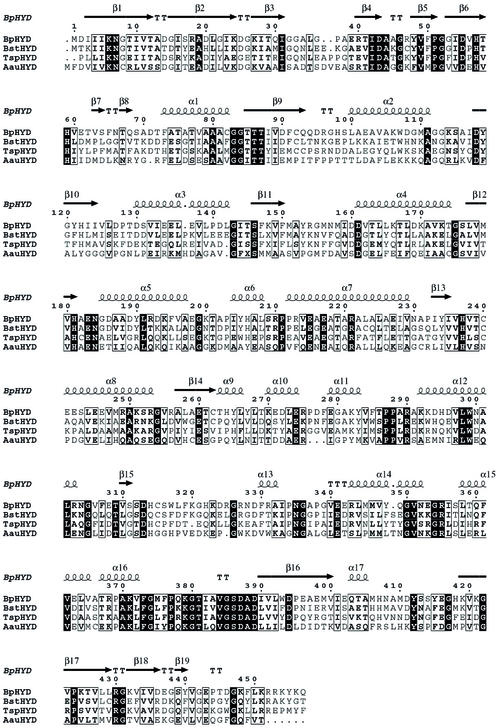
Although HYDBp shares moderate sequence homology with HYDTsp, HYDBst, and HYDAau (Fig. 6 and Table 3), their overall structures and the structures of the catalytic active sites are strikingly similar. Superpositions based on Cα atoms of the monomers reveal RMSDs of 1.15 Å for 1GKP (447 Cα atoms), 1.08 Å for 1GKQ (446 Cα atoms), 1.01 Å for 1K1D (452 Cα atoms), and 1.62 Å for 1GKR (418 Cα atoms) (Table 3). The most significant structural differences are located on the surface loops or in the regions having residue(s) insertion or deletion, including regions 33 to 41 (β3-β4 loop), 62 to 69 (β7-β8), 94 to 99 (β9-α2 loop), 127 to 140 (β10-α3 loop and α3), 275 to 280 (α10-α11 loop), 317 to 322 (β15-α13 loop), 352 to 356 (α14-α15 loop), and 455 to 457 (C terminus). The core residues involved in secondary structures are better conserved than those exposed on the surface. Most of the deletions or insertions are found in solvent-exposed areas. There are small differences in the secondary structure assignments between these enzymes which reflect most likely the minor fluctuations of hydrogen-bonding interactions in different structures rather than real structural differences.
FIG. 6.
Sequence alignment of HYDs from four bacterial organisms with known crystal structures. The secondary structure of HYDBp is placed on top. The alignment was generated with ClustalW (21) and drawn with ESPript (19). Invariant residues are highlighted in black, and conserved residues are shown in boldface type.
On the other hand, superpositions of the tetramers reveal much larger RMSDs of 2.20 Å for 1GKP (1,622 Cα atoms), 1.68 Å for 1GKQ (1,751 Cα atoms), 2.02 Å for 1K1D (1,500 Cα atoms), and 2.46 Å for 1GKR (890 Cα atoms) (Table 3). This indicates that the intersubunit relationships within the tetramer differ slightly in different HYD structures. This is in agreement with the observation that the buried accessible surface areas at the intersubunit interfaces vary in different HYD structures (Table 3). The two HYDTsp structures (1GKP and 1GKQ) with different space groups show relatively smaller RMSDs for the tetramer superposition (0.92 versus 0.41 Å for the monomer superposition). Since oligomerization of HYD is required for its enzymatic activity, the variation of the intersubunit contacts in different HYD structures might be correlated with their enzymatic properties, such as thermostability and enzymatic activity.
It is noteworthy that, although HYDBst is from a thermophile, its enzymatic properties appear more like those of HYDBp than those of HYDTsp. The thermostability of HYDBst is between those of HYDTsp and HYDBp (see below). Sequence comparison shows that HYDBst is more homogenous to HYDBp (51%) than to HYDTsp (40%). There are more structural similarities between HYDBst and HYDBp than between HYDBst and HYDTsp, including conserved amino acid residues, the number of hydrophilic interactions, and the type of residues involved in the formation of hydrophobic cores (see below).
