Abstract
We report here our preliminary results on the use of catalytic antibodies as an approach to neutralizing organophosphorus chemical weapons. A first-generation hapten, methyl-α-hydroxyphosphinate Ha, was designed to mimic the approach of an incoming water molecule for the hydrolysis of exceedingly toxic methylphosphonothioate VX (1a). A moderate protective activity was first observed on polyclonal antibodies raised against Ha. The results were further confirmed by using a mAb PAR 15 raised against phenyl-α-hydroxyphosphinate Hb, which catalyzes the hydrolysis of PhX (1b), a less toxic phenylphosphonothioate analog of VX with a rate constant of 0.36 M−1⋅min−1 at pH 7.4 and 25°C, which corresponds to a catalytic proficiency of 14,400 M−1 toward the rate constant for the uncatalyzed hydrolysis of 1b. This is a demonstration on the organophosphorus poisons themselves that mAbs can catalytically hydrolyze nerve agents, and a significant step toward the production of therapeutically active abzymes to treat poisoning by warfare agents.
Inactivation of extremely toxic organophosphorus chemical weapons has become a subject of major importance. The international control of their proliferation is thwarted by the ease of their synthesis and by the similarity between their chemical precursors and widely used pest-control agents. Their harmful effect is related to their potency to inhibit irreversibly mammalian acetylcholinesterase (AChE) (1–3), the enzyme responsible for regulating the concentration of the neurotransmitter acetylcholine at cholinergic synapses. Mild means of decontamination on the battlefield or in laboratories and tools for their in vivo degradation have both been investigated (4). Common decontamination methods include hydrolysis in strongly alkaline media, oxidation with highly corrosive solutions, or nucleophile-assisted substitution. Genetically engineered cholinesterases (4, 5) or phosphatases (6, 7) probably constitute the most interesting approach described to date to the inactivation of these organophosphorus esters under physiological conditions. Yet activities described so far on VX (1a) remain low. Moreover, these strategies require expensive and time-consuming steps to yield adequate amounts of the engineered enzyme needed, and their in vivo half-life is short, even when humanized.
The ability of antibodies to bind strongly to foreign molecules has long been used therapeutically. Their power to neutralize natural poisons in vivo is still used in the treatment of snake toxins, for example. Advances in the production of mAbs (8) (mAbs are now readily available in gram quantities) and more recently the discovery of catalytic antibodies (9) have revived interest in these proteins and notably in their potential clinical applications. To achieve tailored reactions and to increase the number of chemical reactions available for enzyme-like catalyses, the mimicry of enzyme mechanisms has been studied extensively. The pioneering work of P. G. Schultz (10) and R. A. Lerner (11) demonstrated that it is possible to select, from the huge repertoire of immunoglobulins, antibodies endowed with catalytic properties for a given reaction. Indeed, antibodies that are able to destroy a toxin catalytically rather than simply bind to it should be of great use in therapy. Moreover, it has recently been shown in vivo that such an approach can stop the drug-seeking behavior of cocaine-addicted rats and protect them against an overdose that was lethal for controls (12).
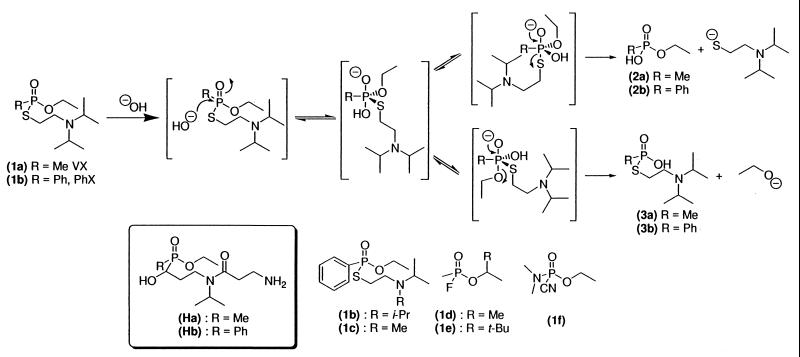
Among the chemical warfare nerve agents, the exceedingly toxic agent VX (1a) displays structural features preventing an easy and straightforward detoxification (13), in contrast with other warfare nerve agents as Sarin (1d), Soman (1e), or Tabun (1f) (Fig. 1), which are less hydrolytically stable. Moreover, some natural or genetically engineered enzymes (somanase, for example) are known to be active against those three organophosphorus poisoning compounds, but not against VX (4–7, 13). VX is thus a target of prior importance in testing this approach to its in vitro and in vivo inactivation. Here we describe our results for a first-generation hapten designed to degrade VX (1a) and its less toxic aromatic analog PhX (1b) (Fig. 1) via the use of catalytic antibodies.
Figure 1.
Hydrolysis reaction, hapten, and organophosphorus poisoning compounds structures.
Materials and Methods
Immunogen Preparation.
The conjugates methyl-α-hydroxyphosphinate Ha-keyhole limpet hemocyanin (KLH) and phenyl-α-hydroxyphosphinate Hb-KLH were prepared by adding 9 μmol of hapten to 3 mg of KLH in 2 ml of 0.1 M phosphate buffer, pH 7.4. Glutaraldehyde aqueous solution (5 μl 25%) was added. After stirring overnight in the dark at 4°C, the mixture was dialyzed against 0.1 M phosphate buffer, pH 7.4, at 4°C. Immunogens Ha- and Hb-KLH were stored at −20°C until use.
Preparation of Enzymatic Tracer.
