Abstract
Protein malnutrition may increase susceptibility to gastrointestinal parasitic infections, possibly as a result of impaired intestinal and/or systemic T helper 2 (Th2) effector responses induced by down-regulation of Th2 cytokines and/or up-regulation of Th1 cytokines. To test this hypothesis, female BALB/c mice (n = 18/diet) were fed a control (24%), marginal (7%), or deficient (3%) protein diet and given a challenge infection with Heligmosomoides polygyrus. The 3% mice had higher worm burdens at 1, 2, and 4 weeks postchallenge infection (pci), lower increases in serum IgE, reduced intestinal eosinophilia, and depressed mucosal mast cell proliferation and activation at 1–2 weeks pci. To determine whether these suppressed effector responses resulted from altered spleen and mesenteric lymph node (MLN) cytokine production, cells were restimulated in vitro with parasite antigen and cytokine concentrations were measured. Deficient MLN cells secreted significantly less IL-4 and more IFN-γ at 1–2 weeks pci than did control MLN cells. Deficient spleen cells also secreted more IFN-γ at 2 weeks pci compared with control spleen cells. From reverse transcription-PCR analyses, the 3% mice also had lower IL-4 mRNA level in spleen and MLN at 1–2 weeks pci. Our study supports the hypothesis that protein malnutrition increases the survival of a nematode parasite by decreasing gut-associated IL-4 (Th2) and increasing IFN-γ (Th1) within 2 weeks pci, leading to reduced intestinal and systemic Th2 effector responses.
Protein malnutrition (PM) and gastrointestinal nematode infections are chronic diseases that frequently coexist in human populations of developing countries. Acquired resistance to intestinal parasitism depends on the magnitude and rapidity of T helper 2 (Th2) cytokine (IL-4, IL-5, IL-10) and effector responses (eosinophilia, IgE, mastocytosis) (1, 2) and the concomitant absence of Th1 responses (IFN-γ, macrophage activity) (3) in both systemic and gut-associated tissues. PM has been shown to increase susceptibility to nematode parasites (4, 5) by depressing blood eosinophilia and IgG1 (5, 6). However, little is known about the consequences of PM on protective immunity in gut-associated lymphoid tissues (GALT), even though immune responses in GALT are the first line of host defense against intestinal parasites and importantly may direct the appropriateness and magnitude of peripheral responses. The rate of protein turnover in the gut mucosa is 25–30 times greater than that observed for muscle (7) and is therefore potentially very sensitive to changes in host protein status (8). Furthermore, the consequences of mild to moderate PM on Th2 immunity in nematode-infected hosts have not been characterized. To distinguish the relative importance of systemic versus intestinal immune responses to the development of host resistance and to ascertain the degree to which PM alters Th2 immunity, we compared gut-associated and systemic cytokine and effector responses in immunized mice given a challenge infection with the mouse nematode, Heligmosomoides polygyrus (Hp).
Materials and Methods
Animals and Nutrition Protocols.
Three-week-old female BALB/c mice (Charles River Breeding Laboratories) were randomly assigned to one of three diets (24%, 7%, 3% protein) (6), offered ad libitum in Mouse Powder Feeders (Lab Products, Quebec, Canada) throughout the experiment. All nutrients (except protein) were identical among the three diets and were fed above the National Research Council (9) mouse requirements to ensure that a 30% reduction in food intake due to PM or infection would not generate other nutrient deficiencies. Food intake and body weight (bwt) gain were measured every 3–4 days. Plasma albumin and blood urea nitrogen were analyzed by using the Abbott Discrete Analyzer as described (6). All procedures were approved by the McGill Animal Care Committee according to the Canadian Council on Animal Care.
Infection Protocols.
Primary infections with Hp are usually chronic, but BALB/c mice, which are intermediate Th2 responders, can develop resistance to a secondary challenge infection when subjected to an anthelmintic-abbreviated immunizing protocol (10). Mice, after 3 weeks of feeding, were intubated orally with 100 infective L3 (11). On days 9 and 14 postprimary infection (ppi), mice were treated orally with pyrantel pamoate (Pfizer; 172 mg/kg bwt) to remove adult parasites from the primary infection. On day 19 ppi, lymphoid organs from mice were assayed for baseline cytokine production. On day 21 ppi, mice were reinfected with 100 L3 (the challenge cohort, C), and previously uninfected mice were infected with 100 L3 to form a primary infection group (P) with which to compare the degree of protection acquired during challenge infection. Worms were recovered by using standard procedures (6) from primary mice (18/diet/day) killed at days 6 and 28 ppi (P6 and P28) and from challenged mice killed at 3, 6, 9, 14, 21, and 28 days postchallenge infection (pci) (C3–C28).
Systemic Effector and MMCP-1 Measurements.
Eosinophilia was measured from tail vein blood by using the Unopette Test (Fisher Scientific). Total serum IgE was measured by two-site ELISA according to manufacturer's directions (PharMingen). Immulon II plates (Dynatech Laboratories) were coated with rat anti-mouse IgE mAb (R35–72) and developed with biotinylated, rat anti-IgE mAb (R35–92) and avidin-peroxidase (Cedarlane Laboratories). Serum mucosal mast cell protein-1 (MMCP-1) was quantitated by two-site ELISA by using sheep anti-MMCP-1 mAb (Moredun Scientific, Peniciuk, Scotland), purified mouse MMCP-1 as standard (Moredun Scientific), and peroxidase-conjugated rabbit anti-MMCP-1 mAb (Moredun Scientific) (27). Parasite-specific IgG1 was determined by direct ELISA with L4 antigen of Hp (11), biotinylated rat anti-mouse IgG1 mAb (PharMingen), and avidin-peroxidase (Cedarlane Laboratories).
