Abstract
Background
Determining the direct cost of providing medical care to patients with HIV/AIDS is important for both short-term and long-term decision-making and for appropriate resource allocation. We aimed to categorize and measure the direct costs of medical care provided to the entire HIV-positive population receiving care in southern Alberta between 1995 and 2001.
Methods
We collected all patient-specific direct costs including the cost of pharmaceutical drugs (HIV and non-HIV drugs), outpatient care (including physician costs and laboratory testing), inpatient (in-hospital) care and home care (acute, long-term, palliative) from primary sources for all patients between April 1995 and April 2001. We determined cost per patient per month (PPPM) adjusted to 2001 Canadian dollars.
Results
Since 1995, the direct cost of providing medical care to patients with HIV/AIDS has increased primarily as a result of increased antiretroviral drug costs both in absolute and in PPPM terms. Mean PPPM expenditures increased from $655 in 1995/96, that is, before the use of highly active antiretroviral therapy (HAART), to $1036 in 1997/98 when HAART was widely used. During the following 3 years, mean overall PPPM costs remained stable. Antiretroviral drugs accounted for 30% ($198 PPPM) of the total cost in 1995/96 increasing to 69% ($775 PPPM) in 2000/01. Inpatient, outpatient and home care costs decreased in both percentage and cost PPPM between 1995/96 and 2000/01 from 26% to 10%, 27% to 14% and 8% to 3% respectively.
Interpretation
The cost of providing medical care to HIV-positive patients continues to increase, although the burden of costs is distributed differently from before the introduction of HAART, with the costs of drug therapy offsetting the costs of inpatient care and home care. Careful consideration of all aspects of direct costing data is needed when any health economic policy issues are examined.
Determining the direct cost (i.e., the health care resources used) of providing medical care to patients with HIV/AIDS is important for both short-term and long-term decision-making and for appropriate resource allocation. The changing nature of the HIV epidemic, as well as the introduction of highly active antiretroviral therapy (HAART) and frequent adjustments in management protocols when using this therapy, mandates continuous monitoring of resources used if accurate projections are to be employed to support both optimal care and prevention programming. Studies of the direct costs of HIV care vary with regard to areas such as the time frame of the work, context of health care delivery, extent of costs examined, methodologies used for data collection and the population studied.1,2,3,4,5,6,7,8,9,10,11,12 Many studies rely on costing information reported by the patient. Because it is difficult to collect sustainable data, studies often report costs that are 3 or 4 years old, do not reflect current needs or trends and cannot be used on an ongoing basis.
In 1989, the government of Alberta established the Southern Alberta Clinic (SAC) to provide multidisciplinary, centralized outpatient HIV care. Regardless of a patient's place of residence within southern Alberta, the SAC provides sole access to provincially funded antiretroviral drugs, viral load testing and CD4 lymphocyte subset monitoring, as well as offering research access and specialty/subspecialty services. Since 1990, a computerized database has been used for the explicit purpose of collecting comprehensive clinical, epidemiologic and socioeconomic information used in the care of each patient. This offers both the data and the framework for detailed costing analysis.
In southern Alberta, the SAC program, all inpatient care and all public home care programs fall within the regional budget. Referrals to community-based home care programs and applications to public and private disability programs are usually made through the SAC. Detailed per patient costing within each portfolio is available. These links to other service providers allow a virtually complete per patient history of HIV-specific health care and associated direct costs to be generated on an ongoing basis.
We established a comprehensive costing data system from primary rather than secondary sources for the entire population living with HIV/AIDS in southern Alberta between April 1995 and April 2001. This direct costing information reflects a “real world” clinical setting and is designed to be sustainable to allow for its ongoing use. We then used this to establish the direct costs of caring for all people with HIV and AIDS within our universal health care system.
