Abstract
Although a role for CD8+ T cells in the pathogenesis of rheumatoid arthritis (RA) has been suggested, the precise nature of their involvement is not fully understood. In the present study we examined the central and effector memory phenotypes of CD4+ and CD8+ T cells in the peripheral blood of patients with RA and systemic lupus erythematosus. Terminally differentiated effector memory CD45RA+CD62L-CD8+ T cells were significantly decreased in RA patients, whereas the central memory CD45RA-CD62L+ CD8+ T-cell population was increased as compared with levels in healthy control individuals. Naïve and preterminally differentiated effector memory CD45RA-CD62L- CD8+ T cells did not differ between RA patients and control individuals. The CD45RA-CD62L+ central memory CD4+ T-cell subpopulation was increased in RA patients, whereas the naïve and effector memory phenotype of CD4+ T cells did not differ between RA patients and control individuals. In patients with systemic lupus erythematosus the distribution of naïve/memory CD4+ and CD8+ T cells did not differ from that in age- and sex-matched control individuals. These findings show that peripheral blood CD8+ T cells from RA patients exhibit a skewed maturation phenotype that suggests a perturbation in the homeostasis of these cells. The central memory CD45RA-CD62L+ CD4+ and CD8+ T-cell numbers were increased in RA, suggesting an accelerated maturation of naïve T cells. The decreased numbers of terminally differentiated CD45RA+CD62L- effector memory CD8+ T cells in peripheral blood of RA patients may reflect increased apoptosis of these cells or enhanced migration of these cells to sites of inflammation, which may play a role in the pathogenesis of RA.
Keywords: CD4, CD8, memory T cells, peripheral blood, rheumatoid arthritis
Introduction
The precise role played by CD8+ T cells in the pathogenesis and inflammation of rheumatoid arthritis (RA) is unclear. In the synovial membrane, the most common IFN-γ-producing cell is the CD8+ T cell, suggesting that this population of T cells plays a major role in macrophage activation and perpetuation of the inflammatory response [1]. CD8+ T cells were recently associated with the presence of germinal centers in RA synovium [2], suggesting a role for CD8+ T cells in the formation or maintenance of those lymphoid structures in the synovium. Further studies indicated that CD8+ T cells exhibit oligoclonality in the peripheral blood [3,4] and synovial fluid of RA patients [5], raising the question of whether this oligoclonality is antigen driven. However, recent studies have indicated that large numbers of virus-specific CD8+ T cells preferentially accumulate in the synovial fluid of RA patients and that these cells are also oligoclonal, suggesting that non-antigen-specific homing may be responsible for the observed oligoclonality of CD8+ T cells in the synovial fluid [6]. Because chemokines such as macrophage inflammatory protein-1α and RANTES (regulated upon activation, normal T-cell expressed and secreted) are expressed in RA synovial tissue [7,8], subsets of CD8+ T cells may be preferentially recruited into the synovial tissue in a non-antigen-specific manner. If the expression of chemokines is also accompanied by a perturbation in CD8+ T-cell homeostasis in the periphery that favors differentiation into cell types that can be recruited into the synovium, then a vicious cycle may be set up in RA in which there is continuous generation of CD8+ T cells that can be recruited into the synovium, resulting in chronic inflammation and joint destruction.
Recently, memory CD8+ T cells were classified into three distinct populations, based on phenotype [9-11]: a central memory population, which is CD45RA-CCR7+CD62L+CD28+IL-2+IFN-γ-; and two effector memory populations, namely the CD45RA-CD62L-CCR7- and the terminally differentiated CD45RA+CD62L-CCR7- populations. The two latter effector memory populations contain perforin, secrete IFN-γ and tumor necrosis factor-α, are cytotoxic, and are capable of rapid effector function after stimulation [9-11].
