Abstract
In trypanosomes, the large mitochondrial genome within the kinetoplast is physically connected to the flagellar basal bodies and is segregated by them during cell growth. The structural linkage enabling these phenomena is unknown. We have developed novel extraction/fixation protocols to characterize the links involved in kinetoplast-flagellum attachment and segregation. We show that three specific components comprise a structure that we have termed the tripartite attachment complex (TAC). The TAC involves a set of filaments linking the basal bodies to a zone of differentiated outer and inner mitochondrial membranes and a further set of intramitochondrial filaments linking the inner face of the differentiated membrane zone to the kinetoplast. The TAC and flagellum-kinetoplast DNA connections are sustained throughout the cell cycle and are replicated and remodeled during the periodic kinetoplast DNA S phase. This understanding of the high-order trans-membrane linkage provides an explanation for the spatial position of the trypanosome mitochondrial genome and its mechanism of segregation. Moreover, the architecture of the TAC suggests that it may also function in providing a structural and vectorial role during replication of this catenated mass of mitochondrial DNA. We suggest that this complex may represent an extreme form of a more generally occurring mitochondrion/cytoskeleton interaction.
INTRODUCTION
The African trypanosome Trypanosoma brucei belongs to a large order of pathogenic and free-living flagellated protozoa designated the kinetoplastida. The majority of these organisms possess a single mitochondrion containing a structural mass of proteins and catenated circular DNA molecules, the kinetoplast. The kinetoplast is essential for survival and cyclical transmission of the parasite between mammalian host and tsetse fly. Early light microscope descriptions of trypanosomes recognized the precise location of the kinetoplast in proximity to the base of the flagellum (Robertson, 1912, 1913). The positions of the flagellum and kinetoplast have become the central features in classifying the different life cycle stages of kinetoplastids (Hoare and Wallace, 1966; Vickerman, 1976). Electron microscopy revealed the kinetoplast to be a highly complex disk-shaped structure located within a distended portion of the mitochondrial matrix adjacent to the flagellum basal bodies (Vickerman, 1973). The T. brucei kinetoplast consists of multiple copies of topologically interlocked circular DNA molecules termed maxicircles and minicircles, along with specific kinetoplast proteins. The maxicircle composition of the T. brucei network is 25–50 copies and represents 10% of the network mass (each maxicircle is 20 kb and all have identical DNA sequence). Maxicircles encode the mitochondrial proteins and some guide RNAs (gRNAs), whereas the minicircles are present in several thousand copies (1 kb in length), are heterogeneous in sequence, and encode gRNAs. The gRNAs are essential for the RNA editing process whereby the maxicircle encoded protein transcripts are modified by the addition or removal of uridine residues to produce the final mRNA molecules (Simpson and Maslov, 1999; Simpson et al., 2000; Cruz-Reyes et al., 2001; Schnaufer et al., 2002; Stuart et al., 2002; Stuart and Panigrahi, 2002).
T. brucei is characterized throughout its life cycle by the possession of a flagellum. The flagellar axoneme is subtended by a basal body and exits the cell body through the flagellar pocket. Cells early in the cell cycle possess a single flagellum and a single basal body with an associated probasal body. During G1/S, the probasal body matures to nucleate the assembly of the new flagellum, and two new probasal bodies are elaborated. The new flagellum extends and the basal bodies move apart and adopt particular positions, ultimately influencing the cell cleavage axis. Trypanosome flagella are characterized by the possession of a paraflagellar rod (PFR). This highly organized structure runs alongside the axoneme from the point of emergence from the cell body (Gull, 1999). The kinetoplast DNA molecules are replicated in a single periodic mitochondrial S phase that is temporally coordinated with nuclear DNA synthesis (Cosgrove and Skeen, 1970; Woodward and Gull, 1990) but the replicated kinetoplast DNA (kDNA) is segregated before mitosis begins. We have shown that this kinetoplast segregation process is mediated by movement apart of the flagellar basal bodies; one daughter kinetoplast moving with the basal body of the old flagellum and one with the basal body of the new flagellum (Robinson and Gull, 1991).
The molecular mechanisms of trypanosome mitochondrial genome replication and its expression by RNA editing are known in some detail. Maxicircles and minicircles replicate simultaneously, but maxicircles seem to replicate within the kinetoplast structure, whereas minicircles leave the kinetoplast and reattach at the two opposing poles of the network. These antipodal sites have also been shown to contain some of the enzymes necessary for kDNA replication (Klingbeil et al., 2001; Morris et al., 2001). Thus, although we have a rapidly growing appreciation of kinetoplast replication we have very little insight to the features responsible for the segregation of the daughter kinetoplasts. We do know that segregation is mediated by the separation of the flagellar basal bodies (Robinson and Gull, 1991; Robinson et al., 1995). In essence, we know how the process functions but we have no information on the structures involved, even though unequivocal evidence for the existence of some form of high-order structural link between the kinetoplast and flagellum has been demonstrated (Robinson and Gull, 1991).
We have now addressed this issue, and the application of particular electron microscopical fixation regimes has allowed the characterization of a tripartite attachment complex (TAC). Our results provide the structural explanation for the fidelity of kinetoplast position and segregation. We suggest that this highly ordered interaction of a mitochondrial “nucleoid” with the cytoskeleton may be an extreme, but informative, case of a more general structural interaction of mitochondria and plastids with elements of their respective cell's cytoskeleton.
MATERIALS AND METHODS
Organisms
Procyclic T. brucei 427 trypanosomes were propagated in SDM 79 medium as described by Brun and Schönenburger (1979). Crithidia fasciculata strain ATTC 1145 was cultivated and prepared for electron microscopy as described by Russell et al. (1984).
Transmission Electron Microscopy
Whole cells were prepared according to Tooze (1985). Briefly, a mid-log phase culture (5 × 106 cells/ml) of cells was fixed in 2% (vol/vol) glutaraldehyde, 2% (wt/vol) paraformaldehyde in 0.1 M sodium cacodylate buffer (SCB), pH 7.2. The cells were then washed in SCB, postfixed with 2% (wt/vol) osmium tetroxide in SCB, stained with 0.5% aqueous magnesium uranyl acetate, dehydrated, and embedded in Spurr's resin (Spurr, 1969). Some samples were prepared by a modification of a protocol (Begg et al., 1978) whereby cells were harvested as described above and washed in 0.1 M phosphate buffer, pH 7.0. They were then fixed for 30 min at room temperature in 2.5% (vol/vol) glutaraldehyde containing 1% tannic acid in 0.1 M phosphate buffer, postfixed in 0.5% osmium tetroxide (wt/vol) in 0.1 M phosphate buffer, pH 6.0, for 20 min at on ice. Samples were then stained with 1% aqueous magnesium uranyl acetate and dehydrated/embedded in resin as detailed above.
Cells were also prepared by simultaneously fixing and extracting with 1% glutaraldehyde, 1% formaldehyde in 0.1% Nonidet P-40 in 0.05 M PIPES, 1 mM EGTA, 0.5 mM MgSO4, pH 6.9, for 1 h at room temperature. All other steps were identical to the modified (Tooze, 1985) procedure described above except en bloc staining with 1% aqueous magnesium uranyl acetate was used. All blocks were sectioned at 50- to 70-nm thickness and stained in 5% (wt/vol) uranyl acetate in 1% acetic acid and 0.4% lead citrate in 0.1 N NaOH. Sections were examined on a 420 transmission electron microscope (Philips).