Methods
We established the cost of providing health care (i.e., neither the “indirect” nor the “social” costs) to patients with HIV within a regional population. The data to evaluate these costs are comprehensive. Because of Canada's socialized health insurance system, we are able to obtain information on the costs of all interventions and pharmaceutical drugs. Since these costs reflect payments for both variable and fixed factors (such as hospital infrastructure), our cost estimates should be interpreted as reflecting average, as opposed to variable or marginal, costs of providing care. We have followed the methodology suggested by Graves and colleagues.13
All individuals over 15 years of age who had received any HIV care between April 1995 and April 2001 were included in our study. As of Apr. 1, 2001, 662 patients were receiving care for HIV; 86% lived within Calgary. Ninety-three percent of HIV care is provided at the central clinic, whereas 7% of patients receive care at the local STD (sexually transmitted disease) clinic or in local correctional facilities. Data for patients who either moved out of the region or died are included for the period of their residence in southern Alberta.
Consistent with the recommendations of Drummond and colleagues14 and the Canadian Coordinating Office for Heath Technology Assessment (CCOHTA),15 direct costs were divided into 4 broad categories: drugs, outpatient clinical care (including physician and laboratory costs), inpatient hospital care and community-based home care. They incorporate the standardized framework recommended by Tolley and Gyldmark16 for the identification and evaluation of costs.
In a series of studies approved by the University of Calgary Conjoint Medical Bioethics Committee, health care use data have been collected monthly for SAC patients since early in 1995. Drug costs and laboratory use and outpatient care costs were derived directly from the SAC database. Home care and hospital costs incurred by our patients were identified by crossreferencing and supplied by the other health services within our region. Services and their costs incurred outside our region were budgeted at “in- region” rates. Unit service costs were calculated from SAC financial information or supplied by other health service providers as appropriate. All unit costs are market values except in the case of inpatient and home care services, which are charge data, namely, standard costs that are charged by public institutions for care. All costs were determined using the all-items consumer price index (CPI), as provided by Statistics Canada, and are reported in Canadian dollars adjusted for inflation to 2001.
Cumulative yearly costs were calculated by summing all costs incurred by patients who used the service during the study period and adjusting for inflation. Mean cost per patient per month (PPPM) was used as a relative comparative measure in order to accommodate the increasing patient population receiving care at the SAC. Cost PPPM was determined by multiplying service use by unit service cost and dividing by the total number of patient months in follow-up during the year.
Drug costs include both antiretroviral (ARV) and nonantiretroviral drugs. ARV drugs are purchased wholesale from manufacturers and are dispensed through the SAC pharmacy. These drug costs were calculated on a per day basis determined by the drug, dose and dosing frequency. The dispensing fee for pharmacists is incorporated in the SAC operating budget and is included here in the outpatient care costs. Research drugs provided free by a pharmaceutical company–sponsored protocol were included at eventual market cost.
Nonantiretroviral drugs include other antiviral, antibiotic and antifungal drugs and other licensed drugs that are primarily used to treat opportunistic infections and other HIV problems, such as drug side effects, in addition to non-HIV related conditions. Non-ARV drugs prescribed either by physicians at the SAC or by the patient's family physician were entered into the central database along with the dosage and start/stop dates of the drug. Non-ARV drug costs were generated from wholesale costs and average commercial markups.
In our framework, outpatient care costing for each patient includes the cost of all SAC visits, the cost of visits to HIV-related specialists, the cost of reported family physician and other physician visits, and the costs of all laboratory testing. Research- associated costs are also included when they offset usual clinic care costs.
The cost of laboratory tests, including all HIV-related laboratory tests (CD4 cell count, viral load determination, serologies and chemistry, as well as other tests that are usually ordered on our regular standardized schedule), was obtained directly from the regional diagnostic laboratory and provincial laboratory services. Costs were calculated as cost per test per patient. The cost of genotypic HIV-resistance tests was not included, because resistance testing was not a funded or widely accepted test in Canada during the study period.
