Abstract
Advanced glycation end-product (AGE)-damaged IgG occurs as a result of hyperglycemia and/or oxidative stress. Autoantibodies to IgG-AGE were previously demonstrated in patients with severe, longstanding rheumatoid arthritis (RA). We investigated whether IgG-AGE and anti-IgG-AGE antibodies were present early in the course of RA and other inflammatory arthropathies. We prospectively followed a cohort of 238 patients with inflammatory arthritis of duration less than 1 year. Patients were evaluated clinically and serologically, and radiographs were obtained at initial and 1-year visits. Sera were assayed for IgG-AGE and anti-IgG-AGE antibodies by enzyme-linked immunosorbent assay (ELISA). Rheumatoid factor (RF) was determined by nephelometry and ELISA. Of all patients, 29% had RF-positive RA, 15% had RF-negative RA, 18% had spondyloarthropathy, and 38% had undifferentiated arthritis. IgG-AGE was present in 19% of patients, and was similar in amount and frequency in all groups. Patients with elevated IgG-AGE levels had significantly higher levels of the inflammatory markers C-reactive protein and erythrocyte sedimentation rate, but there was no correlation with blood glucose levels. Overall, 27% of the patients had IgM anti-IgG-AGE antibodies. These antibodies were highly significantly associated with RFs (P < 0.0001) and with swollen joint count (P < 0.01). In early onset arthritis, IgG damaged by AGE was detected in all patient groups. The ability to make IgM anti-IgG-AGE antibodies, however, was restricted to a subset of RF-positive RA patients with more active disease. The persistence of the anti-IgG-AGE response was more specific to RA, and was transient in the patients with spondyloarthropathy and with undifferentiated arthritis who were initially found to be positive for anti-IgG-AGE antibodies.
Keywords: nonenzymatic glycation, rheumatoid arthritis, rheumatoid factor
Introduction
Rheumatoid arthritis (RA) is characterized by persistent articular and systemic inflammation, along with the production of a number of autoantibodies. Antibodies directed toward the Fc portion of the IgG molecule or rheumatoid factor (RF) are detected in approximately 70% of patients with RA. Indeed, they have become an integral part of the definition of this disease, despite the fact that RFs are found in a small percentage of normal individuals, often transiently, as well as in association with other infectious or rheumatic diseases such as Sjögren's syndrome. Recently, a number of novel RA-associated antibodies have been described that may be more specific for RA than RF, but some of these autoantibodies are present in only a subset of RA patients in early stages of disease [1]. The relationship between the synovial and systemic inflammatory response and production of some of these autoantibodies remains unclear.
During inflammation proteins can be damaged by nonenzymatic glyoxidation [2,3]. Schiff bases are formed after the glucose or oxidized glucose interacts with the surface accessible ε-amino groups (primarily on lysine and arginine). Subsequently, Amadori rearrangements occur with the formation of ketoamine and finally the advanced glycation end-products (AGEs), which are stable. These AGEs include a large number of chemical structures such as pentosidine, Nε-(carboxymethly)lysine, pyrraline, and imadazolone, some of which will cross-link proteins (e.g. pentosidine, which links a lysine to an arginine on separate proteins). Hemoglobin-AGE (HbA1c) is the best studied glycated protein [4] and has been shown to correlate with both microvascular and macrovascular complications in diabetic patients. AGE-damaged proteins are increasingly being implicated in other diseases such as atherosclerosis, amyloidosis, aging (in particular, cartilage and the lens of the eye) [3], and most recently RA [5-10] and osteoarthritis [10,11]. The cross-links that form in cartilage due to pentosidine, which cause the typical yellowing [12], are thought to contribute directly to the joint pathology and increases in urinary AGE detected in patients with osteoarthritis or RA. Such increases may also reflect cartilage degradation.
Not only cartilage but also antibodies can be damaged during inflammation. Previous studies have shown that AGE-damaged IgG can be detected in patients with arthritis of long duration [13-16]. AGE can be detected on both the heavy and light chains of IgG [13,16,17]. In addition, our studies indicated not only that IgG-AGE could be detected in patients with RA, but also that approximately 30–40% of RF-positive patients mounted an immune response to IgG-AGE. One possible explanation for the origins of these antibodies and the link to RFs is that RF-positive B-cells could act as antigen-presenting cells for the damaged IgG and thus stimulate the anti-IgG-AGE response (analogous to epitope spreading) by other antigen-selected B-cells that express a surface immunoglobulin specific for the IgG-AGE. In a previous study of RA patients with longstanding disease [14], the anti-IgG-AGE antibodies were found to correlate significantly with measures of disease activity whereas RFs did not. It was thus of interest to determine whether the anti-IgG-AGE response was only a feature of longstanding inflammation and RF-positive status, or whether such antibodies could be detected in patients with recent onset disease. Thus, in the present study we sought to determine whether AGE-damaged IgG could be detected in patients with early synovitis, and whether this was associated with an anti-IgG-AGE response.
Our findings indicate that IgG-AGE damage can be detected in 14–30% of patients with arthritis (RA, a spondylarthropathy, or undifferentiated arthritis [UA]) of duration less than 1 year. IgG-AGE was found to correlate significantly with markers of inflammation but not with hyperglycemia in these patients. The anti-IgG-AGE response, in contrast, was found to be much more RA specific and correlated highly significantly with the ability to make RFs. In this cohort of patients with early disease, anti-IgG-AGE was found to correlate significantly with the swollen joint count and thus appears to be a marker of disease activity.