Although a linear model of differentiation has been suggested for these memory populations (i.e. central memory T cells CD45RA-CCR7+CD62L+ → effector memory T cells CD45RA-CD62L-CCR7- → effector memory T cells CD45RA+CD62L-CCR7- [10]), the exact relationship between these populations is not fully established. Indeed, Champagne et al. [12] suggested that the differentiation may not be linear at all. The central and effector memory phenotypes of CD4+ and CD8+ T cells in peripheral blood of RA patients are unknown. Determination of these phenotypes in RA may provide important insights into T-cell homeostasis, and we therefore examined the distribution of CD4+ and CD8+ T cells into these subpopulations because such a study may reveal differences in the differentiation of T cells in RA patients. Decreases in some of the subpopulations in peripheral blood may indicate that there is a selective migration of these cells out of the peripheral blood, decreased survival of these cells, or blockade in their differentiation. Perturbations in the homeostasis of memory T cells may play an important role in the pathogenesis of RA by generating effector cells that can contribute to the synovial inflammation of RA.
Patients and methods
Patients
Peripheral blood was obtained from patients with RA, systemic lupus erythematosus (SLE), and healthy control individuals following Drexel University Institutional Review Board approval and obtaining informed consent. The RA group consisted of eight patients (seven women, one man) with an age range of 33–63 years (mean 49 years). All patients in the group were receiving disease-modifying antirheumatic drugs and were clinically stable. The SLE group consisted of 12 women with an age range of 22–68 years (mean 45 years) who were clinically stable. All patients in the two groups met the American College of Rheumatology criteria for SLE and RA, respectively. Patient profiles and characteristics are shown in Table 1. Age- and sex-matched healthy control groups were included for the RA and the SLE patient groups (control group for RA: n = 8, age range 32–61 years [mean 50 years]; and control group for SLE: n = 12, age range 22–61 years [mean 46 years]). No statistically significant difference was found between the age of the RA patient group and the corresponding healthy control group (P > 0.9, by Student's t-test), between the age of the SLE patient group and the corresponding healthy control group (P > 0.9, by Student's t-test), and between the RA patient group and the SLE patient group (P > 0.5, by Student's t-test).
Table 1.
Patient profiles and characteristics
| Patient number/sex/age (years) | Disease duration (years) | Therapy | X-ray findings |
| Patients with rheumatoid arthritis | |||
| 1/F/56 | 1 | MTX, steroids | None |
| 2/F/53 | 5 | MTX, Inf, steroids | Erosions |
| 3/F/46 | 4 | MTX | Erosions |
| 4/F/33 | 3 | Hcq, MTX | Erosions |
| 5/M/63 | 3 | Lef | Erosions, osteopenia |
| 6/F/52 | 2 | Lef, steroids | Erosions |
| 7/F/40 | 6 | Etanercept | Erosions |
| 8/F/50 | 6 | Lef | Erosions |
| Patients with systemic lupus erythematosus | |||
| 11/F/68 | 1 | Hcq, steroids | None |
| 12/F/46 | 5 | Hcq | None |
| 13/F/25 | 5 | Hcq | None |
| 14/F/47 | 9 | Hcq, MTX | None |
| 15/F/22 | 5 | Hcq, steroids | Jaccoud's arthropathy |
| 16/F/38 | 6 | Hcq, steroids | None |
| 17/F/46 | 4 | Hcq, steroids | None |
| 18/F/55 | 10 | Hcq, steroids | None |
| 19/F/45 | 3 | Hcq, steroids | None |
| 20/F/61 | 18 | Hcq | None |
| 21/F/35 | 5 | Hcq, MTX, steroids | None |
| 22/F/53 | 8 | Hcq | None |
F, female; Hcq, hydroxychloroquine; Inf, infliximab; Lef, leflunomide; M, male; MTX, methotrexate.
