Abstract
We previously demonstrated that exogenous expression of a truncated form of the tight junction protein ZO-3 affected junctional complex assembly and function. Current results indicate that this ZO-3 construct influences actin cytoskeleton dynamics more globally. We show that expression of the amino-terminal half of ZO-3 (NZO-3) in Madin-Darby canine kidney cells results in a decreased number of stress fibers and focal adhesions and causes an increased rate of cell migration in a wound healing assay. We also demonstrate that RhoA activity is reduced in NZO-3–expressing cells. We determined that ZO-3 interacts with p120 catenin and AF-6, proteins localized to the junctional complex and implicated in signaling pathways important for cytoskeleton regulation and cell motility. We also provide evidence that NZO-3 interacts directly with the C terminus of ZO-3, and we propose a model where altered interactions between ZO-3 and p120 catenin in NZO-3–expressing cells affect RhoA GTPase activity. This study reveals a potential link between ZO-3 and RhoA-related signaling events.
INTRODUCTION
The tight junction is the structural element of epithelial and endothelial cells that creates a selectively permeable barrier to the free diffusion of solutes, small molecules, and ions through the paracellular pathway. The tight junction is one in a series of intercellular junctions apically located in epithelial and endothelial cells; this tripartite grouping of tight junctions, adherens junctions, and desmosomes is known as the junctional complex.
Coordinated Assembly of Tight Junction and Adherens Junction
A growing body of evidence indicates that the individual junctions within the junctional complex are jointly regulated in assembly and function. These data indicate that the first step in junctional complex formation requires E-cadherin–mediated cell adhesion (Gumbiner et al., 1988), followed by transient ZO-1 localization to the adherens junction before recruitment to the newly forming tight junction (Rajasekaran et al., 1996). However, there is also data to suggest that this hierarchy of junctional complex assembly is not absolute. Balda et al. (1993) observed that treating Madin-Darby canine kidney (MDCK) cells with a diacylglycerol analog in low Ca2+ media induced redistribution of ZO-1, but not E-cadherin, to the junctional membrane. More recently, Troxell et al. (2000) have shown that tight junction assembly is extensive in MDCK cells expressing a mutant E-cadherin protein lacking the extracellular domain required for cell-cell adhesion.
Recent data from our laboratory substantiated the notion of cross-talk between the tight junction and adherens junction (Wittchen et al., 2000). We studied the effects of exogenously expressing the amino-terminal half of the tight junction protein ZO-3 (NZO-3) on tight junction physiology and junctional complex assembly. Expression of this construct in MDCK cells caused a significant delay in transepithelial electrical resistance (TER) recovery in a calcium switch assay, indicating that assembly of tight junction barrier properties was perturbed. Expression of NZO-3 also disrupted the normal recruitment of tight junction proteins and the adherens junction proteins E-cadherin and β-catenin to the plasma membrane during the early stages of junctional complex formation. While many investigators have provided evidence that disruption of adherens junction components negatively regulates the tight junction, these results show that the opposite is also the case: expression of a mutant tight junction protein which disrupts tight junction assembly and physiology can also impede adherens junction assembly.
Actin Cytoskeleton during Junctional Assembly and Barrier Function
We also looked at the effects of NZO-3 expression on the actin cytoskeleton and observed that recruitment of perijunctional F-actin during the junctional assembly process was similarly delayed (Wittchen et al., 2000). It is well established that there is reciprocity between actin filament integrity and junctional physiology. For example, pharmacological perturbation of the F-actin cytoskeleton by cytochalasin D causes a drop in TER across an MDCK cell monolayer (Stevenson and Begg, 1994). Likewise, disassembly of the junctional complex by incubation in calcium-free media disrupts the junction-associated peripheral actin ring (Wittchen et al., 2000).
Dynamic regulation of the actin cytoskeleton is controlled in part by the Rho family of GTPases, members of the Ras superfamily of small GTPases. Remodeling the actin cytoskeleton is critical to cell morphology changes, cytokinesis, substrate adhesion, cell spreading, and migration (Kaibuchi et al., 1999). A key feature of all GTPases is their cyclical activation and deactivation by the binding of GTP followed by the intrinsic GTPase conversion to GDP, thus allowing them to act as molecular switches.
The Rho GTPase family members RhoA, Rac1, and Cdc42 each play a specific role during cytoskeletal reorganization. RhoA controls the formation of stress fibers and focal adhesions based on the observation that microinjection of constitutively active RhoA into fibroblasts induces the formation of stress fibers and focal adhesions (Ridley and Hall, 1992). Furthermore, microinjection of the catalytic domain of Rho-kinase, a downstream effector of RhoA, into fibroblasts also caused the formation of stress fibers and focal adhesions (Amano et al., 1997). In general, the presence of abundant stress fibers and focal adhesions is associated with a nonmotile phenotype characterized by strong cell substratum attachment (Herman et al., 1981; Ridley et al., 1995; Nobes and Hall, 1999). Rac and Cdc42 activities are linked to the formation of membrane protrusions at the leading edge of migrating cells. Rac promotes the formation of lamellipodia, broad actin filament-based extensions of the plasma membrane (Ridley et al., 1992). Cdc42 promotes assembly of filopodia (Nobes and Hall, 1995), actin-based, spike-like protrusions at the cell periphery. Both lamellipodia and filopodia are characteristic components of membrane ruffling, the dynamic movement of plasma membrane at the free edge of cells engaged in cell spreading and migration.
Rho GTPases and Junctional Complex Assembly and Function
There is a growing body of evidence that supports a role for the Rho GTPases in assembly and physiological regulation of the junctional complex in epithelial cells. An early observation that connected tight junction physiology with RhoA activity showed that treatment of epithelial cells with C3 transferase, an inhibitor of RhoA, decreased TER and increased paracellular flux, indicating that tight junction barrier properties were compromised (Nusrat et al., 1995). This inhibition of RhoA also disrupted perijunctional actin formation and caused a redistribution of ZO-1 and occludin away from the cell surface (Nusrat et al., 1995). More recently, Jou et al. (1998) demonstrated that expression of either dominant negative or constitutively active RhoA and Rac in MDCK cells reduced TER and perturbed tight junction fence function, indicated by the unrestricted diffusion of membrane lipids from the apical to the lateral membrane.
The assembly of adherens junctions also seems to involve the RhoA pathway. Inhibition of p160ROCK, a downstream effector of RhoA, prevents movement of E-cadherin, an adherens junction protein, and the tight junction proteins ZO-1 and occludin to the plasma membrane during junctional complex assembly (Walsh et al., 2001). RhoA, Rac1, and Cdc42 have been implicated in promoting cell-cell adhesion (Kuroda et al., 1997; Takaishi et al., 1997), and adherens junction formation seems to require RhoA and Rac1 (Braga et al., 1997; Sander et al., 1999). Furthermore, Rac1 is colocalized with E-cadherin at sites of cell-cell contact during adherens junction assembly and translocates to the cytoplasm if junctions are disrupted by removing calcium (Nakagawa et al., 2001). This group and others also demonstrated that E-cadherin mediated cell-cell adhesion stimulates Rac1 activation (Nakagawa et al., 2001; Noren et al., 2001).
