In the 1950s and 1960s, debate raged as to whether coronary thrombosis was the cause or the consequence of ST-segment elevation myocardial infarction (STEMI). In the 1960s and 1970s, discussion focused on how long to keep patients in bed (a month was not uncommon), whether coronary care units reduced mortality and whether “warning arrhythmias” were worth treating. As recently as the early 1980s, no treatment had been shown to reduce mortality or morbidity in the acute phase of STEMI, nor did we know how to prevent a recurrent event. Anticoagulants moved in and out of favour, depending on the shifting sands of expert opinions. Fibrinolytic therapy was used in many European centres and then was discarded as being ineffective, and even dangerous, on the basis of results from inadequately powered trials. Use of lidocaine was common in the United States and in some European countries but was used sparingly in the United Kingdom and elsewhere. Exercise stress testing was rarely done soon after STEMI because of safety concerns. Early coronary arteriography (in the first few days after STEMI) was rare, even in the late 1980s. Since then, we have seen marked changes in the management of patients with STEMI.1,2,3,4
In 1980, DeWood and colleagues5 reported that about 80% of patients with acute MI had coronary occlusion, measured using coronary arteriography, and that the occlusion was due to an intraluminal thrombus. Later studies showed that the coronary thrombus could be dissolved with intracoronary (and later intravenous) administration of streptokinase, and a meta-analysis demonstrated a reduction in mortality. Experts remained skeptical about these results, in part because meta-analysis was a new tool. This opinion changed, however, when further trials convincingly showed that early administration of thrombolytic therapy in combination with ASA led to a halving of mortality.6,7
Then, in 1986, the results from a small, randomized trial involving 56 patients suggested that percutaneous coronary intervention (PCI) was superior to intracoronary streptokinase therapy in improving left ventricular function.8 After several small trials, a systematic review published in 1997 of 10 trials involving a total of 2606 patients that compared either streptokinase or tissue-type plasminogen activator with primary PCI showed a statistically significant 34% reduction in mortality in favour of PCI (6.5% v. 4.4%; OR 0.66, 95% CI 0.46–0.94; p = 0.02), a 47% reduction in nonfatal reinfarction (5.3% v. 2.9%; OR 0.53, 95% CI 0.34–0.80; p = 0.04) and a substantial reduction in hemorrhagic stroke (1.1% v. 0.1%; OR 0.07, 95% CI 0.0–0.43; p < 0.001) at 30 days.9 This not only translates into an additional 21 lives saved per 1000 patients treated with PCI compared with thrombolytic therapy (and hence 40 to 50 lives saved with PCI compared with no therapy), but PCI avoids 2 of the serious complications of thrombolytic therapy: increased rates of reinfarction and intracranial bleeds. New information has emerged from both registries and randomized clinical trials that confirm the benefits of PCI over thrombolysis.10,11,12,13,14,15
Yet, in 2003, few centres have incorporated primary PCI in preference to thrombolytic therapy for the management of STEMI. Reasons for this include concerns regarding potential delays in transferring patients between institutions; the as yet unrealized promise of higher rates of reperfusion and better outcomes from combining thrombolytic therapy with novel antithrombotic agents such as hirudin and platelet glycoprotein IIb/IIIa inhibitors; and the availability of PCI facilities in only a minority of centres that manage patients with STEMI.
Most randomized clinical trials have been done in well-equipped and well-staffed hospitals. Would the benefits of PCI be maintained if treatment had to be delayed for logistical reasons? Recent trials11,12,13,14 and a meta-analysis15 have shown consistent benefits in the composite end point of death, reinfarction and disabling stroke for patients treated with primary PCI, even when they were transferred to another facility to undergo PCI instead of receiving thrombolytic therapy in the original hospital. In an analysis of 10 randomized trials involving a total of 2635 patients, Zijlstra and colleagues16 found progressively increasing event rates among patients presenting within 2 hours, between 2 and 4 hours or more than 4 hours after symptom onset and treated with thrombolytic therapy, whereas the event rates were consistently lower among those treated with primary PCI. In the DANAMI-2 trial,14 transfer delays of up to 3 hours did not seem to have a significant effect on the efficacy of primary PCI. In the PRAGUE-2 trial,13 patients presenting after 3 hours of onset of symptoms had significant benefit from primary PCI as compared with thrombolysis. To date, a large number of patients have not been treated in any single trial of primary PCI to allow accurate ascertainment of the relation between delays in PCI and mortality.