Flagella Preparation
EDTA was added to 5 ml of a mid-log phase culture to a final concentration of 5 mM. Cells were harvested by centrifugation, washed once in phosphate-buffered saline (PBS), pH 7.2, and extracted on ice for 10 min in 0.5% Triton X-100, 10 mM NaH2PO4, 150 mM NaCl, 1 mM MgCl, pH 7.2. Cytoskeletons were harvested, washed in extraction buffer, and resuspended on ice for 45 min in 10 mM NaH2PO4, 150 mM NaCl, 1 mM MgCl, pH 7.2, containing 1 mM Ca2+. The flagella preparation was then washed twice in PBS and resuspended in 500 μl of PBS and used for immunofluorescence studies.
Immunofluorescence
Immunofluorescence was performed as described by Robinson and Gull (1991). Essentially, cells or kinetoplast–flagella complexes were fixed in methanol, rehydrated in PBS, pH 7.2, and probed with a 1:1 mixture of monoclonal antibodies ROD1 and BBA4 (Woods et al., 1989) followed by fluorescein isothiocyanate-conjugated secondary antibodies. DNA was stained with 4,6-diamidino-2-phenylindole (DAPI) and preparations were visualized using an Axioscope (Carl Zeiss, Jena, Germany).
5-Bromodeoxyuridine (BrdU) Incorporation and Labeling of kDNA–Flagella Complexes
BrdU incorporation into cells and immunofluorescence were performed according to Robinson and Gull (1991) flagellum isolation was performed according to Robinson and Gull (1994).
Drug Treatment
Acriflavine was added to T. brucei cultures to a final concentration of 50 ng/ml. Samples were taken every 4 h and processed for imunofluorescence and DAPI staining. Counts of 300 cells were made at each time point to determine the distribution of cell types in the population.
RESULTS
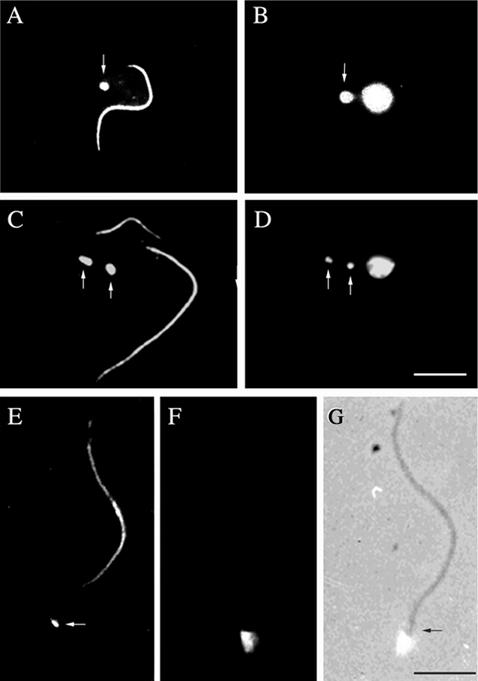
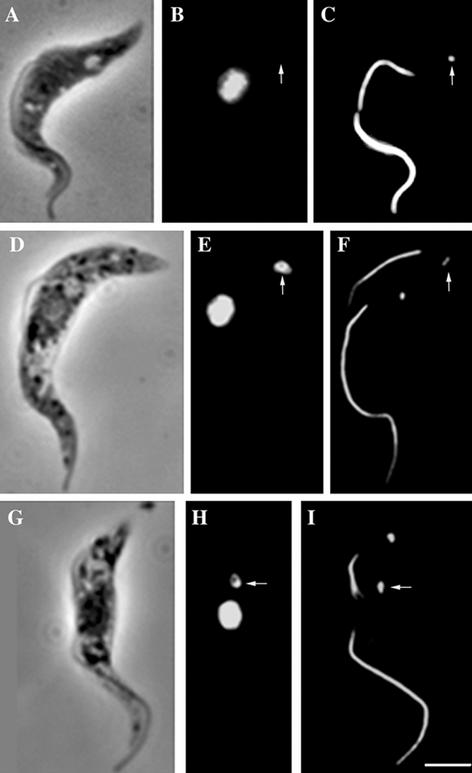
A central feature of trypanosome morphology is the juxtapositioning of the kinetoplast and the flagellum basal body. This configuration is clearly seen in immunofluorescence by using the monoclonal antibody BBA4 to visualize the basal body. Inclusion of the ROD1 monoclonal, which recognizes the paraflagellar rod (PFR), in such preparations allows a visualization of the flagella length and number. The PFR is only present in the flagellum from the point where the axoneme exits the cell. Hence, a useful marker gap exists between the BBA4 basal body staining and the start of the PFR staining by ROD1 (Figure 1, A, C, and E). Figure 1, C and D, shows that when the new flagellum is still relatively short its basal body and the basal body of the old flagellum have already moved apart, so segregating the mitochondrial DNA (Robinson and Gull, 1991). A physical connection between the kinetoplast and the basal body can be demonstrated by isolating the flagella by using detergent and Ca2+ treatment. In such preparations, the mitochondrial DNA in the kinetoplast is still firmly attached to the basal body of the flagellum (Figure 1, E–G). The attachment of the flagellum to the kinetoplast after detergent and Ca2+ treatment confirms the presence of a highly organized connection system.
Figure 1.
Kinetoplasts are physically attached to the flagellum basal bodies and are segregated by them during cell growth. Immunofluorescence of procyclic cells (A–D) or isolated flagella (E–G) by using two monoclonal antibodies, ROD 1 and BBA4, which illustrate the location of the paraflagellar rod within the flagellum in addition to the basal bodies, (arrows in A, C, and E). DAPI staining of the corresponding cells or flagella shows the location of nuclear and/or kinetoplast DNA (arrows in B and D). In E–G, the kinetoplast remains in contact with the isolated flagellum even after cell lysis and cytoskeleton depolymerization, illustrating the physical link between the kinetoplast and flagellum basal bodies via the TAC (E, F, and G). Bars, 5 μm.
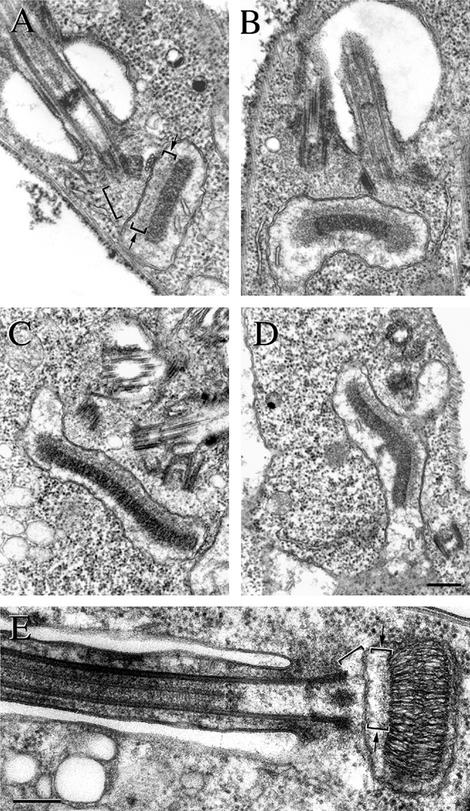
Given this evidence for a physical connection between the flagellar basal body and the kinetoplast, we questioned whether we could detect any form of ultrastructural links between these two organelles. Thin section electron micrographs of T. brucei cells were produced after fixation by a variety of individual protocols. A defined set of structures was detected using this spectrum of fixation regimes but can be exemplified most distinctly by a fixation regime using tannic acid in a modified protocol of Begg et al. (1978). Figure 2A shows an image of a T. brucei procyclic cell, sectioned close to the flagellum pocket area. The section reveals the basal body and a cross section of the disk-shaped kinetoplast within the mitochondrion. Two novel filament systems are defined in this image. First, a set of filaments runs between the proximal end of the basal body and the adjacent outer mitochondrial membrane (large bracket). This set of filaments, which we designate exclusion zone filaments, occupy this defined area and their presence excludes cytoplasmic ribosomes. The plane of the kinetoplast is orthogonal to the longitudinal axis of the flagellum/basal body and so the kinetoplast is seen in transverse section in this figure. The second set of filaments is present only on one face of the kinetoplast and links it with the inner mitochondrial membrane (small brackets and arrows). These filaments, which we designate the unilateral filaments, are always present only on this single face of the kinetoplast. This area is densely packed with fibrous material and this maintains the gap between the kDNA network and the inner mitochondrial membrane with a constant spacing (small brackets and arrows). We observe that the mitochondrial membrane in this zone between the basal body and the kinetoplast exhibits a rather linear profile with few, if any, corrugations. Moreover, cristae were never observed in this zone of the mitochondrion. Cristae, however, were often present projecting into the mitochondrial lumen on the other side of the kinetoplast in Figure 2, A, B, and D. We will refer to this tripartite structure, the exclusion zone filaments, the differentiated mitochondrial membranes, and the unilateral filaments, as the TAC.
