Case 1
A 33-year-old truck driver presented with left shoulder pain dating from a motor vehicle accident 4 years before. His pain was described as “constant” and “constricting,” with “stabbing electric, shock-like” characteristics. It had increased over the past 2 years to intensities between 7 and 10 out of 10 (0 = no pain, 10 = excruciating pain). He had undergone excision of the lateral end of the left clavicle and rotator cuff decompression 3 years earlier, receiving physiotherapy before and after surgery. He had also used heat and a machine providing transcutaneous electric nerve stimulation and had received intra-articular injections of anaesthetic agents and steroids. When first seen in the pain clinic he was taking, on average, one tablet of acetaminophen/codeine/caffeine (300 mg /30 mg /15 mg) and one tablet of acetaminophen/oxycodone (325 mg/5 mg) 5 times daily. He also drank 10 cups of coffee daily, had been unable to sleep more than 3 hours in 24 for the last 2 years and was working night shifts.
Apart from smoking a pack of cigarettes per day, the patient had no other risk factors for addiction, and he described having a supportive wife and 2 daughters. What modifications to his medication regimen and lifestyle might provide him with better pain management?
Case 2
A 40-year-old man came to the pain clinic with a 15-year history of ankylosing spondylitis and a 5-year history of rheumatoid arthritis. His medications for the last 2 years included prednisone (20 mg/d), methotrexate (22.5 mg/wk intravenously) and amitriptyline (50 mg at bedtime). In addition, over the last year he had been taking sustained-release morphine, the dose having been gradually increased from 30 to 115 mg every 12 hours. He had never had good pain control since the onset of the rheumatoid arthritis and had been extremely constipated for several months despite taking two 5-mg tablets of bisacodyl and two 100-mg tablets of docusate sodium 4 times daily, as well as using a Fleet enema intermittently. There was evidence of inflammatory activity in his joints, particularly his right elbow and left knee, and he attended appointments in a wheelchair.
The patient was receiving active management from his rheumatologist (methotrexate and intra-articular steroid injections), had no family or personal history of abusing alcohol or recreational or prescription drugs, and had a supportive wife and 2 young children. What changes to his analgesic regimen might improve his pain management?
The last decade has brought acceptance that opioids can be used as a component of the management of noncancer pain that is chronic (lasting more than 6 months) when other approaches have failed and the quality of life is poor because of the pain. In recent years the annual consumption of morphine, including for noncancer pain, has risen substantially in developed countries.1 Recent guidelines and evidence-based recommendations for the use of opioids in conditions other than acute and cancer pain have advised the use of sustained-release or long-acting opioids, with avoidance of repeated injection, for chronic pain.2,3,4,5,6 Several randomized controlled studies have demonstrated the efficacy of opioids in a variety of noncancer conditions,7,8 including postherpetic neuralgia9 and low back pain.10 The World Health Organization (WHO) analgesic ladder (Fig. 1) has opioids on the 2nd and 3rd steps, to be used when other strategies, such as physiotherapy, massage and therapy with non-opioid medications (acetaminophen, nonsteroidal anti-inflammatory agents, tricyclic antidepressants, anticonvulsants and topical preparations), have failed to provide sufficient pain control and the quality of life is poor because of the pain.11
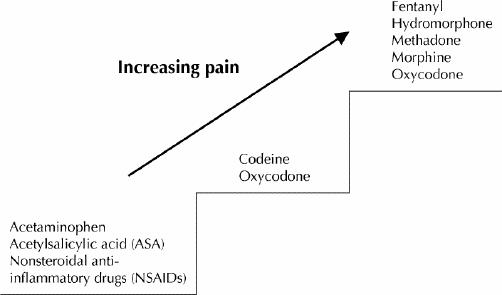
Fig. 1: World Health Organization analgesic ladder.11 Least invasive routes of administration: transdermal and oral.
Perceived barriers to opioid prescribing (Box 1) are still contributing to inadequate pain management. It is now acknowledged that opioids may be appropriate in a subset of the population with a variety of conditions that cause chronic pain, including those that are impossible to diagnose exactly. For many nonmalignant conditions, opioids may allow lowered pain intensity (or more acceptable pain characteristics), an acceptable side effect profile, and enhanced function and quality of life. They may also allow other pain management strategies to become more accessible. This paper discusses approaches to the use of opioids in chronic noncancer pain.
Box 1.