Two proteins that are found at the junctional complex and are linked to signaling pathways involving Ras superfamily GTPases are p120 catenin and AF-6. P120 catenin is known to play a role in cytoskeletal changes linked to both cell junctions and cell motility (Braga, 2000). P120 catenin has dual functions within the cell that directly correlate to its localization. When bound to E-cadherin at the adherens junction, p120 catenin promotes cell adhesion, perhaps by cadherin clustering (Yap et al., 1998). However, increased cytoplasmic p120 catenin causes reduced stress fiber formation and increased cell motility via modulation of the activity of the Rho GTPases (Anastasiadis et al., 2000; Noren et al., 2000). AF-6 is a downstream target of Ras, which also binds to the tight junction protein ZO-1 (Yamamoto et al., 1997).
Because of the close association of actin with the junctional complex of epithelial cells, it is becoming apparent how Rho family members might impact the physiological regulation of fully formed junctions via the actin cytoskeleton. For example, tight junction permeability is thought to be regulated by contraction of the perijunctional actin ring through a contractile force that subtly increases the tension generated between opposing cell surfaces and results in opening of the tight junction (Madara and Pappenheimer, 1987; Hecht et al., 1996). One interesting hypothesis relating to this phenomenon is that rapid cycling of RhoA and Rac between active and inactive forms fine tunes the tension subjected on the peripheral actin ring, thus regulating the degree of tight junction permeability (Jou et al., 1998).
Our previous finding that exogenous expression of a mutant tight junction protein affected actin remodeling during junctional assembly (Wittchen et al., 2000) led to further investigation of the actin cytoskeleton in NZO-3–expressing MDCK cells. The results presented herein identify a more global alteration of actin dynamics in these cells. We show that expression of NZO-3 decreases the number of stress fibers and focal adhesions and increases the rate of cell migration in a wound healing assay. We have identified a potential molecular mechanism underlying these changes and provide evidence for the involvement of RhoA and p120 catenin. The information obtained from these studies sheds light on the mechanisms of cytoskeleton organization by the Rho family of GTPases during junctional assembly and wound healing, and how tight junction elements might influence these processes.
MATERIALS AND METHODS
Cell Lines
Parental MDCK (untransfected), NZO-3/MDCK, CZO-3/MDCK, and FLZO-3/MDCK cell lines have been described previously (Haskins et al., 1998; Wittchen et al., 1999, 2000). Immunofluorescence experiments, wound healing assays, and RhoA activity assays were repeated with two independent NZO-3/MDCK cell lines (clones D5 and B6), and two independent CZO-3/MDCK cell lines (data from one line are shown).
Immunohistochemistry
For staining of subconfluent monolayers, MDCK cell lines were plated on collagen-coated coverslips and allowed to grow until they reached a density of ∼50%. Cells were fixed and permeabilized using 2.5% paraformaldehyde on ice for 30 min followed by incubation with 0.2% Triton X-100/Tris-buffered saline (TBS)+ for 5 min at room temperature. The coverslips were blocked for 15 min at room temperature in TBS+/BLOTTO (5% skim milk powder in TBS + Ca2+ and Mg2+), and costained with anti-vinculin antibody (Zymed Laboratories, South San Francisco, CA) and fluorescein isothiocyanate (FITC)-phalloidin (Sigma-Aldrich, St. Louis, MO) to detect actin filaments. Vinculin localization was visualized with rhodamine anti-mouse secondary antibodies (Jackson Immunoresearch Laboratories, West Grove, PA). After washing 3 × 5 min with TBS + Ca2+ and Mg2+, coverslips were mounted on glass slides by using an antibleaching mounting media (VectaShield; Vector Laboratories, Burlingame, CA), and viewed with an Axioskop fluorescence microscope (Carl Zeiss, Thornwood, NY). Wounded monolayers were fixed 3 h postwounding as described above and stained with rhodamine-phalloidin (Sigma-Aldrich) to detect actin filaments. Images of wounded monolayers were collected and processed identically; representative images shown.
Wound Healing Assays
In vitro wound healing assays were used to assess cell migration. Cells were plated at varying densities, and dishes that had just reached confluence were used. Two linear wounds were scratched in each dish of cells with a p200 pipet tip. Using a phase contrast microscope attached to an MD2 microscope digitizer (Accustage, Minneapolis, MN) to measure stage position, the width of the wound at two different points was measured over a 6-h time period. The average migration rate was calculated by taking the total distance migrated (in micrometers) divided by the total time (in hours). Data were plotted as relative migration rate with the value for parental MDCK cells arbitrarily set to 1 for comparison between experiments. For each cell line, two different wounds were made per dish, and width was measured at two points per wound (n = 4). Data shown are representative of three to six independent experiments.
RhoA Activity Assays
We performed Rho activity assays as described previously (Ren et al., 1999; Noren et al., 2000) with minor modifications. The glutathione S-transferase (GST)-Rho-binding domain (RBD) construct (amino acids 7–89 from rhotekin) was kindly provided by Dr. Keith Burridge (University of North Carolina, Chapel Hill, NC). Cells were serum-starved overnight before performing RhoA activity assays because serum activation of RhoA potentially masks small but physiologically relevant differences in activity (Noren et al., 2001). An ∼80% confluent 10-cm dish of cells was washed in ice-cold HEPES-buffered saline and lysed by scraping in 300 μl of RIPA buffer (50 mM Tris, pH 7.2, 1% Triton X-100, 0.5% sodium deoxycholate, 0.1% SDS, 500 mM NaCl, 10 mM MgCl2, and protease inhibitors [1 μg/ml aprotinin, 1 μg/ml chymostatin, 1 μg/ml leu-peptin, 1 μg/ml pepstatin, and 1 mM Pefabloc SC; Roche Diagnostics, Indianapolis, IN]). Lysates were clarified by centrifugation at 12,000 × g at 4°C for 5 min, and equal volumes of lysates were incubated with 30 μg of GST-RBD beads at 4°C with rotation for 30 min. An aliquot of lysate was reserved for analysis of total RhoA. Beads were washed four times with 1 ml of buffer B (TBS + 1% Triton X-100, 150 mM NaCl, 10 mM MgCl2, and protease inhibitors). The bound fraction (active RhoA) was analyzed by resuspending the beads in 2× gel sample buffer, boiling 5 min, and running on SDS-PAGE. Active RhoA (bound fraction) and total RhoA were analyzed by Western blotting with an anti-RhoA antibody (monoclonal antibody 26C4; Santa Cruz Biotechnology, Santa Cruz, CA). The results were quantified by densitometry of multiple Western blots from four independent experiments. RhoA activity was determined by determining the ratio of the amount of RhoA sedimented by the GST-RBD beads to the total amount of RhoA in the whole cell lysate (active/total) to compare activity of RhoA from different samples.
GST Pull-Down Assays
GST fusion proteins were expressed and purified as described previously (Haskins et al., 1998; Wittchen et al., 1999). Equivalent amounts (estimated by Coomassie Blue staining) of GST-FLZO-3, GST-NZO-3, GST-CZO3, or GST alone were bound to glutathione-Sepharose beads. MDCK cells were lysed in RIPA buffer, and an equal amount of lysate was added to each batch of affinity resin. Batch binding was carried out overnight at 4°C with rotation. Beads were washed 4× with buffer B plus protease inhibitors, resuspended in 2× gel sample buffer, boiled 5 min, and loaded for SDS-PAGE. Blots were incubated with the following antibodies: anti-RhoA (monoclonal antibody 26C4; Santa Cruz Biotechnology), anti-cdc42 (catalog no. sc-87; Santa Cruz Biotechnology), anti-Rac1 (catalog no. R56220; Transduction Laboratories, Lexington, KY), anti-p120catenin (catalog no. P17920; Transduction Laboratories), or anti-AF-6 (catalog no. A60520; Transduction Laboratories).
