Abstract
The intervertebral disc is a cartilaginous structure that resembles articular cartilage in its biochemistry, but morphologically it is clearly different. It shows degenerative and ageing changes earlier than does any other connective tissue in the body. It is believed to be important clinically because there is an association of disc degeneration with back pain. Current treatments are predominantly conservative or, less commonly, surgical; in many cases there is no clear diagnosis and therapy is considered inadequate. New developments, such as genetic and biological approaches, may allow better diagnosis and treatments in the future.
Keywords: back pain, epidemiology, genetics
Introduction
Back pain is a major public health problem in Western industrialized societies. It causes suffering and distress to patients and their families, and affects a large number of people; the point prevalence rates in a number of studies ranged from 12% to 35% [1], with around 10% of sufferers becoming chronically disabled. It also places an enormous economic burden on society; its total cost, including direct medical costs, insurance, lost production and disability benefits, is estimated at €12 billion per annum in the UK and 1.7% of the gross national product in The Netherlands [1,2].
Back pain is strongly associated with degeneration of the intervertebral disc [3]. Disc degeneration, although in many cases asymptomatic [4], is also associated with sciatica and disc herniation or prolapse. It alters disc height and the mechanics of the rest of the spinal column, possibly adversely affecting the behaviour of other spinal structures such as muscles and ligaments. In the long term it can lead to spinal stenosis, a major cause of pain and disability in the elderly; its incidence is rising exponentially with current demographic changes and an increased aged population.
Discs degenerate far earlier than do other musculoskeletal tissues; the first unequivocal findings of degeneration in the lumbar discs are seen in the age group 11–16 years [5]. About 20% of people in their teens have discs with mild signs of degeneration; degeneration increases steeply with age, particularly in males, so that around 10% of 50-year-old discs and 60% of 70-year-old discs are severely degenerate [6].
In this short review we outline the morphology and biochemistry of normal discs and the changes that arise during degeneration. We review recent advances in our understanding of the aetiology of this disorder and discuss new approaches to treatment.
Disc morphology
The normal disc
The intervertebral discs lie between the vertebral bodies, linking them together (Fig. 1). They are the main joints of the spinal column and occupy one-third of its height. Their major role is mechanical, as they constantly transmit loads arising from body weight and muscle activity through the spinal column. They provide flexibility to this, allowing bending, flexion and torsion. They are approximately 7–10 mm thick and 4 cm in diameter (anterior–posterior plane) in the lumbar region of the spine [7,8]. The intervertebral discs are complex structures that consist of a thick outer ring of fibrous cartilage termed the annulus fibrosus, which surrounds a more gelatinous core known as the nucleus pulposus; the nucleus pulposus is sandwiched inferiorly and superiorly by cartilage end-plates.
Figure 1.
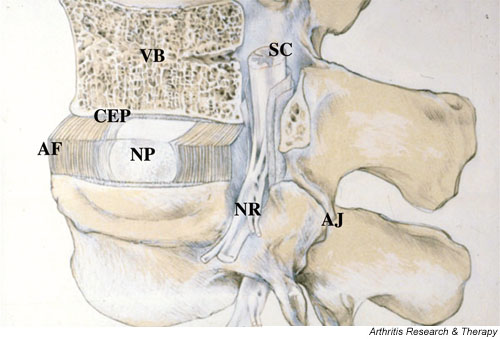
A schematic view of a spinal segment and the intervertebral disc. The figure shows the organization of the disc with the nucleus pulposus (NP) surrounded by the lamellae of the annulus fibrosus (AF) and separated from the vertebral bodies (VB) by the cartilaginous end-plate (CEP). The figure also shows the relationship between the intervertebral disc and the spinal cord (SC), the nerve root (NR), and the apophyseal joints (AJ).
The central nucleus pulposus contains collagen fibres, which are organised randomly [9], and elastin fibres (sometimes up to 150 μm in length), which are arranged radially [10]; these fibres are embedded in a highly hydrated aggrecan-containing gel. Interspersed at a low density (approximately 5000/mm3 [11]) are chondrocyte-like cells, sometimes sitting in a capsule within the matrix. Outside the nucleus is the annulus fibrosus, with the boundary between the two regions being very distinct in the young individual (<10 years).
The annulus is made up of a series of 15–25 concentric rings, or lamellae [12], with the collagen fibres lying parallel within each lamella. The fibres are orientated at approximately 60° to the vertical axis, alternating to the left and right of it in adjacent lamellae. Elastin fibres lie between the lamellae, possibly helping the disc to return to its original arrangement following bending, whether it be flexion or extension. They may also bind the lamellae together as elastin fibres pass radially from one lamella to the next [10]. The cells of the annulus, particularly in the outer region, tend to be fibroblast-like, elongated, thin and aligned parallel to the collagen fibres. Toward the inner annulus the cells can be more oval. Cells of the disc, both in the annulus and nucleus, can have several long, thin cytoplasmic projections, which may be more than 30 μm long [13,14] (WEB Johnson, personal communication). Such features are not seen in cells of articular cartilage [13]. Their function in disc is unknown but it has been suggested that they may act as sensors and communicators of mechanical strain within the tissue [13].
The third morphologically distinct region is the cartilage end-plate, a thin horizontal layer, usually less than 1 mm thick, of hyaline cartilage. This interfaces the disc and the vertebral body. The collagen fibres within it run horizontal and parallel to the vertebral bodies, with the fibres continuing into the disc [8].
The healthy adult disc has few (if any) blood vessels, but it has some nerves, mainly restricted to the outer lamellae, some of which terminate in proprioceptors [15]. The cartilaginous end-plate, like other hyaline cartilages, is normally totally avascular and aneural in the healthy adult. Blood vessels present in the longitudinal ligaments adjacent to the disc and in young cartilage end-plates (less than about 12 months old) are branches of the spinal artery [16]. Nerves in the disc have been demonstrated, often accompanying these vessels, but they can also occur independently, being branches of the sinuvertebral nerve or derived from the ventral rami or grey rami communicantes. Some of the nerves in discs also have glial support cells, or Schwann cells, alongside them [17].
Degenerated discs
During growth and skeletal maturation the boundary between annulus and nucleus becomes less obvious, and with increasing age the nucleus generally becomes more fibrotic and less gel-like [18]. With increasing age and degeneration the disc changes in morphology, becoming more and more disorganized (Fig. 2). Often the annular lamellae become irregular, bifurcating and interdigitating, and the collagen and elastin networks also appear to become more disorganised (J Yu, personal communication).
Figure 2.
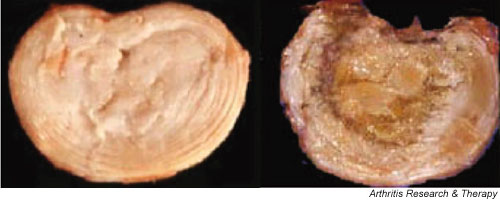
The normal and degenerate lumbar intervertebral disc. The figure shows a normal intervertebral disc on the left. The annulus lamellae surrounding the softer nucleus pulposus are clearly visible. In the highly degenerate disc on the right, the nucleus is desiccated and the annulus is disorganized.
There is frequently cleft formation with fissures forming within the disc, particularly in the nucleus. Nerves and blood vessels are increasingly found with degeneration [15]. Cell proliferation occurs, leading to cluster formation, particularly in the nucleus [19,20]. Cell death also occurs, with the presence of cells with necrotic and apoptotic appearance [21,22]. These mechanisms are apparently very common; it has been reported that more than 50% of cells in adult discs are necrotic [21]. The morphological changes associated with disc degeneration were comprehensively reviewed recently by Boos et al. [5], who demonstrated an age-associated change in morphology, with discs from individuals as young as 2 years of age having some very mild cleft formation and granular changes to the nucleus. With increasing age comes an increased incidence of degenerative changes, including cell death, cell proliferation, mucous degeneration, granular change and concentric tears. It is difficult to differentiate changes that occur solely due to ageing from those that might be considered 'pathological'.
Biochemistry
Normal discs
The mechanical functions of the disc are served by the extracellular matrix; its composition and organization govern the disc's mechanical responses. The main mechanical role is provided by the two major macromolecular components. The collagen network, formed mostly of type I and type II collagen fibrils and making up approximately 70% and 20% of the dry weight of the annulus and nucleus, respectively [23], provides tensile strength to the disc and anchors the tissue to the bone. Aggrecan, the major proteoglycan of the disc [24], is responsible for maintaining tissue hydration through the osmotic pressure provided by its constituent chondroitin and keratan sulphate chains [25]. The proteoglycan and water content of the nucleus (around 50% and 80% of the wet weight, respectively) is greater than in the annulus (approximately 20% and 70% of the wet weight, respectively). In addition, there are many other minor components, such as collagen types III, V, VI, IX, X, XI, XII and XIV; small proteoglycans such as lumican, biglycan, decorin and fibromodulin; and other glycoproteins such as fibronectin and amyloid [26,27]. The functional role of many of these additional matrix proteins and glycoproteins is not yet clear. Collagen IX, however, is thought to be involved in forming cross-links between collagen fibrils and is thus important in maintaining network integrity [28].
