Abstract
In the present study we examined cartilage matrix repair following IL-1-induced matrix depletion. Previous data indicated that, in some cases, chondrocytes can synthesize macromolecules to establish a functional extracellular matrix in response to a matrix-damaging insult or when placed in a three-dimensional environment with inadequate matrix. However, the conditions under which such 'repair' can occur are not entirely clear. Prior studies have shown that chondrocytes in trypsin-depleted young bovine articular cartilage can replenish tissue glycosaminoglycan (GAG) and that the rate of replenishment is relatively uniform throughout the tissue, suggesting that all chondrocytes have similar capacity for repair. In the present study we used the characteristic heterogeneous distribution of matrix depletion in response to IL-1 exposure in order to investigate whether the severity of depletion influenced the rate of GAG replenishment. We used the delayed Gadolinium-Enhanced Magnetic Resonance Imaging of Cartilage (dGEMRIC) method to monitor the spatial and temporal evolution of tissue GAG concentration ([GAG]). For both mild (n=4) and moderate (n=10) IL-1-induced GAG depletion, we observed partial recovery of GAG (80% and 50% of baseline values, respectively) over a 3-week recovery period. During the first 2 weeks of recovery, [GAG] increased homogeneously at 10–15 mg/ml per week. However, during the third week the regions most severely depleted following IL-1 exposure showed negligible [GAG] accumulation, whereas those regions affected the least by IL-1 demonstrated the greatest accumulation. This finding could suggest that the most severely degraded regions do not recover fully, possibly because of more severe collagen damage; this possibility requires further examination.
Keywords: chondrocyte, dGERMIC, glycosaminoglycan, magnetic resonance imaging, regeneration
Introduction
Arthritic diseases are characterized by a progressive loss of extracellular matrix (ECM), which may ultimately lead to frank tissue loss and gross joint damage. The conditions under which chondrocytes can be induced to replenish or repair a depleted matrix (and thereby prevent or slow the disease process) remain unclear. If these conditions were known, then in principal one could design a therapeutic strategy that takes into account the capacity (or absence thereof) for chondrocytes to repair their matrix naturally.
A number of model systems have provided some insights. For instance, IL-1-degraded cartilage tissues and cell suspensions are frequently employed as model systems to study cartilage metabolism under osteoarthritis-like conditions [1-4] and to assess the efficacy of various drug therapies [5-9]. IL-1 signals cells via cell surface receptors to upregulate proteases, matrix metalloproteinases and aggrecanase, while downregulating matrix metalloproteinase inhibition and proteoglycan synthesis [10,11]. The ultimate effect of these metabolic changes include damage to the ECM, loss of ECM components (especially proteoglycan), and alteration of the normal balance of catabolic and anabolic processes that regulate cartilage ECM composition.
With regard to the repair phase, following IL-1-induced cartilage damage, relatively little is known, but partial to complete recovery appears possible [1,3]. In studies of repair, investigators focused on glycosaminoglycan (GAG) – an important and abundant cartilage macromolecule - and used histologic, biochemical, and/or radiolabel incorporation methods to measure GAG concentration ([GAG]) and/or GAG synthesis rates at selected time points. Although these data suggest that repair might be possible, our understanding is limited because of the uncertainty in the temporal changes in matrix composition for a given sample. This uncertainty is a direct consequence of the inherently destructive nature of histologic and biochemical methods; these techniques therefore do not permit monitoring of the spatial and temporal evolution of matrix macromolecules within individual samples/animals. Having to infer temporal spatial information from a cross-section of samples confounds efforts to conduct a detailed analysis of responses in these model systems.
The delayed Gadolinium-Enhanced Magnetic Resonance Imaging of Cartilage (dGEMRIC) method overcomes these problems in that it is nondestructive and can be used to assess quantitatively the spatial distribution of cartilage [GAG] [12,13]. We and others previously reported on the use of dGEMRIC to monitor GAG regeneration in living cartilage tissue over time in trypsin-depleted bovine cartilage [14] and in cell-laden polymer matrices (tissue engineered cartilage) [15]. Those studies clearly demonstrated that dGEMRIC is feasible for long-term [GAG] monitoring, and that its sensitivity is sufficient to permit observation of the spatiotemporal evolution of [GAG]. Each of those studies began with a homogeneously proteoglycan-deficient matrix, in which GAG was replenished over a 5- to 8-week period to levels considered to be within the normal range.
In young bovine articular cartilage (a tissue that many investigators have used to investigate the impact of physical and biochemical factors on ECM metabolism [14,16-21]), IL-1 induces a characteristic spatially heterogeneous depletion of GAG. This young tissue still contains blood vessels, and it is in the perivascular regions that IL-1-induced GAG depletion is most severe. We know from studies investigating the recovery of trypsin-induced homogeneous GAG depletion in young bovine cartilage that the inherent ability of chondrocytes to replenish the matrix is relatively uniform [14]. In the present study, we take advantage of the characteristic spatial heterogeneity of IL-1-induced GAG depletion to study the relative replenishment rates between regions of varying severity of GAG depletion. Accordingly, our goal was to use dGEMRIC to determine whether bovine cartilage explants briefly exposed to IL-1 would recover GAG at a uniform rate, or at a rate that depended on the degree of IL-1-induced GAG depletion. Specifically, we sought to examine recovery after relatively mild and modest degradation – conditions that did not induce a complete loss of tissue GAG.