Direct Binding Experiments
Human p120-3ABC in GST expression vector pGEX5–1 was kindly provided by Dr. Frans van Roy and Dr. J van Hengel (University of Ghent, Ghent, Belgium) (van Hengel et al., 1999). Equivalent amounts of GST and GST-p120 were immobilized on glutathione-Sepharose beads. Histidine-tagged CZO-3 was expressed in Sf9 insect cells by using a baculovirus eukaryotic expression system (Invitrogen, Carlsbad, CA) and purified as described previously (Haskins et al., 1998; Wittchen et al., 1999). Eluted CZO-3 was diluted 1:20 in RIPA buffer, added to GST and GST-p120 beads, and allowed to bind 1 h at 4°C with rotation. Beads were washed 4× with buffer B plus protease inhibitors, resuspended in 2× GSB, and boiled for 5 min before loading for SDS-PAGE. CZO-3 retained by GST-p120 beads was detected by blotting with a polyclonal antibody raised against amino acids 754–898 in the C terminus of ZO-3 (rab5F3 anti-FP2).
For binding of NZO-3 to CZO-3, equivalent amounts (estimated by Coomassie Blue staining) of GST and GST-NZO-3 were immobilized on glutathione-Sepharose beads followed by the addition of histidine-tagged CZO-3. Binding, processing of beads, and detection of bound CZO-3 were carried out as described above.
RESULTS
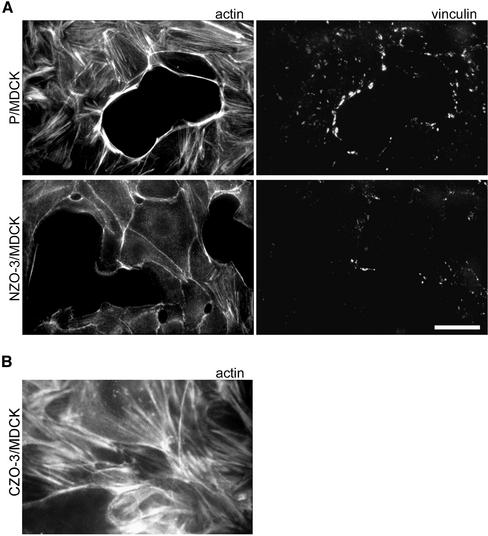
Our previous observation that NZO-3 expression delayed actin recruitment to the perijunctional membrane during junctional complex assembly (Wittchen et al., 2000) prompted us to look at other aspects of actin dynamics in NZO-3–expressing cells. We first noted that NZO-3/MDCK cells contained fewer actin stress fibers than parental untransfected cells or CZO-3–expressing cells. Figure 1A shows NZO-3/MDCK cells and parental MDCK cells plated at subconfluent density stained with rhodamine-phalloidin to visualize F-actin. Thick actin stress fiber bundles are abundant in parental cells, whereas NZO-3/MDCK cells present an almost complete absence of basally located stress fibers. In addition, the limited stress fibers present in NZO-3/MDCK cells are thinner. The perijunctional apical actin ring of NZO-3/MDCK cells is normal compared with parental untransfected cells. Costaining the same cells for vinculin as a marker for focal adhesions revealed that NZO-3/MDCK cells also had fewer and smaller focal adhesions (Figure 1A). Cells expressing CZO-3 contained abundant stress fibers and seemed identical to parental untransfected cells (Figure 1B).
Figure 1.
NZO-3/MDCK cells have fewer stress fibers and focal adhesions than untransfected parental MDCK cells. (A) Parental MDCK (P/MDCK) and NZO-3/MDCK cells were grown to subconfluence and then costained with FITC-phalloidin to visualize F-actin, and anti-vinculin antibodies (rhodamine) as a marker for focal adhesions. NZO-3–expressing cells show a striking decrease in the number of stress fibers and a decrease in number and size of focal adhesions. Bar, 15 μm. (B) CZO-3/MDCK cells stained with FITC-phalloidin to visualize F-actin. These cells show the presence of stress fibers similar to parental MDCK cells.
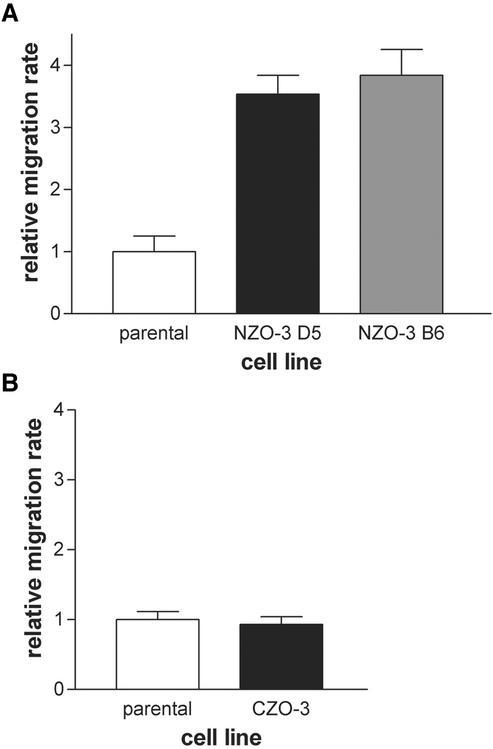
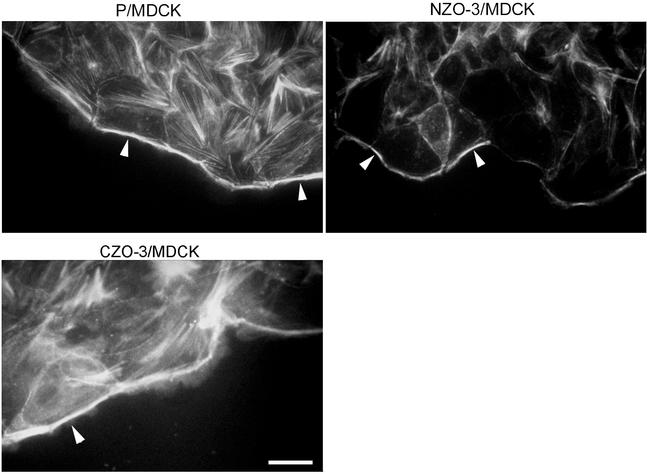
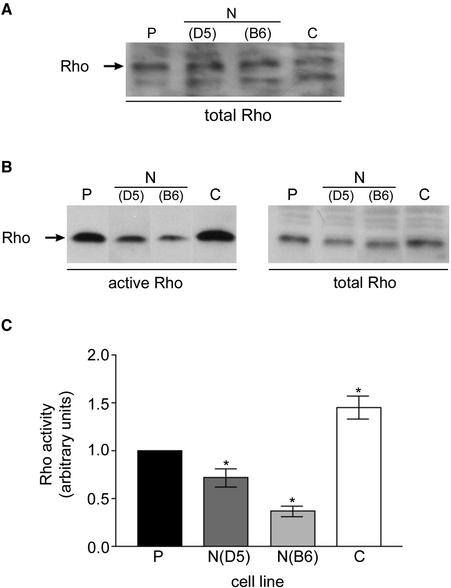
Based on this reduced stress fiber and focal adhesion phenotype, we hypothesized that NZO-3/MDCK cells would migrate faster than parental cells due to weaker attachment to the substratum. Many studies have demonstrated an inverse correlation between the presence of actin stress fibers and increased motility (Herman et al., 1981; Ridley et al., 1995; Nobes and Hall, 1999). We performed in vitro wound healing assays to test our hypothesis. Cell monolayers that had just reached confluence were scratched with a pipet tip to create a linear wound, and the width of the wound at specific locations was measured over a 6-h period. The relative migration rate for two independent NZO-3/MDCK cell lines (B6 and D5) was compared with parental untransfected cells, and we found that both NZO-3–expressing cell lines migrated approximately fourfold faster than parental MDCK cells (Figure 2A). In separate experiments, migration rate of CZO-3/MDCK cells was not significantly different from parental MDCK cells (Figure 2B). When wounded monolayers were stained with phalloidin to visualize actin, not only are the number of stress fibers reduced, as observed in Figure 1, but also there is less F-actin condensed at the wound-facing leading edge of NZO-3/MDCK cells compared with parental cells (Figure 3). CZO-3/MDCK cells showed a similar amount of F-actin at the leading edge to parental MDCK cells (Figure 3). These results identify a global effect on the actin cytoskeleton caused by NZO-3 expression in MDCK cells. Pursuing a possible mechanism through which actin cytoskeleton dynamics is affected, we hypothesized that the Rho family of GTPases might be involved. Given that strong evidence exists that RhoA activity is responsible for the formation of stress fibers and focal adhesions (Ridley and Hall, 1992), we asked