Abstract
The loss of extracellular matrix macromolecules from the cartilage results in serious impairment of joint function. Metalloproteinases called 'aggrecanases' that cleave the Glu373–Ala374 bond of the aggrecan core protein play a key role in the early stages of cartilage destruction in rheumatoid arthritis and in osteoarthritis. Three members of the ADAMTS family of proteinases, ADAMTS-1, ADAMTS-4 and ADAMTS-5, have been identified as aggrecanases. Matrix metalloproteinases, which are also found in arthritic joints, cleave aggrecans, but at a distinct site from the aggrecanases (i.e. Asn341–Phe342). The present review discuss the enzymatic properties of the three known aggrecanases, the regulation of their activities, and their role in cartilage matrix breakdown during the development of arthritis in relation to the action of matrix metalloproteinases.
Keywords: ADAMTS, chondrocytes, matrix metalloproteinases, osteoarthritis
Introduction
Cartilage consists of a relatively small number of chondrocytes and abundant extracellular matrix (ECM) components. While numerous macromolecules have been identified in cartilage, the major constituents are collagen fibrils and aggrecan, a large aggregating proteoglycan [1]. Collagen fibrils consisting mainly of type II collagen and, to a lesser extent, of collagen type IX and type XI form an oriented meshwork that provides the cartilage with tensile strength. Aggrecans fill the interstices of the collagen meshwork by forming large aggregated complexes interacting with hyaluronan and link proteins. Aggrecan monomers are approximately 2.5 million Da and consist of a 250-kDa core protein to which chondroitin sulfate and keratan sulfate glycosaminoglycan (GAG) chains are covalently attached. Aggrecans are highly hydrated because of their negatively charged long polysaccharide chains, and thus provide the cartilage with its ability to resist compressive loads.
Chondrocytes synthesize and catabolize ECM macromolecules, while the matrix in turn functions to maintain the homeostasis of the cellular environment and the structure of cartilage. In diseases such as osteoarthritis (OA) and rheumatoid arthritis (RA), degradation of the ECM exceeds its synthesis, resulting in a net decrease in the amount of cartilage matrix or even in the complete erosion of the cartilage overlying the bone at the joint surface. Although many possible causes of cartilage destruction have been suggested, such as hypoxic conditions and oxygen-derived free radicals [2,3], the primary cause of this process is thought to be an elevation in the activities of proteolytic enzymes. The loss of aggrecan is considered a critical early event of arthritis, occurring initially at the joint surface and progressing to the deeper zones. This is followed by degradation of collagen fibrils and mechanical failure of the tissue.
The matrix metalloproteinases (MMPs) have been considered the main enzymes responsible for degradation of aggrecan and collagens in cartilage [4]. The expression of several MMPs is elevated in cartilage and synovial tissues of patients with RA and OA [4,5]. Those overexpressed in cartilage (e.g. MMP-3, MMP-13 and MMP-14) are considered to be key enzymes in the development of OA, as characteristic lesions develop in the centre of the articular cartilage surface, well away from the synovial membrane, with no infiltration of inflammatory cells [6]. A recently discovered group of metalloproteinases called 'aggrecanases', however, are now thought to also play an important role in aggrecan breakdown. This topic has been covered by several recent reviews [7-11]. In the present article, we describe recent progress in the field and discuss the role of aggrecanases in cartilage matrix degradation in relation to the actions of MMPs.
Discovery of aggrecanases
One well-characterized site that MMPs cleave in the aggrecan core protein is the Asn341–Phe342 bond in the interglobular domain (IGD) between the N-terminal globular domain (G1) and the second globular domain (G2) [12-14] (see Fig. 1). In 1991, however, Sandy et al. [15] reported that when bovine articular cartilage was treated with IL-1, an inflammatory cytokine that evokes cartilage breakdown, aggrecan cleavage occurred at the Glu373–Ala374 bond in the IGD, but not at the Asn341–Phe342 bond. The enzyme responsible for this new proteolytic activity was referred to as 'aggrecanase'.
Figure 1.
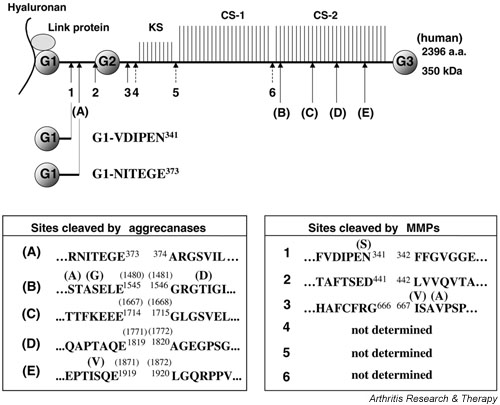
Aggrecan cleaved by aggrecanases and matrix metalloproteinases (MMPs). Aggrecan core protein has three globular domains (G1, G2 and G3). The N-terminal G1 domain interacts with hyaluronan with the help of a link protein. G1-VDIPEN341 and G1-NITEGE373 are G1-bearing N-terminal products generated by MMPs and aggrecanases, respectively. Sites cleaved by aggrecanases are shown as (A)–(E), and sites cleaved by MMPs are shown as 1–6. The dotted arrows are sites predicted based on SDS-PAGE analysis of Little et al. [90] and of Sandy and Verscharen [96]. KS, keratansulfate rich region; CS, chondroitinsulfate rich region. Residues and numbering in parentheses indicate bovine sequences.
Additional hydrolysis found at TAQE1819 ~ AGEG and VSQE1919 ~ LGQR (~ denoting the scissile bond) was also thought to be aggrecanase mediated [16,17]. Aggrecan fragments resulting from the cleavage of the Glu373–Ala374 bond accumulate in the synovial fluids of patients with OA and inflammatory arthritis [18,19], emphasizing the potential importance of aggrecanases in vivo.
The first aggrecanase, called 'aggrecanase 1', was reported by a research group at DuPont in 1999 [20], who subsequently reported a second enzyme, 'aggrecanase 2' [21]. Aggrecanase 1 and aggrecanase 2 are now designated as ADAMTS-4 and ADAMTS-5, respectively. They are zinc metalloproteinases whose structure and domain arrangements are homologous to ADAMTS (a disintegrin and a metalloproteinase domain with thrombospondin motifs) proteins (see [22,23]). More recent studies have shown that ADAMTS-1 also has aggrecanase activity [24,25]. ADAMTS-1 transcripts are found in cartilage [26].
Aggrecanase structure and function
The ADAMTSs and the proteins with a disintegrin and metalloproteinase (ADAMs) belong to the metallopeptidase family M12 [27]. The metalloproteinase domains of ADAMs are related to snake venom metalloproteinases or reprolysins. There are currently 30 ADAM genes [28] and 18 ADAMTS genes known in humans [29].
ADAMs are type I transmembrane proteins with extracellularly located N-termini. Their genes encode an N-terminal signal peptide, a relatively large prodomain (about 170 amino acids), a metalloproteinase domain (about 230 amino acids), a disintegrin domain, a cysteine-rich region usually containing an epidermal growth factor-like domain, and a transmembrane domain followed by a cytoplasmic tail at the C-terminus (Fig. 2). The metalloproteinase domains are well conserved, but only 19 out of 30 have the zinc binding catalytic site consensus sequence HEXXHXXGXXH. Other ADAMs lacking this motif are likely to be proteolytically inactive.
Figure 2.
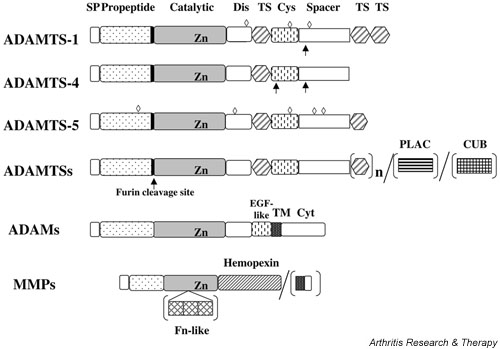
Domain arrangements of ADAMTS, ADAMs and MMPs. N-linked glycosylation sites (◇) and post-translational processing sites of ADAMTS-1 and ADAMTS-4 (↑) are indicated. Some ADAMTSs have PLAC and CUB domain at the C terminus. ADAMs are type I membrane proteins but ADAMTSs lack a transmembrane domain. MMP-2 and MMP-9 have three repeats of a fibronectin type II-like domain and membrane-type MMPs have a transmembrane domain and a cytoplasmic tail. SP, signal peptide; Dis, disintegrin-like domain; TS, thrombospondin type I motif; Cys, cysteine-rich domain; PLAC, proteinase and lacunin domain; CUB, complement C1r/C1s-urchin epidermal growth factor-bone morphogenetic protein-1 domain; TM, transmembrane domain; Fn, fibronectin.