Patients and methods
Patients and control individuals
A cohort of 238 patients was recruited from community physicians to an early synovitis study at the US National Institutes of Health (protocol 94-AR-194). The referrals were derived from over 20 different physicians, most of whom were rheumatologists. All patients had undergone a preliminary rheumatologic evaluation before study entry. Inclusion in the study was based on the presence of persistent synovitis in at least one peripheral joint, which had been present for at least 6 months but less than 1 year. Patients with traumatic, septic, and crystal-induced arthritis were specifically excluded. Patients with well defined diffuse connective tissue diseases such as systemic lupus erythematosus and scleroderma were also excluded.
A tender joint count was determined by assessing for the presence of joint line and/or stress tenderness in 68 peripheral joints, and a swollen joint count was determined by evaluating for effusion and/or synovial thickening in 66 peripheral joints (hips were excluded). The total number of affected joints was calculated based on the presence of either tenderness or swelling in each of the joints examined. Sacroiliitis was defined on the basis of having a history of persistent inflammatory low back or buttock pain in conjunction with tenderness over the sacroiliac joint(s). Radiographic confirmation of sacroiliitis was sought but was not considered to be an essential part of the definition. Enthesitis was considered to be present if the insertion of the Achilles' tendon or the plantar fascia was either swollen or tender on examination, and the patient complained of persistent pain in the hind foot area.
RF was measured using nephelometry (in which a level >20 IU/ml was considered positive) and using enzyme-linked immunosorbent assay (ELISA) as previously described [13,14]. Anteroposterior and lateral radiographs of the hands, wrists, feet, knees, and other affected joints were either available for evaluation or were obtained at the time of assessment. Radiographs were available for analysis in 196 out of the 238 patients in the cohort. The radiographs were evaluated for the presence of erosions by an experienced musculoskeletal radiologist. Patients were deemed to have an erosive arthropathy if one or more definite erosions were demonstrable in any peripheral joint radiograph. No attempt was made to quantify the degree of erosive damage when present. Patients with clinical evidence of sacroiliitis were evaluated using anteroposterior and oblique views of the sacroiliac joints.
The American College of Rheumatology criteria for RA [18] and the European Spondlyarthropathy Study Group criteria [19] were applied to each member of the cohort, based on the clinical, radiographic, and laboratory data obtained. The European Spondlyarthropathy Study Group criteria were slightly modified by considering patients as having an 'asymmetric' arthropathy if they did not meet the American College of Rheumatology symmetry criterion for RA. Because patients were recruited on the basis of having peripheral joint synovitis and not axial disease, the presence of 'inflammatory spinal pain', when present, was insufficient as the only major European Spondlyarthropathy Study Group criterion. Also, alternating buttock pain and sacroiliitis were regarded as one minor criterion rather than two. Patients not fulfilling either set of criteria were classified as having UA for the purposes of the present study, even if a more specific rheumatic diagnosis was suggested clinically. A group of 20 normal individuals (age 32 ± 9.5 years; 14 females and 6 males) served as a control group, and data from that group were used to assign a positive cutoff point only. For the analyses throughout the study, patient groups were compared with each other, and thus the lower age of this normal group was not deemed to be a confounding factor.
Quantification of IgG-AGE in high-molecular-weight complexes
A solid-phase aminophenyl boronic acid (APB)-ELISA [15] was used to measure the IgG-AGE in the high-molecular-weight complexes, which were isolated from serum by a polyethylene glycol precipitation method. Sera were made to a 2.5% final concentration with polyethylene glycol 8000 and incubated for 16 hours at 4°C. After centrifugation at 13 000 g for 15 min, the supernatant was discarded and the precipitate was resuspended back to the original serum volume with phosphate-buffered saline (PBS). The IgG-AGE was measured by ELISA of the AGE proteins that were captured via cis-diols to the solid-phase immobilized APB. APB (Sigma, Oakville, Ontario, Canada) 2 mg/ml in 0.2 mol/l carbonate/bicarbonate buffer (pH 9.4) was reacted with Reacti-Bind maleic anhydride activated polystyrene 96-well plates (Pierce, Rockford, IL, USA) for 16 hours at 37°C. Plates were washed with EPPS buffer (0.15 mol/l NaCl, 0.02 mol/l EPPS [Sigma], and 0.01 mol/l l) MgCl2; pH 8.6) three times. The test samples (100 μl) from the 2.5% polyethylene glycol precipitate (diluted 1/500–1/4000 as necessary to keep the values within the standard curve), positive and negative controls, and an appropriate standard curve using IgG1-AGE (0.625–10 μg/ml), all diluted in EPPS buffer and in duplicate, were incubated for 1 hour at 37°C. After washing the plates three times with PBS/0.1% Tween 20, the plates were blocked with 100 μl 1% goat serum in PBS/Tween 20 for 1 hour at 37°C. The plates were washed three times with PBS/Tween 20.
To detect specifically the bound IgG, 100 μl horse radish peroxidase conjugated F(ab')2 fragments of goat antihuman IgG (heavy chain specific; Jackson, BioCan, Mississauga, Otario, Canada) diluted 1/20000 in PBS/Tween were added and the plates were incubated for 1 hour at 37°C. After washing the plates three times with PBS/Tween, the substrate o-phenylene diamine was added. The reaction was stopped by the addition of 4 mol/l H2SO4 approximately 30 min later. The optical density at 492 nm (reference 690 nm) was measured using an ELISA plate reader (SLT LabInstruments, Fisher, Montreal, Quebec, Canada). The plates could be regenerated once for reuse by a series of washes. These were, in sequence, elution of the AGE-modified proteins with 0.1 mol/l sorbitol (American Chemicals Inc., Montreal, Quebec, Canada; two elutions of 5 min incubation, and then one rinse at room temperature), and then four washes with 0.02 mol/l NaOH, followed by five washes with 0.05 mol/l acetic acid and then 10 washes with distilled water. Plates were stored between uses in distilled water containing a bactericidal agent, namely 0.02% Proclin (Superlco, Sigma). The cutoff for identifying those with elevated levels of IgG-AGE was the mean plus 2 SD of IgG-AGE levels from 20 normal individuals.
