Abstract
Citron kinase is a Rho-effector protein kinase that is related to Rho-associated kinases of ROCK/ROK/Rho-kinase family. Both ROCK and citron kinase are suggested to play a role in cytokinesis. However, no substrates are known for citron kinase. We found that citron kinase phosphorylated regulatory light chain (MLC) of myosin II at both Ser-19 and Thr-18 in vitro. Unlike ROCK, however, citron kinase did not phosphorylate the myosin binding subunit of myosin phosphatase, indicating that it does not inhibit myosin phosphatase. We found that the expression of the kinase domain of citron kinase resulted in an increase in MLC di-phosphorylation. Furthermore, the kinase domain was able to increase di-phosphorylation and restore stress fiber assembly even when ROCK was inhibited with a specific inhibitor, Y-27632. The expression of full-length citron kinase also increased di-phosphorylation during cytokinesis. These observations suggest that citron kinase phosphorylates MLC to generate di-phosphorylated MLC in vivo. Although both mono- and di-phosphorylated MLC were found in cleavage furrows, di-phosphorylated MLC showed more constrained localization than did mono-phosphorylated MLC. Because citron kinase is localized in cleavage furrows, citron kinase may be involved in regulating di-phosphorylation of MLC during cytokinesis.
INTRODUCTION
Small GTPases of Rho family proteins act as molecular switches in various cellular processes including cell motility, cell division, and morphogenesis (Bokoch, 2000; Somlyo and Somlyo, 2000; Ridley, 2001; Settleman, 2001). Their main biological effects are mediated through the regulation and reorganization of the actin cytoskeleton. Rho-kinase/ROK/ROCK is one of the targets of RhoA and is involved in the assembly of stress fibers and focal adhesions in serum stimulated 3T3 cells (Chrzanowska-Wodnicka and Burridge, 1996; Leung et al., 1996; Amano et al., 1997; Ishizaki et al., 1997; Nakano et al., 1999; Watanabe et al., 1999; Totsukawa et al., 2000).
Citron is another target of activated Rho (Di Cunto et al., 1998; Madaule et al., 1998, 2000). There are two variants called citron-N and citron kinase, both of which are produced by the same transcription unit. Citron kinase is a longer variant of citron-N, including an amino-terminal serine/threonine kinase domain. It shares a high degree of structural homology with the ROCK (ROK/Rho-kinase), except that citron kinase has the SH3 binding and PDZ binding domains at its carboxyl-terminus. A shorter variant of citron-N is specifically expressed in the nervous system, and localized to the postsynaptic density, where it forms a stable complex with the membrane-associated guanylate kinase PSD-95 (Furuyashiki et al., 1999; Zhang et al., 1999). The functions of citron-N are unknown, although it has been suggested to link the Rho signaling cascades to NMDA receptor complexes.
Citron kinase has been suggested to play a role in cytokinesis (Madaule et al., 1998; Di Cunto et al., 2000). Narumiya and coworkers have reported that overexpression of citron kinase mutants inhibits cytokinesis, suggesting that it may be a target of RhoA in cytokinesis (Madaule et al., 1998). The involvement of citron kinase in cytokinesis is also supported by the phenotype of mice that are deficient of citron kinase (Di Cunto et al., 2000). Mice lacking citron kinase showed severe ataxia and epilepsy and died within the 3 weeks after birth. Abnormal cytokinesis and massive apoptosis in certain neuronal precursors are suggested to be probable causes of defective neurogenesis. Although those results suggest that citron kinase is involved in cytokinesis, the mechanism is not clear. No physiological substrate of the kinase has been identified, and this is essential to elucidate the actions of citron kinase in cytokinesis and other biological processes.
We found that citron kinase phosphorylated regulatory myosin light chain (MLC) of myosin II at both Ser-19 and Thr-18 in vitro. In vivo, citron kinase generated di-phosphorylated MLC when its kinase domain was expressed in cultured cells. Our results suggest citron kinase may be involved in the regulation of contractile activity and/or organization of cleavage furrows by regulating MLC di-phosphorylation.
MATERIALS AND METHODS
Expression Vectors, Proteins, Reagents, and Antibodies
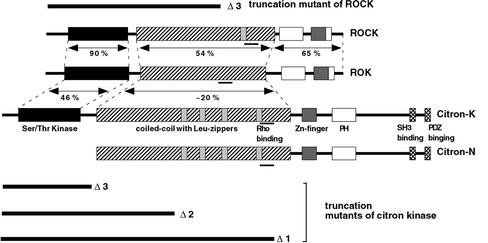
Mammalian expression vectors of pCAG-myc-citron kinase (full-length, Δ1, Δ2, and Δ3 deletion mutants and kinase-deficient mutants), as well as pEGFPC1-citron kinase and pCAG-myc-ROCK1 Δ3 deletion mutant, were described previously (Ishizaki et al., 1997; Madaule et al., 1998; Eda et al., 2001), and the domain structure of citron kinase and its mutants are depicted in Figure 1. Although the Δ3 mutant of citron kinase consists of the kinase domain of citron kinase alone, the Δ2 mutant contains both the kinase domain and half of the coiled-coil domain, which is structurally equivalent to the Δ3 deletion mutant of ROCK. The Δ1 mutant of citron kinase has further extension at the C terminus containing the Rho-binding domain (see Madaule et al., 1998 for detail). A bacterial expression vector of pGEX-2T V14RhoA was kindly provided by Dr. A. Hall.
Figure 1.
Schematic diagram of citron kinase, ROK and ROCK and their truncation mutants. Modified from Figure 1 of Madaule et al. (1998) with permission.
His-tagged, full-length citron kinase was expressed in Baculovirus using the Bac-to-Bac system (Invitrogen, Carlsbad, CA). Full-length citron kinase was cloned at SalI (5′ end) and XhoI (3′ end) of the pENTR A1 Gateway vector and then transferred to the pDEST10 vector (N-terminal His fusion vector). Virus production and citron kinase expression were followed according to the manufacturer's instruction manual. Full-length citron kinase was purified by two steps of sequential affinity column chromatography. Cell lysates of infected Sf9 cells (∼2 × 108) were first bound to a Nickel column and eluted with a linear gradient (50–200 mM) of imidazole. Citron kinase eluted around 80 mM was bound to a GST RhoA column and eluted as a complex of GST-RhoA-citron kinase. About 1–2 μg of purified kinase was prepared.
Nonmuscle myosin II was purified from bovine lung as described (Sellers, 1991). Light chains of myosin II were purified as described (Perrie and Perry, 1970). MLCK was purified from chick gizzard as described (Adelstein and Klee, 1981a). MBS of myosin phosphatase was purified from chick gizzard according to Alessi et al. (1992). Calmodulin was purchased from Sigma (St. Louis, MO). A specific inhibitor of ROCK, Y-27632, was kindly provided by Yoshitomi Pharmaceutical Industries, Ltd. (Oosaka, Japan).
An antimyc polyclonal antibody and an antimyc mAb (9E10) were purchased from Santa Cruz Biotechnology (Santa Cruz, CA) and Covance (Denver, PA), respectively. Chick antimyc polyclonal antibody was purchased from Aves Lab, Inc. (Tigard, OR). Monoclonal and polyclonal antibodies against Ser 19-phosphorylated MLC were described previously (Sakurada et al., 1994; Matsumura et al., 1998). A polyclonal antibody against di-phosphorylated (phosphorylated at both Ser 19 and Thr 18) MLC was described previously (Sakurada et al., 1998).