Biological functions of many ADAMs are not clearly understood. Among those whose function is known are: ADAM-1 and ADAM-2 (fertilin α and fertilin β), which play a role in sperm–egg fusion during fertilization [30]; ADAM-12 (metrin α), which participates in myoblast fusion [31] and which releases heparin-binding epidermal growth factor from the plasma membrane [32]; ADAM-10 (Kuzbanian in Drosophila), which processes Notch and Notch ligand Delta during neural development [33,34]; and ADAM-17 (tumour necrosis factor alpha [TNF-α] converting enzyme), which releases TNF-α, TNF-α receptors and other cell surface molecules [35,36].
ADAMTS proteins are related to ADAMs, but they are not membrane-anchored proteins as they lack a transmembrane domain (Fig. 2). The common domain modules of ADAMTSs are a signal peptide, a prodomain, a metalloproteinase domain, a disintegrin domain, a thrombospondin type I motif, a spacer domain, and a second thrombospondin module of a variable number of repeats at the C-terminal region. Some ADAMTSs have a PLAC (protease and lacunin) domain [37] and a CUB (complement C1r/C1s–urchin epidermal growth factor-bone morphogenetic protein-1) domain [38] at the C-terminus (see Fig. 2).
ADAMTSs have highly selective proteolytic activities (Table 1). ADAMTS-2, ADAMTS-3 and ADAMTS-14 have N-procollagen processing activity [39-41]. ADAMTS-13 cleaves von Willebrand factor, and a decrease in ADAMTS-13 activity results in congenital and acquired thrombotic thrombocytopaenic purpura [42-44]. ADAMTS-1 (METH-1) has been identified as an IL-1-inducible gene in mice, and ADAMTS-1 and ADAMTS-8 (METH-2) have anti-angiogenic activity [45]. The proteolytic activity of ADAMTS-8 has not been investigated. The functions of other ADAMTSs remain unknown.
Table 1.
Biological activities of ADAMTSs
| Enzyme | Substrate | Activity | Reference |
| ADAMTS-1 | Aggrecan, versican, α2-macroglobulin | Cleavage of proteoglycan core proteins, anti-angiogenic | [25,45,50,51] |
| ADAMTS-2 | Procollagen I, procollagen II | Processing of N-propeptide of procollagen | [39] |
| ADAMTS-3 | Procollagen II | Processing of N-propeptide of procollagen | [40] |
| ADAMTS-4 | Aggrecan, versican, brevican | Cleavage of proteoglycan core proteins | [20,52,50] |
| ADAMTS-5 | Aggrecan | Cleavage of aggrecan core protein | [21] |
| ADAMTS-8 | Anti-angiogenic | [45] | |
| ADAMTS-13 | von Willebrand factor | Reduced activity results in thrombotic Thrombocytopaenic purpura | [43] |
| ADAMTS-14 | Procollagen I | Procollagen N-proteinase | [41] |
ADAMTS, a disintegrin and metalloproteinase domain with thrombospondin motif.
Catalytic activity of aggrecanases
Besides the Glu373–Ala374 bond in the IGD, ADAMTS-4 and ADAMTS-5 cleave at least four other sites in the chondroitin sulfate-rich CS-2 region of bovine aggrecan: GELE1480 ~ GRGD, KEEE1667 ~ GLGS, TAQE1771 ~ AGEG, and VSQE1871 ~ LGQR [46-48]. These sites are much more readily cleaved than the Glu373–Ala374 bond [46,47] (Fig. 1). The structural requirements for different rates of hydrolysis are not known, but they may be influenced by the location of polysaccharide chains as well as of amino acid sequences around the cleavage site in the core protein. ADAMTS-1 cleaves the Glu1480–Gly1481 and Glu1871–Leu1872 bonds of bovine aggrecan [25]. In addition, ADAMTS-1 [25] and ADAMTS-4 [49] hydrolyse the Asn341–Phe342 bond at a high enzyme to substrate ratio, suggesting that these two ADAMTSs may also cleave at the so-called 'MMP-cleavage site'. Other substrates include versican and α2-macroglobulin for ADAMTS-1 [50,51], and brevican and versican for ADAMTS-4 [50,52]. When the 'bait region' of α2-macroglobulin is hydrolyzed by a proteinase, α2-macroglobulin entraps the enzyme and sterically hinders it from accessing large protein substrates [53]. It is therefore likely that α2-macroglobulin is an endogenous inhibitor of ADAMTS-1.
ECM components such as collagens, fibronectin and thrombospondin, and general proteinase substrates such as casein and gelatin are not cleaved by ADAMTS-1, ADAMTS-4 or ADAMTS-5 [47]. The highly selective specificity of these enzymes can be attributed to the non-catalytic domains. Tortorella et al. [54], who reported that the ADAMTS-4 proteinase domain alone does not cleave aggrecan core protein, suggest that the thrombospondin type I domain is critical for aggrecan recognition and cleavage. The cleavage of the Glu373–Ala374 bond in the IGD by aggrecanases is enhanced by the presence of keratan sulfate chains in this domain [55]. Little activity is detected when the full-length ADAMTS-4 is incubated with the deglycosylated aggrecan [54], indicating that interaction of polysaccharide chains and the enzyme is important for the aggrecanase activity. A study using a recombinant IGD and its deletion mutants has indicated that at least 32 residues at the N-terminal side of the cleavage site (P residues of substrate) and 13 residues at the C-terminal side (P' residues) are required for aggrecanases to cleave the Glu373–Ala374 bond [56]. MMP-cleaved IGD is no longer susceptible to aggrecanase, whereas aggrecanase-cleaved IGD is hydrolyzed by MMPs at the Asn341–Phe342 bond [57]. Not only the primary sequence, but also the secondary structure of the IGD thus appears to be critical for substrate recognition by aggrecanases.
Post-translational processing of ADAMTSs
ADAMTSs are synthesized as pre-proproteins and are targeted to the secretory pathway. All members possess a furin cleavage site just before the proteinase domain, and therefore they are most probably activated intracellularly by a proprotein convertase and secreted as active enzymes. ADAMTS-1 may undergo further processing extracellularly, with a C-terminal part of the spacer domain and the two thrombospondin type I domains being removed [58] (see Fig. 2). This processing reduces both the affinity of the enzyme for heparin and the ability of the enzyme to suppress endothelial cell proliferation [58]. The mature full-length ADAMTS-4 (75 kDa) is also further processed extracellularly to 60-kDa and 50-kDa forms by MMPs [59]. These additional processing events greatly increase the aggrecanase activity of the enzyme [59], indicating that post-translational processing may be an important regulatory mechanism for this enzyme in vivo.
TIMP-3 as an endogenous inhibitor of aggrecanases
The aggrecanase activity from bovine cartilage is inhibited by TIMP-1 with an IC50 (inhibitor concentration that gives 50% enzyme inhibition) of 210 nM [60]. ADAMTS-1 is inhibited by TIMP-2 and TIMP-3, but only a very high concentration (500 nM) was tested [25]. TIMP-3 is a potent inhibitor of ADAMTS-4 and ADAMTS-5 [61,62]. The recombinant N-terminal inhibitory domain of human TIMP-3 inhibits ADAMTS-4 and ADAMTS-5 with Ki values in the subnanomolar range, considerably lower than those for MMPs [61], indicating that TIMP-3 is a potent endogenous inhibitor of aggrecanase 1 and aggrecanase 2.
TIMPs are generally considered specific inhibitors of MMPs, but TIMP-3 is exceptional in that it also inhibits some other metalloproteinases, such as TNF-α converting enzyme (ADAM-17) [63], ADAM-10 [64] and ADAM-12 [65]. Another unique feature of TIMP-3 is its ability to tightly bind to negatively charged polysaccharides [66]. TIMP-3 is expressed in skeletal tissues during development of mouse embryos [67] and in normal bovine and human chondrocytes and synoviocytes, and the levels of expression are elevated in human OA synovium [68]. TIMP-3 expression in cultured chondrocytes, and synovial fibroblasts is upregulated by transforming growth factor beta [69] or oncostatin M [70]. Treatment of human rheumatoid synovial fibroblasts with the anti-arthritic agent calcium pentosan polysulfate increases TIMP-3 protein levels, without altering its mRNA levels [71]. This increase of TIMP-3 is due to an enhanced transition of the mRNA without affecting the stability and secretion of newly synthesized TIMP-3 [71]. The increase of TIMP-3 production is further augmented by cotreatment of the cells with IL-1 [71]. Calcium pentosan polysulfate inhibits the IL-1-stimulated and retinoic acid-stimulated aggrecan breakdown in bovine articular cartilage [72]. This effect is probably due to an elevated production of TIMP-3 in the cartilage [71] and to direct inhibition of aggrecanase activity [73]. Increased levels of TIMP-3 may therefore be beneficial for protecting cartilage from degradation.
