Abstract
Objective:
To determine the changes in sensation of pressure, 2-point discrimination, and submaximal isometric-force production variability due to cryotherapy.
Design and Setting:
Sensation was assessed using a 2 × 2 × 2 × 3 repeated-measures factorial design, with treatment (ice immersion or control), limb (right or left), digit (finger or thumb), and sensation test time (baseline, posttreatment, or postisometric-force trials) as independent variables. Dependent variables were changes in sensation of pressure and 2-point discrimination. Isometric-force variability was tested with a 2 × 2 × 3 repeated-measures factorial design. Treatment condition (ice immersion or control), limb (right or left), and percentage (10, 25, or 40) of maximal voluntary isometric contraction (MVIC) were the independent variables. The dependent variables were the precision or variability (the standard deviation of mean isometric force) and the accuracy or targeting error (the root mean square error) of the isometric force for each percentage of MVIC.
Subjects:
Fifteen volunteer college students (8 men, 7 women; age = 22 ± 3 years; mass = 72 ± 21.9 kg; height = 183.4 ± 11.6 cm).
Measurements:
We measured sensation in the distal palmar aspect of the index finger and thumb. Sensation of pressure and 2-point discrimination were measured before treatment (baseline), after treatment (15 minutes of ice immersion or control), and at the completion of isometric testing (final). Variability (standard deviation of mean isometric force) of the submaximal isometric finger forces was measured by having the subjects exert a pinching force with the thumb and index finger for 30 seconds. Subjects performed the pinching task at the 3 submaximal levels of MVIC (10%, 25%, and 40%), with the order of trials assigned randomly. The subjects were given a target representing the submaximal percentage of MVIC and visual feedback of the force produced as they pinched the testing device. The force exerted was measured using strain gauges mounted on an apparatus built to measure finger forces.
Results:
Sensation of pressure was less (ie, it took greater pressure to elicit a response) after ice immersion, thumbs were more affected than index fingers, and the decrease was greater in the right limb than the left. Two-point discrimination was not affected by cryotherapy but was higher in the finger than in the thumb under all conditions. Isometric-force variability (standard deviation of mean isometric force) was greater as percentage of force increased from 10% to 40% of MVIC. Targeting accuracy (root mean square error) was decreased at 40% of MVIC. Accuracy and force variability were not affected by cryotherapy.
Conclusions:
The application of cryotherapy and reduced sensation of pressure appear to have little effect on motor control of the digits. These results support the hypothesis that the use of cold is not contraindicated for use as an analgesic before submaximal rehabilitative exercise focusing on restoring neuromuscular control to injured tissues.
Keywords: root mean square error, accuracy, precision, sensation, maximal voluntary isometric contraction
Gripping an object involves submaximal isometric contractions of sufficient force to manipulate the object and prevent it from slipping. Submaximal muscle contraction, regardless of motion, requires sensory input (perception) to provide the necessary information to create precise movements (motor output).1 The human brain receives information about the environment from a variety of sources. Visual and tactile information are examples of stimuli that affect action and performance of a skill.2,3 Other important factors for muscle control include information from proprioceptors such as Golgi tendon organs in the joints and muscle spindles.1,4–6 Although the exact role of each of these proprioceptive inputs in creating muscle contraction is uncertain, each is essential in performing an isometric contraction.1
The measurement of variability in submaximal isometric-force production is based on the mean and standard deviation of the distribution as well as the root mean square error.7 The increased variability in relation to a set of standards is a result of some control problem within the sensorimotor system.8 The source of variability in force production may be related to variations in the state of muscle activity, excitability of motor neurons, and signals from higher nervous centers. It is unclear what the source of the variability is or at what level it must be studied.8
In this study, we measured the variability of a submaximal pinching force. This was accomplished by calculating the average standard deviation of the submaximal force produced during the submaximal isometric-force trials. Variability was also calculated by determining the root mean square error of the mean force produced by each subject.
Several authors9–12 have reported that cold causes reduced dexterity and sensitivity in the hand and fingers. The decrease in manual dexterity could be due to the effect of cold on nerve conduction velocity, proprioception, or muscular function. Nerve conduction velocity in efferent and afferent fibers decreases linearly with decreases in nerve tissue temperature.13 The perception of the stimulus may be altered as afferent pathways are slowed down because of decreased temperature.14 Additionally, motor-unit activation may be altered because of the changes in nerve conduction, possibly causing alterations in the force produced by contracting muscles. If the force produced during a contraction depended on tactile stimulus, a person might experience an increase in force variability (decreased precision) or an increase in targeting error (decreased accuracy) during an isometric contraction after a cryotherapy application.
