A distinctive subfamily of β-sheet proteins are the parallel β-helices, first identified in the pectate lyases used by Erwinia species to infect plant cells (31, 73). This motif involves the processive folding of the polypeptide chain into an elongated coil or solenoid-shaped structure, with the β-strands orthogonal to the long axis. There are currently 12 proteins exhibiting this fold in the protein databank, the majority of which are involved in the recognition or metabolism of polysaccharides or lipopolysaccharides (LPSs). For example, the lateral surface of the parallel β-coils of the P22 tailspike adhesin recognizes and cleaves the LPS O-antigen of Salmonella enterica serovar Typhimurium (59). The solving of the crystal structure of P22 tailspike complexed with octasaccharide by Steinbacher and coworkers (59, 61) revealed the substrate is bound along its entire length within a 21-Å-long groove-like depression on the solvent-exposed face of the parallel β-helix domain. Other parallel β-helices are cell surface proteins involved in bacterial pathogenesis, such as the pertactin of Bordetella pertussis. Bradley et al. proposed that the function of this fold is to generate a long lateral surface for reading the sequences of polysaccharides (10).
During viral infection, the critical first steps of recognition and attachment to a host are mediated by specialized protein adhesins of the virus particle. These first contacts between virus and host occur through protein-protein and/or protein-polysaccharide interactions.
Recently the structures of a set of structural proteins involved in viral adhesion or infection have been solved. These structures are predominantly elongated homotrimers with a fibrous morphology and β-sheet topologies with unusual repetitive folds, including the newly identified triple β-helix (67) and triple β-spiral (65). These folds share some structural and functional similarities to the single β-helices.
We review here the structure of these elongated trimeric proteins, with particular emphasis on the possibility that they have been selected for some of the specialized functions involved in virus attachment and infection. One of these is likely to be the maintenance of an extended active site used to recognize and bind specific sequences on cell surface polysaccharides. These proteins also exhibit relatively high Tm, protease-resistant domains, and stability in the presence of detergent at room temperature. These may reflect their function as external virion proteins that have to survive proteases and denaturing conditions in diverse environments.
Viruses have evolved multiple mechanisms for getting their nucleic acids inside their cellular hosts. These include DNA injection, membrane fusion, and receptor-mediated endocytosis. The elucidation of the structure of the homotrimeric influenza virus hemagglutinin over two decades ago (70) led to an understanding of how pH-driven conformational change inserts a peptide sequence into the host membrane thereby triggering membrane fusion. The trimeric coiled-coil has turned out to be a structural paradigm for many fusogenic viruses (2), including human immunodeficiency virus (HIV) (13).
More recently, high-resolution structures of adhesins from viruses that gain entry to cells by receptor-mediated internalization have been determined. These structures are of the penton fiber from human adenovirus type 2 (Ad2) (65) and the σ1 attachment protein of mammalian reovirus type 3 Dearing (T3D) (15). These proteins bind protein and carbohydrate moieties of host cell surfaces and in many respects are the animal virus analogs of the bacteriophage adhesins.
A group of viruses that infect bacteria, the double-stranded DNA (dsDNA) bacteriophages, anchor themselves to the surface of their hosts and subsequently form a membrane-spanning protein channel that allows the ejection of DNA from the phage capsid into the host cytoplasm. The phage organelle which accomplishes this feat is its tail. High-resolution structures are known for two adhesins from tailed bacteriophage: the bacteriophage P22 tailspike protein (62) and the T4 short tail fiber, gp12 (66). Both of these proteins interact with cell surface polysaccharides. The crystal structures of four other proteins out of the approximately 24 different kinds of subunits (19) that make up the T4 tail apparatus have also been determined.
Table 1 lists the proteins and protein database codes for structural coordinates downloadable from http://www.pdb.org. Views of protein models were generated by using Swiss-PDB (http://us.expasy.org/spdbv/) and rendered by POV-Ray 3.1 (http://www.pov.org). Due to crystallographic convention, many of the files contain a single chain from a biological unit. The relevant biological multimer can be automatically generated by using the EMBL-EBI “Protein Quaternary Structure” query form at http://pqs.ebi.ac.uk/ or by applying the symmetry transformations given in the “Remarks” section of many of the protein data bank files listed.
TABLE 1.
Homotrimeric, β-rich viral proteins whose structures are known
| Protein | Functional namea | Protein database identification | Virus | Reference |
|---|---|---|---|---|
| gp9 | Tailspike C-terminal domain | 1tsp | P22 | 62 |
| gp9 | Tailspike N-terminal domain | 1lkt | P22 | 60 |
| gpwac | Fibritin | 1aa0 | T4 | 64 |
| gp12 | Short tail fiber | 1h6w | T4 | 66 |
| gp5-27* | Tail lysozyme complex | 1k28 | T4 | 33 |
| gp9 | LTF connector-trigger | 1qex | T4 | 38 |
| gp11 | STF connector | 1e16 | T4 | 41 |
| σ1 | σ1 attachment protein | 1kke | Reovirus, T3D | 15 |
| Ad2 fiber | Adenovirus penton fiber | 1qiu | Adenovirus, type 2 | 65 |
LTF, long tail fiber; STF, short tail fiber.
BACTERIOPHAGE T4 SHORT TAIL FIBER
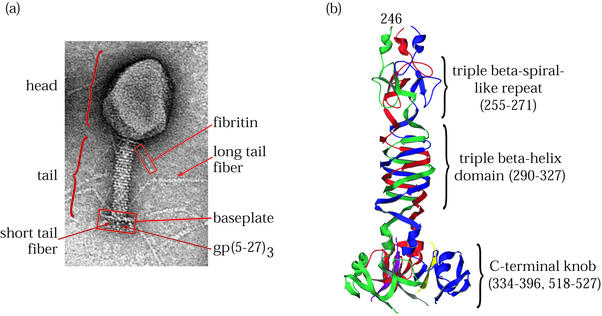
T4 short tail fiber, gp12, is a secondary adhesin that binds irreversibly to the LPS component on the cell surface of Escherichia coli B strains during virus attachment (52). The biologically active protein is an elongated homotrimeric fiber composed of 527 residues. van Raaij et al. determined the crystal structure of a heat- and protease-stable proteolytic fragment of gp12 lacking 84 residues from the N terminus and internal residues 397 to 517 from the C terminus (66, 67). As depicted in Fig. 1, the structure consists of three domains: an N-terminal elongated shaft having a β-strand structure not unlike the triple β-spiral fold of adenovirus fiber (see below), a middle triple β-helix domain, and a globular C-terminal knob composed largely of β-sheets. Residues 255 to 271 contain one of six 17-amino-acid-long complex sequence repeats found in the N-terminal half of gp12 beginning at residue 50 (12, 44). Eight similar repeats are found in gp34, and a single occurrence of this motif is present in gp37; both are homotrimeric components of the T4 long tail fiber (12). In the crystal structure, residues 85 to 245 did not show resolvable electron density, but the presence of repeats in the primary amino acid sequence suggests this region consists of five nearly tandem domains identical to the one β-spiral-like repeat seen at residues 255 to 271.