Measurement of antibodies to IgG-AGE
IgM and IgA anti-IgG-AGE antibodies were detected in serum or plasma by ELISA, as previously described [13,14], with the testing laboratory being blinded to the diagnosis. IgG of all four subclasses, which were fully glycated in vitro, were used at a concentration of 2 μg/ml to coat the wells of an enzyme immunoassay plate (ICN, Montreal, Quebec, Canada). After washing the plates, the sera or plasma, diluted 1:1000 in duplicate, were incubated in the AGE-modified immunoglobulin-coated wells for 2 hours at 37°C. After washing the plates in PBS/Tween (0.1%), the bound antibodies were detected using peroxidase conjugated F(ab')2 fragments of antihuman IgM, or IgA (Jackson) diluted 1/10000 in PBS/Tween. To follow the reactivity over time, and keep consistent results, serum from one normal individual (approximating the mean reactivity) and a positive control was tested each time the assay was performed. After washing the plates three times with PBS/Tween, the substrate o-phenylene diamine was added. The reaction was stopped by the addition of 4 M H2SO4 approximately 30 min later. The optical density at 492 nm (reference 690 nm) was measured, using an ELISA plate reader (SLT LabInstruments). Cutoff values were determined from the sera of 20 normal control individuals, and were the mean plus 2 SD. In order to standardize the optical densities over time, the experimental values obtained were corrected to the positive control that was included in every assay. For selected sera, the titers for reactivity against the IgG-AGE as well as against bovine serum albumin with and without AGE damage were determined.
Because IgG1, IgG2, and IgG4 are structurally very similar, and in general when a sera was positive against IgG1-AGE it was also reactive against IgG2-AGE as well as IgG4-AGE, for clarity only the results for the response to is structurally IgG1-AGE are presented. Because IgG3 quite different (60 amino acid long hinge region versus the 12–15 amino acid hinge in IgG1, IgG2, and IgG4, along with several other differences in the heavy chain), the data for the immune response to IgG3-AGE are also presented. In preliminary studies (not shown), investigations into the specificity of the assay for RFs were done. Sera containing RF and anti-IgG-AGE antibodies were preincubated with either an AGE [Nε-(carboxymethly)lysine] or IgG (which lacked AGE damage) to see if the reactivity could be blocked in the subsequent ELISA. We found that IgG at 1 mg/ml inhibited 30% of the binding of polyclonal RF from one patient with RA to IgG, but it did not inhibit the binding of polyclonal anti-IgG-AGE antibodies to IgG-AGE. Higher concentrations of IgG (4 mg/ml) inhibited the binding of polyclonal RF by up to 90%, and anti-IgG-AGE by 30%, but this could have been because the polyclonal IgG might have had AGE on it. Preincubation with Nε-(carboxymethly)lysine (50 mg/ml) inhibited the binding of polyclonal anti-IgG-AGE antibodies to IgG-AGE by 40% but did not inhibit RF binding to IgG. Lysine at the same concentration did not inhibit either. Thus, this assay appears to measure anti-IgG-AGE antibodies and not simply RFs.
Statistical analysis
Patient groups were compared using analysis of variance or the Kruskal–Wallis test for continuous variables, and using the χ2 test for proportions. The significance levels were adjusted for multiple comparisons using the Bonferroni method where applicable. Statistical analyses were performed using Epi Info statistical software (Centers for Disease Control and Prevention, Atlanta, GA, USA; http://www.cdc.gov/epo/epi/epiinfo.htm) and SPSS statistical software (SPSS Inc., Chicago, IL, USA).
Results
The clinical characteristics of the early synovitis patients are shown in Table 1. A total of 105 patients met the American College of Rheumatology criteria for RA. Of the patients meeting American College of Rheumatology criteria, 69 (66%) were IgM RF positive by nephelometry. Only 10 patients (all with RA) were found to be positive for IgA RFs. Because so few of the cohort members were positive for IgA RFs, no meaningful comparisons were possible with respect to disease parameters, and thus the focus of the present study was on the IgM RF response. A total of 43 patients met the European Spondlyarthropathy Study Group criteria for spondylarthropathy, whereas 90 patients had UA. Females predominated in all groups, ranging from 61% to 73% of the groups. The patients with RA were significantly older than the spondylarthropathy and UA groups. Within the spondylarthropathy group a number of patients with a reactive self-limiting arthritis were identified, but there were also a number of patients with multiple joint involvement that persisted and who were on steroids, thus accounting for the prednisone use in 26%. These patients with multiple joint involvement could be thought of as having 'undifferentiated spondylarthropathies' who did not fit the picture of a reactive type of arthritis.
Table 1.
