Abstract
Although the predominant mechanism of intra-articular hyaluronan (hyaluronic acid) (HA) and hylans for the treatment of pain associated with knee osteoarthritis (OA) is unknown, in vivo, in vitro, and clinical studies demonstrate various physiological effects of exogenous HA. HA can reduce nerve impulses and nerve sensitivity associated with the pain of OA. In experimental OA, this glycosaminoglycan has protective effects on cartilage, which may be mediated by its molecular and cellular effects observed in vitro. Exogenous HA enhances chondrocyte HA and proteoglycan synthesis, reduces the production and activity of proinflammatory mediators and matrix metalloproteinases, and alters the behavior of immune cells. Many of the physiological effects of exogenous HA may be a function of its molecular weight. Several physiological effects probably contribute to the mechanisms by which HA and hylans exert their clinical effects in knee OA.
Keywords: cartilage, hyaluronan, hylan, mechanism of action, osteoarthritis
Introduction
Osteoarthritis (OA), the most common form of arthritis, is a chronic disease characterized by the slow degradation of cartilage, pain, and increasing disability. The disease can have an impact on several aspects of a patient's life, including functional and social activities, relationships, socioeconomic status, body image, and emotional well-being [1]. Currently available pharmacological therapies target palliation of pain and include analgesics (i.e. acetaminophen, cyclooxygenase-2-specific inhibitors, nonselective nonsteroidal anti-inflammatory drugs, tramadol, opioids), intra-articular therapies (glucocorticoids and hyaluronan [hyaluronic acid] [HA]), and topical treatments (i.e. capsaicin, methylsalicylate) [2].
Intra-articular treatment with HA and hylans (see Table 1 for definitions) has recently become more widely accepted in the armamentarium of therapies for OA pain [2]. HA is responsible for the viscoelastic properties of synovial fluid. This fluid contains a lower concentration and molecular weight (MW) of HA in osteoarthritic joints than in healthy ones [3]. Thus, the goal of intra-articular therapy with HA is to help replace synovial fluid that has lost its viscoelastic properties. The efficacy and tolerability of intra-articular HA for the treatment of pain associated with OA of the knee have been demonstrated in several clinical trials [4-14]. Three (hylan G-F 20) to five (sodium hyaluronate) injections can provide relief of knee pain from OA for up to 6 months [6,7,11]. Intra-articular hylan or HA is also generally well tolerated, with a low incidence of local adverse events (from 0% to 13% of patients) [5,6,8,11,12] that was similar to that found with placebo [6,11].
Table 1.
Definition and characteristics of hyaluronan (hyaluronic acid) and hylans
| Definition | Characteristics |
| Hyaluronan (hyaluronic acid) or sodium hyaluronate | Long, nonsulfated, straight chains of variable length |
| Repeating disaccharide unit of N-acetylglucosamine and glucuronic acid | |
| Forms a randomized coil in physiological solvents | |
| Average MW 4–5 million Da | |
| Hylans | Crosslinked hyaluronan chains in which the carboxylic and N-acetyl groups are unaffected |
| MW of Hylan A is 6 million Da | |
| Can be water-insoluble as a gel (e.g. hylan B) or membrane bound |
MW, molecular weight.
Because the residence time of exogenously administered HA in the joint is relatively short, HA probably has physiological effects in the joint that contribute to its effects in the joint over longer periods. The exact mechanism(s) by which intra-articular HA or hylans relieve pain is currently unknown. Improvements in OA with administration of HA have been shown in both electrophysiology and animal pain model studies [15-17]; Gomis A, Pawlak M, Schmidt RF, Belmonte C: Effects of elastoviscous substances on the mechanosensitivity of articular pain receptors. Presented at the Osteoarthritis Research Society International World Congress on Osteoarthritis, September 2001, Washington, DC, USA]. HA treatment has also been shown to have protective effects on cartilage in experimental models of OA [18-20]. In vitro studies also show that HA has beneficial effects on the extracellular matrix, immune cells, and inflammatory mediators [21-26]. This article provides a brief introduction to the pathophysiology of OA and reviews the current scientific literature regarding the physiological effects of HA and hylans, focusing on antinociceptive effects, possible protective effects on cartilage, and effects on molecular and cellular factors involved in OA disease progression. The effects of HA and hylans on these factors may provide insight into the mechanism by which HA and hylans elicit their clinical benefits.
Methods
Relevant literature was identified by searching MEDLINE from 1966 through July 2002. The following search words were used alone and in combination when appropriate: hyaluronan, hyaluronic acid, sodium hyaluronate, hylan, OA, knee, cartilage, synovium, pathophysiology, extracellular matrix, proteoglycans (PGs), aggrecanase, inflammation, immunology, proteases, matrix metalloproteinases (MMPs), cytokines, proinflammatory mediators, nitric oxide (NO), prostaglandins, lymphocytes, nociceptors, and mechanoreceptors. Additional references were located by consulting the bibliographies of MEDLINE sources.
Pathophysiology of osteoarthritis
OA is characterized by a slow degradation of cartilage over several years. In normal cartilage, a delicate balance exists between matrix synthesis and degradation; in OA, however, cartilage degradation exceeds synthesis. The balance between synthesis and degradation is affected by age and is regulated by several factors produced by the synovium and chondrocytes, including cytokines, growth factors, aggrecanases, and MMPs [27-32] (Fig. 1).
Figure 1.
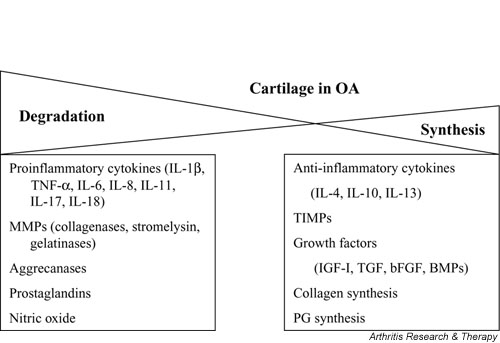
Several factors contribute to the breakdown and synthesis of cartilage. In osteoarthritis (OA), the balance between cartilage degradation and synthesis leans toward degradation. BMP, bone morphogenetic protein; bFGF, basic fibroblastic growth factor; IGF, insulin-like growth factor; IL, interleukin; MMP, matrix metalloproteinase; PG, proteoglycan; TGF, transforming growth factor; TIMP, tissue inhibitor of metalloproteinases; TNF, tumor necrosis factor.
In addition to water, the extracellular matrix is composed of PGs entrapped within a collagenous framework or fibrillary matrix (Fig. 2) [33]. PGs are made up of glycosaminoglycans attached to a backbone made of HA [33]. In OA, the collagen turnover rate increases, the PG content decreases, the PG composition changes, and the water content increases [33]. The size of HA molecules [3] and their concentration [34] in synovial fluid also decrease in OA. A significant PG in articular cartilage is aggrecan, which binds to HA and helps provide the compressibility and elasticity of cartilage [32]. Aggrecan is cleaved by aggrecanases, leading to its degradation and to subsequent erosion of cartilage [34,35]. The loss of aggrecan from the cartilage matrix is one of the first pathophysiological changes observed in OA [32].
Figure 2.
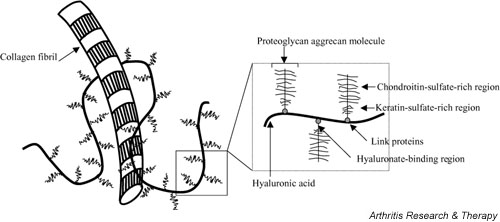
The extracellular matrix of cartilage is composed of proteoglycans attached to a backbone of hyaluronic acid that is intertwined among collagen fibrils. Proteoglycans have both chondroitin-sulfate- and keratin-sulfate-rich regions, and link proteins facilitate binding of aggrecan to hyaluronic acid.
Cytokines produced by the synovium and chondrocytes, especially IL-1 and tumor necrosis factor alpha (TNF-α), are also key players in the degradation of cartilage [29]. IL-1β is spontaneously released from cartilage of OA but not normal cartilage [36]. Both IL-1β and TNF-α stimulate their own production and the production of other cytokines (e.g. IL-8, IL-6, and leukotriene inhibitory factor), proteases, and prostaglandin E2 (PGE2) [30]. Synthesis of the inflammatory cytokines IL-1 and TNF-α and expression of their receptors are enhanced in OA [29-31]. Both cytokines have been shown to potently induce degradation of cartilage in vitro [31]. Other proinflammatory cytokines overexpressed in OA include IL-6, IL-8, IL-11, and IL-17, as well as leukotriene inhibitory factor [30]. The production of the chemokine RANTES (regulated upon activation, normal T-cell expressed and secreted), is also high in OA cartilage compared with normal cartilage, is stimulated by IL-1, and increases the release of PGs from cartilage [37].
Prostaglandins and leukotrienes may also be involved in cartilage destruction in OA. PGE2 is spontaneously produced by OA cartilage [38] and leukotriene B4 is elevated in the synovial fluid of OA [36]. Although IL-1β stimulates the release of PGE2 [39], the role of PGE in cartilage biology is unclear, since studies show both anabolic and catabolic effects of PGE on cartilage [38].
The extracellular matrix in cartilage is degraded by locally produced MMPs. Elevated levels of stromelysin (MMP-3), collagenases (MMP-1, -8, and -13), and gelatinases (MMP-2 and -9) have also been found in chondrocytes or the articular cartilage surface in OA [29,31]. The activity of many MMPs increases in OA by either an increase in their own synthesis, an increased activation by their proenzymes, or decreased activity of their inhibitors [29]. Proinflammatory cytokines, including IL-1, TNF-α, IL-17, and IL-18, increase synthesis of MMPs, decrease MMP enzyme inhibitors, and decrease extracellular matrix synthesis [29]. To further exacerbate the degradative activity in OA, expression levels of tissue inhibitor of metalloproteinases (TIMP)-1 are reduced [29].