The matrix is a dynamic structure. Its molecules are continually being broken down by proteinases such as the matrix metalloproteinases (MMPs) and aggrecanases, which are also synthesized by disc cells [29-31]. The balance between synthesis, breakdown and accumulation of matrix macromolecules determines the quality and integrity of the matrix, and thus the mechanical behaviour of the disc itself. The integrity of the matrix is also important for maintaining the relatively avascular and aneural nature of the healthy disc.
The intervertebral disc is often likened to articular cartilage, and indeed it does resemble it in many ways, particularly in the biochemical components present. However, there are significant differences between the two tissues, one of these being the composition and structure of aggrecan. Disc aggrecan is more highly substituted with keratan sulphate than that found in the deep zone of articular cartilage. In addition, the aggrecan molecules are less aggregated (30%) and more heterogeneous, with smaller, more degraded fragments in the disc than in articular cartilage (80% aggregated) from the same individual [32]. Disc proteoglycans become increasingly difficult to extract from the matrix with increasing age [24]; this may be due to extensive cross-linking, which appears to occur more within the disc matrix than in other connective tissues.
Changes in disc biochemistry with degeneration
The most significant biochemical change to occur in disc degeneration is loss of proteoglycan [33]. The aggrecan molecules become degraded, with smaller fragments being able to leach from the tissue more readily than larger portions. This results in loss of glycosaminoglycans; this loss is responsible for a fall in the osmotic pressure of the disc matrix and so a loss of hydration.
Even in degenerate discs, however, the disc cells can retain the ability to synthesize large aggrecan molecules, with intact hyaluronan-binding regions, which have the potential to form aggregates [24]. Less is known of how the small proteoglycan population changes with disc degeneration, although there is some evidence that the amount of decorin, and more particularly biglycan, is elevated in degenerate human discs as compared with normal ones [34].
Although the collagen population of the disc also changes with degeneration of the matrix, the changes are not as obvious as those of the proteoglycans. The absolute quantity of collagen changes little but the types and distribution of collagens can alter. For example, there may be a shift in proportions of types of collagens found and in their apparent distribution within the matrix. In addition, the fibrillar collagens, such as type II collagen, become more denatured, apparently because of enzymic activity. As with proteoglycans, the triple helices of the collagens are more denatured and ruptured than are those found in articular cartilage from the same individual; the amount of denatured type II collagen increases with degeneration [35,36]. However, collagen cross-link studies indicate that, as with proteoglycans, new collagen molecules may be synthesized, at least early in disc degeneration, possibly in an attempt at repair [37].
Other components can change in disc degeneration and disease in either quantity or distribution. For example, fibronectin content increases with increasing degeneration and it becomes more fragmented [38]. These elevated levels of fibronectin could reflect the response of the cell to an altered environment. Whatever the cause, the formation of fibronectin fragments can then feed into the degenerative cascade because they have been shown to downregulate aggrecan synthesis but to upregulate the production of some MMPs in in vitro systems.
The biochemistry of disc degeneration indicates that enzymatic activity contributes to this disorder, with increased fragmentation of the collagen, proteoglycan and fibronectin populations. Several families of enzymes are capable of breaking down the various matrix molecules of disc, including cathepsins, MMPs and aggrecanases. Cathepsins have maximal activity in acid conditions (e.g. cathepsin D is inactive above pH 7.2). In contrast, MMPs and aggrecanases have an optimal pH that is approximately neutral. All of these enzymes have been identified in disc, with higher levels of, for example, MMPs in more degenerate discs [39]. Cathepsins D and L and several types of MMPs (MMP-1, -2, -3, -7, -8, -9 and -13) occur in human discs; they may be produced by the cells of the disc themselves as well as by the cells of the invading blood vessels. Aggrecanases have also been shown to occur in human disc but their activity is apparently less obvious, at least in more advanced disc degeneration [29,30,40].
Effect of degenerative changes on disc function and pathology
The loss of proteoglycan in degenerate discs [33] has a major effect on the disc's load-bearing behaviour. With loss of proteoglycan, the osmotic pressure of the disc falls [41] and the disc is less able to maintain hydration under load; degenerate discs have a lower water content than do normal age-matched discs [33], and when loaded they lose height [42] and fluid more rapidly, and the discs tend to bulge. Loss of proteoglycan and matrix disorganization have other important mechanical effects; because of the subsequent loss of hydration, degenerated discs no longer behave hydrostatically under load [43]. Loading may thus lead to inappropriate stress concentrations along the end-plate or in the annulus; the stress concentrations seen in degenerate discs have also been associated with discogenic pain produced during discography [44].
Such major changes in disc behaviour have a strong influence on other spinal structures, and may affect their function and predispose them to injury. For instance, as a result of the rapid loss of disc height under load in degenerate discs, apophyseal joints adjacent to such discs (Fig. 1) may be subject to abnormal loads [45] and eventually develop osteoarthritic changes. Loss of disc height can also affect other structures. It reduces the tensional forces on the ligamentum flavum and hence may cause remodelling and thickening. With consequent loss of elasticity [46], the ligament will tend to bulge into the spinal canal, leading to spinal stenosis – an increasing problem as the population ages.
Loss of proteoglycans also influences the movement of molecules into and out of the disc. Aggrecan, because of its high concentration and charge in the normal disc, prevents movement of large uncharged molecules such as serum proteins and cytokines into and through the matrix [47]. The fall in concentration of aggrecan in degeneration could thus facilitate loss of small, but osmotically active, aggrecan fragments from the disc, possibly accelerating a degenerative cascade. In addition, loss of aggrecan would allow increased penetration of large molecules such as growth factor complexes and cytokines into the disc, affecting cellular behaviour and possibly the progression of degeneration. The increased vascular and neural ingrowth seen in degenerate discs and associated with chronic back pain [48] is also probably associated with proteoglycan loss because disc aggrecan has been shown to inhibit neural ingrowth [49,50].
Disc herniation
The most common disc disorder presenting to spinal surgeons is herniated or prolapsed intervertebral disc. In these cases the discs bulge or rupture (either partially or totally) posteriorly or posterolaterally, and press on the nerve roots in the spinal canal (Fig. 1). Although herniation is often thought to be the result of a mechanically induced rupture, it can only be induced in vitro in healthy discs by mechanical forces larger than those that are ever normally encountered; in most experimental tests, the vertebral body fails rather than the disc [51]. Some degenerative changes seem necessary before the disc can herniate; indeed, examination of autopsy or surgical specimens suggest that sequestration or herniation results from the migration of isolated, degenerate fragments of nucleus pulposus through pre-existing tears in the annulus fibrosus [52].
It is now clear that herniation-induced pressure on the nerve root cannot alone be the cause of pain because more than 70% of 'normal', asymptomatic people have disc prolapses pressurizing the nerve roots but no pain [4,53]. A past and current hypothesis is that, in symptomatic individuals, the nerves are somehow sensitized to the pressure [54], possibly by molecules arising from an inflammatory cascade from arachodonic acid through to prostaglandin E2, thromboxane, phospholipase A2, tumour necrosis factor-α, the interleukins and MMPs. These molecules can be produced by cells of herniated discs [55], and because of the close physical contact between the nerve root and disc following herniation they may be able to sensitize the nerve root [56,57]. The exact sequence of events and specific molecules that are involved have not been identified, but a pilot study of sciatic patients treated with tumour necrosis factor-α antagonists is encouraging and supports this proposed mechanism [58,59]. However, care must be exercised in interrupting the inflammatory cascade, which can also have beneficial effects. Molecules such as MMPs, which are produced extensively in prolapsed discs [30], almost certainly play a major role in the natural history of resorbing the offending herniation.
Aetiology of disc degeneration
Disc degeneration has proved a difficult entity to study; its definition is vague, with diffuse parameters that are not always easy to quantify. In addition, there is a lack of a good animal model. There are significant anatomical differences between humans and the laboratory animals that are traditionally used as models of other disorders. In particular, the nucleus differs; in rodents as well as many other mammals, the nucleus is populated by notochordal cells throughout adulthood, whereas these cells disappear from the human nucleus after infancy [60]. In addition, although the cartilage end-plate in humans acts as a growth plate for the vertebral body, in most animals the vertebrae have two growth plates within the vertebral body itself, and the cartilage end-plate is a much thinner layer than that found in humans. Thus, although the study of animals that develop degeneration spontaneously [61,62] and of injury models of degeneration [63,64] have provided some insight into the degenerative processes, most information on aetiology of disc degeneration to date has come from human studies.
