Abstract
Behçet's disease is characterized by recurrent aphthous stomatitis, uveitis, genital ulcers, and skin lesions. The role of the HLA-B*51 gene has been confirmed in recent years, although its contribution to the overall genetic susceptibility to Behçet's disease was estimated to be only 19%. The production of a variety of cytokines by T cells activated with multiple antigens has been shown to play a pivotal role in the activation of neutrophils. As regards the treatment, anti-tumor necrosis factor alpha therapy has been shown to be effective for mucocutaneous symptoms as well as for sight-threatening panuveitis, although a randomized, controlled trial is required.
Keywords: HLA-B51, neutrophil, T lymphocytes, treatment, tumor necrosis factor alpha
Introduction
Behçet's disease is characterized by recurrent aphthous stomatitis, uveitis, genital ulcers, and skin lesions. Since vascular manifestations are common in this disease, it is regarded as vasculitis. However, the predominant histopathological features in the inflamed tissues are infiltration of lymphocytes and monocytes, and sometimes polymorph nuclear leukocytes, through small veins without microscopic changes in the vessel walls. Thrombophilia or thrombophlebilis involving small and large veins is also common, whereas arteritis is rare. In these regards, Behçet's disease is unique compared with other vasculitides.
The clinical characteristics of Behçet's disease are the recurrent episodes of remission and the exacerbation of various symptoms. Chronic sustained inflammation in certain tissues is rare. Recurrent uveitis attacks usually result in the loss of vision that affects profoundly the activity of daily life of the patients. The involvement of the vascular system, of the intestinal system, and of the central nervous system is usually life threatening.
The etiology and pathogenesis of Behçet's disease have not been fully clarified. However, recent investigations have made significant progress in these areas. Moreover, increasing attention has been paid to the effect of anti-tumor necrosis factor alpha therapy in this disease. The present article overviews an update on the etiology, pathogenesis, clinical manifestation, and treatment of Behçet's disease.
Etiology and pathogenesis
Genetics
Behçet's disease has higher prevalence in the countries along the ancient 'Silk Road' from Japan to the Mediterranean region. A number of studies have provided evidence that HLA-B51 is strongly associated with the disease in different ethnic groups [1]. It has been discussed, however, whether HLA-B51 participates in the disease due to a linkage disequilibrium with a nearby gene [2], since the positive ratio of HLA-B51 in Behçet's disease patients is only approximately 60% [3].
Mizuki's group recently proposed that the critical region for Behçet's disease in the human major histocomaptibility complex (MHC) gene could be pinpointed to a 46-kb segment between the MHC class I chain-related gene A (MIC-A) gene and the HLA-B gene [4]. The MIC-A gene is a highly polymorphic member of MHC class I chain (MIC), with more than 20 alleles in terms of amino acid variation in the α1 (exon 2), α2 (exon 3), and α3 (exon 4) domains [5]. MIC-A encodes a cell surface glycoprotein that is not associated with β2-microglobulin, that lacks a CD8 binding site, and that is conformationally stable independent of conventional class I peptide ligands [5]. MIC-A is expressed in a variety of cells, and its expression is regulated by promoter heat shock elements similar to those of hsp70 genes [5]. Analysis of MIC-A genotyping revealed that the frequency of the MIC-A009 allele, coding the extracellular domains of MIC-A, was greatly increased in Japanese patients with Behçet's disease [5]. Stratification and linkage analyses between MIC-A009 and HLA-B51, however, disclosed that the real disease susceptibility gene in Behçet's disease is the HLA-B*51 allele itself. Moreover, MIC-A009 was found to be strongly associated with HLA-B51 as well as HLA-B52, which was not increased in Behçet's disease. It was therefore concluded that the significant increase of the MIC-A009 allele in the Japanese patients is due to a strong linkage disequilibrium with the HLA-B*51 allele [5].
Similar findings on the linkage disequilibrium between the HLA-B*51 allele and the MIC-A allele have been reported in ethnic groups other than Japanese patients [6]. It has thus been disclosed that strong association of the MIC-A A6 allele of the transmembrane region of MIC-A with Behçet's disease results from a strong linkage disequilibrium with the HLA-B*51 allele.
Twenty-four different HLA-B*51 alleles (HLA-B*5101-HLA-B*5124) have now been described. It was therefore possible that there might be disease-specific polymorphisms or mutations within the HLA-B*51 genes. However, analysis with sequencing of the HLA-B*51 genes from Behçet's disease patients and from healthy individuals failed to disclose the difference in the exonic nucleotide sequences [7]. Moreover, no disease-specific polymorphisms or mutations within the HLA-B*51 intronic and promoter/enhancer regions could be associated with Behçet's disease, although there were single nucleotide polymorphisms in these regions both in patients and in controls [7]. These data therefore demonstrated that the HLA-B exonic sequence that encodes the HLA-B*51 allele is the real pathogenic factor in Behçet's disease. This observation in a Japanese population has also been confirmed in different ethnic groups [8].
Gül et al. confirmed the genetic linkage of the HLA-B gene, but not the MIC-A gene, with Behçet's disease using the transmission disequilibrium test [9]. However, the highest contribution of HLA-B to the overall genetic susceptibility to Behçet's disease was estimated to be only 19% in an analysis of a small group of multicase families [9]. This is consistent with the fact that the positive ratio of HLA-B51 in Behçet's disease is approximately 60% [3]. Identification of other susceptibility loci should thus be required. On the contrary, a lower rate of recombination has been observed within the extended MHC region telomeric to the HFE gene, which caused hereditary hemochromatosis, and strong linkage disequilibrium is a feature of this part of the genome [10,11]. Gül et al. have provided evidence of a novel susceptibility locus for Behçet's disease at position D6S285 in 6p22-p23, ~17 cM telomeric to the HLA-B*51 locus, in a linkage study in 28 multicase Turkish families using highly polymorphic microsatellite markers [12]. The identification of the gene in this novel susceptibility locus will make a great contribution to our understanding of the pathogenesis of Behçet's disease.