Method
Culture and degradation protocols
Cartilage–bone cores of 5mm or 7mm in diameter were harvested from young bovine femoropatellar groove articular cartilage within 24 hours of slaughter. After removing the articular surface, three or four 1-mm-thick discs were sliced parallel to the articular surface. A flat edge was made on some of the discs to ease orientation and registration during imaging and analysis. The discs were weighed, and then immediately placed in 2ml sterile culture media in 24-well culture plates. All explants were incubated at 37°C throughout the study.
Culture medium was prepared with low-glucose Dulbecco's modified Eagle medium with 10mmol/l HEPES (GIBCO BRL, Grand Island, NY, USA), 0.1mmol/l nonessential amino acids (Sigma Chemical, St. Louis, MO, USA), 0.4 mmol/l L-proline (Sigma Chemical), and 1mmol/l gadolinium-DTPA (Magnevist, Berlex Imaging, Wayne, NJ, USA). Supplements including: 1% fetal calf serum (GIBCO BRL, Grand Island, NY), 1% l-glutamine (Sigma Chemical), 1% ascorbic acid (Sigma Chemical) and 1% penicillin and streptomycin (Sigma Chemical) were added immediately prior to use. Media was was collected daily and analyzed for GAG release by the dimethylmethylene blue (DMMB) assay using purified shark chondroitin sulfate (Sigma Chemical) as the reference standard.
All samples were cultured in basal media for at least 3 days after harvest. To create samples that have heterogeneously-degraded ECM, samples were exposed to IL-1β (Cistron Biotechnology, Pine Brooks, NJ, USA), which was added each day to the culture medium. Two series of experiments were conducted, each with its own set of controls. In the first series, samples were exposed to 10 ng/ml IL-1 for 3 and 6 days (incurring 'mild' degradation; n=4). The addition of IL-1 to the media began at days 6 and 3, respectively, after harvest, such that all samples began the 'recovery phase' on day 9 after harvest. Control samples were cultured in basal media for the entire experiment. In the second series, samples were exposed to 20 ng/ml IL-1 (n=10) for 6 and 9 days and incurred 'moderate' degradation. In this group the addition of IL-1 addition to the media for all samples started at 3 days after harvest, and therefore samples began the recovery phase at day 9 or 12 after harvest. Again, control samples were cultured in basal media for the entire experiment. Control samples are referred to as 'mild controls' or 'moderate controls', according to the IL-1 series with which they were cultured (n=2 and 2).
Following IL-1 exposure, the samples were transferred to sterile flat-bottomed 10mm nuclear magnetic resonance (NMR) tubes (Wilbur Scientific, Boston, MA, USA) that were custom cut to a length of 5cm, where they were cultured in basal media for the duration of the recovery experiment.
In summary, the experiment comprised three periods: an initial period (before any exposure to IL-1), an exposure period (during which tissue was exposed to IL-1 in preparation for the recovery phase), followed by a recovery period (during which there was no IL-1 present).
Imaging protocols
For imaging, the shortened NMR/culture tubes were joined to full-length NMR tubes via a sterilized rubber stopper inserted into the open ends of both tubes.
All images were acquired on a Bruker 8.45 T magnetic resonance microimaging system (Bruker Instruments, Billerica, MA, USA) with a standard 10mm radiofrequency coil. T1-weighted images in the axial orientation with respect to explant cylindrical geometry were measured weekly post-IL-1 exposure with either an inversion recovery sequence ('mild' series) or a saturation recovery sequence ('moderate' series). The saturation recovery protocol consisted of 10 T1-weighted images measured with time-to-repeat times of 25, 75, 125, 175, 275, 375, 475, 600, 900, and 1800ms. For inversion recovery measurements, images were acquired with nine inversion delays of 16.7, 33.3, 50, 66.7, 100, 150, 250, 400, and 600ms. Both pulse sequences used a time-to-echo of 8.5ms, section thicknesses of 0.5mm, in-plane resolutions of 100μm, and two averages, for a total imaging time of less than 1 hour per sample.
Analysis of GAG release throughout these experiments suggested that, within the range of sensitivity provided by our dimethylmethylene blue assay, GAG release patterns were unaffected by removal from incubation at 37°C to room temperature for 3–6 hours of imaging each week (because all the plugs were out of the incubator for the imaging session).
Tissue T1s without contrast agent were determined spectroscopically using an inversion delay pulse sequence with 12 delays ranging from 0.2 to 10s and a 10mm broadband radiofrequency probe. At the conclusion of each experimental series, two samples (one control and one treated) were equilibrated in gadolinium-free medium, then extracted from media and blotted dry, and placed in an NMR tube. As had previously been observed, we found very little difference in T1 between samples of the same series (<5%) or across series (<10%) [12]. Therefore, the T1 times in the absence of contrast agent of samples from the same series were averaged and these averages used as the reference tissue T1 for all other samples within the same experiment series.