Trials currently evaluating out-of-hospital thrombolysis followed by PCI (facilitated PCI), whereby patients receive a thrombolytic agent or a platelet glycoprotein IIb/IIIa inhibitor, or both, before PCI, will be especially important to our understanding of treatment options in the face of delays to PCI. An alternative would be to consider a strategy of rescue PCI for failed thrombolysis or recurrent ischemia. However, there are few data to show that routine angioplasty after thrombolysis is beneficial, and one should be aware that a previous generation of such trials failed to demonstrate a benefit.17
The combination of thrombolytic therapy with novel antithrombotic agents is thought to be a promising alternative to primary PCI. However, results from trials of such combined treatment have been disappointing.18 There has been little improvement in mortality and some decrease in rates of reinfarction and recurrent ischemia, but these have been counterbalanced by higher rates of major bleeding, including intracranial bleeds, especially in elderly patients. By contrast, primary PCI appears to be more durable and to have fewer complications, even when performed in less specialized centres.19 The question is no longer whether primary PCI is of value but, rather, how do we incorporate it into clinical practice?
Many centres with on-site PCI capability are already offering primary PCI to patients presenting to the emergency department of their own institution during regular working hours, but not during other times. The challenge is to establish primary PCI programs at these institutions, with operators available 24 hours a day, including weekends. This poses formidable challenges, even at centres with PCI facilities. Several questions remain to be addressed:
· Should all STEMI patients be offered primary PCI, or only those at high risk of death or intracranial bleeds and those with contraindications to thrombolytic therapy?
· Who would select patients for primary PCI or thrombolytic therapy? How will on-call teams be organized to achieve rapid responses?
· How will hospitals cope with the increased demand for round-the-clock service?
· What are the implications for human resource needs, training of cardiologists and staff morale?
· Should elective angioplasty (for which there is little evidence of a reduction in mortality or morbidity among patients with stable coronary artery disease) be performed less frequently in order to divert more resources to providing a 24-hour emergent, rapid-response PCI service?
In Canada, the majority of patients with STEMI present to community hospitals without on-site PCI facilities. Although barriers particular to the geography and weather of Canada are undoubtedly part of the reason for delayed implementation of PCI, most Canadians live within 300 km of the 49th parallel. In the DANAMI-2 and PRAGUE-2 studies, some patients were transported to PCI centres more than 100 km away. Where distances are greater, a hybrid system of air and land ambulance transfer, as reported in one recent randomized trial,12 may need to be developed.
Many of the practical questions posed require both regional and local answers. Each region should identify local barriers, including geography, weather conditions, availability of trained personnel and number of hospital beds, and develop cost-effective plans to implement appropriate systems and protocols.
Is the shift toward PCI cost-effective? Establishing a rapid-response PCI team may reduce costs if expensive thrombolytic agents are avoided, bleeding complications are minimized, knowledge of coronary anatomy allows for more effective and rapid risk stratification, patients are discharged early (e.g., day 3 or 4), and more patients return earlier to work.
Another approach to increasing the cost-effectiveness would be to limit primary PCI to high-risk patients, who would potentially derive the most benefit from a more aggressive approach. These include patients with anterior or complicated inferior STEMI, elderly people and patients with shock or heart failure. Objective criteria need to be established, since often it is the younger patient with an uncomplicated clinical presentation who undergoes more invasive procedures compared with the older patient who has heart failure.20
There may be redundancies and inefficiencies within the current system that should be addressed systematically. For instance, in 2 recent reports from Alberta and Ontario, the proportion of individuals who underwent diagnostic catheterization and were found to have normal or mild disease ranged from 20% to 25%.21,22 Although there are potential benefits to defining coronary anatomy in some individuals, better selection of patients for diagnostic catheterization may be warranted. Even a 5% decrease in the rate of “normal angiograms” translates into a significant ability to accommodate the added demands for more primary PCI. Catheterization laboratories should play a more active role in selecting appropriate cases. In patients with stable coronary artery disease, PCI (even with stenting) has not been shown to be superior to aggressive medical therapy in preventing death or myocardial infarction. One may then ask Should we not do more primary PCI procedures for acute STEMI and balance this increase with fewer procedures for chronic coronary disease, especially in patients with little or no symptoms? Trials currently addressing the role of routine PCI combined with aggressive medical therapy versus medical therapy alone may lead to a more selective offering of PCI to patients with stable chronic coronary disease.23
Cardiologists and clinical trialists should be proud of the tremendous progress in proving the value of several useful therapies (thrombolytic therapy, primary PCI, ASA, β-blockers, angiotensin-converting-enzyme inhibitors, lipid-lowering drugs, smoking cessation and hypertension control) and the harm of other strategies (prolonged bedrest, antiarrhythmic therapy). Implementing the useful therapies for all eligible patients will lead to a substantial, favourable impact on the health of our patients. It is time to establish national standards of care for patients with acute MI and for hospitals to document whether their staff adhere to them. The greatest benefits to our patients in the next 2 decades will probably be derived from fully implementing what we already know, in addition to continuing to shed light on our areas of ignorance.