In an attempt to reverse the breakdown of the extracellular matrix, the chondrocytes increase synthesis of matrix components including PGs [29]. Even though this activity increases, a net loss of PG in the upper cartilage layer is seen, because the increased activity has been observed only in the middle and deeper layers of cartilage [29]. Elevated anti-inflammatory cytokines found in the synovial fluid of OA include IL-4, IL-10, and IL-13 [30]. Their role is to reduce production of IL-1, TNF-α, and MMPs, increase TIMP-1, and inhibit prostaglandin release [32,40]. Local production of growth and differentiation factors such as insulin-like growth factor 1, transforming growth factors, fibroblastic growth factors, and bone morphogenetic proteins also stimulate matrix synthesis [29,41].
The production of NO, another inflammatory mediator synthesized by the cartilage in OA and well documented in experimental OA, is stimulated by the proinflammatory cytokines IL-1 and TNF-α [29-31,36]. NO may be involved in cartilage catabolism by inhibiting the synthesis of collagen and PG, enhancing MMP activity, reducing the synthesis of an IL-1 receptor antagonist by chondrocytes, and increasing susceptibility to cell injury (i.e. apoptosis) [30,36,42]. NO can also inhibit the attachment of fibronectin to chondrocytes, thus enhancing PG synthesis [42].
Additionally, NO can induce apoptosis of chondrocytes in OA [30]. Chondrocyte apoptosis occurs in both human and experimental OA and is correlated with the severity of cartilage destruction [42]. Apoptosis of chondrocytes in OA has been shown to have a higher incidence in OA than in normal cartilage, to be present close to the articular surface, and to be significantly correlated with OA grade [43,44]. Death of chondrocytes could easily lead to reduced matrix production, since chondrocytes are the only source of matrix components and their population is not renewed [29]. Depletion of PGs was observed in cartilage areas that contained apoptotic chondrocytes [43]. Cellular products of apoptosis may also contribute to the pathophysiology of OA, because apoptotic cells are not effectively removed from cartilage [29] due to its avascular nature and can cause pathogenetic events such as abnormal cartilage calcification or extracellular matrix degradation [43].
Role of hyaluronan in the synovial fluid
HA is responsible for the viscoelastic quality of synovial fluid that acts as both a lubricant and shock absorber [3]. In synovial fluid, HA coats the surface of the articular cartilage and shares space deeper in the cartilage among collagen fibrils and sulfated PGs [3]. In this respect, HA probably protects the cartilage and blocks the loss of PGs from the cartilage matrix into the synovial space, maintaining the normal cartilage matrix [3]. Similarly, HA may also help prevent invasion of inflammatory cells into the joint space.
In acute and chronic inflammatory processes of the joint, the size of HA molecules decreases at the same time as the number of cells in the joint space increases [3]. In synovial fluid from knee joints in OA, concentrations of HA, glycosaminoglycans, and keratan sulfate are lower than in synovial fluid from normal knee joints [34]. Additionally, experiments using rabbit synovial cells showed that the proinflammatory cytokines IL-1 and TNF-α stimulate the expression of HA synthetase [45], which may contribute to the fragmentation of HA under inflammatory conditions.
Exogenous HA may facilitate the production of newly synthesized HA. When synovial fibroblasts from OA knees were cultured with HA formulations of various MWs (3.4 × 105 to 4.7 × 106), the amount of newly synthesized HA in response to the exogenous HA was both concentration- and MW-dependent [21]. Higher-MW agents stimulated the synthesis of HA more than lower-MW formulations and an optimal concentration was noted for each MW [21].
HA in the synovial fluid binds to chondrocytes via the CD44 receptor [46,47], supporting a role for HA in healthy cartilage. The primary means of retention and anchoring of PG aggregates to chondrocytes is the CD44 HA receptor [48]. When expression of CD44 was suppressed in bovine articular cartilage slices, a near-complete loss of PG staining was observed [48]. A similar decrease in PG staining was found when very small HA molecules were used to block the binding of HA to the CD44 receptor [47]. CD44 adhesion to HA has also been shown to mediate chondrocyte proliferation and function [49].
Hyaluronan and nociception
Relief of knee pain from OA with HA in clinical studies may be due to the effects of HA on nerve impulses and nerve sensitivity. Inflammation of the knee joint influences excitability of nociceptors of articular nerves [15]. In experimental OA, these nerves become hyperalgesic, spontaneously discharge, and are sensitive to non-noxious joint movements [15]. Administration of HA to isolated medial articular nerves from an experimental model of OA significantly decreased ongoing nerve activity as well as movement-evoked nerve activity [15]. In another model, nerve impulses evoked by movement of an inflamed knee were significantly reduced with hylan G-F 20 to about 60% of that of the controls (Gomis A, Pawlak M, Schmidt RF, Belmonte C: Effects of elastoviscous substances on the mechanosensitivity of articular pain receptors. Presented at the Osteoarthritis Research Society International World Congress on Osteoarthritis, September 2001, Washington, DC, USA). These authors reported that HAs with lower MWs had either less of an effect or no effect on nerve impulse frequency. Impulse discharge and firing frequency of activated nerve sensory fibers decreased to 65% and 45% of that of control values, respectively, when hylan was administered [50]. Mechanical forces on stretch-activated ion channels are involved in depolarization of the articular nerve terminal. In the presence of hylan, these ion channels also have decreased mechanical sensitivity (de la Peña E, Pecson B, Schmidt RF, Belmonte C: Effects of hylans on the response characteristics of mechanosensitive ion channels. Presented at the 9th World Congress on Pain, Vienna, Austria 1999).
In a rat model, HA improved the abnormal gait of rats with experimentally induced OA in a dose-dependent manner, indicating an antinociceptive effect of HA [16]. This effect may be mediated through the attenuation of prostaglandin E2 (PGE2) and bradykinin synthesis, since HA inhibited their synthesis in a MW-dependent manner [16]. Further, HA has been shown to induce analgesia in a bradykinin-induced model of joint pain in rats [17]. This analgesic action was also MW-dependent, as significant effects were observed at lower concentrations with a higher-MW formulation than with lower-MW HAs [17].
Lastly, HA may have direct or indirect effects on substance P, which can be involved in pain [51]. Since substance P interacts with excitatory amino acids, prostaglandins, and NO, the effects of HA on these factors can indirectly affect the pharmacology of substance P [51]. Additionally, HA has been shown to inhibit an increased vascular permeability induced by substance P [51].
Molecular and cellular effects of hyaluronan
Many effects of exogenous HA on the extracellular matrix, inflammatory mediators, and immune cells have been reported in in vitro studies. The influence of HA on these factors may contribute to cartilage protection in OA.
Effects of hyaluronan on the extracellular matrix
In vitro experiments indicate that HA administration can enhance the synthesis of extracellular matrix proteins, including chondroitin and keratin sulfate, and PGs (Table 2). In rabbit chondrocytes cultured on collagen gels, HA increased the synthesis of the glycosaminoglycan chondroitin sulfate [52]. Release of keratan sulfate, a PG fragment, into synovial fluid is also suppressed by HA in an ovine model [53]. In a clinical study with HA in which patients served as their own controls, keratin sulfate was lower in more knees treated with HA (10/12) than in knees treated with saline (4/12) [54].
Table 2.
Effects of hyaluronan (hyaluronic acid) and hylans on the extracellular matrix
| Effect | Reference | Experimental model; treatment | MW-dependent | Dose-dependent |
| Enhanced HA synthesis | Smith & Ghosh, 1987 [21] | Synovial fibroblasts of patients with normal joints and with OA; HA of various MWs and concentrations | Yes | Yes |
| Increased synthesis of chondroitin sulfate | Kawasaki et al., 1999 [52] | Rabbit chondrocytes; various HA doses | N/A | Yes |
| Enhanced PG synthesis | Frean et al., 1999 [22] | Equine articular cartilage; various HA doses | N/A | Yes |
| Fukuda et al., 1996 [56] | Bovine articular cartilage; various HA doses | N/A | Yes | |
| Enhanced PG synthesis in the presence of IL-1α | Stöve et al., 2002 [58] | Human chondrocytes from OA knee patients; HA, IL-1α or HA + IL-1α | N/A | N/A |
| Increased production of high-MW PGs | Kikuchi et al., 1996 [57] | Rabbit ligamental cells; various HA doses | N/A | Yes |
| Increased content and influenced distribution of PGs | Kikuchi et al., 2001 [108] | Rabbit chondrocytes; various HA concentrations | N/A | Yes |
| Suppressed PG release from cartilage | Yoshioka et al., 1997 [19] | Rabbit ACL transection; HA (five weekly injections) given 4 weeks PS | N/A | N/A |
| Larsen et al., 1992 [61] | Bovine cartilage explants; HA or hylan | N/A | N/A | |
| Suppressed PG release from cell matrix layer | Shimazu et al., 1993 [59] | Rabbit chondrocytes; various MWs and doses of HA | No | Yes |
| Decreased PG release from cartilage matrix | Morris et al., 1992 [60] | Bovine articular cartilage; various doses of HA with or without IL-1β | N/A | Yes |
| Prevented PG breakdown from cartilage | Ghosh et al., 1995 [53] | Ovine meniscectomy; HA (five weekly injections) given 16 weeks PS; keratan sulfate peptide measured in SF 1 week preinjection and 1 and 4 weeks postinjection | N/A | N/A |
| Protected extracellular matrix from degradation | Abatangelo et al., 1989 [63] | Canine ACL resection (Pond-Nuki); HA given 7 days PS weekly for 6 weeks | N/A | N/A |
ACL, anterior cruciate ligament; HA, hyaluronan (hyaluronic acid); IL, interleukin; MW, molecular weight; OA, osteoarthritis; PG, proteoglycan; PS, postsurgery; SF, synovial fluid.