whether RhoA activity was decreased in NZO-3–expressing MDCK cells. When loaded for equivalent amount of total protein, we observed no difference in the total amount of RhoA protein from whole cell lysates of NZO-3/MDCK cells compared with parental and CZO-3/MDCK cells (Figure 4A). To determine the level of Rho activity, we used an affinity precipitation technique that specifically pulls out active (GTP-bound) RhoA from the total RhoA pool. A representative Western blot of the active RhoA fraction versus the total RhoA from the same lysate is shown in Figure 4B. These results were quantified by densitometry, and the relative activity of RhoA was plotted as a ratio of active RhoA relative to the total amount of RhoA in each sample (Figure 4C). Both NZO-3/MDCK cell lines had a significantly lower amount of active RhoA compared with parental cells (p < 0.05). CZO-3/MDCK cells had significantly higher RhoA activity compared with parental cells; multiple exposures of the Western blot were analyzed to confirm this observation.
Figure 2.
NZO-3/MDCK cells migrate faster than untransfected parental MDCK and CZO-3/MDCK cells in an in vitro wound healing assay. (A) Cell monolayers were scratched with a pipet tip, and wound width was measured over a 6-h period for two separate stable NZO-3–expressing cell lines (D5 and B6) and parental MDCK cells. Chart is representative of six independent experiments. (B) In separate experiments migration rate was compared between parental MDCK cells and CZO-3/MDCK cells (representative of three experiments).
Figure 3.
NZO-3/MDCK cells have reduced F-actin staining at the free edge of the wound. Wounded monolayers were stained with rhodamine-phalloidin to visualize F-actin. Parental MDCK cells and CZO-3/MDCK cells show intense F-actin staining at free edge of cells adjacent to the wound (arrowheads). The amount of F-actin at the wound edge is less in MDCK/NZO-3 cells (arrowheads). Bar, 15 μm.
Figure 4.
RhoA activity is reduced in NZO-3/MDCK compared with untransfected parental cells and CZO-3/MDCK cells. (A) Equivalent amounts of total cell protein were loaded in each lane, and the resultant Western blot was probed with anti-RhoA antibodies. There is no significant difference in the amount of total RhoA protein in each cell line. (B) RhoA activity was assayed by separating active from inactive RhoA. A representative immunoblot of active RhoA retained on GST-RBD affinity beads (left) and total lysate (right; samples are not loaded for equivalent protein) is shown. (C) Rho activity (active Rho/total Rho) for each sample was quantitatively compared using data from four independent experiments (n = 4) and plotted ± SEM Rho activity in parental MDCK cells was arbitrarily set to a value of 1.0. Two independent MDCK/NZO-3 cell lines (D5 and B6) had significantly lower Rho activity (p ≤ 0.05) compared with parental MDCK cells. CZO-3/MDCK cells had significantly higher Rho activity compared with parental MDCK cells.
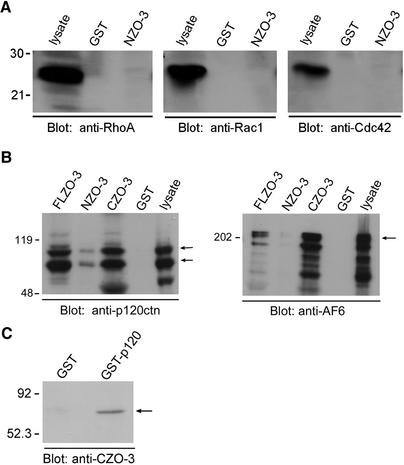
We next performed binding experiments from whole cell lysates to attempt to identify a physical link between the RhoA signaling pathway and the tight junction protein ZO-3. We first looked at whether NZO-3 interacts with any members of the Rho family of GTPases. Using NZO-3 expressed as a GST fusion protein, we performed GST pull-down experiments with MDCK whole cell lysates, and probed the bound fraction with antibodies to RhoA, Rac1, or Cdc42. Figure 5A shows that NZO-3 does not interact with Rho, Rac, or Cdc42 in these assays. However, we did identify two novel protein interactions by using this GST pull-down technique. P120 catenin and AF-6 are proteins that have been localized to the junctional complex and linked to signaling pathways implicated in cytoskeleton regulation and cell motility. Therefore, we determined whether they interacted with any of the ZO-3 constructs. Equivalent amounts (estimated by Coomassie Blue staining) of GST alone, FLZO-3, NZO-3, or CZO-3-GST fusion were bound to glutathione-Sepharose beads and incubated with an equal amount of MDCK cell lysate. Immunoblotting the bound fraction with an anti-p120 catenin antibody revealed that p120 catenin binds to both FLZO-3 and CZO-3, with a low level of binding to NZO-3 (Figure 5B). We also determined whether any of these ZO-3 constructs could bind to the Ras-effector protein AF-6 (Yamamoto et al., 1997). We discovered that the FLZO-3 construct binds to AF-6 and that the binding region is in the C terminus, because AF-6 was retained by the CZO-3 beads but not the NZO-3 beads (Figure 5B). In addition to the GST pull-down experiments showing binding of CZO-3 to p120 catenin and AF-6, we confirmed that the interaction between p120 catenin and CZO-3 was direct by performing direct binding assays by using purified proteins. We found that purified histidine-tagged CZO-3 was retained specifically on affinity resin containing p120 catenin, and not on beads with GST alone (Figure 5C).
Figure 5.