It has been proposed that HYDAau, an l-HYD, diverged early in the evolution from other HYDs (1, 42). Indeed, HYDAau is not only less homologous to d-HYDs in sequence (<30% identity), its overall structure is also less similar to the d-HYD structures, with much higher RMSD values for both the monomer and the tetramer (Fig. 6 and Table 3). In particular, the interface contacts of the tetramer in HYDAau are markedly different from those in the d-HYD structures. The interface between subunits A and B in HYDAau has much fewer interactions than that in the d-HYD structures and buries only 1,334 Å2 of solvent-accessible surface, whereas the interface between subunits A and C is comparable to that in the d-HYD structures and buries 1,734 Å2 of solvent-accessible surface (Table 3).
Insights into the molecular basis of the thermostability of HYDs.
The overall structure, secondary structure topology, and the structure of the catalytic active site of HYDBp are similar to those of HYDBst and HYDTsp, yet their thermostabilities differ evidently. HYDBp is stable at 50°C but shows reduced enzymatic activity at 60°C (see above). HYDBst is stable up to 60°C (37, 48). HYDTsp displays remarkable thermostability and can be used for several hours at 70°C (28). In general, the thermostability of the three d-HYDs can be ordered as follows: HYDTsp > HYDBst > HYDBp.
Many factors have been suggested to influence the thermostability of an enzyme, including compactness of the structure, overall hydrophobicity, amino acid composition, salt bridges, and hydrogen-bonding interactions, etc. (15, 32, 35, 61, 65). The difficulty in identifying the main determinants for thermostability has been partially attributed to limited structural information available on both thermostable and mesostable proteins. The availability of the structures of d-HYDs from both thermophilic and mesophilic species permits a detailed structural comparison.
Amino acid composition.
Protein amino acid composition is considered to be correlated to its thermostability (11, 12, 57, 59). Comparison of the amino acid compositions of HYDBp, HYDBst, and HYDTsp has revealed some interesting differences (Table 4). HYDBp has a relatively lower Pro content (18 of 457 residues) than HYDTsp (22 of 458 residues) (HYDBst contains 18 Pro residues and 460 total residues). Proline is a residue with a rigid conformation and places constraints on the main-chain flexibility of proteins. An increase in the proline content of HYDTsp might constrain its conformational flexibility and, thus, might help HYDTsp to resist unfolding at high temperatures. Another notable difference is the greater Met content (13 of 457 residues) in HYDBp than that in HYDTsp (9 of 458 residues) and HYDBst (8 of 469 residues) (Table 4). The Cys content in HYDBp (5 of 457 residues) is comparable to that in HYDBst (8 of 469 residues) and HYDTsp (6 of 458 residues). Analyses of the positions of Met and Cys in the context of three-dimensional structures of HYDs reveal that HYDBp has many more Met and Cys residues (11 of 18 residues) on the surface than HYDBst (7 of 16 residues) and HYDTsp (6 of 15 residues). Met and Cys residues on the surface can be readily oxidized at high temperatures and decrease the thermostability of proteins (15). The presence of a large number of Met and Cys residues on the HYDBp surface might contribute in part to its lower thermostability.
TABLE 4.
Comparison of properties of different D-HYD structures that may affect the enzyme's thermostability
| HYD structure (PDB entry no.) | Amino acid composition (no. of residues/% of residues)
|
No. of hydrophilic interactions (3.6 Å)a
|
||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Total | Hydro- phobic | Polar | Acidic | Basic | Cys | Met | Pro | Phe | Tyr | Trp | Intersubunits A and B | Intersubunits A and C | Intra- molecule | |
| HYDBp (1NFG) | 457 | 258/56.5 | 217/47.5 | 63/14.0 | 64/14.0 | 5/1.1 | 13/2.8 | 18/3.9 | 16/3.5 | 15/3.3 | 4/0.9 | 6 | 4 | 752 |
| HYDBst (1K1D) | 460 | 259/56.3 | 220/47.8 | 63/13.9 | 59/12.8 | 8/1.7 | 8/1.7 | 18/3.9 | 19/4.1 | 15/3.3 | 5/1.1 | 8 | 3 | 755 |
| HYDTsp (1GKP) | 458 | 259/56.6 | 223/48.7 | 61/13.5 | 62/13.5 | 6/1.3 | 9/2.0 | 22/4.8 | 23/5.0 | 20/4.4 | 4/0.9 | 9 (9) | 3 (3) | 821 (799) |
The hydrophilic interactions were computed by using the program CONTACT (14). For HYDTsp, the numbers of interactions for PDB entry no. 1GKQ are given in parentheses.