Synthetic aggrecanase inhibitors
Synthetic inhibitors designed for MMPs often inhibit aggrecanase activity [74], but some selective inhibitors for aggrecanase have been reported recently. Succinate-based hydroxamic acid compounds containing 3-hydroxyphenyl and cis-(1S)-(2R)-amino-2-indanol moieties have good selectivity for aggrecanases [75]. The best compound has an IC50 value of 12 nM against aggrecanase, with the Ki values for MMP-1, MMP-2 and MMP-9 in a micromolar range (4–33 μM), and it is orally available [75].
Compounds with a biphenylmethyl group in the P1' position show improved potency for aggrecanase with IC50 values in the low nanomolar range [76]. These compounds have excellent selectivity over MMP-1 and MMP-9, but only moderate selectivity over MMP-2. Information about other MMPs and specific ADAMTSs is not available as the aggrecanase enzyme used was not defined in these studies, but once the inhibitory activities of these compounds against each aggrecanase (ADAMTS-1, ADAMTS-4 and ADAMTS-5) and other MMPs are known, they may be useful agents to test the role of aggrecanases and MMPs in various models of cartilage degradation.
Regulation of aggrecanase activity and the expression of ADAMTS-1, ADAMTS-4 and ADAMTS-5
Aggrecanase activity was first described in bovine articular cartilage treated with IL-1 [15], but it is also enhanced in cartilage treated with TNF-α, retinoic acid [7], IL-17 [77], ceramide [78] or the 45 kDa fibronectin fragment containing collagen/gelatin binding motifs [79]. It is therefore reasonable to consider that some ADAMTS genes are transcriptionally regulated. However, reports describing mRNA levels of aggrecanases in response to inductive stimuli are not consistent at present.
For example, the treatment of normal human cartilage in culture with IL-1, TNF-α or retinoic acid increases aggrecanase activity, but it has no effect on mRNA levels for ADAMTS-1, ADAMTS-4 and ADAMTS-5 [26]. This suggests that enhanced aggrecanase activity may be regulated post-transcriptionally or that the increased activity is due to unidentified aggrecanases. On the other hand, human chondrocytes [80], bovine chondrocytes [81], bovine articular cartilage [81,82] and porcine articular cartilage [83] treated with IL-1 increase ADAMTS-4 mRNA levels. In the case of immortalized human chondrocytes, however, the levels of ADAMTS-4 mRNA increase only if treated with IL-1 and oncostatin M, but not with either cytokine alone [84]. Several studies indicate that IL-1 has little or no effect on ADAMTS-5 mRNA levels [80-82]. Two studies report that IL-1 treatment increases ADAMTS-5 mRNA levels in porcine articular cartilage [83] and in immortalized human chondrocytes [84]. The variability and inconsistency among these reports may indicate that the regulatory mechanisms of ADAMTS-4 and ADAMTS-5 transcription and translation depend on the species and age of the tissue and culture conditions of isolated cells. The stability and half-life of the mRNA may also affect results.
Synovial tissues in culture also produce and release soluble aggrecanase activity [48]. However, the treatment of bovine synovium with IL-1 or retinoic acid does not alter mRNA levels of ADAMTS-4 and ADAMTS-5 [48]. Similar results have been obtained for human synoviocytes treated with IL-1 or TNF-α [85], even though these cytokines are potent inducers of MMP production in synoviocytes. Nevertheless, Yamanishi et al. [85] found that transforming growth factor beta significantly increases ADAMTS-4 mRNA in human synoviocytes along with increasing the production of a 90-kDa protein thought to be the precursor form of the enzyme. ADAMTS-5 mRNA is constitutively produced in both RA and OA synoviocytes, and the 70-kDa protein is detected in cell lysates, but neither mRNA nor protein levels are regulated by transforming growth factor beta [85]. These observations again emphasize that the regulation of ADAMTS-4 and ADAMTS-5 genes in response to cytokines and growth factors depends on the cell type.
Other important findings regarding the regulation of aggrecanase activity have been made by Caterson and colleagues, who reported that cyclosporin A and n-3 fatty acids downregulate ADAMTS-4 and/or ADAMTS-5 mRNAs [81,83,86]. Treatment of porcine articlar cartilage with cyclosporin A abrogates the IL-1-enhanced ADAMTS-4 and ADAMTS-5 mRNAs [83]. Supplementation of bovine chondrocytes with n-3 fatty acid reduces the IL-1-inducible mRNAs for ADAMTS-4 and cyclooxygenase 2, but not those for ADAMTS-5 [81]. A similar suppressive effect on ADAMTS-4 mRNA is seen in human OA cartilage treated with n-3 fatty acid along with reduction of aggrecanase activity in the cartilage [86]. Supplementation with n-3 fatty acid also reduced mRNA levels of MMP-3, MMP-13, cyclooxygenase 2, 5-lipoxygenase, 5-lipoxygenase activating protein, TNF-α, IL-1α and IL-1β [86]. The mechanisms by which these genes are regulated by n-3 fatty acid and cyclosporin A are not known, but elucidation of such mechanisms could suggest useful ways to manipulate expression of the genes associated with inflammation and joint destruction.
Aggrecanases versus MMPs in cartilage degradation
Because several MMPs are elevated in arthritic joints [4,5], and because the MMP-generated G1-VDIPEN341 fragment and the aggrecanase-generated G1-NITEGE373 fragment are found in cartilage [87] and synovial fluids [18,19,88] from patients with RA and OA, there is a debate regarding which group of enzymes plays the major role in aggrecan degradation under biological and pathological conditions. In short-term in vitro models of cartilage explants stimulated with IL-1, TNF-α or retinoic acid, aggrecanases appear to be the primary enzymes that degrade aggrecan, at least in the first week [89,90]. Little contribution is made by MMPs although the mRNA levels of MMP-3 and MMP-13 are elevated [89]. After about 3 weeks of incubation, however, MMP-dependent cleavage of aggrecan core protein can be detected, at which time collagen breakdown also starts to occur [90].
Fosang et al. [91] reported that porcine cartilage treated with IL-1 or retinoic acid for 5 days increased the MMP-generated aggrecan fragments in cartilage, but a later report indicated that this is an experimental artefact [92]. Thus, in the in vitro cartilage explant systems, the initial enzymes responsible for degrading aggrecan are aggrecanases, followed by MMPs at a later stage [90]. It is notable, however, that the responses to catabolic stimuli differ in various tissues [93]. Bovine nasal cartilage stimulated with IL-1 or retinoic acid releases GAG primarily due to aggrecanase. In human cartilage, little GAG release is seen with IL-1, but aggrecanase-dependent GAG release is seen with retinoic acid [93]. By contrast, treatment of foetal bovine epiphyseal cartilage with retinoic acid, but not with IL-1, releases GAG without degrading the core protein [93]. This novel mechanism of GAG release is yet to be investigated.
Both G1-VDIPEN341 and G1-NITEGE373 fragments remain in the cartilage by interacting with hyaluronan, and they can be detected by antibodies detecting the C-terminal neoepitope of each fragment (Fig. 1). Using this approach, both MMPs and aggrecanases are shown to contribute to the lysis of aggrecan at distinct sites during the development of the secondary ossification centre in the cartilaginous epiphysis of rat long bone [94]. In normal human cartilage, both neoepitopes are also found and increase with age, but they remain at a steady state after the age of 20–30 years [87]. This probably reflects the much slower turnover rate of the G1 domain (0.027/year with a half-life of 25 years) compared with that of the large aggrecan monomer (0.206/year with a half-life of 3.4 years) [95]. The concentration of the MMP-generated VDIPEN neoepitope in adult joint cartilage represents 15–20% of the resident aggrecan molecules within the matrix, and the proportion of G1-VDIPEN in OA and RA cartilage is about the same as in adult joint cartilage, although high levels of staining are seen in areas of cartilage damage [87]. The distribution of the aggrecanase-generated NITEGE neoepitope is similar to the VDIPEN neoepitope in most cases, but in some cases the NITEGE neoepitope is detected in regions where the VDIPEN neoepitope is not found [87], indicating that two groups of enzymes may function at different sites in cartilage.
More recent studies by Sandy and Verscharen [96] have indicated that normal human adult cartilage contains at least seven main G1-bearing species, which include the full-length, G1-NITEGE373 and G1-VDIPEN341 fragments, and four other fragments (90 kDa, 110 kDa, 160 kDa and 250 kDa after deglycosylation). The latter four fragments (see Fig. 2 for the potential cleavage sites) represent at least 50% of the total core protein, and they are most probably generated by MMPs in vivo. Interestingly, the core protein composition in the cartilage does not change in OA cartilage. Synovial fluids, on the other hand, contain primarily the fragments generated by aggrecanases, and fluids from patients with late-stage OA contain more excessively cleaved fragments. In acutely injured joints there is a marked increase in the ratio of G1-NITEGE to G1-VDIPEN both in the cartilage and synovial fluids. Based on these observations, these investigators propose that excessive aggrecanase activity is destructive to cartilage matrix, whereas MMP activity is nondestructive since it trims mostly the C-terminal region of the aggrecan molecule and much of the GAG-bearing product is retained in the tissue [96] (see Fig. 1).