Demographic and clinical characteristics of patients with early synovitis at initial visit
| Patient group | ||||
| Characteristic | RF+ RA (n = 69) | RF- RA (n = 36) | SpA (n = 43) | UA (n = 90) |
| Caucasian (%) | 77 | 85 | 94 | 82 |
| Females (n) | 43 (61%) | 27 (73%) | 27 (63%) | 60 (67%) |
| Age (years) | 47 ± 12* † | 43 ± 15 | 38 ± 11* | 41 ± 14† |
| Symptom duration (weeks) | 32 ± 18 | 29 ± 26 | 45 ± 59 | 29 ± 19 |
| Swollen joint count | 13 ± 9 | 14 ± 10 | 2 ± 2 | 3 ± 4 |
| Total affected joint count | 19 ± 12 | 19 ± 11 | 5 ± 11 | 5 ± 7 |
| ESR (mm/hour) | 44 ± 27 | 46 ± 33 | 36 ± 30 | 34 ± 29 |
| CRP (mg/dl) | 1.8 ± 1.8 | 2.1 ± 2.2 | 1.9 ± 3.0 | 1.7 ± 2.2 |
| Hemoglobin (mg/dl) | 13 ± 1.5 | 13 ± 1.7 | 13 ± 1.4 | 13 ± 1.6 |
| Glucose (mg/dl) | 109 ± 53 | 99 ± 19 | 99 ± 35 | 97 ± 30 |
| DMARD therapy (%) | 41 | 39 | 19 | 12 |
| Prednisone therapy (%) | 36 | 44 | 26 | 11 |
Values are expressed as mean ± SD, unless otherwise stated. * P < 0.001, †P < 0.05, versus like symbols by analysis of variance or χ2 test, with Yates correction where appropriate. CRP, C-reactive protein; DMARD, disease-modifying antirheumatic drug; ESR, erythrocyte sedimentation rate; RA, rheumatoid arthritis; RF, rheumatoid factor; SpA, spondyloarthropathy; UA, undifferentiated arthritis.
Detection of AGE-damaged IgG in the early synovitis patients
As can be seen from Tables 2 and 3, the IgG-AGE levels were higher in the RA patient group at the initial visit as compared with the other groups studied, whereas the levels were similar in all groups at the year 1 follow-up visit. For both time points, 14–28% of the patients were found to have elevated levels of IgG-AGE. There was a trend for the IgG-AGE amounts to increase in the spondylarthropathy group and, conversely, fall slightly in the RA group at the second visit. This may reflect the lower use of prednisone in the spondylarthropathy patients during the period between visits (Table 4). Interestingly, the patients with elevated IgG-AGE levels were found to have significantly increased levels of the inflammation markers erythrocyte sedimentation rate and C-reactive protein (Table 5). The odds ratio for the association between elevated IgG-AGE positive status and erythrocyte sedimentation rate greater than 50 mm/hour for all 238 patients was 2.45 (95% confidence interval [CI] 1.22–4.96; P = 0094) and for the 105 RA patients it was 3.19 (95% CI 1.13–9.12; P = 0.0024). There was no correlation between IgG-AGE and glucose levels, indicating that it is inflammation and not hyperglycemia that exerts the major influence on AGE formation in the patients with early synovitis. IgG damaged by AGE was found to be associated with total IgG level (r = 0.209; P = 0.0028), although the r value was very low.
Table 2.
AGE-IgG amounts (in relative units) in patients with early synovitis at initial visit and at 1-year follow up
| Patient group | n | AGE-IgG at initial visit | AGE-IgG at 1-year follow up |
| RA | 106 | 2.94 ± 3.37* † | 2.41 ± 2.38 |
| SpA | 43 | 1.82 ± 1.18* | 2.46 ± 2.39 |
| UA | 90 | 1.89 ± 1.52† | 2.18 ± 1.88 |
| Normal | 20 | 1.57 ± 0.74 |
*P < 0.05, †P < 0.05, versus like symbols by nonparametric, Dunn's multiple comparison test. AGE, advanced glycation end-product; RA, rheumatoid arthritis; RF, rheumatoid factor; SpA, spondyloarthropathy; UA, undifferentiated arthritis.
Table 3.
Number positive and frequency for AGE-IgG, rheumatoid factor, and anti-AGE-IgG antibodies in the patient groups at initial visit
| Patient group | |||||
| Serum finding | RF+ RA (n = 70) | RF- RA (n = 36) | SpA (n = 36) | UA (n = 89) | Normal (n = 20) |
| AGE-IgG+ | 14 (20%) | 11 (31%) | 9 (25%) | 13 (15%) | 1(5%) |
| IgM RF+ (by nephlometry) | 70 (100%)* | 0 | 2 (6%) | 16 (18%) | NA |
| IgM RF+ (by ELISA) | 64 (91%)* 1 | 6 (17%)2 | 6 (17%) | 22 (25%) | 1(5%) |
| Anti-AGE-IgG1+ | 27 (39%)* | 5 (14%) | 4 (11%) | 15 (17%) | 0 |
| Anti-AGE-IgG3+ | 28 (40%)* | 4 (11%) | 4 (11%) | 14 (16%) | 0 |
1Fifty-four out of 66 (82%) were rheumatoid factor (RF) positive at the year 1 follow up; 22 out of 34 (6%) remained RF positive at the year 1 follow-up. *P < 0.05, between patient groups by analysis of variance or χ2 test, with Yates correction where appropriate. ELISA, enzyme-linked immunosorbent assay; NA, not available; RA, rheumatoid arthritis; SpA, spondyloarthropathy; UA, undifferentiated arthritis.
Table 4.