Diagnosing pain
Every effort should be made to diagnose and appropriately treat the condition underlying the pain, but pain still requires management before diagnosis and when diagnosis remains elusive. Most pain clinics prefer that patients have had an appropriate diagnostic workup (blood work, imaging, nerve conduction studies) before referral. Pain sites, qualities (throbbing, aching, shooting, burning, paresthesias, stabbing) and intensities should be recorded. Frequency and duration of pain exacerbations, triggers, aggravating and relieving factors, and effect on sleep and mood should be elicited. Several methods of assessing pain intensity exist: numeric scales (0 to 5 or 0 to 10, from no pain to excruciating pain), verbal scales (none, mild, moderate, severe), visual analogue scales and facial series (sad to happy, most often used for children). Scales are only useful in assessing progress within the same person, and they likely incorporate psychosocial influences. The best and worst pain scores should be recorded at each visit, along with an estimate from the patient of the proportion of time at different intensities, including the proportions at the lowest and the average intensities. Functional impairment and changes in impairment caused by pain and pain management should be recorded, with information from family members (with the patient's permission) to corroborate function and status.
Choice of opioid
Opioids act at receptors on the pre- and postsynaptic membranes of neurons in the central and peripheral nervous system. Three major families of opioid receptors have been described: μ, κ and δ.12 Most of the opioids used clinically act on the μ receptors. Work in animal models and cell cultures has demonstrated multiple μ-receptor subtypes, which may support clinical observations that the response to a given opioid, with respect to both efficacy and side effects, can vary widely between individuals, probably owing to genetic polymorphism. About 7% to 10% of white people respond poorly or not at all to codeine; they lack the cytochrome P450 enzyme CYP2D6, which converts codeine to its active metabolites, including morphine.13 No trend has been reported in the literature regarding greater effectiveness of specific opioids for specific pain conditions. A patient cannot be considered unresponsive to opioids until all appropriate opioids have been tried.
Codeine and oxycodone, plain or in combination with acetaminophen, are usually perceived to be on the 2nd step of the WHO analgesic ladder, but any of the strong opioids (fentanyl, hydromorphone, methadone, morphine and oxycodone) can be considered to be on the 2nd or 3rd step because in lower doses they are equivalent to combinations of codeine or oxycodone with acetaminophen.
Titration is usually done with long-acting sustained- release opioids (Table 1) to promote smoother pain relief and improved compliance, though some physicians prefer to titrate short-acting opioids and convert to a sustained-release form when a maintenance dose is achieved. Selection of the initial opioid is based on the criteria shown in Table 2. For example, if the efficacy and side effect profile of an acetaminophen/oxycodone combination had been acceptable, sustained-release oxycodone could be used initially, but if prolonged sedation, nausea or intractable constipation had been present, transdermal fentanyl might be a wiser choice. And if the patient's answers to the CAGE-AID questionnaire15 (Box 2) suggest a history of drug abuse, methadone might be selected and short-acting opioids for “rescue” analgesia not be offered. Once an opioid is chosen, the dose is increased to balance improved pain control and function against side effects of the opioid.
Table 1
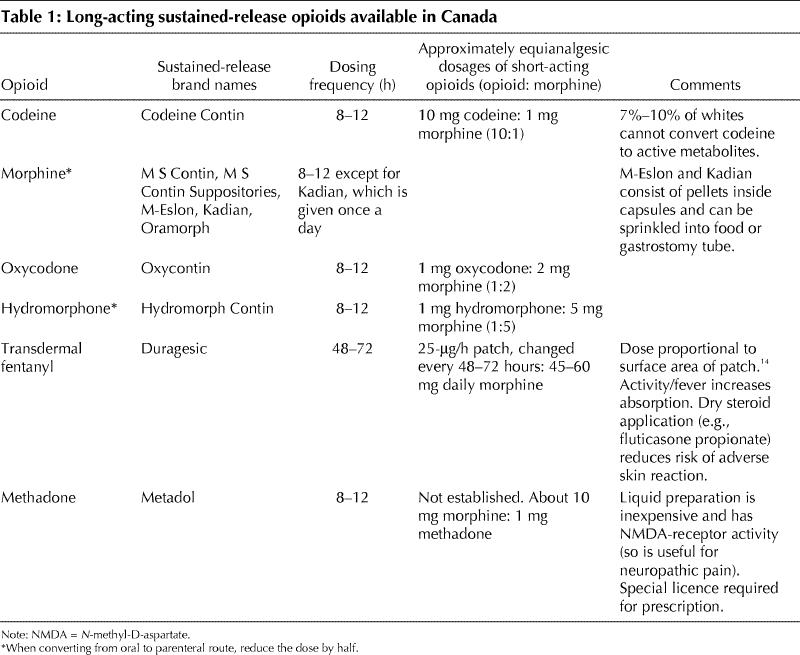
Table 2
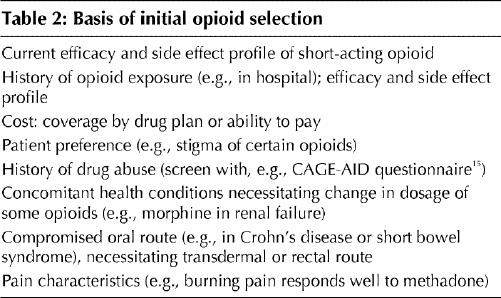
Box 2.