As regards non-MHC genes, increasing attention has been paid to the mutation of the MEFV gene. This gene is linked to familial Mediterranean fever, which has similarities to Behçet's disease in both epidemiology and manifestations. Some mutations in the MEFV gene have been implicated in Behçet's disease, suggesting that they might act as additional susceptibility factors in Behçet's disease [13]. Further studies to delineate the frequency of MEFV gene mutations in Behçet's disease patients in Japan, where familial Mediterranean fever is extremely rare, would be important to confirm the association of the MEFV mutation with the susceptibility of Behçet's disease.
HLA-B*51 is currently the only gene that has been shown to be linked with susceptibility to Behçet's disease. No HLA-B51 restriction of certain peptide antigens has been demonstrated, however, rather obviating the possibility that HLA-B51 might be involved in antigen presentation. HLA-transgenic animal models are quite helpful to explore the relationship between HLA and disease. The occurrence of spontaneous inflammatory disease was thus demonstrated in transgenic rats expressing HLA-B27 and human β2-microglobulin genes [14]. In this regard, it was interesting that a HLA-B*5101 heavy chain transgenic mouse was developed [15]. However, the animal did not develop Behçet's disease-like manifestations, although it did show a very modestly increased neutrophil activity following f-Met-Leu-Phe stimulation compared with control mice [15]. It would be also interesting to try to establish transgenic animal models of HLA-B51 and β2-microglobulin in order to explore the role of HLA-B51 in the pathogenesis of Behçet's disease, since there are some similarities in clinical manifestations between Reiter's syndrome and Behçet's disease.
Immunopathogenesis
The pathergy reaction is a unique feature of Behçet's disease and might be closely related to the pathogenesis. It has been shown that the early pathergy reaction at 4 hours is mediated by neutrophils and lymphocytes without vasculitis, with the rapid accumulation of neutrophils at the needle-prick sites [16]. The dermis at 48 hours of the pathergy reaction was infiltrated predominantly by mononuclear cells composed mainly of T lymphocytes and monocytes/ macrophages, with neutrophils constituting less than 5% of the infiltrating cells [17]. It is thus suggested that hyperchemotaxis of neutrophils might play a role in triggering the reaction, whereas activated T lymphocytes are required for the development of the whole pathergy reaction.
The therapeutic efficacy of cyclosporin A in uveitis of Behçet's disease [18] strongly suggests the involvement of T-cell activation in the pathogenesis of this disease. On the contrary, attention was paid to the role of certain strains of streptococci as an etiologic agent. Patients with Behçet's disease have a significantly higher incidence of tonsillitis and dental caries. Systemic symptoms of Behçet's disease could thus be induced after treatment of dental caries or even by intracutaneous injection of the streptococcal antigens [19]. Accordingly, Streptococcus sanguis-related antigens KTH-1 stimulated in vitro production of IL-6 and IFN-γ by T cells from patients with Behçet's disease [20]. However, Escherichia coli-derived antigens also enhanced the in vitro production of IFN-γ by T cells from the patients, obviating the possibility that T-cell hypersensitivity in Behçet's disease might be specific for streptococcus-related antigens [20].
Lehner and colleagues explored the response of T cells from patients with Behçet's disease to mycobacterium 65-kDa heat shock protein (HSP), since it was disclosed that serum IgA antibodies to the mycobacterial 65-kDa HSP were elevated in Behçet's disease and that a number of monoclonal antibodies of the mycobacterial 65-kDa HSP cross-reacted with selected strains of S. sanguis [21]. They showed that four peptide determinants within the mycobacterial 65-kDa HSP (and the corresponding human HSP peptides) stimulated significantly higher lymphoproliferative responses in Behçet's disease, as compared with the related disease, unrelated disease, and healthy controls [21]. Lehner and colleagues further characterized that the four mycobacterial 65-kDa HSPs and corresponding peptides from human 60-kDa HSP elicited significant γδ T-cell responses in Behçet's disease, as compared with controls [22]. They claimed that T-cell recognition of certain 60-kDa HSP peptides by γδ T cells might be important in the pathogenesis of the disease [22]. Lehner and colleagues also postulated that an immune response to the streptococcal HSP might also be directed to epithelial and other human 60-kDa HSPs, although there was the lack of specificity. It is thus possible that antigens other than these HSP-related peptides might be involved in the pathogenesis of Behçet's disease.
Supporting the role of γδ T cells in the pathogenesis of Behçet's disease, Freysdottir et al. provided evidence for the increased proportion of peripheral blood γδ T cells in Behçet's disease compared with both recurrent aphthous stomatitis and healthy controls [23]. These γδ T cells expressed activation markers, such as CD25, CD69, and CD29, and produced the inflammatory cytokines IFN-γ and tumor necrosis factor alpha (TNF-α) [23].
It has been reported that high numbers of γδ T cells, predominantly Vγ9Vδ2 T cells producing IFN-γ, were recovered from intraocular fluid of Behçet's disease patients but not from control patients [24]. These Vγ9Vδ2 T cells responded to isopentyl pyrophosphate and related non-peptide prenyl pyrophosphates, but not to 65-kDa HSP [24]. Isopentyl pyrophosphate and related prenyl pyrophosphates are essential metabolites for both prokaryotic and eukaryotic cells [25]. It has therefore been suggested that ubiquitous antigens of microbial origin may trigger cross-reactive autoimmune responses in Behçet's disease [25]. The increase in Vγ9Vδ2 T cells has been also found in the peripheral blood of patients with Behçet's disease [26]. These results, however, do not necessarily indicate the specific activation of Vγ9Vδ2 T cells, since human peripheral blood γδ T cells mainly express Vγ9Vδ2 [27]. It is thus possible that the increase in Vγ9Vδ2 T cells might simply reflect the larger fraction of this subset within the pool of γδ T cells.