FIG. 1.
T4 virion and its short tail fiber, gp12. (a) Electron micrograph of bacteriophage T4 showing the locations of structural proteins and features. (b) Ribbon diagram of the T4 short tail fiber structure (67). The C-terminal domain at the bottom of the figure binds irreversibly to the bacterial host cell LPS. Residues 290 to 327 comprise a triple β-helix. A single β-strand motif similar but not identical to a triple β-spiral repeat is seen near the N terminus of the structure at the top. The three subunits are shown in red, green, and blue. (C-terminal strands whose connectivities were not assigned are shown in yellow, purple, and gray.)
The triple β-helix domain, spanning residues 290 to 327, consists of right-handed coils of parallel β-strands in which each turn is composed of strands from each of the three chains. The triple β-helix is triangular in cross section, and the sides are made up of adjacent β-strands composed of hexapeptide quasi-repeats. This motif is stabilized through extensive intrachain hydrogen bonding as well as by a hydrophobic core composed mainly of inwardly pointing stacked aliphatic residues contributed by each of the three subunit chains. The only other observed triple β-helix domain is found in the gp5C domain of the T4 tail lysozyme complex (33) (discussed below).
The C-terminal knob is the most distal part of the molecule relative to the baseplate, where the short tail fiber is attached in the phage (44). This small globular domain is connected to the rest of the molecule by a small α-helix from each strand and is composed of a six-stranded β-sandwich from each of the three chains. In electron micrographs, the C-terminal end of the short tail fiber is observed interacting with the cell surface of the bacterial host (57).
Correct folding of the short tail fiber has been shown to require a phage-encoded dedicated chaperone, gp57, both in vivo (36) and in vitro (11). When assembled onto the baseplate, a specialized complex at the distal end of the bacteriophage tail that connects the long and short tail fibers to the tail, the short tail fibers are stowed in a retracted conformation (21, 44).
During attachment, after sufficient numbers of long tail fibers have bound to the host, the baseplate undergoes a dramatic conformational change from a hexagonal to a star-shaped outline, and the C-terminal ends of the short tail fibers extend away from the baseplate, where they can contact the host cell surface. A kink observed in the free protein corresponding to a flexible hinge domain is believed to enable the short tail fiber to assume the retracted configuration when bound to the baseplate in its hexagonal state (44). This kink may also be responsible for the lack of interpretable electron density between residues 85 and 245 of the shaft domain in the crystal structure of the short tail fiber fragment (66).
BACTERIOPHAGE T4 TAIL LYSOZYME COMPLEX
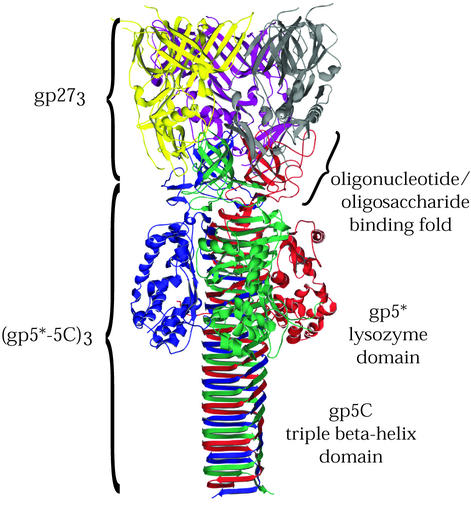
After T4 is firmly attached to its host via its baseplate short tail fibers, the tail contracts, driving a rigid inner tail tube tipped by the tail lysozyme complex across the cell wall and membranes. The T4 tail lysozyme complex is made up of three copies each of two kinds of protein, gp5 and gp27, which together form part of a central hub on the distal face of the baseplate (19). In the fully assembled virus, gp5 is cleaved after residue 351, but the resulting fragments, gp5* and gp5C, remain associated with the virion (32). Kanamaru et al. have solved the crystal structure of the (gp27-gp5*-gp5C)3 complex containing three molecules of gp27 and three copies of the proteolytically processed gp5 (33).
As shown in Fig. 2, the tail lysozyme complex is a structurally intricate, multidomain protein resembling a palm tree in outline. The “fronds” are composed of a trimer of the 319-amino-acid gp27. Each subunit of gp27 has an internal pseudo-two-fold rotational symmetry, such that the trimer, when viewed from above, has the appearance of a hexameric ring. The gp27 ring has the same diameter as the inner tail tube, which it caps. The 351-amino-acid N-terminal fragment of gp5, gp5*, contains three structural domains. An N-terminal five-stranded β-barrel oligosaccharide/oligonucleotide binding fold is proposed to bind to a carbohydrate portion of peptidoglycan, anchoring the lysozyme at the site where the cell is to be punctured. The “coconuts” of the palm tree are the lysozyme domains of gp5* at its C-terminal end. The tail lysozyme domain has sequence and structural similarity to the cytoplasmic T4 lysozyme, gpe. The lysozyme activity of gp5* is essential for phage infectivity (48), whereas gpe is not.
FIG. 2.
T4 tail lysozyme complex (gp27-gp5). Shown is a ribbon diagram of the lysozyme complex structure (33), which contains three copies each of gp27 and gp5. In the mature protein, gp5 is proteolytically cleaved, yielding gp5* and gp5C, which both remain associated with the complex. The three gp5 subunits, composed of the gp5* and gp5C fragments, are shown in red, green, and blue, and the three gp27 subunits are shown in yellow, purple, and gray. Residues of positions 436 to 575 of gp5 are found in the gp5C fragment and form a remarkable seven-turn, triple β-helix.
The C-terminal 234 amino acids of gp5, the gp5C fragment, form the “trunk” and contain a remarkable structural feature: a seven-turn triple β-helix. The triple β-helix domain has a triangular cross section, and the sides are composed of adjacent β-strands composed of, on average, octapeptide quasi-repeats. Each side of the helix is formed by adjacent β-strands from each chain. gp5C is stable in 10% sodium dodecyl-sulfate (SDS) and urea up to 2 M (33).