Some in vivo models of arthritis indicate that MMPs may participate in cartilage destruction. In antigen-induced arthritis and collagen-induced arthritis mouse models, NITEGE neoepitopes are present, but VDIPEN neoepitopes are not, during the early phase of aggrecan depletion [97]. VDIPEN neoepitopes are detected in the antigen-induced arthritis model when aggrecan degradation has progressed, and this coincides with collagenase cleavage of type II collagen [98]. However, cartilage from MMP-3(-/-) mice exhibits neither VDIPEN neoepitopes nor collagenase-cleaved neoepitopes during antigen-induced arthritis, but proteoglycan depletion occurs to a similar extent in MMP-3(-/-) and wild-type mice [98]. The probable mediators of aggrecan degradation are aggrecanases. Nevertheless, cartilage destruction was not observed in MMP-3(-/-) mice even 2 weeks after arthritis induction, suggesting that MMP-3 may play a key role in later progression of cartilage erosion in the antigen-induced arthritis model. By contrast, in the more severe collagen-induced arthritis model, MMP-3(-/-) mice develop arthritis to a similar extent as the wild-type mice, and there is no obvious decrease of VDIPEN epitope [99]. This activity is most probably due to the induction of other MMPs. It is also possible that ADAMTS-1 and ADAMTS-4 [25,49] or cathepsin B [100] released from chondrocytes, in part, participate in this process.
STR/ort mice spontaneously develop OA in the medial tibial cartilage of the knee joint. The lesions are not accompanied by inflammation and they closely resemble those in the knee of human OA [101]. In nonarthritic joints, MMP and aggrecanase neoepitopes map to different locations in cartilage, suggesting that two groups of enzymes function at different sites in normal turnover of aggrecan [102]. When the disease progresses, distributions of VDIPEN and NITEGE neoepitopes become similar, suggesting that both MMPs and aggrecanases play a role in cartilage destruction in STR/ort mice [102].
Concluding remarks
Three ADAMTSs have been identified as aggrecanases. Aggrecan products generated by these metalloproteinases are found in normal, OA and RA cartilage, and in synovial fluids, supporting the notion that these enzymes participate in aggrecan catabolism in the tissue. Since the ADAMTSs have been only recently discovered, however, limited information is available regarding the biological and pathological significance of these enzymes. It is yet to be investigated which and to what extent these ADAMTSs are responsible for cartilage degradation in vivo. It is also not known whether other ADAMTSs can degrade the aggrecan core protein. The ADAMTSs have highly selective substrate specificities, seemingly associated with the noncatalytic domains of these enzymes, as exemplified by ADAMTS-4. An understanding of the molecular interactions mediating such specificities will shed light on the mechanism of action of ADAMTSs on aggrecan and may suggest novel ways of inhibiting aggrecan breakdown.
The regulation of various ADAMTS genes in articular cartilage needs further investigation since data on the expression patterns of these enzymes in response to stimulatory factors are variable. Aggrecanases are also expressed in other tissues [21]. The expression of ADAMTS-1 mRNA increases in the injured motor neurons [103], and aggrecanase-mediated degradation of nerve tissue proteoglycans is seen in mouse brain and peripheral nerves [104], in developing and adult rat spinal cord, and after injury [105]. Levels of ADAMTS-4 mRNA increase in astrocytes treated with β-amyloid [106]. These observations indicate that aggrecanases also play an important role in the catabolism of aggrecan and other aggrecan-like molecules in normal nerves and in neuronal tissue remodelling. Little is known about the promoter regions of ADAMTSs or about the enhancer elements that increase expression. Further studies on this topic may help explain tissue- and age-dependent aggrecanase expression.
Several lines of evidence have been provided that MMPs also function as aggrecan-degrading enzymes in vivo. However, it is yet to be investigated whether MMPs function primarily in the normal turnover of aggrecan or whether they are actively involved in cartilage degradation during disease progression. Elevated levels of MMPs including MMP-3 and MMP-13 are found in OA cartilage, and levels of a number of other MMPs are increased in the rheumatoid synovium, but they are produced as inactive zymogens. Once activated, they may also participate in aggressive aggrecan degradation. As the disease progresses, the local pH of the cartilage may fall [107], and cathepsin B, cathepsin L [108] and cathepsin K [107] from chondrocytes may participate in further cartilage destruction. Several proteinases are therefore likely to be involved in cartilage destruction in the advanced stages of arthritis. To further advance our understanding of the precise in vivo functions of these proteinases in cartilage degradation during the progression of OA and RA, selective inhibitors of each enzyme and the deletion of specific proteinase genes may be necessary. The information obtained by such experiments may also provide useful insights for developing therapeutic agents to prevent progressive destruction of the cartilage matrix.
Competing interests
None declared.
Abbreviations
ADAM = protein with a disintegrin and metalloproteinase domain; ADAMTS = a disintegrin and metalloproteinase domain with thrombospondin motif; ECM = extracellular matrix; G1 = N-terminal globular domain; GAG = glycosaminoglycan; IC50 = inhibitor concentration that gives 50% enzyme inhibition; IGD = interglobular domain; IL = interleukin; Ki = inhibition constant; MMP = matrix metalloproteinase; OA = osteoarthritis; RA = rheumatoid arthritis; TNF-α = tumour necrosis factor alpha.
Acknowledgments
Acknowledgements
The authors thank Dr Linda Troeberg for critically reading the manuscript. This work was supported by the Welcome Trust Grant Number 061709 and NIH Grant AR40994.
References
- Hinegård D, Lorenzo P, Sanxe T. Matrix glycoprotein, proteoglycans, and cartilage. In: Ruddy S, Harris ED Jr, Sledge CB, editor. In Kelly's Textbook of Rheumatology. Philadelphia: WB Saunders Company; 2001. pp. 41–53. [Google Scholar]
- Evans CH, Brown TD. Role of physical and medical aspects in degradating the matrix. In: Woessner JF Jr, Howell DS, editor. In Joint Cartilage Degradation: Basic and Clinical Aspects. New York: Marcel Dekker; 1993. pp. 187–208. [Google Scholar]
- Burkhardt H, Schwingel M, Menninger H, Macartney HW, Tschesche H. Oxygen radicals as effectors of cartilage destruction. Direct degradative effect on matrix components and indirect action via activation of latent collagenase from polymorphonuclear leukocytes. Arthritis Rheum. 1986;29:379–387. doi: 10.1002/art.1780290311. [DOI] [PubMed] [Google Scholar]
- Okada Y. Proteinase and matrix degradation. In: Ruddy S, Harris ED Jr, Sledge CB, editor. In Kelly's Textbook of Rheumatology. Philadelphia: WB Saunders Company; 2001. pp. 55–72. [Google Scholar]
- Tetlow LC, Adlam DJ, Woolley DE. Matrix metalloproteinase and proinflammatory cytokine production by chondrocytes of human osteoarthritic cartilage: associations with degenerative changes. Arthritis Rheum. 2001;44:585–594. doi: 10.1002/1529-0131(200103)44:3<585::AID-ANR107>3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]