The γδ T cells account for only 2–5% of peripheral blood T cells in humans. The importance of αβ T cells in the pathogenesis of a variety of autoimmune diseases has thus been implicated. In this regard, oligoclonal expansion of peripheral blood αβ T cells has been demonstrated in 30–50% of patients with Behçet's disease [28,29]. Since most of the T-cell expansions were reduced in correlation with ameliorated disease activity, a possible involvement of antigen-specific T cells in the pathogenesis was suggested [29]. It is probable that antigen-specific T-cell responses might drive an attack of a variety of symptoms in Behçet's disease. Of note, positive skin reactions to streptococcal-related antigens as well as E. coli-derived antigens and Klebsiella pneumoniae-derived antigens were frequently observed in Behçet's disease [19]. It is thus possible that patients with Behçet's disease might be hypersensitive to multiple antigens rather than to a certain single antigen. In fact, although the oligoclonal T-cell expansions have been reported in an exacerbation phase of Behçet's disease, the recurrent expansion of the same T-cell clone in each attack has not been demonstrated in longitudinal courses of Behçet's disease.
It is postulated alternatively that T cells in Behçet's disease are hypersensitive to a variety of antigens. In this regard, we have demonstrated that T cells from Behçet's disease patients were stimulated to produce IFN-γ with very low concentrations of staphylococcal enterotoxin B and SEC1 that were not able to stimulate T cells from normal individuals or control patients (rheumatoid arthritis) [30]. Since there were no significant differences between Behçet's disease T cells and control T cells in monocyte-independent or monocyte-dependent IFN-γ production stimulated with low or high concentrations of anti-CD3, it was suggested that abnormalities in signal transduction triggered by perturbation of T-cell receptors, but not in that induced by cross-linking of CD3 molecules, might play an important role in the pathogenesis of Behçet's disease [30]. It should be emphasized that our results do not indicate that the superantigen effects are involved in the pathogenesis of Behçet's disease, but that they emphasize the role of hypersensitive signaling through T-cell receptors. Abnormal signal transduction through T-cell receptors might thus explain the hypersensitivity of γδ T cells to 65-kDa HSP or to prenyl pyrophosphates. Since monocytes from Behçet's disease patients could not result in hypersensitivity of control T cells to various antigens [30], it is probable that abnormalities in T cells, but not those in monocytes, play a role.
The increased production of IFN-γ has been demonstrated in Behçet's disease. Accordingly, peripheral Th1 cells were significantly increased in active Behçet's disease [31]. Moreover, serum IL-12 levels were found to be correlated with peripheral Th1 cells and disease progression [31], although the mechanism of increased IL-12 production in Behçet's disease remains unclear. Frassanito et al. postulated that active Behçet's disease might possibly be a disease of antigen-presenting cells, and that T cells may be 'innocent bystanders', since the elevation of IL-12 appeared to be crucial in the pathogenesis [31]. It should be emphasized, however, that hypersensitivity of T cells that lead to T-cell activation might account for the activation of antigen-presenting cells through CD40-CD154 interactions to produce IL-12. In addition, monocytes from Behçet's disease patients could not result in hypersensitivity of control T cells to various antigens [30]. It would therefore be misleading to conclude that the deviation to Th1 responses in Behçet's disease is due to abnormalities in antigen-presenting cells.
It has recently been shown that active Behçet's disease has a higher number of CD4+ T cells containing IFN-γ and CD40 ligand, which are characteristics of Th1 cells [32]. Of note, the elevation of IL-17 in the sera of Behçet's disease has been demonstrated [32]. On the contrary, the production of IL-8, a cytokine that activates neutrophils, by T cells is enhanced in Behçet's disease [33,34]. It should be pointed out that expression of IL-17 has been detected almost exclusively in activated CD4+ and CD8+ T cells [35]. More importantly, IL-17 has been shown to selectively recruit neutrophils to the sites of inflammation [35]. These results suggest that abnormalities in T-cell responses result in hyperreactivity of neutrophils in Behçet's disease through the production a variety of cytokines, including IL-8 and IL-17. It is therefore strongly suggested that neutrophil activation might be sequelae of hypersensitivity of T cells in Behçet's disease.
The immunopathogenesis that is currently postulated is shown in Fig. 1. Primarily, hypersensitivity of T cells (αβ T cells and γδ T cells) to multiple antigens appears to play a critical role in the pathogenesis. The activation of monocytes subsequent to T-cell activation through CD40-CD154 interactions as well as a variety of T-cell-derived cytokines (IFN-γ and TNF-α) may result in the production of IL-12, which leads to the shift to Th1 responses. In consequence of abnormal T-cell activation, neutrophil activation may be triggered by cytokines such as IL-8, IL-17, IFN-γ, and TNF-α. Whereas the roles of costimulation molecules have not been fully explored in Behçet's disease, the presence of anti-CTLA-4 antibody has been reported in a fraction of Behçet's disease patients [36]. Although the presence of this antibody might be possibly involved in abnormal T-cell responses, the antibody might be produced only as a secondary phenomenon of recurrent T-cell activation in Behçet's disease.
Figure 1.
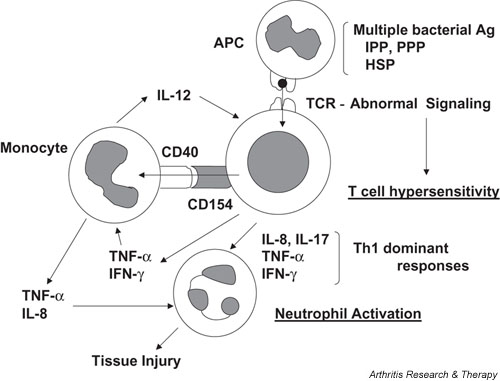
Proposed model of the pathogenesis in Behçet's disease. Ag, antigen; APC, antigen-presenting cells; HSP, heat shock protein; IFN, interferon; IL, interleukin; IPP, isoprenyl pyrophosphate; PPP, prenyl pyrophosphate; TCR, T-cell receptor; Th1, T helper cells type 1; TNF-α, tumor necrosis factor alpha.
Clinical manifestations
Vasculo-Behçet's disease
Involvement of veins and arteries in Behçet's disease is usually called vasculo-Behçet's disease. Venous thrombosis appeared to be the major vascular involvement in 7–33% of patients with Behçet's disease, and represents 85–93% of vasculo-Behçet's disease [35]. Deep vein thrombosis was significantly associated with the male gender and a positive pathergy test [37].