Aromatic residues in hydrophobic cores.
The hydrophobic effect is considered to be the major driving force in protein folding (16). An increase in the overall hydrophobicity of a protein can increase the internal packing (compactness) of its structure, thereby enhancing its thermostability (4, 35, 41). Comparison of the amino acid compositions of HYDBp, HYDBst, and HYDTsp shows no significant difference in the overall hydrophobicity, yet it shows marked differences in the numbers of hydrophobic residues containing large aromatic side chains (Phe, Tyr, and Trp) (Table 4). The content of aromatic residues in HYDTsp (47 of 458 residues) is notably higher than that in HYDBp (35 of 457 residues) and HYDBst (39 of 469 residues). The significance of this difference becomes more evident when viewed in the context of three-dimensional structure. Most of these aromatic residues are buried in the interior and participate in forming the proteins' hydrophobic cores. When a large hydrophobic residue in the protein core is changed to a smaller residue, the protein is usually destabilized to a varying degree (9, 17, 20, 35, 56). This destabilization is primarily due to the reduction in hydrophobicity and the creation of a cavity. Structural comparison indicates that substitution of large aromatic residues in HYDTsp with smaller residues in HYDBp or HYDBst would apparently increase the number and volume of cavities of various size in the interior of the protein structure and conceivably makes HYDBp and HYDBst less stable and more susceptible to high-temperature degradation.
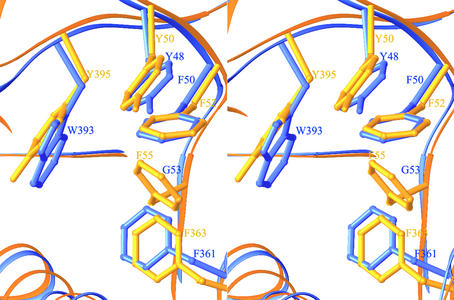
Several hydrophobic cores in the structures of d-HYDs are noteworthy. There are two hydrophobic cores at the interface between the β-sheet domain and the TIM barrel domain. One is located between α-helices α15 and α16 and the β-sheet domain and consists of amino acid residues Tyr48 (β5), Phe50 (β5), Gly53 (β5), Phe361 (α15), and Trp393 (β16) in HYDBp (Fig. 7). The same cluster of residues exist in both HYDBst (Tyr49, Phe51, Gly54, Phe364, and Phe396) and HYDTsp (Tyr50, Phe52, Phe55, Phe363, and Tyr395) (Fig. 7). The replacement of Phe55 in HYDTsp to Gly53 in HYDBp (or Gly54 in HYDBst) creates a cavity in the hydrophobic core and, thus, may decrease the stability of HYDBp (or HYDBst) at high temperatures. Vice versa, mutation of Gly53 to Phe in HYDBp or HYDBst would introduce more hydrophobic interactions and is likely to improve their thermostability. Another hydrophobic core is situated between α-helices α15 and α16 and the TIM barrel domain, comprising amino acid residues Leu266 (α9), Tyr267 (α9), Trp299 (α12), Gly350 (α14), and Tyr415 (β17) in HYDBp. The corresponding residues are Leu268, Val269, Trp301, Gly353, and Phe418 in HYDBst and Phe268, Leu269, Trp301, Tyr351, and Phe417 in HYDTsp. Changing Gly350 in HYDBp to Tyr or Phe is also likely to stabilize the hydrophobic core and might increase its thermostability.
FIG. 7.
Structural comparison of a representative hydrophobic core formed by aromatic residues in HYDBp and HYDTsp. The side chains of HYDBp are shown in blue; those of HYDTsp are shown in gold. Changing Phe55 in HYDTsp to Gly53 creates a cavity in the hydrophobic core and destabilizes the structure.
There are also several hydrophobic cores between the α-helix and β-sheet layers of the TIM barrel domain. One is located near the catalytic active site and is made of amino acid residues Phe73 (α1), Ile88 (β9), Tyr119 (β10), and Tyr121 (β10). The equivalent residues are Phe74, Ile89, Tyr120, and Phe122 in HYDBst and His75, Tyr90, Tyr121, and Phe123 in HYDTsp. Substitution of Ile88 in HYDBp (or Ile89 in HYDBst) to Tyr or Phe could increase the compactness of the hydrophobic core and might increase the thermostability of HYDBp and HYDBst. Another hydrophobic core occurs between α4 and α7 and β12 and β13. HYDTsp has residue Phe224 pointing its aromatic side chain towards the center of the core while both HYDBp (Ala222) and HYDBst (Ala224) have alanine at the equivalent position. Changing Ala222 to Phe might also stabilize the structures of HYDBp and HYDBst.