Figure 2.
TAC can be visualized using transmission electron microscopy. Transmission electron micrographs of thin sections through the basal body and kinetoplast of T. brucei, (A–D) and C. fasciculata (E). The exclusion zone is seen between the basal bodies and the outer mitochondrial membrane (present in all figures, but highlighted by a large bracket in A and E). The unilateral filaments are present only on the flagellar face of the kinetoplast and link it with the inner mitochondrial membrane (A and E small brackets and arrows). (B) A cell that possesses two flagella. The kinetoplast of this cell is characterized by the occurrence of fibrous lobes or at each edge of the kinetoplast disk. Both terminal lobes have a different texture and electron density to the main body of the kinetoplast. Because the cell has two flagella and it seems to be in kinetoplast S phase, it is likely to be at a stage between 0.4 and 0.7 of the unit cell cycle. The unilateral zone fibers do not extend to the edge of the replicating network. The cell in C possesses two flagella, their associated basal bodies, and probasal bodies and is later in kinetoplast S phase than the cell in B. Note that ribosome-free regions have formed in association with the probasal bodies but the unilateral zone fibers do not extend to the edge of the replicating network. (D) Oblique section of the two basal bodies and the two probasal bodies. All three components of the TAC are present at this stage of the cell cycle, but the two TAC sets are further apart and the kinetoplast has a shallow V configuration. The differentiated area of mitochondrial membranes forms two discrete zones. A separate set of exclusion zone filaments connects each of these areas of membrane to the particular basal body set. The micrograph of a C. fasciculata cell in E also shows all three components of the TAC; the exclusion zone (large bracket) and unilateral filaments (small bracket and arrows) are indicated. Bars, 250 nm.
Figure 2B shows a thin section of the flagellar pocket area of a cell, which is some way into its cell cycle because it possesses two flagella. The kinetoplast of this cell is characterized by the occurrence of fibrous lobes at each end of the kinetoplast. Both terminal fibrous lobes have a different texture and electron density to the main body of the kinetoplast. Our interpretation of this micrograph (two flagella in one flagellum pocket with basal bodies not very far apart) suggests that it is likely to be at a stage between 0.4 and 0.7 of the unit cell cycle (Woodward et al., 1995). This period encompasses S phase, and it seems likely that these two antipodal fibrous lobes on the kinetoplast are associated with kinetoplast replication (Abu-Elneel et al., 2001; Drew and Englund, 2001). This form of kinetoplast still exhibits the full TAC however, and we note that the unilateral filaments still connect to the main body of the kinetoplast but not totally to the antipodal lobe structures. This image (Figure 2B) shows the early replication processes of the TAC. Two ribosome-free regions are observed and each region subtends a flagellum basal body. Also, two distinct zones of mitochondrial membrane are present between in the region between the basal bodies and the kinetoplast. Each zone of mitochondrial membrane exhibits a linear profile with few corrugations. Thus, TAC replication occurs while a connection is maintained to the replicating kinetoplast.
After replication the kinetoplast increases in size. Two examples of this form are seen in Figure 2, C and D. The cell in Figure 2C possesses two flagella and their associated basal bodies and probasal bodies. Figure 2C shows an oblique section of the two basal bodies and the two probasal bodies. All three components of the TAC are present at this stage of the cell cycle. The cell in Figure 2D again exhibits two sets of basal and probasal bodies. However, it occurs later in the segregation process because the two sets are further apart. Moreover, in this image the kinetoplast has a shallow “V” configuration, and the differentiated area of mitochondrial membranes that look thickened and more electron dense maintain a linear profile and lack cristae. As such the mitochondrial membranes now clearly form two discrete zones. Likewise, a separate set of exclusion zone filaments connects each of these areas of membrane to their particular basal body set. Figure 2E shows an image of a C. fasciculata cell where the flagellum has been sectioned longitudinally. The TAC can clearly be seen in this cell. The C. fasciculata TAC includes exclusion zone fibers that exclude cytoplasmic ribosomes (large bracket). Also, the mitochondrial membrane exhibits a linear profile in the region adjacent to the exclusion zone fibers (small brackets and arrows), and the unilateral filaments are also present in this trypanosome but seem to be more loosely organized in comparison with those of T. brucei.
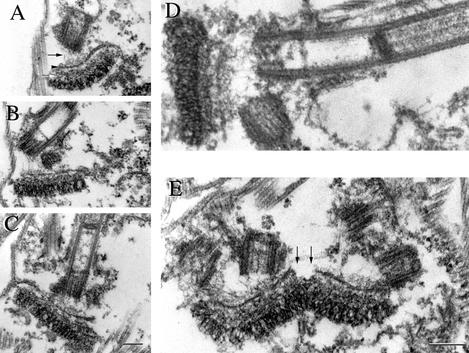
Having obtained detailed data from standard electron microscopy preparations of T. brucei whole cells, we sought improvements in fixation of detergent-extracted cytoskeletons that might allow us even clearer demonstrations of the TAC components when cytoplasmic material was removed by detergent. One particular procedure proved highly informative. Thin section analysis after simultaneous fixation and detergent extraction of cells provided a novel view of the basal body/kinetoplast relationship (Figure 3). This combined detergent/fixation extraction protocol resulted in cytoskeletons where all of the membranous organelles and cytoplasmic components had been removed. However, the components of the TAC, including the specialized area of mitochondrial inner and outer membrane were preserved under these operational conditions. Figure 3, A–E, shows examples of basal bodies with their associated TAC/kinetoplasts. The exclusion zone filaments are more clearly seen in these preparations as 5- to 10-nm electron dense filaments emanating from the proximal end of the basal body (arrow) toward (and linking to) the remnant of the outer mitochondrial membrane. Although all of the normal mitochondrial membranes had disappeared (by detergent extraction), a double line of electron dense material survived at the precise junction between the unilateral filaments and exclusion zone filaments (arrowhead). Remarkably, these mitochondrial membrane remnants remain intact only between the basal bodies and the kinetoplast, whereas no remnants of mitochondrial membrane were observed outside of this region. The unilateral filament set was seen as a tightly packed mass between the inner mitochondrial membrane remnant and the kinetoplast (white arrow). The unilateral filaments retain their packed appearance and membrane-kinetoplast DNA interaction after extraction-fixation treatment. In these preparations, the kinetoplast retains its fibrous, striated, and compact organization that is normally visualized in whole cells. Figure 3E represents an image of this TAC area in a cell where the basal bodies have duplicated (there are two sets of a basal body and probasal body seen in the oblique section). This micrograph shows that at this stage each set of basal bodies is associated with a complete TAC reminiscent of the situation seen in a whole cell preparation (Figure 2D). Interestingly, however, the detergent/fixation protocol emphasizes the distinct difference between the differentiated mitochondrial membrane of the TAC and that of the normal mitochondrial membranes. A distinct gap is seen between the two nascent TACs, where the normal membrane has been removed. We interpret this as indicative of a late stage in the process in TAC segregation.