BACTERIOPHAGE P22 TAILSPIKE PROTEIN
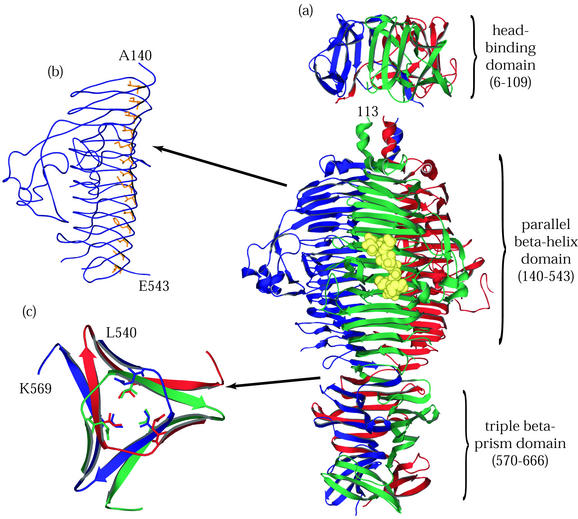
Tailspike, the phage P22 adhesin, is a 215-kDa homotrimer composed of subunits 666 amino acids in length. Steinbacher et al. determined crystallographic structures for stable N-terminal (60) and C-terminal (62) fragments of tailspike whose boundaries had previously been characterized by proteolysis and in vitro refolding analyses (16, 22). As illustrated in Fig. 3, the overall form of tailspike is an oligomer of three elongated subunits containing tandem structural domains laterally bound to each other along a long axis of threefold rotational symmetry.
FIG. 3.
P22 tailspike protein. The illustrations are based on structures determined by Steinbacher and coworkers (59-62). (a) The entire P22 tailspike protein, shown bound to the nonasaccharide from S. enterica serovar 253Ty O-antigen (in yellow space-filling representation). The N-terminal domain is at the top, and the three subunit chains are shown in red, green, and blue. (b) An interior hydrophobic stack from one of the three identical single-chain, parallel β-helices is shown with side chains highlighted in yellow. (c) Residues 540 to 569, viewed from above and showing inwardly pointing hydrophobic residues. This region, which spans the interdigitated domain, forms one turn of a triple-stranded β-helix and is involved in trimer stability.
The structure of the N-terminal 143 residues in each chain is composed of two β-sheets of five and three antiparallel strands. Together, the six sheets form the knob or dome-shaped capsid-binding domain. The N terminus of each subunit is buried within the central threefold axis at the interface between the subunits. The structure of the C-terminal portion of tailspike was determined for residues 109 to 666. Residues 143 to 540 fold into a parallel β-helix. This fold is composed of rungs or coils of β-strands that wrap about each other in solenoid fashion to form extended β-sheet surfaces with prevalent intrachain hydrogen bonding between adjacent β-strands. Many of the inwardly pointing hydrophobic residues of this domain participate in stacking interactions in which equivalent residues in adjacent stands form extensive hydrophobic ridges along the inner faces of the β-sheets (Fig. 3). The C-terminal residues 540 to 666, implicated in oligomer stability and trimerization (39), form extensive interchain hydrogen bonds and packing interactions. The amino acids from positions 540 to 569 make a single turn of a three-stranded β-helix effecting a symmetrical rotational displacement of the C-terminal portions of each subunit with respect to the rest of the chains.
The P22 tailspike recognizes and attaches to the O-antigen moieties of LPSs on the surfaces of its hosts, S. enterica serovars Typhimurium and Enteritidis. Tailspike has endorhamnosidase activity, cleaving the α1,3-O-glycosidic bond between repeating tetrasaccharide units of O-antigen (30). Intervening loops between certain rungs of the parallel β-helix provide additional height to the sides of the binding site. Within the substrate binding site is the glycolytic active site composed of three acidic residues: D392, D395, and Q359 (61).
P22's tailspike protein can distinguish three O-antigen types out of over 1,000 structurally and/or chemically distinct Salmonella O-antigen variants (51). Selective recognition of the sequence of saccharide units in the O-antigen repeat requires contact with structural and chemical determinants well beyond the scissile glycosidic bond. This requirement is reflected in the extensive β-sheet surface of the parallel β-helix domain, which provides a relatively large solvent-exposed surface area for the specific recognition and binding of an elongated, flexible polysaccharide. The binding of the octasaccharide to tailspike buries approximately 775 Å of available surface area per complex (61). This structure-function relationship is common to many of the parallel β-helix proteins (18, 31). The abundance of P22-related phages among collections of phages used in Salmonella typing schemes (56) and occurring as lysogens in wild-type isolates of Salmonella (55) demonstrates a diversity of O-antigen binding specificity among the adhesins of P22-like phage.
ADENOVIRUS FIBER
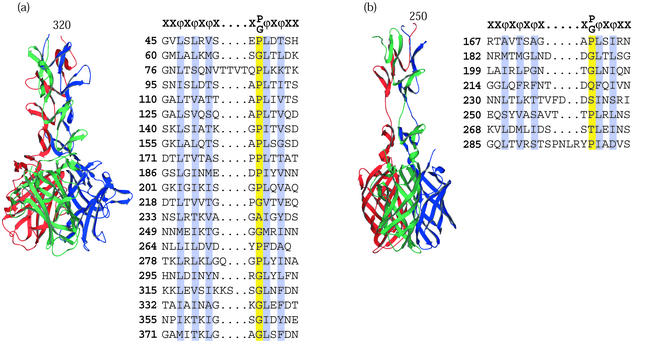
The human adenovirus type 2 (Ad2) adhesin is the penton fiber, which projects from the center of each icosahedral vertex of the virus capsid. Ad2 fiber is a trimer composed of chains 582 amino acids long. Full-length Ad2 fiber contains an N-terminal region that interacts with the virion penton base (24), an elongated central shaft domain (53), and a C-terminal globular receptor-binding domain (43). Limited proteolysis of the Ad2 fiber revealed a stable fragment of the protein (46). van Raaij and coworkers have solved the structure of an Ad fiber fragment consisting of amino acids 319 to 582 (65). The structure and its domainal organization are illustrated in Fig. 4.
FIG. 4.