Nutritional pathways to disc degeneration
One of the primary causes of disc degeneration is thought to be failure of the nutrient supply to the disc cells [65]. Like all cell types, the cells of the disc require nutrients such as glucose and oxygen to remain alive and active. In vitro, the activity of disc cells is very sensitive to extracellular oxygen and pH, with matrix synthesis rates falling steeply at acidic pH and at low oxygen concentrations [66,67], and the cells do not survive prolonged exposure to low pH or glucose concentrations [68]. A fall in nutrient supply that leads to a lowering of oxygen tension or of pH (arising from raised lactic acid concentrations) could thus affect the ability of disc cells to synthesize and maintain the disc's extracellular matrix and could ultimately lead to disc degeneration.
The disc is large and avascular and the cells depend on blood vessels at their margins to supply nutrients and remove metabolic waste [69]. The pathway from the blood supply to the nucleus cells is precarious because these cells are supplied virtually entirely by capillaries that originate in the vertebral bodies, penetrating the subchondral plate and terminating just above the cartilaginous end-plate [16,70]. Nutrients must then diffuse from the capillaries through the cartilaginous end-plate and the dense extracellular matrix of the nucleus to the cells, which may be as far as 8 mm from the capillary bed.
The nutrient supply to the nucleus cells can be disturbed at several points. Factors that affect the blood supply to the vertebral body such as atherosclerosis [71,72], sickle cell anaemia, Caisson disease and Gaucher's disease [73] all appear to lead to a significant increase in disc degeneration. Long-term exercise or lack of it appears to have an effect on movement of nutrients into the disc, and thus on their concentration in the tissue [74,75]. The mechanism is not known but it has been suggested that exercise affects the architecture of the capillary bed at the disc–bone interface. Finally, even if the blood supply remains undisturbed, nutrients may not reach the disc cells if the cartilaginous end-plate calcifies [65,76]; intense calcification of the end-plate is seen in scoliotic discs [77], for instance. Disturbances in nutrient supply have been shown to affect transport of oxygen and lactic acid into and out of the disc experimentally [78] and in patients [79].
Although little information is available to relate nutrient supply to disc properties in patients, a relationship has been found between loss of cell viability and a fall in nutrient transport in scoliotic discs [80,81]. There is also some evidence that nutrient transport is affected in disc degeneration in vivo [82], and the transport of solutes from bone to disc measured in vitro was significantly lower in degenerate than in normal discs [65]. Thus, although there is as yet little direct evidence, it now seems apparent that a fall in nutrient supply will ultimately lead to degeneration of the disc.
Mechanical load and injury
Abnormal mechanical loads are also thought to provide a pathway to disc degeneration. For many decades it was suggested that a major cause of back problems is injury, often work-related, which causes structural damage. It is believed that such an injury initiates a pathway that leads to disc degeneration and finally to clinical symptoms and back pain [83]. Animal models have supported this finding. Although intense exercise does not appear to affect discs adversely [84] and discs are reported to respond to some long-term loading regimens by increasing proteoglycan content [85], experimental overloading [86] or injury to the disc [63,87] can induce degenerative changes. Further support for the role of abnormal mechanical forces in disc degeneration comes from findings that disc levels adjacent to a fused segment degenerate rapidly (for review [88]).
This injury model is also supported by many epidemiological studies that have found associations between environmental factors and development of disc degeneration and herniation, with heavy physical work, lifting, truck-driving, obesity and smoking found to be the major risk factors for back pain and degeneration [89-91]. As a result of these studies, there have been many ergonomic interventions in the workplace [91]. However, the incidence of disc degeneration-related disorders has continued to rise despite these interventions. Over the past decade, as magnetic resonance imaging has refined classifications of disc degeneration [5,92], it has become evident that, although factors such as occupation, psychosocial factors, benefit payments and environment are linked to disabling back pain [93,94], contrary to previous assumptions these factors have little influence on the pattern of disc degeneration itself [95,96]. This illustrates the tenuous relationship between degeneration and clinical symptoms.
Genetic factors in disc degeneration
More recent work suggested that the factors that lead to disc degeneration may have important genetic components. Several studies have reported a strong familial predisposition for disc degeneration and herniation [97-99]. Findings from two different twin studies conducted during the past decade showed heritability exceeding 60% [100,101]. Magnetic resonance images in identical twins, who were discordant for major risk factors such as smoking or heavy work, were very similar with respect to the spinal columns and the patterns of disc degeneration (Fig. 3) [102].
Figure 3.
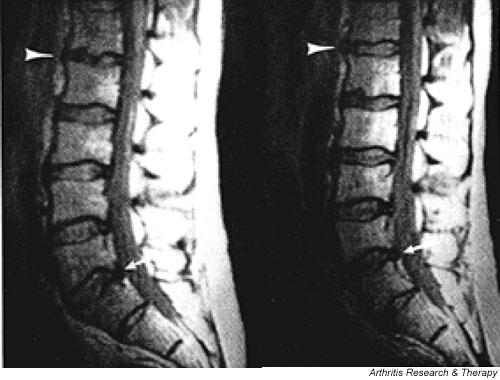
Magnetic resonance images of the lumbar discs of 44-year-old identical twins. Note similarities in the contours of the end-plates, particularly at L1-L2 (white arrow head). The spines also show similar degenerative changes in the disc, particularly at L4-L5 (white arrow). From [102], with kind permission from the authors and publishers.
Genetic predisposition has been confirmed by recent findings of associations between disc degeneration and gene polymorphisms of matrix macromolecules. The approach to date has been via searching for candidate genes, with the main focus being extracellular matrix genes. Although there is a lack of association between disc degeneration and polymorphisms of the major collagens in the disc, collagen types I and II [103], mutations of two collagen type IX genes, namely COL9A2 and COL9A3, have been found to be strongly associated with lumbar disc degeneration and sciatica in a Finnish population [104,105]. The COL9A2 polymorphism is found only in a small percentage of the Finnish population, but all individuals with this allele had disc degenerative disorders, suggesting that it is associated with a dominantly inherited disease. In both these mutations, tryptophan (the most hydrophobic amino acid, which is not normally found in any collagenous domain) substituted for other amino acids, potentially affecting matrix properties [103].
Other genes associated with disc generation have also been identified. Individuals with a polymorphism in the aggrecan gene were found to be at risk for early disc degeneration in a Japanese study [106]. This mutation leads to aggrecan core proteins of different lengths, with an over-representation of core proteins able to bind only a low number of chondroitin sulfate chains among those with severe disc degeneration. Presumably these individuals have a lower chondroitin sulfate content than normal, and their discs will behave similarly to degenerate discs that have lost proteoglycan by other mechanisms. Studies of transgenic mice have also demonstrated that mutations in structural matrix molecules such as aggrecan [107], collagen II [108] and collagen IX [109] can lead to disc degeneration. Mutations in genes other than those of structural matrix macromolecules have also been associated with disc degeneration. A polymorphism in the promoter region of the MMP-3 gene was associated with rapid degeneration in elderly Japanese subjects [110]. In addition, two polymorphisms of the vitamin D receptor gene were the first mutations shown to be associated with disc degeneration [111-114]. The mechanism of vitamin D receptor gene polymorphism involvement in disc degeneration is unknown, but at present it does not appear to be related to differences in bone density [111,112,114].
All of the genetic mutations associated with disc degeneration to date have been found using a candidate gene approach and all, apart from the vitamin D receptor polymorphism, are concerned with molecules that determine the integrity and function of the extracellular matrix. However, mutations in other systems such as signalling or metabolic pathways could lead to changes in cellular activity that may ultimately result in disc degeneration [115]. Different approaches may be necessary to identify such polymorphisms. Genetic mapping, for instance, has identified a susceptibility locus for disc herniation, but the gene involved has not yet been identified [116].
In summary, the findings from these genetic and epidemiological studies point to the multifactorial nature of disc degeneration. It is evident now that mutations in several different classes of genes may cause the changes in matrix morphology, disc biochemistry and disc function typifying disc degeneration. Identification of the genes involved may lead to improved diagnostic criteria; for example, it is already apparent that the presence of specific polymorphisms increase the risk for disc bulge, annular tears, or osteophytes [112,117]. However, because of the evidence for gene–environment interactions [97,114,118], genetic studies in isolation are unlikely to delineate the various pathways of disc degeneration.