Cell Culture, DNA Transfection, and Immunoprecipitation
PtK2 cells were maintained in a 1:1 mixture of DME and F12 medium containing 10% fetal calf serum. BHK, NRK, CHO, and COS7 cells were maintained in DME medium containing 10% fetal calf serum. Transfection was performed using a GeneJuice (Novagene, Madison, WI) or Lipofectamine (Invitrogen, Carlsbad, CA) transfection reagent.
For immunoprecipitation of exogenously expressed citron kinase or ROCK, COS7 cells were transfected with the myc-tagged constructs of citron kinase or ROCK according to manufacturer's instructions. After a 24-h incubation, transfected cells were lysed in an immunoprecipitation buffer containing 30 mM Tris-HCl (pH 7.5), 100 mM NaCl, 5 mM MgCl2, 50 mM sodium pyrophosphate, 1 mM EGTA, 1 mM EDTA, 20 mM beta-glycerophosphate, 1 mM sodium vanadate, 10 mM NaF, 1% Triton X-100, 1 mM DTT, 1 mM PMSF, and a mixture of leupeptin, pepstastin, and chymostatin (10 μg/ml each). Cells were homogenized with a Dounce homogenizer and clarified by centrifugation at 16,000 × g for 15 min. The supernatant was incubated with a myc mAb (10 μg/100-mm dish) for 1–2 h at 4°C. The immunocomplex was precipitated with protein A-Sepharose (Pharmacia Biotech, Inc., Piscataway, NJ) during a 1-h incubation. The immunocomplex was washed three times with the immunoprecipitation buffer, washed three times with a buffer containing 20 mM Tris-HCl (pH 7.5), 50 mM NaCl, and 5 mM MgCl2, and used for kinase assay.
Phosphorylation Assay
The kinase assays were performed in 25 mM Tris-HCl buffer (pH 7.5) containing 50 mM NaCl, 1 mM DTT, 5 mM MgCl2, 0.1 mM ATP (0.1 mCi/ml), 0.1 mM EGTA, enzyme, and varying amounts of MLC or bovine lung myosin II. Kinases used include immunoprecipitated citron kinase and ROCK, purified MLCK, and Baculovirus-expressed full-length citron kinase. The reaction was performed at 30°C for 3–60 min and terminated by the addition of 2× SDS sample buffer. After SDS-PAGE, MLC bands were cut out and counted by the Cerenkov method. Urea/glycerol gel electrophoresis revealed that purified myosin II was not phosphorylated (our unpublished results).
We also used mono-phosphorylated myosin II as a substrate and prepared it as follows: Purified myosin II was incubated with MLCK (5 μg/ml) in 25 mM Tris-HCl buffer (pH 7.5) containing 50 mM NaCl, 1 mM DTT, 5 mM MgCl2, 0.1 mM CaCl2, 1 mM ATP, and calmodulin (5 μg/ml) at room temperature for 30 min. Phosphorylated myosin II was precipitated by overnight dialysis against 20 mM imidazole buffer (pH 6.5) containing 30 mM KCl and 5 mM MgCl2, washed once with the same buffer, and dissolved in 25 mM Tris-HCl (pH 7.5) containing 0.3 M NaCl. Urea/glycerol gel electrophoresis revealed that more than 90% of myosin II was mono-phosphorylated under these conditions. To compare the specificity of a substrate between mono-phosphorylated and unphosphorylated myosin II, unphosphorylated myosin II was prepared in the same way as described above except that ATP was omitted when myosin II was phosphorylated with MLCK.
Immunofluorescence
Immunofluorescence was performed using formaldehyde fixation as described (Yamashiro et al., 1998). Exogenously expressed citron kinase or ROCK was detected by immunofluorescence using antimyc mAb or antimyc polyclonal antibody. Changes in phosphorylation of MLC was examined by immunofluorescence using the polyclonal or monoclonal antibodies against mono-phosphorylated MLC or the polyclonal antibody against di-phosphorylated MLC, as described previously (Totsukawa et al., 2000). F-actin was visualized using fluorescently labeled phalloidin (Sigma). Cells were examined with a Nikon TE 300 inverted microscope. Phase and fluorescence images were taken with a Photometric CoolSnap-fx CCD camera (Roper Scientific Inc., Tucson, AZ) and processed with IPLab image processing software (Scanalytics, Inc., Fairfax, VA).
Other Procedures
SDS-PAGE was performed as described (Blatter et al., 1972) using 12.5% polyacrylamide except that the buffer system of Laemmli (1970) was used. Protein concentrations were determined using an Advanced Protein Assay reagent (Cytoskeleton, Denver, CO) with bovine serum albumin as a standard. Phosphopeptide mapping of phosphorylated MLC was performed as described (Yamakita et al., 1994).
RESULTS
Phosphorylation of Regulatory Light Chain (MLC) of Myosin II by Citron Kinase In Vitro
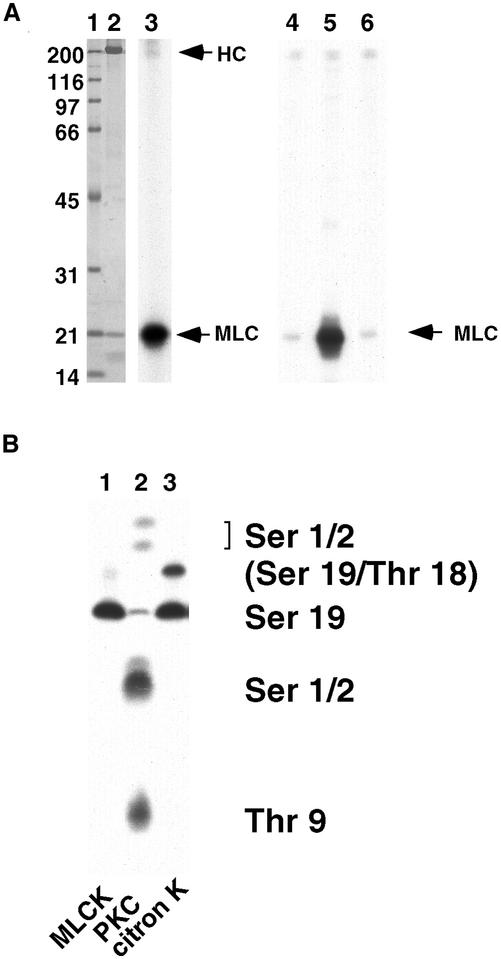
We found that citron kinase phosphorylated MLC in vitro. A Δ1deletion mutant (that contains the N-terminus kinase domain as well as the coiled-coil and the Rho binding domain; Madaule et al., 1998) of myc-tagged citron kinase was expressed in COS7 cells, immunoprecipitated by an antimyc antibody, and used for kinase assay using bovine lung myosin II as a substrate. As Figure 2A shows, immunoprecipitated citron kinase was able to phosphorylate MLC of intact myosin II (lanes 2 and 3). It also phosphorylated isolated MLC (lane 5). As a control, a kinase defective (KD) mutant of citron kinase was immunoprecipitated in the same way and used for its kinase activity. The KD mutant failed to phosphorylate MLC (lane 6). In addition, immunoprecipitates from MOCK-transfected cells did not phosphorylate MLC (lane 4). These results suggest that citron kinase itself, rather than an unknown kinase associated or contaminated with immunoprecipitated citron kinase, phosphorylates MLC. We examined other constructs of citron kinase including mutants with further deletion of the C terminus (Δ2, the kinase domain plus half of the coiled-coil domain, and Δ3, kinase domain alone) as well as full-length citron kinase, which all phosphorylated MLC. As the Δ3 mutant consists of the kinase domain alone, it is unlikely that an unknown kinase associated with this mutant phosphorylates MLC.
