Abstract
UDP-N-acetylmuramic acid:l-alanine ligase (MurC) catalyzes the addition of the first amino acid to the cytoplasmic precursor of the bacterial cell wall peptidoglycan. The crystal structures of Haemophilus influenzae MurC in complex with its substrate UDP-N-acetylmuramic acid (UNAM) and Mg2+ and of a fully assembled MurC complex with its product UDP-N-acetylmuramoyl-l-alanine (UMA), the nonhydrolyzable ATP analogue AMPPNP, and Mn2+ have been determined to 1.85- and 1.7-Å resolution, respectively. These structures reveal a conserved, three-domain architecture with the binding sites for UNAM and ATP formed at the domain interfaces: the N-terminal domain binds the UDP portion of UNAM, and the central and C-terminal domains form the ATP-binding site, while the C-terminal domain also positions the alanine. An active enzyme structure is thus assembled at the common domain interfaces when all three substrates are bound. The MurC active site clearly shows that the γ-phosphate of AMPPNP is positioned between two bound metal ions, one of which also binds the reactive UNAM carboxylate, and that the alanine is oriented by interactions with the positively charged side chains of two MurC arginine residues and the negatively charged alanine carboxyl group. These results indicate that significant diversity exists in binding of the UDP moiety of the substrate by MurC and the subsequent ligases in the bacterial cell wall biosynthesis pathway and that alterations in the domain packing and tertiary structure allow the Mur ligases to bind sequentially larger UNAM peptide substrates.
Novel antimicrobial agents are needed to combat the emergence of bacterial resistance mechanisms for a number of currently prescribed antibiotics (34). Enzymes involved in peptidoglycan biosynthesis are essential for bacterial growth and are present in a majority of pathogenic microorganisms. They are also unique to bacteria. Peptidoglycan synthesis has proven to be a successful target of antimicrobials. Two well-known classes of antibiotics, β-lactams and glycopeptides, interfere with peptidoglycan cross-linking, the last step in peptidoglycan synthesis. Ramoplanin, a member of a new class of antibiotics known as glycolipodepsipeptides, targets MurG and transglycosylase and exhibits antimicrobial activity against gram-positive bacteria (1, 20). The earlier cytoplasmic steps of the peptidoglycan biosynthesis pathway have been less exploited, although in principle they also represent essential and unique targets. Fosfomycin is the only fully developed antibacterial agent to inhibit any of the cytoplasmic steps of peptidoglycan biosynthesis. This natural product antibiotic targets the first enzyme of the pathway, MurA (UDP-N-acetylglucosamine enolpyruvyltransferase) (15). All of the above antimicrobial drugs were identified by whole-cell-based screening approaches. Target-based screens, although only modestly successful to date (27), are enabled by the availability of genomic information for clinically important microorganisms, both gram negative and gram positive. Structure-based drug design will increasingly be enabled by the availability of three-dimensional structures for promising pathway-validated targets. The structure that we report for MurC now makes this target available for structure-based drug design.
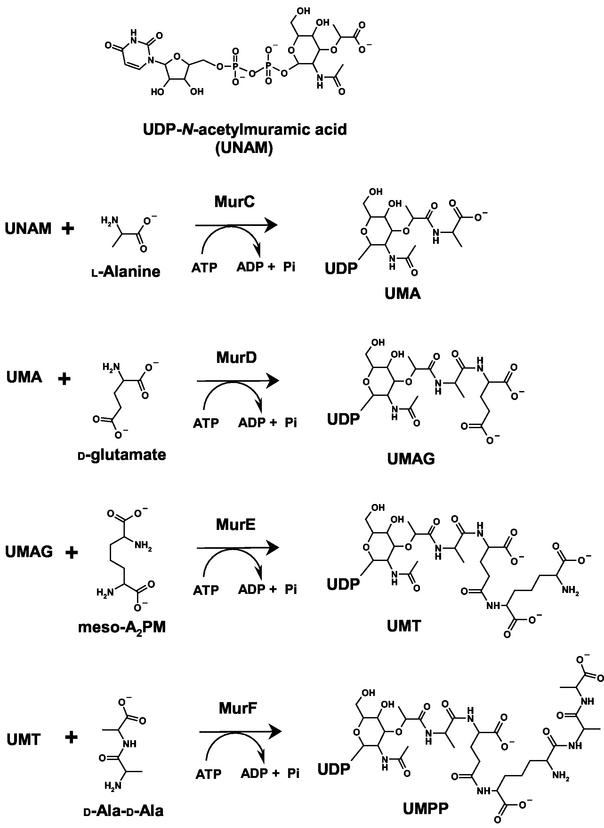
The ATP-dependent ligation of l-alanine (Ala) and UDP-N-acetylmuramic acid (UNAM) to form UDP-N-acetylmuramoyl-l-alanine (UMA) is catalyzed by the enzyme UDP-N-acetylmuramate:l-alanine ligase (MurC). Subsequent amino acids are added to the C terminus of UMA by the related ATP-dependent peptidoglycan synthetases MurD (adding d-glutamate), MurE (adding a diamino acid, i.e., meso-diaminopimelate or l-lysine), and MurF (adding a dipeptide, typically d-Ala-d-Ala) (Fig. 1). These enzymes are all thought to utilize a common reaction mechanism. An acyl-phosphate intermediate is formed via transfer of the γ-phosphate of ATP to the carboxylate of UNAM or the C-terminal carboxylate of UNAM peptide. The amide of the incoming amino acid displaces the phosphate by nucleophilic attack, thereby extending the peptide chain. While MurC function is required for de novo cell wall biosynthesis (E. Realo, M. T. Bocquel, M. Fairley, D. Mengin-Lecruelt, C. Parquet, K. Salah Bey, Y. Taburet, M. Harnois, and J. van Heijenoort, Abstr. 97th Gen. Meet. Am. Soc. Microbiol., abstr. H-2, p. 285, 1997), another related protein, the mpl gene product, adds the tripeptide l-Ala-d-Glu-meso-diaminopimelate to UNAM in a single step, recycling the tripeptide and circumventing MurC, MurD, and MurE in the salvage pathway (16, 24). The mpl gene product is more highly similar to MurC (about 28% identity) than to the other enzymes, consistent with the fact that the bond being formed is the same in the two reactions. This set of enzymes, the murein peptide synthetases, catalyzing nonribosomal peptide synthesis thus represents an interesting sequence of structural evolution and divergence within a pathway. In the context of previously reported structures of MurD (2), MurE (17), and MurF (35), the crystal structures of MurC presented here complete our structural view of this intriguing family of de novo murein peptide ligases.