Figure 3.
Discrete components of the TAC can be visualized after simultaneous fixation and extraction of T. brucei procyclic cells. Transmission electron micrographs of thin sections through the basal body and kinetoplast of T. brucei after simultaneous fixation and detergent extraction resulted in cytoskeletons whereby all membranous organelles are removed but the components of the TAC are preserved. The exclusion zone filaments are seen between the basal bodies and the remaining outer mitochondrial membrane (present in all figures, but highlighted by an arrow in A). The exclusion zone filaments are clearly seen as 5- to 10-nm electron dense filaments emanating from the proximal end of the basal body (arrow) toward and linking the remnant of the outer mitochondrial membrane (A–E). A double line of electron-dense mitochondrial membrane survived detergent extraction at the junction between the unilateral filaments and exclusion zone filaments. Membrane remnants are also observed (present in all figures, but highlighted by an arrowhead in A). Unilateral filaments were seen as a tightly packed mass between the inner mitochondrial membrane remnant and the kinetoplast (present in all figures, but highlighted by a white arrow in A). Figure E represents an image of the TAC in a cell where the basal bodies have duplicated and each set of basal bodies is associated with a complete TAC. Detergent/fixation protocol illustrates the distinct difference between the differentiated mitochondrial membranes within the TAC and that of normal mitochondrial membranes, which are removed by this treatment. A distinct gap is seen between the two nascent TACs, where the normal membrane has been removed (arrows). Bars, 250 nm.
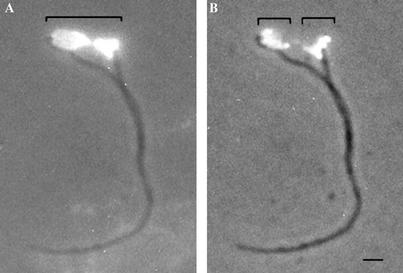
It is important to ask when in the cell cycle the TAC-mediated connection is present, when is it remodeled, and is the kDNA permanently attached or does remodeling involve DNA detachment and reattachment? Our electron microscopy analysis strongly suggests that the kinetoplast/basal body connection is maintained via the TAC during all stages of the cell cycle, but it is important to provide corroborative evidence by using different approaches. The situation during S phase is particularly intriguing because kDNA replication is known to involve the release of at least one component (minicircles) from the kinetoplast complex (Abu-Elneel et al., 2001; Drew and Englund, 2001; Klingbeil et al., 2001; Morris et al., 2001). This raises the question of whether the kinetoplast matrix structure as a whole is still firmly attached to the basal bodies during S phase by means of the TAC. Cells were treated with the thymidine analog BrdU, thus allowing the replicating kinetoplasts to incorporate this proliferation marker. Cells were then detergent extracted and the subpellicular microtubules depolymerized with calcium. These kDNA/flagella preparations were processed for immunofluorescence and probed with anti-BrdU antibodies. We searched for kinetoplasts that had incorporated the BrdU into the replicating network and analyzed the association of the flagella to the spread kDNA networks. Figure 4 illustrates such a BrdU-labeled kDNA/flagellum preparation. DAPI staining and phase contrast imaging (Figure 4A, area under large bracket) show the kinetoplast network to be in mid-to-late stages of replication as observed by the characteristic bilobed appearance, typified by such a replicative form (Robinson and Gull, 1991). Figure 4B shows the same network observed with fluorescent anti-BrdU labeling and phase contrast imaging. This network has two antipodal lobes of BrdU incorporation, located 180o apart (areas under small brackets). Attachment of the flagella, via their basal bodies, to the replicating network is clearly observed at the outermost poles of the network, 180o apart and within the sites where replicated DNA occurs. We conclude from this result that the TAC is present and maintained during kDNA replication. Furthermore, these data show that kinetoplast replication and segregation occurs simultaneously and that replicated DNA occurs within the sites of physical attachment of the flagellum basal bodies to the kDNA network.
Figure 4.
Kinetoplast DNA replication and segregation occurs simultaneously in T. brucei procyclic cells. Procyclic cells were treated with BrdU to allow the replicating kinetoplasts to incorporate this proliferation marker. Cells were detergent extracted, the subpellicular microtubules depolymerized, and the resulting kDNA/flagella preparations probed with anti-BrdU antibodies. (A) kDNA/flagella complex visualized simultaneously with phase contrast and UV light by using the DAPI filter. The spread kDNA is seen lying between the basal bodies of a mature and immature flagellum (area below large bracket). The DAPI staining also shows the kinetoplast network to be in mid-to-late stages of replication as observed by the characteristic bilobed appearance. Anti-BrdU labeling combined with phase contrast of the same complex is shown in B illustrates that the network has two antipodal lobes of BrdU incorporation, located 180o apart (area below small brackets). Attachment of the flagella to the replicating network is clearly observed at the outermost poles of the network, within the sites where replicated DNA occurs. This is the site of the TAC. Bar, 1 μm.
The above-mentioned results show that the TAC is a coherent structure linking the kinetoplastid-located mitochondrial genome to the flagellar basal bodies throughout the cell cycle. The BuDR experiment shows that the new TAC complex associated with the new flagellum is able to gain kDNA at S phase. We next addressed the issue of whether this connection would be disturbed by inhibition of kDNA synthesis and if so, what would be the cellular consequences? Acriflavine is an attractive compound for such studies because it is known to have a selective effect on kDNA replication and has well studied effects on mitochondrial DNA in many other cell types (Simpson, 1968; Tarrago-Litvak et al., 1978; Gillham et al., 1987; Matagne et al., 1989; Agbe and Yielding, 1995). The most striking feature of acriflavine treatment is the rapid rise through 4 and 8 h treatment of a cell type that we term 1K1N (old) and 1K1N (new) (Figure 5). These cells represent >70% of the cell types in the 8-h drug-treated population (Ogbadoyi, 1997). Although these cells have only one kinetoplast, they are, judging by the presence and length of the new flagellum, clearly in an advanced stage of the cell cycle (Figure 5). Although the basal bodies have replicated and segregated and the new flagellum has formed, the mass of kDNA is associated with only one basal body complex (arrow in E and H). In the majority of cases (6:1 ratio at 8 h), this is the basal body subtending the old flagellum and we term these cells 1K1N (old) (Figure 5, G, H, and I). In some cases, mainly at 4-h treatment, the kDNA is stretched between basal bodies (included in counts but not shown). The stretched kDNA supports the previous conclusion that the kinetoplast DNA is attached to the flagellum basal bodies during replication and segregation by means of the TAC; the stretched phenotype being explained by a lack of kDNA decatenation after drug treatment, thus blocking network separation. The kinetoplast is thus pulled or stretched between the segregating basal bodies while attached to the flagellum basal bodies via the TAC. The consequence of the asymmetric segregation of the kinetoplast in the 1K1N (old) and 1K1N (new) cells is the production upon division of a 1K1N sibling and a 0K1N sibling (Figure 5, D–I). These latter cell types are known as dyskinetoplastic trypanosomes and have long been recognized as a feature of DNA-intercalating drug treatment of trypanosomes (Guttman and Eisenman, 1965; Kusel et al., 1967; Laub-Kupersztejn and Thirion, 1969; Simpson, 1972; Zaitseva et al., 1977; Shapiro et al., 1989). Our observations now show that these dyskinetoplastic trypanosomes can form at the very first cell division by asymmetric segregation of the kDNA. Taken with our previous points, this strongly suggests that the TAC/kinetoplast DNA connection is normally remodeled at S phase to take account of the new TAC and replicated kDNA and that this remodeling can be perturbed leaving the kDNA associated with one (usually their old TAC complex). The lack of a mitochondrial genome is clearly lethal in the longer term for these procyclic cells.