Intestinal Mucosal Mast Cell (MMC) and Eosinophil Histology.
Segments of small intestine (0.5 cm long, 10 cm from pylorus) were fixed in Carnoy's fixative for MMC or 4% paraformaldehyde at 4°C overnight for intestinal eosinophils. Paraffin-embedded intestinal sections (5–6 μm thick) were stained with Alcian Blue and counterstained with Safranine O (mast cells) or with 0.5% Chromotrope 2R (eosinophils).
Cell Preparation and Culture.
Spleens and mesenteric lymph nodes (MLN) were passed aseptically through a 60 mesh screen (Sigma). Red blood cells were lysed by NH4Cl. Cell viability was determined by using 0.1% trypan blue in PBS (GIBCO) and was always >90%. Cells were resuspended in complete medium consisting of RPMI 1640 (GIBCO), 10% heat-inactivated FBS (GIBCO), 20 mM Hepes buffer (Sigma), 200 units/ml penicillin (Sigma), 200 μg/ml streptomycin sulfate (Sigma), 25 μg/ml gentamicin (GIBCO), and 50 μM 2-mercaptoethanol. Cell suspensions were plated in complete medium at a final concentration of 5 × 106 cells/ml. Aliquots of 1.0 ml were incubated with L4 antigen (7.5 μg protein/ml) at 37°C in 5% CO2 atmosphere with 90% humidity for 48 h and then centrifuged at 300 × g for 10 min. Supernatants were stored at −80°C until they were assayed for cytokines.
Cytokine ELISAs.
IL-4, IL-5, IL-10, and IFN-γ concentrations were measured by sandwich ELISA according to manufacturer's (PharMingen) directions. After overnight incubation with cytokine standards and supernatants, plates were developed with biotinylated anti-cytokine mAb (PharMingen) and avidin-peroxidaseγ-conjugate (Cedarlane Laboratories). Cytokine concentrations were calculated from standard curves generated by using known amounts of recombinant (r) murine IL-4, rIL-5, rIL-10, and rIFN-γ (PharMingen).
Semiquantitative Reverse Transcription (RT)-PCR.
Cytokine mRNA levels were assayed by semiquantitative RT-PCR under conditions that ensured unsaturable amplification. Concentrations of RNA extracted by Trizol (GIBCO) were determined spectrophotometrically. The RNA was suspended in water, heated to 65°C for 10 min, and reverse-transcribed in a 10 mM mix of four dNTP, 13 μl of 5× RT buffer, 2.6 μl of 0.1 M dithiothreital, 0.26 μl of RNase inhibitor (40 units/μl), 0.52 μl oligo(dT) (0.5 μg/μl), 0.65 μl of Moloney murine leukemia virus reverse transcriptase (200 units/μl) (GIBCO), 36.27 μl distilled water, and 5.2 μl total RNA (0.3 μg/μl). The mix was incubated at 37°C for 1 h and then heated to 95°C for 5 min. A 10-μl aliquot of the RT reaction was used in a 100 μl PCR which contained 0.5 μl of Taq DNA polymerase (GIBCO), 10 μl of 10× PCR buffer, 0.2 μl of 10 mM dNTP, 4 μl of 50 mM MgCl, and 1 μl of forward and reverse primers (50 pm/μl). Primers for IL-4, IL-5, IL-10, and IFN-γ and the reference gene, hypoxanthine phosphoribosyltransferase (HPRT), were selected as described by Svetic et al. (12). The samples were subjected to 29 cycles as follows: 94°C for 0.5 min, 56°C for 1 min, and 72°C for 2 min. A final extension step was performed by heating to 72°C for 8 min. After PCR amplification, a 10-μl aliquot was mixed with 2-μl loading buffer and electrophoresed on a 1.2% agarose gel containing ethidium bromide (0.25 μg/ml). Cytokine mRNA was quantitated by densitometric scanning of the photograph by using Macintosh photoshop and NIH image 160 software. The HPRT mRNA levels did not differ significantly among dietary groups, infection time, and tissues. The mRNA level for each cytokine was normalized to HPRT and expressed as ratio to HPRT mRNA.
Statistical Analyses.
Results are reported as means ± SE. When the variances were nonhomogenous (Barlett's test), data were logarithmically transformed. All data (except nutritional outcomes and eosinophilia) were pooled by infection protocol (primary or challenge) and each was analyzed for main effects of diet, time, and diet × time by using a two-way ANOVA. Repeated measures ANOVA assessed the effects of diet and time on blood eosinophils and one-way ANOVA detected the effects of diet on nutritional outcomes of C28 mice. Because dietary modulation of host immunity may vary in magnitude at different stages of infection, data (worm burdens, antibody titers, histological counts, MMCP-1 concentration, and cytokines) were also analyzed for the main effect of diet at each time point with a one-way ANOVA followed by post hoc pairwise comparisons (Tukey) when diet was significant. All analyses were performed by using sas 6.12 (SAS Institute, Cary, NC) and considered significant at P < 0.05.