FIG. 1.
Cytoplasmic steps of peptidoglycan biosynthesis. The figure shows the biosynthesis of the peptidoglycan precursor catalyzed by Mur ligases. UNAM is synthesized by the first two enzymes in the pathway, MurA and MurB. MurC catalyzes the ATP-dependent ligation of l-alanine onto the carboxylate of UNAM to form UMA. MurD adds d-glutamate to UMA to form UDP-N-acetylmuramoyl-l-alanyl-d-glutamate (UMAG). The third Mur ligase, MurE, adds meso-diaminopimelate to produce UDP-N-acetylmuramoyl:tripeptide (UMT), followed by the last Mur ligase, MurF, which adds the dipeptide d-Ala-d-Ala to create UDP-N-acetylmuramoyl:pentapeptide (UMPP).
MATERIALS AND METHODS
Cloning, expression, and purification.
The murC gene was amplified by PCR from Haemophilus influenzae DNA obtained from the American Type Culture Collection (ATCC 51907D) with the primers murC_HiF (ATGAAACATTCCCACGAAGAAATTAG) and murC_HiR (TTAATTCTTCCAAGATTCAGCCAAGCCAC). The resulting product was cloned into an Escherichia coli kanamycin-resistant arabinose-inducible expression vector (pSX29) to give an open reading frame encoding MKHQHQHQHQHQHQQPL fused directly to the N terminus of the full-length 475-amino-acid gene product identical to the SWISS-PROT reference sequence MURC_HAEIN (P45066). The expression plasmid was transformed into the methionine auxotroph DL41 (18) and expressed in a 96-well fermentor at 24°C. Recombinant MurC was purified with ProBond nickel-chelating resin (Invitrogen, Carlsbad, Calif.) followed by diafiltration into 150 mM NaCl-25 mM Tris, pH 7.9.
Enzymatic synthesis and purification of UNAM.
UNAM was prepared from UDP-N-acetylglucosamine (Sigma-Aldrich Co., St. Louis, Mo.) by enzymatic synthesis with purified recombinant MurA and MurB (A. Brooun, unpublished results). A reaction mixture (100 ml) containing 8 mM UDP-N-acetylglucosamine, 12 mM NADPH, 12 mM phosphoenolpyruvate, and 1 mM dithiothreitol was incubated with 50 pmol of H. influenzae MurA and 50 pmol of Pseudomonas aeruginosa MurB at room temperature. The reaction was monitored by analytical reversed-phase high-pressure liquid chromatography (RP-HPLC) coupled with electrospray mass spectrophotometry. After 24 h, the concentration of NADPH was raised to 24 mM total and the reaction mixture was incubated for an additional 24 h. After 48 h of incubation the yield of UNAM was greater than 90%, as assessed by RP-HPLC mass spectrophotometry, and the reaction mixture was frozen at −80°C prior to purification. UNAM was purified by RP-HPLC on a Mu Bondapak C18 10 μM 125-Å 40- by 100-mm Preppak by Trilink Biotechnologies Inc. (San Diego, Calif.). The final product was >93.5% pure, and the overall reaction yield postpurification was ∼30%. Concentration was determined by UV spectroscopy at 262 nm. UNAM was stored as a 100 mM solution in water at −80°C.
Crystallization and data collection.
Crystals of both native and selenomethionine-substituted MurC can be reproducibly grown from Syrrx's standard crystallization screens. The data presented here, however, were collected exclusively from the selenomethionine-substituted enzyme preparations used for the structure determination. These crystals were obtained at 4°C from 100-nl sitting drop vapor diffusion experiments by mixing 50 nl of 14-mg/ml MurC protein (25 mM Tris [pH 7.9], 150 mM NaCl, 5 mM dithiothreitol) containing 5 mM UNAM substrate, with 50 nl of reservoir solution containing 24% polyethylene glycol 4000, 20% glycerol, 160 mM MgCl2, and 100 mM Tris, pH 8.1, and equilibrated against 50-μl reservoir wells. Crystals grew overnight to approximate dimensions of 0.1 by 0.05 by 0.05 mm3, were harvested directly from the mother liquor with nylon loops, and flash frozen by direct immersion into liquid nitrogen. These crystals belong to the orthorhombic space group P212121 with unit cell dimensions a = 91.5 Å, b = 92.3 Å, and c = 118.2 Å and contain two enzyme molecules in the asymmetric unit. Single-wavelength anomalous dispersion (SAD) data at 2.3-Å resolution were collected at the selenium peak wavelength (0.979 Å) on beamline 5.0.2 of the Advanced Light Source (ALS; Berkeley, Calif.). These data were used to provide the initial fit of the H. influenzae MurC amino acid sequence to the electron density. Subsequent higher-resolution data were collected to 1.85 Å on ALS beamline 5.0.3 and used for further refinement of the model. Cocrystallization conditions for the quaternary complex of MurC with UNAM, l-alanine, Mn2+, and the ATP analogue AMPPNP were identified at 20°C from 100-nl sitting drops. These crystals were reproduced in larger 4-μl sitting drops consisting of 2 μl of 14-mg/ml MurC protein, 5 mM UNAM, 5 mM Ala, 5 mM Mn2+, and 5 mM AMPPNP, with 2 μl of reservoir solution containing 20% polyethylene glycol 4000, 10% isopropanol, and 100 mM HEPES, pH 7.5. These crystals belong to the monoclinic space group P21, a = 74.9 Å, b = 87.3 Å, c = 86.1 Å, and β = 104.9°°, and contain two MurC enzyme molecules in the asymmetric unit. X-ray diffraction data to 1.7-Å resolution were collected at ALS beamline 5.0.3 from a single crystal flash frozen in liquid nitrogen in mother liquor supplemented with 25% ethylene glycol as a cryoprotectant. Data collection statistics are presented in Table 1. Neither of these two crystal forms is similar to a previously reported crystal form of E. coli MurC (11).