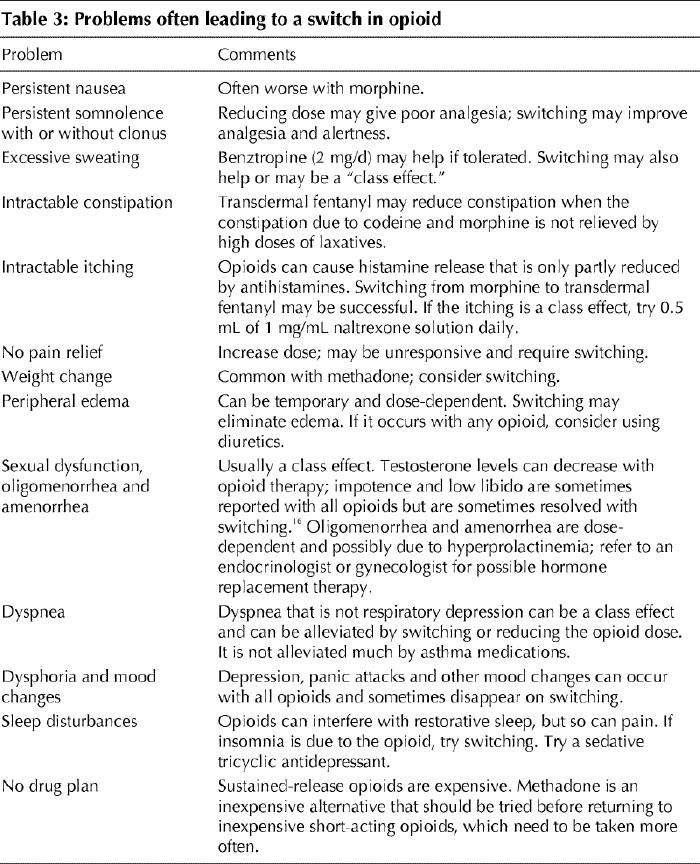
Decisions to switch to alternatives after titration with the initial opioid may be based on the approaches outlined in Table 3. Approximately equianalgesic dosages of other opioids (Table 1) may vary with the individual, and a 10% to 20% decrease in the equianalgesic dose is recommended when switching because different types of opioid receptors are affected by different opioids. Caution should be exercised when switching to methadone because it is about 7 to 10 times more potent than morphine when given long term. Very low doses of methadone (2.5 to 5 mg every 12 hours or, in patients over 65 years of age, 1 mg every 12 hours) are advisable while the dosage of the initial opioid is being tapered. Relatively small doses of methadone can provide excellent analgesia in certain individuals in whom larger doses of alternative opioids have been unsuccessful, but this approach requires slow titration over several weeks, as a reservoir of methadone builds in the patient's fatty and other tissues. Sometimes pain is controlled only after several weeks of a stable dose of methadone.
Table 3
The prescription of opioids for chronic pain in some patients, such as the elderly and those with hepatic or renal disease, deserves special caution because of the potential accumulation of toxic metabolites in these people.17,18 Accumulation of active metabolites such as morphine-3-glucuronide and morphine-6-glucuronide may cause reduced cognitive function, increased anxiety, allodynia and clonus. The accumulation varies with the individual and the mode of drug delivery.19 Often for patients over 65 the initial dose is lower and the titration slower than for younger patients. Impairment of cognitive function due to the accumulation of active metabolites may be alleviated by a switch to transdermal fentanyl,20 which is metabolized in the liver to inactive metabolites.14
Patients may find their function and activity levels enhanced by judicious use of “rescue” or “breakthrough” analgesia. For exacerbations of pain that are spontaneous or related to activity, stress or other factors, rescue analgesia can be provided by a short-acting opioid, taken from a few times a month to several times a day. The dose should be tailored to the maintenance analgesic dose (one-third to one-sixth of the 12-hourly dose), and the frequency should be tailored to the patient's needs and response. Some patients find the effectiveness reduced if the drug is used too frequently. Patients with addictive tendencies or rebound headaches may be best managed with only sustained-release long-acting opioids.