Image processing
MATLAB (The Math Works, Natick, MA, USA) was used to create a T1 map by curve-fitting each T1-weighted image series on a voxel-by-voxel basis. T1 maps were then processed into GAG maps with MATLAB using equations derived from a modified ideal Donnan theory. This dGEMRIC method of relating measured T1 and cartilage [GAG] was previously validated and reported [12,13,22].
The mean [GAG] for a sample at a given time point was computed as the mean [GAG] calculated across all cartilage-containing pixels of the image. The rate of [GAG] accumulation (i.e. the tissue's recovery rate) was calculated as the difference in the mean [GAG] values at specified time points divided by the elapsed time.
As expected, qualitative assessment of images from samples exposed to IL-1 exhibited the characteristic heterogeneity in degree of GAG depletion. In order to quantitate objectively the time course of GAG recovery relative to the degree of GAG depletion, GAG maps were registered using Adobe Photoshop (Adobe Systems, Inc, San Jose, CA, USA) in order to allow chosen regions of interest to be automatically analyzed across multiple images from successive imaging sessions. Registered images were segmented so that tissue regions of relatively high, medium, or low [GAG] were identified in images taken after 3 weeks of recovery. The [GAG] and location of these pixels were tracked in time. High, medium, and low [GAG] regions of the 3-week images were discerned according to the following definitions:
Pixel assigned to 'high' GAG region if:
[GAG]pixel > (mean [GAG]all pixels + 0.5 × SDall pixels)
Pixel assigned to 'low' GAG region if:
[GAG]pixel < (mean [GAG]all pixels - 0.5 × SDall pixels)
Pixel assigned to 'medium' GAG region if not assigned to high or low region
Statistical analysis
Magnetic resonance imaging derived group mean [GAG] changes were assessed using repeated-measures oneway analysis of variance with a compound symmetry variance structure using SAS (SAS Institute Inc., Cary, NC, USA) to test the hypothesis that mean [GAG] in a given sample or regions of a given sample did not change with time. This technique analyzed the significance of time as an effect on weekly [GAG] measurements (or weekly changes in [GAG]) taken from the same samples (or same region of a sample) each week. Paired two-sample Student's t-tests (Microsoft Excel) were used to determine the degree of [GAG] recovery observed with respect to initial [GAG], before exposure to IL-1.
Results
Glycosaminoglycan release into media
The release of GAG into the media was measured daily as a surrogate for monitoring the effect of IL-1 exposure and of ECM stability following IL-1 withdrawal. Control samples from each series had a small rate of release throughout (0.4 ± 0.2 μg/mg initial wet-weight/day), except for a slightly higher release rate (0.7±0.2μg/mg initial wet-weight/day) during the first 2–3 days after harvest. Assuming an initial [GAG] of approximately 5% of wet-weight, this steady-state release rate corresponds to a loss of about 0.6–1%/day.
As expected, during the IL-1 exposure period the exposed samples lost significantly more GAG than did controls, in accordance with the severity of the IL-1 exposure. Those in the 'mild' and 'moderate' groups lost 148±49μg and 433±98μg GAG, respectively, as compared with the 81±5μg and 103±58μg lost during the same period by the control samples. The GAG release rates never dropped to negligible levels during the exposure period, indicating that GAG was not totally depleted from the disks at these exposure levels. Turning to the recovery period, within 1–2 days after cessation of IL-1 exposure the GAG release rates dropped to levels comparable with those of control samples. GAG release persisted at this level (0.3±0.1 and 0.5±0.3μg/mg initial wet-weight/day for 'mild' and 'moderate' groups, respectively) throughout the remainder of the study.
Tissue glycosaminoglycan concentration over time
Use of dGEMRIC allowed for the actual [GAG] in the tissue to be followed over time. The images of the control samples showed relatively stable [GAG] over the culture period (Fig. 1a,1c), with the coefficient of variation (SD/mean over time) for 'bulk' GAG (i.e. GAG averaged over the entire cartilage image slice) ranging from 2% to 12%. One can also see that the bulk [GAG] was considerably different for the 'mild' and 'moderate' control samples. This is probably a reflection of differences between the animals used for each series, a conclusion supported by considering measurements made before IL-1 exposure on a subset of samples (92±11mg/ml for the 'mild' series and 57±6mg/ml for the 'moderate' series; n=6 for each). To provide a relative reference, the mean and SD of untreated samples are represented by the shaded regions in Fig. 2a,2b.
Figure 1.
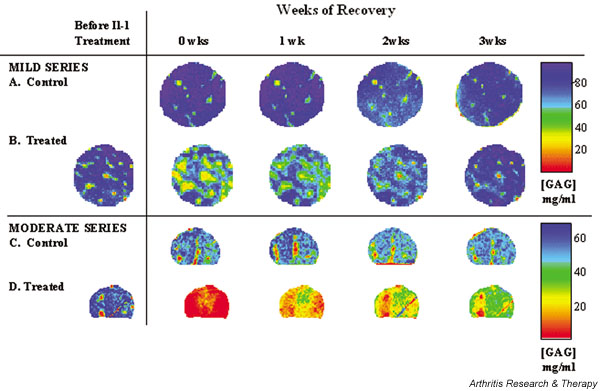
Representative glycosaminoglycan (GAG) map series derived from T1 maps measured on successive weeks. Initial GAG concentration ([GAG]) was substantially different for the two animals (one animal/series), and therefore each series is shown on its own color-scale. (A and C) Control [GAG] is stable (COV varied by ± 2–12%) throughout the recovery period for both series. (B and D) At the beginning of the recovery period (week 0), [GAG] for IL-1-exposed samples is lower than the initial [GAG] and steadily increases over the 3-week recovery period.