Disease-modifying antirheumatic drug and prednisone use in the early synovitis patients at initial visit and year-1 follow up
| Patient group | DMARD at initial visit | DMARD at year 1 follow-up | Prednisone at initial visit | Prednisone at year 1 follow-up |
| RF+ RA | 28/69 (41%) | 46/67 (86%) | 25/69 (36%) | 25/67 (37%) |
| RF- RA | 14/36 (39%) | 27/34 (79%) | 16/36 (44%) | 17/36 (50%) |
| SpA | 8/43 (19%) | 10/37 (29%) | 11/43 (26%) | 3/37 (8%) |
| UA | 11/90 (12%) | 18/79 (23%) | 10/90 (11%) | 9/79 (23%) |
Values are expressed as number taking/total (%). DMARD, disease-modifying antirheumatic drug; RA, rheumatoid arthritis; RF, rheumatoid factor; SpA, spondyloarthropathy; UA, undifferentiated arthritis.
Table 5.
AGE-IgG is associated with the inflammatory response, whereas anti-AGE-IgG is associated with rheumatoid factor positive rheumatoid arthritis
| Serum finding | ||||
| Characteristic | IgG-AGE+ (n = 47) | IgG-AGE- (n = 191) | Anti-IgG-AGE+ (n = 65) | anti-IgG-AGE- (n = 173) |
| Females (%) | 55 | 68 | 71 | 64 |
| Age (years) | 39 ± 12 | 43 ± 14 | 41 ± 13 | 43 ± 14 |
| Swollen joint count | 8.2 ± 8.7 | 7.2 ± 8.9 | 11.0 ± 11* | 6.2 ± 7.6 |
| ESR (mm/hour) | 51 ± 38* | 36 ± 27 | 45 ± 30 | 37 ± 30 |
| CRP (md/dl) | 2.5 ± 3.1* | 1.5 ± 1.8 | 1.7 ± 2.0 | 1.8 ± 2.3 |
| Hemoglobin (mg/dl) | 13.0 ± 1.9 | 12.5 ± 1.5 | 12.7 ± 1.5 | 12.9 ± 1.6 |
| Glucose (mg/dl) | 100 ± 30 | 102 ± 40 | 99 ± 25 | 102 ± 42 |
| IgG (mg/dl) | 1427 ± 529* | 1182 ± 378 | 1252 ± 369 | 1226 ± 443 |
| IgM (mg/dl) | 139 ± 61 | 152 ± 88 | 173 ± 61* | 140 ± 88 |
| IgA (mg/dl) | 285 ± 139 | 236 ± 104 | 282 ± 126 | 232 ± 106 |
| RF+ (%) | 19 (40%) | 69 (36%) | 45 (69%)* | 25% |
| anti-AGE-IgG1+ (n) | 18 (26%) | 40 (21%) | 50 (77%) | - |
| anti-AGE-IgG3+ (n) | 12 (26%) | 38 (20%) | 49 (76%) | - |
Values are expressed as means ± SD, unless otherwise stated. *P < 0.05, by analysis of variance or χ2 test, with Yates correction where appropriate; comparisons are between AGE-IgG+ and AGE-IgG- patients, and between anti-AGE-IgG+ and anti-AGE-IgG- patients. AGE, advanced glycation end-product; CRP, C-reactive protein; ESR, erythrocyte sedimentation rate; RF, rheumatoid factor.
Antibodies to IgG-AGE
As can be seen in Fig. 1, and Tables 3 and 5, antibodies to IgG-AGE were detected in patients primarily with RA. This specificity varied according to the target subclass of IgG, with the immune response higher in the majority of cases to IgG1 (and thus IgG2 and IgG4) than to IgG3 (Fig. 1), and for the representative sera illustrated no reactivity was detected against either BSA or BSA-AGE. Antibodies specific for AGE-damaged IgG1 or IgG3 were found in 11–40% of patients in the different groups, and were highly significantly associated (P < 0.0001) with the presence of RFs (Tables 3 and 5), with odds ratios of 7.32 (95% CI 3.68–14.67) for all the 238 patients and 9.54 (95% CI 2.77–36.12) for the 105 RA patients. Nonetheless, this immune response to damaged IgG was not simply a reflection of the titer of RF because at the initial visit 10 patients (all with RA) had high-titer RF but lacked the anti-IgG-AGE response. At the year 1 follow-up visit, eight patients (all with RA) were found to have a high-titer RF response but no detectable anti-IgG-AGE response. There was a highly significant association between RF positivity by nephelometry and that by ELISA (odds ratio 44.56, 95% CI 17.98–114.51; P < 0.00001). Of those RA patients who were RF positive by nephelometry, 35 out of 69 had antibodies to either IgG1-AGE and/or IgG3-AGE. Our previous studies showed that approximately 50% of RF-positive patients with longstanding disease were anti-IgG-AGE positive [14]. Thus, this sub-population appears to be fairly consistent over time.
Figure 1.
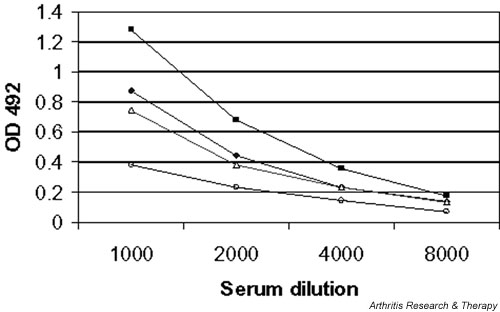
IgM antibodies directed at IgG1-advanced glycation end-product (AGE) or IgG3-AGE in two patients with rheumatoid arthritis (RA) when sera were diluted 1/1000–1/8000: [◆], RA1 anti-IgG1-AGE; [■], RA2 anti-IgG1-AGE; [△], RA1 anti-IgG3-AGE; [○], RA2 anti-IgG3-AGE. Binding to bovine serum albumin (BSA) for RA1 and RA2 (sera diluted 1/500) was 0.031 and 0.078, respectively, and for BSA-AGE for RA1 and RA2 (sera diluted 1/500) 0.029 and 0.025, respectively. OD, optical density.