Injury during athletic participation often requires therapeutic treatment before, during, or after contests or practices. Cryotherapy is a common adjunct to therapeutic exercise; it is used in the initial stages of injury treatment to reduce metabolism,15,16 inflammation,17 and muscle spasm and to control pain.18,19 This modality reduces nerve conduction velocity, decreases muscle-spindle excitability, and reduces local blood flow.18–21 These changes brought about by cryotherapy raise the question of whether the treatment may have deleterious effects on control of movement. A variety of responses to cold application have been reported, including decreased muscle functioning,22–25 no effect on muscle functioning,26 no effect on proprioception or agility,27–29 and decreased maximal isometric contraction.22,23 Investigations of the effect of cold on 2-point discrimination have shown no effect on the lower extremities.29 However, other authors9–12 have shown decreases in sensation of the finger after cold exposure.
The investigations on the effect of cryotherapy on muscle contraction have been conducted on large muscle groups, measuring strength of contraction rather than variability of muscular contraction. Furthermore, the effects of cryotherapy on the hand have not been studied extensively. Our objective was to measure the effects of cryotherapy on sensation of pressure, 2-point discrimination, and isometric-force precision and accuracy in the thumb and index finger.
METHODS
Design
This study was conducted with 2 experimental designs. Sensation was assessed with a 2 × 2 × 2 × 3 repeated-measures factorial design, with treatment (ice immersion or control), limb (right or left), digit (finger or thumb), and sensation test time (baseline, posttreatment, or postisometric-force trials) as independent variables. The dependent variables were changes in sensation of pressure and 2-point discrimination. Isometric-force variability was investigated using a 2 × 2 × 3 repeated-measures factorial design. The treatment condition (ice immersion or control), limb (right or left), and percentage (10, 25, or 40) of maximal voluntary isometric contraction (MVIC) were the independent variables. The dependent variables were the precision or variability (the standard deviation) and the accuracy or targeting error (the root mean square error) of the isometric force for each percentage of MVIC.
Subjects
Fifteen healthy subjects (8 men, 7 women; age = 22 ± 3 years; mass = 72 ± 21.9 kg; height = 183.4 ± 11.6 cm) with no history of neurologic disorders, cold allergy, or frostbite volunteered for the experiment. All subjects provided informed consent. Prior approval for the study was obtained from the university's institutional review board for the protection of human subjects.
Instrumentation
Cutaneous sensation in the index finger and thumb was assessed using Semmes-Weinstein monofilaments (Lafayette Instruments, Lafayette, IN) and the Disk-Criminator (Lafayette Instruments). The monofilaments were used to measure sensitivity to pressure, whereas the Disk-Criminator was used to measure 2-point discrimination. Sensation testing was conducted in approximately 30 seconds.
Two Micro-Measurements Precision Strain Gauges (13 × 5 mm) (Vishay Measurements Group, Shelton, CT) were glued to 2 metal half-cylinders, 5½ × 1¼ × ⅝ in (13.97 × 3.18 × 1.59 cm), the grip bars (Figure 1). This apparatus has been used in previous motor-control research. Subjects pinched the grip bars with the thumb and index finger, and the forces were measured by the strain gauges. The strain-gauge force data were digitized at a sample rate of 100 Hz and collected on a personal computer for analysis. Isometric-force output of the thumb and index finger were measured on separate strain gauges. These values were then summed and displayed on a computer screen. The computer screen simultaneously displayed the target force line.
Figure 1.
Testing apparatus for measuring the isometric gripping strength of the thumb and index finger.
Procedures
The experiment was conducted on 2 consecutive days, with subjects testing at the same time each day. Testing was conducted bilaterally, with each limb tested in both treatment conditions. Therefore, each subject completed each test 4 times (2 times on each hand) and experienced each treatment condition on both limbs (Table).