Hydrophilic interactions.
Biochemical and structural analyses of mesophilic and thermophilic proteins indicate that a large number of hydrophilic interactions (charged ion pairs and hydrogen-bonding interactions) are the essential structural feature responsible for the enhanced thermostability of hyperthermophilic and thermophilic enzymes (18, 32, 63, 65). Comparison of amino acid compositions shows that HYDTsp (223 of 458 residues) contains relatively more polar amino acid residues than HYDBst (220 of 469 residues) and HYDBp (217 of 457 residues) (Table 4). Analyses of the intramolecular hydrophilic interactions indicate that HYDTsp has more hydrophilic interactions than HYDBp and HYDBst (Table 4). There are 44 pairs of salt bridges and 777 hydrogen-bonding interactions (distance, ≤3.6 Å) in HYDTsp. There are only 38 pairs of salt bridges and 714 hydrogen-bonding interactions in HYDBp and 22 pairs of salt bridges and 733 hydrogen-bonding interactions in HYDBst. Thermostable proteins enhance electrostatic interactions through optimal placement of charged amino acid residues within the protein structure, especially on the protein surface, to increase their thermostability (65). Analyses show that in all three d-HYDs, most of the charged amino acid residues (79.5% in HYDBp, 81.1% in HYDBst, and 80.5% in HYDTsp) are located on the protein surface and many of them form networks of salt bridges and hydrogen-bonding interactions. A significantly increased number of hydrophilic interactions on the protein surface in HYDTsp appear to stabilize the structure of HYDTsp and might contribute in part to its high thermostability.
It has been suggested that intersubunit salt bridges and hydrogen-bonding interactions can enhance the thermostability of oligomeric structures (7, 55), and the oligomeric structure appears to contribute to the thermostability of HYDs (48). However, comparison of the hydrophilic interactions at the intersubunit interfaces in the three d-HYD structures reveals only minor differences at the interface of subunits A and B (Table 4). The hydrophilic interactions at the interface of subunits A and C are also comparable. It appears that the hydrophilic interactions at the subunit interfaces might have minor contributions to the differences in the thermostability of different HYDs.
In summary, the difference in thermostability of thermophilic and mesophilic d-HYDs appears to be a combination of contributions from several factors. The more thermostable HYDTsp contains more aromatic residues in the interior of the structure, more salt bridges and hydrogen-bonding interactions, less oxidation-susceptible Met and Cys residues on the protein surface, and more rigid proline residue than HYDBp and HYDBst. These factors are likely to make major contributions to the varying thermostability of these enzymes. This information could be useful in helping to engineer new mesophilic d-HYDs with improved thermostability.
Acknowledgments
We thank Yadong Yu and Yongcheng Lu for help with X-ray data collection and discussion.
The research in J.D.'s laboratory was supported by National Natural Science Foundation of China (NSFC) grants (no. 30125011, 30170223, and 30130080), 863 Hi-Tech Program grants (no. 2001AA235071 and 2002BA711A13), and a Chinese Academy of Sciences grant (no. KSCX1-SW-1), and the work in W.J.'s laboratory was supported by an NSFC grant (no. 30125002) and an 863 Hi-Tech Program grant (no. 2001AA235081). E.A.'s laboratory was sponsored in part by National Institutes of Health grants (no. AI 27690 and GM 66671).