Interestingly, as shown in Fig. 2, the anti-IgG-AGE antibodies response persisted in the RF-positive RA patients at follow-up, whereas those positive for anti-IgG-AGE antibodies in the other groups had lower frequencies of such antibodies at the year 1 follow-up. When the change in the anti-IgG-AGE response was examined (Table 6), it was apparent that only in the RF-positive RA group was there a subset of patients (approximately 20%) who not only had gained this immune response by the year 1 follow-up visit but also maintained it. In contrast in the spondylarthropathy and UA groups, the initial positive anti-IgG-AGE response was lost at follow up. Thus, the anti-IgG-AGE response appears much more specific to RF-positive RA.
Figure 2.
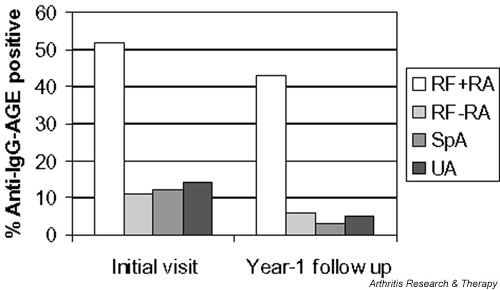
Anti-IgG-advanced glycation end-product (AGE) antibodies persist in rheumatoid factor (RF)-positive rheumatoid arthritis
Table 6.
Change in the anti-IgG-AGE response from initial visit to follow up in the early synovitis cohort
| Patient group | n | Always negative | Positive at initial assessment;negative at follow up | Negative at initial assessment;positive at follow up | Always positive |
| RA RF+ | 68 | 22 (32%) | 17 (25%) | 15 (22%) | 14 (21%) |
| RA RF- | 35 | 32 (91%) | 1 (3%) | 0 (0%) | 2 (6%) |
| SpA | 38 | 32 (84%) | 5 (13%) | 1 (3%) | 0 (0%) |
| UA | 75 | 64 (85%) | 8 (11%) | 1 (2%) | 2 (3%) |
Values are expressed as n (%), unless otherwise stated. AGE, advanced glycation end-product; RA, rheumatoid arthritis; RF, rheumatoid factor; SpA, spondyloarthropathy; UA, undifferentiated arthritis.
Of the clinical features examined, only the swollen joint count was found to be associated with anti-IgG-AGE antibodies (Table 5). When patients with and without anti-IgG-AGE antibodies were examined for erosions at the year 1 follow-up visit, there was trend toward more erosive disease in the anti-IgG-AGE group, but this was not statistically significant (data not shown). Anti-IgG-AGE antibodies were found to be associated with IgA levels (r = 0.304; P = 0.02) for reasons that are as yet unknown.
Discussion
Previous studies of AGE in RA have been cross-sectional in nature and in general conducted in patients with disease of several to many years duration. Thus, it was important to examine a cohort of patients with early synovitis to determine whether AGE-damaged proteins were present near disease onset, in order to determine whether the generation of AGEs could be linked to an ongoing pathogenic process or whether they are merely the consequence of damage in the past. The amount of AGE-damaged proteins present in an individual reflects not only the synthesis of the AGE, which can take weeks to months, but also the clearance of the AGE-damage protein either through normal mechanisms for the protein in question or by specific receptors for AGE, one of which is termed receptor for AGE (RAGE) [20]. The present study shows that the formation of AGE-damaged IgG in this early synovitis cohort is linked to the inflammatory response rather than to hyperglycemia. The latter is key in the AGE associated with diabetes [3]. We did not measure hemoglobin A1c in the present study, but in a previous study it was not found to correlate with IgG-AGE in patients with longstanding RA [14]. Thus, it appears that AGE formation associated with arthritis occurs as a result of oxidative stress. Recent studies have shown that high levels of RAGE expression, which is induced by AGE, can contribute to the inflammatory cycle by the induction of proinflammatory cytokines, via the nuclear factor-κB pathway [21]. No studies have yet examined RAGE expression in the synovium of patients with early synovitis. RAGE appears to play a central role in the arthralgia associated with AGE-modified β2-microglobulin, the latter being a consequence of long-term dialysis [22].
From our studies, the presence of IgG damaged by AGE is not disease specific but occurs in all forms of arthritis, and importantly at an early stage of disease. Approximately 15–30% of the patients had elevated levels of IgG-AGE at their initial visit, and the levels were seen to fluctuate over time because there was no correlation between the amounts of IgG-AGE at the initial and the year 1 follow-up visits. The frequency and levels of IgG-AGE were slightly higher than we previously observed in patients with longstanding disease [14]. Elevations in pentosidine (a specific type of AGE) were previously found to correlate with increased clinical disease activity in RA and with RF levels [6-11]. However, in the present study no clinical parameter of joint disease was found to correlate with levels of IgG-AGE.
Our previous studies found a subset of RA patients with longstanding disease to have antibodies to IgG-AGE [14]. In the present study we extend this observation to patients with early synovitis. Consistent with previous studies, the ability to make anti-IgG-AGE antibodies was strongly linked to RF-positive status. A mechanism that may account for this is that RF-positive B-cells might play a role in antigen presentation of the IgG-AGE and in induction of the immune response to AGEs. This would be analogous to the process of epitope spreading. Recent studies employing mass spectrometry have shown that much of the AGE on IgG is located within the Fab region [17], which would not interfere with the binding of the Fc portion of IgG to the RF.