Subject Testing Order*
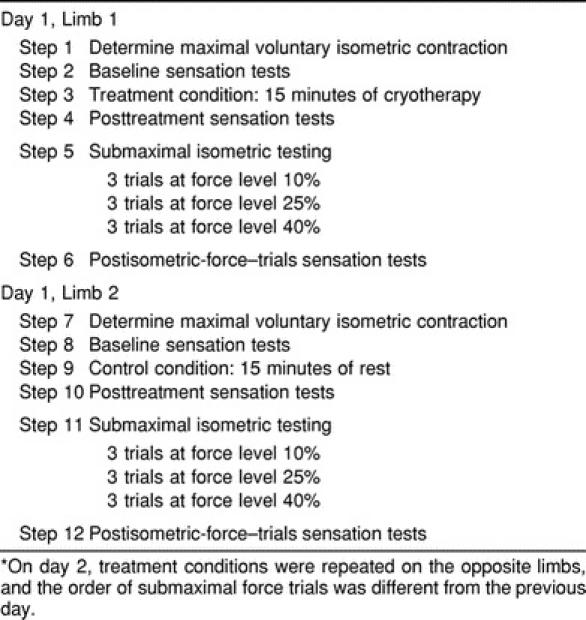
Before each day's testing, subjects sat quietly for 15 minutes to acclimate to the room temperature. Subject testing began with our measuring MVIC generated during a pinching task using the thumb and index finger. Two-point discrimination and sensation of pressure in the index finger and thumb of the limb being tested were then assessed (baseline). After baseline measurements of sensation, the 15-minute treatment condition (cryotherapy or control) was completed. Immediately thereafter, sensation was assessed a second time (posttreatment). Subjects then completed the submaximal isometric-force trials at the 3 different force levels. This was followed by the third assessment of sensation (final).
Immediately after the first limb was tested, these same procedures were completed on the subject's other limb. The treatment condition was the opposite of the one used for the first test, and the order of submaximal isometric-force trials was also different. On day 2, subjects again completed the same testing procedures as on day 1, but the treatment conditions were reversed (cryotherapy condition for the right arm for day 1, control condition for the right arm for day 2). The order of the submaximal isometric-force trials was also different from the day-1 testing sessions.
Subject Positioning
Each subject sat comfortably in a chair in front of a table. The tester positioned the subject's hand and fingers on the testing device, and the positioning remained the same throughout testing. The distal phalanges of the thumb and index finger were in constant contact with the 2 grip bars of the pinch-force measurement apparatus. The third through fifth fingers were in a fully flexed position. The arm was bent at the elbow to about 100° of flexion to allow the subjects to be comfortably seated and in constant contact with the strain-gauge device. Each subject's hand positioning was standardized for both extremities throughout all trials.
Maximal Voluntary Isometric Contraction
The MVIC of the thumb and index finger was measured at the onset of testing. Subjects were asked to maximally press the bars between the thumb and index finger for 10 seconds, repeated 3 times, with the hand on the table in the position described previously. Subjects were instructed to give maximal effort for the entire duration of each of the 3 trials. A mean MVIC was determined after three 10-second trials, using the peak value of MVIC during each of the 3 trials. The force output was displayed on a computer screen for the subjects. The mean MVIC was used to determine the percentages of submaximal force for the submaximal isometric-force testing.
Sensation Testing
Cutaneous sensation was measured in 2 ways: using a monofilament system and the Disk-Criminator. During testing, the subject's hand was placed on the table with a towel under it for padding. The palmar surface of the hand was exposed. The subject's eyes were closed, and he or she was instructed to fully attend to the testing. The filaments and tines were pressed onto the palmar surface of the distal phalanx of the thumb and index finger to assess sensory function of the median nerve.
The order of sensation testing was randomized using a balanced design. The digit tested first was chosen at random. Sensation testing was conducted 3 times before treatment, after the 1-minute treatment, and, finally, after the completion of isometric testing for that hand. The time for sensation testing was less than 1 minute for each of the 3 test times, or less than 3 minutes total.
For sensation-of-pressure testing, the monofilament was pressed against the skin until it bent. The testing apparatus was held in place for 2 seconds and removed for 3 seconds as suggested by van Vliet et al.30 This was repeated 3 times for each filament. The tester prompted the subject on when to respond and to respond with a “yes” or “no” when he or she felt the monofilament. The filaments were numbered by size, with 1.65 being the smallest. The numbers on the filaments correlate to a gram value of pressure needed to bend the filament.31 The smallest monofilament was used first for all testing. Monofilaments were increased in size, using the increments 2.36 (0.02 g), 2.44 (0.04 g), 2.83 (0.07 g), 3.22 (0.16 g), 3.61 (0.4 g), 3.84 (0.6 g), and 4.08 (1.0 g) until the subject had a “yes” response on consecutive increments. The established normal range of sensation for the fingers ranges from 1.65 to 2.83.32 All subjects fell into this range at the beginning of testing.
The Disk-Criminator had 5 levels of discrimination, the first being 0 mm, or 1 point, whereas the rest were 2 points, with distances between the 2 points of 1 mm to 5 mm. A normal 2-point discrimination measurement is 2 mm or less on the fingertips.33 For 2-point discrimination testing, subjects were asked to respond with the number (1 or 2) they felt. Subjects sat quietly with their eyes closed during testing. The tester applied just enough pressure to depress the skin directly below the instrument, and the points contacted the skin at the same time. The placement of 1 or 2 points was randomly mixed. Each subject was assessed 3 times on each of the 5 distances on the Disk-Criminator. The number of correct responses (of 15) was the 2-point discrimination score.