Results
Nutritional Status.
Although mice fed the low-protein diet (3%) ate the same amount of food, hence calories, as mice fed the control diet (Table 1), they ate significantly more food per g bwt than the other two dietary groups (P < 0.0001). However, this higher relative energy intake did not support normal growth; the 3% group gained 71% less bwt than the controls (P < 0.0001), showing that the low-protein diet induced protein but not energy deficiency. In contrast, the marginal protein (7%) mice ate more food than either the 24% or 3% mice (P < 0.0001) and gained comparable bwt as the 24% mice.
Table 1.
Nutritional outcomes of C28 mice
| Parameter*† | 24% | 7% | 3% |
|---|---|---|---|
| Total food (g) | 173 ± 2a | 193 ± 2b | 176 ± 3a |
| Food/bwt/day (g/g bwt/day) | 150 ± 2a | 157 ± 2a | 193 ± 3b |
| Body weight gain‡ | 8.7 ± 0.4a | 8.5 ± 0.4a | 2.5 ± 0.4b |
| MLN weight¶ | 5.1 ± 0.2a | 4.1 ± 0.2b | 3.7 ± 0.2b |
| Spleen weight§ | 5.7 ± 0.2a | 5.3 ± 0.2a | 4.6 ± 0.2b |
| Thymus weight§ | 1.9 ± 0.9 | 1.8 ± 0.8 | 1.8 ± 1.7 |
| Plasma albumin (g/liter) | 25 ± 1a,b | 28 ± 1a | 23 ± 2b |
| BUN (mmol/liter) | 8.8 ± 0.5a | 3.7 ± 0.1b | 1.7 ± 0.1c |
BUN, blood urea nitrogen.
Values are x̄ ± SE.
Different letters, P < 0.05.
Final-initial bwt (g).
Relative to bwt × 10−3.
Protein restriction to 3% and 7% decreased relative MLN weight (P = 0.0007), and the 3% protein diet also lowered relative spleen weight (P = 0.0018). Thymus weight was not affected by protein deficiency. Blood urea nitrogen showed a dose-dependent response to dietary protein level (P < 0.0001), whereas plasma albumin concentration was decreased in mice fed 3% protein compared with the 7% diet (P = 0.0234), but neither differed from those fed 24% protein. The absence of hypoalbuminaemia in the 3% mice despite their reduced body and lymphoid organ growth suggested that plasma albumin concentration is less sensitive to PM than the immunological indices described below.
Parasite Outcomes.
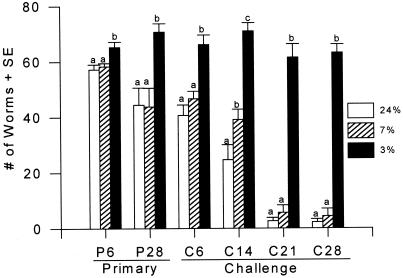
Previously immunized (challenge) mice fed the 24% and 7% protein diets eliminated >95% of Hp by day 28 pci compared with their nonimmunized (primary) counterparts which expelled only 56–60% (Fig. 1). In contrast, worm burdens were significantly higher in mice fed the 3% protein diet during both primary (P = 0.024) and challenge infections (P < 0.0001), thus supporting the observation that PM impaired the development of protective host immunity to Hp.
Figure 1.
Effects of PM on worm burdens after primary or challenge infection with Hp.
Effector Responses.
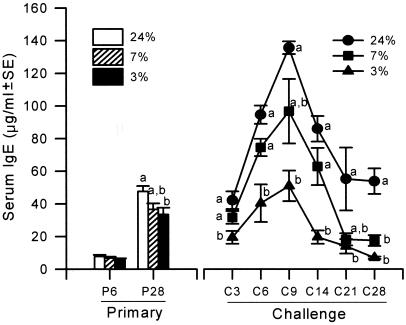
PM significantly lowered serum IgE levels (Fig. 2) on day 28 ppi (P = 0.0336) and throughout the challenge infection (P < 0.0001) but did not affect the timing of secondary IgE responses, which peaked at days 6–9 pci. Serum IgE responses in 7% mice fluctuated between high levels observed in controls and the low titers of 3% mice, thus suggesting a differential response of IgE over time to marginal protein intakes. PM did not impair the parasite-specific IgG1 during primary or challenge infection (data not shown). In all dietary groups, parasite-specific IgG1 rose rapidly early after challenge infection and reached maximal values on day 9 pci (P < 0.05) that exceeded those of the primary infection. Blood eosinophilia was impaired by PM during primary infection (P < 0.001) but not during challenge infection.
Figure 2.
Effects of PM on total serum IgE after primary or challenge infection with Hp.