Beneficial effects on PG synthesis have also been demonstrated in vitro with HA. This glycosaminoglycan has been shown to increase PG synthesis in equine articular cartilage [22], rabbit chondrocytes [55], and bovine articular cartilage treated with IL-1, which has been shown to reduce PG synthesis in vitro [56]. An increase in high-MW PG production was also demonstrated with HA in cells of rabbit ligament [57]. In another study, although HA alone decreased PG production from chondrocytes of patients with knee OA, HA countered the reduction of PG production induced by IL-1α [58]. HA has also been shown to suppress the release of PGs from rabbit chondrocytes [19,59] and bovine articular cartilage [60] in the absence and in the presence of IL-1. Additionally, resorption of PGs from cartilage explants was inhibited with hylan; in these experiments, high-viscosity hylan was more effective than a low-viscosity form [61]. A reduction in collagen gene expression induced by IL-1β in rabbit articular chondrocytes has also been suppressed by HA [62]. In an in vivo model of canine OA, a reduced amount of glycosaminoglycan release was found in hyaluronate-treated joints compared with an increased release in untreated joints [63].
HA has also been shown to suppress cartilage damage by fibronectin fragments in vitro and in vivo. Fragments of fibronectin bind and penetrate cartilage and subsequently increase levels of MMPs and suppress PG synthesis [64]. In explant cultures of human cartilage, HA blocked PG depletion induced by fibronectin fragments [65]. This protective effect was associated with its coating of the articular surface, suppression of fibronectin-fragment-enhanced stromelysin-1 release, increased PG synthesis, and restoration of PGs in damaged cartilage [65]. Similar effects of HA on PGs were observed in bovine articular cartilage in vitro: HA suppressed fibronectin-fragment-mediated PG depletion and partially restored PGs in the damaged cartilage [64]. HA also attenuated the enhanced stromelysin-1 release induced with fragments of fibronectin [64]. When fibronectin fragments were intra-articularly administered into rabbit knees, the decrease in PG content was reduced with HA [66].
Effects of hyaluronan on inflammatory mediators
HA has significant effects on inflammatory mediators, including cytokines, proteases and their inhibitors, and prostaglandins (Table 3), that may translate into cartilage protection. In vitro studies show that HA alters the profile of inflammatory mediators such that the balance between cell matrix synthesis and degradation is shifted away from degradation. The proinflammatory cytokine TNF-α and its receptor were not evident in canine atrophied articular cartilage treated with HA by immunostaining but were observed in untreated cartilage [67]. In the synovium of rabbits in the early development of OA, HA also reduced the expression of IL-1β and stromelysin (MMP-3) [23], two mediators known to play a role in cartilage degradation. In bovine articular chondrocytes, high-MW HA stimulated the production of TIMP-1, the MMP inhibitor [68]. Although HA also stimulated stromelysin activity in the same study, the increase was inconsistent and was less with a high-MW than with a low-MW HA [68]. Further, the stromelysin/TIMP-1 ratio was reduced with the high-MW HA, suggesting a cartilage protective effect [68]. The plasminogen activator system, shown to be active in synovial fibroblasts of rheumatoid arthritis (RA), is also influenced by HA [24]. In synovial fibroblasts from OA and RA patients, HA reduced the secreted antigen and activity of urokinase plasminogen activator, as well as its receptor [24]. Similarly, intra-articular administration of HA decreased urokinase plasminogen activator activity in the synovial fluid of patients who showed clinical improvement [69].
Table 3.
Effects of hyaluronan (hyaluronic acid) and hylans on inflammatory mediators
| Effect | Reference | Experimental model; treatment | MW-dependent | Dose-dependent |
| Reduced levels of prostaglandins and leukotriene | Hirota et al., 1998 [72] | Human synovial fluid of temporomandibular joint before and after injection of HA (2 injections 2 weeks apart) | N/A | N/A |
| Decreased levels of PGE2 | Goto et al., 2001 [73] | Synovial fluid of RA patients collected after five weekly HA injections | N/A | N/A |
| Lowered IL-1-induced PGE2 production | Yasui et al., 1992 [71] | Human synovial cells from an OA patient; various MWs and doses of HA | Yes | Yes |
| Stimulated cAMP production; decreased levels of PGE2 | Punzi et al., 1989 [74] | Synovial fluid of patients with knee-joint effusion before and after injection of HA | N/A | N/A |
| Reduced expression of IL-1 and stromelysin | Takahashi et al., 1999 [23] | Rabbit ACL transection; five weekly injections of HA 4 weeks PS; observations 9 weeks PS | N/A | N/A |
| Suppressed production of TNF-α | Comer et al., 1996 [67] | Atrophied canine articular cartilage; HA with or without TGF-β every 4 days from day 56 to day 92 | N/A | N/A |
| Increased production of TIMP-1, the MMP inhibitor; reduced ratio of stromelysin to TIMP-1 | Yasui et al., 1992 [68] | Bovine articular chondrocytes; HA of various MWs | Yes | N/A |
| Decreased plasminogen activator activity and antigen | Nonaka et al., 2000 [24] | Synovial fibroblasts of OA and RA patients; various doses of HA in vitro | N/A | No |
| Nonaka et al., 1999 [69] | Synovial fluid collected from OA patients before and after injection of HA | N/A | N/A | |
| Reduced arachidonic acid release | Tobetto et al., 1992 [70] | Synovial cells of OA patients; various MWs and doses of HA | Yes | Yes |
| Exhibited antioxidant effects | Fukuda et al., 2001 [77] | Bovine articular chondrocytes; various doses of HA | N/A | Yes |
| Fukuda et al., 1997 [78] | Bovine chondrocytes; various doses of HA | N/A | Yes | |
| Moseley et al., 2002 [75] | In vitro oxidation assay | Yes | Yes | |
| Protected cells from damage due to hydroxyl radicals | Presti & Scott, 1994 [80] | Chicken embryo fibroblasts; various MWs and doses of HAs | Yes | Yes |
| Reduced NO production | Takahashi et al., 2001 [81] | Rabbit ACL transection; five weekly HA injections 4 weeks PS; meniscus and synovial NO production assessed in vitro 9 weeks PS |
ACL, anterior cruciate ligament; HA, hyaluronan (hyaluronic acid); IL-1, interleukin-1; MMP, matrix metalloproteinase; MW, molecular weight; NO, nitric oxide; OA, osteoarthritis; PGE2, prostaglandin E2 ; PS, postsurgery; RA, rheumatoid arthritis; TGF-β, transforming growth factor beta; TIMP-1, tissue inhibitor of metalloproteinases-1; TNF-α, tumor necrosis factor alpha.
Metabolites of arachidonic acid such as various prostaglandins mediate, in part, inflammatory responses. HA reduced arachidonic acid release [70] and IL-1α-induced PGE2 production [71] in a dose- and MW-dependent manner; the higher the MW and concentration, the more potent the inhibition. Intra-articular injection of HA in the temporomandibular joint reduced levels of prostaglandin F2α, 6-keto-prostaglandin F1α, and leukotriene C4 [72]. In synovial fluid from the knees of patients with OA and RA, intra-articular HA reduced the levels of PGE2 [73,74] and stimulated cAMP concentrations, another mechanism by which HA may act in an anti-inflammatory manner [74].
HA also has antioxidant effects in various systems. Most recently, in an in vitro assay it showed such effects that were both MW- and dose-dependent [75]. Using two different antioxidant models, Sato and colleagues found that both HA and one of its components, D-glucuronic acid, reduced the amount of reactive oxygen species [76]. Interleukin-1-induced oxidative stress [77] and superoxide anion [78] in bovine chondrocytes were also reduced with HA in a dose-dependent manner. High-MW HA also protects against the damage to articular chondrocytes by oxygen-derived free radicals, which are known to play a role in the pathogenesis of arthritic disorders [79]. Lastly, in avian embryonic fibroblasts, HA reduced cell damage induced by hydroxyl radicals in a MW- and dose-dependent manner [80].
The effects of HA on NO, well recognized for its role in inflammation, may be tissue specific. Production of NO from the meniscus and synovium of a rabbit OA model was significantly reduced with HA treatment [81]. Other experiments showed that HA did not affect NO production from articular cartilage [82,83]. In hepatic cells, fragments of HA increased the expression of the inducible form of NO synthase, while high-MW HA did not have an effect on its expression [84]. In the synovial fluid of OA, it could be speculated that the presence of HA fragments or low-MW HA may induce the inducible form of NO synthase, consequently increasing NO concentration in the disease state. Introducing a high-MW HA could prevent the production of NO in OA; however, further studies are needed to support this hypothesis.
Effects of hyaluronan on immune cells
Besides altering the production and activity of inflammatory mediators and proteases, HA can change the behavior of immune cells. Its effects on immune cells are summarized in Table 4. HA has been shown to reduce the motility of lymphocytes; this observation occurred with physiological fluids (i.e. synovial fluid and liquid vitreous) containing a high concentration of HA [25]. When the HA in these fluids was digested with hyaluronidase, it no longer inhibited the motility, indicating that the motility inhibition depended on the molecular size and polysaccharide conformation of the molecule [25]. Inhibition of lymphocyte proliferation by HA has also been shown to be dependent on the MW as well as the concentration of HA [85]. Similarly, lymphocyte stimulation in vitro was shown to be suppressed by HA in a MW-dependent manner [86].
Table 4.