Treatment Conditions
Subjects underwent a 15-minute ice-bath immersion of the arm from 1 in (2.54 cm) proximal to the medial epicondyle to the distal end of the fingers. The arms of the control subjects were placed in the empty tub. The ice bath was at a temperature of 10°C at the beginning of testing and was allowed to warm as it would in a practical setting in the athletic training room. The temperature was measured after treatment and adjusted for the next treatment, but records of posttreatment temperatures were not kept. After treatment, subjects towel dried the hand, we tested sensation, and then we began submaximal isometric-force testing. Time from the end of the treatment to the start of the submaximal isometric-force trials was approximately 2 minutes.
Submaximal Isometric-Force Testing
A computer screen was positioned on the desk in front of the subjects so that they could easily see the screen. The computer display provided visual feedback to the subjects of the forces they produced during the entire length of the trial. Each subject's individual target force (a percentage of the maximal force) was displayed as a horizontal line (in red). A second line (in yellow) represented the instant output of the subject's isometric force. Subjects were instructed to match the force output line with the target line. The isometric-force output line (in yellow) moved longitudinally across the screen during the 30-second trial and rose and fell as the subject increased or decreased the pressure on the strain gauges.
For submaximal testing, the subject's hand was positioned in contact with the strain-gauge apparatus on the grip bars in the same position as that used during the MVIC testing. Subjects were instructed to maintain the same positioning of the thumb and index finger throughout testing. The arm and hand remained in the same position as described previously.
Submaximal-force testing required the subjects to match, as accurately as possible, 3 specific isometric target forces: 10%, 25%, and 40% of MVIC. For each target force level, 5 trials of 30 seconds were performed, with 30 seconds' rest between trials. The order of submaximal-force percentages was randomly determined for each subject.
Data Analysis
The mean and standard deviation of the total force produced by the thumb and finger at each percentage of MVIC were calculated using SAS software (version 7.0, SAS Institute Inc, Cary, NC). The trials consisted of 2500 samples collected in the last 25 seconds of each submaximal force trial. The first 500 samples (5 seconds) were removed, so that any differences in the subject's acquisition of the target were not a factor in force variability. The mean force produced at each percentage of MVIC was determined by averaging the 5 trials conducted at each level. The standard deviation for the mean of the 5 trials was used to assess the variability of the subject's ability to maintain the submaximal force. We calculated root mean square error terms for each trial, using the target force and the actual force produced by the subjects during each trial, with the formula ((Σ(x − t)2)/2500), where x = the raw data and t = the target force. The root mean square error during each trial represented the subject's accuracy, the average of the absolute value of over- and underestimating the target during isometric testing. Again, the root mean square error term was averaged for the 5 trials at each percentage of MVIC. The data for the dependent variables (change in sensation of pressure, 2-point discrimination, variability, and root mean square error) were evaluated with an analysis of variance using Tukey-Kramer post hoc tests, with an alpha level of P < .05 set a priori to identify differences among the treatment conditions, percentage of force, arm, digit, and interactions of the 4 variables.
RESULTS
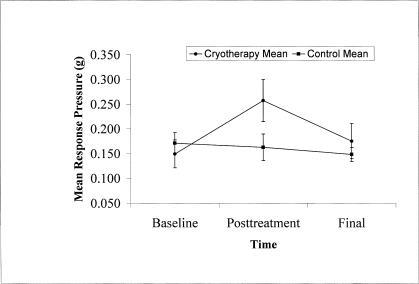
Sensation of pressure was 0.108 g higher after cryotherapy than at baseline (F1,352 = 9.09, P ≤ .003) (Figure 2). The posttreatment sensation of pressure was significantly higher than the baseline and postisometric force trials (F2,352 = 8.75, P ≤ .001, Tukey post hoc test P = .001). The left hand was more discriminating during sensation of pressure testing; that is, the right hand required greater force to elicit a response after cryotherapy (F2,352 = 6.36, P ≤ .012, Tukey post hoc test P = .012) than the left hand. There was also a greater decrease in the pressure sensitivity in the thumb than the finger after cryotherapy (F1,352 = 5.02, P ≤ .026, Tukey post hoc test P = .025).
Figure 2.