TABLE 1.
MurC-Hi crystallographic data collection and refinement statistics
| Type of value | SAD | UNAM | UMA:AMPPNP |
|---|---|---|---|
| Crystallographic data | |||
| Space group | P21 21 21 | P21 21 21 | P21 |
| Unit cell (Å) | |||
| a | 92.2 | 91.5 | 74.9 |
| b | 92.7 | 92.3 | 87.3 |
| c | 118.9 | 118.2 | 86.1 |
| β | 90.0 | 90.0 | 104.9 |
| Wavelength (Å) | 0.979 | 1.00 | 1.00 |
| Resolution (Å) | 2.30 | 1.85 | 1.70 |
| No. of observations | 187,884 | 347,154 | 488,589 |
| No. of unique reflections | 81,484a | 77,944 | 113,461 |
| Completeness (%) | 93.2 (2.30-2.39 Å: 95.6) | 90.0 (1.85-1.89 Å: 93.0) | 96.6 (1.70-1.76 Å: 90.5) |
| I/σI | 11.6 (5.1) | 10.8 (3.0) | 13.4 (2.5) |
| R_sym | 0.055 (0.19) | 0.064 (0.45) | 0.046 (0.54) |
| Mosaicity | 0.38 | 0.45 | 0.80 |
| Wilson B value | 22.1 | 13.3 | 20.4 |
| Overall fom | |||
| Before DM | 0.75 | ||
| After DM | 0.85 | ||
| Refinement | |||
| Resolution (Å) | 25.0-1.85 | 25.0-1.70 | |
| No. of reflections (all data) | 73.241 | 107,740 | |
| Completeness (%) | 90.0 | 96.5 | |
| No. of atoms (total) | 7,930 | 8,051 | |
| R_cryst | 0.169 (1.85-1.90 Å: 0.198) | 0.168 (1.70-1.745 Å: 0.244) | |
| R_free | 0.208 (0.249) | 0.194 (0.262) | |
| Estimated coordinate error (Å) | 0.080 | 0.062 | |
| Fo-Fc correlation coefficient (free) | 0.957 (0.936) | 0.967 (0.957) | |
| rms bond length (Å) | 0.008 | 0.008 | |
| rms bond angle (°) | 1.11 | 1.13 | |
| B_avg (Å2) | |||
| Protein | 11.8 | 11.6 | |
| UNAM/UMA | 29.1 | 25.4 | |
| AMPPNP | NAb | 19.9 | |
| Water | 33.8 | 39.0 |
Freidel mates were not merged for these data.
NA, not applicable.
SAD phasing, model building, and refinement.
SAD X-ray diffraction data were analyzed using the program SHELXD (30) to find the heavy atom substructure, locating 27 out of the 28 total seleniums expected (14 from each MurC enzyme in the asymmetric unit). SAD phases were calculated to 2.8 Å from these selenium positions by use of the SHELXE program. With the use of the selenium positions, the NCS matrix relating the two molecules in the asymmetric unit was determined by manually overlaying the selenium atoms belonging to each molecule with the program Xfit (23). The phases were then extended to 2.1 Å, and the electron density was improved by solvent flattening, histogram matching, and noncrystallographic symmetry averaging with the program DM (8). The resulting experimental phases were of sufficient quality to allow fitting of most of the amino acid sequence to the electron density map by using the program Xfit (23). This model was then refined against the higher-resolution 1.85-Å data with the program REFMAC (25) as implemented within the CCP4 program suite (7) and used as the search model to solve the structure of the monoclinic quaternary complex with the AMoRe program (26). Data in the resolution range 15.0 to 3.5 Å gave a correlation coefficient of 0.628 and an R value of 0.354 for rotating and translating two MurC enzyme molecules into the monoclinic asymmetric unit. This model was then also refined with REFMAC, interspersed with manual inspection and rebuilding as necessary with Xfit. The stereochemical quality of the refined models was assessed with PROCHECK (19). Refinement statistics for these structures are listed in Table 1.
Coordinates.
Coordinates and structure factors for MurC:UNAM (accession code 1P31.pdb) and MurC:UMA:AMPPNP (1P3D.pdb) have been deposited with the Protein Data Bank (PDB).
RESULTS
Quality of structures.
The refined model for the MurC:UNAM complex consists of 931 residues, two UNAM molecules, four Mg2+ ions, and 699 ordered solvent molecules, with a crystallographic R value of 0.169, and an Rfree value of 0.208, for 73,241 unique reflections in the resolution range 25.0 to 1.85 Å. The structure is well ordered throughout, except for the N-terminal HQ tag and first ∼10 residues and last ∼3 residues of both MurC molecules, and possesses good geometry, with root mean square (rms) deviations from ideal values of 0.008 Å for bond lengths and 1.11° for bond angles (Table 1). The two MurC enzymes in the asymmetric unit are in similar overall conformations with 1.17-Å rms deviation between 464 equivalent Cα atoms, mostly due to slight differences in the associations among the three separate domains. With the notable exception of residue Arg 377, which is a key residue for binding the alanine substrate, all of the nonglycine residues in both MurC molecules fall within the allowed regions of the Ramachandran plot of main chain torsion angles, with 94.5% of residues in the most favored regions.