Figure 2.
Phosphorylation of RMLC by citron-kinase in vitro. (A) Phosphorylation of MLC by citron-kinase. Citron kinase Δ1 mutant was expressed in COS7 cells, immunoprecipitated with an antimyc antibody, and assayed for phosphorylation using bovine lung myosin II (lanes 2 and 3) or isolated MLC (lanes 4–6) as a substrate. Lanes 1 and 2, Coomassie blue staining. Lane 1, molecular mass markers (numbers in kilodaltons); lane 2, lung myosin plus immunoprecipitated kinase; lane 3, autoradiograph of lane 2. Lanes 4–6, autoradiograph; lane 4, immunoprecipitates from mock-transfected cells; lane 5, immunoprecipitates of citron kinase; lane 6, immunoprecipitates of kinase-defective citron kinase. HC, heavy chain of myosin II; MLC, myosin light chain. (B) One-dimensional phosphopeptide map. Citron kinase phosphorylated both Ser19 and Thr18. Note that citron kinase produced di-phosphorylated MLC to a higher extent than did MLCK. Protein kinase C phosphorylated Ser1/2 and Thr9.
The sites of MLC phosphorylated by citron kinase were identified by one-dimensional phosphopeptide mapping (Yamakita et al., 1994). As Figure 2B shows, citron kinase generated two phosphopeptides, one corresponding to a peptide phosphorylated at Ser19 and the other to a peptide phosphorylated at both Ser19 and Thr 18 (lane 3). As a control, phosphopeptide patterns of MLC phosphorylated by MLCK or PKC are shown in lanes 1 and 2, respectively. It should be noted that citron kinase produced di-phosphorylated MLC to a higher extent than did MLCK.
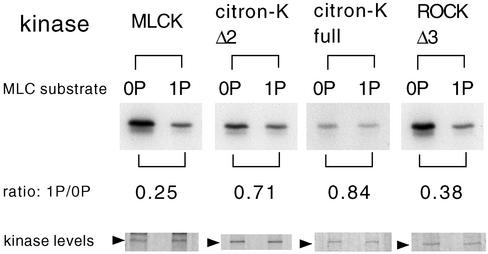
It is possible that citron kinase phosphorylates mono-phosphorylated MLC as well as it does unphosphorylated MLC, thus generating more di-phosphorylated MLC than did MLCK. We thus measured activities of citron kinase, ROCK, and MLCK using both unphosphorylated (0P) and mono-phosphorylated (1P) myosin II as a substrate. As Figure 3 shows, both full-length and mutant citron kinases phosphorylated these two substrates equally well. In contrast, MLCK or ROCK phosphorylated un-phosphorylated MLC much better than they did mono-phosphorylated MLC. The ratios of phosphate incorporation between unphosphorylated and mono-phosphorylated substrate are 0.7–0.8 for citron kinase, 0.2–0.3 for MLCK, and 0.3–0.4 for ROCK.
Figure 3.
Citron kinase phosphorylates mono-phosphorylated MLC as well as it does un-phosphorylated MLC. MLC kinase reactions were performed using either unphosphorylated myosin (0P) or mono-phosphorylated myosin (1P) as a substrate. Myc-tagged, full-length citron kinase, Δ2 citron kinase, or Δ3 ROCK was expressed in COS7 cells, immunoprecipitated by a myc antibody, and used for kinase reactions. Purified MLCK was also used. Ratios of phosphate incorporation between mono-phosphorylated MLC and unphosphorylated MLC are shown. Kinase levels are shown in the bottom panel (Coomassie blue staining). The results are representative of three independent experiments.
Citron Kinase Does Not Phosphorylate MBS of Myosin Phosphatase
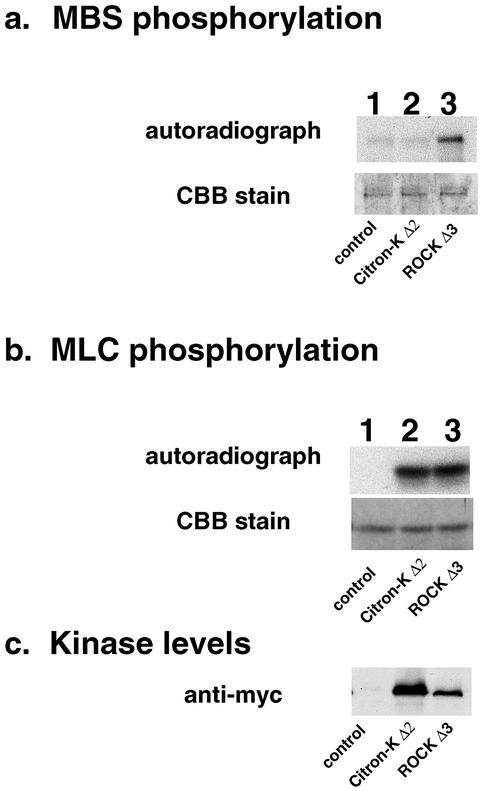
It is well known that ROCK phosphorylates MBS of myosin phosphatase. Because the kinase domain of ROCK shows 46% identity to that of citron kinase (Madaule et al., 1998), we examined whether citron kinase was able to phosphorylate MBS. The kinase domain of myc-tagged ROCK or citron kinase was expressed in COS7 cells, immunoprecipitated, and assayed for their kinase activities using both MLC and MBS as a substrate (Figure 4). As Figure 4a shows, phosphorylation of MBS by citron kinase (lane 2) was as low as that of mock transfection (lane 1). On the other hand, ROCK phosphorylated MBS (lane 3). The lack of phosphate incorporation into the MBS by citron kinase is neither due to inactive kinase activity of immunoprecipitated citron kinase nor poor expression of citron kinase. Both citron kinase and ROCK phosphorylated MLC to a similar extent (lanes 2 and 3 of Figure 4b). In addition, Western blotting with a myc antibody (c) revealed that the expression level of citron kinase (lane 2) in COS7 cells was ∼5 times higher than that of ROCK (lane 3). These results indicate that the activity of citron kinase toward MBS is insignificant.
Figure 4.
Citron kinase does not significantly phosphorylate MBS. The kinase domain mutants (Δ2) of citron kinase and ROCK (Δ3) were expressed in COS7 cells, immunoprecipitated, and assayed for their kinase activities using MBS (a) or MLC (b) as a substrate. Both citron kinase (lane 2) and ROCK (lane 3) phosphorylated MLC to a similar extent (b). However, only ROCK phosphorylated MBS (lane 3 of a). Lane 1 is control without kinase. The levels of kinase used are shown in c, which were detected by immunoblot using the antimyc antibody. CBB, Coomassie brilliant blue. The results are representative of three independent experiments.