Posttreatment (15-minute ice bath) mean pressure of sensation was greater (*P = .001) in the treatment group, meaning the ability to sense pressure in the digits was less. Sensation of pressure was not different between conditions at baseline and final, nor was there a difference between tests in control subjects.
Two-point discrimination was not affected by cryotherapy, being greater in the finger than in the thumb (F1,352 = 18.17, P < .01, Tukey post hoc test P = .005).
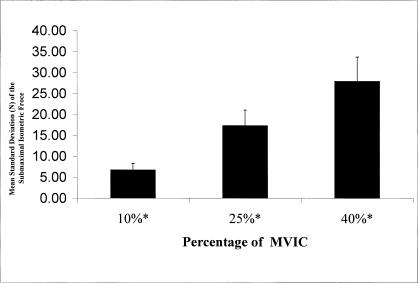
Cryotherapy had no effect on precision (force variability F2,168 = 0.42, P > .05) or accuracy (root mean square error F2,168 = 0.54, P = .05). The variability of the force produced increased as the percentage of force increased (F2,168 = 65.36, P < .010; 40% > 25% > 10%, Tukey post hoc test P = .001) (Figure 3).
Figure 3.
Targeting precision decreased as the percentage of the submaximal isometric force increased. *Standard deviation increased as percentage of maximal voluntary isometric contraction increased (P < .01). Targeting precision was not affected by treatment condition, and there was no difference due to arm tested. MVIC indicates maximal voluntary isometric contraction.
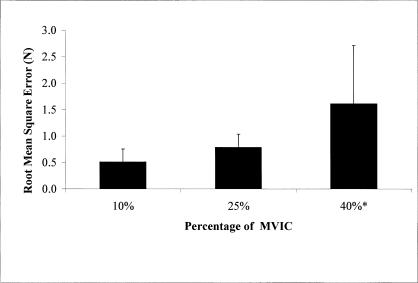
Accuracy (root mean square error) also increased as the target forces increased (F2,168 = 25.73, P < .01) (Figure 4); 40% of MVIC was greater than 25% and 10% (Tukey post hoc test P = .001).
Figure 4.
Targeting accuracy decreased as the percentage of submaximal isometric force increased. Root mean square error increased as the percentage of maximal voluntary isometric contraction increased from 25% to 40% (*P < .01). The targeting error was not affected by the treatment condition and was not different in the 10% and 25% conditions. MVIC indicates maximal voluntary isometric contraction.
DISCUSSION
The goals of this study were to examine the effect of cryotherapy on the sensory perception in the index finger and thumb and on submaximal isometric-force production. We hypothesized that the sensation of pressure would decrease and 2-point discrimination would become less accurate after a 15-minute period of cooling in the 10°C ice bath. We also hypothesized that decreased tissue temperatures would increase the variability of the mean isometric force produced by the thumb and index finger.
Apparently a 15-minute ice-bath immersion did not affect force-production targeting error (accuracy and precision) under the conditions of this study. Understanding the interaction of accuracy (root mean square error) and precision (standard deviation) allows one to understand variability and error measurements. A person who is able to hit a target 9 out of 10 times is very accurate, but if that same person hits the same spot on the target only 1 time out of 10, he or she is very imprecise. Another person who hits the same point 8 out of 10 times but outside of the target is considered precise but inaccurate.
Our results are similar to a previous investigation of grip coordination and force with the thumb and index finger. Sharp and Newell7 also reported that as the target force increased (from 10% to 50% of MVIC), root mean square error (N) and the standard deviation of the submaximal force increased. Root mean square error and the variability were significantly different among 5%, 10%, 30%, and 50% of MVIC. Our root mean square values were lower than theirs: 0.8 and 1.6 N at 25% and 40% of MVIC, whereas their values were approximately 3 and 8 N at 30% and 50% of MVIC, respectively. The difference in methods and devices used for the 2 studies may have contributed to these differences. Our subjects performed five 30-second trials, whereas their subjects performed ten 6-second trials. Both sets of authors made corrections for the error at the initiation of each trial by removing a portion of each trial while the subjects acquired the target force. We removed the first 5 seconds, whereas they7 only collected data once the subject achieved the target. The likeliest reason for the differences, however, is the equipment used. The designs were similar, but they used individual load cells for each digit, and we did not. The load cells may have resulted in more accurate measurements of the forces generated by the thumb and index finger.
The increase in variability (decreased precision) and targeting error (decreased accuracy) may have been due to fatigue; however, we did not measure fatigue. Several of the subjects stated that the 40% MVIC trials were the most difficult to perform. While watching the subjects perform the 40% trials, we noted that it was harder to accurately hit the target than during the 10% trials in the first few seconds of each trial. The longer subjects maintained a higher level of force, the greater the potential for fatigue. This fatigue may be the result of a depletion of muscle adenosine triphosphate when greater demand is placed on the muscles for longer periods of time.