- Dean DD, Martel-Pelletier J, Pelletier JP, Howell DS, Woessner JF., Jr Evidence for metalloproteinase and metalloproteinase inhibitor imbalance in human osteoarthritic cartilage. J Clin Invest. 1989;84:678–685. doi: 10.1172/JCI114215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caterson B, Flannery CR, Hughes CE, Little CB. Mechanisms involved in cartilage proteoglycan catabolism. Matrix Biol. 2000;19:333–344. doi: 10.1016/s0945-053x(00)00078-0. [DOI] [PubMed] [Google Scholar]
- Mort JS, Billington CJ. Articular cartilage and changes in arthritis: matrix degradation. Arthritis Res. 2001;3:337–341. doi: 10.1186/ar325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagase H. Aggrecanase in cartilage matrix breakdown. Connective Tissue. 2001;33:27–32. [Google Scholar]
- Arner EC. Aggrecanase-mediated cartilage degradation. Curr Opin Pharmacol. 2002;2:322–329. doi: 10.1016/s1471-4892(02)00148-0. [DOI] [PubMed] [Google Scholar]
- Roughley PJ, Mort JS. Catabolism of proteoglycans. In: Iozzo RV, editor. In Proteoglygans Structure, Biology, and Molecular Interactions. New York: Marcel Dekker; 2000. pp. 93–113. [Google Scholar]
- Fosang AJ, Neame PJ, Hardingham TE, Murphy G, Hamilton JA. Cleavage of cartilage proteoglycan between G1 and G2 domains by stromelysins. J Biol Chem. 1991;266:15579–15582. [PubMed] [Google Scholar]
- Fosang AJ, Neame PJ, Last K, Hardingham TE, Murphy G, Hamilton JA. The interglobular domain of cartilage aggrecan is cleaved by PUMP, gelatinases, and cathepsin B. J Biol Chem. 1992;267:19470–19474. [PubMed] [Google Scholar]
- Flannery CR, Lark MW, Sandy JD. Identification of a stromelysin cleavage site within the interglobular domain of human aggrecan. Evidence for proteolysis at this site in vivo in human articular cartilage. J Biol Chem. 1992;267:1008–1014. [PubMed] [Google Scholar]
- Sandy JD, Neame PJ, Boynton RE, Flannery CR. Catabolism of aggrecan in cartilage explants. Identification of a major cleavage site within the interglobular domain. J Biol Chem. 1991;266:8683–8685. [PubMed] [Google Scholar]
- Ilic MZ, Handley CJ, Robinson HC, Mok MT. Mechanism of catabolism of aggrecan by articular cartilage. Arch Biochem Biophys. 1992;294:115–122. doi: 10.1016/0003-9861(92)90144-l. [DOI] [PubMed] [Google Scholar]
- Loulakis P, Shrikhande A, Davis G, Maniglia CA. N-terminal sequence of proteoglycan fragments isolated from medium of interleukin-1-treated articular-cartilage cultures. Putative site(s) of enzymic cleavage. Biochem J. 1992;284:589–593. doi: 10.1042/bj2840589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandy JD, Flannery CR, Neame PJ, Lohmander LS. The structure of aggrecan fragments in human synovial fluid. Evidence for the involvement in osteoarthritis of a novel proteinase which cleaves the Glu 373–Ala 374 bond of the interglobular domain. J Clin Invest. 1992;89:1512–1516. doi: 10.1172/JCI115742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lohmander LS, Neame PJ, Sandy JD. The structure of aggrecan fragments in human synovial fluid. Evidence that aggrecanase mediates cartilage degradation in inflammatory joint disease, joint injury, and osteoarthritis. Arthritis Rheum. 1993;36:1214–1222. doi: 10.1002/art.1780360906. [DOI] [PubMed] [Google Scholar]
- Tortorella MD, Burn TC, Pratta MA, Abbaszade I, Hollis JM, Liu R, Rosenfeld SA, Copeland RA, Decicco CP, Wynn R, Rockwell A, Yang F, Duke JL, Solomon K, George H, Bruckner R, Nagase H, Itoh Y, Ellis DM, Ross H, Wiswall BH, Murphy K, Hillman MC, Jr, Hollis GF, Arner EC. Purification and cloning of aggrecanase-1: a member of the ADAMTS family of proteins. Science. 1999;284:1664–1666. doi: 10.1126/science.284.5420.1664. [DOI] [PubMed] [Google Scholar]
- Abbaszade I, Liu RQ, Yang F, Rosenfeld SA, Ross OH, Link JR, Ellis DM, Tortorella MD, Pratta MA, Hollis JM, Wynn R, Duke JL, George HJ, Hillman MC, Jr, Murphy K, Wiswall BH, Copeland RA, Decicco CP, Bruckner R, Nagase H, Itoh Y, Newton RC, Magolda RL, Trzaskos JM, Burn TC. Cloning and characterization of ADAMTS11, an aggrecanase from the ADAMTS family. J Biol Chem. 1999;274:23443–23450. doi: 10.1074/jbc.274.33.23443. [DOI] [PubMed] [Google Scholar]
- Tang BL. ADAMTS: a novel family of extracellular matrix proteases. Int J Biochem Cell Biol. 2001;33:33–44. doi: 10.1016/s1357-2725(00)00061-3. [DOI] [PubMed] [Google Scholar]
- Cal S, Obaya AJ, Llamazares M, Garabaya C, Quesada V, Lóez-Otín C. Cloning, expression analysis, and structural characterization of seven novel human ADAMTSs, a family of metalloproteinases with disintegrin and thrombospondin-1 domains. Gene. 2002;283:49–62. doi: 10.1016/s0378-1119(01)00861-7. [DOI] [PubMed] [Google Scholar]
- Kuno K, Okada Y, Kawashima H, Nakamura H, Miyasaka M, Ohno H, Matsushima K. ADAMTS-1 cleaves a cartilage proteoglycan, aggrecan. FEBS Lett. 2000;478:241–245. doi: 10.1016/s0014-5793(00)01854-8. [DOI] [PubMed] [Google Scholar]
- Rodríguez-Manzaneque JC, Westling J, Thai SN, Luque A, Knäuper V, Murphy G, Sandy JD, Iruela-Arispe ML. ADAMTS1 cleaves aggrecan at multiple sites and is differentially inhibited by metalloproteinase inhibitors. Biochem Biophys Res Commun. 2002;293:501–508. doi: 10.1016/S0006-291X(02)00254-1. [DOI] [PubMed] [Google Scholar]
- Flannery CR, Little CB, Hughes CE, Caterson B. Expression of ADAMTS homologues in articular cartilage. Biochem Biophys Res Commun. 1999;260:318–322. doi: 10.1006/bbrc.1999.0909. [DOI] [PubMed] [Google Scholar]
- Rawling ND, O'Brien EA, Barrett AJ. MEROPS: the protease database. Nucleic Acids Res. 2002;70:343–346. doi: 10.1093/nar/30.1.343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- http://www.gene.ucl.ac.uk/nomenclature/genefamily/metallo.html
- http://www.gene.ucl.ac.uk/nomenclature/genefamily/adamts.html
- Blobel CP, Wolfsberg TG, Turck CW, Myles DG, Primakoff P, White JM. A potential fusion peptide and an integrin ligand domain in a protein active in sperm-egg fusion. Nature. 1992;356:248–252. doi: 10.1038/356248a0. [DOI] [PubMed] [Google Scholar]
- Yagami-Hiromasa T, Sato T, Kurisaki T, Kamijo K, Nabeshima Y, Fujisawa-Sehara A. A metalloprotease-disintegrin participating in myoblast fusion. Nature. 1995;377:652–656. doi: 10.1038/377652a0. [DOI] [PubMed] [Google Scholar]
- Asakura M, Kitakaze M, Takashima S, Liao Y, Ishikura F, Yoshinaka T, Ohmoto H, Node K, Yoshino K, Ishiguro H, Asanuma H, Sanada S, Matsumura Y, Takeda H, Beppu S, Tada M, Hori M, Higashiyama S. Cardiac hypertrophy is inhibited by antagonism of ADAM12 processing of HB-EGF: metalloproteinase inhibitors as a new therapy. Nat Med. 2002;8:35–40. doi: 10.1038/nm0102-35. [DOI] [PubMed] [Google Scholar]
- Pan D, Rubin GM. Kuzbanian controls proteolytic processing of Notch and mediates lateral inhibition during Drosophila and vertebrate neurogenesis. Cell. 1997;90:271–280. doi: 10.1016/s0092-8674(00)80335-9. [DOI] [PubMed] [Google Scholar]
- Qi H, Rand MD, Wu X, Sestan N, Wang W, Rakic P, Xu T, Artavanis-Tsakonas S. Processing of the notch ligand delta by the metalloprotease Kuzbanian. Science. 1999;283:91–94. doi: 10.1126/science.283.5398.91. [DOI] [PubMed] [Google Scholar]
- Black RA, Rauch CT, Kozlosky CJ, Peschon JJ, Slack JL, Wolfson MF, Castner BJ, Stocking KL, Reddy P, Srinivasan S, Nelson N, Boiani N, Schooley KA, Gerhart M, Davis R, Fitzner JN, Johnson RS, Paxton RJ, March CJ, Cerretti DP. A metalloproteinase disintegrin that releases tumour-necrosis factor-alpha from cells. Nature. 1997;385:729–733. doi: 10.1038/385729a0. [DOI] [PubMed] [Google Scholar]