Kinetic Analysis of Citron Kinase
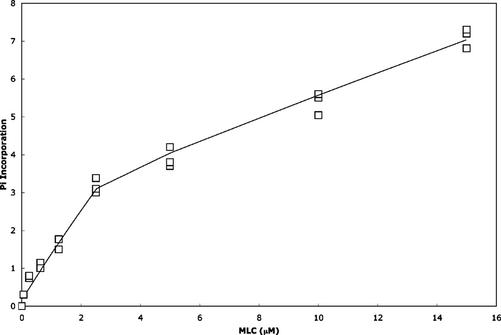
To estimate the molecular activity and Km of citron kinase, full-length citron kinase complexed with constitutively active GST-RhoA was used for phosphorylation with varying concentrations of MLC (Figure 5). The apparent Km and molecular activity of full-length citron kinase were 6.6 ± 1.0 μM and 0.3 ± 0.09 s–1, respectively. The Km value is comparable to the values (0.9–2.5 μM) reported for Rho-kinase and those (5–52 μM) of MLCK (Adelstein and Klee, 1981b; Gallagher et al., 1991; Amano et al., 1996; Feng et al., 1999). On the other hand, the molecular activity of citron kinase is lower than the values reported for Rho-kinase (0.67–2 s–1) and MLCK (2–65 s–1; Adelstein and Klee, 1981b; Gallagher et al., 1991; Amano et al., 1996; Feng et al., 1999). When intact myosin was used as a substrate, full-length citron kinase was able to phosphorylate MLC up to 0.5–0.7 mol/mol of MLC.
Figure 5.
Phosphorylation of MLC by Baculovirus-expressed full-length citron kinase. Full-length citron kinase (5 ng/μl) was incubated at 30°C for 5 min with varying concentrations (0.25–15 μM) of MLC. Pi incorporation is in pmol.
Increase in MLC Di-phosphorylation by the Expression of the Kinase Domain of Citron Kinase in Cultured Cells
To further test whether citron kinase phosphorylates MLC, cultured cells were transfected with myc-tagged citron kinase and stained with the phospho-MLC specific antibodies. Immunofluorescence with the phospho-MLC specific antibodies has allowed us to determine changes in MLC phosphorylation in transfected cells (detected by a myc antibody; Totsukawa et al., 2000). We used two antibodies, one specific to mono-phosphorylated MLC and the other to di-phosphorylated MLC. It should be noted that the antibody against mono-phosphorylated MLC did not recognize di-phosphorylated MLC and the antibody against di-phosphorylated MLC did not recognize mono-phosphorylated MLC. Actin organization was also examined by phalloidin staining.
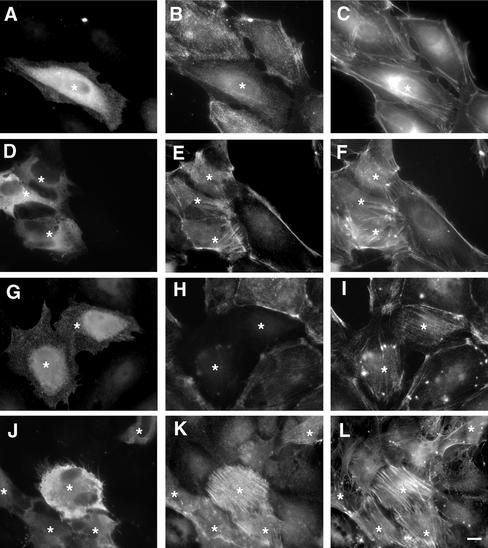
We examined the effects of the Δ2 mutant of citron kinase on MLC phosphorylation of PTK cells (Figure 6). This mutant, which consists of the kinase domain and a part of the coiled-coil domain (see Figure 1), distributed diffusely throughout the cytoplasm (asterisk in A, D, and G; Eda et al., 2001). Surprisingly, when transfected cells were stained with the antibody specific to mono-phosphorylated MLC, they showed lower staining (asterisk in B). Quantitative measurement showed that staining intensity with the mono-phosphorylated antibody was reduced to 45 ± 25% of the control (n = 30). However, phalloidin staining revealed that transfected cells retained well-developed stress fibers (asterisk in C). Because stress fiber formation depends on actomyosin contractility and MLC phosphorylation (Chrzanowska-Wodnicka and Burridge, 1996; Totsukawa et al., 2000) and because citron kinase produced di-phosphorylated MLC in vitro, we thought myosin may be di-phosphorylated. Indeed, transfected cells (asterisks in E) exhibited significantly higher staining with the antibody specific to di-phosphorylated MLC, indicating that di-phosphorylation was increased. Again phalloidin staining (F) revealed that transfected cells had well-developed stress fibers. To confirm the changes in MLC phosphorylation, transfected cells were triple-labeled with the antibodies against myc (G), mono- (H), and di-phosphorylated MLC (I). The increase in di-phosphorylation (I) with simultaneous decrease in mono-phosphorylation (H) was clearly seen in transfected cells (asterisks in G). A similar result was obtained with Δ3 mutant of citron kinase (which contains the kinase domain alone). These observations indicate that the expression of kinase domain of citron kinase caused an increase in MLC di-phosphorylation.
Figure 6.
Di-phosphorylation of MLC in vivo by citron kinase. PTK cells were first transfected with Δ2 citron kinase and then immunolabeled with the specific antibodies against myc (A, D, G, and J), against mono-phosphorylated MLC (B and H), and against di-phosphorylated MLC (E, I, and K). F-actin was also visualized by labeling with fluorescent phalloidin (C, F, and L). Asterisks indicate cells expressing myc-tagged mutant citron kinase. Cells in J–L were treated with a specific inhibitor of ROCK, Y-27632, for 30 min before immunofluorescence staining. The expression of Δ2 citron kinase increased di-phosphorylation of MLC (asterisks in E, I, and K) but decreased mono-phosphorylation (asterisk in B and H). Bar, 10 μm.
The increase in di-phosphorylation becomes more evident when ROCK activity is blocked by a specific inhibitor, Y-27632. The inhibition of ROCK is known to result in the disassembly of stress fibers (Ishizaki et al., 2000). As Figure 6, J–L shows, however, cells expressing Δ2 citron kinase (asterisks in J) retained well-developed stress fibers (asterisks in L). At the same time, these transfected cells clearly exhibited an increase in MLC di-phosphorylation (asterisks in K), indicating that di-phosphorylation efficiently restores the assembly of stress fibers. In contrast, surrounding cells expressing no citron kinase lost staining with the antibody against di-phosphorylated MLC (K), and stress fibers of these nontransfected cells were disassembled (L). These results indicate that citron kinase is able to generate di-phosphorylation of MLC even when myosin phosphatase is activated by the inhibition of ROCK.