Inpatient care costs were obtained directly from Calgary Health Region's Patient Activity and Costing System (PACS) by crossreferencing our patient database with PACS. It includes the costs associated with inpatient stays as well as emergency department visits in the hospitals. All admissions to hospital for patients with HIV were included regardless of admitting diagnosis. Mean costs for all hospital admissions were categorized into whether the most responsible diagnosis was for an HIV-related or non-HIV-related condition. Inpatient care costs were calculated by this system on a per patient per day basis based upon the length of the stay in hospital. Costs include both the direct costs associated with patient care (i.e., supplies, equipment, salaries, nursing supervision, and laboratory and diagnostic testing) and indirect costs associated with hospital overheads (i.e., administration and support services). Because PACS does not include physician fees, the estimated costs of chart review and inhospital physician visit and consultation were calculated based on a mean per patient per day cost of $125. The cost of all medications for hospital inpatients excluding ARV drugs that are in the SAC budget are included in inpatient care costs. When direct costing data were not available for the occasional patient admitted elsewhere in southern Alberta, but outside our regional costing system, a mean cost of $756 per day was used for a patient with a primary diagnosis of HIV, and $685 for other diagnoses based on the mean daily costs within our region.
The cost of community-based home care includes the cost of nursing surveillance for all 3 categories of service offered: acute, long-term and palliative. It excludes the cost of drugs that are captured in the clinic database. Costs were obtained on a per patient basis directly from primary sources through the Calgary Region Home Care Program and were calculated on a per day basis based on the length of stay and the services provided.
Results
Sociodemographic, clinical and treatment characteristics of the study population are presented in Table 1. The number of people being treated for HIV/AIDS in southern Alberta increased from 453 in 1995/96 to 654 in 2000/01. In the same period, this population became more diverse, with a greater proportion of females (8% to 13%) and older individuals (33% to 49% aged > 40 years). In 1995/96, 71% of people with HIV/AIDS reported their risk category as being MSM (men who have sex with men), whereas in 2000/01 only 58% did. The proportion of individuals in the risk categories of having heterosexual sex and practising injection drug use increased from 10% and 15% to 17% and 22% respectively from 1995/96 to 2000/01. Mean CD4 cell counts increased from 300 х 106/L for all patients in 1995/96 to 448 х 106/L in 2000/01; fewer patients had AIDS (12% versus 3%). The number of patients with ARV regimens containing 3 or more drugs increased from 23% to 62%, whereas the proportion of patients not receiving ARV therapy also increased from 26% in 1996/97 to 36% in 2000/01 reflecting decreasing use of monotherapy and dual therapy.
Table 1

Mean costs PPPM for each costing category are listed in Table 2 and shown in Fig. 1. The largest increase in mean costs occurred between 1995/96 and 1998/99 when HAART was introduced and implemented on a widespread basis. Total mean costs rose from $655 to $1111 PPPM, an increase of 70%. Mean costs PPPM have increased by less than 1% since 1998.
Table 2
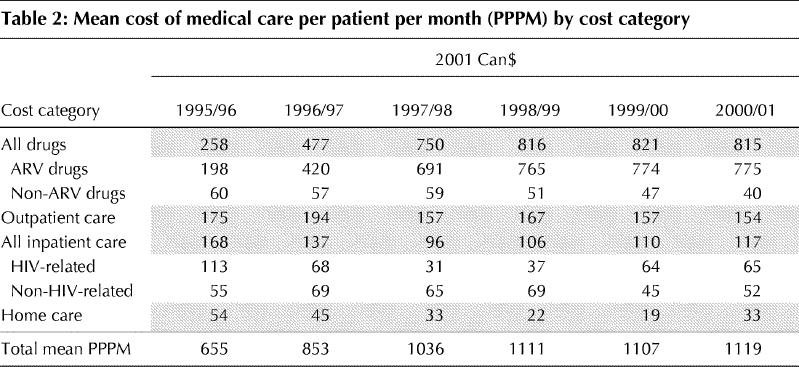
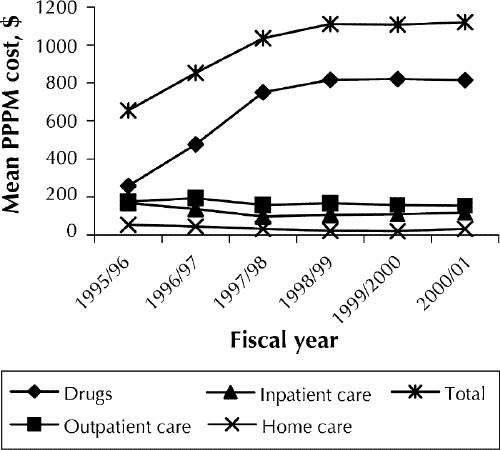
Fig. 1: Mean cost of medical care for patients with HIV/AIDS per patient per month (PPPM) by cost category.