A number of studies have explored the pathogenesis of thrombophilia in Behçet's disease. Neither deficiency in protein C, in protein S, in factor V Leiden and in antithrombin III nor resistance to activated protein C and anticardiolipin antibody levels seemed to be correlated with vascular thrombosis in Behçet's disease [37,38]. There were increased thrombin generation, fibrinolysis, and thrombomodulin in Behçet's disease, but these abnormalities were not related to thrombosis [38]. These results therefore suggest that thrombophilia in Behçet's disease might be related more to inflammation than to clotting disorder.
Recent studies have disclosed the occurrence of anti-endothelial cell antibodies in Behçet's disease [39]. It has been demonstrated, moreover, that increased E-selectin expression was observed when endothelial cells were incubated with sera from patients with active Behçet's disease or with sera from patients with anti-endothelial cell antibodies and high levels of myeloperoxydase, or with purified myeloperoxydase itself [39]. Since neutrophils from active Behçet's disease release increased amounts of myeloperoxydase [39], it is probable that neutrophil activation as well as the expression of anti-endothelial cell antibodies might play an important role in the development of endothelial inflammatory damages, leading to thrombophilia.
Arterial involvement, although rare, does occur in Behçet's disease. The arterial manifestations in Behçet's disease resemble those of Takayasu's arteritis, including arterial occlusion and aneurysm formation. Histopathological studies revealed that the number of vasa vasorum with infiltration of neutrophils and lymphocytes was significantly increased in vasculo-Behçet's disease compared with in Takayasu's arteritis and other inflammatory aneurysms [40]. It was therefore suggested that arterial involvement in vasculo-Behçet's disease might be caused by a neutrophilic vasculitis targeting the vasa vasorum, leading to degeneration of the arterial wall [40].
Neuro-Behçet's syndrome
The neurological involvement in Behçet's disease is either caused by primary neural parenchymal lesions (neuro-Behçet's syndrome) or is secondary to major vascular involvement [41,42]. The latter type is rarely complicated with the parenchymal lesions and should be called vasculo-Behçet's disease [41]. This vasculo-Behçet's disease type generally has a better prognosis compared with the parenchymal type [41].
The most commonly involved area in neuro-Behçet's syndrome is the brain stem, but spinal cord lesions, hemisphere lesions and meningoencephalitis also occur [42]. Among a variety of signs and symptoms, pyramidal tract signs are most frequently observed [41,42]. Although a lot of efforts have been made, the etiology and pathogenesis of neuro-Behçet's syndrome still remain unclear. In addition, factors determining prognosis and appropriate treatment have not been delineated.
We have recently disclosed that neuro-Behçet's syndrome can be classified as acute type and as chronic progressive type [43]. Acute neuro-Behçet's syndrome is characterized by acute meningoencephalitis with or without focal lesions, presenting high-intensity areas in T2-weighted images or fluid attenuated inversion recovery (FLAIR) images on magnetic resonance imaging scans [43]. Cyclosporin A is frequently associated with acute neuro-Behçet's syndrome, at least among the Japanese patients [44]. Acute neuro-Behçet's syndrome responds to steroid therapy, and is usually self-limiting.
By contrast, the chronic progressive type of neuro-Behçet's syndrome is characterized by intractable, slowly progressive dementia, ataxia and dysarthria, with persistent elevation of cerebrospinal fluid IL-6 activity (> 20 pg/ml) [45]. Most patients (approximately 90%) in our series with the chronic progressive type of neuro-Behçet's syndrome were HLA-B51-positive, and they had history of attacks of acute type neuro-Behçet's syndrome prior to the development of progressive neurological symptoms [45].
It should therefore be pointed out that the two types of neuro-Behçet's syndrome are currently considered to represent different stages rather than independent clinical entities. In fact, we have recently experienced some patients who displayed prolonged elevation of cerebrospinal fluid IL-6 activity following the acute type neuro-Behçet's syndrome. It is therefore suggested that the appropriate treatment of such patients can prevent progression of neurological symptoms, although further studies are required to confirm this point. Of note, chronic progressive neuro-Behçet's syndrome is resistant to conventional treatment with corticosteroid, with cyclophosphamide, or with azathioprine. Recent studies, however, suggest the efficacy of low-dose weekly methotrexate in the chronic progressive type of neuro-Behçet's syndrome [46].
Treatment
Abnormal activation of neutrophil functions has been recognized in the pathogenesis of Behçet's disease [47]. Colchicine has been widely used as a basic drug for treatment of Behçet's disease based on the claim that colchicine exerts beneficial effects through inhibition of neutrophil functions [47]. The results of a 2-year randomized, double-blind, placebo-controlled study have recently demonstrated that colchicine significantly reduced the occurrence of arthritis in both female and male patients, whereas it reduced the occurrence of genital ulcers and erythema nodosum only in female patients [48]. This possibly reflects less severe disease among the women [48]. Since T-cell abnormalities have been shown to be involved upstream of neutrophil activation in the pathogenesis of Behçet's disease (Fig. 1), it is conceivable that inhibition of neutrophil functions by colchicine might not be sufficient for treatment of more severe manifestations. In this regard, cyclosporin A, an inhibitor of T-cell function, has been shown to be effective in suppressing an attack of uveitis, one of the most severe manifestations of Behçet's disease [18]. However, the efficacy of cyclosporin A is not still satisfactory in sight-threatening uveitis in Behçet's disease. Moreover, the neurotoxicity of cyclosporin A, which leads to the occurrence of acute type neuro-Behçet's syndrome, has been found in as many as 25.5% of patients [44]. The use of cyclosporin A in Behçet's disease is therefore being limited.
Several groups have reported the beneficial effects of IFN-α in Behçet's disease. Alpsoy et al. demonstrated, in a 3-month randomized, placebo-controlled, double-blinded study, that IFN-α2a is effective for the treatment of the mucocutaneous lesions in Behçet's disease [49]. In this trial, five of six patients in the IFN-α2a-treated group versus one of three patients in the placebo group showed an improvement in ocular manifestations [49]. However, a double-blinded control study with larger numbers of patients would be required to demonstrate the efficacy in the treatment of uveitis in Behçet's disease.