The refined model for the quaternary complex of MurC:UNAM:AMPPNP:Ala consists of 923 residues, two UNAM:Ala molecules, two AMPPNP molecules, four Mn2+ ions, and 788 ordered solvent molecules. The model has been refined to a crystallographic R value of 0.168, and an Rfree value of 0.194, for 107,740 unique reflections in the resolution range 25.0 to 1.70 Å. This structure also contains disordered residues at the termini. The rms deviations from ideality for the bond lengths are 0.008 Å and 1.13° for bond angles. The two MurC molecules in the asymmetric unit superimpose within 0.36-Å rms deviation for 459 equivalent Cα positions. The two molecules in the MurC:UNAM complex structure superimpose upon these essentially identical enzyme molecules within 0.33 and 1.12 Å, respectively. Again, all of the nonglycine residues, with the exception of Arg 377, are in allowed regions of the Ramachandran plot.
Global protein structure.
MurC is a three-domain α/β protein of approximate overall dimensions 60 by 60 by 45 Å (Fig. 2). Each domain is formed sequentially from contiguous amino acid residues in the sequence. Domain 1, from the N terminus to residue 118, consists of a central five-stranded, all-parallel β-sheet and four surrounding α-helices (Fig. 2). This fold is reminiscent of the dinucleotide-binding, or Rossmann, fold (29) seen in many nucleotide-binding proteins (31). This domain binds the nucleotide moiety of the UNAM substrate, with the glycine-rich loop between β1 and α1 contacting the phosphate groups of the UDP (Fig. 2). A similar UDP-binding domain is seen in the structure of MurD (PDB entry 2UAG) (2, 3) with 73 structurally equivalent Cα atoms within 2.3-Å rms deviation.
FIG. 2.
Stereo view of the MurC global fold, domain organization, and binding of UNAM and AMPPNP. The Cα backbone trace of MurC is shown with α-helices colored blue, β-sheets colored orange, and loop regions colored according to the individual domains (domain 1, UDP binding, gold; domain 2, ATP binding, green; domain 3, ligand binding, purple). The binding of the UNAM (red), AMPPNP (blue), and metal ions (pink spheres) is also shown.
The second, central domain of MurC is the largest of the three domains, spanning residues 119 to 324, with a central seven-stranded, mostly parallel β-sheet surrounded by five helical segments and flanked by a smaller antiparallel, three-stranded β-sheet (Fig. 2). This is a common fold seen in many ATP-binding proteins (9), including all four of the Mur ligases. This ATP-binding domain provides the bulk of the interactions with the adenine ring and phosphates of the AMPPNP in the quaternary complex structure. Domains that are topologically equivalent to this central MurC domain are present in the other Mur ligases, with rms deviations of 2.3 Å for 138 equivalent Cα positions between MurC and MurD, 2.1 Å for 121 equivalent Cα atoms between MurC and MurE (PDB entry 1E8C) (17), and 1.9 Å for 128 superposed Cα atoms between MurC and MurF (PDB entry 1GG4) (35).
The third and C-terminal domain of MurC contains residues 315 to 473 and consists of a central six-stranded β-sheet with one antiparallel and five parallel β-strands, flanked by five α-helices. This domain contains a classic Rossmann dinucleotide-binding fold and sits atop the central, ATP-binding domain, providing contacts to the ribose sugar and α-phosphate group of the AMPPNP. Residues from this ligand-binding domain provide key interactions that orient and position the incoming amino acid ligand with the growing peptidoglycan chain. Although there are significant differences in the lengths and orientations of the α-helices and loops connecting the β-strands, this domain is also conserved among all of the Mur ligases. MurD contains 97 equivalent Cα atoms with an rms deviation from MurC of 2.6 Å, MurE has 106 equivalent Cα atoms within an rms deviation of 2.2 Å, and MurF has 107 Cα atoms within an rms deviation of 2.7 Å.
MurC complex with UNAM.
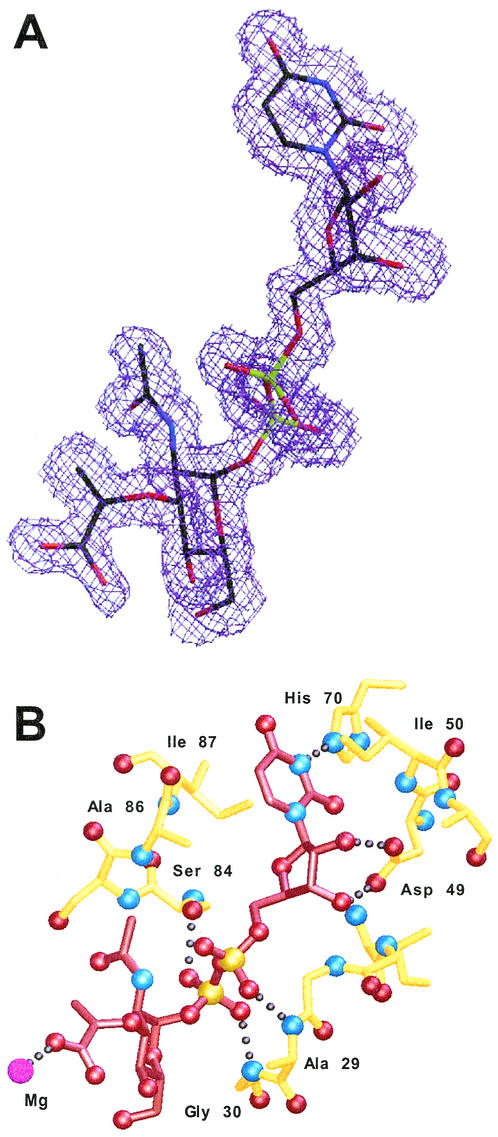
The electron density is clear and unambiguous for the substrate UNAM in the MurC:UNAM complex (Fig. 3A). MurC binds the UDP half of the UNAM substrate in an extended conformation with the uracil ring in an anti conformation and inserted between Ile 50 on one side and Ala 86 and Ile 87 on the other (Fig. 3B). A hydrogen bond is formed between the uracil N1 atom and the side chain Nɛ2 of His 70, whereas the ribose ring is in a C2′-endo sugar pucker and is positioned by a pair of hydrogen bonds from the side chain Oδ1 and Oδ2 atoms of Asp 49 to the O2′ and O3′ ribose oxygens. The α-phosphate of the UDP group forms a hydrogen bond through a phosphate oxygen to the main chain nitrogen atom of Ala 29, while the β-phosphate oxygens form two hydrogen bonds with the polypeptide backbone nitrogen of Gly 30 and the side chain Oγ atom of Ser 84. The side chain of Arg 107 lies near the UNAM phosphates and could counterbalance their negative charges, although there is no direct interaction between them. The N-acetylmuramic acid portion of the UNAM substrate extends into the cleft formed among all three MurC domains but does not make any direct, specific interactions with MurC residues. One of the N-acetylmuramic acid carboxylate atoms, however, forms a ligand to an active-site metal ion in the MurC:UNAM structure (Fig. 3B).
