Abstract
Rab GTPases are regulators of membrane traffic. Rabs specifically associate with target membranes via the attachment of (usually) two geranylgeranyl groups in a reaction involving Rab escort protein and Rab geranylgeranyl transferase. In contrast, related GTPases are singly prenylated by CAAX prenyl transferases. We report that di-geranylgeranyl modification is important for targeting of Rab5a and Rab27a to endosomes and melanosomes, respectively. Transient expression of EGFP-Rab5 mutants containing two prenylatable cysteines (CGC, CC, CCQNI, and CCA) in HeLa cells did not affect endosomal targeting or function, whereas mono-cysteine mutants (CSLG, CVLL, or CVIM) were mistargeted to the endoplasmic reticulum (ER) and were nonfunctional. Similarly, Rab27aCVLL mutant is also mistargeted to the ER and transgenic expression on a Rab27a null background (Rab27aash) did not rescue the coat color phenotype, suggesting that Rab27aCVLL is not functional in vivo. CAAX prenyl transferase inhibition and temperature-shift experiments further suggest that Rabs, singly or doubly modified are recruited to membranes via a Rab escort protein/Rab geranylgeranyl transferase-dependent mechanism that is distinct from the insertion of CAAX-containing GTPases. Finally, we show that both singly and doubly modified Rabs are extracted from membranes by RabGDIα and propose that the mistargeting of Rabs to the ER results from loss of targeting information.
INTRODUCTION
Protein prenylation is a common posttranslational lipid modification that involves the covalent addition of C15 farnesyl or more commonly C20 geranylgeranyl (GG) to carboxyl-terminal cysteine residues (Glomset and Farnsworth, 1994; Seabra, 1998). Prenylation is required for proteins to associate with cellular membranes. In the absence of prenyl modification, the affected proteins remain cytosolic and are unable to function properly.
Three distinct protein prenyltransferases have been identified in eukaryotic cells: protein farnesyl transferase (PFT), protein geranylgeranyl transferase type-I (PGGT), and Rab geranylgeranyl transferase (RGGT) (Casey and Seabra, 1996). The first two enzymes are also called CAAX prenyltransferases due to their ability to recognize proteins containing a CAAX motif on their carboxyl terminus. In this motif, C is a cysteine, A is an aliphatic amino acid and X is a methionine, serine, glutamine, or alanine for PFT substrates (e.g., Ras and nuclear lamins), or a leucine or phenylalanine for PGGT substrates (e.g., Rho and Rac). Unlike the CAAX prenyltransferases, RGGT only recognizes Rab substrates in the context of a 1:1 complex with a Rab escort protein (REP) (Andres et al., 1993; Anant et al., 1998; Seabra, 2000). REP recognizes newly synthesized Rabs and presents them to RGGT, which then catalyzes the transfer of GG groups to Rabs (Seabra, 2000; Thoma et al., 2001b,c). Alternatively, newly synthesized Rabs can associate with a preformed REP:RGGT complex (Thoma et al., 2001a)
After prenylation in the cytosol, CAAX-containing GTPases are first targeted to the endoplasmic reticulum (ER) and only subsequently to their site of action (Choy et al., 1999; Apolloni et al., 2000; Michaelson et al., 2001). This common ER entry site seems to be a consequence of the specific localization of the two enzymes that modify the CAAX-GTPases downstream of the prenyltransferases (Dai et al., 1998; Schmidt et al., 1998). These are a prenyl-CAAX protease (Rce1 in mammals, RCE1 or AFC1 in Saccharomyces cerevisiae) that cleaves the last three amino acids (AAX) in the CAAX motif and an isoprenyl cysteine carboxyl methyltransferase (Icmt in mammals, STE14 in S. cerevisiae) that methylates the newly exposed prenylated cysteine. The CAAX motif alone, when fused to EGFP, is able to target this otherwise soluble protein to the ER and Golgi (Choy et al., 1999). Transport of the CAAX proteins from the ER to the plasma membrane requires a second signal within the hypervariable region of these proteins, which is palmitoylation for H-Ras and N-Ras, or a polybasic amino acid sequence for K-Ras (Hancock et al., 1991). This second signal is responsible for different trafficking routes. H-Ras and N-Ras are targeted to the plasma membrane via the classical exocytic pathway, whereas K-Ras travels via an alternative path, which seems to be microtubule dependent (Choy et al., 1999; Apolloni et al., 2000). These mechanisms seem to apply to Rho proteins except that some Rho proteins associate with the cytosolic protein RhoGDI, which adds an additional level of complexity to Rho membrane association (Michaelson et al., 2001).