New therapies
Current treatments attempt to reduce pain rather than repair the degenerated disc. The treatments used presently are mainly conservative and palliative, and are aimed at returning patients to work. They range from bedrest (no longer recommended) to analgesia, the use of muscle relaxants or injection of corticosteroids, or local anaesthetic and manipulation therapies. Various interventions (e.g. intradiscal electrotherapy) are also used, but despite anecdotal statements of success trials thus far have found their use to be of little direct benefit [119]. Disc degeneration-related pain is also treated surgically either by discectomy or by immobilization of the affected vertebrae, but surgery is offered only to one in every 2000 back pain episodes in the UK; the incidence of surgical treatment is five times higher in the USA [93]. The success rates of all these procedures are generally similar. Although a recent study indicated that surgery improves the rate of recovery in well selected patients [120], 70–80% of patients with obvious surgical indications for back pain or disc herniation eventually recover, whether surgery is carried out or not [121,122].
Because disc degeneration is thought to lead to degeneration of adjacent tissues and be a risk factor in the development of spinal stenosis in the long term, new treatments are in development that are aimed at restoring disc height and biomechanical function. Some of the proposed biological therapies are outlined below.
Cell based therapies
The aim of these therapies is to achieve cellular repair of the degenerated disc matrix. One approach has been to stimulate the disc cells to produce more matrix. Growth factors can increase rates of matrix synthesis by up to fivefold [123,124]. In contrast, cytokines lead to matrix loss because they inhibit matrix synthesis while stimulating production of agents that are involved in tissue breakdown [125]. These proteins have thus provided targets for genetic engineering. Direct injection of growth factors or cytokine inhibitors has proved unsuccessful because their effectiveness in the disc is short-lived. Hence gene-therapy is now under investigation; it has the potential to maintain high levels of the relevant growth factor or inhibitor in the tissue. In gene therapy, the gene of interest (e.g. one responsible for producing a growth factor such as transforming growth factor-β or inhibiting interleukin-1) is introduced into target cells, which then continue to produce the relevant protein (for review [126]). This approach has been shown to be technically feasible in the disc, with gene transfer increasing transforming growth factor-β production by disc cells in a rabbit nearly sixfold [127]. However, this therapy is still far from clinical use. Apart from the technical problems of delivery of the genes into human disc cells, the correct choice of therapeutic genes requires an improved understanding of the pathogenesis of degeneration. In addition, the cell density in normal human discs is low, and many of the cells in degenerate discs are dead [21]; stimulation of the remaining cells may be insufficient to repair the matrix.
Cell implantation alone or in conjunction with gene therapy is an approach that may overcome the paucity of cells in a degenerate disc. Here, the cells of the degenerate disc are supplemented by adding new cells either on their own or together with an appropriate scaffold. This technique has been used successfully for articular cartilage [128,129] and has been attempted with some success in animal discs [130]. However, at present, no obvious source of clinically useful cells exists for the human disc, particularly for the nucleus, the region of most interest [131]. Moreover, conditions in degenerate discs, particularly if the nutritional pathway has been compromised [65], may not be favourable for survival of implanted cells. Nevertheless, autologous disc cell transfer has been used clinically in small groups of patients [132], with initial results reported to be promising, although few details of the patients or outcome measures are available.
At present, although experimental work demonstrates the potential of these cell-based therapies, several barriers prevent the use of these treatments clinically. Moreover, these treatments are unlikely to be appropriate for all patients; some method of selecting appropriate patients will be required if success with these therapies is to be realized.
Conclusion
Disorders associated with degeneration of the intervertebral disc impose an economic burden similar to that of coronary heart disease and greater than that of other major health problems such as diabetes, Alzheimer's disease and kidney diseases [1,133]. New imaging technologies, and advances in cell biology and genetics promise improved understanding of the aetiology, more specific diagnoses and targeted treatments for these costly and disabling conditions. However, the intervertebral disc is poorly researched, even in comparison with other musculoskeletal systems (Table 1). Moreover, the research effort in, for instance, the kidney in comparison with that in the disc is completely disparate to the relative costs of the disorders associated with each organ and the number of people affected. Unless more research attention is attracted to interverterbal disc biology, little will come from these new technologies, and back pain will remain as it is at present – a poorly diagnosed and poorly treated syndrome that reduces the quality of life of a significant proportion of the population.
Table 1.
Comparison between numbers of papers published in different research areas
| Intervertebral disc | Tendon | Cartilage | Kidney | |
| Metabolism | 696 | 2758 | 14,873 | 193,929 |
| Biomechanics | 769 | 3572 | 3996 | 16,275 |
The table gives the results of a literature search on PubMed in January 2003 using the keywords in the left-hand column together with each of the column headings. No sorting of references was performed.
Competing interests
None declared.
Abbreviations
MMP = matrix metalloproteinase.
Acknowledgments
Acknowledgement
The authors thank the Arthritis Research Campaign for support (U0511).
References
- Maniadakis N, Gray A. The economic burden of back pain in the UK. Pain. 2000;84:95–103. doi: 10.1016/S0304-3959(99)00187-6. [DOI] [PubMed] [Google Scholar]
- van Tulder MW, Koes BW, Bouter LM. A cost-of-illness study of back pain in The Netherlands. Pain. 1995;62:233–240. doi: 10.1016/0304-3959(94)00272-G. [DOI] [PubMed] [Google Scholar]
- Luoma K, Riihimaki H, Luukkonen R, Raininko R, Viikari-Juntura E, Lamminen A. Low back pain in relation to lumbar disc degeneration. Spine. 2000;25:487–492. doi: 10.1097/00007632-200002150-00016. [DOI] [PubMed] [Google Scholar]
- Boden SD, Davis DO, Dina TS, Patronas NJ, Wiesel SW. Abnormal magnetic-resonance scans of the lumbar spine in asymptomatic subjects. A prospective investigation. J Bone Joint Surg [Am] 1990;72:403–408. [PubMed] [Google Scholar]
- Boos N, Weissbach S, Rohrbach H, Weiler C, Spratt KF, Nerlich AG. Classification of age-related changes in lumbar intervertebral discs: 2002 Volvo Award in basic science. Spine. 2002;27:2631–2644. doi: 10.1097/00007632-200212010-00002. [DOI] [PubMed] [Google Scholar]
- Miller J, Schmatz C, Schultz A. Lumbar disc degeneration: Correlation with Age, Sex, and Spine Level in 600 Autopsy Specimens. Spine. 1988;13:173–178. [PubMed] [Google Scholar]
- Twomey LT, Taylor JR. Age changes in lumbar vertebrae and intervertebral discs. Clin Orthop. 1987;224:97–104. [PubMed] [Google Scholar]