Viral attachment fibers that have triple β-spiral repeats. (a) Structure of the human Ad2 penton fiber (65) showing four triple β-spiral repeats (residues 320 to 392) in the N-terminal shaft domain and the CAR binding C-terminal knob (residues 399 to 582). (b) Model of the reovirus σ1 attachment protein structure (15) showing three triple β-spiral repeats (residues 246 to 309) and the C-terminal knob (residues 310 to 455). The two knob proximal repeats are separated by a flexible spacer. The knob is a β-barrel composed of two key motifs shown as Greek letters. Both complexes are shown with the N-terminal domains at the top and the chains colored red, green, and blue. For both proteins, alignments of previously defined 15-amino-acid repeats are shown, conserved hydrophobic or structural residues are highlighted, and a canonical repeat motif is given at the top of each alignment.
The shaft domain contains a repetitive fold of unusual topology, the triple β-spiral, which corresponds to 15-residue repeats observed in the amino acid sequence (17, 29, 63). Four triple β-spiral structural repeats are seen in the structure's shaft domain, but the full-length molecule likely contains 21 repeats. It has been noted (65) that the sequence repeats as they are currently recognized do not directly correlate with the repeated structural elements of the fiber shaft domain. In essence, the repeats are a half-repeat out of register (i.e., the latter half of one repeat and the first half of the next repeat constitute a single structural repeat).
The shaft domain is stabilized by hydrogen bonds between β-strands in each repeat, between repeats within a chain, and between chains. Conserved hydrophobic residues at functionally identical positions within each repeat are oriented towards the central axis of the molecule, forming an extended hydrophobic core. The three chains wrap about the long axis with an overall left-handed rotational displacement of the chains about the three-fold axis where approximately seven repeats constitute a full turn.
Adenovirus attachment is a multi-step process. Adenovirus binds to cells through a primary interaction between the C-terminal, eight-stranded β-sandwich knob domain of the fiber and the coxsackievirus-adenovirus receptor (CAR) (4), a transmembrane protein of undefined function. This interaction is seen in a crystal structure of the fiber knob domain of Ad12 complexed with a soluble domain of the human CAR protein solved by Bewley and coworkers (6).
Recent data demonstrate that adenovirus fiber can use cell surface carbohydrates as cooperative receptors during the initial binding of virus to cell. The C-type adenoviruses Ad2 and Ad5 can bind heparan-sulfated glycosaminoglycans (HS GAGs) during infection (23). Sialic acid can functionally substitute for the CAR protein in the D-type adenoviruses Ad8, Ad19, and Ad37, and this interaction is correlated with sequences in the knob domain (1). Which fiber domain binds to HS GAG remains unknown. Attachment of adenovirus to host cells concludes with internalization of the virus via interactions between RGD sequences in the penton base, a capsid component that surrounds the N-terminal domain of the fiber, and integrins on the cell surface (49).
REOVIRUS σ1 ATTACHMENT PROTEIN
The reovirus σ1 attachment protein is very similar in structure and domainal organization to the adenovirus fiber protein. The full-length protein is composed of three chains 455 amino acids in length and has a fibrous tail or shaft domain and a globular head domain. The N-terminal 167 amino acids contain canonical heptad repeats (26) predicted to form a triple α-helical coiled-coil (15). σ1 is anchored to the virus at its N-terminal end (27). Chappell et al. determined the crystal structure of a trypsinized σ1 fragment consisting of residues 246 to 255, whose features are illustrated in Fig. 4 (15). Residues 246 to 309 span three repeats of a triple β-spiral. Eight such repeats make up the shaft domain of the full-length σ1 protein. A flexible spacer between repeats at residues 291 to 294 introduces asymmetry into the crystallized trimer, resulting in a tilting of the knob domain with respect to the long axis of the complex. σ1 uses junction adherens molecule (JAM) as the viral receptor and binds via a globular knob domain composed of a β-barrel from each of the three chains (3).
Reovirus binds sialic acid through a single N-terminal proximal triple β-spiral repeat (14). Reovirus has also been shown to degrade peptidoglycan and mucin (8). This enzymatic activity is probably used to penetrate the mucin layer covering the intestinal epithelia during peroral infection. Limited sequence similarity between the N-terminal domain coiled-coil region of σ1 and C-type lysozyme has been observed (7), but how this similarity relates a fibrous trimer to a globular monomeric protein is unknown.
Reovirus probably undergoes proteolytic cleavage during normal infection to make infectious subvirion particles (9). Removal of lambda and mu outer proteins allows the fiber to extend from the core (50). A conformational change in the σ1 protein possibly allows this extension to occur, since it is unlikely that the fiber extends all the way into the viral core. σ1 assembles cotranslationally through an N-terminal triple α-helical coiled-coil domain (28). Assembly of C-terminal domains requires the host chaperone heat shock protein 70 (hsp70) and ATP (42).
BACTERIOPHAGE T4 TAIL COMPONENTS: GP9, GP11, AND FIBRITIN
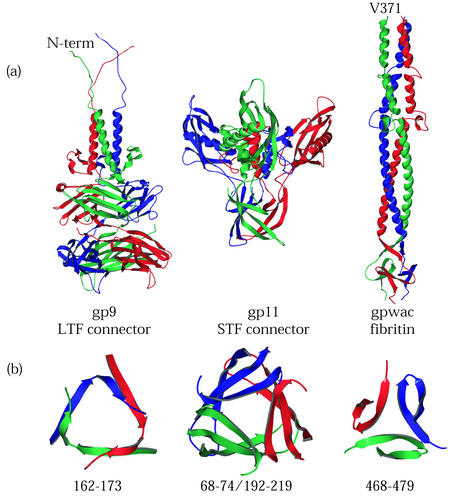
The structures of the T4 tail proteins gp9 and gp11 have been solved (38, 41). gp9 and gp11 are the interface between the fibers and the arm proteins of the baseplate complex at the distal end of the bacteriophage T4 tail (19). They are found on the outer vertices of the hexagonal baseplate. gp11 is required to connect the short tail fiber to the baseplate. The function of gp9 is twofold: to secure the long tail fiber to the baseplate and to convey binding information from the fibers to the baseplate complex (20).
Fibritin, the gene product of the T4 whiskers antigen control gene (wac), is a largely α-helical triple coiled-coil containing a triple β-propeller-like domain at its C terminus (64). Fibritin in the virion is bound to the collar region at the junction between the head and the tail and is required for efficient assembly of the long tail fiber to the baseplate (71).