- Moss ML, Jin SL, Milla ME, Bickett DM, Burkhart W, Carter HL, Chen WJ, Clay WC, Didsbury JR, Hassler D, Hoffman CR, Kost TA, Lambert MH, Leesnitzer MA, McCauley P, McGeehan G, Mitchell J, Moyer M, Pahel G, Rocque W, Overton LK, Schoenen F, Seaton T, Su JL, Warner J, Willard D, Becherer JD. Cloning of a disintegrin metalloproteinase that processes precursor tumour-necrosis factor-alpha. Nature. 1997;385:733–736. doi: 10.1038/385733a0. [DOI] [PubMed] [Google Scholar]
- Nardi JB, Martos R, Walden KK, Lampe DJ, Robertson HM. Expression of lacunin, a large multidomain extracellular matrix protein, accompanies morphogenesis of epithelial monolayers in Manduca sexta. Insect Biochem Mol Biol. 1999;29:883–897. doi: 10.1016/s0965-1748(99)00064-8. [DOI] [PubMed] [Google Scholar]
- Bork P, Beckmann G. The CUB domain. A widespread module in developmentally regulated proteins. J Mol Biol. 1993;231:539–545. doi: 10.1006/jmbi.1993.1305. [DOI] [PubMed] [Google Scholar]
- Colige A, Beschin A, Samyn B, Goebels Y, van Beeumen J, Nusgens BV, Lapière CM. Characterization and partial amino acid sequencing of a 107-kDa procollagen I N-proteinase purified by affinity chromatography on immobilized type XIV collagen. J Biol Chem. 1995;270:16724–16730. doi: 10.1074/jbc.270.28.16724. [DOI] [PubMed] [Google Scholar]
- Fernandes RJ, Hirohata S, Engle JM, Colige A, Cohn DH, Eyre DR, Apte SS. Procollagen II amino propeptide processing by ADAMTS-3. Insights on dermatosparaxis. J Biol Chem. 2001;276:31502–31509. doi: 10.1074/jbc.M103466200. [DOI] [PubMed] [Google Scholar]
- Colige A, Vandenberghe I, Thiry M, Lambert CA, van Beeumen J, Li SW, Prockop DJ, Lapière CM, Nusgens BV. Cloning and characterization of ADAMTS-14, a novel ADAMTS displaying high homology with ADAMTS-2 and ADAMTS-3. J Biol Chem. 2002;277:5756–5766. doi: 10.1074/jbc.M105601200. [DOI] [PubMed] [Google Scholar]
- Levy GG, Nichols WC, Lian EC, Foroud T, McClintick JN, McGee BM, Yang AY, Siemieniak DR, Stark KR, Gruppo R, Sarode R, Shurin SB, Chandrasekaran V, Stabler SP, Sabio H, Bouhassira EE, Upshaw JD, Jr, Ginsburg D, Tsai HM. Mutations in a member of the ADAMTS gene family cause thrombotic thrombocytopenic purpura. Nature. 2001;413:488–494. doi: 10.1038/35097008. [DOI] [PubMed] [Google Scholar]
- Furlan M, Robles R, Galbusera M, Remuzzi G, Kyrle PA, Brenner B, Krause M, Scharrer I, Aumann V, Mittler U, Solenthaler M, Lammle B. von Willebrand factor-cleaving protease in thrombotic thrombocytopenic purpura and the hemolytic-uremic syndrome. N Engl J Med. 1998;339:1578–1584. doi: 10.1056/NEJM199811263392202. [DOI] [PubMed] [Google Scholar]
- Tsai HM, Lian EC. Antibodies to von Willebrand factor-cleaving protease in acute thrombotic thrombocytopenic purpura. N Engl J Med. 1998;339:1585–1594. doi: 10.1056/NEJM199811263392203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vazquez F, Hastings G, Ortega MA, Lane TF, Oikemus S, Lombardo M, Iruela-Arispe ML. METH-1, a human ortholog of ADAMTS-1, and METH-2 are members of a new family of proteins with angio-inhibitory activity. J Biol Chem. 1999;274:23349–23357. doi: 10.1074/jbc.274.33.23349. [DOI] [PubMed] [Google Scholar]
- Tortorella MD, Pratta M, Liu RQ, Austin J, Ross OH, Abbaszade I, Burn T, Arner E. Sites of aggrecan cleavage by recombinant human aggrecanase-1 (ADAMTS-4). J Biol Chem. 2000;275:18566–18573. doi: 10.1074/jbc.M909383199. [DOI] [PubMed] [Google Scholar]
- Tortorella MD, Liu RQ, Burn T, Newton RC, Arner E. Characterization of human aggrecanase 2 (ADAM-TS5): substrate specificity studies and comparison with aggrecanase 1 (ADAM-TS4). Matrix Biol. 2002;21:499–511. doi: 10.1016/s0945-053x(02)00069-0. [DOI] [PubMed] [Google Scholar]
- Vankemmelbeke MN, Holen I, Wilson AG, Ilic MZ, Handley CJ, Kelner GS, Clark M, Liu C, Maki RA, Burnett D, Buttle DJ. Expression and activity of ADAMTS-5 in synovium. Eur J Biochem. 2001;268:1259–1268. doi: 10.1046/j.1432-1327.2001.01990.x. [DOI] [PubMed] [Google Scholar]
- Westling J, Fosang AJ, Last K, Thompson VP, Tomkinson KN, Hebert T, McDonagh T, Collins-Racie LA, LaVallie ER, Morris EA, Sandy JD. ADAMTS4 cleaves at the aggrecanase site (Glu373–Ala374) and secondarily at the matrix metalloproteinase site (Asn341–Phe342) in the aggrecan interglobular domain. J Biol Chem. 2002;277:16059–16066. doi: 10.1074/jbc.M108607200. [DOI] [PubMed] [Google Scholar]
- Sandy JD, Westling J, Kenagy RD, Iruela-Arispe ML, Verscharen C, Rodriguez-Mazaneque JC, Zimmermann DR, Lemire JM, Fischer JW, Wight TN, Clowes AW. Versican V1 proteolysis in human aorta in vivo occurs at the Glu441–Ala442 bond, a site that is cleaved by recombinant ADAMTS-1 and ADAMTS-4. J Biol Chem. 2001;276:13372–13378. doi: 10.1074/jbc.M009737200. [DOI] [PubMed] [Google Scholar]
- Kuno K, Terashima Y, Matsushima K. ADAMTS-1 is an active metalloproteinase associated with the extracellular matrix. J Biol Chem. 1999;274:18821–18826. doi: 10.1074/jbc.274.26.18821. [DOI] [PubMed] [Google Scholar]
- Matthews RT, Gary SC, Zerillo C, Pratta M, Solomon K, Arner EC, Hockfield S. Brain-enriched hyaluronan binding (BEHAB)/brevican cleavage in a glioma cell line is mediated by a disintegrin and metalloproteinase with thrombospondin motifs (ADAMTS) family member. J Biol Chem. 2000;275:22695–22703. doi: 10.1074/jbc.M909764199. [DOI] [PubMed] [Google Scholar]
- Barret AJ. α2-Macroglobulin. Methods Enzymol. 1981;80:737–754. doi: 10.1016/s0076-6879(81)80056-0. [DOI] [PubMed] [Google Scholar]
- Tortorella M, Pratta M, Liu RQ, Abbaszade I, Ross H, Burn T, Arner E. The thrombospondin motif of aggrecanase-1 (ADAMTS-4) is critical for aggrecan substrate recognition and cleavage. J Biol Chem. 2000;275:25791–25797. doi: 10.1074/jbc.M001065200. [DOI] [PubMed] [Google Scholar]
- Pratta MA, Tortorella MD, Arner EC. Age-related changes in aggrecan glycosylation affect cleavage by aggrecanase. J Biol Chem. 2000;275:39096–39102. doi: 10.1074/jbc.M006201200. [DOI] [PubMed] [Google Scholar]
- Hörber C, Buttner FH, Kern C, Schmiedeknecht G, Bartnik E. Truncation of the amino-terminus of the recombinant aggrecan rAgg1mut leads to reduced cleavage at the aggrecanase site. Efficient aggrecanase catabolism may depend on multiple substrate interactions. Matrix Biol. 2000;19:533–543. doi: 10.1016/s0945-053x(00)00113-x. [DOI] [PubMed] [Google Scholar]
- Mercuri FA, Maciewicz RA, Tart J, Last K, Fosang AJ. Mutations in the interglobular domain of aggrecan alter matrix metalloproteinase and aggrecanase cleavage patterns. Evidence that matrix metalloproteinase cleavage interferes with aggrecanase activity. J Biol Chem. 2000;275:33038–33045. doi: 10.1074/jbc.275.42.33038. [DOI] [PubMed] [Google Scholar]
- Rodríguez-Manzaneque JC, Milchanowski AB, Dufour EK, Leduc R, Iruela-Arispe ML. Characterization of METH-1/ADAMTS1 processing reveals two distinct active forms. J Biol Chem. 2000;275:33471–33479. doi: 10.1074/jbc.M002599200. [DOI] [PubMed] [Google Scholar]
- Gao G, Westling J, Thompson VP, Howell TD, Gottschall PE, Sandy JD. Activation of the proteolytic activity of ADAMTS4 (aggrecanase-1) by C-terminal truncation. J Biol Chem. 2002;277:11034–11041. doi: 10.1074/jbc.M107443200. [DOI] [PubMed] [Google Scholar]
- Arner EC, Pratta MA, Trzaskos JM, Decicco CP, Tortorella MD. Generation and characterization of aggrecanase. A soluble, cartilage-derived aggrecan-degrading activity. J Biol Chem. 1999;274:6594–6601. doi: 10.1074/jbc.274.10.6594. [DOI] [PubMed] [Google Scholar]