NZO-3 does not interact with RhoA, Rac, or Cdc42 from MDCK cell lysates; CZO-3 binds AF-6 and p120 catenin. (A) MDCK cell lysate was added to affinity columns containing immobilized GST-NZO-3 or GST alone, and the bound fractions were immunoblotted with anti-RhoA, anti-Rac, and anti-Cdc42 antibodies. Presence of each GTPase in the lysate was confirmed by immunoblotting an aliquot of lysate. None of the GTPases tested were retained by NZO-3 or GST alone. (B) Equivalent amounts of full-length ZO-3 (FLZO-3), NZO-3, CZO-3 or GST alone immobilized on glutathione-Sepharose were incubated with MDCK lysate. The bound fraction was immunoblotted with anti-p120 catenin or anti-AF-6 antibodies. Both p120 catenin and AF-6 bind to FLZO-3 and CZO-3, with negligible binding to NZO-3. (C) Equivalent amounts of GST or GST-p120 were immobilized on glutathione-Sepharose beads; purified 6-histidine–tagged CZO-3 was then added to each affinity column. The bound fractions were immunoblotted with an antibody that recognizes the C terminus of ZO-3. CZO-3 is specifically retained on GST-p120 beads, and not to GST alone.
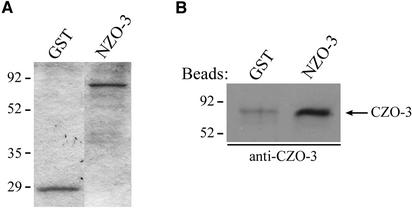
The question of how exogenous expression of NZO-3 alters the activity of RhoA despite the fact that it is the C-terminal half of ZO-3 that binds p120 catenin and AF-6 might be answered by the presence of intramolecular interactions within ZO-3. These intramolecular interactions may subsequently regulate intermolecular interactions of ZO-3. To address this possibility, we determined whether the amino-terminal half of ZO-3 can interact with the C-terminal half. This interaction could theoretically occur via the C-terminal SH3 domain and a consensus PXXP binding motif (Ren et al., 1993) found in the proline-rich region of the N-terminal half of the molecule. Equal amounts of purified GST and NZO-3/GST were immobilized on glutathione-Sepharose beads (Figure 6A), followed by the addition of purified histidine-tagged CZO-3. The bound fraction was immunoblotted with an antibody specific for the C terminus of ZO-3. Figure 6B shows that CZO-3 binds directly to NZO-3 substantially above background.
Figure 6.
NZO-3 binds directly to CZO-3. (A) Coomassie Blue-stained gel showing equal amounts of GST and GST-NZO-3 present on the beads. (B) After the addition of purified histidine-tagged CZO-3 to GST and GST-NZO-3 beads, the bound fraction was probed with an antibody specific for the C-terminal half of ZO-3. CZO-3 is retained on the NZO-3–containing beads. A small amount of nonspecific binding occurs with GST alone.
DISCUSSION
In this study, we expanded our previous findings on the effects of exogenously expressing NZO-3 in MDCK cells. We previously found that expression of this construct delays assembly of the junctional complex, including the recruitment of tight and adherens junction proteins and F-actin to the forming junctional membrane (Wittchen et al., 2000). Herein, we show more global effects on F-actin dynamics in NZO-3–expressing cells. NZO-3/MDCK cells have fewer actin stress fibers and fewer and smaller focal adhesions than untransfected parental cells or cells transfected with the C-terminal half of ZO-3. NZO-3–expressing cells also migrate faster than parental MDCK cells in a wound healing assay. Because Rho GTPases are known to be important regulators of actin cytoskeletal dynamics, we explored the potential relationships between NZO-3 expression and Rho GTPase activity and found that NZO-3–expressing cells have lower RhoA activity compared with parental MDCK cells and CZO-3/MDCK cells. We further investigated the binding interactions of the ZO-3 protein constructs and found that although ZO-3 does not seem to interact with RhoA itself, CZO-3 interacts with p120 catenin as well as the Ras target protein AF-6 from MDCK whole cell lysates.
NZO-3 Expression Affects the Actin Cytoskeleton and Increases Cell Migration
Our observation that NZO-3/MDCK cells have fewer stress fibers compared with parental untransfected MDCK cells (Figure 1) initiated this investigation. That this was observed in multiple clonal cell lines expressing NZO-3 and that the stress fibers of CZO-3/MDCK cells are indistinguishable from parental cells indicates that the observation in NZO-3/MDCK cells is specific. Because the actin cytoskeleton is tethered to focal adhesions via actin stress fiber bundles, it is not surprising that the number and size of focal adhesions are also diminished in NZO-3/MDCK cells (Figure 1). The meaning of the result that NZO-3/MDCK cells have less actin staining at the free edge of the wound compared with parental MDCK cells (Figure 3) is unclear, because migrating cells generally have a prominent F-actin condensation in the leading edge lamellipodia (Waterman-Storer et al., 1999). It could be that although there is less F-actin visible at the leading edge in NZO-3/MDCK cells, F-actin that is present may be more dynamic with a more rapid turnover and less availability for binding phalloidin. Membrane ruffling activity at the leading edge is controlled by the activities of Rac1 and Cdc42; however, we were unable to detect consistent differences in the activity of these GTPases in NZO-3/MDCK cells versus parental MDCK cells.
The phenotype of fewer actin stress fibers and focal adhesions suggested that NZO-3/MDCK cells might be more readily motile. There is a well-established correlation between cell motility and reduced actin stress fibers and focal adhesions; these F-actin structures are typically considered characteristics of a nonmotile state (Herman et al., 1981; Ridley et al., 1995; Nobes and Hall, 1999). Wound healing assays are one way of measuring the migratory behavior of cells. During wound closure cells migrate to fill the empty region, either in connection with each other, or in some cases individual cells break off to migrate individually. The former condition typifies the migration of the MDCK epithelial cells observed in this study, where there is coordinated migration of a cell sheet in which cell-cell interactions are maintained. We observed that NZO-3/MDCK cells migrated faster than control MDCK cells, correlating well with the reduced amount of actin stress fibers and focal adhesions.
Involvement of the Rho Family of GTPases
Because the Rho GTPases are classical mediators of actin cytoskeleton remodeling during events such as cell migration, it was of interest to determine whether there were differences in their activity in the NZO-3–expressing cells. Specifically, we looked at RhoA activity because of its well-established role in stress fiber and focal adhesion formation. We found that RhoA activity was lower in NZO-3/MDCK cells compared with parental MDCK cells and CZO-3/MDCK cells (Figure 4B). Two independent NZO-3/MDCK cell lines were used to confirm this observation. It is interesting to note that CZO-3/MDCK cells had significantly higher RhoA activity compared with parental cells, even though there was no difference in the number or thickness of stress fibers or the rate of cell migration during wound closure (Figures 1B and 2B). One explanation is that there is a maximum threshold level of RhoA activation; activity exceeding this will not have further additive effects on stress fibers or migration behavior. Alternatively, negative feedback mechanisms may exist to counteract the phenotype produced by increased RhoA signaling.
Linking ZO-3 with RhoA Signaling Pathway: Binding Interactions
Identifying novel binding partners for ZO-3 in general will provide information about its cellular function, and in context of the observations presented herein, this can provide information on the molecular mechanism underlying the phenotypes we observe in NZO-3/MDCK cells. One potential mechanism by which NZO-3 expression could decrease the activity of RhoA is by interacting with the GTPase and either inhibiting its activity directly, or indirectly, by preventing it from binding to its guanine nucleotide exchange factors (GEFs). We tested whether NZO-3 could bind to RhoA from MDCK whole cell lysate and could not detect an interaction (Figure 5A). It is also possible that the NZO-3 interaction with the RhoA GTPases is transient or with such low affinity that binding is undetectable in the in vitro binding assays performed. Alternatively, NZO-3 might interact with a Rho GDP-dissociation inhibitor, keeping RhoA in an inactive (GDP-bound) state; we tested this by using GST pull-down experiments and found that NZO-3 does not interact with Rho GDI (our unpublished data).