Thalidomide is a drug that virtually disappeared from clinical use after its teratogenicity was demonstrated in the 1960s. The results of a randomized, double-blind, placebo-controlled trial for 24 weeks demonstrated that thalidomide is effective for treating the mucocutaneous lesions, including oral and genital ulcers, and follicular lesions in adult patients with Behçet's disease, although the effect diminished rapidly after discontinuation of treatment [50]. The beneficial effects of thalidomide have also been reported in pediatric patients with Behçet's disease [51]. However, awareness of the danger of axonal neuropathy and teratogenesis at all times during thalidomide therapy is mandatory.
It has been demonstrated that γδ T cells in Behçet's disease are activated in vivo and produce large amounts of TNF-α [23,26]. It has also been shown that thalidomide inhibits transcription of TNF-α [52]. Infliximab, a chimeric monoclonal antibody to TNF-α, has been demonstrated to be an effective therapy for Crohn's disease [53] and rheumatoid arthritis [54]. Accumulating reports on patients with Behçet's disease showed that infliximab was effective in the treatment of intractable orogenital ulceration [55], of skin lesions [56], and of gastrointestinal lesions [57]. It has also been disclosed that infliximab is a rapid and effective therapy for sight-threatening panuveitis in Behçet's disease [58]. Infliximab administration thus leads to a rapid and effective suppression of acute ocular inflammation, and the remission of the uveitis remained for as long as 28 days after infliximab administration in all five patients [58]. Etanercept is also now being used in Behçet's disease. A controlled study with larger numbers of patients for longer periods of time would be required to demonstrate the efficacy of tumor necrosis factor blockade on visual outcome and extraocular manifestations in patients with Behçet's disease.
As mentioned earlier, low-dose weekly methotrexate has been shown to be effective in patients with the chronic progressive type of neuro-Behçet's disease [46]. It has also been shown that methotrexate has beneficial effects in ocular manifestations in Behçet's disease [59]. Further studies to explore the efficacy of methotrexate in various manifestations in Behçet's disease would be worthwhile.
Conclusion
Significant progress has been made in recent years in the etiology and pathogenesis of Behçet's disease. The role of the HLA-B*51 gene has thus been confirmed, although its contribution to the overall genetic susceptibility to Behçet's disease was estimated to be only 19%. In this regard, identification of a novel gene located in 6p22-p23, telomeric to the MHC region, would be quite important.
The mechanism of neutrophil activation in Behçet's disease was unclear. The results of recent studies have confirmed that the production of a variety of cytokines by T cells activated with multiple antigens plays a pivotal role in the activation of neutrophils. The mechanism of T-cell hypersensitivity and the role of genetic factors need to be clarified. As regards treatment, anti-TNF-α therapy has been shown to be effective for mucocutaneous symptoms as well as for sight-threatening panuveitis in Behçet's disease, although a controlled study with larger numbers of patients is required. Taking into consideration the natural course of Behçet's disease, that the severity of the disease activity declines as years go by after the onset, the use of anti-TNF-α therapy could be limited within several years, thus decreasing the occurrence of adverse effects.
Competing interests
None declared.
Abbreviations
HSP = heat shock protein; IFN = interferon; IL = interleukin; MHC = major histocompatibility complex; MIC-A = MHC class I chain-related gene A; Th1 = T helper cells type 1; TNF-α = tumor necrosis factor alpha.
References
- Ohno S, Ohguchi M, Hirose S, Matsuda H, Wakisaka A, Aizawa M. Close association of HLA-Bw51 with Behcet's disease. Arch Ophthalmol. 1982;100:1455–1458. doi: 10.1001/archopht.1982.01030040433013. [DOI] [PubMed] [Google Scholar]
- Mizuki N, Ohno S, Sato T, Ishihara M, Miyata S, Nakamura S, Naruse T, Mizuki H, Tsuji K, Inoko H. Microsatellite polymorphism between the tumor necrosis factor and HLA-B genes in Behcet's disease. Hum Immunol. 1995;43:129–135. doi: 10.1016/0198-8859(94)00159-n. [DOI] [PubMed] [Google Scholar]
- Mizuki N, Ota M, Katsuyama Y, Yabuki K, Ando H, Shiina T, Nomura E, Onari K, Ohno S, Inoko H. HLA-B*51 allele analysis by the PCR-SBT method and a strong association of HLA-B*5101 with Japanese patients with Behcet's disease. Tissue Antigens. 2001;58:181–184. doi: 10.1034/j.1399-0039.2001.580306.x. [DOI] [PubMed] [Google Scholar]