- Roberts S, Menage J, Urban JPG. Biochemical and structural properties of the cartilage end-plate and its relation to the intervertebral disc. Spine. 1989;14:166–174. doi: 10.1097/00007632-198902000-00005. [DOI] [PubMed] [Google Scholar]
- Inoue H. Three-dimensional architecture of lumbar intervertebral discs. Spine. 1981;6:139–146. doi: 10.1097/00007632-198103000-00006. [DOI] [PubMed] [Google Scholar]
- Yu J, Winlove CP, Roberts S, Urban JP. Elastic fibre organization in the intervertebral discs of the bovine tail. J Anat. 2002;201:465–475. doi: 10.1046/j.1469-7580.2002.00111.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maroudas A, Nachemson A, Stockwell R, Urban J. Some factors involved in the nutrition of the intervertebral disc. J Anat. 1975;120:113–130. [PMC free article] [PubMed] [Google Scholar]
- Marchand F, Ahmed AM. Investigation of the laminate structure of lumbar disc anulus fibrosus. Spine. 1990;15:402–410. doi: 10.1097/00007632-199005000-00011. [DOI] [PubMed] [Google Scholar]
- Errington RJ, Puustjarvi K, White IR, Roberts S, Urban JP. Characterisation of cytoplasm-filled processes in cells of the intervertebral disc. J Anat. 1998;192:369–378. doi: 10.1046/j.1469-7580.1998.19230369.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruehlmann SB, Rattner JB, Matyas JR, Duncan NA. Regional variations in the cellular matrix of the annulus fibrosus of the intervertebral disc. J Anat. 2002;201:159–171. doi: 10.1046/j.1469-7580.2002.00080.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts S, Eisenstein SM, Menage J, Evans EH, Ashton IK. Mechanoreceptors in intervertebral discs. Morphology, distribution, and neuropeptides. Spine. 1995;20:2645–2651. doi: 10.1097/00007632-199512150-00005. [DOI] [PubMed] [Google Scholar]
- Crock HV, Goldwasser M, Yoshizawa H. Vascular anatomy related to the intervertebral disc. In: Ghosh P, editor. Biology of the Intervertebral Disc. Boca Raton: CRC Press; 1991. pp. 109–133. [Google Scholar]
- Johnson WE, Evans H, Menage J, Eisenstein SM, El Haj A, Roberts S. Immunohistochemical detection of Schwann cells in innervated and vascularized human intervertebral discs. Spine. 2001;26:2550–2557. doi: 10.1097/00007632-200112010-00007. [DOI] [PubMed] [Google Scholar]
- Buckwalter JA. Aging and degeneration of the human intervertebral disc. Spine. 1995;20:1307–1314. doi: 10.1097/00007632-199506000-00022. [DOI] [PubMed] [Google Scholar]
- Johnson WEB, Eisenstein SM, Roberts S. Cell cluster formation in degenerate lumbar intervertebral discs is associated with increased disc cell proliferation. Connect Tissue Res. 2001;42:197–207. doi: 10.3109/03008200109005650. [DOI] [PubMed] [Google Scholar]
- Hastreiter D, Ozuna RM, Spector M. Regional variations in certain cellular characteristics in human lumbar intervertebral discs, including the presence of alpha-smooth muscle actin. J Orthop Res. 2001;19:597–604. doi: 10.1016/S0736-0266(00)00069-3. [DOI] [PubMed] [Google Scholar]
- Trout JJ, Buckwalter JA, Moore KC. Ultrastructure of the human intervertebral disc: II. Cells of the nucleus pulposus. Anat Rec. 1982;204:307–314. doi: 10.1002/ar.1092040403. [DOI] [PubMed] [Google Scholar]
- Gruber HE, Hanley EN. Analysis of aging and degeneration of the human intervertebral disc – Comparison of surgical specimens with normal controls. Spine. 1998;23:751–757. doi: 10.1097/00007632-199804010-00001. [DOI] [PubMed] [Google Scholar]
- Eyre DR, Muir H. Quantitative analysis of types I and II collagens in the human intervertebral disc at various ages. Biochimica et Biophysica Acta. 1977;492:29–42. doi: 10.1016/0005-2795(77)90211-2. [DOI] [PubMed] [Google Scholar]
- Johnstone B, Bayliss MT. The large proteoglycans of the human intervertebral disc. Changes in their biosynthesis and structure with age, topography, and pathology. Spine. 1995;20:674–684. doi: 10.1097/00007632-199503150-00008. [DOI] [PubMed] [Google Scholar]
- Urban JP, Maroudas A, Bayliss MT, Dillon J. Swelling pressures of proteoglycans at the concentrations found in cartilaginous tissues. Biorheology. 1979;16:447–464. doi: 10.3233/bir-1979-16609. [DOI] [PubMed] [Google Scholar]
- Roberts S, Menage J, Duance V, Wotton S, Ayad S. 1991 Volvo award in basic sciences: Collagen types around the cells of the intervertebral disc and cartilage end plate: An immunolocalization study. Spine. 1991;16:1030–1038. [PubMed] [Google Scholar]
- Melrose J, Ghosh P, Taylor TK. A comparative analysis of the differential spatial and temporal distributions of the large (aggrecan, versican) and small (decorin, biglycan, fibromodulin) proteoglycans of the intervertebral disc. J Anat. 2001;198:3–15. doi: 10.1046/j.1469-7580.2001.19810003.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eyre DR, Wu JJ, Fernandes RJ, Pietka TA, Weis MA. Recent developments in cartilage research: matrix biology of the collagen II/IX/XI heterofibril network. Biochem Soc Trans. 2001;30:893–899. doi: 10.1042/bst0300893. [DOI] [PubMed] [Google Scholar]
- Sztrolovics R, Alini M, Roughley PJ, Mort JS. Aggrecan degradation in human intervertebral disc and articular cartilage. Biochem J. 1997;326:235–241. doi: 10.1042/bj3260235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts S, Caterson B, Menage J, Evans EH, Jaffray DC, Eisenstein SM. Matrix metalloproteinases and aggrecanase: their role in disorders of the human intervertebral disc. Spine. 2000;25:3005–3013. doi: 10.1097/00007632-200012010-00007. [DOI] [PubMed] [Google Scholar]
- Weiler C, Nerlich AG, Zipperer J, Bachmeier BE, Boos N. 2002 SSE Award Competition in Basic Science: expression of major matrix metalloproteinases is associated with intervertebral disc degradation and resorption. Eur Spine J. 2002;11:308–320. doi: 10.1007/s00586-002-0472-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donohue PJ, Jahnke MR, Blaha JD, Caterson B. Characterization of link protein(s) from human intervertebral-disc tissues. Biochem J. 1988;251:739–747. doi: 10.1042/bj2510739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyons G, Eisenstein SM, Sweet MB. Biochemical changes in intervertebral disc degeneration. Biochim Biophys Acta. 1981;673:443–453. doi: 10.1016/0304-4165(81)90476-1. [DOI] [PubMed] [Google Scholar]
- Inkinen RI, Lammi MJ, Lehmonen S, Puustjarvi K, Kaapa E, Tammi MI. Relative increase of biglycan and decorin and altered chondroitin sulfate epitopes in the degenerating human intervertebral disc. J Rheumatol. 1998;25:506–514. [PubMed] [Google Scholar]
- Antoniou J, Steffen T, Nelson F, Winterbottom N, Hollander AP, Poole RA, Aebi M, Alini M. The human lumbar intervertebral disc: evidence for changes in the biosynthesis and denaturation of the extracellular matrix with growth, maturation, ageing, and degeneration. J Clin Invest. 1996;98:996–1003. doi: 10.1172/JCI118884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hollander AP, Heathfield TF, Liu JJ, Pidoux I, Roughley PJ, Mort JS, Poole AR. Enhanced denaturation of the alpha (II) chains of type-II collagen in normal adult human intervertebral discs compared with femoral articular cartilage. J Orthop Res. 1996;14:61–66. doi: 10.1002/jor.1100140111. [DOI] [PubMed] [Google Scholar]
- Duance VC, Crean JK, Sims TJ, Avery N, Smith S, Menage J, Eisenstein SM, Roberts S. Changes in collagen cross-linking in degenerative disc disease and scoliosis. Spine. 1998;23:2545–2551. doi: 10.1097/00007632-199812010-00009. [DOI] [PubMed] [Google Scholar]
- Johnson SL, Aguiar DJ, Ogilvie JW. Fibronectin and its fragments increase with degeneration in the human intervertebral disc. Spine. 2000;25:2742–2747. doi: 10.1097/00007632-200011010-00005. [DOI] [PubMed] [Google Scholar]