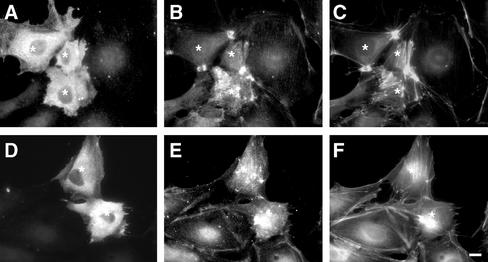
To compare the effects of citron kinase with those of ROCK, we examined how the expression of the kinase domain of ROCK (Δ3 mutant) alters MLC phosphorylation (Figure 7). As revealed by phalloidin staining (C and F), expression of ROCK frequently induced the formation of characteristic stellar stress fibers (C), which is consistent with the previous reports (Leung et al., 1996; Amano et al., 1997; Ishizaki et al., 1997). These stellar stress fibers were highly stained with the antibodies against either mono- (B) or di-phosphorylated MLC (E). The increase in both mono- and di-phosphorylation of MLC by ROCK overexpression is probably due to the two activities of ROCK: ROCK can directly phosphorylate MLC (Amano et al., 1996; Totsukawa et al., 2000) and at the same time inhibit myosin phosphatase (Kimura et al., 1996).
Figure 7.
Increases in mono- and di-phosphorylation by the expression of ROCK. PTK cells were transfected with Δ3 mutant of ROCK (equivalent to Δ2 mutant of citron kinase) and then stained with antimyc (A and D), anti–mono-phosphorylated MLC (B), and anti–di-phosphorylated MLC (E) antibodies. F-actin structure was visualized by labeling with fluorescent phalloidin (C and F). Cells expressing myc-tagged mutant ROCK are indicated by asterisks. Note that ROCK increases both mono- and di-phosphorylation of MLC. Stellar stress fibers were frequently formed. Bar, 10 μm.
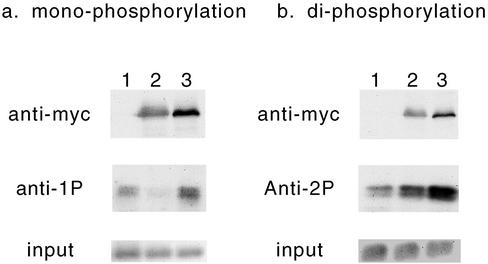
The changes in MLC phosphorylation by citron kinase or by ROCK were confirmed biochemically using Western blotting (Figure 8). BHK cells were used for this purpose because they are more efficient for transfection. Cells were transfected with either citron kinase Δ2 mutant or ROCK Δ3 mutant. Total cell lysates were then immunoblotted using the antibodies specific to mono- or di-phosphorylated MLC (middle panels) as well as the myc antibody to detect levels of expression (top panels). Similar amounts of lysates were loaded for each lane (bottom panels). Cells transfected with citron kinase showed lower reactivity to the antibody against mono-phosphorylated MLC (lane 2 of Figure 8a) than did mock-transfected cells (lane 1). On the other hand, the immunoblot with the antibody against di-phosphorylated MLC revealed higher reactivity with citron kinase-transfected cells (lane 2 of Figure 8b) than with mock-transfected cells (lane 1). ROCK-transfection increased both mono- and di-phosphorylation of MLC to a great extent (lane 3 of Figure 8, a and b, respectively), which is probably due to the inhibition of myosin phosphatase by ROCK. These data are consistent with the immunofluorescence observations (Figures 6 and 7).
Figure 8.
Increase in di-phosphorylation and decrease in mono-phosphorylation of MLC by expression of citron kinase. BHK cells were first transfected with Δ2 mutant of citron kinase or Δ3 mutant of ROCK, then total cell lysates were immunoblotted with antibodies against mono-phosphorylated MLC (middle panel of a) or against di-phosphorylated MLC (middle panel of b). Lane 1, mock-transfection; lane 2, transfection with Δ 2 citron kinase mutant; lane 3, transfection with Δ3 ROCK mutant. The same lysates were also immunoblotted with antimyc antibody to show levels of expression (top panels). Coomassie blue staining of histone H3 of total cell lysates are shown in the lower panels to indicate the loading.
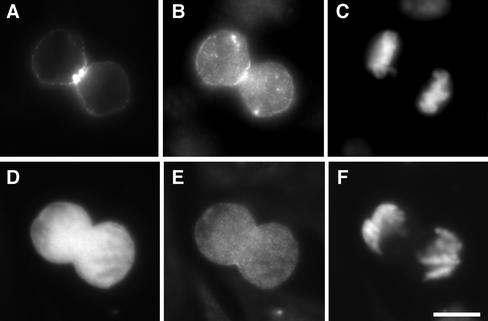
We then examined whether the expression of full-length citron kinase alters MLC phosphorylation. Previous work has demonstrated that, although full-length citron kinase is present as protein aggregates in interphase cells, it becomes dispersed during prophase and localized in cleavage furrows during cytokinesis (Eda et al., 2001). Because RhoA is likely to activate citron kinase during cytokinesis, we examined whether the expression of full-length citron kinase alters MLC di-phosphorylation during cytokinesis. To this end, we chose CHO cells because CHO cells are relatively easily synchronized and show good transfection efficiency. As Figure 9 shows, we have expressed GFP-tagged full-length citron kinase in synchronized CHO cells (A) and stained them with the antibody against di-phosphorylated MLC (B) and DAPI (C). As a control, GFP alone was expressed (D) and stained with the anti–di-phosphorylated MLC antibody (E) and DAPI (F). GFP-citron kinase was localized in cleavage furrows (A) where strong staining of MLC di-phosphorylation was observed (B). Quantitative analyses of immunofluorescence revealed that staining intensities of di-phosphorylation more than doubled. On the other hand, control cells expressing GFP alone (D) showed di-phosphorylation indistinguishable from untransfected cells (E).
Figure 9.
Di-phosphorylation of MLC during cytokinesis by the expression of full-length citron kinase. CHO cells were transfected with GFP-full-length citron kinase (A–C) or GFP alone (D–F) and synchronized for cell division. A and D, GFP. Cleaving cells were labeled with the anti–di-phosphorylated MLC antibody (B and E) and with DAPI (C and F).
In interphase cells, GFP-tagged citron kinase showed punctate localization, as reported previously (Madaule et al., 1998; Eda et al., 2001). Such cells showed no changes in MLC mono- or di-phosphorylation (our unpublished results). This lack of effects is likely due to the fact that the localization of full-length citron kinase is constrained to protein aggregates (Eda et al., 2001), which would restrict the access of the kinase to a substrate.
Localization of Mono- and Di-phosphorylated MLC during Cell Division
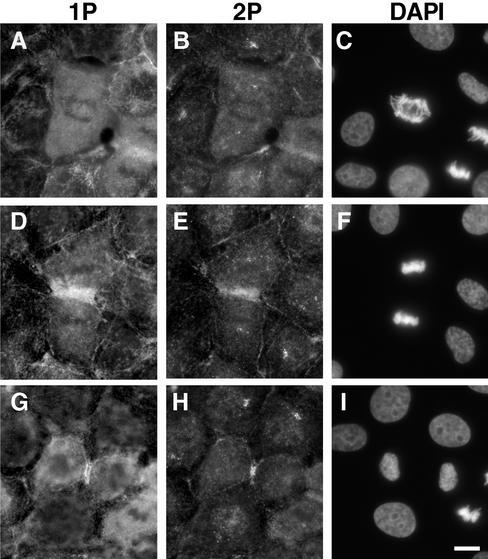
We examined the localization of di-phosphorylated MLC during cell division. Figure 10 shows double-labeled immunofluorescence localization of both mono-phosphorylated (A, D, and G) and di-phosphorylated (B, E, and H) MLC in NRK cells at different stages of cytokinesis. From metaphase (our unpublished results) to early anaphase (A–C), both mono- and di-phosphorylated MLC showed diffuse staining though anti–di-phosphorylated MLC antibody stained centrioles. At telophase (D–F), both mono- (D) and di-phosphorylated (E) MLC colocalized at cleavage furrows. It is interesting to note that di-phosphorylated MLC showed more constrained localization in cleavage furrows than did mono-phosphorylated MLC. At late telophase (G and H), the constrained localization of di-phosphorylated MLC became clearer.