Effects of hyaluronan (hyaluronic acid) and hylans on immune cells
| Effect | Reference | Experimental model; treatment | MW-dependent | Dose-dependent |
| Reduced lymphocyte motility | Balazs & Darzynkiewicz, 1973 [25] | Macrophages from various species; high- and low-MW HA | Yes | Yes |
| Inhibited lymphocyte proliferation | Peluso et al., 1990 [85] | Human mononuclear cells; high- and low-MW HA | Yes | Yes |
| Suppressed lymphocyte stimulation | Darzynkiewicz & Balazs, 1971 [86] | Human lymphocytes; various MWs and doses of HA | Yes | Yes |
| Inhibited macrophage phagocytosis and cell motility | Balazs et al., 1981 [26] | Mouse macrophages; human and equine synovial fluid | N/A | N/A |
| Forrester & Balazs, 1980 [87] | Mouse macrophages; high- and low-MW HAs | Yes | Yes | |
| Inhibited phagocytosis and degranulation of neutrophils | Pisko et al., 1983 [88] | Human neutrophils; various doses of HA | N/A | Yes |
| Reduced PMN leukocyte migration and activation | Partsch et al., 1989 [89] | PMN cells from OA patients; various HA doses | N/A | Yes |
| Inhibited cartilage degradation associated with neutrophil adhesion | Tobetto et al., 1993 [92] | Rat peritoneal neutrophils exposed to bovine cartilage | Yes | Yes |
| Suppressed neutrophil aggregation and adhesion | Forrester ackie, 1981 [93] | Rabbit neutrophils; various HA doses and MWs | Yes | Yes |
| Stimulated PMN leukocyte phagocytosis, adherence, and migration | Håkansson et al., 1980 [91] | Human PMN leukocytes from patients with impaired host resistance | N/A | N/A |
HA, hyaluronan (hyaluronic acid); MW, molecular weight; OA, osteoarthritis; PMN, polymorphonuclear.
Leukocyte function, including phagocytosis, adherence, and mitogen-activated stimulation, can be modulated by HA. Both human and equine synovial fluids have been shown to inhibit macrophage phagocytosis, an effect that was dependent on the viscosity of the fluid [26]. Similarly, high-MW HA inhibited macrophage phagocytosis in a dose-dependent manner, while a low-MW hyaluronate did not inhibit phagocytosis [87]. Neutrophil phagocytosis was also significantly inhibited by HA at a concentration of 4 mg/ml (close to that of normal synovial fluid) but not at 1 mg/ml [88].
The function of the polymorphonuclear (PMN) leukocyte is also influenced by HA. All concentrations of HA tested reduced PMN leukocyte migration in a dose-dependent manner [89]. HA also inhibited PMN leukocyte migration induced by leukotriene B4, a potent chemotactic factor [89]. Additionally, activation of PMN leukocytes, as measured by superoxide generation, was inhibited with hylan concentrations greater than 0.5 mg/ml [61]. The degree of this inhibition was directly correlated with the viscosity of the hylan sample [61]. Finally, HA has been shown to increase the negative charge and number of hydrophobic sites on the cell surface of PMN leukocytes [90], which may alter cell–cell communication in a way that has yet to be determined. In contrast, HA has been shown to stimulate PMN leukocyte function both in vitro and in vivo [91]. These conflicting results may be due to the fact that in the latter study the leukocytes were isolated from patients with impaired host resistance.
Cartilage degradation associated with neutrophils has been associated with neutrophil adhesion to cartilage in vitro [92]. HA was shown to inhibit this neutrophil-induced cartilage degradation in a dose- and MW-dependent fashion [92]. Neutrophil aggregation and adhesion were also inhibited by HA in a dose- and MW-dependent manner, but this inhibition was not dependent on HA viscosity [93].
Cartilage effects of hyaluronan and hylans
The effects of HA and hylans on cartilage histology are well documented in experimental animal studies, but strong clinical trial data is lacking (Table 5).
Table 5.
Effects of hyaluronan (hyaluronic acid) and hylans on cartilage
| Effect | Reference | Experimental model/treatment/endpoints | MW-dependent |
| Suppressed cartilage degeneration | Shimizu et al., 1998 [18] | Rabbit ACL transection; HA (five weekly injections) or crosslinked HA (three weekly injections) 4 weeks PS; observations 9 weeks PS | Yes |
| Listrat et al., 1997 [10] | Clinical study of OA patient (n = 36; 1 year); three weekly HA injections every 3 months; arthroscopic evaluation 1 year from study start | N/A | |
| Prevented cartilage damage | Ghosh et al., 1995 [53] | Ovine meniscectomy; HA (five weekly injections) given 16 weeks PS; joint articular cartilage histologically graded 5 weeks after last injection | N/A |
| Prevented cartilage damage; maintained normal morphology | Schiavinato et al., 1989 [20] | Pond-Nuki canine OA model; HA given 1–7 weeks PS or 7–17 weeks PS; observations 7, 13, and 17 weeks PS | N/A |
| Preserved cartilage histology and smoothness | Yoshimi et al., 1994 [102] | Rabbit ACL resection; HA (various MWs) injections one week PS weekly until assessments were made (6 or 12 weeks) | Yes |
| Sakakibara et al., 1994 [103] | Rabbits immobilized at the onset of HA administration; HA (various MWs) twice/week for 5 weeks; observations 1–6 weeks after immobilization | Yes | |
| Fu et al., 2001 [94] | Immobilization-induced cartilage degradation in rabbits; six weekly injections of HA with remobilization; assessments 1 week after the last injection | Yes | |
| Improved superficial cartilage layer and synovial membrane morphology; reduced synovial thickness and inflammation | Frizziero et al., 1998 [104] | Clinical study of OA patients (n = 40); HA (five weekly injections); cartilage and synovial biopsies and arthroscopy performed at baseline and 6 months after first injection | N/A |
| Improved synovium structure and synoviocyte morphology; reduced inflammatory cells in the synovium | Pasquali Ronchetti et al., 2001 [105] | Clinical study of patients with primary and secondary OA (n = 99); HA (five weekly injections) or MP (three weekly injections); synovial biopsies 2–3 weeks pretreatment and 6 months post-treatment | N/A |
| Improved superficial cartilage compactness and thickness; increased chondrocyte density (HA better results than MP for most parameters); improved chondrocyte morphology | Guidolin et al., 2001 [106] | Clinical study of OA patients (n = 24); HA (five weekly injections) or MP (three weekly injections); biopsies taken 6 months from treatment initiation | N/A |
| Prevented cartilage damage; maintained cartilage thickness, area, and smoothness | Yoshioka et al., 1997 [19] | Rabbit ACL transection; HA given 4 weeks PS; assessed femoral condyles 9 weeks after surgery | N/A |
| Prevented cartilage damage; maintained cartilage thickness, area, smoothness, and surface uniformity | Shimizu et al., 1998 [95] | Rabbit ACL transection; HA given 4 weeks PS; assessed femoral condyles 21 weeks after surgery | N/A |
| Maintained cartilage smoothness; prevented deep fissures and cracks in the cartilage surface | Wenz et al., 2000 [101] | Severe and resect canine ACL (Pond-Nuki); HA (five weekly injections) given 3, 6, or 12 weeks PS; assessed patella and patella/knee 5 weeks after last injection | N/A |
| Enhanced meniscal regeneration; inhibited cartilage deterioration | Kobayashi et al., 2000 [96] | Rabbit partial meniscectomy; HA (five weekly injections) 1 week PS; assessed meniscus and tibial cartilage 6 months PS | N/A |
| Reduced disease severity | Marshall et al., 2000 [99] | Canine OA models; hylan G-F 20 (three weekly injections) given 2 months PS; assessed 6 months after treatment | N/A |
| Accelerated migration of synovial cells; enhanced migration of chondrocytes when coincubated with bFGF | Maniwa et al., 2001 [107] | Rabbit synovial cells and chondrocytes incubated with HA, bFGF, HA + bFGF | N/A |
ACL, anterior cruciate ligament; bFGF, basic fibroblastic growth factor; HA, hyaluronan (hyaluronic acid); MP, methylprednisolone; MW, molecular weight; OA, osteoarthritis; PS, postsurgery.
Experimental OA studies
Histological data demonstrate a protective effect of HA on cartilage in various animal models of experimental OA. Overall, the therapeutic use of HA has been shown to reduce the severity of OA and to maintain cartilage thickness, area, and surface smoothness. In rabbits with cartilage degeneration from immobilization, HA reduced the area of cartilage ulceration observed and prevented loss of chondrocytes [94]. Several beneficial effects of HA on cartilage have also been demonstrated in experimental OA induced by anterior cruciate ligament transection in rabbits. In general, the grade of cartilage damage 9 weeks after treatment was less severe in animals treated with HA than in animals given the vehicle only or not treated [19]. When compared with the cartilage of nonsurgical contralateral controls, the cartilage of HA-treated joints was equal in thickness and area, while cartilage thickness and area in vehicle-treated and untreated joints were significantly less than in controls [19]. Additionally, surface roughness was significantly less in HA-treated animals than in vehicle-treated and untreated animals [19]. A 21-week study found similar protective effects on cartilage [95]. Even after 6 months, HA has been shown to enhance meniscal regeneration and inhibit cartilage degradation in rabbits with partial meniscectomy [96]. The grade of OA tended to be less severe in animals given HA than in those give the vehicle only, and more intense immunostaining for glycosaminoglycans was observed with HA treatment compared with the vehicle [96].
Other studies investigating the effects of HA on meniscus injury and repair in rabbits found no differences attributable to treatment. However, this may have been due to the timing of HA treatment [97,98]. In these studies, HA was administered 1 week after surgery [97,98], as opposed to 4 weeks after surgery in the other studies just mentioned [19,95]. Similarly, in other studies using a canine OA model, hylan reduced disease severity when animals were treated 2 months after surgery [99]; however, the effects on cartilage when hylan or HA was injected immediately after surgery were similar to those of the vehicle [99,100]. When HA was given 3, 6, or 12 weeks after anterior cruciate ligament resection in dogs (Pond-Nuki OA model), the cartilage was smooth and did not display deep fissures or cracks as in the placebo-treated animals [101]. Another study using the Pond-Nuki model showed that HA treatment significantly reduced OA progression as measured by a reduced OA grade in comparison with controls [20].