Previous research on the targeting error and variability associated with submaximal forces of the fingers suggests that the increase in error is due to a lack of coordination of the digits.7 As the force increases, grip coordination becomes increasingly more difficult and leads to greater error and variability. It appears that there is a specific grip configuration for higher forces. Increasing the number of digits used in the task may result in a decrease in targeting error and variability at higher force levels, but many digits may also increase the variability. Sharp and Newell7 reported that the grip had a significantly reduced error when 3 and 4 digits were used to grip the device rather than 2 or 5 digits.
Several authors27–29,34 have suggested that cryotherapy has no effect on closed or open kinetic chain proprioception. We observed no increase in targeting error after cryotherapy, indicating that proprioception was not affected by the cold. The mechanoreceptors, muscle spindles, and Golgi tendon organs are still capable of providing input for the brain to stimulate motor endplates to contract muscle fibers and produce the proper amount of force to accurately achieve the target. However, the role of vision was not measured in this study. The dominance of vision may have overridden any effects of reduced proprioception.
Sensation After Cryotherapy
Greater pressure was needed to obtain a sensory response after cold application. This suggests that decreased temperatures affect the sensory receptors, a possibility supported by previous research in which authors9,11 reported a decrease in sensation in the index finger after rapid cooling.
The thermal sensory fibers are intensely stimulated by the near-freezing temperatures of the ice bath.35 Simultaneously, nocioceptor stimulation results in the sensation of pain experienced at low temperatures. Continuous stimulation of a receptor causes the receptor to adapt to the stimulus by increasing its response threshold and, thus, firing less frequently.35 This increase in threshold may explain the response during the sensation-of-pressure testing. However, mechanoreceptors and nocioceptors are independent receptors, so an increase in pain-receptor threshold may not cause an increased threshold in mechanoreceptors after ice application. A possible explanation is the stimulus of polymodal nocioceptors, which respond to thermal and mechanical noxious stimuli simultaneously.35 These nocioceptors may allow the sensation threshold to increase in both types of receptors. Additionally, the afferent output from different nerve endings is transported to the spine by the same dorsal root ganglion neurons, the primary afferent pathway. It is quite possible that the reduction of action-potential transmission as the result of decreased nerve conduction velocity and increased receptor threshold lead to an increase in pressure needed to stimulate a response after ice is applied.13
The fingertips are the most sensitive area of the body.35 With the high level of receptor acuity in the digits, it may be possible to have an increase in mean response during the sensation-of-pressure testing that is not clinically significant. The increase in mean response (0.033 g) was not large enough to reach the level of the next highest monofilament, from 3.22 (0.16 g) to 3.61 (0.4 g). Subjects were still responding to the 3.22 monofilament, but more errors occurred at this level than before the ice bath. A more sensitive test is indicated for further study of the decrease in sensitivity to pressure after cryotherapy.
The results of the 2-point discrimination tests suggest a difference in the sensitivity of the finger and thumb35 and between right and left35 but not between dominant and nondominant hands.32 The right finger and left thumb were better able to detect smaller distances in 2-point discrimination. Our results, however, suggest that the finger, on either hand, is better able to discriminate smaller distances and is better at 2-point discrimination. We also found that cryotherapy did not affect 2-point discrimination. This concurs with previously reported data that suggested sensory perception on the sole of the foot was not altered by cold in 2-point discrimination.29 The fact that 2-point discrimination was not affected may be because of the highly sensitive receptors located in the digits of the hand.
Meissner corpuscles and Merkel disks are located in the fingers in large numbers and have relatively small receptive fields, approximately 2 to 4 mm in diameter.35 The large number of receptors in close proximity may account for the ability of the receptors to distinguish such a small difference in distance, 2 mm. Again the clinical significance of these findings is that cryotherapy does not affect 2-point discrimination.
Effect of Cryotherapy on Variance of Force Production
Variance of force production was also not affected by cooling, indicating that subjects were as precise in cold and no-cold conditions. As we observed with the variability data, as force increased, the standard deviation increased. Ulnar and median motor nerve conduction is decreased after 30 minutes of cooling at a higher temperature than used in this study.13,14 We believed that a decrease in motor-nerve conduction velocity would decrease reaction time, increase mean force variability, and decrease the precision of force production. However, our results do not indicate that this occurs, and decreasing nerve conduction velocity in the hand and forearm may not alter motor function as measured in this study.