REFERENCES
- 1.Abendroth, J., K. Niefind, O. May, M. Siemann, C. Syldatk, and D. Schomburg. 2002. The structure of L-hydantoinase from Arthobacter aurescens leads to an understanding of dihydropyrimidinase substrate and enantio specificity. Biochemistry 41:8589-8597. [DOI] [PubMed] [Google Scholar]
- 2.Abendroth, J., K. Niefind, and D. Schomburg. 2002. X-ray structure of a dihydropyrimidinase from Thermus sp. at 1.3 Å resolution. J. Mol. Biol. 320:143-156. [DOI] [PubMed] [Google Scholar]
- 3.Altenbuchner, J., M. Siemann-Herzberg, and C. Syldatk. 2001. Hydantoinases and related enzymes as biocatalysts for the synthesis of unnatural chiral amino acids. Curr. Opin. Biotechnol. 12:559-563. [DOI] [PubMed] [Google Scholar]
- 4.Argos, P., M. G. Rossmann, U. M. Grau, H. Zuber, G. Frank, and J. D. Tratschin. 1979. Thermal stability and protein structure. Biochemistry 18:5698-5703. [DOI] [PubMed] [Google Scholar]
- 5.Benini, S., W. R. Rypniewski, K. S. Wilson, S. Miletti, S. Ciurli, and S. Mangani. 2000. The complex of Bacillus pasteurii urease with acetohydroxamate anion from X-ray data at 1.55 Å resolution. J. Biol. Inorg. Chem. 5:110-118. [DOI] [PubMed] [Google Scholar]
- 6.Benini, S., W. R. Rypniewski, K. S. Wilson, S. Miletti, S. Ciurli, and S. Mangani. 1999. A new proposal for urease mechanism based on the crystal structures of the native and inhibited enzyme from Bacillus pasteurii: why urea hydrolysis costs two nickels. Structure 7:205-216. [DOI] [PubMed] [Google Scholar]
- 7.Bogin, O., I. Levin, Y. Hacham, S. Tel-Ir, M. Peretz, F. Frolow, and Y. Burstein. 2002. Structural basis for the enhanced thermal stability of alcohol dehydrogenase mutants from the mesophilic bacterium Clostridium beijerinckii: contribution of salt bridging. Protein Sci. 11:2561-2574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Brunger, A. T., P. D. Adams, G. M. Clore, W. L. Delano, P. Gros, R. W. Grosse-Kunstleve, J.-S. Jiang, J. Kuszewski, M. Nilges, N. S. Pannu, R. J. Read, L. M. Rice, T. Simonson, and G. L. Warren. 1998. Crystallography & NMR system: a new software suite for macromolecular structure determination. Acta Crystallogr. D 54:905-921. [DOI] [PubMed] [Google Scholar]
- 9.Buckle, A. M., P. Cramer, and A. R. Fersht. 1996. Structural and energetic responses to cavity-creating mutations in hydrophobic cores: observation of a buried water molecule and the hydrophilic nature of such hydrophobic cavities. Biochemistry 35:4298-4305. [DOI] [PubMed] [Google Scholar]
- 10.Carson, M. 1987. Ribbon models of macromolecules. J. Mol. Graph. 5:103-106. [Google Scholar]
- 11.Chakravarty, S., and R. Varadarajan. 2000. Elucidation of determinants of protein stability through genome sequence analysis. FEBS Lett. 470:65-69. [DOI] [PubMed] [Google Scholar]
- 12.Chakravarty, S., and R. Varadarajan. 2002. Elucidation of factors responsible for enhanced thermal stability of proteins: a structural genomics based study. Biochemistry 41:8152-8161. [DOI] [PubMed] [Google Scholar]
- 13.Cheon, Y. H., H. S. Kim, K. H. Han, J. Abendroth, K. Niefind, D. Schomburg, J. Wang, and Y. Kim. 2002. Crystal structure of D-hydantoinase from Bacillus stearothermophilus: insight into the stereochemistry of enantioselectivity. Biochemistry 41:9410-9417. [DOI] [PubMed] [Google Scholar]
- 14.Collaborative Computational Project, Number 4. 1994. The CCP4 suite: programs for protein crystallography. Acta Crystallogr. D 50:760-763. [DOI] [PubMed] [Google Scholar]
- 15.Danson, M. J., and D. W. Hough. 1998. Structure, function and stability of enzymes from Archaea. Trends Microbiol. 6:307-314. [DOI] [PubMed] [Google Scholar]
- 16.Dill, K. A. 1990. Dominant forces in protein folding. Biochemistry 29:7133-7155. [DOI] [PubMed] [Google Scholar]