Figure 2.
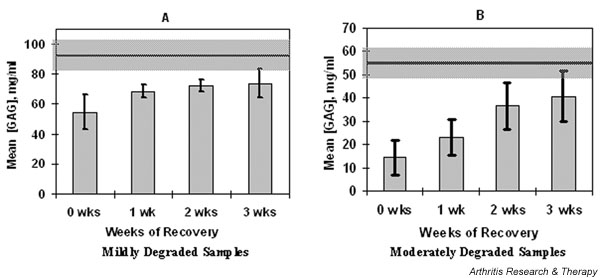
Mean glycosaminoglycan concentration ([GAG]) in IL-1-exposed samples measured at weekly intervals using delayed gadolinium enhanced magnetic resonance imaging of cartilage (dGEMRIC). The mean [GAG] increase with recovery time for samples subjected to (A) mild (3 and 6 days of 10 ng/ml IL-1, n = 4; P = 0.06) or (B) moderate (6 and 9 days of 20 ng/ml IL-1, n = 10; P = 0.0001) degradation, and then permitted to recover for 3 weeks in culture. The mean [GAG] for a given sample at a given time point was computed as the mean of [GAG] measured across all pixels of the image; error bars are ± SD between sample means. Shaded regions represent initial [GAG]; 92 ± 11 mg/ml for 'mild' series and 57 ± 6 mg/ml for 'moderate' series.
Images of the exposed samples acquired immediately following cessation of IL-1 revealed a much lower [GAG] than the initial values, and the [GAG] in exposed samples increased with time of recovery (Figs 1 and 2). Over the 3-week recovery period following IL-1 exposure, [GAG] increased in all samples exposed to IL-1 (Fig. 1b,1d and Fig. 2, n = 14; P < 0.0001). In the 'mild' group the [GAG] increased by 19 ± 5 mg/ml (n = 4; P = 0.06) whereas in the 'moderate' group [GAG] increased by 26 ± 11 mg/ml (n = 10; P < 0.0001).
Given the variation in initial [GAG], we evaluated the percentage degree of recovery only for those samples for which we obtained an 'initial' image before any exposure to IL-1 (n=4 'mild' and n=4 'moderate'). In these cases the [GAG] after 3 weeks of recovery did not reach the initial levels. The [GAG] in the 'mild' group reached 77 ± 19% of initial [GAG] (n=4; P=0.019) and the 'moderate' group reached 49 ± 11% of initial [GAG] (n=4; P= 0.00003).
Regional analysis of glycosaminoglycan concentration recovery
The mean rate of [GAG] recovery (increase in [GAG]/time) averaged across all pixels of IL-1-degraded samples remained steady throughout 3 weeks of post-IL-1-exposure culture at a rate of 1–2 mg/ml per day (1.2±0.9mg/ml per day). However, looking specifically at the spatial distribution of [GAG], considerable differences in weekly [GAG] recovery are clearly evident across different regions of the same samples.
In both the 'mild' and 'moderate' groups, heterogeneous degradation patterns prevailed, with greatest degradation occurring in perivascular regions (Fig. 1c,1d), although the degradation in the 'moderate' group was more severe and homogeneous than in the 'mild' group (the control images and abundant experience with this model system supports the implicit assumption here that the initial [before IL-1] distribution of GAG was homogeneous).
From a qualitative examination of the recovery images, it can also be appreciated that the regions with relatively low [GAG] after 3 weeks are also the regions that had relatively low [GAG] at the beginning of the recovery period, immediately after IL-1 exposure (Figs 1 and 3).
Figure 3.
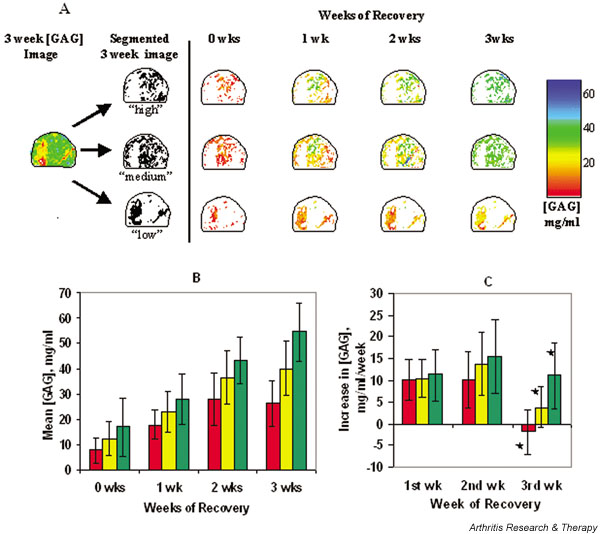
(A) Example of regional analysis scheme. For samples in the 'moderate' group, glycosaminoglycan concentration ([GAG]) maps measured after 3 weeks of recovery were segmented into 'low', 'medium', and 'high' regions, as specified in the Methods section under Image processing (so that the set of pixels defined as 'low' represented the regions of tissue that recovered the least during the 3-week recovery period and the set defined as 'high' represented tissue that recovered the most). The mean [GAG] of these three regions were followed in time. At each time point, segmented images were analyzed separately to assess whether GAG contents and recovery rates were comparable. (B) Weekly mean [GAG] ± SD of regions defined as 'low' (red), 'medium' (yellow), or 'high' (green), according to the process illustrated in panel A. (C) Weekly changes in mean [GAG] ± SD are shown for each region. Rate of [GAG] recovery is independent of absolute [GAG] for the first 2 weeks of culture after IL-1 exposure. 'Low', 'medium', and 'high' GAG regions recover at statistically different rates during the third week following IL-1 exposure (* P < 0.0001). All mean [GAG] values and recovery rates are derived from a total of 10 samples.