FIG. 3.
UNAM and the UDP recognition pocket of MurC. (A) Omit Fo-Fc electron density contoured at the 2σ level for the UNAM in the MurC:UNAM complex structure. UNAM is shown with black carbons, red oxygens, blue nitrogens, and green phosphorus atoms. (B) MurC interactions with the UNAM substrate. Hydrogen-bonding interactions from domain 1 MurC residues (yellow) to the UNAM (red) are shown.
ATP binding and active-site structural chemistry.
Consistent with biochemical kinetic results that indicate that ATP is the first substrate to bind (12), ATP binding likely assembles the Mur ligases into an active conformation. The MurC ATP-binding site lies at the interface between the second and third domains with key interactions with the adenine ring and α- and β-phosphates provided by residues in the ATP-binding domain. The AMPPNP is well ordered, possessing average temperature values of 18.7 and 22.5 Å2 in the two molecules, and all of the atoms are clearly visible in the electron density map, including the γ-phosphate at the enzyme active site (Fig. 4A). The adenine ring of AMPPNP inserts into a pocket between the peptide backbone of Gly 128 and the side chain of His 291, forming a pair of hydrogen bonds between the adenine N7 and exocyclic N6 atoms and the side chain Nδ2 and Oδ1 atoms, respectively, of the strictly conserved residue Asn 295 (Fig. 4B).
FIG. 4.
MurC interactions with the ATP analogue AMPPNP. (A) Omit Fo-Fc electron density contoured at the 2σ level for the AMPPNP and Mn2+ ions in the MurC:UMA:AMPPNP complex. AMPPNP is shown with black carbons, red oxygens, blue nitrogens, and green phosphorus atoms. The Mn2+ ions are shown as pink spheres. (B) MurC interactions with bound AMPPNP. The ATP analogue is shown in dark blue. MurC residues from domain 2 (light blue) recognize the adenine ring, and the P-loop residues from residues 128 to 131 bind the phosphate oxygens. MurC residues from domain 3 (pink) interact with the ribose moiety and α-phosphate.
The ligand-binding domain caps the bound AMPPNP and provides three specific interactions. The ribose sugar of the AMPPNP is anchored by hydrogen bonds between the ribose O2′ atom and Thr 356 Oγ, and from O2′ and O3′ to the side chain Oδ1 and Oδ2 atoms of Asp 345, while the side chain of Arg 326 balances the negative charge of the α-phosphate of AMPPNP through a hydrogen bond formed from its Nɛ atom to a phosphate oxygen. All of the remaining interactions with the AMPPNP phosphate groups are furnished by residues from the ATP-binding domain, including the side chain Nɛ of Lys 129, which contacts a γ-phosphate oxygen, and the peptide backbone nitrogens of four consecutive residues in the P-loop between βX and αY (Gly 128, Lys 129, Thr 130, and Thr 131). Thr 131 forms a hydrogen bond to a second α-phosphate oxygen, and Thr 130 and Lys 129 contact two oxygens of the β-phosphate group of the AMPPNP (Fig. 4B).
The position of the γ-phosphate is constrained between two active-site metal ions. The first metal ion corresponds to the Mg2+ ion seen in the MurC:UNAM complex, with Nɛ2 of His 198 and a γ-phosphate oxygen forming direct metal ligands, and additional water-mediated interaction with the side chains of Glu 176, Asp 197, and His 348. The second metal-ion site is occupied accompanying AMPPNP binding (Fig. 4B). This metal bridges two AMPPNP β- and γ-phosphate oxygens and is bound by the side chains of Thr 130 and Glu 173. The position of this phosphate could not be clearly determined from complexes of MurD with ADP (3), and as MurC, and likely all of the Mur ligases, proceed through an acyl-phosphate intermediate (14), the exact position of the reactive γ-phosphate is critical for understanding the active-site structural chemistry. Surprisingly, despite the fact that the quaternary complex contained nonhydrolyzable AMPPNP and the γ-phosphate position is fully occupied, the electron density clearly shows that the alanine ligand has been added to the UNAM to form the product UMA (Fig. 5A). The alanine portion of UMA is positioned by two arginine residues of the ligand-binding domain, Arg 377 and Arg 380, that form hydrogen bonds from their positively charged, side chain guanidiniums to the carboxylate atoms of the alanine. Specificity for alanine derives from a shallow pocket formed by the side chains of His 348, His 376, Tyr 346, and Ala 459. The shallow depth of this pocket precludes binding of larger amino acids, and its hydrophobic characteristics would discourage binding of small polar amino acids. Curiously, the two crystallographically independent UMA molecules are in different conformations. Although the alanine and muramoyl portions of the UMA superimpose, the carboxylate portion of the muramic acid is in a similar conformation as that seen in the MurC:UNAM complex in one case, whereas in the other molecule it is rotated 180° from this orientation. This latter conformation may represent an early product release step.