Figure 10.
Localization of mono- and di-phosphorylated MLC during cell division. NRK cells at different stages of cell division were stained with the antibodies against mono-phosphorylated MLC (A, D, and G), di-phosphorylated MLC (B, E, and H), and DAPI (C, F, and I). A–C, early anaphase; D–F, telophase; G–I, late telophase. Note that di-phosphorylated MLC in cleavage furrows is more constrained than is mono-phosphorylated MLC. Bar, 10 μm.
DISCUSSION
We have shown here that citron kinase, like ROCK, is able to function as an MLC kinase. In vitro, citron kinase phosphorylates MLC at both Ser19 and Thr18. The expression of the kinase domain causes di-phosphorylation of MLC in a variety of cells. To our knowledge, MLC is the first physiological substrate of citron kinase. Because citron kinase is localized in cleavage furrows (Madaule et al., 1998; Kosako et al., 1999; Kosako et al., 2000; Fukata et al., 2001), it is likely to activate myosin during cytokinesis. The presence of the two RhoA-activated MLC kinases, citron kinase and ROCK, in cleavage furrows may explain why the ROCK inhibitor, Y-27632, does not effectively inhibit cytokinesis (Madaule et al., 2000). The direct phosphorylation of MLC by citron kinase may also account for abnormal contractility of “push and pull” movements during cytokinesis when a kinase active mutant of citron kinase is overexpressed (Madaule et al., 2000).
Although both ROCK and citron kinase phosphorylate MLC, there are two important differences in their activities. First, citron kinase does not phosphorylate MBS of myosin phosphatase (Figure 4) and thus does not inhibit myosin phosphatase activity. In contrast, ROCK phosphorylates MBS, inhibiting the activity of myosin phosphatase. This indicates that ROCK would block the turnover of MLC phosphorylation, making most MLC to be the phosphorylated form of MLC. Both immunofluorescence (Figure 6) and biochemical analysis (Figure 8) support this notion. When constitutively active ROCK is expressed in cells, stellar actin fibers are assembled (Figure 7), which is likely to be a result of contraction of actomyosin due to a very high extent of MLC phosphorylation (Figure 8). In contrast, citron kinase would not block turnover of MLC phosphorylation. Consistent with this notion, cells expressing the kinase domain of citron kinase exhibit parallel stress fibers with apparently normal morphology (Figure 6).
Second, the expression of citron kinase in cells resulted in an increase in di-phosphorylation of MLC but a decrease in MLC mono-phosphorylation (Figure 6). This is in contrast to the expression of ROCK in which both mono- and di-phosphorylation were increased (Figure 7). The increase in di-phosphorylated MLC by the citron kinase domain is apparently explained by the result that citron kinase phosphorylated monophosphorylated MLC as well as it did un-phosphorylated MLC (Figure 3). This ability of citron kinase may also explain the decrease in mono-phosphorylation of MLC because citron kinase would effectively convert mono-phosphorylated myosin to di-phosphorylated myosin.
Other interpretations are also possible. For example, citron kinase may activate a myosin phosphatase that is specific for mono-phosphorylated myosin, although no such phosphatase has been known. Another possibility is that citron kinase phosphorylates and inhibits other major MLC kinases. If citron kinase, for example, inhibited MLCK, then mono-phosphorylated MLC may be reduced. We found, however, that citron kinase did not phosphorylate MLCK or ROCK, which are believed to be two major MLC kinases inside cells. Further studies are necessary to determine whether citron kinase modulates other MLC kinases including PAK (Chew et al., 1998; Zeng et al., 2000) and ZIP kinase (Murata-Hori et al., 1999, 2001).
The activity of citron kinase is likely to be regulated in a cell cycle–dependent manner. Citron kinase has been reported to be present as protein aggregates in interphase cells (Eda et al., 2001), and as such, it may not be active. In contrast, ROCK is diffusely localized in interphase cells. ROCK is, at least in part, active as its activity is known to be essential for the formation of stress fibers (Leung et al., 1996; Amano et al., 1997; Ishizaki et al., 1997; Nakano et al., 1999; Watanabe et al., 1999; Totsukawa et al., 2000). During prophase, citron kinase becomes dispersed and is translocated to cleavage furrows during cytokinesis (Eda et al., 2001). Because Rho A has been reported to be greatly activated during cytokinesis (Kimura et al., 2000), citron kinase is likely to become activated. This activation probably accounts, at least in part, for the di-phosphorylation of MLC at cleavage furrows (Figures 9 and 10) although other kinases such as ROCK may also be involved in the di-phosphorylation of MLC.
Di-phosphorylated myosin showed more constrained localization at cleavage furrows than did mono-phosphorylated myosin (Figure 10). Although di-phosphorylated myosin showed twofold higher ATPase activity than mono-phosphorylated myosin (Ikebe and Hartshorne, 1985), the velocity of actin filaments in an in vitro motility assay is the same for both types of phosphorylated myosin (Umemoto et al., 1989). On the other hand, di-phosphorylation significantly increases thick filament assembly and actomyosin superprecipitation (Ikebe and Hartshorne, 1985), suggesting that di-phosphorylated myosin may play a role in cross-linking of actin filaments rather than stimulation of motor activity. Further studies are required to elucidate the exact role of di-phosphorylation of MLC in cleavage furrows.
It now appears that three different kinases including MLCK, ROCK, and citron kinase are all localized in cleavage furrows (Madaule et al., 1998; Kosako et al., 1999, 2000; Poperechnaya et al., 2000; Fukata et al., 2001; Chew et al., 2002) and are functioning as MLC kinases. In addition, MBS of myosin phosphatase phosphorylated by Rho-kinase (ROCK) is also localized in cleavage furrows (Kawano et al., 1999). These results reinforce the importance of the regulation of MLC phosphorylation during cytokinesis. An important question is why citron kinase–deficient mice show a cytokinesis defect in some neuronal precursor cells even though these cells express abundant ROCK (Di Cunto et al., 2000). This observation indicates that citron kinase plays a role distinct from ROCK. As discussed above, citron kinase, unlike ROCK, does not block turnover of MLC phosphorylation while generating di-phosphorylated MLC. This ability of citron kinase to allow turnover may be important for certain cells to complete cytokinesis. For example, ROCK may be activated by Rho to a much higher extent in the absence of citron kinase than in its presence because Rho may not be shared between ROCK and citron kinase. Such overactivation of ROCK may result in the MLC phosphorylation to a very high extent while blocking dephosphorylation of MLC or turnover of MLC phosphorylation. Blockage of MLC dephosphorylation may hinder the proper execution of cytokinesis because cytokinesis is known to be associated with simultaneous contraction and disassembly of contractile rings, and the disassembly is likely to require MLC dephosphorylation or turnover. It is also quite possible that citron kinase controls cytokinesis by phosphorylating unique substrates that ROCK cannot phosphorylate. Future studies should be directed at elucidating whether citron kinase indeed regulates MLC di-phosphorylation during cytokinesis in a way distinct from ROCK or whether citron kinase has other substrates that are critical in regulating other aspects of cell division.