Figure 5.
Acriflavine blocks the postreplication mitochondrial genome linkage to the TAC. Phase contrast (A, D, and G), DAPI staining (B, E, and H), and immunofluorescence labeling (C, F, and I) of acriflavine-treated T. brucei cells was done using two monoclonal antibodies ROD 1 and BBA4. This indicates the location of the paraflagellar rod and the basal bodies after acriflavine treatment. DAPI staining of cells show the location of the nucleus and/or kinetoplast DNA. The cell in A, B, and C is dyskinetoplastic with no kDNA present (B, arrow). The ROD 1/BBA4 staining of this dyskinetoplastic cell shows the presence of a flagellum and a corresponding basal body (C, arrow). The cell in D, E, and F shows normal morphology when viewed by phase contrast microscopy, but the DAPI and immunofluorescence images show that the cell has one kinetoplast and two flagella. The single kinetoplast of this cell is associated with the new flagellum basal body (E and F, arrows). The cell in G, H, and I also has a “normal” morphology by phase contrast but again the DAPI and immunofluorescence images show that the cell has one kinetoplast and two flagella. In this case, the kinetoplast is associated with the old flagellum basal body. Bar, 5 μm.
DISCUSSION
Protists exhibit some of the most highly ordered examples of organelle positioning observed within eukaryotic cells, and the kinetoplast is a particular example. The catenated kDNA mass is located at a particular position within the mitochondrion and that site is maintained at a particular position within the cell. There is increasing evidence that mitochondrial positioning and inheritance are effected by physical and functional association with the cytoskeleton (Yaffe, 1999a,b; Berger and Yaffe, 2000). Genes identified from mutational analysis of mitochondrial inheritance encode proteins that fall into two main groups: those likely to be associated with the cytoskeleton and those associated with the outer mitochondrial membrane (Jones and Fangman, 1992; Guan et al., 1993; Sogo and Yaffe, 1994; Berger et al., 1997; Hales and Fuller, 1997; Shepard and Yaffe, 1999; Fekkes et al., 2000). In some cases, the evidence of a cytoskeletal association points to proteins that influence mitochondrial shape and transmission to microtubules and at other times to actin microfilaments (McConnell et al., 1990; McConnell and Yaffe, 1993; Hermann et al., 1997; Otsuga et al., 1998; Sesaki and Jensen, 1999). Moreover, there is evidence that mitochondrial outer membrane proteins such as Mmm1p are also involved in processes that control the distribution and stability of the DNA nucleoid at the face of the inner mitochondrial membrane (Hobbs et al., 2001). Also, the intermembrane space protein Mgm1p is implicated in mitochondrial structure, fusion, and inheritance (Wong et al., 2000). Such macromolecular complexes involving crosstalk between mitochondrial membranes and the mitochondrial genome are reminiscent of the structural features of the TAC that we describe in trypanosomes. The T. brucei genome project is, as yet, incomplete. However, there is good evidence for the existence of homologs of proteins such as Mgm1p and others in the T. brucei GSS database (our unpublished observations), suggesting wide evolutionary conservation of such components.
Although genetic evidence for molecular components influencing links between the cytoskeleton and the mitochondrion has increased, our results provide a reciprocal view whereby such discrete structural linkages have been visualized in the electron microscope.
Exclusion Zone Filaments of TAC
The proximity of the flagellum basal body and kinetoplast was noted in classical descriptions in the early years of the last century and now forms a definition of the stages of trypanosomatids (Hoare and Wallace, 1966). Traditional fixation techniques showed little structure at the most proximal region of the flagellum basal bodies. Indeed, Vickerman (1969) remarked “a definite structural nexus between the basal body of the flagellum and the mitochondrial envelope of the kinetoplast has not become apparent by electron microscopy even though the two are linked in morphogenesis.” Vickerman (1973) recognized the fact that some physical material might be present because he noted a “zone of exclusion,” whereby ribosomes were not present between the mitochondrion and basal bodies. Souto-Padron et al. (1984) used quick-freeze deep-etch approach to visualize filaments in this general area. Our approach using different fixation regimes has allowed us to visualize for the first time a defined substructure of filaments within this area and we have termed them the exclusion zone filaments. Our results showing that these filaments are present under conditions of detergent extraction is consistent with the idea that it is this set of filaments that forms and maintains the connection.
Unilateral Kinetoplast Filaments of TAC
Because the kinetoplast is maintained at a specific position yet the single mitochondrion extends along the length of the cell (Vickerman, 1965; Simpson, 1972; Vickerman et al., 1988), there is a strong suggestion that some structure links the kDNA within the mitochondrial lumen to a particular position on the inner mitochondrial membrane. Moreover, this structure should presumably be positioned directly opposite the basal bodies. The presence of the filament system, which we have termed the unilateral kinetoplast filaments, provides the structural explanation for this positioning mechanism. The identification of the unilateral kinetoplast filaments also raises the possibility that such a “local” differentiated face of the kinetoplast disk may play a role in organizing and directing structural features of kDNA replication.
Differentiated Mitochondrial Membrane of TAC
There are four distinct features of the mitochondrial membrane in the area between the basal bodies and the kinetoplast. Both membranes often seem to be more parallel than in other areas of the mitochondrion. Also, this zone exhibits no cristae, and the membrane “survives” detergent extraction. Finally, the area emerges as two separated zones when the replicated basal bodies separate. The nature of the detergent-resistant link between the two filament components of the TAC present on either side of the mitochondrial membranes is unknown. One can rehearse possible architectures ranging from a “molecular rivet” spanning both mitochondrial membranes to an oligomeric protein/lipid raft complex. Whatever the nature of the transmembrane connection, it must allow maintenance of a discrete membrane potential that is required for protein import and is vital to the parasite.
Replication and Segregation
The TAC is present at all parts of the cell cycle and duplicates along with the basal bodies at the periodic kDNA S phase. Our electron microscopy reveals two dense structures, possibly akin to the “fibrous knots” described in another context by Rudzinska and Vickerman (1968) at either end of the kinetoplast during this S phase. Although the unilateral filaments of the TAC are still present along the main kinetoplast, they do not seem to connect so directly with these two terminal structures, which we term the fibrous lobes. Our correlation of the known cell cycle markers in T. brucei and the presence of these fibrous lobes place their occurrence within S phase. Replication of the T. brucei kDNA network occurs with maxicircles replicating within the structure, whereas minicircles leave and reattach at the two opposing poles of the network (Ferguson et al., 1994; Robinson and Gull, 1994; Abu-Elneel et al., 2001; Drew and Englund, 2001; Klingbeil et al., 2001; Morris et al., 2001). In addition to replicated minicircles, these antipodal sites contain some of the enzymes necessary for kDNA replication. We suggest that the fibrous lobes are the ultrastructural correlates of these antipodal sites and are part of the mechanism whereby replicated kDNA is reincorporated and connected with the new and old TACs. This raises the question of whether the TAC components are inherited in a conservative or semiconservative manner. One rationale for probasal body association with the TAC in pre-S phase cells is that it will therefore be connected to the internal unilateral filaments (and therefore kinetoplast) before it matures to form the new flagellum.