However, by performing in vitro binding assays, we found that FLZO-3 and CZO-3, but not NZO-3, interact with p120 catenin from MDCK lysates (Figure 5B). This is the first demonstration of a tight junction protein interacting with p120 catenin, although both ZO-1 and ZO-2 interact with α-catenin (Itoh et al., 1997, 1999). The dual roles of p120 catenin in cell-cell adhesion and cell migration make it an ideal candidate to link tight junction elements and RhoA signaling in the NZO-3/MDCK cell line. Work by Noren et al. (2000) has shown that cytoplasmic p120 catenin binds to Vav-2, a GEF activator of Rac and Cdc42, suggesting a link between p120 catenin and the Rho GTPase family proteins. Furthermore, they have shown that increasing the soluble pool of p120 catenin results in disassembly of focal adhesions and stress fibers. This overexpression of p120 catenin causes increased cell motility, with correspondingly decreased RhoA activity and increased Rac and Cdc42 activity (Noren et al., 2000; Grosheva et al., 2001). We compared by immunoblot the relative levels of cytoplasmic versus membrane-associated p120 in parental MDCK cells and cells expressing NZO-3 or CZO-3. Using several methods of fractionation, we found that ∼90% of total cellular p120 is membrane associated and ∼10% is soluble in both cell lines (our unpublished data). It is possible that the difference in cytoplasmic p120 levels is too small to detect by our methods yet is still enough to cause a phenotypic effect. Other investigators have confirmed that there is a direct link between p120 catenin and RhoA by showing in vitro that p120 catenin can directly inhibit RhoA activation; preventing GDP dissociation from RhoA by binding to Rho-GDP and acting to sequester it from its activating GEF (Anastasiadis et al., 2000).
We also found that FLZO-3 and CZO-3 interact with the Ras target AF-6 (Figure 5B). AF-6 is known to bind to another tight junction protein (ZO-1), via its Ras-binding domain, and activated Ras can compete with ZO-1 for binding to AF-6 (Yamamoto et al., 1997). When epithelial cells are transformed with Ras, they acquire a fibroblastic, depolarized phenotype (Schoenenberger et al., 1991). In conjunction with this, a correlation between Ras activation and junctional complex disruption has been established (Mercer, 2000). In Ras-transformed MDCK cells, TER is low, indicating disrupted barrier properties, and occludin, claudin-1, and ZO-1 are not localized to the cell membrane at tight junctions. TER and localization of occludin, claudin-1, and ZO-1 to tight junctions could be restored by treating Ras-transformed cells with the mitogen-activated protein kinase kinase inhibitor PD98059 (Chen et al., 2000). Our results provide more evidence for a linkage between the Ras signaling pathway and junctional complex regulation via the interaction of AF-6 and ZO-3. That AF-6 and p120 catenin have both been localized to the junctional complex and have been linked to signaling pathways implicated in cytoskeleton regulation and cell motility underscores the potential significance of their binding to ZO-3 and involvement in the phenotype observed in the NZO-3–expressing cells.
Possible Mechanism to Link NZO-3 Expression to Rho Pathway
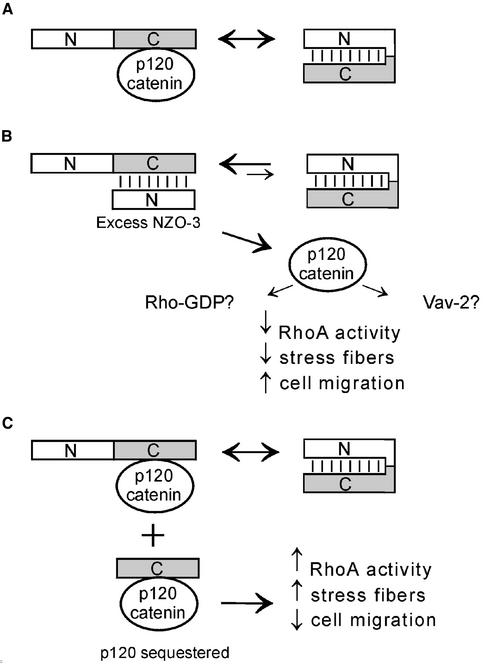
A reasonable model that explains how exogenous expression of NZO-3 alters the activity of RhoA, whereas it is the C-terminal half of ZO-3 that binds p120 catenin and AF-6, is not immediately intuitive. However, intramolecular interactions within ZO-3 could explain regulatory interactions involving ZO-3 and p120 catenin. In parental MDCK cells (Figure 7A), endogenous ZO-3 could exist in two states: a “closed” conformation where the N-terminal half binds to the C-terminal half, or an “open” state that allows for interaction with other partners such as p120 catenin. However, in NZO-3/MDCK cells excess exogenous NZO-3 could inhibit the binding of other proteins, including p120 catenin, to the C-terminal half of endogenous ZO-3 (Figure 7B). P120 catenin would then available to inhibit RhoA signaling, either indirectly via Vav-2 activation (Noren et al., 2000), or directly, by sequestering RhoA from its activating GEFs (Anastasiadis et al., 2000). Based on previous results (Noren et al., 2000; Grosheva et al., 2001), the end result of this p120-mediated decrease in RhoA activity is a decrease in the amount of stress fibers and increased motility, similar to what we observed in this study. Finally, in the case of CZO-3/MDCK cells (Figure 7C), p120 catenin could either bind to the C terminus of endogenous ZO-3 or to the exogenous CZO-3 fragment. Binding of p120 catenin to the CZO-3 fragment could sequester p120 catenin and prevent it from interacting with other binding partners (e.g., Vav2 and Rho-GDP) in the cytoplasm, providing an explanation for the relative increase in RhoA activity observed in CZO-3/MDCK cells (Figure 4B). The model in Figure 7 suggests a mechanism whereby conformational changes of native ZO-3 may be used in situ to regulate cytoskeletal dynamics important for normal junctional complex physiology and cellular events such as migration.
Figure 7.
Hypothetical model for RhoA inhibition in NZO-3/MDCK cells. (A) Parental MDCK cells: The C terminus of endogenous ZO-3 could bind to p120 catenin, or to the N terminus of the same molecule (intramolecular interaction). (B) NZO-3/MDCK cells: Exogenously expressed NZO-3 competes with p120 catenin for binding to the C terminus of endogenous ZO-3. P120 catenin is then available to interact with other proteins such as Vav-2 or Rho-GDP, which can down-regulate RhoA. (C) CZO-3/MDCK cells: p120 catenin could bind to either the C terminus of endogenous ZO-3 or to the CZO-3 construct, thus sequestering it from binding to other effectors and resulting in up-regulation of RhoA.