Flow cytometry
Heparinized venous blood from RA patients, SLE patients and healthy control individuals was collected, and peripheral blood mononuclear cells were freshly isolated by Ficoll-Hypaque (Amersham Pharmacia Biotech, Uppsala, Sweden). The following monoclonal antibody combinations were used to characterize the phenotypes of T cells: anti-CD45RA-FITC/anti-CD3-PE/anti-CD62L-CyChrome/ anti-CD4-APC; and anti-CD45RA-FITC/anti-CD3-PE/anti-CD62L-CyChrome/anti-CD8-APC (PharMingen, San Diego, CA, USA). Briefly, 106 peripheral blood mononuclear cells were stained with each combination of antibodies in Hanks buffered saline solution (Cellgro, Herndon, VA, USA), 3% fetal bovine serum, and 0.02% NaN3 for 15 min on ice; washed twice with Hanks buffered saline solution, 3% fetal bovine serum and 0.02% NaN3; and fixed with 1% paraformaldehyde. Analysis was performed on a FACS-Calibur (Becton Dickinson, San Jose, CA, USA) using FlowJo software (TreeStar, San Carlos, CA, USA).
Statistical analysis
Statistical analysis was performed using Mann–Whitney U test, Student's t-test, linear regression, and Shapiro–Wilk W test for normality. P < 0.05 was considered statistically significant. The JMP statistical analysis program was used (SAS, Cary, NC, USA).
Results
Naïve and memory subpopulations of CD4+ and CD8+ T cells from RA and SLE patients were compared with those in healthy control individuals to determine T-cell maturation differences between those groups.
As compared with the healthy control group, RA patients had fewer CD45RA+CD62L+ CD4+ naïve T cells (32 ± 4.8% in RA patients [n = 8] and 42 ± 6.5% in healthy controls [n = 8], respectively), although this difference was not statistically significant (Fig. 1a,1b). The CD45RA-CD62L+ CD4+ central memory T-cell population was significantly increased in RA patients (50 ± 3.7% [n = 8]) as compared with the healthy control group (38 ± 4.4% [n = 8]; P < 0.05; Fig. 1a,1b). No differences were found in the CD45RA-CD62L-CD4+ effector memory population (15 ± 2.2% for RA patients and 18 ± 2.6% for healthy controls [n = 8 each]) or in the terminally differentiated CD45RA+CD62L- CD4+ effector memory population (1.7 ± 0.5% for RA patients and 2.2 ± 0.6% for healthy controls [n = 8 each]; Fig. 1a,1b).
Figure 1.
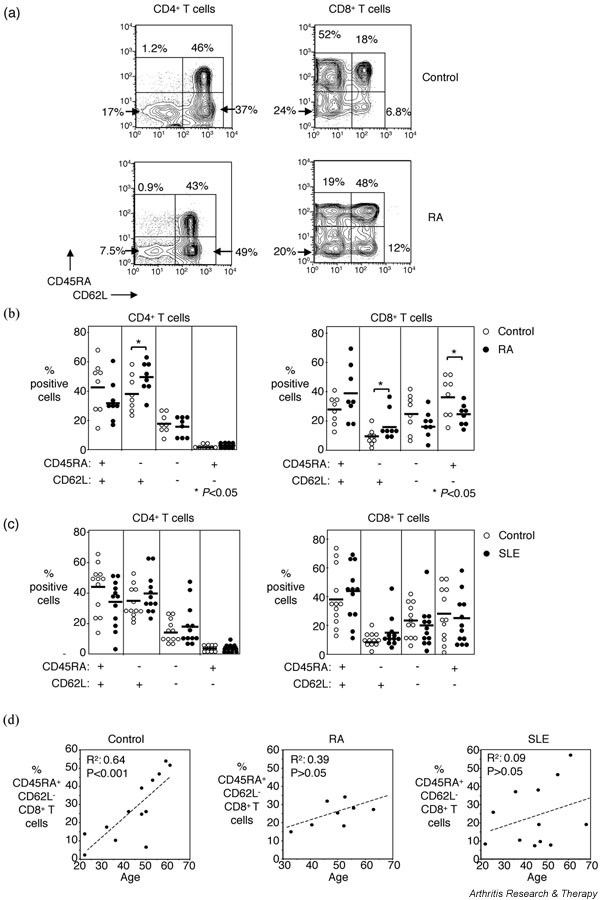
Naïve and memory CD4+ and CD8+ T-cell subpopulations in patients with rheumatoid arthritis (RA), patients with systemic lupus erythematosus (SLE), and healthy control individuals. (a) Representative flow cytometry showing naïve and memory subpopulations of CD4+ and CD8+ T cells from one RA patient and a sex- and age-matched control individual. (b) Pooled data showing naïve and memory subpopulations of CD4+ and CD8+ T cells from RA patients (n = 8) and control individuals (n = 8). Horizontal lines indicate means. (c) Pooled data showing naïve and memory subpopulations of CD4+ and CD8+ T cells from SLE patients (n = 12) and control individuals (n = 12). Horizontal lines indicate means. (d) The correlation between age and CD45RA+CD62L- terminally differentiated CD8+ T cells from control individuals (n = 13), RA patients (n = 8), and SLE patients (n = 12) is shown. The P values were calculated using Mann–Whitney U test and Student's t-test for panel b and linear regression for panel d.