To examine this observation more quantitatively and to assess whether the rates of GAG accumulation were correspondingly heterogeneous, we examined separately the [GAG] accumulation in three 'regions', namely those with 'high', 'medium' and 'low' [GAG] at week 3 (as specified above, under Image processing), which is illustrated for one sample in Fig. 3a. Interestingly, regions of 'low' [GAG] appeared to recover at the same rate as did regions of 'medium' or 'high' [GAG] during the first 2 weeks of post-IL-1-exposure culture. In the first 2 weeks of recovery, all regions recovered at a rate of 10–15mg/ml per week. During the third week the recovery patterns for the three regions differed significantly (P<0.001), with the 'low' regions showing negligible [GAG] accumulation and the 'high' regions the greatest accumulation (Fig. 3a,3c).
Discussion
The present study clearly demonstrates that bovine cartilage explants can, at least partially, recover from IL-1-induced degradation by synthesizing new GAG but that the ultimate rate of recovery may be dependent on the degree of initial depletion. By monitoring the spatially localized changes in [GAG] over a 3-week recovery period, we showed that [GAG] increases significantly with time in post-IL-1-exposure culture, with the early recovery (first 2 weeks) being independent of absolute [GAG] but the later recovery (third week) occurring only in regions with higher [GAG].
With respect to the spatial heterogeneity in recovery rates, we are not aware of any histologic (or other) data describing the apparent dependence of the rate of [GAG] replenishment on the initial state of the ECM. We previously showed that spontaneous recovery from complete trypsin-induced GAG loss occurs uniformly throughout the tissue, showing no significant spatial heterogeneity in recovery rates and nearly complete recovery to the initial state in approximately 5 weeks [14]. Those data suggest that the capacity for cells to synthesize new matrix is uniform throughout the tissue. Thus, the differential response seen here is presumably due to the state of the ECM immediately after IL-1 exposure.
Here, we consider [GAG] as a surrogate for defining the ECM state immediately following IL-1 exposure. [GAG] itself is one direct measure of ECM state. In the context of the present study, [GAG] might also serve as a surrogate for the state of other ECM macromolecules, such as collagen. Given the broad spectrum of IL-1-induced enzymes, we consider that regions of the tissue where [GAG] is more severely affected by IL-1 may also be regions that experience greater damage to the collagen network, as compared with regions that are more resistant to the effects of IL-1. Our finding that the regions that experienced the least recovery were those most severely affected by IL-1 could be a consequence of these same regions sustaining more significant damage to the ECM network. This conclusion is consistent with findings regarding IL-1-induced degradation in rabbits, in which recovery rates decreased with apparent severity of degradation [1].
Interestingly, it is in the latter phase of recovery that a discrepancy in recovery rates becomes evident. During the early phases of recovery, the rate of [GAG] accumulation in the regions with the lowest [GAG] was comparable to the rates of recovery in regions with the highest [GAG]. The mechanism for this heterogeneity is unclear. It could be a manifestation of a corresponding distribution of IL-1-induced changes in chondrocyte metabolism or viability [23], or it could be a manifestation of a corresponding distribution of damage to the collagen scaffold, which in turn limits the ability to replenish [GAG]. Kruijsen et al. [24] showed that both the severity and chronicity of antigen-induced inflammation determined the degree of chondrocyte killing in their in vivo murine model of arthritis. Their studies showed that chondrocyte death was most highly correlated with the degree of joint inflammation present 14 days after arthritis induction. That finding suggests that sustained exposure to IL-1, a proinflammatory agent, may also cause chondrocyte death. However, the fact that the early recovery phase in the present study showed no heterogeneity makes cell death a less likely cause of limited [GAG] replenishment.
A limitation (and obvious next step) to this study is that we do not have independent information about the integrity of the collagen matrix. Magnetic resonance imaging techniques are actively being developed to image the collagen component of tissue, which can be incorporated into future studies [25]. Human osteoarthritic tissue is, by histologic measures, spatially heterogeneous in both collagen damage and degree of proteoglycan depletion. The notion that the differential ability to replenish GAG fully is related to the differential state of the collagen matrix has been suggested by others, based in part on the finding that GAG depletion corresponds with regions that are positive for the col3/4 epitope, which is indicative of collagen damage [6,26]. Although we lack specific information on the state of the collagen matrix, the differences observed in the present study (and, indeed, the demonstrated ability to evaluate regional differences in response) suggest that dGEMRIC and model systems such as these may be useful for establishing a better understanding of the capacity of cartilage to repair osteoarthritis-like degradation.