This hypothetical mechanism involving regulation of protein function via intramolecular interactions is supported by previous studies with other proteins. For example, ezrin (an ERM family protein) exists in two conformationally distinct states: a “dormant” state, which is a closed conformation where the N terminus of the protein binds to the C terminus in a head-to-tail manner; and an “active” state that opens up the protein and exposes otherwise masked binding sites to allow other intermolecular binding interactions to take place (Bretscher et al., 2000). In the case of ezrin, the active state is able to interact with the membrane via its N terminus and the cytoskeleton via its C terminus (Bretscher et al., 1997). Another example of intramolecular regulatory interactions is found in vinculin. In this case, the loss of the intramolecular interaction of the head domain and tail domain accounts for the increased affinity for talin of the head domain compared with intact vinculin (Johnson and Craig, 1994). Disruption of the head–tail interaction also reveals an otherwise hidden F-actin binding site in the tail region of vinculin (Johnson and Craig, 1995). Finally, SAP97 (a membrane-associated guanylate kinase family protein like ZO-3), has an N-terminal domain that interacts with the SH3 and GUK domain of the same molecule, thus altering other protein–protein interactions (Wu et al., 2000).
In summary, our results indicate a novel link between the tight junction protein ZO-3 and cell signaling pathways regulating the actin cytoskeleton and migration of epithelial cells. Expression of the N-terminal half of ZO-3 caused a noticeable decrease in the amount of stress fibers and fewer and smaller focal adhesions. This phenotype was accompanied by an increased migratory ability of these cells. We correlated this actin phenotype and increased migration with decreased RhoA activity in NZO-3/MDCK cells. We also show that the C-terminal half of ZO-3 binds to both p120 catenin and AF-6, providing a mechanistic link between ZO-3 and the observed phenotype. Finally, we provide direct binding evidence that NZO-3 can interact with the C terminus of ZO-3, and we hypothesize a model where altered binding interactions involving ZO-3, p120 catenin, and possibly AF-6 in NZO-3–expressing cells negatively influence RhoA GTPase activity. This study reveals a potential link between the tight junction protein ZO-3 and Rho GTPase-related signaling events.
Acknowledgments
We thank Keith Burridge, Moira Glerum, Lijie Gu, Tom Hobman, Paul Melançon, Nikki Noren, Dave Pilgrim, and Cal Roskelley for providing reagents and/or advice. This work was supported by grants from the Canadian Institutes of Health Research, the Kidney Foundation of Canada, and the Canadian Association of Gastroenterology/Janssen-Ortho. E.S.W. was supported by a Canadian Institutes of Health Research studentship and Alberta Heritage Foundation for Medical Research Studentship award.
Article published online ahead of print. Mol. Biol. Cell 10.1091/mbc.E02-08-0486. Article and publication date are at www.molbiolcell.org/cgi/doi/10.1091/mbc.E02-08-0486.
References
- Amano, M., Chihara, K., Kimura, K., Fukata, Y., Nakamura, N., Matsuura, Y., and Kaibuchi, K. (1997). Formation of actin stress fibers and focal adhesions enhanced by Rho-kinase. Science 275, 1308–1311. [DOI] [PubMed] [Google Scholar]
- Anastasiadis, P.Z., Moon, S.Y., Thoreson, M.A., Mariner, D.J., Crawford, H.C., Zheng, Y., and Reynolds, A.B. (2000). Inhibition of RhoA by p120 catenin. Nat. Cell Biol. 2, 637–644. [DOI] [PubMed] [Google Scholar]
- Balda, M.S., Gonzalez-Mariscal, L., Matter, K., Cereijido, M., and Anderson, J.M. (1993). Assembly of the tight junction: the role of diacylglycerol. J. Cell Biol. 123, 293–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braga, V. (2000). The crossroads between cell-cell adhesion and motility. Nat. Cell Biol. 2, E182–E184. [DOI] [PubMed] [Google Scholar]
- Braga, VM., Machesky, L.M., Hall, A., and Hotchin, N.A. (1997). The small GTPases Rho and Rac are required for the establishment of cadherin-dependent cell-cell contacts. J. Cell Biol. 137, 1421–1431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bretscher, A., Reczek, D., and Berryman, M. (1997). Ezrin: a protein requiring conformational activation to link microfilaments to the plasma membrane in the assembly of cell surface structures. J. Cell Sci. 110, 3011–3018. [DOI] [PubMed] [Google Scholar]
- Bretscher, A., Chambers, D., Nguyen, R., and Reczek, D. (2000). ERM-Merlin and EBP50 protein families in plasma membrane organization and function. Annu. Rev. Cell Dev. Biol. 16, 113–143. [DOI] [PubMed] [Google Scholar]
- Chen, Y., Lu, Q., Schneeberger, E.E., and Goodenough, D.A. (2000). Restoration of tight junction structure and barrier function by down-regulation of the mitogen-activated protein kinase pathway in ras-transformed Madin-Darby canine kidney cells. Mol. Biol. Cell 11, 849–862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grosheva, I, Shtutman, M., Elbaum, M., and Bershadsky, A.D. (2001). p120 catenin affects cell motility via modulation of activity of Rho-family GTPases: a link between cell-cell contact formation and regulation of cell locomotion. J. Cell Sci. 114, 695–707. [DOI] [PubMed] [Google Scholar]
- Gumbiner, B., Stevenson, B.R., and Grimaldi, A. (1988). The role of the cell adhesion molecule uvomorulin in the formation and maintenance of the epithelial junctional complex. J. Cell Biol. 107, 1575–1587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haskins, J., Gu, L., Wittchen, E.S., Hibbard, J., and Stevenson, B.R. (1998). ZO-3, a novel member of the MAGUK protein family found at the tight junction, interacts with ZO-1 and occludin. J. Cell Biol. 141, 199–208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hecht, G., Pestic, L., Nikcevic, G., Koutsouris, A., Tripuraneni, J., Lorimer, D.D., Nowak, G., Guerriero, V., Jr., Elson, E.L., and Lanerolle, P.D. (1996). Expression of the catalytic domain of myosin light chain kinase increases paracellular permeability. Am. J. Physiol. 271, C1678–C1684. [DOI] [PubMed] [Google Scholar]
- Herman, I.M., Crisona, N.J., and Pollard, T.D. (1981). Relation between cell activity and the distribution of cytoplasmic actin and myosin. J. Cell Biol. 90, 84–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Itoh, M., Nagafuchi, A., Moroi, S., and Tsukita, S. (1997). Involvement of ZO-1 in cadherin-based cell adhesion through its direct binding to alpha catenin and actin filaments. J. Cell Biol. 138, 181–192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Itoh, M., Morita, K., and Tsukita, S. (1999). Characterization of ZO-2 as a MAGUK family member associated with tight as well as adherens junctions with a binding affinity to occludin and alpha catenin. J. Biol. Chem. 274, 5981–5986. [DOI] [PubMed] [Google Scholar]
- Johnson, R.P., and Craig, S.W. (1994). An intramolecular association between the head and tail domains of vinculin modulates talin binding. J. Biol. Chem. 269, 12611–12619. [PubMed] [Google Scholar]
- Johnson, R.P., and Craig, S.W. (1995). F-actin binding site masked by the intramolecular association of vinculin head and tail domains. Nature 373, 261–264. [DOI] [PubMed] [Google Scholar]