These T4 tail proteins are stable. gp9 remains trimeric in the presence of 1% SDS (38). gp11 remains trimeric after incubation in 2% SDS and electrophoresis under standard denaturing conditions (40). Fibritin is resistant to denaturation by SDS at room temperature, and the basis of this stability resides in the C-terminal, propeller-like trimerization domain, also called the “foldon.” Fusions of this domain to HIV gp120 (72), collagen (25), and the T4 long tail fiber (45) have been shown to impart stability to the fusion proteins and, in the case of long tail fiber, overcome the need for gp57, the virally encoded chaperone of fiber assembly.
THE ROLE OF TRIPLE β-SPIRALS AND TRIPLE β-HELICES IN HOST CELL RECOGNITION
When the long tail fibers of phage T4 were first recognized as cell attachment organelles, it was generally believed that their extended character was needed to reach receptors that were far apart on the cell surface. This interpretation was carried forward to other elongated adhesins; the P22 tailspike, and the penton fiber of adenovirus. However the discovery that the lateral surface of the P22 tailspike was the active site for recognition, that the chondroitinases were parallel β-helices, raised the possibility that the length of these proteins represented the need to read saccharide sequences and not to extend the reach of the adhesins.
Is the triple β-spiral a widespread carbohydrate interaction motif? Bacteriophage SF6 of Bacillus subtilis (not to be confused with the Shigella phage Sf6) contains a gene whose product is a protein 316 amino acids in length with 98% identity to residues 1 to 316 of reovirus σ1 attachment protein's shaft domain. The bacteriophage lysozyme gene was cloned as a restriction fragment from purified phage DNA and identified on the basis of its lysozyme activity (68). Another phage protein, gp26 of phage P22, shows limited homology to σ1 in the putative N-terminal coiled-coil domain (P. Weigele, personal observation). gp26 is a component of the phage neck and is seen in electron micrographs as a central fiber protruding from the distal end of P22's short tail. Interestingly, phage SP6 has a lambda-like tail with a central tail fiber at its distal end (58), similar in arrangement to the lambda tail-tip protein gpJ (35, 69) and having the same dimensions as the shaft domain of the reovirus σ1 attachment protein.
The shared homologies between proteins from the segmented dsRNA reovirus, dsDNA bacteriophage, and the conserved structural similarity with Ad2 fiber suggest that either that sequences encoding the triple β-spiral have spread laterally among deeply divergent virus types, or the triple β-spiral is in fact an ancient protein fold.
The similarity of SF6 lysozyme to the distal tail fiber in P22 also suggests a possibility that many phage attachment complexes contain proteins with peptidoglycan-binding or -degrading activity as a general strategy for penetrating the cell wall to gain access to the inner membrane. Such is the case for the bacteriophage PRD1 (54), which contains a transglycosylase as part of its attachment machinery; the bacteriophage T4 tail, which has the gp27-gp5 lysozyme complex at its distal tip (34); and T7, which uses an injected viral transglycosylase, gp16, to enhance the efficiency of infection (47).
FOLDING AND ASSEMBLY OF HOMOTRIMERIC ADHESINS
As with coiled-coils and collagens, the native conformation of this class of elongated homotrimers can only be achieved after chain association. The hydrophobic cores of the native trimeric structures contain residues contributed by each of the three chains. The intertwining topological paths that the chains follow in space require that these homotrimers be built from partially folded subunits.
The folding and assembly of the P22 tailspike have been extensively studied both in vitro and in vivo (5, 22). After release from the ribosomes, the chains fold into partially folded monomers that probably have the parallel β-coils already formed. These partially folded chains associate into a protrimer intermediate in which the chains are associated but not fully folded. This protrimer species then undergoes further conformational change to form the thermostable and detergent-resistant native trimer. Amino acid substitutions in the triple β-helix domain trap the chains in the protrimer conformation (39). These protrimer-like species lack the stability of the native trimer, indicating that it is the intertwining of the three chains into the triple β-helix that clamps the native trimer into a detergent-resistant conformation. Currently, we are performing systematic mutagenesis of the P22 tailspike triple β-helix domain in order to better understand the molecular basis of the transition from protrimer to native trimer folding. Similar metastable multimeric intermediates may well be employed in the folding and assembly of the other proteins discussed.
Although the intermediates in the folding and assembly of the T4 short tail fiber have not been investigated, this information is encoded in part by a dedicated, virally encoded chaperone, the product of gene 57 (37). Other complex proteins, such as the σ1 attachment protein, require host-encoded chaperones for their folding and assembly.
WHY DO THESE ADHESINS HAVE INTERTWINED TRIPLE-STRAND FOLDS?
These proteins are unusually stable or have domains that are. Figure 5B presents triangular cross sections of some of the T4 tail proteins at points in the molecule suggested to contribute to their stability. These regions are trimerization interfaces. Their cross sections resemble the small triple β-helix domain of tailspike. The juxtaposition of these cross-sections is not meant to suggest they are the same fold. Rather, the stability of these molecules may be the result of these β-annular or interdigitated interfaces satisfying at least two of three conditions: (i) displaying extensive hydrogen bonding between chains, (ii) having a hydrophobic core common to the three chains, and (iii) acting as a point of rotational displacement so that each subunit is partially twisted about the long symmetry axis of its quaternary structure.
FIG. 5.
T4 tail proteins. (a) Ribbon diagrams of gp9, gp11, and gpwac from structures determined by Kostyuchenko et al. (38), Tao et al. (64), and Leiman et al. (41), respectively. gp9 connects the long tail fibers to the baseplate. gp11 connects the short tail fibers to the baseplate. Fibritin, the wac gene product, is found in the virus as a long “whisker” attached to the T4 neck and plays a role in long tail fiber assembly and retraction. Ribbon diagrams are displayed with N-terminal domains at the top and monomers in red, green, and blue. (b) Ribbon diagrams of the β-annulus domains displayed below the diagrams of the proteins in which they are found. Each annular domain is a cross section of the protein viewed from above.
It is not clear why these species are trimeric, rather than dimeric or tetrameric. It is interesting that although coiled-coils come in dimeric, trimeric, tetrameric, and pentameric forms, the viral hemagglutinins identified so far are all trimeric. Regardless of the number of chains involved, the generation of a highly stable, long solvent-exposed, β-stranded surface may well provide a very effective platform for recognizing a specific sequence of carbohydrate found on cell surface LPS and glycoproteins.
Acknowledgments
This work was supported by NIH grant GM17,980 and NSF grant EIA0225609.