The rate of GAG replenishment observed in this study compares well with published synthesis data. Using sulfate incorporation over periods of less than 24 hours, we and others have reported sulfate incorporation ratios between 0.06 and 0.13nmol/mg wet-weight/hour for young bovine cartilage [27-29]. These incorporation rates imply GAG synthesis rates of 6–13 mg/ml tissue water per week (assuming 1 sulfate per disaccharide, 502 g/mol disaccaride, and 0.8ml tissue water/g wet-weight). By comparison, we observed GAG accumulation rates of 4–14mg/ml per week, as inferred from the change in [GAG]. These values also compare well with the 2–7.3mg/ml per week rates of GAG replenishment seen in young bovine cartilage explants recovering from trypsin-induced GAG depletion [14]. Comparison of these GAG accumulation rates with the rate of GAG release into the culture medium clearly suggests that at least 75% of the newly synthesized GAG is retained by the tissue. (By contrast, in control tissue the amount of GAG synthesized is roughly equivalent to the amount released into the medium.)
We do not have the ability to determine the regional variations in synthesis and loss. Although we clearly observed regional variations in [GAG] accumulation, it is important to appreciate that these differences could arise by regional differences in synthesis or in loss, or both.
Looking more generally at attempts to evaluate [GAG] recovery in IL-1-degraded cartilage, our data are consistent with the temporal progression seen in other model systems in which recovery occurs and is measurable within the first few weeks after a [GAG]-depleting intervention. For example, Takegami et al. [9] reported [GAG] recovery in alginate cultures of human intervertebral disc cells pre-exposed to 0.5ng/ml IL-1 for 3 days. During the first 2 weeks of post-IL-1-exposure culture, those investigators observed [GAG] recovery rates of approximately 4%/day with very little change in [GAG] observed during the third week, when [GAG] levels reached about 85% of the control level. In an in vivo rabbit knee joint subjected to intra-articular injections of IL-1, Page Thomas et al. [3] used SO4 uptake and toluidine blue staining to observe GAG losses of 25–60% in several cartilage sites within the knee, with gradual recovery over the subsequent 3–4 weeks. Arner [1] also examined in vivo GAG synthesis and accumulation in rabbits following intra-articular injections of IL-1. Using dimethylmethylene blue assay and sulfate incorporation, Arner found that both single and multiple injections of IL-1 led to an initial depression in GAG synthesis rate and a slight drop in tissue [GAG] for 4 days after IL-1 exposure. These changes were followed by enhanced synthesis (relative to controls), with a commensurate increase in tissue [GAG] over the subsequent 2 weeks as the content of GAG in the tissue approached 90% of control levels. In those in vivo systems it appears that GAG loss continues for 4–7 days after IL-1 exposure [1]. This is longer than the 1–2 days seen in the present study (in which GAG loss into the medium returned to control levels), and is probably a consequence of IL-1 clearing more quickly from the in vitro environment.
Thus, in widely different model systems – including that reported here – it appears that after an IL-1-induced GAG-depleting intervention tissue can reaccumulate GAG and does so most rapidly during the first few weeks. However, unlike the study described here, in which both spatial and temporal changes in [GAG] were monitored, in the studies described above it was not possible to derive more specific spatial information because the destructive nature of the [GAG] measurements required that time course information be inferred from averages of separate animals/samples harvested at different time points.
Our data can also be compared with those from other studies in which magnetic resonance methods were used to monitor changes in cartilage tissue during culture. Our group previously observed spatially uniform recovery of explants following trypsin-induced GAG depletion using dGEMRIC, with the increases in [GAG] occurring most rapidly during the first week and slowing considerably after 3 weeks [14]. Williams et al. [15] used the same method to monitor GAG accumulation in tissue engineered cartilage over 6 weeks, and observed relatively steady GAG accumulation over the entire period, with the bulk of the accumulation occurring at the periphery of the explant. The initial state of the cell/polymer construct was presumably uniform, and the heterogeneous [GAG] accumulation was attributed to differences in the biophysical environment. Potter et al. [30] observed the growth of tissue engineered over a period of 4 weeks using proton NMR without any additional contrast agent. The relative changes in T1 and T2 times of those studies tracked the histologic finding that GAG increased for the first 3 weeks and then remained relatively constant. Collectively, those studies and the present one illustrate spatial and temporal variations in GAG accumulation in native, treated, and tissue engineered cartilage. Much work, of course, remains if we are to begin to understand the biochemical, biophysical, and structural factors that underlie the differential behavior. Furthermore, much work remains to determine the generalizability of the behavior in these model systems involving young tissue to behavior of cartilage in vivo in older humans.
Conclusion
In the present study we demonstrated that chondrocytes, in a matrix degraded by IL-1 exposure, partially replenished the GAG, and the most severely degraded regions replenished less fully than did other regions. Future studies are underway to examine whether heterogeneity in replenishment rate is seen in human osteoarthritic tissue. The study provides additional evidence that the in vitro dGEMRIC method is a practical means for studying GAG homeostasis and events that disturb it. Ultimately, such studies provide the foundation for evaluating the effects of therapeutic interventions on [GAG] degradation or regeneration in vitro or in vivo [13].