- Crean JK, Roberts S, Jaffray DC, Eisenstein SM, Duance VC. Matrix metalloproteinases in the human intervertebral disc: role in disc degeneration and scoliosis. Spine. 1997;22:2877–2884. doi: 10.1097/00007632-199712150-00010. [DOI] [PubMed] [Google Scholar]
- Ariga K, Yonenobu K, NAKASE T, Kaneko M, Okuda S, Uchiyama Y, Yoshikawa H. Localization of cathepsins D, K, and L in degenerated human intervertebral discs. Spine. 2001;26:2666–2672. doi: 10.1097/00007632-200112150-00007. [DOI] [PubMed] [Google Scholar]
- Urban JPG, McMullin JF. Swelling pressure of the lumbar intervertebral discs: influence of age, spinal level, composition and degeneration. Spine. 1988;13:179–187. doi: 10.1097/00007632-198802000-00009. [DOI] [PubMed] [Google Scholar]
- Frobin W, Brinckmann P, Kramer M, Hartwig E. Height of lumbar discs measured from radiographs compared with degeneration and height classified from MR images. Eur Radiol. 2001;11:263–269. doi: 10.1007/s003300000556. [DOI] [PubMed] [Google Scholar]
- Adams MA, McNally DS, Dolan P. 'Stress' distributions inside intervertebral discs. The effects of age and degeneration. J Bone Joint Surg Br. 1996;78:965–972. doi: 10.1302/0301-620x78b6.1287. [DOI] [PubMed] [Google Scholar]
- McNally DS, Shackleford IM, Goodship AE, Mulholland RC. In vivo stress measurement can predict pain on discography. Spine. 1996;21:2580–2587. doi: 10.1097/00007632-199611150-00007. [DOI] [PubMed] [Google Scholar]
- Adams MA, Dolan P, Hutton WC, Porter RW. Diurnal changes in spinal mechanics and their clinical significance. J Bone Joint Surg Br. 1990;72:266–270. doi: 10.1302/0301-620X.72B2.2138156. [DOI] [PubMed] [Google Scholar]
- Postacchini F, Gumina S, Cinotti G, Perugia D, DeMartino C. Ligamenta flava in lumbar disc herniation and spinal stenosis. Light and electron microscopic morphology. Spine. 1994;19:917–922. doi: 10.1097/00007632-199404150-00009. [DOI] [PubMed] [Google Scholar]
- Maroudas A. Biophysical chemistry of cartilaginous tissues with special reference to solute and fluid transport. Biorheology. 1975;12:233–248. doi: 10.3233/bir-1975-123-416. [DOI] [PubMed] [Google Scholar]
- Freemont AJ, Peacock TE, Goupille P, Hoyland JA, O'Brien J, Jayson M-IV. Nerve ingrowth into diseased intervertebral disc in chronic back pain. Lancet. 1997;350:178–181. doi: 10.1016/s0140-6736(97)02135-1. [DOI] [PubMed] [Google Scholar]
- Johnson WE, Caterson B, Eisenstein SM, Hynds DL, Snow DM, Roberts S. Human intervertebral disc aggrecan inhibits nerve growth in vitro. Arthritis Rheum. 2002;46:2658–2664. doi: 10.1002/art.10585. [DOI] [PubMed] [Google Scholar]
- Melrose J, Roberts S, Smith S, Menage J, Ghosh P. Increased nerve and blood vessel ingrowth associated with proteoglycan depletion in an ovine anular lesion model of experimental disc degeneration. Spine. 2002;27:1278–1285. doi: 10.1097/00007632-200206150-00007. [DOI] [PubMed] [Google Scholar]
- Adams MA, Hutton WC. Prolapsed intervertebral disc. A hyperflexion injury 1981 Volvo Award in Basic Science. Spine. 1982;7:184–191. [PubMed] [Google Scholar]
- Moore RJ, Vernon-Roberts B, Fraser RD, Osti OL, Schembri M. The origin and fate of herniated lumbar intervertebral disc tissue. Spine. 1996;21:2149–2155. doi: 10.1097/00007632-199609150-00018. [DOI] [PubMed] [Google Scholar]
- Boos N, Rieder R, Schade V, Spratt KF, Semmer N, Aebi M. 1995 Volvo Award in clinical sciences. The diagnostic accuracy of magnetic resonance imaging, work perception, and psychosocial factors in identifying symptomatic disc herniations. Spine. 1995;20:2613–2625. doi: 10.1097/00007632-199512150-00002. [DOI] [PubMed] [Google Scholar]
- Cavanaugh JM. Neural mechanisms of lumbar pain. Spine. 1995;20:1804–1809. doi: 10.1097/00007632-199508150-00011. [DOI] [PubMed] [Google Scholar]
- Kang JD, Georgescu HI, McIntyre-Larkin L, Stefanovic-Racic M, Donaldson WF, Evans CH. Herniated lumbar intervertebral discs spontaneously produced matrix metalloproteinases, nitric oxide, interleukin-6, and prostaglandin E2. Spine. 1996;21:271–277. doi: 10.1097/00007632-199602010-00003. [DOI] [PubMed] [Google Scholar]
- Kawakami M, Tamaki T, Weinstein JN, Hashizume H, Nishi H, Meller ST. Pathomechanism of pain-related behavior produced by allografts of intervertebral disc in the rat. Spine. 1996;21:2101–2107. doi: 10.1097/00007632-199609150-00009. [DOI] [PubMed] [Google Scholar]
- Olmarker K, Rydevik B. Disc Herniation and sciatica; the basic science platform. In: Gunzburg R, Szpalski M, editor. In Lumbar Disc Herniation. Philadelphia: Lippincott Williams & Wilkins; 2002. pp. 31–37. [Google Scholar]
- Haro H, Kato T, Komori H, Osada M, Shinomiya K. Vascular endothelial growth factor (VEGF)-induced angiogenesis in herniated disc resorption. J Orthop Res. 2002;20:409–415. doi: 10.1016/S0736-0266(01)00150-4. [DOI] [PubMed] [Google Scholar]
- Korhonen T, Karppinen J, Malmivaara A, Paimela L, Kyllonen E, Lindgren KA, et al. Treatment of sciatica with infliximab, a monocloncal humanised climaeric antibody against TNF [abstract 14]. ISSLS Spring Proceedings. 2002. http://www.spinejournal.com
- Butler WF. Comparative anatomy and development of the mammalian disc. In: Ghosh P, editor. In The Biology of the Intervertebral Disc. Boca Raton: CRC Press; 1989. pp. 84–108. [Google Scholar]
- Moskowitz RW, Ziv I, Denko CW, Boja B, JoneS PK, Adler JH. Spondylosis in sand rats: a model of intervertebral disc degeneration and hyperostosis. J Orthop Res. 1990;8:401–411. doi: 10.1002/jor.1100080312. [DOI] [PubMed] [Google Scholar]
- Ghosh P, Taylor TK, Yarroll JM. Genetic factors in the maturation of the canine intervertebral disc. Res Vet Sci. 1975;19:304–311. [PubMed] [Google Scholar]
- Osti OL, Vernon-Roberts B, Fraser RD. 1990 Volvo Award in experimental studies. Anulus tears and intervertebral disc degeneration. An experimental study using an animal model. Spine. 1990;15:762–767. doi: 10.1097/00007632-199008010-00005. [DOI] [PubMed] [Google Scholar]
- Lipson SJ, Muir H. Vertebral osteophyte formation in experimental disc degeneration. Morphologic and proteoglycan changes over time. Arthritis Rheum. 1980;23:319–324. doi: 10.1002/art.1780230309. [DOI] [PubMed] [Google Scholar]
- Nachemson A, Lewin T, Maroudas A, Freeman MAF. In vitro diffusion of dye through the end-plates and annulus fibrosus of human lumbar intervertebral discs. Acta Orthop Scand. 1970;41:589–607. doi: 10.3109/17453677008991550. [DOI] [PubMed] [Google Scholar]
- Ishihara H, Urban JP. Effects of low oxygen concentrations and metabolic inhibitors on proteoglycan and protein synthesis rates in the intervertebral disc. J Orthop Res. 1999;17:829–835. doi: 10.1002/jor.1100170607. [DOI] [PubMed] [Google Scholar]
- Ohshima H, Urban JPG. Effect of lactate concentrations and pH on matrix synthesis rates in the intervertebral disc. Spine. 1992;17:1079–1082. doi: 10.1097/00007632-199209000-00012. [DOI] [PubMed] [Google Scholar]
- Horner HA, Urban JP. 2001 Volvo Award Winner in Basic Science Studies: effect of nutrient supply on the viability of cells from the nucleus pulposus of the intervertebral disc. Spine. 2001;26:2543–2549. doi: 10.1097/00007632-200112010-00006. [DOI] [PubMed] [Google Scholar]
- Holm S, Maroudas A, Urban JP, Selstam G, Nachemson A. Nutrition of the intervertebral disc: solute transport and metabolism. Connect Tissue Res. 1981;8:101–119. doi: 10.3109/03008208109152130. [DOI] [PubMed] [Google Scholar]
- Urban JP, Holm S, Maroudas A. Diffusion of small solutes into the intervertebral disc: as in vivo study. Biorheology. 1978;15:203–221. doi: 10.3233/bir-1978-153-409. [DOI] [PubMed] [Google Scholar]
- Kauppila LI, McAlindon T, Evans S, Wilson PW, Kiel D, Felson DT. Disc degeneration/back pain and calcification of the abdominal aorta. A 25-year follow-up study in Framingham. Spine. 1997;22:1642–1647. doi: 10.1097/00007632-199707150-00023. [DOI] [PubMed] [Google Scholar]