The extent of beneficial effect on cartilage observed with HA may largely depend on the MW of the HA formulation. In a study of OA in rabbits, cartilage degeneration was less in all HA groups tested, but was significantly less with HA with a MW of 2.02 × 106 than HA with a MW of 9.5 × 105 [102]. Similarly, the histology of articular cartilage and synovial tissue was significantly better with an HA of MW = 2.02 × 106 than with an HA of MW = 9.8 × 105 [103]. Shimizu and colleagues found that the protective effects of hylan G-F 20 (80% hylan A [MW = 6.0 × 106], 20% hylan B gel) and an HA of MW 8 × 105 on articular cartilage were similar to each other, but better than those observed with HAs of MW 5–7 × 105 or 3.6 × 106 [18].
Clinical trials
Until now, the effects of HA on cartilage have not been demonstrated in any randomized, placebo-controlled trials. Results from trials of other types of study design presented here warrant further study in more rigorous trials. In an open-label study (n = 40) of five weekly injections of HA, both the cartilage and the synovial membrane were improved when measured 6 months after the injection [104]. In the nine patients with grade II OA who were assessed, the thickness of the superficial amorphous cartilage layer improved significantly between the baseline and final evaluations [104]. A significant reduction in the thickness of the synovial membrane and in the number of infiltrating mononuclear cells indicated reduced inflammation of the synovium [104]. In a study where patients were randomized to conventional therapy and then arthroscopically evaluated for severity of chondropathy, cartilage deterioration was observed in both control and HA groups, but was significantly less in the HA group as measured by an investigator overall visual analog score and the Société Française d'Arthroscopie (SFA) scoring system [10]. Although these initial clinical trials have several limitations, including an open-label design, unblinded evaluation, lack of appropriate controls, and small sample size, the data from these studies warrant further study of the effects of HA on cartilage protection and disease progression in more rigorous, prospective, randomized, controlled, double-blind clinical studies.
The effects of sodium hyaluronate or methylprednisolone acetate on articular cartilage and the synovium have also been compared in a clinical setting [105,106]. In the synovium of HA-treated knees, the number and aggregation of synoviocytes decreased, and both treatments reduced the number of inflammatory cells, including macrophages, lymphocytes, and mast cells [105]. Sodium hyaluronate (five weekly injections) also significantly improved the compactness and thickness of the amorphous superficial cartilage layer 6 months after treatment, in comparison with baseline [106]. Cartilage changes with methylprednisolone acetate 6 months after treatment were not significantly different from baseline, and the thickness of the superficial amorphous layer was significantly improved with HA compared with intra-articular steroid [106]. Chondrocyte density was also significantly higher with HA compared with baseline and steroid treatment [106]. Lastly, many of the morphometric parameters of the chondrocytes were significantly better with HA than with methylprednisolone [106]. Because the migration and proliferation of chondrogenic precursor cells to the site of cartilage injury are necessary for cartilage repair, an in vitro study examined the effects of HA and basic fibroblastic growth factor on the migration of rabbit synovial cell and chondrocyte migration [107]. The rate of synovial cell migration was enhanced with HA alone, and HA increased chondrocyte migration in the presence of this growth factor [107].
Conclusions
The clinical effects of HA on pain associated with OA of the knee are probably mediated by several factors. In vitro and in vivo studies indicate that HA can enhance PG synthesis and prevent its release from the cell matrix. Regarding inflammation, HA suppresses the production and activity of proinflammatory mediators and proteases as well as altering the function of certain immune cells. Histological evidence shows that HA prevents the degradation of cartilage and may promote its regeneration. Collectively, the physiological effects of intra-articular HA reviewed here support a multifactorial mechanism for HA and hylans in the treatment of pain from knee OA. Future studies investigating the effects of HA and hylans on cartilage in well-controlled clinical studies may help determine whether intra-articular HA and hylans aid in chondroprotection and slowing the progression of disease, and whether the MW of the HA formulation contributes to its efficacy.
Competing interests
LWM has been a paid consultant for Wyeth, Genzyme and Sanofi-Synthelab, companies that market intra-articular hyaluronan products.
Abbreviations
HA = hyaluronan (hyaluronic acid); IL = interleukin; MMP = matrix metalloproteinases; MP = methylprednisolone; MW = molecular weight; NO = nitric oxide; OA = osteoarthritis; PG = proteoglycan; PGE2 = prostaglandin E2; PMN = polymorphonuclear; RA = rheumatoid arthritis; TIMP = tissue inhibitor of metalloproteinases; TNF-α = tumor necrosis factor alpha.
References
- Carr AJ. Beyond disability: measuring the social and personal consequences of osteoarthritis. Osteoarthritis Cartilage. 1999;7:230–238. doi: 10.1053/joca.1998.0154. [DOI] [PubMed] [Google Scholar]
- American College of Rheumatology Subcommittee on Osteoarthritis Guidelines Recommendations for the medical management of osteoarthritis of the hip and knee. Arthritis Rheum. 2000;43:1905–1915. doi: 10.1002/1529-0131(200009)43:9<1905::AID-ANR1>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- Balazs E. The physical properties of synovial fluid and the specific role of hyaluronic acid. In: Helfet AJ, editor. In Disorders of the Knee. Philadelphia: J B Lippincott; 1982. pp. 61–74. [Google Scholar]
- Adams ME, Atkinson MH, Lussier AJ, Schulz JI, Siminovitch KA, Wade JP, Zummer M. The role of viscosupplementation with hylan G-F 20 (Synvisc) in the treatment of osteoarthritis of the knee: a Canadian multicenter trial comparing hylan G-F 20 alone, hylan G-F 20 with non-steroidal anti-inflammatory drugs (NSAIDs) and NSAIDs alone. Osteoarthritis Cartilage. 1995;3:213–225. doi: 10.1016/s1063-4584(05)80013-5. [DOI] [PubMed] [Google Scholar]
- Lussier A, Cividino AA, McFarlane CA, Olszynski WP, Potashner WJ, De Medicis R. Viscosupplementation with hylan for the treatment of osteoarthritis: findings from clinical practice in Canada. J Rheumatol. 1996;23:1579–1585. [PubMed] [Google Scholar]
- Wobig M, Dickhut A, Maier R, Vetter G. Viscosupplementation with hylan G-F 20: a 26-week controlled trial of efficacy and safety in the osteoarthritic knee. Clin Ther. 1998;20:410–423. doi: 10.1016/S0149-2918(98)80052-0. [DOI] [PubMed] [Google Scholar]
- Scale D, Wobig M, Wolpert W. Viscosupplementation of osteoarthritic knees with hylan: a treatment schedule study. Curr Ther Res. 1994;55:220–232. [Google Scholar]
- Wobig M, Beks P, Dickhut A, Maier R, Vetter G. Open-label multicenter trial of the safety and efficacy of viscosupplementation with hylan G-F 20 (Synvisc) in primary osteoarthritis of the knee. J Clin Rheumatol. 1999;5(suppl):S24–S31. [Google Scholar]
- Raynauld JP, Torrance G, Band P, Goldsmith C, Tugwell P, Walker V, Schultz M, Bellamy N. A prospective, randomized, pragmatic, health outcomes trial evaluating the incorporation of hylan G-F into the treatment paradigm for patients with knee osteoarthritis (Part 1 of 2): clinical results. Osteoarthritis Cartilage. 2002;10:506–517. doi: 10.1053/joca.2002.0798. [DOI] [PubMed] [Google Scholar]
- Listrat V, Ayral X, Patarnello F, Bonvarlet JP, Simonnet J, Amor B, Dougados M. Arthroscopic evaluation of potential structure modifying activity of hyaluronan (Hyalgan) in osteoarthritis of the knee. Osteoarthritis Cartilage. 1997;5:153–160. doi: 10.1016/s1063-4584(97)80010-6. [DOI] [PubMed] [Google Scholar]
- Altman RD, Moskowitz R. Intraarticular sodium hyaluronate (Hyalgan) in the treatment of patients with osteoarthritis of the knee: a randomized clinical trial. Hyalgan Study Group. J Rheumatol. 1998;25:2203–2212. [PubMed] [Google Scholar]