Challenges to opioid prescribing
Between 3% and 17% of people are potential addicts; these individuals usually have a family history or a personal history of addiction.21 Addicts crave a substance, often initially seeking its euphoric effects but later taking the drug just to feel normal, and will do anything to acquire it, despite causing themselves harm. However, people who use opioids for chronic pain usually experience physical dependence, which is different from addiction in that those with physical dependence usually experience withdrawal symptoms if they abruptly stop taking the drug instead of gradually tapering the dosage. The CAGE-AID15 and SISAP22 questionnaires (Boxes 2 and 3) are useful in the initial history-taking among patients with chronic pain to identify those at higher risk of addiction. Those at higher risk may be treated with opioids as long as controls such as stricter boundaries are defined in a signed written contract (Table 4)23 and steps such as using methadone as the opioid of choice or dispensing smaller quantities at defined intervals (e.g., weekly or biweekly at the local pharmacy) are in place.
Box 3.

Table 4

Opioids are often perceived as imperfectly controlling chronic nonmalignant pain. Pain intensities often are not reduced below 4 or 5 on a scale of 0 to 10, even when doses are increased to the point at which side effects are unacceptable. In contrast, pain intensities in acute and cancer pain can often be lowered to 0 to 3 out of 10. This refractoriness of chronic noncancer pain often leads physicians to conclude that psychologic factors are at play. However, experimental work in animals and cell cultures has demonstrated that depleted opioid receptors on damaged nerves, changes in the receptor-binding sites and down-regulation of the receptors may be the cause of the observed reduction in opioid efficacy.12,24 It is also probable that genetic polymorphism in humans influences pain perception and response to medications.13,25
Although many patients report somewhat reduced efficacy after the first month of opioid administration, a small percentage appear to become markedly tolerant (the drugs appear to become almost entirely ineffective over relatively short periods, such as weeks). Opioid rotation will likely be effective in these patients because a different subtype of receptor will respond to the new opioid while the receptors that had previously responded recover.12 Anecdotal evidence suggests that combining opioids to lower pain intensity, change pain characteristics and retard the development of tolerance may be more effective than using a single drug by targetting a broader range of opioid receptors in those refractory to therapy with a single opioid.
There is no evidence in the literature to suggest that driving be denied to patients receiving opioid therapy for chronic pain26 except during titration to establish the maintenance dose and when there is a higher likelihood of cognitive dysfunction, as with older patients. A decision to ban driving depends on the judgement of the treating physician, in collaboration with the patient and family. A medical driving test may be considered.27
Long-term effects of opioids
To date, there are few reports of toxic effects in organs from long-term opioid use in nonaddicts. When opioids are used responsibly at stable doses, a balance between pain control and side effects can be established, and these drugs can be considered safer than nonsteroidal anti-inflammatory agents or large quantities (> 2 g/d) of acetaminophen.28 There is one report of the potential for cardiac arrhythmias in female patients on high-dose methadone therapy for chronic pain.29 It is therefore advisable to do electrocardiography and measure the serum levels of electrolytes and magnesium before and after methadone therapy is started in patients who are likely to be receiving a high dose (> 200 mg/d).
Cases revisited
Case 1
The patient likely had neuropathic pain resulting from damaged nerves and ischemic pain resulting from constriction by scar tissue of the blood supply of nerves around the site of trauma and surgery. Sleep deprivation likely was contributing to his reduced ability to cope with pain. He was therefore asked to taper his coffee intake to 2 cups per day and change his night shifts to day shifts. His medication was switched from acetaminophen/codeine/caffeine and acetaminophen/oxycodone preparations to sustained-release oxycodone, titrated to 30 mg every 8 hours, with up to 4 acetaminophen/ oxycodone tablets (325 mg/5 mg) daily for rescue analgesia. For the neuropathic component of his pain, gabapentin therapy (300 mg at bedtime) was started, but he was unable to tolerate it because of somnolence that persisted even with a low dose. Therefore, gabapentin was switched to nortriptyline, titrated from 10 to 75 mg, before bedtime. Currently his pain intensity varies between 3 and 6 out of 10. He now sleeps 8 of every 24 hours, is alert during waking hours and exercises at a gym, with only gentle stretching of the shoulder. His wife reports that he is much more affable with his family and colleagues. His doses of medication have been consistent for 4 years. He is permitted to drive for work in Ontario but not in the United States because of the opioid medication.