- Kauppila LI. Prevalence of stenotic changes in arteries supplying the lumbar spine. A postmortem angiographic study on 140 subjects. Ann Rheum Dis. 1997;56:591–595. doi: 10.1136/ard.56.10.591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones JP. Subchondral osteonecrosis can conceivably cause disk degeneration and 'primary' osteoarthritis. In: Urbaniak JR, Jones JP, editor. Osteonecrosis. Park Ridge, Illinois: American Academy of Orthopedic Surgeons; 1997. pp. 135–142. [Google Scholar]
- Holm S, Nachemson A. Variation in the nutrition of the canine intervertebral disc induced by motion. Spine. 1983;8:866–874. doi: 10.1097/00007632-198311000-00009. [DOI] [PubMed] [Google Scholar]
- Holm S, Nachemson A. Nutritional changes in the canine intervertebral disc after spinal fusion. Clin Orthop. 1982;169:243–258. [PubMed] [Google Scholar]
- Roberts S, Urban JPG, Evans H, Eisenstein SM. Transport properties of the human cartilage end-plate in relation to its composition and calcification. Spine. 1996;21:415–420. doi: 10.1097/00007632-199602150-00003. [DOI] [PubMed] [Google Scholar]
- Roberts S, Menage J, Eisenstein SM. The cartilage end-plate and intervertebral disc in scoliosis: calcification and other sequelae. J Orthop Res. 1993;11:747–757. doi: 10.1002/jor.1100110517. [DOI] [PubMed] [Google Scholar]
- Holm S, Nachemson A. Nutrition of the intervertebral disc: acute effects of cigarette smoking. An experimental animal study. Ups J Med Sci. 1988;93:91–99. doi: 10.1517/03009734000000042. [DOI] [PubMed] [Google Scholar]
- Urban MR, Fairbank JC, Etherington PJ, Loh FL, Winlove CP, Urban JP. Electrochemical measurement of transport into scoliotic intervertebral discs in vivo using nitrous oxide as a tracer. Spine. 2001;26:984–990. doi: 10.1097/00007632-200104150-00028. [DOI] [PubMed] [Google Scholar]
- Urban MR, Fairbank JC, Bibby SR, Urban JP. Intervertebral disc composition in neuromuscular scoliosis: changes in cell density and glycosaminoglycan concentration at the curve apex. Spine. 2001;26:610–617. doi: 10.1097/00007632-200103150-00010. [DOI] [PubMed] [Google Scholar]
- Bibby SR, Fairbank JC, Urban MR, Urban JP. Cell viability in scoliotic discs in relation to disc deformity and nutrient levels. Spine. 2002;27:2220–2228. doi: 10.1097/00007632-200210150-00007. [DOI] [PubMed] [Google Scholar]
- Bartels EM, Fairbank JCT, Winlove CP, Urban JPG. Oxygen and lactate concentrations measured in vivo in the intervertebral discs of scoliotic and back pain patients. Spine. 1998;23:1–8. doi: 10.1097/00007632-199801010-00001. [DOI] [PubMed] [Google Scholar]
- Allan DB, Waddell G. An historical perspective on low back pain and disability. Acta Orthop Scand Suppl. 1989;234:1–23. doi: 10.3109/17453678909153916. [DOI] [PubMed] [Google Scholar]
- Puustjarvi K, Takala T, Wang W, Tammi M, Helminen H, Inkinen R. Proteoglycans in the interverterbal disc of young dogs following strenuous running exercise. Conn Tiss Res. 1993;30:1–16. doi: 10.3109/03008209409061974. [DOI] [PubMed] [Google Scholar]
- Iatridis JC, Mente PL, Stokes IA, Aronsson DD, Alini M. Compression-induced changes in intervertebral disc properties in a rat tail model. Spine. 1999;24:996–1002. doi: 10.1097/00007632-199905150-00013. [DOI] [PubMed] [Google Scholar]
- Lotz JC, Colliou OK, Chin JR, Duncan NA, Liebenberg E. 1998 Volvo Award winner in biomechanical studies – Compression-induced degeneration of the intervertebral disc: An in vivo mouse model and finite-element study. Spine. 1998;23:2493–2506. doi: 10.1097/00007632-199812010-00004. [DOI] [PubMed] [Google Scholar]
- Lipson SJ, Muir H. Experimental intervertebral disc degeneration: morphologic and proteoglycan changes over time. Arthritis Rheum. 1981;24:12–21. doi: 10.1002/art.1780240103. [DOI] [PubMed] [Google Scholar]
- Eck JC, Humphreys SC, Hodges SD. Adjacent-segment degeneration after lumbar fusion: a review of clinical, biomechanical, and radiologic studies. Am J Orthop. 1999;28:336–340. [PubMed] [Google Scholar]
- Heliovaara M. Risk factors for low back pain and sciatica. Ann Med. 1989;21:257–264. doi: 10.3109/07853898909149202. [DOI] [PubMed] [Google Scholar]
- Deyo RA, Bass JE. Lifestyle and Low-Back Pain: the influence of smoking and obesity. Spine. 1989;14:501–506. doi: 10.1097/00007632-198905000-00005. [DOI] [PubMed] [Google Scholar]
- Pope MH, Goh KL, Magnusson ML. Spine ergonomics. Annu Rev Biomed Eng. 2002;4:49–68. doi: 10.1146/annurev.bioeng.4.092101.122107. [DOI] [PubMed] [Google Scholar]
- Thompson JP, Pearce RH, Schechter MT, Adams ME, Tsang IKY, Bishop PB. Preliminary evaluation of a scheme for grading the gross morphology of the human intervertebral disc. Spine. 1990;15:411–415. doi: 10.1097/00007632-199005000-00012. [DOI] [PubMed] [Google Scholar]
- Waddell G. The Back Pain Revolution. Edinburgh: Churchill Livingstone; 1998. [Google Scholar]
- Elfering A, Semmer N, Birkhofer D, Zanetti M, Hodler J, Boos N. Risk factors for lumbar disc degeneration: a 5-year prospective MRI study in asymptomatic individuals. Spine. 2002;27:125–134. doi: 10.1097/00007632-200201150-00002. [DOI] [PubMed] [Google Scholar]
- Battie MC, Videman T, Gibbons LE, Manninen H, Gill K, Pope M, Kaprio J. Occupational driving and lumbar disc degeneration: a case-control study. Lancet. 2002;360:1369–1374. doi: 10.1016/S0140-6736(02)11399-7. [DOI] [PubMed] [Google Scholar]
- Videman T, Battie MC. The influence of occupation on lumbar degeneration. Spine. 1999;24:1164–1168. doi: 10.1097/00007632-199906010-00020. [DOI] [PubMed] [Google Scholar]
- Heikkila JK, Koskenvuo M, Heliovaara M, Kurppa K, Riihimaki H, Heikkila K, Rita H, Videman T. Genetic and environmental factors in sciatica. Evidence from a nationwide panel of 9365 adult twin pairs. Ann Med. 1989;21:393–398. doi: 10.3109/07853898909149227. [DOI] [PubMed] [Google Scholar]
- Matsui H, Kanamori M, Ishihara H, Yudoh K, Naruse Y, Tsuji H. Familial predisposition for lumbar degenerative disc disease. A case-control study. Spine. 1998;23:1029–1034. doi: 10.1097/00007632-199805010-00013. [DOI] [PubMed] [Google Scholar]
- Varlotta GP, Brown MD, Kelsey JL, Golden AL. Familial predisposition for herniation of a lumbar disc in patients who are less than twenty-one years old. J Bone Joint Surg Am. 1991;73:124–128. [PubMed] [Google Scholar]
- Battie MC, Videman T, Gibbons LE, Fisher LD, Manninen H, Gill K. 1995 Volvo Award in clinical sciences. Determinants of lumbar disc degeneration. A study relating lifetime exposures and magnetic resonance imaging findings in identical twins. Spine. 1995;20:2601–2612. [PubMed] [Google Scholar]
- Sambrook PN, MacGregor AJ, Spector TD. Genetic influences on cervical and lumbar disc degeneration: a magnetic resonance imaging study in twins. Arthritis Rheum. 1999;42:366–372. doi: 10.1002/1529-0131(199902)42:2<366::AID-ANR20>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- Battie MC, Haynor DR, Fisher LD, Gill K, Gibbons LE, Videman T. Similarities in degenerative findings on magnetic resonance images of the lumbar spines of identical twins. J Bone Joint Surg Am. 1995;77:1662–1670. doi: 10.2106/00004623-199511000-00004. [DOI] [PubMed] [Google Scholar]
- Ala-Kokko L. Genetic risk factors for lumbar disc disease. Ann Med. 2002;34:42–47. doi: 10.1080/078538902317338634. [DOI] [PubMed] [Google Scholar]
- Annunen S, Paassilta P, Lohiniva J, Perala M, Pihlajamaa T, Karppinen J, Tervonen O, Kroger H, Lahde S, Vanharanta H, Ryhanen L, Goring HH, Ott J, Prockop DJ, Ala-Kokko L. An allele of COL9A2 associated with intervertebral disc disease. Science. 1999;285:409–412. doi: 10.1126/science.285.5426.409. [DOI] [PubMed] [Google Scholar]