- Dixon ASJ, Jacoby RK, Berry H, Hamilton EBD. Clinical trial of intra-articular injection of sodium hyaluronate in patients with osteoarthritis of the knee. Curr Med Res Opin. 1988;11:205–213. doi: 10.1185/03007998809114237. [DOI] [PubMed] [Google Scholar]
- Petrella RJ, DiSilvestro MD, Hildebrand C. Effects of hyaluronate sodium on pain and physical functioning in osteoarthritis of the knee. A randomized, double-blind, placebo-controlled clinical trial. Arch Intern Med. 2002;162:292–298. doi: 10.1001/archinte.162.3.292. [DOI] [PubMed] [Google Scholar]
- Dougados M, Nguyen M, Listrat V, Amor B. High molecular weight sodium hyaluronate (hyalectin) in osteoarthritis of the knee: a 1 year placebo-controlled trial. Osteoarthritis Cartilage. 1993;1:97–103. doi: 10.1016/s1063-4584(05)80024-x. [DOI] [PubMed] [Google Scholar]
- Pozo MA, Balazs EA, Belmonte C. Reduction of sensory responses to passive movements of inflamed knee joints by hylan, a hyaluronan derivative. Exp Brain Res. 1997;116:3–9. doi: 10.1007/pl00005742. [DOI] [PubMed] [Google Scholar]
- Aihara S, Murakami N, Ishii R, Kariya K, Azuma Y, Hamada K, Umemoto J, Maeda S. [Effects of sodium hyaluronate on the nociceptive response of rats with experimentally induced arthritis]. Nippon Yakurigaku Zasshi. 1992;100:359–365. doi: 10.1254/fpj.100.359. [DOI] [PubMed] [Google Scholar]
- Gotoh S, Onaya J, Abe M, Miyazaki K, Hamai A, Horie K, Tokuyasu K. Effects of the molecular weight of hyaluronic acid and its action mechanisms on experimental joint pain in rats. Ann Rheum Dis. 1993;52:817–822. doi: 10.1136/ard.52.11.817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimizu C, Kubo T, Hirasawa Y, Coutts RD, Amiel D. Histomorphometric and biochemical effect of various hyaluronans on early osteoarthritis. J Rheumatol. 1998;25:1813–1819. [PubMed] [Google Scholar]
- Yoshioka M, Shimizu C, Harwood FL, Coutts RD, Amiel D. The effects of hyaluronan during the development of osteoarthritis. Osteoarthritis Cartilage. 1997;5:251–260. doi: 10.1016/s1063-4584(97)80021-0. [DOI] [PubMed] [Google Scholar]
- Schiavinato A, Lini E, Guidolin D, Pezzoli G, Botti P, Martelli M, Cortivo R, De Galateo A, Abatangelo G. Intraarticular sodium hyaluronate injections in the Pond-Nuki experimental model of osteoarthritis in dogs. II. Morphological findings. Clin Orthop. 1989;241:286–299. [PubMed] [Google Scholar]
- Smith MM, Ghosh P. The synthesis of hyaluronic acid by human synovial fibroblasts is influenced by the nature of the hyaluronate in the extracellular environment. Rheumatol Int. 1987;7:113–122. doi: 10.1016/0732-8893(87)90028-9. [DOI] [PubMed] [Google Scholar]
- Frean SP, Abraham LA, Lees P. In vitro stimulation of equine articular cartilage proteoglycan synthesis by hyaluronan and carprofen. Res Vet Sci. 1999;67:183–190. doi: 10.1053/rvsc.1999.0328. [DOI] [PubMed] [Google Scholar]
- Takahashi K, Goomer RS, Harwood F, Kubo T, Hirasawa Y, Amiel D. The effects of hyaluronan on matrix metalloproteinase-3 (MMP-3), interleukin-1beta (IL-1beta), and tissue inhibitor of metalloproteinase-1 (TIMP-1) gene expression during the development of osteoarthritis. Osteoarthritis Cartilage. 1999;7:182–190. doi: 10.1053/joca.1998.0207. [DOI] [PubMed] [Google Scholar]
- Nonaka T, Kikuchi H, Ikeda T, Okamoto Y, Hamanishi C, Tanaka S. Hyaluronic acid inhibits the expression of u-PA, PAI-1, and u-PAR in human synovial fibroblasts of osteoarthritis and rheumatoid arthritis. J Rheumatol. 2000;27:997–1004. [PubMed] [Google Scholar]
- Balazs EA, Darzynkiewicz Z. The effect of hyaluronic acid on fibroblasts, mononuclear phagocytes and lymphocytes. Biology and Fibroblasts. 1973;66:237–252. [Google Scholar]
- Balazs EA, Briller S, Denlinger JL. Na-hyaluronate molecular size variations in equine and human arthritic synovial fluids and the effect on phagocytic cells. Semin Arthritis Rheum. 1981;11:141–143. [Google Scholar]
- Ghosh P, Cheras PA. Vascular mechanisms in osteoarthritis. Best Pract Res Clin Rheumatol. 2001;15:693–710. doi: 10.1053/berh.2001.0188. [DOI] [PubMed] [Google Scholar]
- Lotz M. Cytokines in cartilage injury and repair. Clin Orthop. 2001;S391:S108–S115. doi: 10.1097/00003086-200110001-00011. [DOI] [PubMed] [Google Scholar]
- Sandell LJ, Aigner T. Articular cartilage and changes in arthritis. An introduction: cell biology of osteoarthritis. Arthritis Res. 2001;3:107–113. doi: 10.1186/ar148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelletier JP, Martel-Pelletier J, Abramson SB. Osteoarthritis, an inflammatory disease: potential implication for the selection of new therapeutic targets. Arthritis Rheum. 2001;44:1237–1247. doi: 10.1002/1529-0131(200106)44:6<1237::AID-ART214>3.0.CO;2-F. [DOI] [PubMed] [Google Scholar]
- Poole AR. An introduction to the pathophysiology of osteoarthritis. Front Biosci. 1999;4:D662–D670. doi: 10.2741/poole. [DOI] [PubMed] [Google Scholar]
- Tortorella MD, Malfait AM, Deccico C, Arner E. The role of ADAM-TS4 (aggrecanase-1) and ADAM-TS5 (aggrecanase-2) in a model of cartilage degradation. Osteoarthritis Cartilage. 2001;9:539–552. doi: 10.1053/joca.2001.0427. [DOI] [PubMed] [Google Scholar]
- Bland JH, Cooper SM. Osteoarthritis: a review of the cell biology involved and evidence for reversibility. Management rationally related to known genesis and pathophysiology. Semin Arthritis Rheum. 1984;14:106–133. doi: 10.1016/0049-0172(84)90002-7. [DOI] [PubMed] [Google Scholar]
- Belcher C, Yaqub R, Fawthrop F, Bayliss M, Doherty M. Synovial fluid chondroitin and keratan sulphate epitopes, glycosaminoglycans, and hyaluronan in arthritic and normal knees. Ann Rheum Dis. 1997;56:299–307. doi: 10.1136/ard.56.5.299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tortorella MD, Burn TC, Pratta MA, Abbaszade I, Hollis JM, Liu R, Rosenfeld SA, Copeland RA, Deccico CP, Wynn R, Rockwell A, Yang F, Duke JL, Solomon K, George H, Bruckner R, Nagase H, Itoh Y, Ellis DM, Ross H, Wiswall BH, Murphy K, Hillman MC, Jr, Hollis GF, Newton RC, Magolda RL, Trzaskos JM, Arner EC. Purification and cloning of aggrecanase-1: a member of the ADAMTS family of proteins. Science. 1999;284:1664–1666. doi: 10.1126/science.284.5420.1664. [DOI] [PubMed] [Google Scholar]
- Abramson SB, Attur M, Amin AR, Clancy R. Nitric oxide and inflammatory mediators in the perpetuation of osteoarthritis. Curr Rheumatol Rep. 2001;3:535–541. doi: 10.1007/s11926-001-0069-3. [DOI] [PubMed] [Google Scholar]
- Alaaeddine N, Olee T, Hashimoto S, Creighton-Achermann L, Lotz M. Production of the chemokine RANTES by articular chondrocytes and role in cartilage degradation. Arthritis Rheum. 2001;44:1633–1643. doi: 10.1002/1529-0131(200107)44:7<1633::AID-ART286>3.0.CO;2-Z. [DOI] [PubMed] [Google Scholar]
- Amin AR, Dave M, Attur M, Abramson SB. COX-2, NO, and cartilage damage and repair. Curr Rheumatol Rep. 2000;2:447–453. doi: 10.1007/s11926-000-0019-5. [DOI] [PubMed] [Google Scholar]
- Amin AR, Attur M, Abramson SB. Nitric oxide synthase and cyclooxygenases: distribution, regulation, and intervention in arthritis. Curr Opin Rheumatol. 1999;11:202–209. doi: 10.1097/00002281-199905000-00009. [DOI] [PubMed] [Google Scholar]
- Lotz M, Blanco FJ, Kempis J, Dudler J, Maier R, Villiger PM, Geng Y. Cytokine regulation of chondrocyte functions. J Rheumatol. 1995;22:104–108. [PubMed] [Google Scholar]
- Clemmons DR, Busby WH, Garmong A, Schultz DR, Howell DS, Altman RD, Karr R. Inhibition of insulin-like growth factor binding protein 5 proteolysis in articular cartilage and joint fluid results in enhanced concentrations of insulin-like growth factor 1 and is associated with improved osteoarthritis. Arthritis Rheum. 2002;46:694–703. doi: 10.1002/art.10222. [DOI] [PubMed] [Google Scholar]
- Lotz M, Hashimoto S, Kuhn K. Mechanisms of chondrocyte apoptosis. Osteoarthritis Cartilage. 1999;7:389–391. doi: 10.1053/joca.1998.0220. [DOI] [PubMed] [Google Scholar]
- Hashimoto S, Ochs RL, Setsuro K, Lotz M. Linkage of chondrocyte apoptosis and cartilage degradation in human osteoarthritis. Arthritis Rheum. 1998;41:1632–1638. doi: 10.1002/1529-0131(199809)41:9<1632::AID-ART14>3.0.CO;2-A. [DOI] [PubMed] [Google Scholar]
- Hashimoto S, Takahashi K, Amiel D, Coutts RD, Lotz M. Chondrocyte apoptosis and nitric oxide production during experimentally induced osteoarthritis. Arthritis Rheum. 1998;41:1266–1274. doi: 10.1002/1529-0131(199807)41:7<1266::AID-ART18>3.0.CO;2-Y. [DOI] [PubMed] [Google Scholar]