Case 2
The patient was considered to have mainly nociceptive pain from intractable inflammation. His sustained-release morphine was switched to transdermal fentanyl, titrated from 125 to 200 μg/h. The change resulted in good relief from excessive constipation (such that he needed only 2 bisacodyl and 2 docusate sodium tablets daily) and improved pain control for the next 4 months. Despite the stability of his disease, his analgesia became less effective, and the opioid medication was rotated to sustained-release oxycodone, titrated from 80 to 160 mg every 12 hours. Pain control was restored for the next 2 months. He was assessed by his rheumatologist for a trial of infliximab (a new disease-modifying antirheumatic drug), for which the prednisone dosage needed to be titrated down to 10 mg/d; in this process, his pain was not managed even with an increase in the sustained-release oxycodone to 200 mg every 12 hours. He became somnolent and exhibited clonus, and his right elbow and left knee swelled and became more painful. He was admitted to hospital for pain management and joint aspirations, and the opioid was switched to a subcutaneous hydromorphone infusion, 5 to 8 mg/h. He experienced no pain relief, remaining somnolent with clonus. A switch to oral methadone, titrated to 15 mg every 8 hours, led to pain relief, and he regained alertness and stopped having clonus. He was discharged from hospital a few days later.
Comment
Opioids can be beneficial and safe as a component of the long-term management of noncancer pain. Restrictions on their use have lifted in Canada in the last decade. Physicians are acquiring increased expertise in their use, and opioids are beginning to be perceived as less invasive than recurrent surgical intervention, such as repeated spinal surgery, if they provide adequate relief at a stable dosage. Avenues to be explored include using opioid combinations to improve efficacy, devising strategies to eliminate analgesic tolerance, and developing new analgesics without undesirable opioid side effects, such as constipation, and new drugs that enhance endogenous opioid levels. As education about opioids increases and outdated prejudices decline, fewer patients with chronic noncancer pain will be denied a trial of opioid therapy when pain control is poor and causing suffering.
Footnotes
This article has been peer reviewed.
Competing interests: Dr. Gardner-Nix receives speaker fees from Janssen-Ortho Inc. and Purdue Pharma, companies that manufacture some of the products mentioned in this article.
Correspondence to: Dr. Jacqueline Gardner-Nix, Pain Management Program, Sunnybrook and Women's College Health Sciences Centre, Rm. MG 630, 2075 Bayview Ave., Toronto ON M4N 3M5; fax 416 480-4772; jackie.gardner@sympatico.ca
References
- 1.Clausen TG. International opioid consumption. Acta Anaesthesiol Scand 1997; 41: 162-5. [DOI] [PubMed]
- 2.Jovey RD, Ennis J, Gardner-Nix J, Goldman B, Hays H, Lynch M, et al. Use of opioid analgesics for the treatment of chronic noncancer pain - a consensus statement and guidelines from the Canadian Pain Society. Pain Res Manage 2003; 8(Suppl A):3A-28A. [DOI] [PubMed]
- 3.College of Physicians and Surgeons of Alberta. Guidelines for management of chronic non-malignant pain. Edmonton: College of Physicians and Surgeons of Alberta; 1993.
- 4.American Academy of Pain Medicine and American Pain Society. The use of opioids for the treatment of chronic pain. A consensus statement from the American Academy of Pain Medicine and the American Pain Society. Clin J Pain 1997;13:6-8. [PubMed]
- 5.American Geriatric Society clinical practice guidelines: the management of chronic pain in older persons. Geriatrics 1998;53(Suppl 3):S6-7. [PubMed]
- 6.Evidence-based recommendations for medical management of chronic non-malignant pain: reference guide for physicians. Toronto: College of Physicians and Surgeons of Ontario; 2000.