Inheritance and Dyskinetoplastic Cells
Dyskinetoplastic strains of trypanosomes have been described both as natural occurrences and as the result of experimental insults (Guttman and Eisenman, 1965; Kusel et al., 1967; Laub-Kupersztejn and Thirion, 1969; Riou and Saucier, 1979; Schnaufer et al., 2002). However, recent results show that knockdown of certain mitochondrial functions (such as RNA editing) is a lethal event even in bloodstream forms (Schnaufer et al., 2001). Simpson (1972) noted that in Leishmania tarentolae low concentrations of acriflavine produced a selective inhibition of kDNA synthesis for a few generations whereon one daughter trypanosome retained all of the remaining kDNA. However, in higher concentrations of the drug, trypanosomes produced a one-step “all and none” type of kinetoplast segregation. He hypothesized that in such cases the dye interferes with the distribution of kDNA between daughters as well as inhibiting DNA replication (Simpson, 1972). We support this view and suggest that the TAC provides an explanation of these events. We envisage the all and none form of division resulting from a failure of replication and segregation such that one basal body segregates without an attached kinetoplast. This form of division would result because the exclusion zone filaments have been formed in the previous cell cycle, but new unilateral filaments would need to be formed (or remodeled) in the present cell cycle. The production (within one cell cycle) and cellular consequences of missegregation reinforces our view that segregation of the basal bodies as well as kinetoplast replication and segregation are major events influencing cell cycle checkpoint control (Ploubidou et al., 1999).
Replicating free minicircle intermediates of C. fasciculata have been visualized almost exclusively situated at the flagellum face of the kinetoplast (Drew and Englund, 2001). Some kDNA binding proteins are localized to the whole kinetoplast network, whereas others such as a DNA primase and a minicircle binding protein localize to specific zones (Abeliovich et al., 1993; Hines and Ray, 1998; Johnson and Englund, 1998; Abu-Elneel et al., 1999, 2001). The unilateral filaments may provide the basis for a structural anisometry plus a focused matrix necessary for ordered enzymatic functions required for replication of the network.
Kinetoplast Positioning and Segregation Are Mediated by TAC
The existence of some “link” between the kinetoplast and basal body of the flagellum has been speculated upon in many previous descriptions of trypanosome cell biology. Our use of specific fixation regimes has allowed us to visualize the TAC, a cytoplasmic filament system, a differentiated zone of mitochondrial membrane, and a lumenal mitochondrial filament system that we suggest represents a highorder system responsible for both kinetoplast positioning and segregation (Figure 6). This transmembrane structure provides the explanation for why the kinetoplast maintains its position close to the basal body in the various trypanosomes. Our view is that this complex may represent an extreme form of a more generalized mitochondrion/cytoskeleton interaction and as such is informative in its component structure and complexity. We suggest that the complex TAC is seen at an extreme level in trypanosomes because of their need to ensure absolute efficiency in the segregation of their single mass of mitochondrial DNA. The TAC is a component of the kinetoplast positioning and segregation machinery as seen by our electron microscopy and immunofluorescence studies on whole cells, cytoskeletons, and kinetoplast/flagellum complexes. We suggest that this structure is present in many, if not all, pathogenic trypanosomes as well as nonpathogenic kinetoplastida such as C. fasciculata.
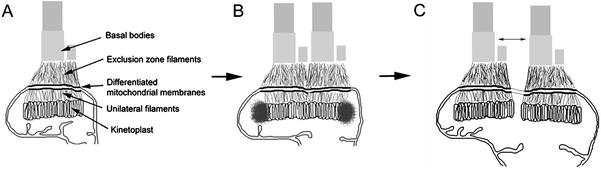
Figure 6.
Schematic diagram of the TAC complex in trypanosomes. (A) Basal bodies, kinetoplast, and the components of the TAC (exclusion zone filaments, differentiated mitochondrial membranes, and unilateral filaments) in a trypanosome in G1 of the cell cycle. In this period, there is a single flagellum, a basal body, and a probasal body. (B) Organization of the S phase TAC. When the cell enters S phase, discrete fibrous lobes occur at the poles of the kinetoplast, the probasal body matures into a basal body and subtends the new flagellum, and two new probasal bodies are formed. Two nascent TAC complexes are discernible at this period of the cell cycle. (C) Period where movement apart of the flagella basal bodies segregates the replicated kinetoplast DNA. Note that the position and orientation of the basal bodies have been idealized in this two-dimensional cartoon.
Our electron microscopy shows a novel mitochondrial DNA/cytoskeleton interaction, the TAC. We show that the basal body complexes of the flagellum cytoskeleton are firmly attached to the kinetoplast via the TAC. We also show that this TAC linkage must undergo some form of remodeling during kinetoplast S phase, because acriflavine inhibition results in basal bodies that segregate without any associated kDNA. Given the need for an efficient bipolar, kinetoplast segregation mechanism then the evolution of a TAC link, not merely to elements of the cytoskeleton but thence to the unit flagellar basal bodies and their replication/segregation cycle, seems to be an elegant example of the hitch-hikers guide to the cytoskeleton (Gull, 2001). We suggest that this complex may represent an extreme form of a more generally occurring mitochondrion/cytoskeleton interaction.
Acknowledgments
This work was supported by a Program Grant (to K.G.) from The Wellcome Trust. K.G. is a Wellcome Trust Principal Fellow and D.R.R. held a Wellcome Trust Career Development Fellowship. This investigation received financial support from the United Nations Developmental Program/World Bank/World Health Organization Special Program for Research and Training in Tropical Diseases. The work was also assisted by an equipment grant from The Wellcome Trust.
Article published online ahead of print. Mol. Biol. Cell 10.1091/mbc.E02-08-0525. Article and publication date are at www.molbiolcell.org/cgi/doi/10.1091/mbc.E02-08-0525.
Abbreviations used: kDNA, kinetoplast DNA; TAC, tripartite attachment complex.