β See related article page 35
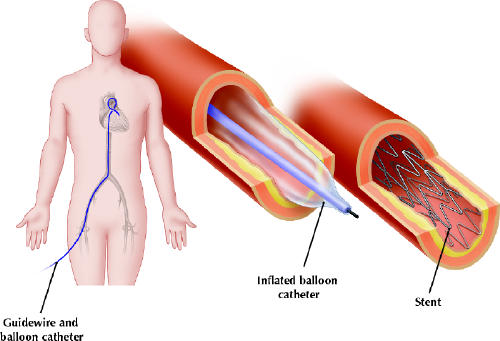
Figure. Percutaneous coronary interventions are performed to open narrowed or blocked coronary arteries. A catheter is inserted under local anesthetic into the femoral artery or into the radial or brachial artery and is then steered through the central arteries to the heart. A tiny balloon-tipped catheter is moved up to the point of severe narrowing and the balloon inflated to open up the artery. A stent, often used to keep the unclogged artery open, is mounted on the balloon-tipped catheter and is put in place by inflation of the balloon. [Reproduced from CMAJ 2002;166(1):51-61.] Photo by: Christine Kenney
Footnotes
The treatment of ST-segment elevation myocardial infarction has undergone profound changes since the bedrest era of the 1960s. Most recently, the use of percutaneous coronary intervention (PCI) has been shown to be superior to thrombolysis. However, to be effective, PCI must be done as soon as possible after MI. Patients in large urban areas of Canada may have access to PCI, but what about those in most other areas? We asked 2 groups of authors to comment on the gap between evidence and implementation as well as the barriers to round-the-clock PCI capability in Canada and how they can be overcome. The perspective of William Ghali and coauthors follows this comment.
Contributors: Both authors contributed substantially to the conception and writing of the commentary and gave final approval of the version to be published.
Competing interests: None declared.
Correspondence to: Dr. Madhu K. Natarajan, 2nd floor, McMaster Clinic, Hamilton Health Sciences — General Site, 237 Barton St. E, Hamilton ON L8L 2X2; fax 905 527-2337; natarajm@ccc.mcmaster.ca
References
- 1.Fitchett D, Goodman S, Langer A. New advances in the management of acute coronary syndromes: 1. Matching treatment to risk. CMAJ 2001;164(9):1309-16. [PMC free article] [PubMed]
- 2.Armstrong PW. New advances in the management of acute coronary syndromes: 2. Fibrinolytic therapy for acute ST-segment elevation myocardial infarction. CMAJ 2001;165(6):791-7. [PMC free article] [PubMed]
- 3.Buller CE, Carere RG. New advances in the management of acute coronary syndromes: 3. The role of catheter-based procedures. CMAJ 2002;166(1):51-61. [PMC free article] [PubMed]
- 4.Ageno W, Turpie AGG. New advances in the management of acute coronary syndromes: 4. Low-molecular-weight heparins. CMAJ 2002;166(7):919-24. [PMC free article] [PubMed]
- 5.DeWood MA, Spores J, Notske R, Mouser LT, Burroughs R, Golden MS, et al. Prevalence of total coronary occlusion during the early hours of transmural myocardial infarction. N Engl J Med 1980;303:897-902. [DOI] [PubMed]
- 6.Gruppo Italiano per lo Studio della Spreptochinasi nell'Infarcto Miocardico (GISSI). Effectiveness of intravenous thrombolytic treatment in acute myocardial infarction. Lancet 1986;1:397-402. [PubMed]
- 7.ISIS-2 (Second International Study of Infarct Survival) Collaborative Group. Randomised trial of intravenous streptokinase, oral aspirin, both, or neither among 17 187 cases of suspected acute myocardial infarction: ISIS-2. Lancet 1988;2:349-60. [PubMed]
- 8.O'Neill W, Timmis GC, Bourdillon PD, Lai P, Ganghadarhan V, Walton J Jr, et al. A prospective randomized clinical trial of intracoronary streptokinase versus coronary angioplasty for acute myocardial infarction. N Engl J Med 1986;314:812-8. [DOI] [PubMed]
- 9.Weaver WD, Simes RJ, Betriu A, Grines CL, Zijlstra F, Garcia E, et al. Comparison of primary coronary angioplasty and intravenous thrombolytic therapy for acute myocardial infarction. JAMA 1997;278:2093-8. [PubMed]