- Ota M, Mizuki N, Katsuyama Y, Tamiya G, Shiina T, Oka A, Ando H, Kimura M, Goto K, Ohno S, Inoko H. The critical region for Behcet disease in the human major histocompatibility complex is reduced to a 46-kb segment centromeric of HLA-B, by association analysis using refined microsatellite mapping. Am J Hum Genet. 1999;64:1406–1410. doi: 10.1086/302364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mizuki N, Ota M, Katsuyama Y, Yabuki K, Ando H, Goto K, Nakamura S, Bahram S, Ohno S, Inoko H. Association analysis between the MIC-A and HLA-B alleles in Japanese patients with Behcet's disease. Arthritis Rheum. 1999;42:1961–1966. doi: 10.1002/1529-0131(199909)42:9<1961::AID-ANR23>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- Cohen R, Metzger S, Nahir M, Chajek-Shaul T. Association of the MIC-A gene and HLA-B51 with Behcet's disease in Arabs and non-Ashkenazi Jews in Israel. Ann Rheum Dis. 2002;61:157–160. doi: 10.1136/ard.61.2.157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sano K, Yabuki K, Imagawa Y, Shiina T, Mizuki N, Ohno S, Kulski JK, Inoko H. The absence of disease-specific polymorphisms within the HLA-B51 gene that is the susceptible locus for Behcet's disease. Tissue Antigens. 2001;58:77–82. doi: 10.1034/j.1399-0039.2001.580202.x. [DOI] [PubMed] [Google Scholar]
- Kera J, Mizuki N, Ota M, Katsuyama Y, Pivetti-Pezzi P, Ohno S, Inoko H. Significant associations of HLA-B*5101 and B* and lack of association of class II alleles with Behcet's disease in Italian patients. Tissue Antigens. 5108;54:565–571. doi: 10.1034/j.1399-0039.1999.540605.x. [DOI] [PubMed] [Google Scholar]
- Gül A, Hajeer AH, Worthington J, Barrett JH, Ollier WE, Silman AJ. Evidence for linkage of the HLA-B locus in Behcet's disease, obtained using the transmission disequilibrium test. Arthritis Rheum. 2001;44:239–241. doi: 10.1002/1529-0131(200101)44:1<239::AID-ANR31>3.0.CO;2-X. [DOI] [PubMed] [Google Scholar]
- Feder JN, Gnirke A, Thomas W, Tsuchihashi Z, Ruddy DA, Basava A, Dormishian F, Domingo R, Jr, Ellis MC, Fullan A, Hinton LM, Jones NL, Kimmel BE, Kronmal GS, Lauer P, Lee VK, Loeb DB, Mapa FA, McClelland E, Meyer NC, Mintier GA, Moeller N, Moore T, Morikang E, Prass CE, Quintana L, Starnes SM, Schatzman RC, Brunke KJ, Drayna DT, Risch NJ, Bacon BR, Wolff RK. A novel MHC class I-like gene is mutated in patients with hereditary haemochromatosis. Nat Genet. 1996;13:399–408. doi: 10.1038/ng0896-399. [DOI] [PubMed] [Google Scholar]
- Malfroy L, Roth MP, Carrington M, Borot N, Volz A, Ziegler A, Coppin H. Heterogeneity in rates of recombination in the 6-Mb region telomeric to the human major histocompatibility complex. Genomics. 1997;43:226–231. doi: 10.1006/geno.1997.4800. [DOI] [PubMed] [Google Scholar]
- Gül A, Hajeer AH, Worthington J, Ollier WE, Silman AJ. Linkage mapping of a novel susceptibility locus for Behcet's disease to chromosome 6p22-23. Arthritis Rheum. 2001;44:2693–2696. doi: 10.1002/1529-0131(200111)44:11<2693::aid-art449>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- Touitou I, Magne X, Molinari N, Navarro A, Quellec AL, Picco P, Seri M, Ozen S, Bakkaloglu A, Karaduman A, Garnier JM, Demaille J, Kone-Paut I. MEFV mutations in Behcet's disease. Hum Mutat. 2000;16:271–272. doi: 10.1002/1098-1004(200009)16:3<271::AID-HUMU16>3.0.CO;2-A. [DOI] [PubMed] [Google Scholar]
- Hammer RE, Maika SD, Richardson JA, Tang JP, Taurog JD. Spontaneous inflammatory disease in transgenic rats expressing HLA-B27 and human beta 2m: an animal model of HLA-B27-associated human disorders. Cell. 1990;63:1099–1112. doi: 10.1016/0092-8674(90)90512-d. [DOI] [PubMed] [Google Scholar]
- Takeno M, Kariyone A, Yamashita N, Takiguchi M, Mizushima Y, Kaneoka H, Sakane T. Excessive function of peripheral blood neutrophils from patients with Behcet's disease and from HLA-B51 transgenic mice. Arthritis Rheum. 1995;38:426–433. doi: 10.1002/art.1780380321. [DOI] [PubMed] [Google Scholar]
- Ergun T, Gurbuz O, Harvell J, Jorizzo J, White W. The histopathology of pathergy: a chronologic study of skin hyperreactivity in Behcet's disease. Int J Dermatol. 1998;37:929–933. doi: 10.1046/j.1365-4362.1998.00474.x. [DOI] [PubMed] [Google Scholar]
- Gul A, Esin S, Dilsen N, Konice M, Wigzell H, Biberfeld P. Immunohistology of skin pathergy reaction in Behcet's disease. Br J Dermatol. 1995;132:901–907. doi: 10.1111/j.1365-2133.1995.tb16946.x. [DOI] [PubMed] [Google Scholar]
- Masuda K, Nakajima A, Urayama A, Nakae K, Kogure M, Inaba G. Double-masked trial of cyclosporin versus colchicine and long-term open study of cyclosporin in Behcet's disease. Lancet. 1989;i:1093–1096. doi: 10.1016/s0140-6736(89)92381-7. [DOI] [PubMed] [Google Scholar]
- The Behcet's Disease Research Committee of Japan Skin hypersensitivity to streptococcal antigens and the induction of systemic symptoms by the antigens in Behcet's disease – a multicenter study. J Rheumatol. 1989;16:506–511. [PubMed] [Google Scholar]
- Hirohata S, Oka H, Mizushima Y. Streptococcal-related antigens stimulate production of IL6 and interferon-gamma by T cells from patients with Behcet's disease. Cell Immunol. 1992;140:410–419. doi: 10.1016/0008-8749(92)90207-6. [DOI] [PubMed] [Google Scholar]
- Pervin K, Childerstone A, Shinnick T, Mizushima Y, van der Zee R, Hasan A, Vaughan R, Lehner T. T cell epitope expression of mycobacterial and homologous human 65-kilodalton heat shock protein peptides in short term cell lines from patients with Behcet's disease. J Immunol. 1993;151:2273–2282. [PubMed] [Google Scholar]