REFERENCES
- 1.Arnberg, N., P. Pring-Åkerblom, and G. Wadell. 2002. Adenovirus type 37 uses sialic acid as a cellular receptor on Chang C cells. J. Virol. 76:8834-8841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Baker, K. A., R. E. Dutch, R. A. Lamb, and T. S. Jardetzky. 1999. Structural basis for paramyxovirus-mediated membrane fusion. Mol. Cell 3:309-319. [DOI] [PubMed] [Google Scholar]
- 3.Barton, E. S., J. C. Forrest, J. L. Connolly, J. D. Chappell, Y. Liu, F. J. Schnell, A. Nusrat, C. A. Parkos, and T. S. Dermody. 2001. Junction adhesion molecule is a receptor for reovirus. Cell 104:441-451. [DOI] [PubMed] [Google Scholar]
- 4.Bergelson, J. M., J. A. Cunningham, G. Droguett, E. A. Kurt-Jones, A. Krithivas, J. S. Hong, M. S. Horwitz, R. L. Crowell, and R. W. Finberg. 1997. Isolation of a common receptor for Coxsackie B viruses and adenoviruses 2 and 5. Science 275:1320-1323. [DOI] [PubMed] [Google Scholar]
- 5.Betts, S., and J. King. 1999. There's a right way and a wrong way: in vivo and in vitro folding, misfolding and subunit assembly of the P22 tailspike. Struct. Fold Des. 7:R131-R139. [DOI] [PubMed] [Google Scholar]
- 6.Bewley, M. C., K. Springer, Y. B. Zhang, P. Freimuth, and J. M. Flanagan. 1999. Structural analysis of the mechanism of adenovirus binding to its human cellular receptor, CAR. Science 286:1579-1583. [DOI] [PubMed] [Google Scholar]
- 7.Bisaillon, M., and G. Lemay. 1999. Computational sequence analysis of mammalian reovirus proteins. Virus Genes 18:13-37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bisaillon, M., S. Senechal, L. Bernier, and G. Lemay. 1999. A glycosyl hydrolase activity of mammalian reovirus sigma1 protein can contribute to viral infection through a mucus layer. J. Mol. Biol. 286:759-773. [DOI] [PubMed] [Google Scholar]
- 9.Bodkin, D. K., M. L. Nibert, and B. N. Fields. 1989. Proteolytic digestion of reovirus in the intestinal lumens of neonatal mice. J. Virol. 63:4676-4681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bradley, P., L. Cowen, M. Menke, J. King, and B. Berger. 2001. BETAWRAP: successful prediction of parallel beta-helices from primary sequence reveals an association with many microbial pathogens. Proc. Natl. Acad. Sci. USA 98:14819-14824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Burda, M. R., and S. Miller. 1999. Folding of coliphage T4 short tail fiber in vitro. Analyzing the role of a bacteriophage-encoded chaperone. Eur. J. Biochem. 265:771-778. [DOI] [PubMed] [Google Scholar]
- 12.Cerritelli, M. E., J. S. Wall, M. N. Simon, J. F. Conway, and A. C. Steven. 1996. Stoichiometry and domainal organization of the long tail-fiber of bacteriophage T4: a hinged viral adhesin. J. Mol. Biol. 260:767-780. [DOI] [PubMed] [Google Scholar]
- 13.Chan, D. C., D. Fass, J. M. Berger, and P. S. Kim. 1997. Core structure of gp41 from the HIV envelope glycoprotein. Cell 89:263-273. [DOI] [PubMed] [Google Scholar]
- 14.Chappell, J. D., V. L. Gunn, J. D. Wetzel, G. S. Baer, and T. S. Dermody. 1997. Mutations in type 3 reovirus that determine binding to sialic acid are contained in the fibrous tail domain of viral attachment protein σ1. J. Virol. 71:1834-1841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chappell, J. D., A. E. Prota, T. S. Dermody, and T. Stehle. 2002. Crystal structure of reovirus attachment protein sigma1 reveals evolutionary relationship to adenovirus fiber. EMBO J. 21:1-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chen, B., and J. King. 1991. Thermal unfolding pathway for the thermostable P22 tailspike endorhamnosidase. Biochemistry 30:6260-6269. [DOI] [PubMed] [Google Scholar]
- 17.Chroboczek, J., R. W. Ruigrok, and S. Cusack. 1995. Adenovirus fiber. Curr. Top. Microbiol. Immunol. 199:163-200. [DOI] [PubMed] [Google Scholar]
- 18.Ciccarelli, F. D., R. R. Copley, T. Doerks, R. B. Russell, and P. Bork. 2002. CASH—a beta-helix domain widespread among carbohydrate-binding proteins. Trends Biochem. Sci. 27:59-62. [DOI] [PubMed] [Google Scholar]
- 19.Coombs, D. H., and F. Arisaka. 1994. T4 tail structure and function, p. 259-281. In J. D. Karam, J. W. Drake, K. N. Kreuzer, G. Mosig, D. H. Hall, F. A. Eiserling, L. W. Black, E. K. Spicer, E. Kutter, K. Carlson, and E. S. Miller (ed.), Molecular biology of bacteriophage T4. American Society for Microbiology, Washington, D.C.