- 7.Arkinstall W, Sandler A, Goughnour B, Babul N, Harsanyi Z, Darke AC. Efficacy of controlled-release codeine in chronic non-malignant pain: a randomized, placebo-controlled clinical trial. Pain 1995;62:169-78. [DOI] [PubMed]
- 8.Moulin DE, Iezzi A, Amireh R, Sharpe WK, Boyd D, Merskey H. Randomised trial of oral morphine for chronic non-cancer pain. Lancet 1996;347: 143-7. [DOI] [PubMed]
- 9.Watson CP, Babul N. Efficacy of oxycodone in neuropathic pain: a randomized controlled trial in postherpetic neuralgia. Neurology 1998;50:1837-41. [DOI] [PubMed]
- 10.Jamison RN, Raymond SA, Slawsby EA, Nedeljkovic SS, Katz NP. Opioid therapy for chronic non-cancer back pain. A randomized prospective study. Spine 1998;23:2591-600. [DOI] [PubMed]
- 11.World Health Organization. Cancer pain relief. 2nd ed. Geneva: WHO; 1996.
- 12.Pasternak GW. The pharmacology of mu analgesics: from patients to genes. Neuroscientist 2001;7:220-31. [DOI] [PubMed]
- 13.Eckhardt K, Li S, Ammon S, Schanzle G, Mikus G, Eichelbaum M. Same incidence of adverse drug events after codeine administration irrespective of the genetically determined differences in morphine formation. Pain 1998;76:27-33. [DOI] [PubMed]
- 14.Duragesic [product monograph]. Compendium of pharmaceuticals and specialties. Ottawa: Canadian Pharmacists Association; 2003. p. 541-3.
- 15.Brown RL, Rounds LA. Conjoint screening questionnaires for alcohol and other drug abuse: criterion validity in primary care practice. Wis Med J 1995; 94: 135-40. [PubMed]
- 16.Daniell HW. Hypogonadism in men consuming sustained-action oral opioids. J Pain 2002;3:377-84. [DOI] [PubMed]
- 17.Parmar MS. Safe drug prescribing [letter]. CMAJ 2002;166:1651. [PMC free article] [PubMed]
- 18.Kappel J, Calissi P. Nephrology: 3. Safe drug prescribing for patients with renal insufficiency. CMAJ 2002;166:473-7. [PMC free article] [PubMed]
- 19.Smith MT. Neuroexcitatory effects of morphine and hydromorphone: evidence implicating the 3-glucuronide metabolites. Clin Exp Pharmacol Physiol 2000; 27:524-8. [DOI] [PubMed]
- 20.McNamara P. Opioid switching from morphine to transdermal fentanyl for toxicity reduction in palliative care. Palliat Med 2002;16:425-34. [DOI] [PubMed]
- 21.Fishbain DA. Report on the prevalence of drug/alcohol abuse and dependence in chronic pain patients (CPPs). Subst Use Misuse 1996;31:945-6. [DOI] [PubMed]
- 22.Coombs RB, Jarry JL, Santhiapillai AC, Abrahamsohn RV, Atance CM. The SISAP: a new screening instrument for identifying potential opioid abuse in the management of chronic nonmalignant pain in general medical practice. Pain Res Manage 1996;1:155-62.
- 23.Gitlin MC. Contracts for opioid administration for the management of chronic pain: a reappraisal. J Pain Symptom Manage 1999;18:6-8. [DOI] [PubMed]
- 24.Yaksh TL. Analgesia pain and tolerance: the St. John's discussion. Pain Res Manage 2000;5:19-22.
- 25.Mogil JS. The genetic mediation of individual differences in sensitivity to pain and its inhibition. Proc Natl Acad Sci U S A 1999;96:7744-51. [DOI] [PMC free article] [PubMed]
- 26.Galski T, Williams JB, Ehle HT. Effects of opioids on driving ability. J Pain Symptom Manage 2000;19:200-8. [DOI] [PubMed]
- 27.The Canadian Medical Association. Determining medical fitness to drive: a guide for physicians. 6th ed. Ottawa: The Association; 2000.
- 28.Garcia Rodriguez LA, Hernandez-Diaz S. Relative risk of upper gastrointestinal complications among users of acetaminophen and nonsteroidal anti-inflammatory drugs. Epidemiology 2001:12:570-6. [DOI] [PubMed]
- 29.Hays H, Woodroffe MA. High dosing methadone and a possible relationship to serious cardiac arrhythmias. Pain Res Manage 2001;6:64. [PubMed]