- Tanimoto K, Ohno S, Fujimoto K, Honda K, Ijuin C, Tanaka N, Doi T, Nakahara M, Tanne K. Proinflammatory cytokines regulate the gene expression of hyaluronic acid synthetase in cultured rabbit synovial membrane cells. Connect Tissue Res. 2001;42:187–195. doi: 10.3109/03008200109005649. [DOI] [PubMed] [Google Scholar]
- Salter DM, Godolphin JL, Gourlay MS, Lawson MF, Hughes DE, Dunne E. Analysis of human articular chondrocyte CD44 isoform expression and function in health and disease. J Pathol. 1996;179:396–402. doi: 10.1002/(SICI)1096-9896(199608)179:4<396::AID-PATH606>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- Knudson W, Loeser RF. CD44 and integrin matrix receptors participate in cartilage homeostasis. Cell Mol Life Sci. 2002;59:36–44. doi: 10.1007/s00018-002-8403-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chow G, Nietfeld JJ, Knudson CB, Knudson W. Antisense inhibition of chondrocyte CD44 expression leading to cartilage chondrolysis. Arthritis Rheum. 1998;41:1411–1419. doi: 10.1002/1529-0131(199808)41:8<1411::AID-ART10>3.0.CO;2-Z. [DOI] [PubMed] [Google Scholar]
- Ishida O, Tanaka Y, Morimoto I, Takigawa M, Eto S. Chondrocytes are regulated by cellular adhesion through CD44 and hyaluronic acid pathway. J Bone Miner Res. 1997;12:1657–1663. doi: 10.1359/jbmr.1997.12.10.1657. [DOI] [PubMed] [Google Scholar]
- Belmonte C, Pozo MA, Balazs EA. Modulation by hyaluronan and its derivatives (hylans) of sensory nerve activity signaling articular pain. In: Laurent T, editor. In Chemistry, Biology and Medical Applications of Hyaluronan and Its Derivatives Proceedings of the Wenner-Gren Foundation International Symposium. London: Portland Press; 1998. pp. 205–217. [Google Scholar]
- Moore AR, Willoughby DA. Hyaluronan as a drug delivery system for diclofenac: a hypothesis for mode of action. Int J Tissue React. 1995;17:153–156. [PubMed] [Google Scholar]
- Kawasaki K, Ochi M, Uchio Y, Adachi N, Matsusaki M. Hyaluronic acid enhances proliferation and chondroitin sulfate synthesis in cultured chondrocytes embedded in collagen gels. J Cell Physiol. 1999;179:142–148. doi: 10.1002/(SICI)1097-4652(199905)179:2<142::AID-JCP4>3.0.CO;2-Q. [DOI] [PubMed] [Google Scholar]
- Ghosh P, Holbert C, Read R, Armstrong S. Hyaluronic acid (hyaluronan) in experimental osteoarthritis. J Rheumatol Suppl. 1995;43:155–157. [PubMed] [Google Scholar]
- Creamer P, Sharif M, George E, Meadows K, Cushnaghan J, Shinmei M, Dieppe P. Intra-articular hyaluronic acid in osteoarthritis of the knee: an investigation into mechanisms of action. Osteoarthritis Cartilage. 1994;2:133–140. doi: 10.1016/s1063-4584(05)80063-9. [DOI] [PubMed] [Google Scholar]
- Kikuchi T, Yamada H, Shimmei M. Effect of high molecular weight hyaluronan on cartilage degeneration in a rabbit model of osteoarthritis. Osteoarthritis Cartilage. 1996;4:99–110. doi: 10.1016/s1063-4584(05)80319-x. [DOI] [PubMed] [Google Scholar]
- Fukuda K, Dan H, Takayama M, Kumano F, Saitoh M, Tanaka S. Hyaluronic acid increases proteoglycan synthesis in bovine articular cartilage in the presence of interleukin-1. J Pharmacol Exp Ther. 1996;277:1672–1675. [PubMed] [Google Scholar]
- Kikuchi T, Sakuta T, Yamaguchi T. Effects of hyaluronan on cell proliferation and proteoglycan synthesis in rabbit ligamental cells. Int J Tissue React. 1996;18:87–95. [PubMed] [Google Scholar]
- Stove J, Gerlach C, Huch K, Gunther KP, Puhl W, Scharf HP. Effects of hyaluronan on proteoglycan content of osteoarthritic chondrocytes in vitro. J Orthop Res. 2002;20:551–555. doi: 10.1016/S0736-0266(01)00141-3. [DOI] [PubMed] [Google Scholar]
- Shimazu A, Jikko A, Iwamoto M, Koike T, Yan W, Okada Y, Shinmei M, Nakamura S, Kato Y. Effects of hyaluronic acid on the release of proteoglycan from the cell matrix in rabbit chondrocyte cultures in the presence and absence of cytokines. Arthritis Rheum. 1993;36:247–253. doi: 10.1002/art.1780360217. [DOI] [PubMed] [Google Scholar]
- Morris EA, Wilcon S, Treadwell BV. Inhibition of interleukin 1-mediated proteoglycan degradation in bovine articular cartilage explants by addition of sodium hyaluronate. Am J Vet Res. 1992;53:1977–1982. [PubMed] [Google Scholar]
- Larsen NE, Lombard KM, Parent EG, Balazs EA. Effect of hylan on cartilage and chondrocyte cultures. J Orthop Res. 1992;10:23–32. doi: 10.1002/jor.1100100104. [DOI] [PubMed] [Google Scholar]
- Goto H, Onodera T, Hirano H, Shimamura T. Hyaluronic acid suppresses the reduction of α2(VI) collagen gene expression caused by interleukin-1β in cultured rabbit articular chondrocytes. Tohoku J Exp Med. 1999;187:1–13. doi: 10.1620/tjem.187.1. [DOI] [PubMed] [Google Scholar]
- Abatangelo G, Botti P, Del Bue M, Gei G, Samson JC, Cortivo R, De Galateo A, Martelli M. Intraarticular sodium hyaluronate injections in the Pond-Nuki experimental model of osteoarthritis in dogs. I. Biochemical results. Clin Orthop. 1989;241:278–285. [PubMed] [Google Scholar]
- Homandberg GA, Hui F, Wen C, Kuettner KE, Williams JM. Hyaluronic acid suppresses fibronectin fragment mediated cartilage chondrolysis: I. In vitro. Osteoarthritis Cartilage. 1997;5:309–319. doi: 10.1016/s1063-4584(97)80035-0. [DOI] [PubMed] [Google Scholar]
- Kang Y, Eger W, Koepp H, Williams JM, Kuettner KE, Homandberg GA. Hyaluronan suppresses fibronectin fragment-mediated damage to human cartilage explant cultures by enhancing proteoglycan synthesis. J Orthop Res. 1999;17:858–869. doi: 10.1002/jor.1100170611. [DOI] [PubMed] [Google Scholar]
- Williams JM, Plaza V, Hui F, Wen C, Kuettner KE, Homandberg GA. Hyaluronic acid suppresses fibronectin fragment mediated cartilage chondrolysis: II. In vivo. Osteoarthritis Cartilage. 1997;5:235–240. doi: 10.1016/s1063-4584(97)80019-2. [DOI] [PubMed] [Google Scholar]
- Comer JS, Kincaid SA, Baird AN, Kammermann JR, Hanson RR, Jr, Ogawa Y. Immunolocalization of stromelysin, tumor necrosis factor (TNF) alpha, and TNF receptors in atrophied canine articular cartilage treated with hyaluronic acid and transforming growth factor beta. Am J Vet Res. 1996;57:1488–1496. [PubMed] [Google Scholar]
- Yasui T, Akatsuka M, Tobetto K, Umemoto J, Ando T, Yamashita K, Hayakawa T. Effects of hyaluronan on the production of stromelysin and tissue inhibitor of metalloproteinase-1 (TIMP-1) in bovine articular chondrocytes. Biomed Res. 1992;13:343–348. [Google Scholar]
- Nonaka T, Kikuchi H, Shimada W, Itagene H, Ikeda T, Hamanishi C, Tanaka S. Effects of hyaluronic acid on fibrinolytic factors in the synovial fluid (in vivo). Pathophysiology. 1999;6:41–44. doi: 10.1016/S0928-4680(98)00031-5. [DOI] [Google Scholar]
- Tobetto K, Yasui T, Ando T, Hayaishi M, Motohashi N, Shinogi M, Mori I. Inhibitory effects of hyaluronan on [14C]arachidonic acid release from labeled human synovial fibroblasts. Jpn J Pharmacol. 1992;60:79–84. doi: 10.1254/jjp.60.79. [DOI] [PubMed] [Google Scholar]
- Yasui T, Akatsuka M, Tobetto K, Hayaishi M, Ando T. The effect of hyaluronan on interleukin-1 alpha-induced prostaglandin E2 production in human osteoarthritic synovial cells. Agents Actions. 1992;37:155–156. doi: 10.1007/BF01987905. [DOI] [PubMed] [Google Scholar]
- Hirota W. Intra-articular injection of hyaluronic acid reduces total amounts of leukotriene C4, 6-keto-prostaglandin F1alpha, prostaglandin F2alpha and interleukin-1beta in synovial fluid of patients with internal derangement in disorders of the temporomandibular joint. Br J Oral Maxillofac Surg. 1998;36:35–38. doi: 10.1016/s0266-4356(98)90745-8. [DOI] [PubMed] [Google Scholar]
- Goto M, Hanyu T, Yoshio T, Matsuno H, Shimizu M, Murata N, Shiozawa S, Matsubara T, Yamana S, Matsuda T. Intra-articular injection of hyaluronate (SI-6601D) improves joint pain and synovial fluid prostaglandin E2 levels in rheumatoid arthritis: a multicenter clinical trial. Clin Exp Rheumatol. 2001;19:377–383. [PubMed] [Google Scholar]
- Punzi L, Schiavon F, Cavasin F, Ramonda R, Gambari PF, Todesco S. The influence of intra-articular hyaluronic acid on PGE2 and cAMP of synovial fluid. Clin Exp Rheumatol. 1989;7:247–250. [PubMed] [Google Scholar]
- Moseley R, Leaver M, Walker M, Waddington RJ, Parsons D, Chen WYJ, Embery G. Comparison of the antioxidant properties of HYAFF®-11p75 AQUACEL® and hyaluronan towards reactive oxygen species in vitro. Biomaterials. 2002;23:2255–2264. doi: 10.1016/S0142-9612(01)00360-X. [DOI] [PubMed] [Google Scholar]