- 20.Crowther, R. A. 1980. Mutants of bacteriophage T4 that produce infective fibreless particles. J. Mol. Biol. 137:159-174. [DOI] [PubMed] [Google Scholar]
- 21.Crowther, R. A., E. V. Lenk, Y. Kikuchi, and J. King. 1977. Molecular reorganization in the hexagon to star transition of the baseplate of bacteriophage T4. J. Mol. Biol. 116:489-523. [DOI] [PubMed] [Google Scholar]
- 22.Danner, M., A. Fuchs, S. Miller, and R. Seckler. 1993. Folding and assembly of phage P22 tailspike endorhamnosidase lacking the N-terminal, head-binding domain. Eur. J. Biochem. 215:653-661. [DOI] [PubMed] [Google Scholar]
- 23.Dechecchi, M. C., P. Melotti, A. Bonizzato, M. Santacatterina, M. Chilosi, and G. Cabrini. 2001. Heparan sulfate glycosaminoglycans are receptors sufficient to mediate the initial binding of adenovirus types 2 and 5. J. Virol. 75:8772-8780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Devaux, C., M. L. Caillet-Boudin, B. Jacrot, and P. Boulanger. 1987. Crystallization, enzymatic cleavage, and the polarity of the adenovirus type 2 fiber. Virology 161:121-128. [DOI] [PubMed] [Google Scholar]
- 25.Frank, S., R. A. Kammerer, D. Mechling, T. Schulthess, R. Landwehr, J. Bann, Y. Guo, A. Lustig, H. P. Bachinger, and J. Engel. 2001. Stabilization of short collagen-like triple helices by protein engineering. J. Mol. Biol. 308:1081-1089. [DOI] [PubMed] [Google Scholar]
- 26.Fraser, R. D. B., D. B. Furlong, B. L. Trus, M. L. Nibert, B. N. Fields, and A. C. Steven. 1990. Molecular structure of the cell-attachment protein of reovirus: correlation of computer-processed electron micrographs with sequence-based predictions. J. Virol. 64:2990-3000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Furlong, D. B., M. L. Nibert, and B. N. Fields. 1988. Sigma 1 protein of mammalian reoviruses extends from the surfaces of viral particles. J. Virol. 62:246-256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gilmore, R., M. C. Coffey, G. Leone, K. McLure, and P. W. Lee. 1996. Co-translational trimerization of the reovirus cell attachment protein. EMBO J. 15:2651-2658. [PMC free article] [PubMed] [Google Scholar]
- 29.Green, N. M., N. G. Wrigley, W. C. Russell, S. R. Martin, and A. D. McLachlan. 1983. Evidence for a repeating cross-beta sheet structure in the adenovirus fibre. EMBO J. 2:1357-1365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Iwashita, S., and S. Kanegasaki. 1976. Enzymic and molecular properties of base-plate parts of bacteriophage P22. Eur. J. Biochem. 65:87-94. [DOI] [PubMed] [Google Scholar]
- 31.Jenkins, J., and R. Pickersgill. 2001. The architecture of parallel beta-helices and related folds. Prog. Biophys. Mol. Biol. 77:111-175. [DOI] [PubMed] [Google Scholar]
- 32.Kanamaru, S., N. C. Gassner, N. Ye, S. Takeda, and F. Arisaka. 1999. The C-terminal fragment of the precursor tail lysozyme of bacteriophage T4 stays as a structural component of the baseplate after cleavage. J. Bacteriol. 181:2739-2744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kanamaru, S., P. G. Leiman, V. A. Kostyuchenko, P. R. Chipman, V. V. Mesyanzhinov, F. Arisaka, and M. G. Rossmann. 2002. Structure of the cell-puncturing device of bacteriophage T4. Nature 415:553-557. [DOI] [PubMed] [Google Scholar]
- 34.Kao, S.-H., and W. H. McClain. 1980. Baseplate protein of bacteriophage T4 with both structural and lytic functions. J. Virol. 34:95-103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Katsura, I. 1983. Tail assembly and injection, p. 331-346. In R. W. Hendrix (ed.), Lambda II. Cold Spring Harbor Laboratory, Cold Spring Harbor, N.Y.
- 36.King, J., and U. K. Laemmli. 1973. Bacteriophage T4 tail assembly: structural proteins and their genetic identification. J. Mol. Biol. 75:315-337. [DOI] [PubMed] [Google Scholar]
- 37.King, J., and U. K. Laemmli. 1971. Polypeptides of the tail fibres of bacteriophage T4. J. Mol. Biol. 62:465-477. [DOI] [PubMed] [Google Scholar]
- 38.Kostyuchenko, V. A., G. A. Navruzbekov, L. P. Kurochkina, S. V. Strelkov, V. V. Mesyanzhinov, and M. G. Rossmann. 1999. The structure of bacteriophage T4 gene product 9: the trigger for tail contraction. Struct. Fold Des. 7:1213-1222. [DOI] [PubMed] [Google Scholar]
- 39.Kreisberg, J. F., S. D. Betts, C. Haase-Pettingell, and J. King. 2002. The interdigitated beta-helix domain of the P22 tailspike protein acts as a molecular clamp in trimer stabilization. Protein Sci. 11:820-830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kurochkina, L. P., P. G. Leiman, S. Y. Venyaminov, and V. V. Mesyanzhinov. 2001. Expression and properties of bacteriophage T4 gene product 11. Biochemistry (Moscow) 66:141-146. [DOI] [PubMed] [Google Scholar]
- 41.Leiman, P. G., V. A. Kostyuchenko, M. M. Shneider, L. P. Kurochkina, V. V. Mesyanzhinov, and M. G. Rossmann. 2000. Structure of bacteriophage T4 gene product 11, the interface between the baseplate and short tail fibers. J. Mol. Biol. 301:975-985. [DOI] [PubMed] [Google Scholar]
- 42.Leone, G., M. C. Coffey, R. Gilmore, R. Duncan, L. Maybaum, and P. W. Lee. 1996. C-terminal trimerization, but not N-terminal trimerization, of the reovirus cell attachment protein is a posttranslational and Hsp70/ATP-dependent process. J. Biol. Chem. 271:8466-8471. [DOI] [PubMed] [Google Scholar]
- 43.Louis, N., P. Fender, A. Barge, P. Kitts, and J. Chroboczek. 1994. Cell-binding domain of adenovirus serotype 2 fiber. J. Virol. 68:4104-4106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Makhov, A. M., B. L. Trus, J. F. Conway, M. N. Simon, T. G. Zurabishvili, V. V. Mesyanzhinov, and A. C. Steven. 1993. The short tail-fiber of bacteriophage T4: molecular structure and a mechanism for its conformational transition. Virology 194:117-127. [DOI] [PubMed] [Google Scholar]
- 45.Miroshnikov, K. A., E. I. Marusich, M. E. Cerritelli, N. Cheng, C. C. Hyde, A. C. Steven, and V. V. Mesyanzhinov. 1998. Engineering trimeric fibrous proteins based on bacteriophage T4 adhesins. Protein Eng. 11:329-332. [DOI] [PubMed] [Google Scholar]
- 46.Mitraki, A., A. Barge, J. Chroboczek, J. P. Andrieu, J. Gagnon, and R. W. Ruigrok. 1999. Unfolding studies of human adenovirus type 2 fibre trimers. Evidence for a stable domain. Eur. J. Biochem. 264:599-606. [DOI] [PubMed] [Google Scholar]