It appears that cryotherapy does not affect the targeting precision or force variability of contracting muscles. The application of ice before submaximal exercise, such as during cryokinetics or a cryostretch routine, should not decrease the athlete's ability to maintain a given level of muscle contraction. We did not, however, address MVIC after cryotherapy. Maximal contraction after cryotherapy is reduced,22 and activities requiring maximal contraction may be affected. During the initial stages of rehabilitation, however, the athlete rarely contracts the muscle maximally. Therefore, we conclude that the use of cryotherapy to treat pain and muscle spasm before submaximal rehabilitative therapeutic exercise is not contraindicated.36
CONCLUSIONS
Cryotherapy does not increase isometric-force targeting error or mean force standard deviation. Sensation of pressure was decreased after cryotherapy, whereas 2-point discrimination was unaltered, suggesting that a more sensitive method of testing may be necessary to determine the exact impact of cold on sensation. The use of cryotherapy as an analgesic before submaximal closed chain upper extremity exercise does not appear to be contraindicated.
REFERENCES
- 1.Sainburg RL, Poizner H, Ghez C. Loss of proprioception produces deficits in interjoint coordination. J Neurophysiol. 1993;70:2136–2147. doi: 10.1152/jn.1993.70.5.2136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Srinivasan MA, Lamotte RH. Tactual discrimination of softness. J Neurophysiol. 1995;73:88–101. doi: 10.1152/jn.1995.73.1.88. [DOI] [PubMed] [Google Scholar]
- 3.Akamatsu M. The influence of combined visual and tactile information on finger and eye movments during shape tracing. Ergonomics. 1992;5:647–660. doi: 10.1080/00140139208967844. [DOI] [PubMed] [Google Scholar]
- 4.Shumway-Cook A, Woollacott M. Motor Control: Theory and Practical Applications. Philadelphia, PA: Williams & Wilkins; 1995. [Google Scholar]
- 5.Bevan L, Cordo P, Carlton L, Carlton M. Proprioceptive coordination of movement sequences: discrimination of joint angle versus angular distance. J Neurophysiol. 1994;71:1862–1872. doi: 10.1152/jn.1994.71.5.1862. [DOI] [PubMed] [Google Scholar]
- 6.Cordo P, Carlton L, Bevan L, Carlton M, Kerr GK. Proprioceptive coordination of movement sequences: role of velocity and position information. J Neurophysiol. 1994;71:1848–1861. doi: 10.1152/jn.1994.71.5.1848. [DOI] [PubMed] [Google Scholar]
- 7.Sharp WE, Newell KM. Coordination of grip configurations as a function of force output. J Mot Behav. 2000;32:73–82. doi: 10.1080/00222890009601361. [DOI] [PubMed] [Google Scholar]
- 8.Newell K, Corocos DM. Variability in Motor Control. Champaign, IL: Human Kinetics; 1993. [Google Scholar]
- 9.Morton R, Provins KA. Finger numbness after acute local exposure to cold. J Appl Physiol. 1960;15:149–154. doi: 10.1152/jappl.1960.15.1.149. [DOI] [PubMed] [Google Scholar]
- 10.Mackworth NH. Finger numbness in very cold winds. J Appl Physiol. 1953;5:533–543. doi: 10.1152/jappl.1953.5.9.533. [DOI] [PubMed] [Google Scholar]
- 11.Provins KA, Morton R. Tactile discrimination and skin temperature. J Appl Physiol. 1960;15:155–160. doi: 10.1152/jappl.1960.15.1.155. [DOI] [PubMed] [Google Scholar]
- 12.Teichner W. Manual dexterity in the cold. J Appl Physiol. 1957;11:333–338. doi: 10.1152/jappl.1957.11.3.333. [DOI] [PubMed] [Google Scholar]
- 13.Halar EM, Delisa JA, Soine TL. Nerve conduction studies in upper extremities: skin temperature corrections. Arch Phys Med Rehabil. 1983;64:412–416. [PubMed] [Google Scholar]
- 14.Zankel HT. Effect of physical agents on motor conduction velocity of the ulnar nerve. Arch Phys Med Rehabil. 1966;47:787–792. [PubMed] [Google Scholar]
- 15.Merrick MA. Secondary injury after musculoskeletal trauma: a review and update. J Athl Train. 2002;37:209–217. [PMC free article] [PubMed] [Google Scholar]