- Jou, T.S., Schneeberger, E.E., and Nelson, W.J. (1998). Structural and functional regulation of tight junctions by RhoA and Rac1 small GTPases. J. Cell Biol. 142, 101–115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaibuchi, K., Kuroda, S., and Amano, M. (1999). Regulation of the cytoskeleton and cell adhesion by the Rho family GTPases in mammalian cells. Annu. Rev. Biochem. 68, 459–486. [DOI] [PubMed] [Google Scholar]
- Kuroda, S., Fukata, M., Fujii, K., Nakamura, T., Izawa, I., and Kaibuchi, K. (1997). Regulation of cell-cell adhesion of MDCK cells by Cdc42 and Rac1 small GTPases. Biochem. Biophys. Res. Commun. 240, 430–435. [DOI] [PubMed] [Google Scholar]
- Madara, J.L., and Pappenheimer, J.R. (1987). Structural basis for physiological regulation of paracellular pathways in intestinal epithelia. J. Membr. Biol. 100, 149–164. [DOI] [PubMed] [Google Scholar]
- Mercer, J.A. (2000). Intercellular junctions: downstream and upstream of Ras? Semin. Cell Dev. Biol. 11, 309–314. [DOI] [PubMed] [Google Scholar]
- Nakagawa, M., Fukata, M., Yamaga, M., Itoh, N., and Kaibuchi, K. (2001). Recruitment and activation of Rac1 by the formation of E-cadherin-mediated cell-cell adhesion sites. J. Cell Sci. 114, 1829–1838. [DOI] [PubMed] [Google Scholar]
- Nobes, C.D., and Hall, A. (1995). Rho, rac, and cdc42 GTPases regulate the assembly of multimolecular focal complexes associated with actin stress fibers, lamellipodia, and filopodia. Cell 81, 53–62. [DOI] [PubMed] [Google Scholar]
- Nobes, C., and Hall, A. (1999). Rho GTPases control polarity, protrusion, and adhesion during cell movement. J. Cell Biol. 144, 1235–1244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noren, N.K., Liu, B.P., Burridge, K., and Kreft, B. (2000). p120 catenin regulates the actin cytoskeleton via Rho family GTPases. J. Cell Biol. 150, 567–580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noren, N.K., Niessen, C.M., Gumbiner, B.M., and Burridge, K. (2001). Cadherin engagement regulates Rho family GTPases. J. Biol. Chem. 276, 33305–33308. [DOI] [PubMed] [Google Scholar]
- Nusrat, A., Giry, M., Turner, J.R., Colgan, S.P., Parkos, C.A., Carnes, D., Lemichez, E., Boquet, P., and Madara, J.L. (1995). Rho protein regulates tight junctions and perijunctional actin organization in polarized epithelia. Proc. Natl. Acad. Sci. USA 92, 10629–10633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajasekaran, A.K., Hojo, M., Huima, T., and Rodriguez-Boulan, E. (1996). Catenins and zonula occludens-1 form a complex during early stages in the assembly of tight junctions. J. Cell Biol. 132, 451–463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ren, R., Mayer, B.J., Cicchetti, P., and Baltimore, D. (1993). Identification of a ten-amino acid proline-rich SH3 binding site. Science 259, 1157–1161. [DOI] [PubMed] [Google Scholar]
- Ren, X.D., Kiosses, W.B., and Schwartz, M.A. (1999). Regulation of the small GTP-binding protein Rho by cell adhesion and the cytoskeleton. EMBO J. 18, 578–585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ridley, A.J., Comoglio, P.M., and Hall, A. (1995). Regulation of scatter factor/hepatocyte growth factor responses by Ras, Rac, and Rho in MDCK cells. Mol. Cell. Biol. 15, 1110–1122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ridley, A.J., and Hall, A. (1992). The small GTP-binding protein Rho regulates the assembly of focal adhesions and actin stress fibers in response to growth factors. Cell 70, 389–399. [DOI] [PubMed] [Google Scholar]
- Ridley, A.J., Paterson, H.F., Johnston, C.L., Diekmann, D., and Hall, A. (1992). The small GTP-binding protein rac regulates growth factor-induced membrane ruffling. Cell 70, 401–410. [DOI] [PubMed] [Google Scholar]
- Sander, E.E., ten Klooster, J.P., van Delft, S., van der Kammen, R.A., and Collard, J.G. (1999). Rac downregulates Rho activity: reciprocal balance between both GTPases determines cellular morphology and migratory behavior. J. Cell Biol. 147, 1009–1022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schoenenberger, C.A., Zuk, A., Kendall, D., and Matlin, K.S. (1991). Multilayering and loss of apical polarity in MDCK cells transformed with viral K-ras. J. Cell Biol. 112, 873–889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevenson, B.R., and Begg, D.A. (1994). Concentration-dependent effects of cytochalasin D on tight junctions and actin filaments in MDCK epithelial cells. J. Cell Sci. 107, 367–375. [DOI] [PubMed] [Google Scholar]
- Takaishi, K., Sasaki, T., Kotani, H., Nishioka, H., and Takai, Y. (1997). Regulation of cell-cell adhesion by rac and rho small G proteins in MDCK cells. J. Cell Biol. 139, 1047–1059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Troxell, M.L., Gopalakrishnan, S., McCormack, J., Poteat, B.A., Pennington, J., Garringer, S.M., Schneeberger, E.E., Nelson, W.J., and Marrs, J.A. (2000). Inhibiting cadherin function by dominant mutant E-cadherin expression increases the extent of tight junction assembly. J. Cell Sci. 113, 985–996. [DOI] [PubMed] [Google Scholar]
- van Hengel, J., Vanhoenacker, P., Staes, K., and van Roy, F. (1999). Nuclear localization of the p120(ctn) Armadillo-like catenin is counteracted by a nuclear export signal and by E-cadherin expression. Proc. Natl. Acad. Sci. USA 96, 7980–7985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walsh, S.V., Hopkins, A.M., Chen, J., Narumiya, S., Parkos, C.A., and Nusrat, A. (2001). Rho kinase regulates tight junction function and is necessary for tight junction assembly in polarized intestinal epithelia. Gastroenterology 121, 566–579. [DOI] [PubMed] [Google Scholar]
- Waterman-Storer, C.M., Worthylake, R.A., Liu, B.P., Burridge, K., and Salmon, E.D. (1999). Microtubule growth activates Rac1 to promote lamellipodial protrusion in fibroblasts. Nat. Cell Biol. 1, 45–50. [DOI] [PubMed] [Google Scholar]
- Wittchen, E.S., Haskins, J., and Stevenson, B.R. (1999). Protein interactions at the tight junction. Actin has multiple binding partners, and ZO-1 forms independent complexes with ZO-2 and ZO-3. J. Biol. Chem. 274, 35179–35185. [DOI] [PubMed] [Google Scholar]
- Wittchen, E.S., Haskins, J., and Stevenson, B.R. (2000). Exogenous expression of the amino terminal half of the tight junction protein ZO-3 perturbs junctional complex assembly. J. Cell Biol. 151, 825–836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu, H., Reissner, C., Kuhlendahl, S., Coblentz, B., Reuver, S., Kindler, S., Gundelfinger, E.D., and Garner, C.C. (2000). Intramolecular interactions regulate SAP97 binding to GKAP. EMBO J. 19, 5740–5751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto, T., Harada, N., Kano, K., Taya, S.-I., Canaani, E., Matsuura, Y., Mizoguchi, A., Ide, C., and Kaibuchi, K. (1997). The ras target AF-6 interacts with ZO-1 and serves as a peripheral component of tight junctions in epithelial cells. J. Cell Biol. 139, 785–795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yap, A.S., Niessen, C.M., and Gumbiner, B.M. (1998). The juxtamembrane region of the cadherin cytoplasmic tail supports lateral clustering, adhesive strengthening, and interaction with p120ctn. J. Cell Biol. 141, 779–789. [DOI] [PMC free article] [PubMed] [Google Scholar]