In contrast to the IgG-AGE elevations, which were not found to be disease specific, the anti-IgG-AGE response was much more specific to RA, and in particular to RF-positive RA. Indeed, at the initial visit, whereas a small percentage of patients with UA or spondyloarthropathy were found to have anti-IgG-AGE antibodies, this immune response appeared to be largely transient in these groups only, with the loss of this specificity by the year 1 follow-up visit. In contrast, in the RF-positive RA group, not only did approximately 20% gain this specificity at the year 1 follow-up visit (versus 0–3% in the other groups) but also a much higher proportion maintained this specificity at follow up than in any other group. Thus, the anti-IgG-AGE response is much more specific to RF-positive RA.
In the overall cohort, the anti-IgG-AGE response was associated with the swollen joint count, which probably reflects the increased joint count seen in RA, because within RA there was no significant correlation noted in these patients with early synovitis. In our previous study in patients with longstanding disease [14], there was a significant correlation between the anti-IgG-AGE response and swollen joint count, whereas the RF response was not a useful biomarker for current disease activity. In the latter study the anti-IgG-AGE response was also linked to a physician-assessed disease activity score (visual analogue score). The latter type of evaluation was not conducted with the early synovitis cohort. Nonetheless, it does appear that, with respect to current disease activity, the level of anti-IgG-AGE antibodies represents a useful biomarker. RFs detected in other early synovitis cohorts have been shown to be predictive of long-term radiologic damage [23-25], and thus it will be of interest to follow this cohort over time to determine whether the anti-IgG-AGE antibodies will also be predictive of more severe disease in this subset of RA patients.
Conclusion
In early onset arthritis, IgG damaged by AGE, as a result of inflammation, was detected in all patient groups. The ability to make anti-IgG-AGE antibodies, however, was restricted to a subset of RF-positive RA patients with more active disease. The persistence of the anti-IgG-AGE response was found to be more specific to RA and was transient in the patients with spondyloarthropathy or UA who were initially found to be positive for anti-IgG-AGE antibodies. These studies demonstrated that this immune response to IgG-AGE occurs early in RA pathogenesis, and give important insight into the consequences of inflammation in this disease setting.
Competing interests
None declared.
Abbreviations
AGE = advanced glycation end-product; APG = aminophenyl boronic acid; CI = confidence interval; ELISA = enzyme-linked immunosorbent assay; PBS = phosphate-buffered saline; RA = rheumatoid arthritis; RAGE = receptor for advanced glycation end-products; RF = rheumatoid factor; UA = undifferentiated arthritis.
Acknowledgments
Acknowledgements
The authors would like to thank Dr H Ralph Schumacher for his invaluable contributions to this study, and Dr Gabor Illei for critical reading of the manuscript. Supported in part from a NIAMS Intramural Research Program.
References
- Goldbach-Mansky R, Lee J, McCoy A, Hoxworth J, Yarboro C, Smolen JS, Steiner G, Rosen A, Zhang C, Menard HA, Zhou ZJ, Palosuo T, Van Venrooij WJ, Wilder RL, Klippel JH, Schumacher HR, Jr, EI-Gabalawy HS. Rheumatoid arthritis associated autoantibodies in patients with synovitis of recent onset. Arthritis Res. 2000;2:236–243. doi: 10.1186/ar93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh R, Barden A, Mori T, Beilin L. Advanced glycation end-products: a review. Diabetologia. 2001;44:129–146. doi: 10.1007/s001250051591. [DOI] [PubMed] [Google Scholar]
- Ulrich P, Cerami A. Protein glycation, diabetes, and aging. Recent Prog Horm Res. 2001;56:1–21. doi: 10.1210/rp.56.1.1. [DOI] [PubMed] [Google Scholar]
- Makita Z, Vlassara H, Rayfield E, Cartwright K, Friedman E, Rodby R, Cerami A, Bucala R. Hemoglobin-AGE: a circulating marker of advanced glycosylation. Science. 1992;258:651–653. doi: 10.1126/science.1411574. [DOI] [PubMed] [Google Scholar]
- Chen JR, S Takahashi, M Suzuki, K Kushida, S Miyamoto, Inoue T. Pentosidine in synovial fluid in osteoarthritis and rheumatoid arthritis: relationship with disease activity in rheumatoid arthritis. J Rheumatol. 1998;25:2440–2444. [PubMed] [Google Scholar]
- Furumitsu Y, Inaba M, Yukioka K, Yukioka M, Kumeda Y, Azuma Y, Ohta T, Ochi T, Nishizawa Y, Morii H. Levels of serum and synovial fluid pyridinium crosslinks in patients with rheumatoid arthritis. J Rheumatol. 2000;27:64–70. [PubMed] [Google Scholar]
- Miyata T, Ishiguro N, Yasuda Y, Ito T, Nangaku M, Iwata H, Kurokawa K. Increased pentosidine, an advanced glycation end product, in plasma and synovial fluid from patients with rheumatoid arthritis and its relation with inflammatory markers. Biochem Biophys Res Comm. 1998;244:45–49. doi: 10.1006/bbrc.1998.8203. [DOI] [PubMed] [Google Scholar]