The enzymatic tracer was prepared by covalent linkage of hapten Hb to the G4 form of AChE as follows:
Thiolation of the G4 form of AChE: 50 nmol S-acetyl thioglyocolic acid N-hydroxysuccinimide dissolved in 20 μl dimethylformamide (DMF) was added to 300 μg G4 in 380 μl of 0.1 M phosphate buffer, pH 7.4. After 1 h at room temperature, 100 μl of 0.8 M hydroxylamine aqueous solution was added. After 25 min of reaction, thiolated G4 was purified on a 20 cm × 1 cm G25 Sephadex column (elution buffer: 0.1 M sodium phosphate buffer, pH 6.0, containing 5 × 10−3 M EDTA and 0.01% sodium azide, degazed with nitrogen bubbling). AChE-containing fractions were gathered and stored at −80°C.
A 10-fold excess of maleimidated-Hb (prepared by reaction of a 0.5 M DMF solution of Hb with 1 equivalent of 4-(maleimidomethyl)cyclohexanecarboxylic acid N-hydroxysuccinimide ester and a catalytic amount of Et3N) dissolved in 0.1 M sodium phosphate buffer, pH 6.0, containing 5 × 10−3 M EDTA and 0.01% sodium azide were added to the previously prepared thiolated G4 form of AChE. After 5 h of reaction at 30°C, enzymatic tracer was purified and stored as described elsewhere (14).
Generation and Purification of mAbs.
Three Biozzi mice each received a s.c. injection of 100 μl of 0.5 mg/ml Hb-KLH conjugate emulsified in complete Freund's adjuvant. Booster injections were given at 2-week intervals. After receiving a final i.v. injection of 100 μl of Hb-KLH, the spleen cells of the mouse presenting the highest antiserum titer for Hb-AChE conjugate were fused with NS1 myeloma cells as described previously (8). Hybridoma supernatants were screened for their capacity to bind Hb-AChE conjugate by using enzyme immunoassay (EIA) as described previously (15). After the cloning of selected hybridoma cells, mAbs were expanded as ascitic fluids in nude mice.
mAbs were purified with standard protein A chromatography: 40 ml of binding buffer (aqueous 0.1 M borate/0.15 M NaCl, pH 9.0) and 1 ml of bound protein A gel were added to a 5-ml sample of ascitic fluid. After overnight incubation at 4°C, the protein A gel was decanted, applied to a column, and washed with binding buffer until the absorbance of the eluent returned to the baseline (at 280 nm). Elution buffer (0.1 M citrate buffer, pH 5.0) was applied to the column, and 2-ml fractions were collected. IgG-containing fractions were collected and dialyzed against an appropriate buffer. Purified mAb solutions were stored at −20°C until use.
Competitive EIA Procedure; Antibody Affinity Measurement.
Competitive EIA was performed as previously described (16) by using 0.1 M phosphate buffer (pH 7.4) containing 0.15 M NaCl, 10−3 M EDTA, 0.1% BSA, and 0.01% sodium azide (EIA buffer). Briefly, 96-well microtiter plates coated with affinity-purified goat polyclonal anti-mouse IgGs were used to ensure separation between bound and free tracer fractions. The reagents were dispensed in the following order: 50 μl of standard or buffer, 50 μl of diluted selected antibody, and 50 μl of enzymatic tracer. The plates were reacted overnight at 4°C and washed before addition to each well of 200 μl of Ellman's reagent [7.5 × 10−4 M acetylthiocholine iodide, 2.5 × 10−4 M 5,5′dithiobis(2-nitrobenzoic acid), 15 mM NaCl] in 0.1 M phosphate buffer. After 2 h of enzymatic reaction, the absorbance at 414 nm of each well was measured. Results were expressed in terms of B/Bo (%), where B and Bo represent the amount of solid phase-bound tracer in the presence and absence of competitor, respectively. Fifty percent B/Bo values (concentration of competitor for which the solid phase-bound tracer activity is half of Bo) can be considered as a good approximation to the Kd value of the tested product toward the antibody when Kd>[Ab]>[tracer].
Competitive ELISA Procedure.
Competitive ELISA was performed on 96-well microtiter plates coated with 50 nM Ha- or Hb-BSA conjugate solutions in 0.05 M phosphate buffer prepared as previously described for the KLH conjugates, and remaining free sites on the microtiter plates were then saturated by using EIA buffer (containing 0.1% free BSA). The reagents were dispensed in the following order: 50 μl of standard or buffer, 50 μl of diluted selected antibody. The plates were reacted overnight at 4°C and washed before addition to each well of 100 μl of AChE covalently bound affinity-purified goat polyclonal anti-mouse IgGs 2 Ellman units/ml in EIA buffer. The plates were reacted 4 h at room temperature and washed before addition to each well of 200 μl of Ellman's reagent [7.5 × 10−4 M acetylthiocholine iodide/2.5 × 10−4 M 5,5′dithiobis(2-nitrobenzoic acid)/15 mM NaCl] in 0.1 M phosphate buffer. After 2 h of enzymatic reaction, the absorbance at 414 nm of each well was measured.
HPLC-Monitored Kinetics Assays.
Fifty microliters of 2–300 mM solutions of (1b) in CH3CN was added to 950 μl of a 16.8-μM solution of mAb PAR 15 in 0.1 N Tris⋅HCl buffer at pH 7.4. HPLC separation of the crude reaction mixture was achieved on a C8 reverse-phase Zorbax SB-C8 5-μm column. Flow rate: 1.5 ml/min; column temperature: 35°C; elution conditions: isocratic water/methanol/TFA 60/40/0.1. The phosphinic acid was quantified by UV absorbance measurement at 264 nm. Retention times: PhX (1b): 6.8 min; phosphinic acid 2b: 5.3 min.; phosphinic acid 3b: 4.1 min.