- 47.Moak, M., and I. J. Molineux. 2000. Role of the Gp16 lytic transglycosylase motif in bacteriophage T7 virions at the initiation of infection. Mol. Microbiol. 37:345-355. [DOI] [PubMed] [Google Scholar]
- 48.Nakagawa, H., F. Arisaka, and S. Ishii. 1985. Isolation and characterization of the bacteriophage T4 tail-associated lysozyme. J. Virol. 54:460-466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Nemerow, G. R., and P. L. Stewart. 1999. Role of αv integrins in adenovirus cell entry and gene delivery. Microbiol. Mol. Biol. Rev. 63:725-734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Nibert, M. L., J. D. Chappell, and T. S. Dermody. 1995. Infectious subvirion particles of reovirus type 3 Dearing exhibit a loss in infectivity and contain a cleaved σ1 protein. J. Virol. 69:5057-5067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Orskov, F., and I. Orskov. 1983. From the National Institutes of Health. Summary of a workshop on the clone concept in the epidemiology, taxonomy, and evolution of the Enterobacteriaceae and other bacteria. J. Infect. Dis. 148:346-357. [DOI] [PubMed] [Google Scholar]
- 52.Riede, I. 1987. Receptor specificity of the short tail fibres (gp12) of T-even type Escherichia coli phages. Mol. Gen. Genet. 206:110-115. [DOI] [PubMed] [Google Scholar]
- 53.Ruigrok, R. W., A. Barge, C. Albiges-Rizo, and S. Dayan. 1990. Structure of adenovirus fibre. II. Morphology of single fibres. J. Mol. Biol. 215:589-596. [DOI] [PubMed] [Google Scholar]
- 54.Rydman, P. S., and D. H. Bamford. 2000. Bacteriophage PRD1 DNA entry uses a viral membrane-associated transglycosylase activity. Mol. Microbiol. 37:356-363. [DOI] [PubMed] [Google Scholar]
- 55.Schicklmaier, P., E. Moser, T. Wieland, W. Rabsch, and H. Schmieger. 1998. A comparative study on the frequency of prophages among natural isolates of Salmonella and Escherichia coli with emphasis on generalized transducers. Antonie Leeuwenhoek 73:49-54. [DOI] [PubMed] [Google Scholar]
- 56.Schmieger, H. 1999. Molecular survey of the Salmonella phage typing system of Anderson. J. Bacteriol. 181:1630-1635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Simon, L. D., and T. F. Anderson. 1967. The infection of Escherichia coli by T2 and T4 bacteriophages as seen in the electron microscope. I. Attachment and penetration. Virology 32:279-297. [DOI] [PubMed] [Google Scholar]
- 58.Steensma, H. Y., and J. Blok. 1979. Effect of calcium ions on the infection of Bacillus subtilis by bacteriophage SF 6. J. Gen. Virol. 42:305-314. [DOI] [PubMed] [Google Scholar]
- 59.Steinbacher, S., U. Baxa, S. Miller, A. Weintraub, R. Seckler, and R. Huber. 1996. Crystal structure of phage P22 tailspike protein complexed with Salmonella sp. O-antigen receptors. Proc. Natl. Acad. Sci. USA 93:10584-10588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Steinbacher, S., S. Miller, U. Baxa, N. Budisa, A. Weintraub, R. Seckler, and R. Huber. 1997. Phage P22 tailspike protein: crystal structure of the head-binding domain at 2.3 Å, fully refined structure of the endorhamnosidase at 1.56 Å resolution, and the molecular basis of O-antigen recognition and cleavage. J. Mol. Biol. 267:865-880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Steinbacher, S., S. Miller, U. Baxa, A. Weintraub, and R. Seckler. 1997. Interaction of Salmonella phage P22 with its O-antigen receptor studied by X-ray crystallography. Biol. Chem. 378:337-343. [DOI] [PubMed] [Google Scholar]
- 62.Steinbacher, S., R. Seckler, S. Miller, B. Steipe, R. Huber, and P. Reinemer. 1994. Crystal structure of P22 tailspike protein: interdigitated subunits in a thermostable trimer. Science 265:383-386. [DOI] [PubMed] [Google Scholar]
- 63.Stouten, P. F., C. Sander, R. W. Ruigrok, and S. Cusack. 1992. New triple-helical model for the shaft of the adenovirus fibre. J. Mol. Biol. 226:1073-1084. [DOI] [PubMed] [Google Scholar]
- 64.Tao, Y., S. V. Strelkov, V. V. Mesyanzhinov, and M. G. Rossmann. 1997. Structure of bacteriophage T4 fibritin: a segmented coiled coil and the role of the C-terminal domain. Structure 5:789-798. [DOI] [PubMed] [Google Scholar]
- 65.van Raaij, M. J., A. Mitraki, G. Lavigne, and S. Cusack. 1999. A triple beta-spiral in the adenovirus fibre shaft reveals a new structural motif for a fibrous protein. Nature 401:935-938. [DOI] [PubMed] [Google Scholar]
- 66.van Raaij, M. J., G. Schoehn, M. R. Burda, and S. Miller. 2001. Crystal structure of a heat- and protease-stable part of the bacteriophage T4 short tail fibre. J. Mol. Biol. 314:1137-1146. [DOI] [PubMed] [Google Scholar]
- 67.van Raaij, M. J., G. Schoehn, M. Jaquinod, K. Ashman, M. R. Burda, and S. Miller. 2001. Identification and crystallisation of a heat- and protease-stable fragment of the bacteriophage T4 short tail fibre. Biol. Chem. 382:1049-1055. [DOI] [PubMed] [Google Scholar]
- 68.Verma, M. 1986. Molecular cloning and sequencing of lysozyme gene of bacteriophage SF6 of Bacillus subtilis. Curr. Microbiol. 13:299-301. [Google Scholar]
- 69.Wang, J., M. Hofnung, and A. Charbit. 2000. The C-terminal portion of the tail fiber protein of bacteriophage lambda is responsible for binding to LamB, its receptor at the surface of Escherichia coli K-12. J. Bacteriol. 182:508-512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Wilson, I. A., J. J. Skehel, and D. C. Wiley. 1981. Structure of the haemagglutinin membrane glycoprotein of influenza virus at 3 Å resolution. Nature 289:366-373. [DOI] [PubMed] [Google Scholar]
- 71.Wood, W. B., and M. P. Conley. 1979. Attachment of tail fibers in bacteriophage T4 assembly: role of the phage whiskers. J. Mol. Biol. 127:15-29. [DOI] [PubMed] [Google Scholar]
- 72.Yang, X., J. Lee, E. M. Mahony, P. D. Kwong, R. Wyatt, and J. Sodroski. 2002. Highly stable trimers formed by human immunodeficiency virus type 1 envelope glycoproteins fused with the trimeric motif of T4 bacteriophage fibritin. J. Virol. 76:4634-4642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Yoder, M. D., N. T. Keen, and F. Jurnak. 1993. New domain motif: the structure of pectate lyase C, a secreted plant virulence factor. Science 260:1503-1507. [DOI] [PubMed] [Google Scholar]