- 17.Eriksson, A. E., W. A. Baase, X. J. Zhang, D. W. Heinz, M. Blaber, E. P. Baldwin, and B. W. Matthews. 1992. Response of a protein structure to cavity-creating mutations and its relation to the hydrophobic effect. Science 255:178-183. [DOI] [PubMed] [Google Scholar]
- 18.Goldman, A. 1995. How to make my blood boil. Structure 3:1277-1279. [DOI] [PubMed] [Google Scholar]
- 19.Gouet, P., E. Courcelle, D. I. Stuart, and F. Metoz. 1999. ESPript: multiple sequence alignments in PostScript. Bioinformatics 15:305-308. [DOI] [PubMed] [Google Scholar]
- 20.Grimsley, G. R., K. L. Shaw, L. R. Fee, R. W. Alston, B. M. Huyghues-Despointes, R. L. Thurlkill, J. M. Scholtz, and C. N. Pace. 1999. Increasing protein stability by altering long-range coulombic interactions. Protein Sci. 8:1843-1849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Higgins, D., J. Thompson, T. Gibson, J. D. Thompson, D. G. Higgins, and T. J. Gibson. 1994. CLUSTAL W: improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position-specific gap penalties and weight matrix choice. Nucleic Acids Res. 22:4673-4680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Holm, L., and C. Sander. 1997. An evolutionary treasure: unification of a broad set of amidohydrolases related to urease. Proteins 28:72-82. [PubMed] [Google Scholar]
- 23.Jabri, E., M. B. Carr, R. P. Hausinger, and P. A. Karplus. 1995. The crystal structure of urease from Klebsiella aerogenes. Science 268:998-1004. [PubMed] [Google Scholar]
- 24.Jabri, E., and P. A. Karplus. 1996. Structures of the Klebsiella aerogenes urease apoenzyme and two active-site mutants. Biochemistry 35: 10616-10626. [DOI] [PubMed] [Google Scholar]
- 25.Janin, J., and C. Chothia. 1990. The structure of protein-protein recognition sites. J. Biol. Chem. 65:16027-16030. [PubMed] [Google Scholar]
- 26.Jones, T. A., J. Y. Zou, S. W. Cowan, and M. Kjeldgaard. 1991. Improved methods for building protein models in electron density maps and the location of errors in these models. Acta Crystallogr. A 47:110-119. [DOI] [PubMed] [Google Scholar]
- 27.Kabsch, W., and C. Sander. 1983. Dictionary of protein secondary structure: pattern recognition of hydrogen-bonded and geometrical features. Biopolymers 22:2577-2637. [DOI] [PubMed] [Google Scholar]
- 28.Keil, O., M. P. Schneider, and J. P. Rasor. 1995. New hydantoinases from thermophilic microorganisms-synthesis of enantiomerically pure D-amino acids. Tetrahedron 6:1257-1260. [Google Scholar]
- 29.Kim, G. J., Y. H. Cheon, and H. S. Kim. 2000. Directed evolution of a novel N-carbamylase/D-hydantoinase fusion enzyme for functional expression with enhanced stability. Biotechnol. Bioeng. 68:211-217. [PubMed] [Google Scholar]
- 30.Kim, G. J., J. H. Park, D. C. Lee, H. S. Ro, and H. S. Kim. 1997. Primary structure, sequence analysis, and expression of the thermostable D-hydantoinase from Bacillus stearothermophilus SD1. Mol. Gen. Genet. 255:152-156. [DOI] [PubMed] [Google Scholar]
- 31.Kim, G. L., and H. S. Kim. 1998. C-terminal regions of D-hydantoinases are nonessential for catalysis, but affect the oligomeric structure. Biochem. Biophys. Res. Commun. 243:96-100. [DOI] [PubMed] [Google Scholar]
- 32.Ladenstein, R., and G. Antranikian. 1998. Proteins from hyperthermophiles: stability and enzymatic catalysis close to the boiling point of water. Adv. Biochem. Eng. Biotechnol. 61:38-85. [DOI] [PubMed] [Google Scholar]
- 33.Laskowski, R. A., M. W. MacArthur, D. S. Moss, and J. M. Thornton. 1993. PROCHECK: a program to check the stereochemical quality of protein structures. J. Appl. Crystallogr. 26:283-291. [Google Scholar]