AChE Inhibition Quantification.
For covalent inhibitors: 50 μl of dilutions of the solution to be tested and 50 μl of AChE (0.2 Ellman unit/ml in EIA buffer) were reacted for 2 h in a 96-well microtiter plate. After 2 h, 100 μl of a 2-fold concentrated Ellman's reagent [15 × 10−4 M acetylthiocholine iodide, 5 × 10−4 M 5,5′dithiobis(2-nitrobenzoic acid), 30 mM NaCl] in 0.1 M phosphate buffer, pH 7.4, was dispensed into each well. After 30 min of enzymatic reaction, the absorbance at 414 nm of each well was measured and fitted to a standard curve established with known concentrations of the inhibitor to determine the amount of inhibitor in the solution to be tested.
For reversible inhibitors: 90 μl of dilutions of the solution to be tested, and 90 μl of AChE (0.2 Ellman unit/ml in EIA buffer) were reacted overnight at 4°C in a 96-well microtiter plate. After 14 h, 25 μl of a 10-fold concentrated Ellman's reagent [75 × 10−4 M acetylthiocholine iodide/25 × 10−4 M 5,5′dithiobis (2-nitrobenzoic acid)/150 mM NaCl] in 0.1 M phosphate buffer, pH 7.4, was dispensed into each well. After 10 min of enzymatic reaction, the absorbance at 414 nm of each well was measured and fitted to a standard curve established with known concentrations of the inhibitor to determine the amount of inhibitor in the solution to be tested.
VX Neutralization by Anti-Ha Antisera.
Extremely toxic nerve agent VX (1a) was obtained from Centre d'Etudes du Bouchet and cautiously manipulated there. One hundred microliters of organophosphorus nerve agent VX (1a) (1.25 nM in EIA buffer) was mixed with 100 μl diluted (1/100 in EIA buffer) BALB/c mouse sera. After 18 h of reaction at room temperature, 200 μl of a 0.6 nM solution of AChE was added, and after 4 h, the remaining esterase activity was measured as previously described. Other VX and AChE concentrations were tested in the 0.2–2 nM range for VX and 0.2–0.8 nM for AChE. They all gave similar results. Sera of immunized BALB/c mice were collected 7 days after the third of three boosts (every 15 days).
Results and Discussion
Few antibodies endowed with phosphatase-like activities have been described to date, and the problem of mimicking the putative pentacoordinated anionic phosphorane transition state has led to modest (17–21) or controversial (22–26) catalytic efficiencies (for ref. 22 and 23, no control experiments were entertained, not even hapten inhibition of the catalytic activity). A slightly more successful strategy based on a pentacoordinated metallochelate has been recently reported (21). Phosphoranes structurally close to VX proved to be unstable in aqueous medium (25); we thus first chose hapten Ha bearing an αhydroxyphosphinate moiety, because the best results described so far for the selection of catalytic antibodies endowed with phosphatase-like activity by Scalan et al. (17) are based on such a strategy. As described, such compounds, which are known to be good inhibitors of natural phosphatases (26, 27), should mimic the early approach of an incoming hydroxide ion to the phosphorus center.
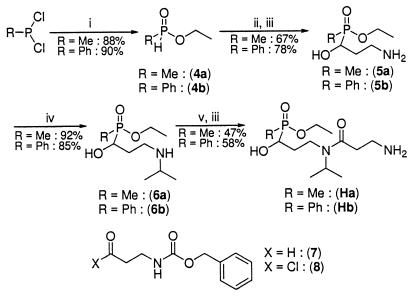
Hapten Ha was thus synthesized in six steps and in a 25.5% global yield from methyl dichlorophosphonite as previously described (25) [Fig. 2; see supplemental data (www.pnas.org)]. The key step is an Abramov–Pudovik (28) addition of the anion of the corresponding phosphinite 4a to aldehyde 7.
Figure 2.
Hapten synthesis. (i) EtOH, THF, 0°C; (ii) (7), EtN(i-Pr)2, DME, 50°C; (iii) H2, Pd/C 5%, EtOH/H2O 95/5; (iv) (a) acetone, MgSO4; (b) H2, Pd/C 5%, MeOH/acetone 50/50; (v) (8) Et3N, CH2Cl2.