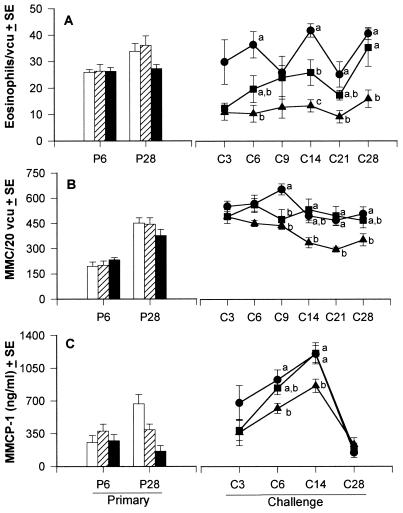
Despite weekly fluctuations, the numbers of intestinal eosinophils during challenge infection (Fig. 3A) showed an overall dose response to the dietary protein level (P < 0.0001). Marked increases in gut eosinophilia were apparent in challenged mice fed 24% protein but were not observed in the 7% or 3% groups, which had even lower intestinal eosinophilia compared with their primary counterparts. Intestinal MMC counts (Fig. 3B) were higher in challenged mice than in early primary (P6) mice, reflecting a strong capacity for acquired MMC response to Hp. Overall, the 3% protein diet decreased MMC numbers during a challenge (P < 0.0001) but not primary infection. The 7% mice maintained intermediate MMC responses during challenge infection; their MMC numbers were lower compared with 24% mice only on day 9 pci, and at other pci time points were either similar to 24% or intermediate between the 24% and 3% mice. High MMC numbers in challenged mice fed 24% persisted throughout the challenge infection period but dropped in the 3% group after day 9 pci (P = 0.0015), indicating that 3% mice had reduced gut mastocytosis compared with the 24% mice on day 9 pci and successively thereafter.
Figure 3.
Effects of PM on the numbers of intestinal eosinophils (A) and MMC (B), and serum MMCP-1 concentration (C) after primary or challenge infection with Hp.
Overall, the secretory capacity of MMC as measured by the concentration of serum MMCP-1 (Fig. 3C) was not different among the dietary groups after primary or challenge infection. However, PM significantly inhibited the increase in MMCP-1 on day 6 pci (P = 0.018) and day 14 pci (P = 0.008). In all dietary groups, MMCP-1 levels peaked on day 14 pci (P < 0.05), which followed peak intestinal MMC numbers (in 24% mice on day 9 pci) and maximal serum IgE and parasite-specific IgG1 responses (day 9 pci), demonstrating that PM altered the magnitude but not kinetics of Ig-dependent MMC activation and secretion.
Cytokine Responses to L4 Antigen in Vitro.
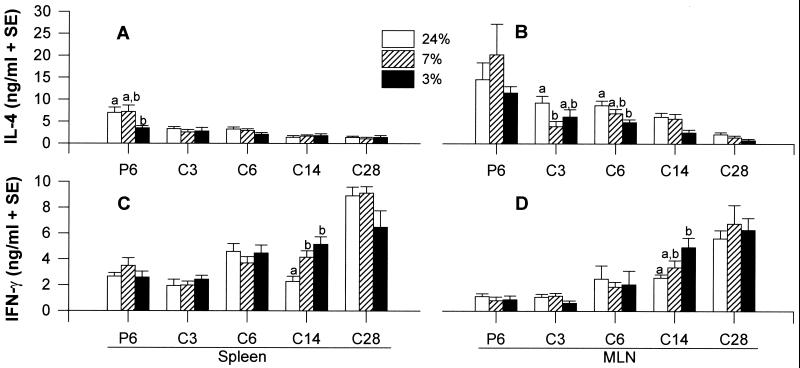
Th2 cytokine (IL-4, IL-5, IL-10) and Th1 cytokine (IFN-γ) production by spleen and MLN were measured to determine whether impaired effector responses during PM were associated with perturbations in intestinal and/or splenic cytokine production (Fig. 4). In general, Th2 cytokine secretion was higher in the first week of primary infection compared with all pci time points (with the exception of IL-5 in spleen), indicating a strong priming of CD4+ cells to secrete Th2-type cytokines. Also, the Th2 cytokine profiles after challenge infection in all dietary groups showed that the response was greater in MLN than in spleen, peaked by 1 week pci in MLN, and declined rapidly thereafter. Effects of PM on secondary Th2 cytokine production were detected only in IL-4 secretion by MLN (P = 0.0004). Also, we observed differences in mucosal IL-4 production among dietary groups immediately before challenge infection. In these primed mice, MLN cells of 24% mice produced more IL-4 (8.66 ± 0.71 ng/ml) compared with the 3% group (3.98 ± 0.96 ng/ml) whereas mice fed 7% protein secreted an intermediate level (5.16 ± 2.43 ng/ml). After challenge infection, the MLN of the 7% protein group secreted less IL-4 at day 3 pci compared with their 24% protein counterparts (P = 0.0195). Similarly at day 6 pci, IL-4 production was lower in MLN of mice fed 3% protein compared with mice fed 24% protein (P = 0.0125). The profiles of IL-5 and IL-10 production in spleen and MLN were similar to IL-4, but were not affected by PM during challenge infection (data not shown).
Figure 4.
Effects of PM on IL-4 and IFN-γ production in spleen (A and C) or in MLN (B and D) after primary (P) or challenge (C) infection with Hp.
IFN-γ secretion by both spleen and MLN (Fig. 4 C and D) increased significantly throughout the challenge infection in all dietary groups (P < 0.05), except in the spleens of mice fed 3% protein. Despite a lack of an overall significant effect of diet on IFN-γ production in spleen or MLN, mice fed 3% protein secreted more IFN-γ compared with mice fed 24% protein in spleen (P = 0.0003) and in MLN (P = 0.023) on day 14 pci. Thus, PM did not alter the inherent capacity of spleen or MLN cells to synthesize IFN-γ but increased IFN-γ production at the onset of worm expulsion in a challenge infection.