- 16.Merrick MA, Rankin JM, Andres FA, Hinman CL. A preliminary examination of cryotherapy and secondary injury in skeletal muscle. Med Sci Sports Exerc. 1999;31:1516–1521. doi: 10.1097/00005768-199911000-00004. [DOI] [PubMed] [Google Scholar]
- 17.Knight KL. The effects of hypothermia on inflammation and swelling. Athl Train J Natl Athl Train Assoc. 1976;11:7–10. [Google Scholar]
- 18.Knight KL. Cryotherapy in Sport Injury Management. Champaign, IL: Human Kinetics; 1995. [Google Scholar]
- 19.Prentice W. Therapeutic Modalities in Sports Medicine. 2nd ed. St Louis, MO: Mosby; 1990. [Google Scholar]
- 20.Ho SS, Coel MN, Kagawa R, Richardson AB. The effects of ice on blood flow and bone metabolism in knees. Am J Sports Med. 1994;22:537–540. doi: 10.1177/036354659402200417. [DOI] [PubMed] [Google Scholar]
- 21.Ho SS, Illgen RL, Meyer RW, Torok PJ, Cooper MD, Reider B. Comparison of various icing times in decreasing bone metabolism and blood flow in the knee. Am J Sports Med. 1995;23:74–76. doi: 10.1177/036354659502300112. [DOI] [PubMed] [Google Scholar]
- 22.Coppin EG, Livingstone SD, Kuehn LA. Effects on handgrip strength due to arm immersion in a 10 degree C water bath. Aviat Space Environ Med. 1978;49:1322–1326. [PubMed] [Google Scholar]
- 23.McGown HL. Effects of cold application on maximal isometric contraction. Phys Ther. 1967;47:185–192. doi: 10.1093/ptj/47.3.185. [DOI] [PubMed] [Google Scholar]
- 24.Cross KM, Wilson RW, Perrin DH. Functional performance following an ice immersion to the lower extremity. J Athl Train. 1996;31:113–116. [PMC free article] [PubMed] [Google Scholar]
- 25.Ruiz DH, Myrer JW, Durrant E, Fellingham GW. Cryotherapy and sequential exercise bouts following cryotherapy on concentric and eccentric strength in the quadriceps. J Athl Train. 1993;28:320–323. [PMC free article] [PubMed] [Google Scholar]
- 26.Evans TA, Ingersoll CD, Knight KL, Worrell T. Agility following the application of cold therapy. J Athl Train. 1995;30:231–234. [PMC free article] [PubMed] [Google Scholar]
- 27.Thieme HA, Ingersoll CD, Knight KL, Ozmun JC. Cooling does not affect knee proprioception. J Athl Train. 1996;31:8–11. [PMC free article] [PubMed] [Google Scholar]
- 28.LaRiviere J, Osternig LR. The effect of ice immersion on joint position sense. J Sport Rehabil. 1994;3:58–67. [Google Scholar]
- 29.Ingersoll CD, Knight KL, Merrick MA. Sensory perception of the foot and ankle following therapeutic applications of heat and cold. J Athl Train. 1992;27:231–234. [PMC free article] [PubMed] [Google Scholar]
- 30.van Vliet D, Novak CB, Mackinnon SE. Duration of contact time alters cutaneous pressure threshold measurements. Ann Plast Surg. 1993;31:335–339. doi: 10.1097/00000637-199310000-00010. [DOI] [PubMed] [Google Scholar]
- 31.Dellon AL, Mackinnon SE, Brandt KE. The markings of the Semmes-Weinstein nylon monofilaments. J Hand Surg Am. 1993;18:756–757. doi: 10.1016/0363-5023(93)90333-X. [DOI] [PubMed] [Google Scholar]
- 32.Hage JJ, van der Steen LP, de Groot PJ. Difference in sensibility between the dominant and nondominant index finger as tested using the Semmes-Weinstein monofilaments pressure aesthesiometer. J Hand Surg Am. 1995;20:227–229. doi: 10.1016/s0363-5023(05)80012-7. [DOI] [PubMed] [Google Scholar]
- 33.Aronson A, Bastron J, Brown J. Clinical Examinations in Neurology. 3rd ed. Philadelphia, PA: WB Saunders; 1971. The sensory examination. [Google Scholar]
- 34.Gerig B. The effects of cryotherapy upon ankle proprioception. J Athl Train. 1990;25:119. [Google Scholar]
- 35.Kandel ER, Schwartz JH, Jessell TM. Principles of Neural Science. 3rd ed. Norwalk, CT: Appleton and Lange; 1991. [Google Scholar]
- 36.Knight K, Brucker J, Stoneman P, Rubley M. Muscle injury management with cryotherapy. Athl Ther Today. 2000;5(4):26–30. [Google Scholar]