- Hasan A, Fortune F, Wilson A, Warr K, Shinnick T, Mizushima Y, van der Zee R, Stanford MR, Sanderson J, Lehner T. Role of gamma delta T cells in pathogenesis and diagnosis of Behcet's disease. Lancet. 1996;347:789–794. doi: 10.1016/s0140-6736(96)90868-5. [DOI] [PubMed] [Google Scholar]
- Freysdottir J, Lau S, Fortune F. Gammadelta T cells in Behcet's disease (BD) and recurrent aphthous stomatitis (RAS) Clin Exp Immunol. 1999;118:451–457. doi: 10.1046/j.1365-2249.1999.01069.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verjans GM, van Hagen PM, van der Kooi A, Osterhaus AD, Baarsma GS. Vgamma9Vdelta2 T cells recovered from eyes of patients with Behcet's disease recognize non-peptide prenyl pyrophosphate antigens. J Neuroimmunol. 2002;130:46–54. doi: 10.1016/s0165-5728(02)00208-4. [DOI] [PubMed] [Google Scholar]
- De Libero G. Sentinel function of broadly reactive human gamma delta T cells. Immunol Today. 1997;18:22–26. doi: 10.1016/s0167-5699(97)80010-2. [DOI] [PubMed] [Google Scholar]
- Yamashita N, Kaneoka H, Kaneko S, Takeno M, Oneda K, Koizumi H, Kogure M, Inaba G, Sakane T. Role of gammadelta T lymphocytes in the development of Behcet's disease. Clin Exp Immunol. 1997;107:241–247. doi: 10.1111/j.1365-2249.1997.274-ce1159.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schondelmaier S, Wesch D, Pechhold K, Kabelitz D. V gamma gene usage in peripheral blood gamma delta T cells. Immunol Lett. 1993;38:121–126. doi: 10.1016/0165-2478(93)90176-3. [DOI] [PubMed] [Google Scholar]
- Direskeneli H, Eksioglu-Demiralp E, Kibaroglu A, Yavuz S, Ergun T, Akoglu T. Oligoclonal T cell expansions in patients with Behcet's disease. Clin Exp Immunol. 1999;117:166–170. doi: 10.1046/j.1365-2249.1999.00931.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esin S, Gul A, Hodara V, Jeddi-Tehrani M, Dilsen N, Konice M, Andersson R, Wigzell H. Peripheral blood T cell expansions in patients with Behcet's disease. Clin Exp Immunol. 1997;107:520–527. doi: 10.1046/j.1365-2249.1997.d01-947.x. [DOI] [PubMed] [Google Scholar]
- Hirohata S, Hashimoto T. Abnormal T cell responses to bacterial superantigens in Behcet's disease (BD) Clin Exp Immunol. 1998;112:317–324. doi: 10.1046/j.1365-2249.1998.00572.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frassanito MA, Dammacco R, Cafforio P, Dammacco F. Th1 polarization of the immune response in Behcet's disease: a putative pathogenetic role of interleukin-12. Arthritis Rheum. 1999;42:1967–1974. doi: 10.1002/1529-0131(199909)42:9<1967::AID-ANR24>3.0.CO;2-Z. [DOI] [PubMed] [Google Scholar]
- Hamzaoui K, Hamzaoui A, Guemira F, Bessioud M, Hamza M, Ayed K. Cytokine profile in Behcet's disease patients. Relationship with disease activity. Scand J Rheumatol. 2002;31:205–210. doi: 10.1080/030097402320318387. [DOI] [PubMed] [Google Scholar]
- Mantas C, Direskeneli H, Oz D, Yavuz S, Akoglu T. IL-8 producing cells in patients with Behcet's disease. Clin Exp Rheumatol. 2000;18:249–251. [PubMed] [Google Scholar]
- Kaneko S, Suzuki N, Yamashita N, Nagafuchi H, Nakajima T, Wakisaka S, Yamamoto S, Sakane T. Characterization of T cells specific for an epitope of human 60-kD heat shock protein (hsp) in patients with Behcet's disease (BD) in Japan. Clin Exp Immunol. 1997;108:204–212. doi: 10.1046/j.1365-2249.1997.3611265.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witowski J, Pawlaczyk K, Breborowicz A, Scheuren A, Kuzlan-Pawlaczyk M, Wisniewska J, Polubinska A, Friess H, Gahl GM, Frei U, Jorres A. IL-17 stimulates intraperitoneal neutrophil infiltration through the release of GRO alpha chemokine from mesothelial cells. J Immunol. 2000;165:5814–5821. doi: 10.4049/jimmunol.165.10.5814. [DOI] [PubMed] [Google Scholar]
- Matsui T, Kurokawa M, Kobata T, Oki S, Azuma M, Tohma S, Inoue T, Yamamoto K, Nishioka K, Kato T. Autoantibodies to T cell costimulatory molecules in systemic autoimmune diseases. J Immunol. 1999;162:4328–4335. [PubMed] [Google Scholar]
- Houman MH, Ben Ghorbel I, Khiari Ben Salah I, Lamloum M, Ben Ahmed M, Miled M. Deep vein thrombosis in Behcet's disease. Clin Exp Rheumatol. 2001;19(suppl 24):S48–S50. [PubMed] [Google Scholar]
- Espinosa G, Font J, Tassies D, Vidaller A, Deulofeu R, Lopez-Soto A, Cervera R, Ordinas A, Ingelmo M, Reverter JC. Vascular involvement in Behcet's disease: relation with thrombophilic factors, coagulation activation, and thrombomodulin. Am J Med. 2002;112:37–43. doi: 10.1016/s0002-9343(01)01048-8. [DOI] [PubMed] [Google Scholar]
- Triolo G, Accardo-Palumbo A, Triolo G, Carbone MC, Ferrante A, Giardina E. Enhancement of endothelial cell E-selection expression by sera from patients with active Behcet's disease: moderate correlation with anti-endothelial cell antibodies and serum myeloperoxidase levels. Clin Immunol. 1999;91:330–337. doi: 10.1006/clim.1999.4687. [DOI] [PubMed] [Google Scholar]