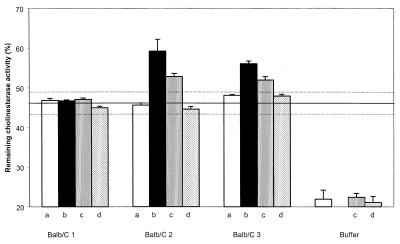
Hydrogenolysis of the carbobenzyloxy protective group cleanly yielded primary amine 5a, which under reductive amination conditions was transformed into a secondary amine 6a. Successive amidation with acylchloride 8 and deprotection gave hapten Ha. Hapten Ha was then covalently coupled to KLH for immunization and to BSA for ELISA. VX is an exceedingly toxic product (LD50 = 8 μg/kg i.v., rabbit, and 28 μg/kg percutaneous, rabbit) (13). Common experimental methods to monitor its hydrolysis compatible with an aqueous and protein medium (NMR or HPLC, for instance) require the manipulation of amounts of VX that are too important for a first screening. Yet, the very efficient AChE inhibition displayed by VX (1a) allows direct measurement of the inactivation of VX with no HPLC experiments. Indeed, within minutes (calculated rate constant of inhibition: 8 × 107 M−1⋅min−1) (29), VX stoichiometrically modifies the catalytic active site of AChE, resulting in an irreversible inactivation of the enzyme (30). Thus, direct measurement of the remaining catalytic activity of a defined solution of AChE (Electrophorus Electricus) incubated with an unknown quantity of VX (1a) allows precise quantification of the inhibitor. Using this methodology, we evaluated the in vitro protection afforded by immunization with hapten Ha by directly testing the efficiency of polyclonal anti-Ha antisera in limiting AChE inhibition by VX. Three BALB/c mice were immunized, and the affinity for the hapten was measured by competitive ELISA. We observed a strong immunological reaction (antisera were used 1/100,000 diluted in the ELISA experiment), but the affinities (Kd) of the polyclonal anti-Ha antibodies (after the third boost) appeared low as estimated by competitive ELISA (15 μM, 28 μM, and 10 μM for the three mice, respectively). However, two of the three mouse antisera (1/100 diluted in EIA buffer) collected after three successive immunizations with Ha-KLH conjugate displayed notable protective activity against VX (1a) (Fig. 3).
Figure 3.
Remaining AChE activity of a 0.6 nM AChE solution in EIA buffer treated with a 0.625 nM VX solution pretreated for 18 h with the corresponding mouse serum (1/100 diluted in EIA buffer) (a) before immunization, (b) after immunization, (c) after immunization and in presence of 50 μM hapten Ha, and (d) after immunization and in presence of 150 μM hapten Ha. Continuous and dashed lines represent means ± 2 SD for sera of 18 BALB/c mice preimmune or immunized with Hb, 5a, 5b, or unrelated haptens.
Control experiments by using buffer alone showed that hydrolysis led to 20% degradation of VX (1a) after overnight reaction. AChE inhibition by VX (1a) was significantly reduced in the presence of antiserum, very likely because of the presence of endogenous cholinesterases. We measured equivalent amounts of cholinesterase activity in nonimmune, preimmune, and immune sera. As determined by the Ellman method (31), this activity was calculated to be 4.3 ± 0.8 units/ml and is summarized in Table 1 for the anti-Ha, -Hb, -5a, and -5b antisera.
Table 1.
Estimated amount of cholinesterases in the mouse sera
| Mouse | Hapten | Preimmune | Immunized |
|---|---|---|---|
| Balb/C 1 | Ha | 39.2 ± 3 | 38.8 ± 5 |
| Balb/C 2 | Ha | 38.9 ± 2.5 | 39.6 ± 3 |
| Balb/C 3 | Ha | 36.3 ± 3 | 40.9 ± 2.5 |
| Balb/C 4 | Hb | 35.2 ± 3 | 42.5 ± 3 |
| Balb/C 5 | Hb | 43.6 ± 3 | 39.2 ± 3 |
| Balb/C 6 | Hb | 48.4 ± 3 | 52.8 ± 3 |
| Balb/C 7 | 5a | 38.2 ± 3 | 40.5 ± 3 |
| Balb/C 8 | 5a | 41.5 ± 3 | 40.3 ± 3 |
| Balb/C 9 | 5a | 43.2 ± 3 | 42.1 ± 3 |
| Balb/C 10 | 5b | 35.8 ± 3 | 46.4 ± 3 |
| Balb/C 11 | 5b | 46.2 ± 3 | 51.4 ± 5 |
| Balb/C 12 | 5b | 48.4 ± 3 | 44.0 ± 3 |
Rate of hydrolysis of acetylthiocholine in 1:100 diluted sera in EIA buffer was determined by the Ellman method and expressed in 10−3 Ellman units/ml.
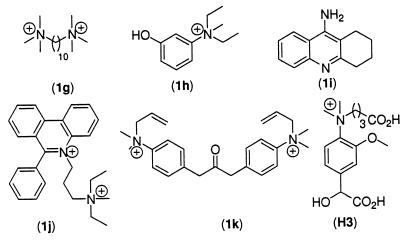
A significant additive effect against VX (1a) poisoning activity was observed with the serum of two of the three mice immunized with hapten Ha; see Fig. 1. Control experiments were performed with: • Sera of 15 BALB/c mice preimmune or immunized with haptens Hb, 5a, and 5b related to hapten Ha (Fig. 2). • Sera of 3 BALB/c mice preimmune or immunized with hapten H3 (32) (Fig. 4), related neither to VX (1a) nor to hapten Ha.
Figure 4.
H3 and AChE inhibitors structures.
Altogether, these sera showed no significant difference from the three sera of mice before immunization (average activity ± 2 SD displayed as dashed lines in Fig. 3).
To confirm that the activity measured is related to the immunization process, we proceeded first to the same experiment in the presence of various concentrations of hapten Ha. If low concentrations (10 μM) of Ha did not show significant activity on the protective activity of the mice sera, higher concentration (50 μM) significantly lowered the protective activity. Finally, at a 150-μM concentration in Ha, the whole protective activity disappeared (Fig. 3), whereas no difference was observed either with the buffer or with the preimmune mice sera. The protective activity is thus related either to the binding site of the polyclonal antibodies or to another enzyme also weakly inhibited by Ha.