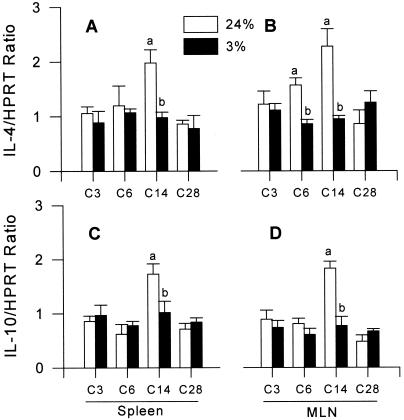
IL-4 mRNA levels in spleen (P = 0.0204) and MLN (P = 0.0256) of mice fed 3% protein showed no increase and remained low after Hp reinfection compared with mice fed 24% protein (Fig. 5 A and B). Suppressed IL-4 gene expression during PM was detected on day 14 pci in spleen and on days 6 and 14 pci in MLN (P < 0.05). Also, IL-10 mRNA levels in spleen and MLN (Fig. 5 C and D) were lower in 3% mice compared with 24% mice at day 14 pci (P < 0.05), but did not differ overall throughout the challenge infection. The levels of IL-5 and IFN-γ mRNA (data not shown) were not altered by PM during challenge infection.
Figure 5.
Effects of PM on IL-4 and IL-10 mRNA expression by semiquantitative RT-PCR in spleen (A and C) or in MLN (B and D) after challenge infection with Hp.
Discussion
This study is a comprehensive investigation of PM on the magnitude and kinetics of Th2 immune responses in mice infected with a gastrointestinal nematode parasite. Contrary to convention, PM did not suppress all immune processes but exerted differential site- and type-specific effects on cytokine and effector responses to a challenge infection with Hp. To illustrate this, four important findings are noted: (i) PM was more detrimental to gut-associated immunity than to systemic responses; (ii) PM down-regulated gut-associated but not splenic IL-4 production early after challenge infection and did not affect secondary responses of other Th2 cytokines; (iii) PM up-regulated IFN-γ production at 2 weeks pci, which may be a critical period for the expression of protective Th2 immunity; and (iv) PM decreased levels of MMC, IgE, and gut eosinophils but not parasite-specific IgG1 or blood eosinophils. Additionally, the level of dietary protein influenced the degree of acquired immunity and parasite survival during reinfection with Hp. Our study supports the hypothesis that PM prolongs the survival of a nematode parasite by decreasing gut-associated IL-4 (Th2) and increasing IFN-γ (Th1) early in the infection, leading to reduced intestinal and systemic Th2 effector responses.
Acquired immunity to secondary infections with nematode parasites is associated with Th2 cytokine and effector responses (1, 2), although the precise mechanisms by which these Th2 mediators exert their protective effect are unclear. Most previous studies (2, 13) have examined effector responses in circulating blood, yet these may not reflect responses occurring at the intestinal site of nematode infection. For example, >99% of intestinal IgE raised against Trichinella spiralis is produced locally in the gut (14) and intestinal MMC responses to Hp are associated with arrested larval development and damage of young adult worms (15, 16). In our study, the higher worm burdens in Hp-challenged mice fed low protein were associated with lower intestinal but not peripheral eosinophilia as well as diminished MMC proliferation and activation, thus supporting the concept that effector responses in GALT may be important during acquired resistance to nematode parasites. More importantly, our results substantiate the view that gut mucosal immunity is more susceptible to PM than are systemic lymphoid tissues or levels of circulating immune mediators.
We investigated whether the impaired effector responses observed during PM were associated with dysregulated cytokine production. IL-4 is a critical cytokine directing host resistance to nematode infections by promoting the differentiation of Th cells to the Th2 phenotype (17), generation of memory Th2 cells (18), IgE isotype switch and proliferation (19, 20), mast cell FcɛRI expression (21), transport of IgE into intestine of nematode-infected mice (22), and expulsion of established Hp infection (23). Recently, IL-3 and IL-13 have been implicated as key immunoregulatory cytokines in the Th2 responses to some nematode parasites (22), but their distinct role in Hp infection is not yet known. Given the prerequisite of IL-4 for Th2 immunity, the decreased IL-4 mRNA and protein in MLN of mice fed 3% protein may have been responsible in part for their impaired Th2 effector responses and prolonged survival of Hp during challenge infection. Also, the finding that PM suppressed intestinal but not splenic IL-4 synthesis suggested that acquired resistance to Hp is influenced more by gut-associated cytokines than by systemic responses and that PM compromises intestinal cytokine production to a greater extent than that of splenic production. In contrast to IL-4, PM did not impair IL-5 and IL-10 production even though it reduced IL-10 mRNA levels. This finding suggested that relatively small amounts of IL-4 permit synthesis of other Th2 cytokines and that PM may affect gene expression without impairing translation of mRNA to protein. Moreover, these results emphasize the need to reappraise the functional role of IL-5 and IL-10 in host protection. The expression of IL-10 mRNA does not correlate with other Th2 mRNA levels in primary Hp infection of normal mice (12), and its major effect may be in suppressing Th1 rather than enhancing the development of Th2 cells (24). Similarly, IL-5 may not be required by the host to eliminate nematode parasites even though it promotes eosinophilia (25, 26). Taken together, the differential effects on Th2 cytokines suggest that PM impairs specific components of Th2 cytokine response (IL-4 production in GALT) that are important for acquired immunity to Hp.