Competing interests
None declared.
Abbreviations
dGEMRIC = delayed gadolinium enhanced magnetic resonance imaging of cartilage; ECM = extracellular matrix; GAG = glycosaminoglycan; IL = interleukin; NMR = nuclear magnetic resonance.
Acknowledgments
Acknowledgements
The authors gratefully acknowledge the generous MATLAB coding assistance of Joseph Samosky. Funding was provided in part by the NIH, grant #AR42773; NIH Shared Instrumentation, grant #RR14792; and the Edwin Hood Taplin professorship.
References
- Arner EC. Effect of animal age and chronicity of interleukin-1 exposure on cartilage proteoglycan depletion in vivo. J Orthop Res. 1994;12:321–330. doi: 10.1002/jor.1100120304. [DOI] [PubMed] [Google Scholar]
- Beekman B, Verzijl N, de Roos JA, TeKoppele JM. Matrix degradation by chondrocytes cultured in alginate: IL-1 beta induces proteoglycan degradation and proMMP synthesis but does not result in collagen degradation. Osteoarthritis Cartilage. 1998;6:330–340. doi: 10.1053/joca.1998.0132. [DOI] [PubMed] [Google Scholar]
- Page Thomas DP, King B, Stephens T, Dingle JT. In vivo studies of cartilage regeneration after damage induced by catabolin/interleukin-1. Ann Rheum Dis. 1991;50:75–80. doi: 10.1136/ard.50.2.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patwari P, Kurz B, Sandy JD, Grodzinsky AJ. Mannosamine inhibits aggrecanase-mediated changes in the physical properties and biochemical composition of articular cartilage. Arch Biochem Biophys. 2000;374:79–85. doi: 10.1006/abbi.1999.1538. [DOI] [PubMed] [Google Scholar]
- Badger AM, Cook MN, Swift BA, Newman-Tarr TM, Gowen M, Lark M. Inhibition of interleukin-1-induced proteoglycan degradation and nitric oxide production in bovine articular cartilage/chondrocyte cultures by the natural product, hymenialdisine. J Pharmacol Exp Ther. 1999;290:587–593. [PubMed] [Google Scholar]
- Billinghurst RC, Wu W, Ionescu M, Reiner A, Dahlberg L, Chen J, van Wart H, Poole AR. Comparison of the degradation of type II collagen and proteoglycan in nasal and articular cartilages induced by interleukin-1 and the selective inhibition of type II collagen cleavage by collagenase. Arthritis Rheum. 2000;43:664–672. doi: 10.1002/1529-0131(200003)43:3<664::AID-ANR24>3.0.CO;2-D. [DOI] [PubMed] [Google Scholar]
- McCarty MF, Russell AL. Niacinamide therapy for osteoarthritis: does it inhibit nitric oxide synthase induction by interleukin 1 in chondrocytes? Med Hypotheses. 1999;53:350–360. doi: 10.1054/mehy.1998.0792. [DOI] [PubMed] [Google Scholar]
- Caron JP, Fernandes JC, Martel-Pelletier J, Tardif G, Mineau F, Geng C, Pelletier JP. Chondroprotective effect of intraarticular injections of interleukin-1 receptor antagonist in experimental osteoarthritis. Suppression of collagenase-1 expression. Arthritis Rheum. 1996;39:1535–1544. doi: 10.1002/art.1780390914. [DOI] [PubMed] [Google Scholar]
- Takegami K, Masuda K, An H, Kamada H, Peitryla D, Thonar I. The effect of osteogenic protein-1 on intervertebral disc cells pre-exposed to interleukin-1. 46th Annual Meeting Proceedings of the Orthopaedic Research Society. Transactions of the Orthopaedic Research Society, Orlando, FL. 2000;25:338. [Google Scholar]
- Smith RL. Degradative enzymes in osteoarthritis. Front Biosci. 1999;4:D704–712. doi: 10.2741/a388. [DOI] [PubMed] [Google Scholar]
- Malemud C, Hering T. Regulation of the chondrocytes in osteoarthritis. In: Adolphe M Boca Raton, editor. In Biological Regulation of the Chondrocytes. CRC Press, Inc; 1992. pp. 295–320. [Google Scholar]
- Bashir A, Gray ML, Burstein D. Gd-DTPA2- as a measure of cartilage degradation. Magn Reson Med. 1996;36:665–673. doi: 10.1002/mrm.1910360504. [DOI] [PubMed] [Google Scholar]
- Bashir A, Gray ML, Hartke J, Burstein D. Nondestructive imaging of human cartilage glycosaminoglycan concentration by MRI. Magn Reson Med. 1999;41:857–865. doi: 10.1002/(sici)1522-2594(199905)41:5<857::aid-mrm1>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- Allen RG, Burstein D, Gray ML. Monitoring glycosaminoglycan replenishment in cartilage explants with gadolinium-enhanced magnetic resonance imaging. J Orthop Res. 1999;17:430–436. doi: 10.1002/jor.1100170320. [DOI] [PubMed] [Google Scholar]