The protective activity of the mice sera toward other AChE inhibitors, interacting either with the “peripheral” anionic site or with the catalytic site, was also assayed. The water stable inhibitors decamethonium (1g), edrophonium‡‡ (1h), tacrine (1i), propidium (1j), and BW284C51 (1k) (Fig. 4) were first mixed with the mice sera at concentrations close to their IC50 value and then added to a 0.6 nM AChE solution. No significant difference in the measured cholinesterase activity was observed by using preimmune or immune sera, even after 24 h incubation with the mice sera.
Two other warfare agents, sarin (1d) and soman (1e), which inhibit AChE through the same irreversible modification of the catalytic site as VX, were also tested. For both compounds a 2 nM solution was incubated with the mice sera and further reacted with 0.6 nM AChE once the remaining organophosphorus compound was estimated to be ≈0.5 nM (i.e., 45 min for soman, 90 min for sarin). In both cases, no difference was observed between preimmune and immunized mice (keeping in mind that reaction time is shorter than with VX). This experiment allows us to exclude contamination with a naturally occurring phosphatase.
Finally, to assert the selectivity of the hydrolysis, the protective activity was tested on PhX (1b) and the related compound 1c, which showed the same hydrolytic reactivity as VX (1a) in the buffer and also inhibited AChE (IC50 = 20 ± 2 nM for 1b and 24 ± 3 nM for 1c, as compared with 0.3 ± 0.05 nM for 1a, with the same incubation time with Electrophorus Electricus AChE of 2 h at room temperature; for PhX 1b and related compound 1c, the inhibition is slowly reversible; see below). After 18 h reaction at room temperature, none of the mice sera exhibited noticeable protective activity against those two inhibitors. Interestingly, the affinities (Kd) of the polyclonal anti-Ha antibodies to those AChE inhibitors appeared particularly low as estimated by competitive ELISA (0.6 mM, 1.3 mM, and 1 mM for 1b; 1.1 mM, 1.0 mM, and 0.6 mM for 1c and the three mice, respectively). Those inhibitors thus seem not to be recognized properly by the polyclonal antibodies and accordingly not to be proper substrates for the degradation by the “catalytic” antibodies.
The small volume of sera collected at each bleeding from immunized mice precluded any further purification, and we were thus unable to characterize the protective effect in more detail. At this step, it is not possible to distinguish between a passive neutralization process and a catalytic degradation of VX (1a). To discriminate between these two possibilities, we resorted to the use of mAbs. To avoid the manipulation of dangerously high amounts of exceedingly toxic poisoning compound VX (1a), we produced these mAbs against a hapten Hb that bears a strongly antigenic aromatic moiety instead of the methyl moiety. The mAbs activity was tested against PhX (1b), a VX analog but a less potent AChE inhibitor. Moreover, with PhX (1b), no irreversible aging phenomenon (30) was detected, and two successive overnight dialyses of an AChE solution fully inhibited by PhX (1b) allowed the total recovery of the enzyme activity. Hb was synthesized in six steps and in a 35% global yield following the same synthetic scheme as for Ha [Fig. 2; see supplemental data (www.pnas.org)] and then covalently coupled to KLH for immunization, to BSA for ELISA, or to AChE for use as enzymatic tracer in enzyme immunoassay. Three Biozzi mice were immunized with the Hb-KLH conjugate, and mAbs were produced by using conventional procedures (33). Hybridoma supernatants were screened by testing their ability to bind the Hb-BSA conjugate immobilized on microtitration plates. Thirteen hybridoma cell lines were stabilized and expanded as ascitic fluids.
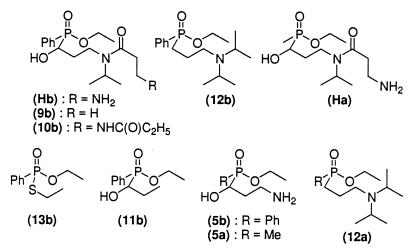
The 13 mAbs were then fully characterized by using a competitive EIA and a series of hapten analogs (Fig. 5), as previously described (32).
Figure 5.
Hapten analogs.
Standard curves were established with each of these compounds for each mAb, and the corresponding 50% B/Bo values were compared with that of Hb to evaluate the relative affinity of the analogs. It is worth noting that this is only a rough estimation of relative affinities, and that only 10-fold differences can be unambiguously interpreted. The results of these experiments are given in Table 2.
Table 2.