- Paassilta P, Lohiniva J, Goring HH, Perala M, Raina SS, Karppinen J, Hakala M, Palm T, Kroger H, Kaitila I, Vanharanta H, Ott J, Ala-Kokko L. Identification of a novel common genetic risk factor for lumbar disk disease. JAMA. 2001;285:1843–1849. doi: 10.1001/jama.285.14.1843. [DOI] [PubMed] [Google Scholar]
- Kawaguchi Y, Osada R, Kanamori M, Ishihara H, Ohmori K, Matsui H, Kimura T. Association between an aggrecan gene polymorphism and lumbar disc degeneration. Spine. 1999;24:2456–2460. doi: 10.1097/00007632-199912010-00006. [DOI] [PubMed] [Google Scholar]
- Watanabe H, Nakata K, Kimata K, Nakanishi I, Yamada Y. Dwarfism and age-associated spinal degeneration of heterozygote cmd mice defective in aggrecan. Proc Natl Acad Sci USA. 1997;94:6943–6947. doi: 10.1073/pnas.94.13.6943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li SW, Prockop DJ, Helminen H, Fassler R, Lapvetelainen T, Kiraly K, Peltarri A, Arokoski J, Lui H, Arita M. Transgenic mice with targeted inactivation of the Col2 alpha 1 gene for collagen II develop a skeleton with membranous and periosteal bone but no endochondral bone. Genes Dev. 1995;9:2821–2830. doi: 10.1101/gad.9.22.2821. [DOI] [PubMed] [Google Scholar]
- Kimura T, Nakata K, Tsumaki N, Miyamoto S, Matsui Y, Ebara S, Ochi T. Progressive degeneration of articular cartilage and intervertebral discs. An experimental study in transgenic mice bearing a type IX collagen mutation. Int Orthop. 1996;20:177–181. doi: 10.1007/s002640050058. [DOI] [PubMed] [Google Scholar]
- Takahashi M, Haro H, Wakabayashi Y, Kawa-uchi T, Komori H, Shinomiya K. The association of degeneration of the intervertebral disc with 5a/6a polymorphism in the promoter of the human matrix metalloproteinase-3 gene. J Bone Joint Surg Br. 2001;83:491–495. doi: 10.1302/0301-620x.83b4.11617. [DOI] [PubMed] [Google Scholar]
- Kawaguchi Y, Kanamori M, Ishihara H, Ohmori K, Matsui H, Kimura T. The association of lumbar disc disease with vitamin-D receptor gene polymorphism. J Bone Joint Surg Am. 2002;84-A:2022–2028. doi: 10.2106/00004623-200211000-00018. [DOI] [PubMed] [Google Scholar]
- Videman T, Gibbons LE, Battie MC, Maravilla K, Vanninen E, Leppavuori J, Kaprio J, Peltonen L. The relative roles of intragenic polymorphisms of the vitamin D receptor gene in lumbar spine degeneration and bone density. Spine. 2001;26:E7–E12. doi: 10.1097/00007632-200102010-00003. [DOI] [PubMed] [Google Scholar]
- Videman T, Leppavuori J, Kaprio J, Battie MC, Gibbons LE, Peltonen L, Koskenvuo M. Intragenic polymorphisms of the vitamin D receptor gene associated with intervertebral disc degeneration. Spine. 1998;23:2477–2485. doi: 10.1097/00007632-199812010-00002. [DOI] [PubMed] [Google Scholar]
- Jones G, White C, Sambrook P, Eisman J. Allelic variation in the vitamin D receptor, lifestyle factors and lumbar spinal degenerative disease. Ann Rheum Dis. 1998;57:94–99. doi: 10.1136/ard.57.2.94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Superti-Furga A, Bonafe L, Rimoin DL. Molecular-pathogenetic classification of genetic disorders of the skeleton. Am J Med Genet. 2001;106:282–293. [PubMed] [Google Scholar]
- Zortea M, Vettori A, Trevisan CP, Bellini S, Vazza G, Armani M, Simonati A, Mostacciuolo ML. Genetic mapping of a susceptibility locus for disc herniation and spastic paraplegia on 6q23.3-q24.1. J Med Genet. 2002;39:387–390. doi: 10.1136/jmg.39.6.387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sambrook PN, MacGregor AJ, Spector TD. Genetic influences on cervical and lumbar disc degeneration: a magnetic resonance imaging study in twins. Arthritis Rheum. 1999;42:366–372. doi: 10.1002/1529-0131(199902)42:2<366::AID-ANR20>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- Solovieva S, Lohiniva J, Leino-Arjas P, Raininko R, Luoma Section SK, Ala-Kokko L, Riihimaki H. COL9A3 gene polymorphism and obesity in intervertebral disc degeneration of the lumbar spine: evidence of gene-environment interaction. Spine. 2002;27:2691–2696. doi: 10.1097/00007632-200212010-00008. [DOI] [PubMed] [Google Scholar]
- Saal JA, Saal JS. Intradiscal electrothermal treatment for chronic discogenic low back pain: prospective outcome study with a minimum 2-year follow-up. Spine. 2002;27:966–973. doi: 10.1097/00007632-200205010-00017. [DOI] [PubMed] [Google Scholar]
- Fritzell P, Hagg O, Wessberg P, Nordwall A. 2001 Volvo Award Winner in Clinical Studies: lumbar fusion versus nonsurgical treatment for chronic low back pain: a multicenter randomized controlled trial from the Swedish Lumbar Spine Study Group. Spine. 2001;26:2521–2532. doi: 10.1097/00007632-200112010-00002. [DOI] [PubMed] [Google Scholar]
- Weber H. A controlled, prospective study with ten years of observation. Spine. 1983;8:131–140. [PubMed] [Google Scholar]
- Gibson JN, Grant IC, Waddell G. Surgery for lumbar disc prolapse. Cochrane Database Syst Rev. 2000. p. CD001350. [DOI] [PubMed]
- Thompson JP, Oegema TR, Bradford DS. Stimulation of mature canine intervertebral disc by growth factors. Spine. 1991;16:253–260. doi: 10.1097/00007632-199103000-00001. [DOI] [PubMed] [Google Scholar]
- Osada R, Ohshima H, Ishihara H, Yudoh K, Sakai K, Matsui H, Tsuji H. Autocrine/paracrine mechanism of insulin-like growth factor-1 secretion, and the effect of insulin-like growth factor-1 on proteoglycan synthesis in bovine intervertebral discs. J Orthop Res. 1996;14:690–699. doi: 10.1002/jor.1100140503. [DOI] [PubMed] [Google Scholar]
- Takegami K, Thonar EJ, An HS, Kamada H, Masuda K. Osteogenic protein-1 enhances matrix replenishment by intervertebral disc cells previously exposed to interleukin-1. Spine. 2002;27:1318–1325. doi: 10.1097/00007632-200206150-00014. [DOI] [PubMed] [Google Scholar]
- Hall RA, Kang JD, Kang JD. Degeneration, repair, and regeneration of the intervertebral disc. Curr Opinion Rheum. 2000;11:413–420. [Google Scholar]
- Nishida K, Kang JD, Gilbertson LG, Moon SH, Suh JK, Vogt MT, Robbins PD, Evans CH. Modulation of the biologic activity of the rabbit intervertebral disc by gene therapy: An in vivo study of adenovirus-mediated transfer of the human transforming growth factor beta 1 encoding gene. Spine. 1999;24:2419–2425. doi: 10.1097/00007632-199912010-00002. [DOI] [PubMed] [Google Scholar]
- Roberts S, Hollander AP, Caterson B, Menage J, Richardson JB. Matrix turnover in human cartilage repair tissue in autologous chondrocyte implantation. Arthritis Rheum. 2001;44:2586–2598. doi: 10.1002/1529-0131(200111)44:11<2586::aid-art439>3.0.co;2-i. [DOI] [PubMed] [Google Scholar]
- Brittberg M. Autologous chondrocyte transplantation. Clin Orthop. 1999;367(suppl):S147–S155. doi: 10.1097/00003086-199910001-00016. [DOI] [PubMed] [Google Scholar]
- Gruber HE, Johnson TL, Leslie K, Ingram JA, Martin D, Hoelscher G, Banks D, Phieffer L, Coldham G, Hanley EN., Jr Autologous intervertebral disc cell implantation: a model using Psammomys obesus, the sand rat. Spine. 2002;27:1626–1633. doi: 10.1097/00007632-200208010-00007. [DOI] [PubMed] [Google Scholar]
- Alini M, Roughley PJ, Antoniou J, Stoll T, Aebi M. A biological approach to treating disc degeneration: not for today, but maybe for tomorrow. Eur Spine J. 2002;11(suppl 2):S215–S220. doi: 10.1007/s00586-002-0485-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ganey TM, Meisel HJ. A potential role for cell-based therapeutics in the treatment of intervertebral disc herniation. Eur Spine J. 2002;11 Suppl 2:S206–S214. doi: 10.1007/s00586-002-0494-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maetzel A, Li L. The economic burden of low back pain: a review of studies published between 1996 and 2001. Best Pract Res Clin Rheumatol. 2002;16:23–30. doi: 10.1053/berh.2001.0204. [DOI] [PubMed] [Google Scholar]