FIG. 5.
MurC interactions with UMA and the structural basis for alanine specificity. (A) Omit Fo-Fc electron density contoured at the 2σ level for the UMA product in the MurC:UMA:AMPPNP complex. UMA is shown with black carbons, red oxygens, blue nitrogens, and green phosphorus atoms, with the attached alanine in the lower right. (B) Stereo view of the MurC active site and interactions that dictate alanine specificity. The phosphates of the AMPPNP (blue bonds), bound Mn2+ ions (large purple spheres), and key domain 2 residues (green bonds) are shown along with a portion of the UMA product (red bonds) and key alanine specificity-determining residues from domain 3 (purple bonds). Arg 377 and Arg 380 from the ligand-binding domain orient the alanine through interactions with its carboxylate, while His 348, His 376, Tyr 346, and Ala 459 form a shallow hydrophobic pocket that complements the short alanine side chain.
DISCUSSION
The Mur ligases are a functionally and evolutionarily related, structurally conserved superfamily of enzymes (5, 13). This protein family includes folylpolyglutamate synthetase (32), the CapB protein (21), and cyanophycin synthetase (36), enzymes that perform similar chemical reactions as those of the Mur ligases—the synthesis of an amide or peptide bond via an acyl-phosphate intermediate with hydrolysis of ATP to ADP. This nonribosomal peptide bond synthesis proceeds through an initial phosphorylation of a substrate carboxylate, followed by the nucleophilic attack of the amine of the amino acid ligand to form a tetrahedral intermediate, and the subsequent collapse of this intermediate to yield the reaction products. For the Mur ligases, biochemical data from oxygen isotope exchange experiments support the formation of an acyl-phosphate intermediate (14, 33), and the effectiveness of phosphinate inhibitors that mimic the tetrahedral transition state (10, 22, 28) provides support for the second step in the reaction. The present results for MurC are consistent with the reaction mechanism proposed from structural analysis of MurD complexes with substrates and ADP (3) and particularly highlight the importance of the two divalent metal ions to facilitate the reaction. Site-directed mutagenesis of E. coli MurC (5) identifies three residues as being critical for catalysis. The analogous residues in H. influenzae MurC are Lys 129, which bridges the β- and γ-phosphates of AMPPNP; Glu 173, which directly ligates the second metal ion; and Glu 352, which binds the first metal ion via a water molecule. This latter metal ion bridges an AMPPNP γ-phosphate oxygen and a UNAM carboxylate oxygen, serving not only to neutralize the negative charges of these groups but also to activate the carboxylate for a direct SN2 displacement reaction. The fact that we observe the reaction product, UMA, in the crystal obtained from solutions containing UNAM, AMPPNP, Mn2+, and alanine suggests that the reaction can proceed, albeit slowly, without forming an acyl-phosphate. Prolonged incubation of the reactants in the crystal lattice likely overcame a kinetic barrier to peptide bond formation.
The modular, three-domain architecture of the Mur ligases allows for active, or “closed,” tertiary enzyme structures to form when the complex is fully assembled with bound ATP and peptidoglycan precursor substrates. Crystal structures of MurD (4) and MurF (35), determined in the substrate-free form, are in an “open” conformation with domain-packing arrangements that do not form productive substrate-binding sites. We also observe a slight ∼10° closing of the third MurC domain relative to the central domain when comparing the MurC:UNAM complex structure with that determined with bound AMPPNP. The third, C-terminal domain of the Mur ligases plays a dual role in both capping the ATP-binding site and also inserting a loop into the enzyme active site to position the appropriate amino acid substrate. Thus, the ATP- and ligand-binding domains are structurally conserved in all of the Mur ligases (Fig. 6) with the structural variability between them concentrated in the N-terminal UDP-binding domain. The lack of significant sequence conservation in the ligand-binding domain likely reflects the fact that each Mur ligase binds a distinct ligand, and, in fact, two of the three specific contacts to the AMPPNP made by residues from this domain (Arg 326 and Asp 345, Fig. 4) are absolutely conserved in the Mur ligase family.
FIG. 6.
Structural alignment of the Mur ligases. The individual domains from the structure of MurC were superimposed on the same domains from the structures of MurD, MurE, and MurF with the program SEQUOIA (6). For domain 1, the UDP-binding domain, only MurC and MurD were aligned, as the equivalent domains in the other Mur ligases are structurally distinct. Residues in MurD, MurE, and MurF deemed to be structurally superimposable with MurC are shown as capitalized equivalences. Residues identical in all four enzymes are shaded.
The three-domain architecture allows the Mur ligases to bind and orient the similar, yet consecutively longer, peptidoglycan precursor substrates with respect to the ATP- and ligand-binding domains and enzyme active site. The MurC UDP-binding domain is homologous to the same domain of MurD. In order to accommodate the longer substrate, MurD widens the cleft between domains by rotating this domain ∼30° away from the ATP-binding domain, relative to the domain arrangement seen in MurC (Fig. 7). Although the UDP-binding domain seen in MurE shares no structural homology with MurC or MurD, a more pronounced domain rotation further widens the interdomain cleft of MurE to accommodate the longer substrate.
FIG. 7.
Tertiary structure rearrangements in substrate-bound MurC and MurD structures. The structures of MurC and MurD are shown superimposed according to their central, ATP-binding domains (bottom), viewed with the ligand-binding domains behind, and the UDP-binding domains (top). The substrates are shown in red. The topologically equivalent UDP-binding domain of MurD rotates ∼30° relative to the same domain arrangement seen in MurC.