Relative affinities, μM, of the different mAbs with regard to series of Hb analogs, as determined by competitive EIA
| mAb | Hb | 9b | 10b | 12b | Ha | 13b | 11b | 5b | 5a | 12a |
|---|---|---|---|---|---|---|---|---|---|---|
| PAR 2 | 3.3 | ND | ND | 36 | 186 | ND | ND | 750 | 4,200 | ND |
| PAR 10 | 0.57 | 0.48 | 0.95 | 23 | 58 | 75 | 143 | 345 | 2,450 | 4,300 |
| PAR 11 | 0.59 | 0.45 | 0.82 | 23.5 | 54.5 | 67 | 145 | 303 | 2,650 | 3,800 |
| PAR 12 | 0.75 | 0.47 | 0.78 | 41 | 31 | 154 | 78 | 206 | 1,130 | 5,300 |
| PAR 13 | 0.42 | 0.39 | 0.46 | 12 | 18 | 37 | 17 | 66 | 860 | 7,850 |
| PAR 14 | 0.43 | 0.48 | 0.45 | 18 | 34 | 37.5 | 26 | 54.5 | 915 | 6,700 |
| PAR 15 | 0.71 | 0.49 | 0.85 | 19 | 69 | 88 | 169 | 340 | 2,980 | 3,800 |
| PAR 16 | 1.0 | 0.75 | 1.45 | 29 | 109 | 243.5 | 255 | 514 | 3,240 | 8,600 |
| PAR 17 | 0.52 | 0.50 | 0.58 | 13 | 19.5 | 38 | 22 | 63 | 940 | 5,200 |
| PAR 18 | 0.88 | 0.70 | 1.24 | 32 | 83 | 64 | 187 | 505 | 3,610 | 3,400 |
| PAR 19 | 0.76 | 0.60 | 0.82 | 37 | 37 | 120 | 95 | 209 | 1,140 | 9,700 |
| PAR 20 | 1.02 | 0.51 | 0.84 | 58.5 | 38 | 126 | 118 | 332 | 1,370 | 5,550 |
| PAR 21 | 0.56 | 0.25 | 0.88 | 26 | 18.5 | 154 | 79 | 219 | 1,480 | 6,800 |
Results are expressed in terms of B/Bo (50%) (see Materials and Methods). Because 12a and 13b are AChE inhibitors (IC50 = 2.7 ± 0.3 μM and IC50 = 48 ± 5 μM), affinity was determined by two-step competitive ELISA and not by EIA, in order to prevent the simultaneous presence in the assay of AChE and its unbound inhibitors. ND, not determined.
Taken together, these results demonstrated that the binding sites of most of the antibodies cover the entire surface of the haptenic molecule, excepting the aliphatic linker (Hb, 9, and 10 display equivalent affinities), and that the α-hydroxyphosphinate moiety is essential for binding. It is worth noting that Ha, with a methyl group at the phosphorus, is poorly recognized, with an apparent affinity 100 times lower than Hb. The same gap in affinity can be observed between 12a and 12b. We also evaluated the antibody affinities for the products and the substrate 1b of the reaction (Table 3). The 13 mAbs have an ideal specificity profile, with affinity for the hapten > affinity for the substrate > affinity for the product.
Table 3.
Relative affinities, μM, of the different mAbs with regard to the substrate and products of the hydrolysis reaction, determined by competitive ELISA
| mAb | PhX 1b | 2b | 3b |
|---|---|---|---|
| PAR 2 | ND | 4,010 | 910 |
| PAR 10 | 240 | 650 | 490 |
| PAR 11 | 135 | 620 | 420 |
| PAR 12 | 170 | >20,000 | 4,100 |
| PAR 13 | 62.5 | 750 | 890 |
| PAR 14 | 120 | 690 | 7,400 |
| PAR 15 | 204 | 1,250 | 406 |
| PAR 16 | 120 | 1,200 | 270 |
| PAR 17 | 170 | 1,400 | 520 |
| PAR 18 | 180 | 1,280 | 370 |
| PAR 19 | 65 | >20,000 | 6,400 |
| PAR 20 | 225 | >10,000 | >10,000 |
| PAR 21 | 215 | >10,000 | 2,700 |
Results are expressed in terms of 50% B/Bo. Because 1b and 3b are AChE inhibitors (IC50 = 20 ± 2 nM and IC50 = 24.6 ± 3 μM), affinity was determined by two-step competitive ELISA and not by EIA. ND, not determined.
Release of the nontoxic O-ethyl phenyl phosphonic acid 2b when 1b was brought into contact with the 13 mAbs was monitored by HPLC. PhX hydrolysis appears to be extremely sensitive to temperature, pH, and the buffer. For instance, the phosphate buffer was shown to enhance hydrolysis up to 10-fold as compared with hydrolysis in the Tris⋅HCl buffer at the same pH, concentration, and temperature. This confirms the hypothesis of a phosphonate-based mechanism for the autocatalytic hydrolysis of VX in the presence of stoichiometric or substoichiometric amounts of water, as claimed by Y. C. Yang (34). To overcome this hydrolysis mechanism, thiophosphonate hydrolysis was performed in 0.1 M Tris⋅HCl buffer at pH 7.4, with 5% CH3CN (vol/vol), at a finely controlled temperature of 25°C (kuncat = 2.8 × 10−5⋅min−1). Two antibodies, PAR 15 and PAR 16, displayed significant activity in the hydrolysis of 1b. Because this activity of PAR 15, but not of PAR 16, was fully inhibited by the hapten Hb, the former mAb was selected for further studies.
Because of the limited solubility of 1b under the reaction conditions (5 mg/ml, 15 mM), we were unable to reach complete saturation. Yet kinetic experiments showed that mAb PAR 15 displayed multiple turnovers; moreover, a Lineweaver–Burk plot was perfectly linear and enabled us to evaluate the ratio kcat/Km = 0.36 M−1⋅min−1, and thus a catalytic proficiency (kcat/Km)/kuncat = 14,400 M−1 [because of the limited solubility of PhX (1b), the steady-state Km and kcat values cannot be obtained independently].
The following controls were performed to confirm that the observed catalytic activity was actually related to the presence and binding capacity of mAb PAR 15.
(i) Mab PAR 15 from various batches of ascetic fluids was affinity purified one to three times on a protein A column or successively purified on a protein A column followed by sieve molecular size exclusion chromatography by using Sephadex G75 gel. Whatever the preparation, activity was recovered together with the antibody protein, yielding solutions with equivalent specific activities (results not shown). The purity of these preparations was attested by SDS/PAGE analysis under reducing and nonreducing conditions.
(ii) None of the control reactions performed with other mAbs raised against hapten Hb or with irrelevant mAbs purified under the same conditions as PAR 15 displayed catalytic activity.