References
- Abeliovich, H., Tzfati, Y., and Shlomai, J. (1993). A trypanosomal CCHC-type zinc finger protein which binds the conserved universal sequence of kinetoplast DNA minicircles: isolation and analysis of the complete cDNA from Crithidia fasciculata. Mol. Cell. Biol. 13, 7766–7773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abu-Elneel, K., Kapeller, I., and Shlomai, J. (1999). Universal minicircle sequence-binding protein, a sequence-specific DNA-binding protein that recognizes the two replication origins of the kinetoplast DNA minicircle. J. Biol. Chem. 274, 13419–13426. [DOI] [PubMed] [Google Scholar]
- Abu-Elneel, K., Robinson, D.R., Drew, M.E., Englund, P.T., and Shlomai, J. (2001). Intramitochondrial localization of universal minicircle sequence-binding protein, a trypanosomatid protein that binds kinetoplast minicircle replication origins. J. Cell Biol. 153, 725–733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agbe, A., and Yielding, K.L. (1995). Kinetoplasts play an important role in the drug responses of Trypanosoma brucei. J. Parasitol. 81, 968–973. [PubMed] [Google Scholar]
- Begg, D.A., Rodewald, R., and Rebhun, L.I. (1978). The visualization of actin filament polarity in thin sections. Evidence for the uniform polarity of membrane-associated filaments. J. Cell Biol. 79, 846–852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berger, K.H., Sogo, L.F., and Yaffe, M.P. (1997). Mdm12p, a component required for mitochondrial inheritance that is conserved between budding and fission yeast. J. Cell Biol. 136, 545–553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berger, K.H., and Yaffe, M.P. (2000). Mitochondrial DNA inheritance in Saccharomyces cerevisiae. Trends Microbiol. 8, 508–513. [DOI] [PubMed] [Google Scholar]
- Brun, R., and Schönenburger, M. (1979). Cultivation and in vitro cloning of procyclic forms of Trypanosoma brucei in a semi-defined medium. Acta Tropica 36, 289–292. [PubMed] [Google Scholar]
- Cosgrove, W.B., and Skeen, M.J. (1970). The cell cycle in Crithidia fasciculata. Temporal relationships between synthesis of deoxyribonucleic acid in the nucleus and in the kinetoplast. J. Protozool. 17, 172–177. [DOI] [PubMed] [Google Scholar]
- Cruz-Reyes, J., Zhelonkina, A., Rusche, L., and Sollner-Webb, B. (2001). Trypanosome RNA editing: simple guide RNA features enhance U deletion 100-fold. Mol. Cell. Biol. 21, 884–892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drew, M., and Englund, P.T. (2001). Intramitochondrial location and dynamics of Crithidia fasciculata kinetoplast minicircle replication intermediates. J. Cell. Biol. 153, 735–744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fekkes, P., Shepard, K.A., and Yaffe, M.P. (2000). Gag3p, an outer membrane protein required for fission of mitochondrial tubules. J. Cell Biol. 151, 333–334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferguson, M.L., Torri, A.F., Pérez-Morga, D., Ward, D.C., and Englund, P.T. (1994). Kinetoplast DNA replication: mechanistic differences between Trypanosoma brucei and Crithidia fasciculata. J. Cell Biol. 126, 631–639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gillham, N.W., Boynton, J.E., and Harris, E.H. (1987). Specific elimination of mitochondrial DNA from Chlamydomonas by intercalating dyes. Curr. Genet. 12, 41–47. [DOI] [PubMed] [Google Scholar]
- Guan, K.L., Farh, L., Marshall, T.K., and Deschenes, R.J. (1993). Normal mitochondrial structure and genome maintenance in yeast requires the dynamin-like product of the MGM1 gene. Curr. Genet. 24, 141–148. [DOI] [PubMed] [Google Scholar]
- Gull, K. (1999). The cytoskeleton of trypanosomatid parasites. Annu. Rev. Microbiol. 53, 629–655. [DOI] [PubMed] [Google Scholar]
- Gull, K. (2001). Protist tubulins: new arrivals, evolutionary relationships and insights to cytoskeletal function. Curr. Opin. Microbiol. 4, 427–432. [DOI] [PubMed] [Google Scholar]
- Guttman, H.N., and Eisenman, R.N. (1965). Acriflavin-induced loss of kinetoplast deoxyribonucleic acid in Crithidia fasciculata (Culex pipiens strain). Nat. Cell. Biol. 207, 1280–1281. [DOI] [PubMed] [Google Scholar]
- Hales, K.G., and Fuller, M.T. (1997). Developmentally regulated mitochondrial fusion mediated by a conserved, novel, predicted GTPase. Cell. 90, 121–129. [DOI] [PubMed] [Google Scholar]
- Hermann, G.J., King, E.J., and Shaw, J.M. (1997). The yeast gene, MDM20, is necessary for mitochondrial inheritance and organization of the actin cytoskeleton. J. Cell Biol. 137, 141–153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hines, J.C., and Ray, D.S. (1998). The Crithidia fasciculata KAP1 gene encodes a highly basic protein associated with kinetoplast DNA. Mol. Biochem. Parasitol. 94, 41–52. [DOI] [PubMed] [Google Scholar]
- Hoare, C.A., and Wallace, F.G. (1966). Developmental stages of trypanosomatid flagellates: a new terminology. Nature 212, 1385–1386. [Google Scholar]
- Hobbs, A.E., Srinivasan, M., McCaffery, J.M., and Jensen, R.E. (2001). Mmm1p, a mitochondrial outer membrane protein, is connected to mitochondrial DNA (mtDNA) nucleoids and required for mtDNA stability. J. Cell Biol. 152, 401–410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson, C.E., and Englund, P.T. (1998). Changes in organization of Crithidia fasciculata kinetoplast DNA replication proteins during the cell cycle. J. Cell Biol. 143, 911–919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones, B.A., and Fangman, W.L. (1992). Mitochondrial DNA maintenance in yeast requires a protein containing a region related to the GTP-binding domain of dynamin. Genes Dev. 6, 380–389. [DOI] [PubMed] [Google Scholar]
- Klingbeil, M.M., Drew, M.E., Liu, Y., Morris, J.C., Motyka, S.A., Saxowsky, T.T., Wang, Z., and Englund, P.T. (2001). Unlocking the secrets of trypanosome kinetoplast DNA network replication. Protist 152, 255–262. [DOI] [PubMed] [Google Scholar]
- Kusel, J.P., Moore, K.E., and Weber, M.M. (1967). The ultrastructure of Crithidia fasciculata and morphological changes induced by growth in acriflavin. J. Protozool. 14, 283–296. [DOI] [PubMed] [Google Scholar]
- Laub-Kupersztejn, R., and Thirion, J. (1969). [Effects of acriflavin and ethidium bromide on incorporation of radioactive precursors into nucleic acids and proteins of the trypanosomide Crithidia luciliae]. Arch. Int. Physiol. Biochim. 77, 566–568. [PubMed] [Google Scholar]
- Matagne, R.F., Michel-Wolwertz, M.R., Munaut, C., Duyckaerts, C., and Sluse, F. (1989). Induction and characterization of mitochondrial DNA mutants in Chlamydomonas reinhardtii. J. Cell Biol. 108, 1221–1226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McConnell, S.J., Stewart, L.C., Talin, A., and Yaffe, M.P. (1990). Temperature-sensitive yeast mutants defective in mitochondrial inheritance. J. Cell Biol. 111, 967–976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McConnell, S.J., and Yaffe, M.P. (1993). Intermediate filament formation by a yeast protein essential for organelle inheritance. Science 260, 687–689. [DOI] [PubMed] [Google Scholar]
- Morris, J.C., Drew, M.E., Klingbeil, M.M., Motyka, S.A., Saxowsky, T.T., Wang, Z., and Englund, P.T. (2001). Replication of kinetoplast DNA: an update for the new millennium. Int. J. Parasitol. 31, 453–458. [DOI] [PubMed] [Google Scholar]
- Ogbadoyi, E.O. (1997). Structural Studies on Nuclear and Mitochondrial Segregation in Trypanosoma brucei. Ph.D Thesis. Manchester, United Kingdom: University of Manchester.