In the CD8+ T-cell population, 39 ± 6.2% were CD45RA+CD62L+ naïve cells for the RA patients and 28 ± 3.4% for the healthy control group (Fig. 1a,1b). The central memory CD45RA-CD62L+ CD8+ T-cell population was significantly increased in RA patients (17 ± 3.5% [n = 8]) as compared with the healthy control group (9 ± 1.8% [n = 8]; P < 0.05; Fig. 1a,1b). No difference was found between patients and healthy control group in the CD45RA-CD62L- CD8+ effector memory populations (18 ± 3.2% for RA patients and 25 ± 4.5% for healthy controls [n = 8]), whereas the CD45RA+CD62L- CD8+ terminally differentiated effector memory population was significantly decreased in RA patients (26 ± 2.4%) as compared with healthy controls (38 ± 4.8% [n = 8]; P < 0.05; Fig. 1a,1b).
No significant differences were found when CD4+ and CD8+ T cells of SLE patients were compared with the CD4+ and CD8+ T cells of matched healthy control individuals (Fig. 1c). In the CD4+ T-cell population, 35 ± 4.6% of cells from SLE patients and 45 ± 4.7% in the healthy controls exhibited a naïve phenotype; the central memory phenotype was expressed by 42 ± 3.8% of the CD4+ T cells from SLE patients (n = 12) and in 37 ± 3.1% of the CD4+ T cells from healthy controls (n = 12). Of the CD4+ T cells, 20 ± 3.6% and 16 ± 2.0% were effector memory cells in the SLE and healthy control groups (n = 12 in each), respectively, and only a very small population of the cells were terminally differentiated effector memory CD4+ T cells in SLE patients (2.4 ± 0.9%) and healthy controls (1.7 ± 0.5%; Fig. 1c). The CD8+ T-cell compartment of SLE patients consisted of 42 ± 5.6% CD45RA+CD62L+ naïve cells, 14 ± 2.9% CD45RA-CD62L+ central memory, 20 ± 4.1% CD45RA-CD62L- effector memory, and 24 ± 4.9% CD45RA+CD62L- terminally differentiated effector memory CD8+ T cells (n = 12; Fig. 1c). In the healthy control group, 39 ± 5.8% CD45RA+CD62L+ naïve cells, 9 ± 1.3% CD45RA-CD62L+ central memory, 23 ± 3.4% CD45RA-CD62L- effector memory, and 29 ± 5.2% CD45RA+CD62L- terminally differentiated effector memory CD8+ T cells were found (n = 12; Fig. 1c).
A positive correlation was found between the age and the percentage of CD45RA+CD62L- terminally differentiated effector memory CD8+ T cells in the healthy control group (r2 = 0.64 [n = 13]; P < 0.001; Fig. 1d), indicating that this effector population increases with age. However, no such correlation was detected in RA and SLE patients (Fig. 1d). Finally, the frequency of CD45RA+CD62L- CD8+ T cells did not correlate with disease duration or treatment in either RA or SLE patients (data not shown).
Discussion
The present study shows that the differentiation of peripheral blood CD8+ T cells is skewed in patients with RA and results in an increase in central memory CD45RA-CD62L+ CD8+ T cells, with a concomitant decrease in terminally differentiated effector memory CD45RA+CD62L-CD8+ T cells. The increase in central memory CD45RA-CD62L+ T cells was also found in the CD4+ T-cell population in RA patients. This skewed differentiation was not observed in healthy age-matched control individuals and in SLE patients, indicating that this perturbation in homeostasis of T cells is a specific feature of RA.