(iii) The initial rate of the catalyzed reaction was proportional to the antibody concentration (in the 0.5–20 μM range).
(iv) Hydrolytic activity of 16 μM mAb PAR 15 was fully inhibited by 50 μM hapten Hb [Kd = 0.71 μM for PAR 15 (Table 2)] and 50 μM related compound 9b [Fig. 5 and Kd = 0.49 μM for PAR 15 (Table 2)].
(v) Hydrolytic activity of mAb PAR 15 is fully conserved when set in the presence of EDTA (EDTA up to 1 mM), which further excludes any contamination with a small amount of metal ion-dependent phosphatase or esterase.
In basic medium, release of still toxic thiophosphonic acids 3a or 3b (Fig. 1) also occurs during hydrolysis. This O-Et side chain hydrolysis represents ≈15% when VX (1a) and ≈20% when PhX (1b) react with concentrated HO2 at ambient temperature (35). We checked that thiophosphonic acid 3b was not released at pH 7.4 under the antibody-catalyzed hydrolysis conditions.
The pH dependence of the catalytic activity of mAb PAR 15 was also studied. The optimum activity was shown to occur at pH = 7.4. The catalytic activity was negligible above pH = 8.2 and below pH = 6.8. This pH dependence suggests that the catalysis is related to the presence of two ionizable residues with similar pKas in the binding pocket of mAb PAR 15, one required in the acidic form and the other one in the basic form.
It is worth noting that although the 13 mAbs all show a very similar binding profile, only one of them, PAR 15, displayed a significant catalytic activity. This observation confirms the fact that binding profile has no predictive value for catalysis.
Despite the limited affinity observed for Hb analogs bearing a methyl group instead of a phenyl group at the phosphorus (Table 2), we tested the effect of mAb PAR 15 on VX (1a). As expected, only a very low neutralization effect was observed (results not shown).
Conclusions
We have demonstrated that polyclonal antibodies elicited against Ha were able to neutralize highly toxic phosphonothioate VX (1a) under physiological conditions. We have also demonstrated that this protective activity was specifically related to the immunization process and did not apply toward other structurally related and not related organophosphorus poisoning chemical warfare. We then obtained 13 mAbs recognizing the immunizing hapten Hb with affinities ranging from 0.4 to 3 μM. Affinity substantially decreases when the phenyl moiety is replaced by methyl and when the α-hydroxy group is removed. Under these conditions, it is not surprising that these mAbs bind VX poorly and have a very low protective effect against this nerve agent.
On the other hand, one mAb, PAR 15, demonstrated a significant catalytic activity with a VX analog, the less toxic substrate PhX (1b). To envisage any animal or clinical application, it is clear that a higher affinity for substrates and higher catalytic activity than those observed with anti-Ha and anti-Hb antibodies are required. However, this work demonstrated that mAbs can catalytically hydrolyze organophosphorus poisons and represents significant progress toward the production of therapeutically active abzymes to treat poisoning by warfare agents.
This work is the first step in the process leading to in vivo detoxification via both serotherapy (with mAb PAR 15) and immunotherapy with immunization with a transition state analog (polyclonal anti-Ha antibodies) We are presently working on charged haptens and on new immunization strategies to reach higher rates of catalysis to select antibodies more useful for clinical application.
Supplementary Material
Acknowledgments
This work was financially supported by the Délégation Générale de L'Armement. We are indebted to P. Lamourette and M. Plaisance for technical expertise in mAb preparation.
Abbreviations
- EIA
enzyme immunoassay
- AChE
acetylcholinesterase
- KLH
keyhole limpet hemocyanin
Footnotes
Edrophonium, ethyl(m-hydroxyphenyl)dimethylammonium chloride; tacrine, 9-amino-1,2,3,4-tetrahydroacridine; propidium, 3,8-diamino-5-[3′diethylmethylammonio)propyl]6-phenylphenantridinium iodide; BW284C51, 1,5 bis-[p-(allyl-N,N-dimethylammonio)phenyl]penata-3-one.