- Otsuga, D., Keegan, B.R., Brisch, E., Thatcher, J.W., Hermann, G.J., Bleazard, W., and Shaw, J.M. (1998). The dynamin-related GTPase, Dnm1p, controls mitochondrial morphology in yeast. J. Cell Biol. 143, 333–349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ploubidou, A., Robinson, D.R., Docherty, R.C., Ogbadoyi, E.O., and Gull, K. (1999). Evidence for novel cell cycle checkpoints in trypanosomes: kinetoplast segregation and cytokinesis in the absence of mitosis. J. Cell Sci. 112, 4641–4650. [DOI] [PubMed] [Google Scholar]
- Riou, G.F., and Saucier, J.M. (1979). Characterization of the molecular components in kinetoplast-mitochondrial DNA of Trypanosoma equiperdum. Comparative study of the dyskinetoplastic and wild strains. J. Cell Biol. 82, 248–263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robertson, M. (1912). Notes on the polymorphism of Trypanosoma gambiense in the blood and its relation to the exogenoous cycle in Glossina palpalis. Proc. R. Soc. Lond. B Biol. Sci. 85, 527–593. [Google Scholar]
- Robertson, M. (1913). Notes on the life history of Trypanosoma gambiense, with a brief reference to the cycles of Trypanosoma nanum and Trypanosoma percorum and in Glossina palpalis. Phil. Trans. R. Soc. B. 203, 161–136. [Google Scholar]
- Robinson, D.R., and Gull, K. (1991). Basal body movements as a mechanism for mitochondrial genome segregation in the trypanosome cell cycle. Nature 352, 731–733. [DOI] [PubMed] [Google Scholar]
- Robinson, D.R., and Gull, K. (1994). The configuration of DNA replication sites within the Trypanosoma brucei kinetoplast. J. Cell Biol. 126, 641–648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson, D.R., Sherwin, T., Ploubidou, A., Byard, E.H., and Gull, K. (1995). Microtubule polarity and dynamics in the control of organelle positioning, segregation, and cytokinesis in the trypanosome cell cycle. J. Cell Biol. 128, 1163–1172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudzinska, M.A., and Vickerman, K. (1968). Infectious blood diseases of man and animals. In: Diseases Caused by Protista, vol. 1, ed. D. Weinman and M. Ristic, New York. London: Academic Press, 217–306. [Google Scholar]
- Russell, D.G., Miller, D., and Gull, K. (1984). Tubulin heterogeneity in the trypanosme Crithidia fasciculata. Mol. Cell. Biol. 4, 779–790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schnaufer, A., Domingo, G.J., and Stuart, K. (2002). Natural and induced dyskinetoplastic trypanosomatids: how to live without mitochondrial DNA. Int. J. Parasitol. 32, 1071–1084. [DOI] [PubMed] [Google Scholar]
- Schnaufer, A., Panigrahi, A.K., Panicucci, B., Igo, R.P.J., Wirtz, E., Salavati, R., and Stuart, K. (2001). An RNA ligase essential for RNA editing and survival of the bloodstream form of Trypanosoma brucei. Science 16, 2159–2162. [DOI] [PubMed] [Google Scholar]
- Sesaki, H., and Jensen, R.E. (1999). Division versus fusion: Dnm1p and Fzo1p antagonistically regulate mitochondrial shape. J. Cell Biol. 147, 699–706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shapiro, T.A., Klein, V.A., and Englund, P.T. (1989). Drug-promoted cleavage of kinetoplast DNA minicircles. Evidence for type II topoisomerase activity in trypanosome mitochondria. J. Biol. Chem. 264, 4173–4178. [PubMed] [Google Scholar]
- Shepard, K.A., and Yaffe, M.P. (1999). The yeast dynamin-like protein, Mgm1p, functions on the mitochondrial outer membrane to mediate mitochondrial inheritance. J. Cell Biol. 144, 711–720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simpson, L. (1968). Effect of acriflavin on the kinetoplast of Leishmania tarentolae. Mode of action and physiological correlates of the loss of kinetoplast DNA. J. Cell Biol. 37, 660–682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simpson, L. (1972). The kinetoplast of the hemoflagellates. Int. Rev. Cytol. 32, 139–207. [Google Scholar]
- Simpson, L., and Maslov, D.A. (1999). Evolution of the U-insertion/deletion RNA editing in mitochondria of kinetoplastid protozoa. Ann. N.Y. Acad. Sci. 870, 190–205. [DOI] [PubMed] [Google Scholar]
- Simpson, L., Thiemann, O.H., Savill, N.J., Alfonzo, J.D., and Maslov, D.A. (2000). Evolution of RNA editing in trypanosome mitochondria. Proc. Nat. Acad. Sci. USA 97, 6986–6993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sogo, L.F., and Yaffe, M.P. (1994). Regulation of mitochondrial morphology and inheritance by Mdm10p, a protein of the mitochondrial outer membrane. J. Cell Biol. 126, 1361–1373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Souto-Padron, T., de Souza, W., and Heuser, J.E. (1984). Quickfreeze, deep-etch rotary replication of Trypanosoma cruzi and Herpetomonas megaseliae. J. Cell Sci. 69, 167–178. [DOI] [PubMed] [Google Scholar]
- Spurr, A.R. (1969). A low-viscosity epoxy resin embedding medium for electron microscopy. J. Ultrastruct. Res. 26, 31–43. [DOI] [PubMed] [Google Scholar]
- Stuart, K., Panigrahi, A.K., Schnaufer, A., Drozdz, M., Clayton, C., and Salavati, R. (2002). Composition of the editing complex of Trypanosoma brucei. Phil. Trans. R. Soc. Lond. B 357, 71–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stuart, K., and Panigrahi, A.K. (2002). RNA editing: complexity and complications. Mol. Microbiol. 45, 591–596. [DOI] [PubMed] [Google Scholar]
- Tarrago-Litvak, L., Viratelle, O., Darriet, D., Dalibart, R., Graves, P.V., and Litvak, S. (1978). The inhibition of mitochondrial DNA polymerase gamma from animal cells by intercalating drugs. Nucleic Acids Res. 5, 2197–2210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tooze, J. (1985). Blocked coated pits in AtT20 cells results from endocytotis of budding retrovirions. J. Cell Biol. 101, 1713–1723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vickerman, K. (1965). Polymorphism and mitochondrial activity in sleeping sickness trypanosomes. Nature 208, 762–766. [DOI] [PubMed] [Google Scholar]
- Vickerman, K. (1969). On the surface coat and flagellum adhesion in trypanosomes. J. Cell Sci. 6, 365–383. [DOI] [PubMed] [Google Scholar]
- Vickerman, K. (1973). The mode of attachment of Trypanosoma vivax in the proboscis of the tsetse fly Glossina fuscipes: an ultrastructural study of the epimastigote stage of the trypanosome. J. Protozool. 20, 394–404. [DOI] [PubMed] [Google Scholar]
- Vickerman, K. (1976). The diversity of the kineoplastid flagellates. In: Biology of the Kinetoplastida, vol. 1, ed. W.H.R. Lumsden and D.A. Evans, London: Academic press, 1–34. [Google Scholar]
- Vickerman, K., Tetley, L., Hendry, K.A., and Turner, C.M. (1988). Biology of African trypanosomes in the tsetse fly. Biol. Cell 64, 109–119. [DOI] [PubMed] [Google Scholar]
- Wong, E.D., Wagner, J.A., Gorsich, S.W., McCaffery, J.M., Shaw, J.M., and Nunnari, J. (2000). The dynamin-related GTPase, Mgm1p, is an intermembrane space protein required for maintenance of fusion competent mitochondria. J. Cell Biol. 151, 341–344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woods, A., Sherwin, T., Sasse, R., MacRae, T.H., Baines, A.J., and Gull, K. (1989). Definition of individual components within the cytoskeleton of Trypanosoma brucei by a library of monoclonal antibodies. J. Cell Sci. 93, 491–500. [DOI] [PubMed] [Google Scholar]
- Woodward, R., Carden, M.J., and Gull, K. (1995). Immunological characterization of cytoskeletal proteins associated with the basal body, axoneme and flagellum attachment zone of Trypanosoma brucei. Parasitology 111, 77–85. [DOI] [PubMed] [Google Scholar]
- Woodward, R., and Gull, K. (1990). Timing of nuclear and kinetoplast DNA replication and early morphological events in the cell cycle of Trypanosoma brucei. J. Cell Sci. 95, 49–57. [DOI] [PubMed] [Google Scholar]
- Yaffe, M.J. (1999a). Dynamic mitochondria. Nat. Cell. Biol. 1, 149–150. [DOI] [PubMed] [Google Scholar]
- Yaffe, M.P. (1999b). The machinery of mitochondrial inheritance and behavior. Science 283, 1493–1497. [DOI] [PubMed] [Google Scholar]
- Zaitseva, G.N., Kolesnikov, A.A., and Shirshov, A.T. (1977). The genetic system of kinetoplasts in trypanosomatides. Mol. Cell. Biochem. 14, 47–54. [DOI] [PubMed] [Google Scholar]