Although the naïve/memory phenotype of T cells has previously been investigated in RA in numerous studies using CD45RA and CD45RO expression as markers of naïve and memory cells, respectively, that approach has suffered from the limitation that large numbers of CD45RA+ CD8+ T cells are actually effector memory cells [10,13]. The CD45RA/CD45RO oversimplification has also resulted in rather confusing conclusions regarding T-cell homeostasis, such as defects in primary T-cell homeostasis based on reduced T-cell receptor excision circle (TREC) levels in naïve CD4+ T cells (defined as CD45RO-) in RA patients [14]. Our findings suggest that reduced TREC levels in the CD45RO- CD4+ T-cell population may not be due to a reduction in TRECs in naïve cells but rather to reduced TRECs in the CD45RA+CD45RO-CD62L- effector memory CD4+ T cells. It should be noted that previous studies have reported 'false naïve' CD45RA+ populations of CD4+ and CD8+ T cells in peripheral blood of RA patients [15]; however, the nature of these cells, the exact phenotype, and the significance was not known at that time.
Our finding that peripheral blood CD8+ T cells exhibit increased central memory phenotype and decreased terminally differentiated effector memory phenotype suggests that the peripheral blood homeostasis of CD8+ T cells is perturbed in RA. Perturbations in CD8+ T-cell maturation have been shown for HIV-specific CD8+ T cells, in which there is an accumulation of preterminally differentiated CD45RA-CD62L- CD8+ T cells [12,16], and such a lack of differentiation may result in functional or homing defects. In RA we found a decrease in terminally differentiated CD45RA+CD62L- CD8+ T cells with a concomitant increase in the CD45RA-CD62L+ central memory population. If one accepts the linear model of differentiation [10], which we note has been challenged [12], then our findings indicate that in RA there may be an accelerated differentiation of naïve cells into central memory CD4+ and CD8+ T cells. This accelerated differentiation may be due to a non-antigen-specific effect in RA that differentiates all peripheral T cells irrespective of their specificity, or it may actually reflect an antigen-specific expansion of T cells potentially driven by autoantigen.
The decrease in CD45RA+CD62L- effector memory CD8+ T cells in peripheral blood we found in RA patients may reflect a decrease in the survival of these cells. It should be noted, however, that peripheral blood T cells from RA patients do not exhibit an increase in apoptosis in in vitro cultures, which is in contrast to synovial membrane T cells [17,18]. This may suggest that the skewed phenotype of the CD45RA+CD62L- effector memory CD8+ T cells is more likely due to an increase in the migration of these cells into sites of inflammation. However, a blockade of the differentiation of central memory CD45RA-CD62L+ CD8+ T cells into effector memory CD8+ T cells would also result in an increase in the central memory population with a concomitant decrease in the effector T cells, as observed in the present study.
Studies of the phenotype of CD8+ T cells in the synovial membrane and fluid may shed light as to whether this skewed phenotype is also found in these sites or whether there is an enrichment for CD45RA+CD62L-CD8+ T cells, indicating increased recruitment into the inflamed synovium in RA. Inflammation and production of chemokines such as macrophage inflammatory protein-1α and RANTES [7,8] in the synovium may result in preferential recruitment of such effector memory CD8+ T cells (which are important contributors to IFN-γ production) and subsequent macrophage activation, because terminally differentiated CD45RA+CD62L- CD8+ T cells have been shown to express higher levels of perforin and may be more potent effector cells [10]. The question arises of whether the observed skewed differentiation of CD8+ T cells in RA patients is due to medication, especially steroids. As shown in Table 1, 38% of the RA patients and 58% of the SLE patients were receiving steroid treatment. However, the skewed memory phenotype was only observed in the RA patients, suggesting that this treatment is not responsible for the differences in CD4+ and CD8+ T-cell phenotypes.