Mean PPPM costs for all drugs increased from $258 in 1995/96 to $816 in 1998/99 and had changed very little through 2000/01. In 1995/96 ARV drugs accounted for 77% of all drug costs and 30% of overall costs, whereas non-ARV drugs accounted for 23% of drugs costs and 9% of overall costs. These figures have shifted significantly in the last 5 years, with ARV drugs accounting for 95% of drug costs and 69% of total costs in 2000/01. The mean cost PPPM for ARV drugs in 1995/96 was $198, increasing to $765 in 1998/99. The increase in mean costs is due primarily to an increase in the number of ARV drugs per regimen (see Table 1). From 1998 to 2001, the mean cost PPPM for ARV drugs remained largely unchanged. The cost of non-ARV drugs decreased from a mean of $60 per patient month in 1995/96 to $40 in 2000/01.
Outpatient care costs, including both visits to the SAC and to physicians outside the clinic, decreased from 27% of total costs in 1995/96 to 14% in 2000/01. Mean clinic outpatient care costs PPPM decreased from $175 in 1995/96 to $154 in 2000/01.
Total inpatient care costs were $168 PPPM in 1995/96, accounting for 26% of total direct HIV costs. The cost PPPM for our patients with an HIV-related admitting diagnosis was twice as high ($113 v. $55) as that for patients with another diagnosis. By 1997/98 total inpatient care costs decreased to $96 PPPM, with costs for patients with an HIV-related admitting diagnosis decreasing to $31 PPPM, which was less than half of the cost PPPM for individuals with other diagnoses. In 2000/01, however, inpatient care costs increased to $117 PPPM, with costs per month for HIV-related diagnoses accounting for 56% of the total cost. This increase is driven by the high in- hospital costs of a small number of patients whose initial HIV diagnoses were made during a lengthy stay in hospital.
Home care costs accounted for 8% of the total direct care costs of HIV medical care in 1995/96 and decreased to 3% in 2000/01. The cost PPPM decreased from $54 in 1995/96 to $33 in 2000/01.
Interpretation
We found that the direct per patient costs of providing medical care for HIV-positive patients increased significantly from 1995 to 2001, primarily because of the widespread implementation of HAART. In 1995/96 the mean cost PPPM was $655, with drug costs accounting for 39% of total costs. By 1998/99, the mean cost PPPM increased to $1111, with drug costs accounting for 73% of total costs. From 1998/99 to 2000/01, the mean cost PPPM remained relatively unchanged. Part of the increased costs of HAART have been offset by decreased costs elsewhere (e.g., admissions to hospital, palliative home care), reflecting the improved physical health of our patients and the decreasing need for close medical supervision. This is indicated by an increase in the mean CD4 lymphocyte count in our active patients at year's end from 300 х 106/L (15%) in 1995 to 448 х 106/L (24%) in 2001, which is accepted as a marker of improved health and decreased risk of HIV- related medical problems.