- 10.Magid DJ, Calonge BN, Rumsfeld JS, Canto JG, Frederick PD, Every NR, et al. Relation between hospital primary angioplasty volume and mortality for patients with acute MI treated with primary angioplasty vs thrombolytic therapy. JAMA 2000;284:3131-8. [DOI] [PubMed]
- 11.Widimsky P, Groch L, Zelizko M, Aschermann M, Bednar F, Suryapranata H. Multicentre randomized trial comparing transport to primary angioplasty vs immediate thrombolysis vs combined strategy for patients with acute myocardial infarction presenting to a community hospital without a catheterization laboratory. The PRAGUE Study. Eur Heart J 2000;21:823-31. [DOI] [PubMed]
- 12.Grines CL, Westerhausen DR, Grines LL, Hanlon JT, Logemann TL, Niemela M, et al. A randomized trial of transfer for primary angioplasty versus on-site thrombolysis in patients with high-risk myocardial infarction. J Am Coll Cardiol 2002;39:1713-9. [DOI] [PubMed]
- 13.Widimsky P, Budesinsky T, Vorac D, Groch L, Zelizko M, Aschermann M, et al; PRAGUE Study Group Investigators. Long distance transport for primary angioplasty vs immediate thrombolysis in acute myocardial infarction. Final results of the randomized national multicentre trial — PRAGUE-2. Eur Heart J 2003;24(1):94-104. [DOI] [PubMed]
- 14.The Danish multicenter randomized study on thrombolytic therapy versus acute coronary angioplasty in acute myocardial infarction (DANAMI-2). Available: www.danami-2.dk/index.htm (accessed 2003 June 4).
- 15.Keeley EC, Boura JA, Grines CL. Primary angioplasty versus intravenous thrombolytic therapy for acute myocardial infarction: a quantitative review of 23 randomised trials. Lancet 2003;361:13-20. [DOI] [PubMed]
- 16.Zijlstra F, Patel A, Jones M, Grines CL, Ellis S, Garcia E, et al. Clinical characteristics and outcome of patients with early (<2 h), intermediate (2-4 h) and late (>4 h) presentation treated by primary coronary angioplasty or thrombolytic therapy for acute myocardial infarction. Eur Heart J 2002;23:550-7. [DOI] [PubMed]
- 17.Michels KB, Yusuf S. Does PTCA in acute myocardial infarction affect mortality and reinfarction rates? Circulation 1995;91:476-85. [DOI] [PubMed]
- 18.Topol EJ; GUSTO V Investigators. Reperfusion therapy for acute myocardial infarction with fibrinolytic therapy or combination reduced fibrinolytic therapy and platelet glycoprotein IIb/IIIa inhibition: the GUSTO V randomised trial. Lancet 2001;357:1905-14. [DOI] [PubMed]
- 19.Aversano T, Aversano LT, Passamani E, Knatterud GL, Terrin ML, Williams DO, et al. Thrombolytic therapy vs primary percutaneous coronary intervention for myocardial infarction in patients presenting to hospitals without on-site cardiac surgery: a randomized controlled trial. JAMA 2002;287:1943-51. [DOI] [PubMed]
- 20.Yusuf S, Flather M, Pogue J, Hunt D, Varigos J, Piegas L, et al; OASIS Registry Investigators. Variations between countries in invasive cardiac procedures and outcomes in patients with suspected unstable angina or myocardial infarction without initial ST elevation. OASIS (Organisation to Assess Strategies for Ischaemic Syndromes) Registry Investigators. Lancet 1998;352:507-14. [DOI] [PubMed]
- 21.Natarajan MK, Mehta SR, Holder DH, Goodhart DR, Gafni A, Shilton D, et al. The risks of waiting for cardiac catheterization: a prospective study [published erratum appears in CMAJ 2003;168(2):152]. CMAJ 2002;167(11):1233-40. [PMC free article] [PubMed]
- 22.Hemmel BR, Ghali WA, Quan H, Brant R, Norris CM, Taub KJ, Knudtson ML; APPROACH Investigators. Poor long-term survival after coronary angiography in patients with renal insufficiency. Am J Kidney Dis 2001;37(1):64-72. [DOI] [PubMed]
- 23.Chiquette E, Chilton R. Aggressive medical management of coronary artery disease versus mechanical revascularization. Curr Atheroscler Rep 2003;5(2):118-23. [DOI] [PubMed]