Findings from the present preliminary study show that peripheral blood CD8+ T cells in RA exhibit a skewed effector memory phenotype. This skewed phenotype was not found in CD4+ T cells in RA and was not seen in age-matched healthy control individuals or in SLE patients. The skewed phenotype may be a result of accelerated differentiation and migration into sites of inflammation. An understanding of the mechanisms that are involved in this skewed differentiation of effector memory CD8+ T cells may prove valuable in elucidating the pathogenesis of RA.
Conclusion
In peripheral blood of RA patients a skewed homeostasis of CD8+ T cells was found, with an increase in central memory and a decrease in terminally differentiated effector memory T cells (Fig. 2). This skewed T-cell phenotype was not found in healthy age- and sex-matched control individuals or in patients with SLE. Reduction in peripheral blood effector memory CD8+ T cells in RA may indicate an increase in the migration of these cells into sites of inflammation, and therefore may contribute to ongoing synovial inflammation.
Figure 2.
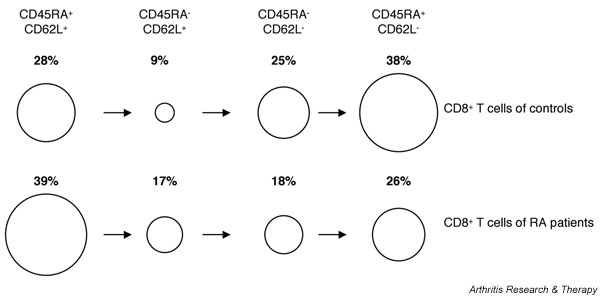
Representation of skewed CD8+ T-cell phenotype in patients with rheumatoid arthritis (RA) as compared with sex- and age-matched healthy control individuals, indicating the relative sizes of the different naïve and memory populations of CD8+ T cells. Percentages refer to the proportions of different naïve/memory population of total CD8+ T cells.
Competing interests
None declared.
Abbreviations
IFN = interferon; IL = interleukin; RA = rheumatoid arthritis; RANTES = regulated upon activation, normal T-cell expressed and secreted; SLE = systemic lupus erythematosus; TREC = T-cell receptor excision circle.
Acknowledgments
Acknowledgment
This work was supported by National Institutes of Health grants R01 AI46719 and R01 AI52005 to PDK.
References
- Morita Y, Yamamura M, Kawashima M, Harada S, Tsuji K, Shibuya K, Maruyama K, Makino H. Flow cytometric single-cell analysis of cytokine production by CD4+ T cells in synovial tissue and peripheral blood from patients with rheumatoid arthritis. Arthritis Rheum. 1998;41:1669–1676. doi: 10.1002/1529-0131(199802)41:2<306::AID-ART15>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- Kang YM, Zhang X, Wagner UG, Yang H, Beckenbaugh RD, Kurtin PJ, Goronzy JJ, Weyand CM. CD8 T cells are required for the formation of ectopic germinal centers in rheumatoid synovitis. J Exp Med. 2002;195:1325–1336. doi: 10.1084/jem.20011565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitzgerald JE, Ricalton NS, Meyer AC, West SG, Kaplan H, Behrendt C, Kotzin BL. Analysis of clonal CD8+ T cell expansions in normal individuals and patients with rheumatoid arthritis. J Immunol. 1995;154:3538–3547. [PubMed] [Google Scholar]
- Wang EC, Lawson TM, Vedhara K, Moss PA, Lehner PJ, Borysiewicz LK. CD8high+ (CD57+) T cells in patients with rheumatoid arthritis. Arthritis Rheum. 1997;40:237–248. doi: 10.1002/art.1780400208. [DOI] [PubMed] [Google Scholar]
- Masuko-Hongo K, Sekine T, Ueda S, Kobata T, Yamamoto K, Nishioka K, Kato T. Long-term persistent accumulation of CD8+ T cells in synovial fluid of rheumatoid arthritis. Ann Rheum Dis. 1997;56:613–621. doi: 10.1136/ard.56.10.613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fazou C, Yang H, McMichael AJ, Callan MF. Epitope specificity of clonally expanded populations of CD8+ T cells found within the joints of patients with inflammatory arthritis. Arthritis Rheum. 2001;44:2038–2045. doi: 10.1002/1529-0131(200109)44:9<2038::AID-ART353>3.0.CO;2-1. [DOI] [PubMed] [Google Scholar]