The effect of PM on kinetics of cytokine responses indicated that dietary protein is required at specific time points for coordinated activation of host immunity. Cytokines are likely most effective in promoting effector responses when the parasite is in its L4 stage (1–10 days pci), which stimulates the most intense expression of acquired immunity in mice (27). Indeed, the higher levels of Th2 cytokines in MLN compared with the spleen and their gradual decline after 14 days pci reflect the strong antigenic signals given to GALT by the tissue-dwelling larvae. Adult worms are less potent inducers of intestinal inflammation, therefore acquired resistance to nematode parasites requires elevated Th2 cytokine production in GALT before Hp matures and emerges into the gut lumen. During challenge infection with Hp, PM modified IL-4 production by MLN only during the first week pci in a selective manner depending on the severity of restriction. The MLN cells of 7% mice were able to rebound from initial impaired IL-4 synthesis on day 3 pci, whereas the MLN cells of the protein-deficient mice were unable to secrete high levels of IL-4 at day 6 pci, which likely contributed to their subsequently impaired IgE and MMC responses on day 9 pci. Moreover, immediately before challenge infection, we found that IL-4 levels in MLN were higher in primed mice fed 24% protein than in mice fed 3% protein. After reinfection, gut-associated IL-4 production in 24% mice remained elevated for 1 week pci, whereas it failed to increase in 3% mice from a low baseline level. Thus, reduced Th2 effector responses in the 3% mice may have resulted from their inability to initiate and/or sustain IL-4 production on challenge with Hp.
The reciprocal amounts of IL-4 and IFN-γ, which are mutually antagonistic for Th cell differentiation and effector activity (24), may have altered the magnitude of Th2 immunity raised against Hp. IL-4 inhibits cytokine synthesis by Th1 cells and macrophage function (28), whereas IFN-γ suppresses Th2 immunity and reverses host resistance to nematode parasites (3, 29). We found that PM decreased gut-associated IL-4 (mRNA and protein) and increased both gut-associated and splenic IFN-γ (protein). Increased IFN-γ during challenge infection coincided with decreasing intestinal IL-4 and thus may be a by-product of decreasing antigenic stimulation for Th2 immune responses. Although we did not detect effects of diet on IFN-γ gene expression, it is possible that PM altered other steps in the IFN-γ synthesis pathway such as mRNA translation or posttranslational modification. At day 14 pci, IFN-γ production was significantly higher in spleen and MLN of mice fed 3% protein compared with controls. During the initial 2 weeks pci, a critical time for the infected host to develop Th2 responses, the up-regulated IFN-γ combined with down-regulated IL-4 may have contributed to the lower effector responses during PM. Mechanisms by which PM could up-regulate nonprotective IFN-γ and simultaneously down-regulate protective IL-4 are unknown, but these results suggest that PM alters the balance of Th2/Th1 cytokine responses that influence IgE-dependent effector mechanisms during challenge infection with Hp.
IgE production is an important host response during helminth infections (1), and as expected, serum IgE levels increased substantially in challenge compared with primary infection. However, this nematode-elicited elevation in IgE was absent in the 3% mice which also had decreased numbers of MMC and eosinophils in the gut mucosa. These results reiterate the concept that protective immunity to nematode parasites requires maximal induction of IgE production concomitant with mucosal mastocytosis and gut eosinophilia. The lower MMCP-1 levels, which reflect the secretory capacity of intestinal MMC, likely implicate a functional consequence of the decreased serum IgE detected in 3% mice. Inasmuch as MMC activation and degranulation depend on expression of IgE receptors and acquisition of IgE by mast cells, the lower serum MMCP-1 concentration observed in 3% mice at days 6 and 14 pci may be due to both lower MMC numbers and reduced IgE levels in GALT. Lowered serum MMCP-1 levels accurately indicate the immunosuppressive effects of PM on MMC secretory capacity given that the intestine is the major source of systemically secreted MMCP-1 and that serum levels correlate positively with concentrations in intestinal lumen of nematode-infected mice (30). Lower IgE responses, reduced MMC numbers in situ, and depressed MMC secretion were apparent by day 9 pci in mice fed 3% protein, and these defects occurred before the onset of parasite expulsion (day 14 pci) in the immunocompetent mice fed 24% protein. Thus, prolonged worm survival in mice fed 3% protein may be attributed in part to the reduced IgE-dependent MMC proliferative and secretory responses observed early (<2 weeks pci) after challenge infection.