- 34.Lee, B., and F. M. Richards. 1971. The interpretation of protein structures: estimation of static accessibility. J. Mol. Biol. 55:379-400. [DOI] [PubMed] [Google Scholar]
- 35.Lee, B., and G. Vasmatzis. 1997. Stabilization of protein structures. Curr. Opin. Biotechnol. 8:423-428. [DOI] [PubMed] [Google Scholar]
- 36.Lee, S. G., D. C. Lee, S. P. Hong, M. H. Sung, and H. S. Kim. 1995. Thermostable D-hydantoinase from thermophilic Bacillus stearothermophilus SD-1: characteristics of purified enzyme. Appl. Microbiol. Biotechnol. 43:270-276. [Google Scholar]
- 37.Lee, S. G., D. C. Lee, and H. S. Kim. 1997. Purification and characterization of thermostable D-hydantoinase from thermophilic Bacillus stearothermophilus SD-1. Appl. Biochem. Biotechnol. 62:251-266. [DOI] [PubMed] [Google Scholar]
- 38.Lee, S. G., D. C. Lee, M. H. Sung, and H. S. Kim. 1994. Isolation of thermostable D-hydantoinase-producing thermophilic Bacillus sp. SD-1. Biotechnol. Lett. 16:461-466. [Google Scholar]
- 39.Luzzati, V. 1952. Traitement statistique des erreurs dans la determination des structures cristallines. Acta Crystallogr. 5:802-810. [Google Scholar]
- 40.Matthews, B. W. 1968. Solvent content of protein crystals. J. Mol. Biol. 33:491-497. [DOI] [PubMed] [Google Scholar]
- 41.Matthews, B. W., H. Nicholson, and W. J. Becktel. 1987. Enhanced protein thermostability from site-directed mutations that decrease the entropy of unfolding. Proc. Natl. Acad. Sci. USA 84:6663-6667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.May, O., A. Habenicht, R. Mattes, C. Syldatk, and M. Siemann. 1998. Molecular evolution of hydantoinases. Biol. Chem. 379:743-747. [PubMed] [Google Scholar]
- 43.May, O., P. T. Nguyen, and F. H. Arnold. 2000. Inverting enantioselectivity by directed evolution of hydantoinase for improved production of L-methionine. Nat. Biotechnol. 18:317-320. [DOI] [PubMed] [Google Scholar]
- 44.May, O., M. Siemann, M. Pietzsch, M. Kiess, R. Mattes, and C. Syldatk. 1998. Substrate-dependent enantioselectivity of a novel hydantoinase from Arthrobacter aurescens DSM 3745: purification and characterization as a new member of cyclic amidases. J. Biotechnol. 61:1-13. [DOI] [PubMed] [Google Scholar]
- 45.May, O., M. Siemann, M. G. Siemann, and C. Syldatk. 1998. Catalytic and structural function of zinc for the hydantoinase from Arthrobacter aurescens DSM 3745. J. Mol. Catal. B4:211-218.
- 46.May, O., M. Siemann, M. G. Siemann, and C. Syldatk. 1998. The hydantoin amidohydrolase from Arthrobacter aurescens DSM 3745 is a zinc metalloenzyme. J. Mol. Catal. B 5:367-370. [Google Scholar]
- 47.Moller, A., C. Syldatk, M. Schulze, and F. Wagner. 1988. Stereo- and substrate-specificity of a D-hydantoinase and a D-N-carbamyl-amino acid amidohydrolase of Arthrobacter crystallopoietes AM2. Enzyme Microb. Technol. 10:618-625. [Google Scholar]
- 48.Mukohara, Y., T. Ishikawa, K. Watabe, and H. Nakamura. 1994. A thermostable hydantoinase of Bacillus stearothermophilus NS1122A: cloning, sequencing, and high expression of the enzyme gene, and some properties of the expressed enzyme. Biosci. Biotechnol. Biochem. 58:1621-1626. [DOI] [PubMed] [Google Scholar]
- 49.Ogawa, J., and S. Shimizu. 2002. Industrial microbial enzymes: their discovery by screening and use in large-scale production of useful chemicals in Japan. Curr. Opin. Biotechnol. 13:367-375. [DOI] [PubMed] [Google Scholar]
- 50.Park, J. H., G. J. Kim, and H. S. Kim. 2000. Production of D-amino acid using whole cells of recombinant Escherichia coli with separately and coexpressed D-hydantoinase and N-carbamoylase. Biotechnol. Prog. 16:564-570. [DOI] [PubMed] [Google Scholar]