- Patel DD, Zachariah JP, Whichard LP. CXCR3 and CCR5 ligands in rheumatoid arthritis synovium. Clin Immunol. 2001;98:39–45. doi: 10.1006/clim.2000.4957. [DOI] [PubMed] [Google Scholar]
- Brennan FM, Cope AP, Katsikis P, Gibbons DL, Maini RN, Feldmann M. Selective immunosuppression of tumour necrosis factor-alpha in rheumatoid arthritis. Chem Immunol. 1995;60:48–60. [PubMed] [Google Scholar]
- Hamann D, Baars PA, Rep MH, Hooibrink B, Kerkhof-Garde SR, Klein MR, van Lier RA. Phenotypic and functional separation of memory and effector human CD8+ T cells. J Exp Med. 1997;186:1407–1418. doi: 10.1084/jem.186.9.1407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sallusto F, Lenig D, Forster R, Lipp M, Lanzavecchia A. Two subsets of memory T lymphocytes with distinct homing potentials and effector functions. Nature. 1999;401:708–712. doi: 10.1038/44385. [DOI] [PubMed] [Google Scholar]
- Tussey L, Speller S, Gallimore A, Vessey R. Functionally distinct CD8+ memory T cell subsets in persistent EBV infection are differentiated by migratory receptor expression. Eur J Immunol. 2000;30:1823–1829. doi: 10.1002/1521-4141(200007)30:7<1823::AID-IMMU1823>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- Champagne P, Ogg GS, King AS, Knabenhans C, Ellefsen K, Nobile M, Appay V, Rizzardi GP, Fleury S, Lipp M, Förster R, Rowland-Jones S, Sékaly R-P, McMichael AJ, Pantaleo G. Skewed maturation of memory HIV-specific CD8 T lymphocytes. Nature. 2001;410:106–110. doi: 10.1038/35065118. [DOI] [PubMed] [Google Scholar]
- Roederer M, Dubs JG, Anderson MT, Raju PA, Herzenberg LA. CD8 naive T cell counts decrease progressively in HIV-infected adults. J Clin Invest. 1995;95:2061–2066. doi: 10.1172/JCI117892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koetz K, Bryl E, Spickschen K, O'Fallon WM, Goronzy JJ, Weyand CM. T cell homeostasis in patients with rheumatoid arthritis. Proc Natl Acad Sci USA. 2000;97:9203–9208. doi: 10.1073/pnas.97.16.9203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neidhart M, Pataki F, Schonbachler J, Bruhlmann P. Flow cytometric characterisation of the 'false naïve' (CD45RA+, CD45RO-, CD29 bright+) peripheral blood T-lymphocytes in health and in rheumatoid arthritis. Rheumatol Int. 1996;16:77–87. doi: 10.1007/BF01816439. [DOI] [PubMed] [Google Scholar]
- Mueller YM, De Rosa SC, Hutton JA, Witek J, Roederer M, Altman JD, Katsikis PD. Increased CD95/Fas-induced apoptosis of HIV-specific CD8(+) T cells. Immunity. 2001;15:871–882. doi: 10.1016/s1074-7613(01)00246-1. [DOI] [PubMed] [Google Scholar]
- Hasunuma T, Hoa TT, Aono H, Asahara H, Yonehara S, Yamamoto K, Sumida T, Gay S, Nishioka K. Induction of Fas-dependent apoptosis in synovial infiltrating cells in rheumatoid arthritis. Int Immunol. 1996;8:1595–1602. doi: 10.1093/intimm/8.10.1595. [DOI] [PubMed] [Google Scholar]
- Hoa TT, Hasunuma T, Aono H, Masuko K, Kobata T, Yamamoto K, Sumida T, Nishioka K. Novel mechanisms of selective apoptosis in synovial T cells of patients with rheumatoid arthritis. J Rheumatol. 1996;23:1332–1337. [PubMed] [Google Scholar]