The selective impact of PM on host immunity was further evidenced in the graded responses to varying levels of dietary protein. Our results suggest that a certain degree of physiological adaptation occurs with marginal malnutrition but that there is a threshold of protein deficiency below which immune function is significantly compromised. Compared with the controls, mice fed 7% protein successfully expelled their parasites and showed intermediate but adequate levels of IgE, MMC numbers, and eosinophilia. These 7% mice adapted to their marginal protein level by increasing caloric intake which may have spared their endogenous proteins (31) for synthesis of immunological proteins and maintenance of the gut mucosal barrier. In contrast, the 3% mice could not ingest enough food to compensate for their low protein intake and as a result exhibited multiple defects in host immunity at both the intestinal and peripheral level. This finding suggests that increased energy intake may have a beneficial impact on host immunity during mild to moderate protein restriction (31). Furthermore, a comparison of immunological outcomes between the 7% and 3% groups may help clarify the importance of Th2 effectors to helminthic immunity. Previous studies in which a single Th2 mechanism was inhibited by neutralizing antibodies (2) or by the deletion of a cytokine-specific gene (25, 32) were inconclusive as to whether IgE, MMC, or eosinophils were functionally important in host resistance but suggested that there are some redundancies in host immune function. Our study provides evidence that the combination of several defective immune defenses synergistically impedes development of host resistance. The 7% mice were capable of eliciting intermediate Th2 effector responses that were apparently effective for rapid parasite expulsion, whereas the 3% mice had lower gut-associated IL-4, higher IFN-γ, and consistently impaired elevations of IgE, MMC, and intestinal eosinophilia which cumulatively may have resulted in their higher parasite burdens during challenge infection.
In summary, our study demonstrated that PM promoted parasite survival by altering cytokine and effector responses in a selective manner depending on the specific tissue compartment, the level of protein restriction and the time point of infection. We found that dietary PM was more detrimental to gut-associated than splenic IL-4 production during the first week pci and this may have been responsible for the subsequent decreased IgE, MMC, and gut eosinophil responses. In addition, we showed that immunocompetence is a sensitive indicator of protein status and that homeostatic mechanisms enabled the host to maintain plasma albumin concentration at the expense of host immune function. Based on our results, we suggest that PM exerts its adverse effects at the following steps along the cascade of immunological responses to a Th2-inducing parasite. Adequate intake of protein is necessary for mRNA expression and protein synthesis of IL-4 in GALT but does not affect IL-5 or IL-10 production which appeared to be normal even in mice with limited synthesis of IL-4. This decreased IL-4 combined with increased IFN-γ contributed to reduced levels of IgE, MMC, and gut eosinophils and in turn to prolonged parasite survival in the malnourished mice. Although some effects attributed to lower IL-4 may be mediated by IL-13, IL-4-independent pathways for Th2 immunity in nematode infection have not been demonstrated consistently (22, 32). That intestinal MMC and eosinophil responses were impaired during PM despite adequate production of IL-5 and IL-10 suggests that dietary protein affects cytokine-independent pathways in the cellular proliferation or trafficking of immune effectors. Finally, the integration of PM into the Hp-mouse system provided evidence that, among the many cytokines and effectors elicited by Hp, the set of IL-4-dependent, IgE-mediated MMC and eosinophil responses in the gut mucosa is more critical to host protection than IL-5, IL-10, peripheral eosinophils, or IgG1. Broadly, we conclude that PM predisposes individuals to acquire higher levels of nematode infection by suppressing specific Th2 immunity at the intestinal level. By advancing the understanding of the immunological mechanisms underlying nematode parasitism in PM, our results reveal immunological targets and nutritional strategies that may help improve the natural abilities of the host immune system to resist gastrointestinal nematode parasites.
Acknowledgments
We thank Farzaneh Jalili for her technical assistance. This research was supported by the Natural Sciences Engineering and Research Council of Canada and by Fonds pour la Formation de Chercheurs et l' Aide à la Recherche, a Quebec provincial funding agency.
Abbreviations
- bwt
body weight
- GALT
gut-associated lymphoid tissues
- HPRT
hypoxanthine phosphoribosyltransferase
- Hp
Heligmosomoides polygyrus