Surprisingly, the manner in which the UDP of the bound substrate is recognized differs in these enzymes. Our MurC crystal structures show that the uracil of the UNAM substrate binds with its hydrophilic, hydrogen-bonding edge inserted into a shallow pocket on the enzyme surface, with both of the ribose O2′ and O3′ oxygens forming hydrogen bonds with the charged side chain of a conserved aspartic acid residue (Fig. 8A). MurD, however, binds the uracil in an opposite orientation with the hydrophobic C-5-C-6 edge of the ring inserted and the ribose oxygens directed away from the enzyme and exposed to solvent (Fig. 8B). The structure of MurE has also been determined with peptidoglycan substrate bound (17). Despite sharing no structural homology with either MurC or MurD in the UDP-binding domain, the mode of MurE UDP recognition is similar to that observed for MurC with the hydrogen-bonding edge of the uracil and ribose oxygens directed towards the enzyme's binding pocket (Fig. 8C). Thus, the structural results for the Mur ligases reveal substantial diversity in the shapes and chemical environments of their UDP-binding pockets, suggesting that antimicrobial agents could be designed that mimic these enzyme-UDP interactions and which independently target individual enzymes within the cell wall biosynthesis pathway. Such antibacterial cocktails that target several or all four Mur ligases could effectively circumvent bacterial resistance mechanisms.
FIG. 8.
Stereo views of the UDP-binding pockets of MurC, MurD, and MurE. The bound UDP portions of the respective substrates are shown along with the molecular surfaces of the UDP-binding pockets for MurC (A), MurD (B), and MurE (C).
Acknowledgments
We acknowledge Bernard Collins, David Hosfield, Matt Maison, Mario Matsusalem, Jacek Nowakowski, William Spencer, and others at Syrrx for their contributions toward the completion of this project. This work is partly based on diffraction experiments conducted at the ALS. We also thank the staff at the ALS for their excellent support.
The ALS is supported by the Director, Office of Science, Office of Basic Energy Sciences, Materials Sciences Division, of the U.S. Department of Energy under contract no. DE-AC03-76SF00098 at Lawrence Berkeley National Laboratory.
REFERENCES
- 1.Auger, G., M. Crouvoisier, M. Caroff, J. van Heijenoort, and D. Blanot. 1997. Synthesis of an analogue of the lipoglycopeptide membrane intermediate I of peptidoglycan biosynthesis. Lett. Peptide Sci. 4:371-376. [Google Scholar]
- 2.Bertrand, J. A., G. Auger, E. Fanchon, L. Martin, D. Blanot, J. van Heijenoort, and O. Dideberg. 1997. Crystal structure of UDP-N-acetylmuramoyl-l-alanine:d-glutamate ligase from Escherichia coli. EMBO J. 16:3416-3425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bertrand, J. A., G. Auger, L. Martin, E. Fanchon, D. Blanot, D. Le Beller, J. van Heijenoort, and O. Dideberg. 1999. Determination of the MurD mechanism through crystallographic analysis of enzyme complexes. J. Mol. Biol. 289:579-590. [DOI] [PubMed] [Google Scholar]
- 4.Bertrand, J. A., E. Fanchon, L. Martin, L. Chantalat, G. Auger, D. Blanot, J. van Heijenoort, and O. Dideberg. 2000. “Open” structures of MurD: domain movements and structural similarities with folylpolyglutamate synthetase. J. Mol. Biol. 301:1257-1266. [DOI] [PubMed] [Google Scholar]
- 5.Bouhss, A., D. Mengin-Lecreulx, D. Blanot, J. van Heijenoort, and C. Parquet. 1997. Invariant amino acids in the Mur peptide synthetases of bacterial peptidoglycan synthesis and their modification by site-directed mutagenesis in the UDP-MurNAc:l-alanine ligase from Escherichia coli. Biochemistry 36:11556-11563. [DOI] [PubMed] [Google Scholar]
- 6.Bruns, C. M., I. Hubatsch, M. Ridderström, B. Mannervik, and J. A. Tainer. 1999. Human glutathione transferase A4-4 crystal structures and mutagenesis reveal the basis of high catalytic efficiency with toxic lipid peroxidation products. J. Mol. Biol. 288:427-439. [DOI] [PubMed] [Google Scholar]
- 7.Collaborative Computational Project 4. 1994. The CCP4 suite: programs for protein crystallography. Acta Crystallogr. Sect. D 50:760-763. [DOI] [PubMed] [Google Scholar]
- 8.Cowtan, K. D., and P. Main. 1998. Miscellaneous algorithms for density modification. Acta Crystallogr. Sect. D 54:487-493. [DOI] [PubMed] [Google Scholar]
- 9.Denessiouk, K. A., and M. S. Johnson. 2000. When fold is not important: a common structural framework for adenine and AMP binding in 12 unrelated protein families. Proteins Struct. Funct. Genet. 38:310-326. [PubMed] [Google Scholar]
- 10.El Zoeiby, A., F. Sanschagrin, and R. C. Levesque. 2003. Structure and function of the Mur enzymes: development of novel inhibitors. Mol. Microbiol. 47:1-12. [DOI] [PubMed] [Google Scholar]
- 11.Emanuele, J. J., Jr., H. Jin, B. L. Jacobson, C. Y. Chang, H. M. Einspahr, and J. J. Villafranca. 1996. Kinetic and crystallographic studies of Escherichia coli UDP-N-acetylmuramate:l-alanine ligase. Protein Sci. 5:2566-2574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Emanuele, J. J., Jr., H. Jin, J. Yanchunas, Jr., and J. J. Villafranca. 1997. Evaluation of the kinetic mechanism of Escherichia coli uridine diphosphate-N-acetylmuramate:l-alanine ligase. Biochemistry 36:7264-7271. [DOI] [PubMed] [Google Scholar]