- Kashiwagi M, Tortorella M, Nagase H, Brew K. TIMP-3 is a potent inhibitor of aggrecanase 1 (ADAM-TS4) and aggrecanase 2 (ADAM-TS5). J Biol Chem. 2001;276:12501–12504. doi: 10.1074/jbc.C000848200. [DOI] [PubMed] [Google Scholar]
- Hashimoto G, Aoki T, Nakamura H, Tanzawa K, Okada Y. Inhibition of ADAMTS4 (aggrecanase-1) by tissue inhibitors of metalloproteinases (TIMP-1, 2, 3 and 4). FEBS Lett. 2001;494:192–195. doi: 10.1016/s0014-5793(01)02323-7. [DOI] [PubMed] [Google Scholar]
- Amour A, Slocombe PM, Webster A, Butler M, Knight CG, Smith BJ, Stephens PE, Shelley C, Hutton M, Knäuper V, Docherty AJ, Murphy G. TNF-alpha converting enzyme (TACE) is inhibited by TIMP-3. FEBS Lett. 1998;435:39–44. doi: 10.1016/s0014-5793(98)01031-x. [DOI] [PubMed] [Google Scholar]
- Amour A, Knight CG, Webster A, Slocombe PM, Stephens PE, Knäuper V, Docherty AJ, Murphy G. The in vitro activity of ADAM-10 is inhibited by TIMP-1 and TIMP-3. FEBS Lett. 2000;473:275–279. doi: 10.1016/s0014-5793(00)01528-3. [DOI] [PubMed] [Google Scholar]
- Loechel F, Fox JW, Murphy G, Albrechtsen R, Wewer UM. ADAM 12-S cleaves IGFBP-3 and IGFBP-5 and is inhibited by TIMP-3. Biochem Biophys Res Commun. 2000;278:511–515. doi: 10.1006/bbrc.2000.3835. [DOI] [PubMed] [Google Scholar]
- Yu WH, Yu S, Meng Q, Brew K, Woessner JF., Jr TIMP-3 binds to sulfated glycosaminoglycans of the extracellular matrix. J Biol Chem. 2000;275:31226–31232. doi: 10.1074/jbc.M000907200. [DOI] [PubMed] [Google Scholar]
- Apte SS, Hayashi K, Seldin MF, Mattei MG, Hayashi M, Olsen BR. Gene encoding a novel murine tissue inhibitor of metalloproteinases (TIMP), TIMP-3, is expressed in developing mouse epithelia, cartilage, and muscle, and is located on mouse chromosome 10. Dev Dyn. 1994;200:177–197. doi: 10.1002/aja.1002000302. [DOI] [PubMed] [Google Scholar]
- Su S, Grover J, Roughley PJ, DiBattista JA, Martel-Pelletier J, Pelletier JP, Zafarullah M. Expression of the tissue inhibitor of metalloproteinases (TIMP) gene family in normal and osteoarthritic joints. Rheumatol Int. 1999;18:183–191. doi: 10.1007/s002960050083. [DOI] [PubMed] [Google Scholar]
- Su S, DiBattista JA, Sun Y, Li WQ, Zafarullah M. Up-regulation of tissue inhibitor of metalloproteinases-3 gene expression by TGF-beta in articular chondrocytes is mediated by serine/threonine and tyrosine kinases. J Cell Biochem. 1998;70:517–527. [PubMed] [Google Scholar]
- Li WQ, Zafarullah M. Oncostatin M up-regulates tissue inhibitor of metalloproteinases-3 gene expression in articular chondrocytes via de novo transcription, protein synthesis, and tyrosine kinase- and mitogen-activated protein kinase-dependent mechanisms. J Immunol. 1998;161:5000–5007. [PubMed] [Google Scholar]
- Takizawa M, Ohuchi E, Yamanaka H, Nakamura H, Ikeda E, Ghosh P, Okada Y. Production of tissue inhibitor of metalloproteinases 3 is selectively enhanced by calcium pentosan polysulfate in human rheumatoid synovial fibroblasts. Arthritis Rheum. 2000;43:812–820. doi: 10.1002/1529-0131(200004)43:4<812::AID-ANR11>3.0.CO;2-Y. [DOI] [PubMed] [Google Scholar]
- Munteanu SE, Ilic MZ, Handley CJ. Calcium pentosan polysulfate inhibits the catabolism of aggrecan in articular cartilage explant cultures. Arthritis Rheum. 2000;43:2211–2218. doi: 10.1002/1529-0131(200010)43:10<2211::AID-ANR8>3.0.CO;2-D. [DOI] [PubMed] [Google Scholar]
- Munteanu SE, Ilic MZ, Handley CJ. Highly sulfated glycosaminoglycans inhibit aggrecanase degradation of aggrecan by bovine articular cartilage explant cultures. Matrix Biol. 2002;21:429–440. doi: 10.1016/s0945-053x(02)00034-3. [DOI] [PubMed] [Google Scholar]
- Arner EC, Pratta MA, Decicco CP, Xue CB, Newton RC, Trzaskos JM, Magolda RL, Tortorella MD. Aggrecanase. A target for the design of inhibitors of cartilage degradation. Ann NY Acad Sci. 1999;878:92–107. doi: 10.1111/j.1749-6632.1999.tb07676.x. [DOI] [PubMed] [Google Scholar]
- Yao W, Wasserman ZR, Chao M, Reddy G, Shi E, Liu RQ, Covington MB, Arner EC, Pratta MA, Tortorella M, Magolda RL, Newton R, Qian M, Ribadeneira MD, Christ D, Wexler RR, Decicco CP. Design and synthesis of a series of (2R)-N(4)-hydroxy-2-(3-hydroxybenzyl)-N(1)-[(1S,2R)-2-hydroxy-2,3-dihydro-1H-inden-1-yl]butanediamide derivatives as potent, selective, and orally bioavailable aggrecanase inhibitors. J Med Chem. 2001;44:3347–3350. doi: 10.1021/jm015533c. [DOI] [PubMed] [Google Scholar]
- Yao W, Chao M, Wasserman ZR, Liu RQ, Covington MB, Newton R, Christ D, Wexler RR, Decicco CP. Potent P1' biphenylmethyl substituted aggrecanase inhibitors. Bioorg Med Chem Lett. 2002;12:101–104. doi: 10.1016/s0960-894x(01)00704-1. [DOI] [PubMed] [Google Scholar]
- Cai L, Yin JP, Starovasnik MA, Hogue DA, Hillan KJ, Mort JS, Filvaroff EH. Pathways by which interleukin 17 induces articular cartilage breakdown in vitro and in vivo. Cytokine. 2001;16:10–21. doi: 10.1006/cyto.2001.0939. [DOI] [PubMed] [Google Scholar]
- Sabatini M, Thomas M, Deschamps C, Lesur C, Rolland G, de Nanteuil G, Bonnet J. Effects of ceramide on aggrecanase activity in rabbit articular cartilage. Biochem Biophys Res Commun. 2001;283:1105–1110. doi: 10.1006/bbrc.2001.4920. [DOI] [PubMed] [Google Scholar]
- Stanton H, Ung L, Fosang AJ. The 45 kDa collagen-binding fragment of fibronectin induces matrix metalloproteinase-13 synthesis by chondrocytes and aggrecan degradation by aggrecanases. Biochem J. 2002;364:181–190. doi: 10.1042/bj3640181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bau B, Gebhard PM, Haag J, Knorr T, Bartnik E, Aigner T. Relative messenger RNA expression profiling of collagenases and aggrecanases in human articular chondrocytes in vivo and in vitro. Arthritis Rheum. 2002;46:2648–2657. doi: 10.1002/art.10531. [DOI] [PubMed] [Google Scholar]
- Curtis CL, Hughes CE, Flannery CR, Little CB, Harwood JL, Caterson B. n-3 fatty acids specifically modulate catabolic factors involved in articular cartilage degradation. J Biol Chem. 2000;275:721–724. doi: 10.1074/jbc.275.2.721. [DOI] [PubMed] [Google Scholar]
- Tortorella MD, Malfait AM, Deccico C, Arner E. The role of ADAM-TS4 (aggrecanase-1) and ADAM-TS5 (aggrecanase-2) in a model of cartilage degradation. Osteoarthritis Cartilage. 2001;9:539–552. doi: 10.1053/joca.2001.0427. [DOI] [PubMed] [Google Scholar]
- Little CB, Hughes CE, Curtis CL, Jones SA, Caterson B, Flannery CR. Cyclosporin A inhibition of aggrecanase-mediated proteoglycan catabolism in articular cartilage. Arthritis Rheum. 2002;46:124–129. doi: 10.1002/1529-0131(200201)46:1<124::aid-art10121>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]
- Koshy PJ, Lundy CJ, Rowan AD, Porter S, Edwards DR, Hogan A, Clark IM, Cawston TE. The modulation of matrix metalloproteinase and ADAM gene expression in human chondrocytes by interleukin-1 and oncostatin M: a time-course study using real-time quantitative reverse transcription-polymerase chain reaction. Arthritis Rheum. 2002;46:961–967. doi: 10.1002/art.10212. [DOI] [PubMed] [Google Scholar]
- Yamanishi Y, Boyle DL, Clark M, Maki RA, Tortorella MD, Arner EC, Firestein GS. Expression and regulation of aggrecanase in arthritis: the role of TGF-beta. J Immunol. 2002;168:1405–1412. doi: 10.4049/jimmunol.168.3.1405. [DOI] [PubMed] [Google Scholar]