- Sato H, Takahashi T, Ide H, Fukushima T, Tabata M, Sekine F, Kobayashi K, Negishi M, Niwa Y. Antioxidant activity of synovial fluid, hyaluronic acid, and two subcomponents of hyaluronic acid. Synovial fluid scavenging effect is enhanced in rheumatoid arthritis patients. Arthritis Rheum. 1988;31:63–71. doi: 10.1002/art.1780310110. [DOI] [PubMed] [Google Scholar]
- Fukuda K, Oh M, Asada S, Hara F, Matsukawa M, Otani K, Hamanishi C. Sodium hyaluronate inhibits interleukin-1-evoked reactive oxygen species of bovine articular chondrocytes. Osteoarthritis Cartilage. 2001;9:390–392. doi: 10.1053/joca.2000.0400. [DOI] [PubMed] [Google Scholar]
- Fukuda K, Takayama M, Ueno M, Oh M, Asada S, Kumano F, Tanaka S. Hyaluronic acid inhibits interleukin-1-induced superoxide anion in bovine chondrocytes. Inflamm Res. 1997;46:114–117. doi: 10.1007/s000110050132. [DOI] [PubMed] [Google Scholar]
- Kvam BJ, Fragonas E, Degrassi A, Kvam C, Matulova M, Pollesello P, Zanetti F, Vittur F. Oxygen-derived free radical (ODFR) action on hyaluronan (HA), on two HA ester derivatives, and on the metabolism of articular chondrocytes. Exp Cell Res. 1995;218:79–86. doi: 10.1006/excr.1995.1133. [DOI] [PubMed] [Google Scholar]
- Presti D, Scott JE. Hyaluronan-mediated protective effect against cell damage caused by enzymatically produced hydroxyl (OH) radicals is dependent on hyaluronan molecular mass. Cell Biochem Funct. 1994;12:281–288. doi: 10.1002/cbf.290120409. [DOI] [PubMed] [Google Scholar]
- Takahashi K, Hashimoto S, Kubo T, Hirasawa Y, Lotz M, Amiel D. Hyaluronan suppressed nitric oxide production in the meniscus and synovium of rabbit osteoarthritis model. J Orthop Res. 2001;19:500–503. doi: 10.1016/S0736-0266(00)90024-X. [DOI] [PubMed] [Google Scholar]
- Takahashi K, Hashimoto S, Kubo T, Hirasawa Y, Lotz M, Amiel D. Effect of hyaluronan on chondrocyte apoptosis and nitric oxide production in experimentally induced osteoarthritis. J Rheumatol. 2000;27:1713–1720. [PubMed] [Google Scholar]
- Tung JT, Venta PJ, Caron JP. Inducible nitric oxide expression in equine articular chondrocytes: effects of antiinflammatory compounds. Osteoarthritis Cartilage. 2002;10:5–12. doi: 10.1053/joca.2001.0476. [DOI] [PubMed] [Google Scholar]
- Rockey DC, Chung JJ, McKee CM, Noble PW. Stimulation of inducible nitric oxide synthase in rat liver by hyaluronan fragments. Hepatology. 1998;27:86–92. doi: 10.1002/hep.510270115. [DOI] [PubMed] [Google Scholar]
- Peluso GF, Perbellini A, Tajana GF. The effect of high and low molecular weight hyaluronic acid on mitogen-induced lymphocyte proliferation. Curr Ther Res. 1990;47:437–443. [Google Scholar]
- Darzynkiewicz Z, Balazs EA. Effect of connective tissue inter-cellular matrix on lymphocyte stimulation. Exp Cell Res. 1971;66:113–123. doi: 10.1016/s0014-4827(71)80018-6. [DOI] [PubMed] [Google Scholar]
- Forrester JV, Balazs EA. Inhibition of phagocytosis by high molecular weight hyaluronate. Immunology. 1980;40:435–446. [PMC free article] [PubMed] [Google Scholar]
- Pisko EJ, Turner RA, Soderstrom LP, Panetti M, Foster SL, Treadway WJ. Inhibition of neutrophil phagocytosis and enzyme release by hyaluronic acid. Clin Exp Rheumatol. 1983;1:41–44. [PubMed] [Google Scholar]
- Partsch G, Schwarzer C, Neumuller J, Dunky A, Petera P, Broll H, Ittner G, Jantsch S. Modulation of the migration and chemotaxis of PMN cells by hyaluronic acid. Z Rheumatol. 1989;48:123–128. [PubMed] [Google Scholar]
- Dahlgren C, Bjorksten B. Effect of hyaluronic acid on polymorphonuclear leucocyte cell surface properties. Scand J Haematol. 1982;28:376–380. doi: 10.1111/j.1600-0609.1982.tb00542.x. [DOI] [PubMed] [Google Scholar]
- Håkansson L, Hällgren R, Venge P. Regulation of granulocyte function by hyaluronic acid. In vitro and in vivo effects on phagocytosis, locomotion, and metabolism. J Clin Invest. 1980;66:298–305. doi: 10.1172/JCI109857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tobetto K, Nakai K, Akatsuka M, Yasui T, Ando T, Hirano S. Inhibitory effects of hyaluronan on neutrophil-mediated cartilage degradation. Connect Tissue Res. 1993;29:181–190. doi: 10.3109/03008209309016825. [DOI] [PubMed] [Google Scholar]
- Forrester JV, Lackie JM. Effect of hyaluronic acid on neutrophil adhesion. J Cell Sci. 1981;50:329–344. doi: 10.1242/jcs.50.1.329. [DOI] [PubMed] [Google Scholar]
- Fu LL, Maffulli N, Chan KM. Intra-articular hyaluronic acid following knee immobilisation for 6 weeks in rabbits. Clin Rheumatol. 2001;20:98–103. doi: 10.1007/s100670170078. [DOI] [PubMed] [Google Scholar]
- Shimizu C, Yoshioka M, Coutts RD, Harwood FL, Kubo T, Hirasawa Y, Amiel D. Long-term effects of hyaluronan on experimental osteoarthritis in the rabbit knee. Osteoarthritis Cartilage. 1998;6:1–9. doi: 10.1053/joca.1997.0086. [DOI] [PubMed] [Google Scholar]
- Kobayashi K, Amiel M, Harwood FL, Healey RM, Sonoda M, Moriya H, Amiel D. The long-term effects of hyaluronan during development of osteoarthritis following partial meniscectomy in a rabbit model. Osteoarthritis Cartilage. 2000;8:359–365. doi: 10.1053/joca.1999.0310. [DOI] [PubMed] [Google Scholar]
- Sonoda M, Harwood FL, Amiel ME, Moriya H, Temple M, Chang DG, Lottman LM, Sah RL, Amiel D. The effects of hyaluronan on tissue healing after meniscus injury and repair in a rabbit model. Am J Sports Med. 2000;28:90–97. doi: 10.1177/03635465000280012801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sonoda M, Harwood FL, Wada Y, Moriya H, Amiel D. The effects of hyaluronan on the meniscus and on the articular cartilage after partial meniscectomy. Am J Sports Med. 1997;25:755–762. doi: 10.1177/036354659702500606. [DOI] [PubMed] [Google Scholar]
- Marshall KW, Manolopoulos V, Mancer K, Staples J, Damyanovich A. Amelioration of disease severity by intraarticular hylan therapy in bilateral canine osteoarthritis. J Orthop Res. 2000;18:416–425. doi: 10.1002/jor.1100180313. [DOI] [PubMed] [Google Scholar]
- Smith GN, Jr, Myers SL, Brandt KD, Mickler EA. Effect of intra-articular hyaluronan injection in experimental canine osteoarthritis. Arthritis Rheum. 1998;41:976–985. doi: 10.1002/1529-0131(199806)41:6<976::AID-ART4>3.0.CO;2-R. [DOI] [PubMed] [Google Scholar]
- Wenz W, Breusch SJ, Graf J, Stratmann U. Ultrastructural findings after intraarticular application of hyaluronan in a canine model of arthropathy. J Orthop Res. 2000;18:604–612. doi: 10.1002/jor.1100180413. [DOI] [PubMed] [Google Scholar]
- Yoshimi T, Kikuchi T, Obara T, Yamaguchi T, Sakakibara Y, Itoh H, Iwata H, Miura T. Effects of high-molecular-weight sodium hyaluronate on experimental osteoarthrosis induced by the resection of rabbit anterior cruciate ligament. Clin Orthop. 1994;298:296–304. [PubMed] [Google Scholar]
- Sakakibara Y, Miura T, Iwata H, Kikuchi T, Yamaguchi T, Yoshimi T, Itoh H. Effect of high-molecular-weight sodium hyaluronate on immobilized rabbit knee. Clin Orthop Rel Res. 1994;299:282–292. [PubMed] [Google Scholar]
- Frizziero L, Govoni E, Bacchini P. Intra-articular hyaluronic acid in the treatment of osteoarthritis of the knee: clinical and morphological study. Clin Exp Rheumatol. 1998;16:441–449. [PubMed] [Google Scholar]
- Pasquali Ronchetti I, Guerra D, Taparelli F, Boraldi F, Bergamini G, Mori G, Zizzi F, Frizziero L. Morphological analysis of knee synovial membrane biopsies from a randomized controlled clinical study comparing the effects of sodium hyaluronate (Hyalgan) and methylprednisolone acetate (Depomedrol) in osteoarthritis. Rheumatology. 2001;40:158–169. doi: 10.1093/rheumatology/40.2.158. [DOI] [PubMed] [Google Scholar]
- Guidolin DD, Ronchetti IP, Lini E, Guerra D, Frizziero L. Morphological analysis of articular cartilage biopsies from a randomized, clinical study comparing the effects of 500–730 kDa sodium hyaluronate (Hyalgan) and methylprednisolone acetate on primary osteoarthritis of the knee. Osteoarthritis Cartilage. 2001;9:371–381. doi: 10.1053/joca.2000.0398. [DOI] [PubMed] [Google Scholar]
- Maniwa S, Ochi M, Motomura T, Nishikori T, Chen J, Naora H. Effects of hyaluronic acid and basic fibroblast growth factor on motility of chondrocytes and synovial cells in culture. Acta Orthop Scand. 2001;72:299–303. doi: 10.1080/00016470152846664. [DOI] [PubMed] [Google Scholar]
- Kikuchi T, Yamada H, Fujikawa K. Effects of high molecular weight hyaluronan on the distribution and movement of proteoglycan around chondrocytes cultured in alginate beads. Osteoarthritis Cartilage. 2001;9:351–356. doi: 10.1053/joca.2000.0395. [DOI] [PubMed] [Google Scholar]