References
- 1.Taylor P. In: Anticholinesterase Agent in the Pharmacological Basis of Therapeutics. Goodman Gilman A, Rall T W, Nied A S, Taylor P, editors. New York: Pergamon; 1990. [Google Scholar]
- 2.Bunton C A. In: Chemical Warfare in Macmillan Encyclopedia of Chemistry. Lagowski J J, editor. Vol. 1. New York: Macmillan; 1997. pp. 343–346. [Google Scholar]
- 3.Yang Y C, Baker J A, Ward J R. Chem Rev. 1992;92:1729–1743. [Google Scholar]
- 4.Millard C B, Lokridge O, Broomfield C A. Biochemistry. 1995;34:15925–15933. doi: 10.1021/bi00049a007. [DOI] [PubMed] [Google Scholar]
- 5.Millard C B, Lokridge O, Broomfield C A. Biochemistry. 1998;37:237–247. doi: 10.1021/bi972057c. [DOI] [PubMed] [Google Scholar]
- 6.Masson P, Josse D, Lockridge O, Viguie N, Taupin C, Buhler C. J Physiol. 1998;92:357–362. doi: 10.1016/S0928-4257(99)80005-9. [DOI] [PubMed] [Google Scholar]
- 7.Josse D, Xie W H, Renault F, Rochu D, Schopfer L M, Masson P, Lockridge O. Biochemistry. 1999;38:2816–2825. doi: 10.1021/bi982281h. [DOI] [PubMed] [Google Scholar]
- 8.Kohler G, Milstein C. Nature (London) 1975;256:495–497. doi: 10.1038/256495a0. [DOI] [PubMed] [Google Scholar]
- 9.Reymond J L. Top Curr Chem. 1999;200:59–93. [Google Scholar]
- 10.Pollack S J, Jacobs J W, Schultz P G. Science. 1986;234:1570–1573. doi: 10.1126/science.3787262. [DOI] [PubMed] [Google Scholar]
- 11.Tramontano A, Janda K D, Lerner R A. Science. 1986;234:1566–1570. doi: 10.1126/science.3787261. [DOI] [PubMed] [Google Scholar]
- 12.Mets B, Winger G, Cabrera C, Seo S, Jamdar S, Yang G, Zhao K, Briscoe R J, Almonte R, Woods, et al. Proc Natl Acad Sci USA. 1998;95:10176–10181. doi: 10.1073/pnas.95.17.10176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yang Y-C. Acc Chem Res. 1999;32:109–115. [Google Scholar]
- 14.Pradelles P, Antoine C, Lellouche J P, Maclouf J. Methods Enzymol. 1990;187:82–89. doi: 10.1016/0076-6879(90)87012-r. [DOI] [PubMed] [Google Scholar]
- 15.Pradelles P, Grassi J, Maclouf J. Anal Chem. 1985;57:1170–1173. doi: 10.1021/ac00284a003. [DOI] [PubMed] [Google Scholar]
- 16.Pradelles P, Grassi J, Charbardes D, Guiso N. Anal Chem. 1989;61:447–453. doi: 10.1021/ac00180a014. [DOI] [PubMed] [Google Scholar]
- 17.Scanlan P G, Prudent J R, Schultz P G. J Am Chem Soc. 1991;113:9397–9398. [Google Scholar]
- 18.Rosenblum J S, Lo L-C, Li T, Janda K D, Lerner R A. Angew Chem Int Ed Engl. 1995;34:2275–2277. [Google Scholar]
- 19.Lavey B J, Janda K D. J Org Chem. 1996;61:7633–7636. doi: 10.1021/jo960629d. [DOI] [PubMed] [Google Scholar]
- 20.Weiner D P, Weinmann T, Wolfe M M, Wentworth P, Janda K D. J Am Chem Soc. 1997;119:4088–4089. [Google Scholar]
- 21.Wentworth P, Liu Y Q, Wentworth A D, Fan P, Foley M J, Janda K D. Proc Natl Acad Sci USA. 1998;95:5971–5975. doi: 10.1073/pnas.95.11.5971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yli-Kauhaluoma J, Humppi T, Yliniemela A. Acta Chem Scand. 1999;53:473–479. doi: 10.3891/acta.chem.scand.53-0473. [DOI] [PubMed] [Google Scholar]
- 23.Brimfeld A A, Lenz D E, Maxwell D M, Broomfield C A. Chem Biol Interactions. 1993;87:95–102. doi: 10.1016/0009-2797(93)90029-x. [DOI] [PubMed] [Google Scholar]
- 24.Thomas N R. Nat. Prod. Reports. 1996. 479–511. [DOI] [PubMed] [Google Scholar]
- 25.Renard P Y, Vayron P, Taran F, Mioskowski C. Tetrahedron Lett. 1999;40:281–284. [Google Scholar]
- 26.Frechette R F, Ackerman A, Beers S, Look R, Moore J. Bioorg Med Chem Letters. 1997;7:2169–2172. [Google Scholar]
- 27.Schwender C F, Berrs S A, Malloy E, Demarest K, Minor L, Lau K H W. Bioorg Med Chem Lett. 1995;5:1801–1806. [Google Scholar]
- 28.Cherkasov R A, Galkin V I, Khabibullina A B, Al Kurdi K. Phosphorus Sulfur Silicon Relat Elem. 1990;49–50:61–64. [Google Scholar]
- 29.Grognet J M, Ardouin T, Istin M, Vandais A, Noel J P, Rima G, Satge J, Pradel C, Sentenac-Roumanou H, Lion C. Arch Toxicol. 1993;67:66–71. doi: 10.1007/BF02072038. [DOI] [PubMed] [Google Scholar]
- 30.Vigny M, Bon S, Massoulié J, Leterrier F. Eur J Biochem. 1978;85:317–323. doi: 10.1111/j.1432-1033.1978.tb12241.x. [DOI] [PubMed] [Google Scholar]
- 31.Ellman G L, Courtney K D, Andres V, Featherstone R M. Biochem Pharmacol. 1961;7:88–95. doi: 10.1016/0006-2952(61)90145-9. [DOI] [PubMed] [Google Scholar]
- 32.Taran F, Renard P Y, Bernard H, Mioskowski C, Frobert Y, Pradelles P, Grassi J. J Am Chem Soc. 1998;120:3332–3339. [Google Scholar]
- 33.Grassi J, Frobert Y, Lamourette P, Lagoutte B. Anal Biochem. 1988;138:436–450. doi: 10.1016/0003-2697(88)90341-7. [DOI] [PubMed] [Google Scholar]
- 34.Yang Y C, Szafraniec L L, Beaudry W T, Rohrbaugh D K, Procell L R, Samuel J B. J Org Chem. 1996;61:8407–8413. [Google Scholar]
- 35.Yang Y C, Berg F J, Szafraniec L L, Beaudry W T, Bunton C A, Kumar A. J Chem Soc Perkin Trans. 1997;2:607–613. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.