- Williams S, Obradovic B, Burstien D, Freed L, Gray M, Langer R, Vunjak-Novakovic G. MRI measurements of fixed charge density as a measure of glycosminoglycan content and distribution in tissue engineered cartilage. 44th Annual Meeting Proceedings, Orthopaedic Research Society. Transactions of the Orthopaedic Research Society, New Orleans, LA. 1998;23:203. [Google Scholar]
- Bonassar LJ, Grodzinsky AJ, Frank EH, Davila SG, Bhaktav NR, Trippel SB. The effect of dynamic compression on the response of articular cartilage to insulin-like growth factor-I. J Orthop Res. 2001;19:11–17. doi: 10.1016/S0736-0266(00)00004-8. [DOI] [PubMed] [Google Scholar]
- Ragan PM, Badger AM, Cook M, Chin VI, Gowen M, Grodzinsky AJ, Lark MW. Down-regulation of chondrocyte aggrecan and type-II collagen gene expression correlates with increases in static compression magnitude and duration. J Orthop Res. 1999;17:836–842. doi: 10.1002/jor.1100170608. [DOI] [PubMed] [Google Scholar]
- Larsen NE, Lombard KM, Parent EG, Balazs EA. Effect of hylan on cartilage and chondrocyte cultures. J Orthop Res. 1992;10:23–32. doi: 10.1002/jor.1100100104. [DOI] [PubMed] [Google Scholar]
- Sah RL, Kim YJ, Doong JY, Grodzinsky AJ, Plaas AH, Sandy JD. Biosynthetic response of cartilage explants to dynamic compression. J Orthop Res. 1989;7:619–636. doi: 10.1002/jor.1100070502. [DOI] [PubMed] [Google Scholar]
- Schafer SJ, Luyten FP, Yanagishita M, Reddi AH. Proteoglycan metabolism is age related and modulated by isoforms of platelet-derived growth factor in bovine articular cartilage explant cultures. Arch Biochem Biophys. 1993;302:431–438. doi: 10.1006/abbi.1993.1236. [DOI] [PubMed] [Google Scholar]
- Luyten FP, Yu YM, Yanagishita M, Vukicevic S, Hammonds RG, Reddi AH. Natural bovine osteogenin and recombinant human bone morphogenetic protein-2B are equipotent in the maintenance of proteoglycans in bovine articular cartilage explant cultures. J Biol Chem. 1992;267:3691–3695. [PubMed] [Google Scholar]
- Bashir A, Gray ML, Boutin RD, Burstein D. Glycosaminoglycan in articular cartilage: in vivo assessment with delayed Gd(DTPA)(2-)-enhanced MR imaging. Radiology. 1997;205:551–558. doi: 10.1148/radiology.205.2.9356644. [DOI] [PubMed] [Google Scholar]
- Nerucci F, Fioravanti A, Cicero MR, Collodel G, Marcolongo R. Effects of chondroitin sulfate and interleukin-1beta on human chondrocyte cultures exposed to pressurization: a biochemical and morphological study. Osteoarthritis Cartilage. 2000;8:279–287. doi: 10.1053/joca.1999.0302. [DOI] [PubMed] [Google Scholar]
- Kruijsen MW, van den Berg WB, van de Putte LB. Influence of the severity and duration of murine antigen-induced arthritis on cartilage proteoglycan synthesis and chondrocyte death. Arthritis Rheum. 1985;28:813–819. doi: 10.1002/art.1780280713. [DOI] [PubMed] [Google Scholar]
- Gray ML, Burstein D, Xia Y. Biochemical (and functional) imaging of articular cartilage. Semin Musculoskelet Radiol. 2001;5:329–343. doi: 10.1055/s-2001-19043. [DOI] [PubMed] [Google Scholar]
- Hollander AP, Pidoux I, Reiner A, Rorabeck C, Bourne R, Poole AR. Damage to type II collagen in aging and osteoarthritis starts at the articular surface, originates around chondrocytes, and extends into the cartilage with progressive degeneration. J Clin Invest. 1995;96:2859–2869. doi: 10.1172/JCI118357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sah R-Y. Biophysical Regulation of Matrix Synthesis, Assembly, and Degradation in Dynamically Compressed Calf Cartilage. Cambridge, MA: Massachusetts Institute of Technology. 1990.
- Hascall VC, Handley CJ, McQuillan DJ, Hascall GK, Robinson HC, Lowther DA. The effect of serum on biosynthesis of proteoglycans by bovine articular cartilage in culture. Arch Biochem Biophys. 1983;224:206–223. doi: 10.1016/0003-9861(83)90205-9. [DOI] [PubMed] [Google Scholar]
- Boustany NN, Gray ML, Black AC, Hunziker EB. Correlation between synthetic activity and glycosaminoglycan concentration in epiphyseal cartilage raises questions about the regulatory role of interstitial pH. J Orthop Res. 1995;13:733–739. doi: 10.1002/jor.1100130513. [DOI] [PubMed] [Google Scholar]
- Potter K, Butler JJ, Adams C, Fishbein KW, McFarland EW, Horton WE, Spencer RG. Cartilage formation in a hollow fiber bioreactor studied by proton magnetic resonance microscopy. Matrix Biol. 1998;17:513–523. doi: 10.1016/s0945-053x(98)90099-3. [DOI] [PubMed] [Google Scholar]