Our study found 2 significant patterns in the direct costs of medical care given to HIV-positive patients since 1995. The first is the predicted increase in costs associated with HAART as a result of an increased number of drugs per regimen and the cost of individual agents. Because of the efficacy of HAART, the costs of inpatient care, home care and drugs for managing HIV-related illness have decreased.
The second pattern remains more problematic. Total costs for medical care, including the cost of drugs PPPM, appear to have stabilized during the past 3 years. Changing medical practices such as delaying the onset of therapy, treatment interruptions and multidrug regimens for resistant viruses may have differentially affected costs.17,18 We identified 3 trends that affect direct costs (Table 1): fewer patients are receiving ARV therapy, patients on ARV therapy are receiving more drugs per regimen and patients are “healthier,” using an overall increase in mean CD4 cell count as a proxy for improved health. The combination of these trends cumulatively may have produced the stability seen in PPPM costs when the population is viewed as a whole. This costing framework will allow these cost drivers to be assessed separately.
The costing data have been generated from the HIV epidemic in southern Alberta. Costs may vary with differing epidemic profiles, as well as by the differing models of providing inpatient and outpatient HIV services from province to province within Canada. Costs reflect only the time period described here and can only assist in broadly predicting future directions in costing trends. Various characteristics of the HIV epidemic in general, changing guidelines for HAART regimens and the use of new technologies such as resistance testing will all influence future costs. We have not looked at the cost-effectiveness and the impact of HAART on productivity that has been reported elsewhere;19,20 however, the costing data presented in this study are comprehensive and accurately reflect the direct costs of providing medical care over the studied time period, because they are derived from primary costing data and not from self-reported costs provided by the patient. Changes in treatment regimens (e.g., treatment interruptions) and patients lost to follow-up (i.e., moved or died) are accounted for.
The changing nature of both the sociodemographic profile of the HIV epidemic and treatment decisions affect the short-term and long-term costs of the treatment and management of HIV care. Careful consideration of these trends is needed when any health economic policy issues are examined.
β See related article page 120
Footnotes
This article has been peer reviewed.
Contributors: All authors contributed to the concept and design of the study, data acquisition and interpretation, and writing of the manuscript. All 3 authors gave their approval of the version to be published.
Acknowledgements: We gratefully acknowledge the contributions of Edith Lundigan (Health Information Services, Calgary Health Region), Richard Schorn (Calgary Home Care Program) and Jinell Mah Ming (SAC Pharmacy) for providing complete and accurate costing data.
This study was funded by a grant from the Canadian Institutes of Health Research.
Competing interests: None declared for Drs. Krentz and Auld. Dr. Gill has had affiliations and competing interests with Glaxo Wellcome, Bristol-Myers Squibb, Hoffman-La Roche, DuPont, Abbott Laboratories, Boehringer Ingelheim, Chiron, Pfizer, Eli Lily, Agouron and Merck Frosst.
Correspondence to: Dr. Hartmut B. Krentz, Southern Alberta Clinic, 213–906 8th Avenue SW, Calgary AB T2P 1H9; fax 403 262-4893; Hartmut.Krentz@CalgaryHealthRegion.ca
References
- 1.Bozzette SA, Joyce G, McCaffrey D, Leibowitz A, Morton SC, Berry SH, et al. Expenditures for the care of HIV-infected patients in the era of highly active antiretroviral therapy. N Engl J Med 2001;344(11):817-23. [DOI] [PubMed]
- 2.Wallace MR, Tasker SA, Shinohara T, Hill HE, Chapman GD, Miller LK. The changing economics of HIV care. AIDS Patient Care STDS 2001;15:25-9. [DOI] [PubMed]
- 3.Gebo KA, Chaisson RE, Folkemer JG, Bartlett JG, Moore RD. Costs of HIV medical care in the era of highly active antiretroviral therapy. AIDS 1999;13 (8): 963-9. [DOI] [PubMed]
- 4.Keiser P, Nassar N, Kvanli MB, Turner D, Smith JW, Skiest D. Long-term impact of highly active antiretroviral therapy on HIV-related health care costs. J Acquir Immune Defic Syndr 2001;27(1):14-9. [DOI] [PubMed]
- 5.Roberts R, Rydman R, Gorosh K, Weinstein RA. Actual costs of HIV/AIDS care [abstract 498]. 8th Conference on Retrovirus and Opportunistic Infections; 2001 Feb 4–8; Chicago.