- 51.Park, J. H., G. J. Kim, S. G. Lee, and H. S. Kim. 1998. Biochemical properties of thermostable D-hydantoinase from Bacillus thermocatenulatus GH-2. Ann. N. Y. Acad. Sci. 864:337-340. [DOI] [PubMed] [Google Scholar]
- 52.Pflugrath, J. W. 1999. The finer things in X-ray diffraction data collection. Acta Crystallogr. D 55:1718-1725. [DOI] [PubMed] [Google Scholar]
- 53.Syldatk, C., A. Laufer, R. Muller, and H. Hoke. 1990. Production of optically pure D- and L-amino acids by bioconversion of D,L-5-monosubstituted hydantoin derivatives. Adv. Biochem. Eng. Biotechnol. 41:31-75. [Google Scholar]
- 54.Syldatk, C., O. May, J. Altenbuchner, R. Mattes, and M. Siemann. 1999. Microbial hydantoinases—industrial enzymes from the origin of life? Appl. Microbiol. Biotechnol. 51:293-309. [DOI] [PubMed] [Google Scholar]
- 55.Szilagyi, A., K. L. Kovacs, G. Rakhely, and P. Zavodszky. 2002. Homology modeling reveals the structural background of the striking difference in thermal stability between two related [NiFe]hydrogenases. J. Mol. Model. 8:58-64. [DOI] [PubMed] [Google Scholar]
- 56.Tanako, K., Y. F. Yamagata, S., and K. Yutani. 1997. Contribution of the hydrophobic effect of the stability of human lysozyme: calorimetric studies and X-ray structural analyses of the nine valine to alanine mutants. Biochemistry 36:688-698. [DOI] [PubMed] [Google Scholar]
- 57.Tekaia, F., E. Yeramian, and B. Dujon. 2002. Amino acid composition of genomes, lifestyles of organisms, and evolutionary trends: a global picture with correspondence analysis. Gene 297:51-60. [DOI] [PubMed] [Google Scholar]
- 58.Thoden, J. B., G. N. J. Phillips, T. M. Neal, F. M. Raushel, and H. M. Holden. 2001. Molecular structure of dihydroorotase: a paradigm for catalysis through the use of a binuclear metal center. Biochemistry 40:6989-6997. [DOI] [PubMed] [Google Scholar]
- 59.Thompson, M. J., and D. Eisenberg. 1999. Transproteomic evidence of a loop-deletion mechanism for enhancing protein thermostability. J. Mol. Biol. 290:595-604. [DOI] [PubMed] [Google Scholar]
- 60.Vanhooke, J. L., M. M. Benning, F. M. Raushel, and H. M. Holden. 1996. Three-dimensional structure of the zinc-containing phosphotriesterase with the bound substrate analog diethyl 4-methylbenzylphosphonate. Biochemistry 35:6020-6025. [DOI] [PubMed] [Google Scholar]
- 61.Vieille, C., and G. J. Zeikus. 2001. Hyperthermophilic enzymes: sources, uses, and molecular mechanisms for thermostability. Microbiol. Mol. Biol. Rev. 65:1-43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Vogels, G. D., and C. van der Drift. 1976. Degradation of purines and pyrimidines by microorganisms. Bacteriol. Rev. 40:403-468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Vogt, G., S. Woell, and P. Argos. 1997. Protein thermal stability, hydrogen bonds, and ion pairs. J. Mol. Biol. 269:631-643. [DOI] [PubMed] [Google Scholar]
- 64.Wilms, B., A. Wiese, C. Syldatk, R. Mattes, and J. Altenbuchner. 2001. Development of an Escherichia coli whole cell biocatalyst for the production of L-amino acids. J. Biotechnol. 86:19-30. [DOI] [PubMed] [Google Scholar]
- 65.Xiao, L., and B. Honig. 1999. Electrostatic contributions to the stability of hyperthermophilic proteins. J. Mol. Biol. 289:1435-1444. [DOI] [PubMed] [Google Scholar]
- 66.Xu, Z., W. H. Jiang, R. S. Jiao, and Y. L. Yang. 2002. Cloning, sequencing and high expression in Escherichia coli of D-hydantoinase gene from Burkholderia pickettii. Sheng Wu Gong Cheng Xue Bao 18:149-154. [PubMed] [Google Scholar]
- 67.Yamashiro, A., K. Yokozeki, H. Kano, and K. Kubota. 1998. Enzymatic production of L-amino acids from the corresponding 5′-substituted hydantoins by a newly isolated bacterium Bacillus brevis AJ-12299. Agric. Biol. Chem. 52:2851-2856. [Google Scholar]