- Kobayashi M, Ito M, Nakagawa A, Matsushita M, Nishikimi N, Sakurai T, Nimura Y. Neutrophil and endothelial cell activation in the vasa vasorum in vasculo-Behcet disease. Histopathology. 2000;36:362–371. doi: 10.1046/j.1365-2559.2000.00859.x. [DOI] [PubMed] [Google Scholar]
- Serdaroglu P. Behçet's disease and the nervous system. J Neurol. 1998;245:197–205. doi: 10.1007/s004150050205. [DOI] [PubMed] [Google Scholar]
- Kidd D, Steuer A, Denman AM, Rudge P. Neurological complications in Behcet's syndrome. Brain. 1999;122:2183–2194. doi: 10.1093/brain/122.11.2183. [DOI] [PubMed] [Google Scholar]
- Kawai M, Hirohata S. Cerebrospinal fluid beta(2)-microglobulin in neuro-Behcet's syndrome. J Neurol Sci. 2000;179:132–139. doi: 10.1016/s0022-510x(00)00403-2. [DOI] [PubMed] [Google Scholar]
- Kotake S, Higashi K, Yoshikawa K, Sasamoto Y, Okamoto T, Matsuda H. Central nervous system symptoms in patients with Behcet disease receiving cyclosporine therapy. Ophthalmology. 1999;106:586–589. doi: 10.1016/S0161-6420(99)90120-3. [DOI] [PubMed] [Google Scholar]
- Hirohata S, Isshi K, Oguchi H, Ohse T, Haraoka H, Takeuchi A, Hashimoto T. Cerebrospinal fluid interleukin-6 in progressive Neuro-Behcet's syndrome. Clin Immunol Immunopathol. 1997;82:12–17. doi: 10.1006/clin.1996.4268. [DOI] [PubMed] [Google Scholar]
- Hirohata S, Suda H, Hashimoto T. Low-dose weekly methotrexate for progressive neuropsychiatric manifestations in Behcet's disease. J Neurol Sci. 1998;159:181–185. doi: 10.1016/s0022-510x(98)00165-8. [DOI] [PubMed] [Google Scholar]
- Matsumura N, Mizushima Y. Leucocyte movement and colchicine treatment in Behcet's disease [letter] Lancet. 1975;2:813. doi: 10.1016/s0140-6736(75)80031-6. [DOI] [PubMed] [Google Scholar]
- Yurdakul S, Mat C, Tuzun Y, Ozyazgan Y, Hamuryudan V, Uysal O, Senocak M, Yazici H. A double-blind trial of colchicine in Behcet's syndrome. Arthritis Rheum. 2001;44:2686–2692. doi: 10.1002/1529-0131(200111)44:11<2686::aid-art448>3.0.co;2-h. [DOI] [PubMed] [Google Scholar]
- Alpsoy E, Durusoy C, Yilmaz E, Ozgurel Y, Ermis O, Yazar S, Basaran E. Interferon alfa-2a in the treatment of Behcet disease: a randomized placebo-controlled and double-blind study. Arch Dermatol. 2002;138:467–471. doi: 10.1001/archderm.138.4.467. [DOI] [PubMed] [Google Scholar]
- Hamuryudan V, Mat C, Saip S, Ozyazgan Y, Siva A, Yurdakul S, Zwingenberger K, Yazici H. Thalidomide in the treatment of the mucocutaneous lesions of the Behcet syndrome. A randomized, double-blind, placebo-controlled trial. Ann Intern Med. 1998;128:443–450. doi: 10.7326/0003-4819-128-6-199803150-00004. [DOI] [PubMed] [Google Scholar]
- Kari JA, Shah V, Dillon MJ. Behcet's disease in UK children: clinical features and treatment including thalidomide. Rheumatology (Oxford) 2001;40:933–938. doi: 10.1093/rheumatology/40.8.933. [DOI] [PubMed] [Google Scholar]
- Tseng S, Pak G, Washenik K, Pomeranz MK, Shupack JL. Rediscovering thalidomide: a review of its mechanism of action, side effects, and potential uses. J Am Acad Dermatol. 1996;35:969–979. doi: 10.1016/s0190-9622(96)90122-x. [DOI] [PubMed] [Google Scholar]
- Targan SR, Hanauer SB, van Deventer SJ, Mayer L, Present DH, Braakman T, DeWoody KL, Schaible TF, Rutgeerts PJ. A short-term study of chimeric monoclonal antibody cA2 to tumor necrosis factor alpha for Crohn's disease. Crohn's Disease cA2 Study Group. N Engl J Med. 1997;337:1029–1035. doi: 10.1056/NEJM199710093371502. [DOI] [PubMed] [Google Scholar]
- Maini R, St Clair EW, Breedveld F, Furst D, Kalden J, Weisman M, Smolen J, Emery P, Harriman G, Feldmann M, Lipsky PE. Infliximab (chimeric anti-tumour necrosis factor alpha monoclonal antibody) versus placebo in rheumatoid arthritis patients receiving concomitant methotrexate: a randomised phase III trial. ATTRACT Study Group. Lancet. 1999;354:1932–1939. doi: 10.1016/s0140-6736(99)05246-0. [DOI] [PubMed] [Google Scholar]
- Robertson LP, Hickling P. Treatment of recalcitrant orogenital ulceration of Behcet's syndrome with infliximab. Rheumatology (Oxford) 2001;40:473–474. doi: 10.1093/rheumatology/40.4.473. [DOI] [PubMed] [Google Scholar]
- Goossens PH, Verburg RJ, Breedveld FC. Remission of Behcet's syndrome with tumour necrosis factor alpha blocking therapy [concise report] Ann Rheum Dis. 2001;60:637. doi: 10.1136/ard.60.6.637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hassard PV, Binder SW, Nelson V, Vasiliauskas EA. Anti-tumor necrosis factor monoclonal antibody therapy for gastrointestinal Behcet's disease: a case report. Gastroenterology. 2001;120:995–999. doi: 10.1053/gast.2001.22556. [DOI] [PubMed] [Google Scholar]
- Sfikakis PP, Theodossiadis PG, Katsiari CG, Kaklamanis P, Markomichelakis NN. Effect of infliximab on sight-threatening panuveitis in Behcet's disease. Lancet. 2001;358:295–296. doi: 10.1016/s0140-6736(01)05497-6. [DOI] [PubMed] [Google Scholar]
- Davatchi F, Shahram F, Chams H, Jamshidi AR, Nadji A, Chams C, Akbarian M, Gharibdoust F. High dose methotrexate for ocular lesions of Behçet's disease. Preliminary short term results of 23 patients. Book of Abstracts 10th International Conference on Behçet's Disease; Berlin. p. 69. June 27–29, 2002.