- 6.Saaverdia JA, Magis C, Molina R, Gontes ML, Del Rio C, Bronfman M. Economic impact of AIDS medical care in Mexico [abstract 24140]. XII International Conference on AIDS; 1998 June 28–July 31; Geneva.
- 7.Anis AH, Hogg RS, Yip B, Wang XH, Montaner JS, O'Shaughnessy MV, et al. Average annual drug cost and its determinants in a population based cohort of HIV-positive adult men and women. Pharmacoeconomics 1998; 13 (3): 327-36. [DOI] [PubMed]
- 8.Beck EL, Mandalia S, Parmar D, Miners A, Youle M, Gazzard B, et al. Population and individual costs of HIV service provision: the impact of HAART 1996–1999 [abstract]. Can J Infect Dis 2002:13(Suppl A):234P.
- 9.Youle M, Trueman P, Simpson K. Health economics in HIV disease. A review of the European literature. Pharmacoeconomics 1999;15(Suppl 1):1-12. [DOI] [PubMed]
- 10.Decock RA, Deporter AM, De Graeve D, Colebunders R. Direct costs of health care for HIV/AIDS patients in Belgium. AIDS Care 2001;13(6):721-31. [DOI] [PubMed]
- 11.Kyriopoulos JE, Geitona MA, Paparizos VA, Kyriakis KK, Botsi CA, Stavrianeas NG. The impact of new antiretroic therapeutic schemes on the cost for AIDS treatment in Greece. J Med Syst 2001;25(1):73-80. [DOI] [PubMed]
- 12.Kimura H. Cost of HIV treatment in highly active antiretroviral therapy in Japan. Nippon Rinsho 2002;60(4):813-6. [PubMed]
- 13.Graves N, Walker D, Raine R, Hutchings A, Roberts JA. Cost data for individual patients included in clinical studies: No amount of statistical analysis can compensate for inadequate costing methods. Health Econ 2002;11(8):735-9. [DOI] [PubMed]
- 14.Drummond M, Stoddart G, Torrance G. Methods for the economic evaluation of healthcare programs. New York: Oxford University Press; 1987.
- 15.Baladi JF. A guidance document for the costing process. Version 1.0. Ottawa: Canadian Coordinating Office for Heath Technology Assessment; 1996.
- 16.Tolley K, Gyldmark M. The treatment and care costs of people living with HIV infection or AIDS: development of a standardised cost framework for Europe. Health Policy 1993;24:55-70. [DOI] [PubMed]
- 17.Krentz HB, Gill MJ. Economic and budgetary implications of a decreasing interest in initiating early antiretroviral therapy seen over a 7-year period. [abstract]. Can J Infect Dis 2002:13(Suppl A):440P.
- 18.Krentz HB, Donaldson C, Gill MJ, Weber M, Auld C. Cost of antiretroviral drugs before and after the introduction of HAART [abstract]. Can J Infect Dis 2001:12(Suppl B):447P.
- 19.Sendi P, Bucher H, Harr T, Craig B, Schwietert M, Pfluger D, et al. Cost effectiveness of highly active antiretroviral therapy in HIV-infected patients. AIDS 1999;13:1115-22. [DOI] [PubMed]
- 20.Sendi P, Gafni A, Birch S. Highly active antiretroviral therapy: pharmacoeconomic issues in the management of HIV infection. Pharmacoeconomics 2001;19(7):709-13. [DOI] [PubMed]