- Curtis CL, Rees SG, Little CB, Flannery CR, Hughes CE, Wilson C, Dent CM, Otterness IG, Harwood JL, Caterson B. Pathologic indicators of degradation and inflammation in human osteoarthritic cartilage are abrogated by exposure to n-3 fatty acids. Arthritis Rheum. 2002;46:1544–1553. doi: 10.1002/art.10305. [DOI] [PubMed] [Google Scholar]
- Lark MW, Bayne EK, Flanagan J, Harper CF, Hoerrner LA, Hutchinson NI, Singer II, Donatelli SA, Weidner JR, Williams HR, Mumford RA, Lohmander LS. Aggrecan degradation in human cartilage. Evidence for both matrix metalloproteinase and aggrecanase activity in normal, osteoarthritic, and rheumatoid joints. J Clin Invest. 1997;100:93–106. doi: 10.1172/JCI119526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fosang AJ, Last K, Maciewicz RA. Aggrecan is degraded by matrix metalloproteinases in human arthritis. Evidence that matrix metalloproteinase and aggrecanase activities can be independent. J Clin Invest. 1996;98:2292–2299. doi: 10.1172/JCI119040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Little CB, Flannery CR, Hughes CE, Mort JS, Roughley PJ, Dent C, Caterson B. Aggrecanase versus matrix metalloproteinases in the catabolism of the interglobular domain of aggrecan in vitro. Biochem J. 1999;344:61–68. [PMC free article] [PubMed] [Google Scholar]
- Little CB, Hughes CE, Curtis CL, Janusz MJ, Bohne R, Wang-Weigand S, Taiwo YO, Mitchell PG, Otterness IG, Flannery CR, Caterson B. Matrix metalloproteinases are involved in C-terminal and interglobular domain processing of cartilage aggrecan in late stage cartilage degradation. Matrix Biol. 2002;21:271–88. doi: 10.1016/s0945-053x(02)00004-5. [DOI] [PubMed] [Google Scholar]
- Fosang AJ, Last K, Stanton H, Weeks DB, Campbell IK, Hardingham TE, Hembry RM. Generation and novel distribution of matrix metalloproteinase-derived aggrecan fragments in porcine cartilage explants. J Biol Chem. 2000;275:33027–33037. doi: 10.1074/jbc.M910207199. [DOI] [PubMed] [Google Scholar]
- Stanton H, Fosang AJ. Matrix metalloproteinases are active following guanidine hydrochloride extraction of cartilage: generation of DIPEN neoepitope during dialysis. Matrix Biol. 2002;21:425–428. doi: 10.1016/s0945-053x(02)00035-5. [DOI] [PubMed] [Google Scholar]
- Sztrolovics R, White RJ, Roughley PJ, Mort JS. The mechanism of aggrecan release from cartilage differs with tissue origin and the agent used to stimulate catabolism. Biochem J. 2002;362:465–472. doi: 10.1042/0264-6021:3620465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee ER, Lamplugh L, Davoli MA, Beauchemin A, Chan K, Mort JS, Leblond CP. Enzymes active in the areas undergoing cartilage resorption during the development of the secondary ossification center in the tibiae of rats ages 0–21 days: I. Two groups of proteinases cleave the core protein of aggrecan. Dev Dyn. 2001;222:52–70. doi: 10.1002/dvdy.1168. [DOI] [PubMed] [Google Scholar]
- Maroudas A, Bayliss MT, Uchitel-Kaushansky N, Schneiderman R, Gilav E. Aggrecan turnover in human articular cartilage: use of aspartic acid racemization as a marker of molecular age. Arch Biochem Biophys. 1998;350:61–71. doi: 10.1006/abbi.1997.0492. [DOI] [PubMed] [Google Scholar]
- Sandy JD, Verscharen C. Analysis of aggrecan in human knee cartilage and synovial fluid indicates that aggrecanase (ADAMTS) activity is responsible for the catabolic turnover and loss of whole aggrecan whereas other protease activity is required for C-terminal processing in vivo. Biochem J. 2001;358:615–626. doi: 10.1042/0264-6021:3580615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Meurs JB, van Lent PL, Holthuysen AE, Singer II, Bayne EK, van den Berg WB. Kinetics of aggrecanase- and metalloproteinase-induced neoepitopes in various stages of cartilage destruction in murine arthritis. Arthritis Rheum. 1999;42:1128–1139. doi: 10.1002/1529-0131(199906)42:6<1128::AID-ANR9>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- van Meurs J, van Lent P, Stoop R, Holthuysen A, Singer I, Bayne E, Mudgett J, Poole R, Billinghurst C, van der Kraan P, Buma P, van den Berg W. Cleavage of aggrecan at the Asn341-Phe342 site coincides with the initiation of collagen damage in murine antigen-induced arthritis: a pivotal role for stromelysin 1 in matrix metalloproteinase activity. Arthritis Rheum. 1999;42:2074–2084. doi: 10.1002/1529-0131(199910)42:10<2074::AID-ANR7>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- Mudgett JS, Hutchinson NI, Chartrain NA, Forsyth AJ, McDonnell J, Singer II, Bayne EK, Flanagan J, Kawka D, Shen CF, Stevens K, Chen H, Trumbauer M, Visco DM. Susceptibility of stromelysin 1-deficient mice to collagen-induced arthritis and cartilage destruction. Arthritis Rheum. 1998;41:110–121. doi: 10.1002/1529-0131(199801)41:1<110::AID-ART14>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- Mort JS, Magny MC, Lee ER. Cathepsin B: an alternative protease for the generation of an aggrecan 'metalloproteinase' cleavage neoepitope. Biochem J. 1998;335:491–494. doi: 10.1042/bj3350491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mason RM, Chambers MG, Flannelly J, Gaffen JD, Dudhia J, Bayliss MT. The STR/ort mouse and its use as a model of osteoarthritis. Osteoarthritis Cartilage. 2001;9:85–91. doi: 10.1053/joca.2000.0363. [DOI] [PubMed] [Google Scholar]
- Chambers MG, Cox L, Chong L, Suri N, Cover P, Bayliss MT, Mason RM. Matrix metalloproteinases and aggrecanases cleave aggrecan in different zones of normal cartilage but colocalize in the development of osteoarthritic lesions in STR/ort mice. Arthritis Rheum. 2001;44:1455–1465. doi: 10.1002/1529-0131(200106)44:6<1455::AID-ART241>3.0.CO;2-J. [DOI] [PubMed] [Google Scholar]
- Sasaki M, Seo-Kiryu S, Kato R, Kita S, Kiyama H. A disintegrin and metalloprotease with thrombospondin type1 motifs (ADAMTS-1) and IL-1 receptor type 1 mRNAs are simultaneously induced in nerve injured motor neurons. Brain Res Mol Brain Res. 2001;89:158–163. doi: 10.1016/s0169-328x(01)00046-8. [DOI] [PubMed] [Google Scholar]
- Singer II, Scott S, Kawka DW, Bayne EK, Weidner JR, Williams HR, Mumford RA, Lark MW, McDonnell J, Christen AJ, Moore VL, Mudgett JS, Visco DM. Aggrecanase and metalloproteinase-specific aggrecan neo-epitopes are induced in the articular cartilage of mice with collagen II-induced arthritis. Osteoarthritis Cartilage. 1997;5:407–418. doi: 10.1016/s1063-4584(97)80045-3. [DOI] [PubMed] [Google Scholar]
- Lemons ML, Sandy JD, Anderson DK, Howland DR. Intact aggrecan and fragments generated by both aggrecanse and metalloproteinase-like activities are present in the developing and adult rat spinal cord and their relative abundance is altered by injury. J Neurosci. 2001;21:4772–4781. doi: 10.1523/JNEUROSCI.21-13-04772.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Satoh K, Suzuki N, Yokota H. ADAMTS-4 (a disintegrin and metalloproteinase with thrombospondin motifs) is transcriptionally induced in beta-amyloid treated rat astrocytes. Neurosci Lett. 2000;289:177–180. doi: 10.1016/s0304-3940(00)01285-4. [DOI] [PubMed] [Google Scholar]
- Konttinen YT, Mandelin J, Li TF, Salo J, Lassus J, Liljestrom M, Hukkanen M, Takagi M, Virtanen I, Santavirta S. Acidic cysteine endoproteinase cathepsin K in the degeneration of the superficial articular hyaline cartilage in osteoarthritis. Arthritis Rheum. 2002;46:953–960. doi: 10.1002/art.10185. [DOI] [PubMed] [Google Scholar]
- Lang A, Horler D, Baici A. The relative importance of cysteine peptidases in osteoarthritis. J Rheumatol. 2000;27:1970–1979. [PubMed] [Google Scholar]