Acknowledgments
We thank Yoshitomi Pharmaceutical Industries, Ltd. For providing Y-27632, and Dr. F. Deis (Rutgers) for critical reading of this manuscript. The work is supported by the National Institutes of Health Grant CA42742 (to F.M.), American Heart Association grant (to S.Y.), and the Bush Memorial Fund. F.M. is a member of the Cancer Institute of New Jersey. G.T. is supported by a fellowship from the Leukemia Research Foundation and the American Heart Association.
Article published online ahead of print. Mol. Biol. Cell 10.1091/mbc.E02-07-0427. Article and publication date are at www.molbiolcell.org/cgi/doi/10.1091/mbc.E02-07-0427.
Abbreviations used: MBS, myosin binding subunit or myosin targeting subunit of myosin phosphatase; MLC, regulatory light chain of myosin II; ROCK, Rho-associated kinase, Rho-kinase or ROK.
References
- Adelstein, R.S., and Klee, C.B. (1981a). Purification and characterization of smooth muscle myosin light chain kinase. J. Biol. Chem. 256, 7501–7509. [PubMed] [Google Scholar]
- Adelstein, R.S., and Klee, C.B. (1981b). Purification and characterization of smooth muscle myosin light chain kinase. J. Biol. Chem. 256, 7501–7509. [PubMed] [Google Scholar]
- Alessi, D., MacDougall, L.K., Sola, M.M., Ikebe, M., and Cohen, P. (1992). The control of protein phosphatase-1 by targetting subunits. The major myosin phosphatase in avian smooth muscle is a novel form of protein phosphatase-1. Eur. J. Biochem. 210, 1023–1035. [DOI] [PubMed] [Google Scholar]
- Amano, M., Chihara, K., Kimura, K., Fukata, Y., Nakamura, N., Matsuura, Y., and Kaibuchi, K. (1997). Formation of actin stress fibers and focal adhesions enhanced by Rho-kinase. Science 275, 1308–1311. [DOI] [PubMed] [Google Scholar]
- Amano, M., Ito, M., Kimura, K., Fukata, Y., Chihara, K., Nakano, T., Matsuura, Y., and Kaibuchi, K. (1996). Phosphorylation and activation of myosin by Rho-associated kinase (Rho-kinase). J. Biol. Chem. 271, 20246–20249. [DOI] [PubMed] [Google Scholar]
- Blatter, D.P., Garner, F., Van Slyke, K., and Bradley, A. (1972). Quantitative electrophoresis in polyacrylamide gels of 2–40%. J. Chromatogr. 64, 147–155. [Google Scholar]
- Bokoch, G.M. (2000). Regulation of cell function by Rho family GTPases. Immunol. Res. 21, 139–148. [DOI] [PubMed] [Google Scholar]
- Chew, T.-L., Wolf, W.A., Gallagher, P.J., Matsumura, F., and Chisholm, R.L. (2002). A fluorescent resonant energy transfer-based bio-sensor reveals transient and regional myosin light chain kinase activation in lamella and cleavage furrows. J. Cell Biol. 156, 543–553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chew, T.L., Masaracchia, R.A., Goeckeler, Z.M., and Wysolmerski, R.B. (1998). Phosphorylation of non-muscle myosin II regulatory light chain by p21-activated kinase (gamma-PAK). J. Muscle Res. Cell Motil. 19, 839–854. [DOI] [PubMed] [Google Scholar]
- Chrzanowska-Wodnicka, M., and Burridge, K. (1996). Rho-stimulated contractility drives the formation of stress fibers and focal adhesions. J. Cell Biol. 133, 1403–1415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Cunto, F., Calautti, E., Hsiao, J., Ong, L., Topley, G., Turco, E., and Dotto, G.P. (1998). Citron rho-interacting kinase, a novel tissue-specific ser/thr kinase encompassing the Rho-Rac-binding protein Citron. J. Biol. Chem. 273, 29706–29711. [DOI] [PubMed] [Google Scholar]
- Di Cunto, F. et al. (2000). Defective neurogenesis in citron kinase knockout mice by altered cytokinesis and massive apoptosis. Neuron 28, 115–127. [DOI] [PubMed] [Google Scholar]
- Eda, M., Yonemura, S., Kato, T., Watanabe, N., Ishizaki, T., Madaule, P., and Narumiya, S. (2001). Rho-dependent transfer of Citron-kinase to the cleavage furrow of dividing cells. J. Cell Sci. 114, 3273–3284. [DOI] [PubMed] [Google Scholar]
- Feng, J. et al. (1999). Rho-associated kinase of chicken gizzard smooth muscle. J. Biol. Chem. 274, 3744–3752. [DOI] [PubMed] [Google Scholar]
- Fukata, Y., Amano, M., and Kaibuchi, K. (2001). Rho-Rho-kinase pathway in smooth muscle contraction and cytoskeletal reorganization of non-muscle cells. Trends Pharmacol. Sci. 22, 32–39. [DOI] [PubMed] [Google Scholar]
- Furuyashiki, T., Fujisawa, K., Fujita, A., Madaule, P., Uchino, S., Mishina, M., Bito, H., and Narumiya, S. (1999). Citron, a Rho-target, interacts with PSD-95/SAP-90 at glutamatergic synapses in the thalamus. J. Neurosci. 19, 109–118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallagher, P.J., Herring, B.P., Griffin, S.A., and Stull, J.T. (1991). Molecular characterization of a mammalian smooth muscle myosin light chain kinase. J. Biol. Chem. 266, 23936–23944. [PMC free article] [PubMed] [Google Scholar]
- Ikebe, M., and Hartshorne, D.J. (1985). Phosphorylation of smooth muscle myosin at two distinct sites by myosin light chain kinase. J. Biol. Chem. 260, 10027–10031. [PubMed] [Google Scholar]
- Ishizaki, T., Naito, M., Fujisawa, K., Maekawa, M., Watanabe, N., Saito, Y., and Narumiya, S. (1997). p160ROCK, a Rho-associated coiled-coil forming protein kinase, works downstream of Rho and induces focal adhesions. FEBS Lett. 404, 118–124. [DOI] [PubMed] [Google Scholar]
- Ishizaki, T., Uehata, M., Tamechika, I., Keel, J., Nonomura, K., Maekawa, M., and Narumiya, S. (2000). Pharmacological properties of Y-27632, a specific inhibitor of rho-associated kinases. Mol. Pharmacol. 57, 976–983. [PubMed] [Google Scholar]
- Kawano, Y., Fukata, Y., Oshiro, N., Amano, M., Nakamura, T., Ito, M., Matsumura, F., Inagaki, M., and Kaibuchi, K. (1999). Phosphorylation of myosin-binding subunit (MBS) of myosin phosphatase by Rho-kinase in vivo. J. Cell Biol. 147, 1023–1038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimura, K. et al. (1996). Regulation of myosin phosphatase by Rho and Rho-associated kinase (Rho-kinase) [see comments]. Science 273, 245–248. [DOI] [PubMed] [Google Scholar]