- Rodriquez-Garcia J, Requena JR, Rodriguez-Segade R. Increased concentrations of serum pentosidine in rheumatoid arthritis. Clin Chem. 1998;44:250–255. [PubMed] [Google Scholar]
- Takahashi M, Suzuki M, Kushida K, Miyamoto S, Inoue T. Relationship between pentosidine levels in serum and urine and activity in rheumatoid arthritis. Br J Rheumatol. 1997;36:637–642. doi: 10.1093/rheumatology/36.6.637. [DOI] [PubMed] [Google Scholar]
- Torchiana EEM, Murgo A, Re KA, Paresce E, DeGiovanni R, Sansone L, Carrabba M. Pyridinium crosslinks in osteoarthritis and rheumatoid arthritis: a predictive factor? [abstract]. J Rheumatol. 1998;25(suppl 52):42. [Google Scholar]
- Pokharna HK, Monnier V, Boja B, Moskowitz RW. Lysyl oxidase and Maillard reaction-mediated cross-links in ageing and osteoarthritic rabbit cartilage. J Orthop Res. 1995;13:13–21. doi: 10.1002/jor.1100130105. [DOI] [PubMed] [Google Scholar]
- Monnier VM, Kohn RR, Cerami A. Accelerated age-related browning of human collagen in diabetes mellitus. Proc Natl Acad Sci USA. 1984;81:583–587. doi: 10.1073/pnas.81.2.583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ligier S, Fortin PR, Newkirk MM. A new antibody in rheumatoid arthritis (RA) targeting glycated IgG: IgM anti-IgG-AGE. Br J Rheumatol. 1998;37:1307–1314. doi: 10.1093/rheumatology/37.12.1307. [DOI] [PubMed] [Google Scholar]
- Lucey M, MM Newkirk, Neville C, Lepage K, Fortin PR. Association between IgM-anti-IgG-AGE autoantibody and disease activity in rheumatoid arthritis. J Rheumatol. 2000;27:319–323. [PubMed] [Google Scholar]
- Newkirk MM, Lepage K, Niwa T, Rubin L. Advanced glycation endproducts (AGE) on IgG, a target for circulating antibodies in North American Indians with Rheumatoid Arthritis (RA). Cell Mol Biol. 1998;44:1129–1138. [PubMed] [Google Scholar]
- Tai W-H, Newkirk MM. An autoantibody targeting glycated IgG is associated with elevated serum immune complexes in rheumatoid arthritis (RA). Clin Exp Immunol. 2000;120:188–193. doi: 10.1046/j.1365-2249.2000.01188.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lapolla A, Fedele D, Garbeglio M, Martano L, Tonani R, Seraglia R, Favretto D, Fedrigo MA, Traldi P. Matrix-assisted laser desorption/ionization mass spectrometry, enzymatic digestion, and molecular modeling in the study of nonenzymatic glycation of IgG. J Am Soc Mass Spectrom. 2000;11:153–159. doi: 10.1016/S1044-0305(99)00134-8. [DOI] [PubMed] [Google Scholar]
- Arnett FC, Edworthy SM, Bloch DA, McShane DJ, Fries JF, Cooper NS, Healey LA, Kaplan SR, Liang MH, Luthra HS, et al. The American Rheumatism Association 1987 revised criteria for the classification of rheumatoid arthritis. Arthritis Rheum. 1988;31:315–324. doi: 10.1002/art.1780310302. [DOI] [PubMed] [Google Scholar]
- Dougados M, van der Linden S, Juhlin R, Huitfeldt B, Amor B, Calin A, Cats A, Dijkmans B, Olivieri I, Pasero G, et al. The European Spondylarthropathy Study Group preliminary criteria for the classification of spondylarthropathy. Arthritis Rheum. 1991;34:1218–1227. doi: 10.1002/art.1780341003. [DOI] [PubMed] [Google Scholar]
- Schmidt AM, Hofmann M, Taguchi A, Yan SD, Stern DM. RAGE: a multiligand receptor contributing to the cellular response in diabetic vasculopathy and inflammation. Semin Thromb Hemost. 2000;26:485–493. doi: 10.1055/s-2000-13204. [DOI] [PubMed] [Google Scholar]
- Yeh CH, Sturgis L, Haidacher J, Zhang XN, Sherwood SJ, Bjercke RJ, Juhasz O, Crow MT, Tilton RG, Denner L. Requirement for p38 and p44/p42 mitogen-activated protein kinases in RAGE-mediated nuclear factor-kappaB transcriptional activation and cytokine secretion. Diabetes. 2001;50:1495–1504. doi: 10.2337/diabetes.50.6.1495. [DOI] [PubMed] [Google Scholar]
- Hou FF, Reddan DN, Seng WK, Owen WF., Jr Pathogenesis of beta(2)-microglobulin amyloidosis: role of monocytes/ macrophages. Semin Dial. 2001;14:135–139. doi: 10.1046/j.1525-139X.2001.00034.x. [DOI] [PubMed] [Google Scholar]
- Kaltenhauser S, Wagner U, Schuster E, Wassmuth R, Arnold S, Seidel W, Troltzsch M, Loeffler M, Hantzschel H. Immunogenetic markers and seropositivity predict radiological progression in early rheumatoid arthritis independent of disease activity. J Rheumatol. 2001;28:735–744. [PubMed] [Google Scholar]
- Rau R, Herborn G, Zueger S, Fenner H. The effect of HLA-DRB1 genes, rheumatoid factor, and treatment on radiographic disease progression in rheumatoid arthritis over 6 years. J Rheumatol. 2000;27:2566–2575. [PubMed] [Google Scholar]
- Aman S, Paimela L, Leirisalo-Repo M, Risteli J, Kautiainen H, Helve T, Hakala M. Prediction of disease progression in early rheumatoid arthritis by ICTP, RF and CRP. A comparative 3-year follow-up study. Rheumatology. 2000;39:1009–1013. doi: 10.1093/rheumatology/39.9.1009. [DOI] [PubMed] [Google Scholar]