- 13.Eveland, S. S., D. L. Pompliano, and M. S. Anderson. 1997. Conditionally lethal Escherichia coli murein mutants contain point defects that map to regions conserved among murein and folyl poly-γ-glutamate ligases: identification of a ligase superfamily. Biochemistry 36:6223-6229. [DOI] [PubMed] [Google Scholar]
- 14.Falk, P. J., K. M. Ervin, K. S. Volk, and H.-T. Ho. 1996. Biochemical evidence for the formation of a covalent acyl-phosphate linkage between UDP-N-acetylmuramate and ATP in the Escherichia coli UDP-N-acetylmuramate:l-alanine ligase-catalyzed reaction. Biochemistry 35:1417-1422. [DOI] [PubMed] [Google Scholar]
- 15.Gadebusch, H. H., E. O. Stapley, and S. B. Zimmerman. 1992. The discovery of cell wall active antibacterial antibiotics. Crit. Rev. Biotechnol. 12:225-243. [DOI] [PubMed] [Google Scholar]
- 16.Goodell, E. W. 1985. Recycling of murein by Escherichia coli. J. Bacteriol. 163:305-310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gordon, E., B. Flouret, L. Chantalat, J. van Heijenoort, D. Mengin-Lecreulx, and O. Dideberg. 2001. Crystal structure of UDP-N-acetylmuramoyl-l-alanyl-d-glutamate:meso-diaminopimelate ligase from Escherichia coli. J. Biol. Chem. 276:10999-11006. [DOI] [PubMed] [Google Scholar]
- 18.Hendrickson, W. A., J. R. Horton, and D. M. LeMaster. 1990. Selenomethionyl proteins produced for analysis by multiwavelength anomalous diffraction (MAD): a vehicle for direct determination of three-dimensional structure. EMBO J. 9:1665-1672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Laskowski, R. A., M. W. MacArthur, D. S. Moss, and J. M. Thornton. 1993. Procheck: a program to check the stereochemical quality of protein structures. J. Appl. Crystallogr. 26:283-291. [Google Scholar]
- 20.Lo, M. C., H. Men, A. Branstrom, J. Helm, N. Yao, R. Goldman, and S. Walker. 2000. A new mechanism of action proposed for Ramoplanin. J. Am. Chem. Soc. 122:3540-3541. [Google Scholar]
- 21.Makino, S., I. Uchida, N. Terakado, C. Sasakawa, and M. Yoshikawa. 1989. Molecular characterization and protein analysis of the cap region, which is essential for encapsulation in Bacillus anthracis. J. Bacteriol. 171:722-730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Marmor, S., C. P. Petersen, F. Reck, W. Yang, N. Gao, and S. L. Fisher. 2001. Biochemical characterization of a phosphinate inhibitor of Escherichia coli MurC. Biochemistry 40:12207-12214. [DOI] [PubMed] [Google Scholar]
- 23.McRee, D. E. 1999. XtalView/Xfit—a versatile program for manipulating atomic coordinates and electron density. J. Struct. Biol. 125:156-165. [DOI] [PubMed] [Google Scholar]
- 24.Mengin-Lecreulx, D., J. van Heijenoort, and J. T. Park. 1996. Identification of the mpl gene encoding UDP-N-acetylmuramate:l-alanyl-γ-d-glutamyl-meso-diaminopimelate ligase in Escherichia coli and its role in recycling of cell wall peptidoglycan. J. Bacteriol. 178:5347-5352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Murshudov, G. N., A. A. Vagin, and E. J. Dodson. 1997. Refinement of macromolecular structures by the maximum-likelihood method. Acta Crystallogr. Sect. D 53:240-255. [DOI] [PubMed] [Google Scholar]
- 26.Navaza, J. 1994. AMoRe: an automated package for molecular replacement. Acta Crystallogr. Sect. A 50:157-163. [Google Scholar]
- 27.Projan, S. J. 2002. New (and not so new) antibacterial targets—from where and when will the novel drugs come? Curr. Opin. Pharmacol. 2:513-522. [DOI] [PubMed] [Google Scholar]
- 28.Reck, F., S. Marmor, S. Fisher, and M. A. Wuonola. 2001. Inhibitors of the bacterial cell wall biosynthesis enzyme MurC. Bioorg. Med. Chem. Lett. 11:1451-1452. [DOI] [PubMed] [Google Scholar]
- 29.Rossmann, M. G., and P. Argos. 1981. Protein folding. Annu. Rev. Biochem. 50:497-532. [DOI] [PubMed] [Google Scholar]
- 30.Schneider, T. R., and G. M. Sheldrick. 2002. Substructure solution with SHELXD. Acta Crystallogr. Sect. D 58:1772-1779. [DOI] [PubMed] [Google Scholar]
- 31.Schulz, G. E. 1992. Binding of nucleotides by proteins. Curr. Opin. Struct. Biol. 2:61-67. [Google Scholar]
- 32.Sun, X., A. L. Bognar, E. N. Baker, and C. A. Smith. 1998. Structural homologies with ATP and folate-binding enzymes in the crystal structure of folylpolyglutamate synthetase. Proc. Natl. Acad. Sci. USA 95:6647-6652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Vaganay, S., M. E. Tanner, J. van Heijenoort, and D. Blanot. 1996. Study of the reaction mechanism of the d-glutamic acid-adding enzyme from Escherichia coli. Microb. Drug Resist. 2:51-54. [DOI] [PubMed] [Google Scholar]
- 34.Walsh, C. 2000. Molecular mechanisms that confer antibacterial drug resistance. Nature 406:775-781. [DOI] [PubMed] [Google Scholar]
- 35.Yan, Y., S. Munshi, B. Leiting, M. S. Anderson, J. Chrzas, and Z. Chen. 2000. Crystal structure of Escherichia coli UDPMurNAc-tripeptide d-alanyl-d-alanine-adding enzyme (MurF) at 2.3 Å resolution. J. Mol. Biol. 304:435-445. [DOI] [PubMed] [Google Scholar]
- 36.Ziegler, K., A. Diener, C. Herpin, R. Richter, R. Deutzmann, and W. Lockau. 1998. Molecular characterization of cyanophycin synthetase, the enzyme catalyzing the biosynthesis of the cyanobacterial reserve material multi-l-arginyl-poly-l-aspartate (cyanophycin). Eur. J. Biochem. 254:154-159. [DOI] [PubMed] [Google Scholar]