- Kimura, K., Tsuji, T., Takada, Y., Miki, T., and Narumiya, S. (2000). Accumulation of GTP-bound RhoA during cytokinesis and a critical role of ECT2 in this accumulation. J. Biol. Chem. 275, 17233–17236. [DOI] [PubMed] [Google Scholar]
- Kosako, H. et al. (1999). Specific accumulation of Rho-associated kinase at the cleavage furrow during cytokinesis: cleavage furrow-specific phosphorylation of intermediate filaments. Oncogene 18, 2783–2788. [DOI] [PubMed] [Google Scholar]
- Kosako, H., Yoshida, T., Matsumura, F., Ishizaki, T., Narumiya, S., and Inagaki, M. (2000). Rho-kinase/ROCK is involved in cytokinesis through the phosphorylation of myosin light chain and not ezrin/radixin/moesin proteins at the cleavage furrow. Oncogene 19, 6059–6064. [DOI] [PubMed] [Google Scholar]
- Laemmli, U.K. (1970). Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227, 680–685. [DOI] [PubMed] [Google Scholar]
- Leung, T., Chen, X.Q., Manser, E., and Lim, L. (1996). The p160 RhoA-binding kinase ROK alpha is a member of a kinase family and is involved in the reorganization of the cytoskeleton. Mol. Cell. Biol. 16, 5313–5327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madaule, P., Eda, M., Watanabe, N., Fujisawa, K., Matsuoka, T., Bito, H., Ishizaki, T., and Narumiya, S. (1998). Role of citron kinase as a target of the small GTPase Rho in cytokinesis. Nature 394, 491–494. [DOI] [PubMed] [Google Scholar]
- Madaule, P., Furuyashiki, T., Eda, M., Bito, H., Ishizaki, T., and Narumiya, S. (2000). Citron, a Rho target that affects contractility during cytokinesis. Microsc. Res. Tech. 49, 123–126. [DOI] [PubMed] [Google Scholar]
- Matsumura, F., Ono, S., Yamakita, Y., Totsukawa, G., and Yamashiro, S. (1998). Specific localization of serine 19 phosphorylated myosin II during cell locomotion and mitosis of cultured cells. J. Cell Biol. 140, 119–129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murata-Hori, M., Fukuta, Y., Ueda, K., Iwasaki, T., and Hosoya, H. (2001). HeLa ZIP kinase induces diphosphorylation of myosin II regulatory light chain, and reorganization of actin filaments in nonmuscle cells. Oncogene. 20, 8175–8183. [DOI] [PubMed] [Google Scholar]
- Murata-Hori, M., Suizu, F., Iwasaki, T., Kikuchi, A., and Hosoya, H. (1999). ZIP kinase identified as a novel myosin regulatory light chain kinase in HeLa cells. FEBS Lett. 451, 81–84. [DOI] [PubMed] [Google Scholar]
- Nakano, K., Takaishi, K., Kodama, A., Mammoto, A., Shiozaki, H., Monden, M., and Takai, Y. (1999). Distinct actions and cooperative roles of ROCK and mDia in Rho small G protein-induced reorganization of the actin cytoskeleton in Madin-Darby canine kidney cells. Mol. Biol. Cell 10, 2481–2491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perrie, W.T., and Perry, S.V. (1970). An electrophoretic study of the low-molecular-weight components of myosin. Biochem. J. 119, 31–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poperechnaya, A., Varlamova, O., Lin, P.J., Stull, J.T., and Bresnick, A.R. (2000). Localization and activity of myosin light chain kinase isoforms during the cell cycle. J. Cell Biol. 151, 697–708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ridley, A.J. (2001). Rho family proteins: coordinating cell responses. Trends Cell Biol. 11, 471–477. [DOI] [PubMed] [Google Scholar]
- Sakurada, K., Ikuhara, T., Seto, M., and Sasaki, Y. (1994). An antibody for phosphorylated myosin light chain of smooth muscle: application to a biochemical study. J. Biochem. (Tokyo) 115, 18–21. [DOI] [PubMed] [Google Scholar]
- Sakurada, K., Seto, M., and Sasaki, Y. (1998). Dynamics of myosin light chain phosphorylation at Ser19 and Thr19/Ser19 in smooth muscle cells in culture. Am. J. Physiol. (Cell Physiol. 43) 274, c1563–c1572. [DOI] [PubMed] [Google Scholar]
- Sellers, J.R. (1991). Regulation of cytoplasmic and smooth muscle myosin. Curr. Opin. Cell Biol. 3, 98–104. [DOI] [PubMed] [Google Scholar]
- Settleman, J. (2001). Rac'n Rho: the music that shapes a developing embryo. Dev. Cell 1, 321–331. [DOI] [PubMed] [Google Scholar]
- Somlyo, A.P., and Somlyo, A.V. (2000). Signal transduction by G-proteins, rho-kinase and protein phosphatase to smooth muscle and non-muscle myosin II. J. Physiol. 522 (Pt 2), 177–185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Totsukawa, G., Yamakita, Y., Yamashiro, S., Hartshorne, D.J., Sasaki, Y., and Matsumura, F. (2000). Distinct roles of ROCK (Rho-kinase) and MLCK in spatial regulation of MLC phosphorylation for assembly of stress fibers and focal adhesions in 3T3 fibroblasts. J. Cell Biol. 150, 797–806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Umemoto, S., Bengur, A.R., and Sellers, J.R. (1989). Effect of multiple phosphorylations of smooth muscle and cytoplasmic myosins on movement in an in vitro motility assay. J. Biol. Chem. 264, 1431–1436. [PubMed] [Google Scholar]
- Watanabe, N., Kato, T., Fujita, A., Ishizaki, T., and Narumiya, S. (1999). Cooperation between mDia1 and ROCK in Rho-induced actin reorganization. Nat. Cell Biol. 1, 136–143. [DOI] [PubMed] [Google Scholar]
- Yamakita, Y., Yamashiro, S., and Matsumura, F. (1994). In vivo phosphorylation of regulatory light chain of myosin II during mitosis of cultured cells. J. Cell Biol. 124, 129–137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamashiro, S., Yamakita, Y., Ono, S., and Matsumura, F. (1998). Fascin, an actin-bundling protein, induces membrane protrusions and increases cell motility of epithelial cells. Mol. Biol. Cell 9, 993–1006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeng, Q., Lagunoff, D., Masaracchia, R., Goeckeler, Z., Cote, G., and Wysolmerski, R. (2000). Endothelial cell retraction is induced by PAK2 monophosphorylation of myosin II. J. Cell Sci. 113(Pt 3), 471–482. [DOI] [PubMed] [Google Scholar]
- Zhang, W., Vazquez, L., Apperson, M., and Kennedy, M.B. (1999). Citron binds to PSD-95 at glutamatergic synapses on inhibitory neurons in the hippocampus. J. Neurosci. 19, 96–108. [DOI] [PMC free article] [PubMed] [Google Scholar]