- L3
third-stage larvae
- L4
fourth-stage larvae
- MLN
mesenteric lymph nodes
- MMC
mucosal mast cells
- MMCP-1
MMC protease-1
- PM
protein malnutrition
- ppi
postprimary infection
- pci
postchallenge infection
- Th
T helper
References
- 1.Monroy F G, Enriquez F J. Parasitol Today. 1992;8:49–53. doi: 10.1016/0169-4758(92)90084-f. [DOI] [PubMed] [Google Scholar]
- 2.Urban J F, Katona I M, Paul W E, Finkelman F D. Proc Natl Acad Sci USA. 1991;88:3513–3517. doi: 10.1073/pnas.88.13.5513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Urban J F, Madden K B, Cheever A W, Trotta P P, Katona I M, Finkelman F D. J Immunol. 1993;151:7086–7094. [PubMed] [Google Scholar]
- 4.Keymer A E, Tarlton A E. Parasitology. 1991;103:121–126. doi: 10.1017/s0031182000059369. [DOI] [PubMed] [Google Scholar]
- 5.Slater A F G, Keymer A E. Parasite Immunol. 1988;10:507–522. doi: 10.1111/j.1365-3024.1988.tb00239.x. [DOI] [PubMed] [Google Scholar]
- 6.Boulay M, Scott M E, Conly S L, Stevenson M M, Koski K G. Parasitology. 1998;116:449–462. doi: 10.1017/s0031182098002431. [DOI] [PubMed] [Google Scholar]
- 7.Nakshabendi I M, Obeidat W, Russell R I, Downie S, Smith K, Rennie M J. Am J Physiol. 1995;269:E996–E999. doi: 10.1152/ajpendo.1995.269.6.E996. [DOI] [PubMed] [Google Scholar]
- 8.Wykes L J, Fiorotto M, Burrin D G, Del Rosario M, Frazer M E, Pond W G, Jahoor F. J Nutr. 1996;126:1481–1488. doi: 10.1093/jn/126.5.1481. [DOI] [PubMed] [Google Scholar]
- 9.National Research Council (NRC) Nutrient Requirements of Laboratory Animals. 4th Ed. Washington, DC: National Academy of Sciences; 1995. [Google Scholar]
- 10.Behnke J M, Robinson M. Parasite Immunol. 1985;7:235–253. doi: 10.1111/j.1365-3024.1985.tb00073.x. [DOI] [PubMed] [Google Scholar]
- 11.Shi H N, Scott M E, Stevenson M M, Koski K G. J Nutr. 1998;128:20–27. doi: 10.1093/jn/128.1.20. [DOI] [PubMed] [Google Scholar]
- 12.Svetic A, Madden K B, Zhou X D, Lu P, Katona I M, Finkelman F D, Urban J F, Gause W C. J Immunol. 1993;150:3434–3441. [PubMed] [Google Scholar]
- 13.Finkelman F D, Katona I M, Urban J F, Holmes J, Ohara J, Tung A S, Sample J, Paul W E. J Immunol. 1988;141:2335–2341. [PubMed] [Google Scholar]
- 14.Negrao-Correa D, Adams L S, Bell R G. J Immunol. 1996;157:4037–4044. [PubMed] [Google Scholar]
- 15.Wahid F N, Behnke J M. Parasite Immunol. 1993;15:401–413. doi: 10.1111/j.1365-3024.1993.tb00625.x. [DOI] [PubMed] [Google Scholar]
- 16.Dehlawi M S, Wakelin D, Behnke J M. Parasite Immunol. 1987;9:187–194. doi: 10.1111/j.1365-3024.1987.tb00499.x. [DOI] [PubMed] [Google Scholar]
- 17.Rincon M, Anguita J, Nakamura T, Fikrig E, Flavell R A. J Exp Med. 1997;185:461–469. doi: 10.1084/jem.185.3.461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bradley L M, Yoshimoto K, Swain S L. J Immunol. 1995;155:1713–1724. [PubMed] [Google Scholar]
- 19.Zhang X H, Werner-Favre C, Tang H Y, Brouwers N, Bonnefoy J Y, Zubler R H. J Immunol. 1991;147:3001–3004. [PubMed] [Google Scholar]
- 20.Katona I M, Urban J F, Kang S S, Paul W E, Finkelman F D. J Immunol. 1991;146:4215–4221. [PubMed] [Google Scholar]
- 21.Toru H, Ra C, Nonyama S, Suzuki K, Yata J-I, Nakahata T. Int Immunol. 1996;8:1367–1373. doi: 10.1093/intimm/8.9.1367. [DOI] [PubMed] [Google Scholar]
- 22.Ramaswamy K, Hakimi J, Bell R G. J Exp Med. 1994;180:1793–1803. doi: 10.1084/jem.180.5.1793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Urban J F, Maliszewski C R, Madden K B, Katona I M, Finkelman F D. J Immunol. 1995;154:4675–4684. [PubMed] [Google Scholar]
- 24.Mosmann T R, Sad S. Immunol Today. 1996;17:138–146. doi: 10.1016/0167-5699(96)80606-2. [DOI] [PubMed] [Google Scholar]
- 25.Herndon F J, Kayes S G. J Immunol. 1992;149:3642–3647. [PubMed] [Google Scholar]
- 26.Finkelman F D, Shea-Donohue T, Goldhill J, Sullivan C A, Morris S C, Madden K B, Gause W C, Urban J F. Annu Rev Immunol. 1997;15:505–533. doi: 10.1146/annurev.immunol.15.1.505. [DOI] [PubMed] [Google Scholar]
- 27.Wahid F N, Behnke J M. Int J Parasitol. 1992;22:699–710. doi: 10.1016/0020-7519(92)90118-5. [DOI] [PubMed] [Google Scholar]
- 28.Tanaka T, Hu-Li J, Seder R A, Fazekas de St. Groth B, Paul W E. Proc Natl Acad Sci USA. 1993;90:5914–5918. doi: 10.1073/pnas.90.13.5914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Maggi E, Parronchi P, Manetti R, Simonelli C, Piccinni M-P, Rugiu F S, de Carli M, Ricci M, Romagnani S. J Immunol. 1992;148:2142–2147. [PubMed] [Google Scholar]
- 30.Wastling J M, Scudamore C L, Thornton E M, Newlands G F J, Miller H R P. Immunology. 1997;90:308–313. doi: 10.1046/j.1365-2567.1997.00155.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Waterlow J C. Annu Rev Nutr. 1986;6:495–526. doi: 10.1146/annurev.nu.06.070186.002431. [DOI] [PubMed] [Google Scholar]
- 32.Takamoto M, Ovington K S, Behm C A, Sugane K, Young I G, Matthae K I. Immunology. 1997;90:511–517. doi: 10.1046/j.1365-2567.1997.00208.x. [DOI] [PMC free article] [PubMed] [Google Scholar]