Much less is known about how Rab proteins associate with membranes and the role of the prenylation motif in the process. There are six different carboxyl-terminal motifs on the 60 known human Rab proteins, XXXCC, XCCXX, XX- CXC, CCXXX, XXCCX and XCXXX (Pereira-Leal and Seabra, 2000, 2001). Most Rab proteins contain two carboxyl-terminal cysteines, both of which undergo geranylgeranylation (Farnsworth et al., 1994). One example is Rab5a (terminating in the sequence XCCXX), a ubiquitous Rab involved in the regulation of early endocytic traffic (Zerial and McBride, 2001). Activated Rab5a interacts with >20 proteins, reflecting the complexity of its mechanism of action (Christoforidis et al., 1999). Proposed functions for Rab5 include promoting homotypic early endosome fusion, regulating clathrin-dependent endocytosis at the plasma membrane, integrating signal transduction with endocytosis, and regulating the motility of early endosomes along microtubules (Tall et al., 2001; Zerial and McBride, 2001). Another example of a doubly geranylgeranylated Rab is Rab27a (terminating in the sequence XXCXC), a cell-type specific Rab that localizes to melanosomes, melanin-containing granules within melanocytes, and other lysosome-related organelles (Marks and Seabra, 2001). Rab27a regulates the transport of melanosomes to the periphery of melanocytes via interaction with melanophilin and myosin Va (Matesic et al., 2001; Fukuda et al., 2002; Hume et al., 2002; Provance et al., 2002; Strom et al., 2002; Wu et al., 2002). In cytotoxic T lymphocytes, Rab27a regulates a late step in the regulated secretion of lytic granules (Haddad et al., 2001; Stinchcombe et al., 2001). Consequently, mutations in Rab27a result in partial albinism and immunodeficiency with hemophagocytic syndrome, a disease called Griscelli Syndrome (Seabra et al., 2002).
Intriguingly, some Rabs possess only one prenylatable carboxyl-terminal cysteine. Most, but not all, mono-cysteine–containing Rabs contain sequences that do not conform to the CAAX motif. For example, Rab13 possesses a CSLG motif, which is unlikely to serve as substrate for the CAAX prenyl transferases. Conversely, Rab8 contains a CVLL motif, which may be geranylgeranylated by both RGGT and PGGT in vitro. Nevertheless, the majority of Rab8 seem to be geranylgeranylated in vivo via the REP/RGGT pathway (Wilson et al., 1998).
The functional significance for the existence of different prenylation motifs and their role in Rab membrane targeting remains obscure. Geranylgeranylation itself is essential for membrane attachment but must serve as a rather nonspecific signal as all Rabs are geranylgeranylated. Replacement of the di-cysteine GGCC carboxyl-terminal sequence in Rab1b for a mono-cysteine motif CLLL did not seem to affect targeting of Rab1b to the endoplasmic reticulum (ER) and Golgi apparatus, suggesting that the prenylation motif is not important for Rab targeting (Overmeyer et al., 2001). Carboxyl methylation could potentially confer specificity to the membrane association of Rabs because only those containing the XXCXC motif are methylated by isoprenylcysteine methyltransferase (Icmt) (Newman et al., 1992; Giannakouros et al., 1993; Li and Stahl, 1993; Smeland et al., 1994; Bergo et al., 2001). However, loss of Icmt activity (and consequently loss of Rab methylation) does not lead to mistargeting of Rab6 in Icmt—/— cells (Bergo et al., 2001). The present studies were designed to address the importance of the prenylation motif in Rab membrane targeting.
MATERIALS AND METHODS
Plasmid Constructs
Human Rab5a cDNA was subcloned into the pEGFPC3 vector (BD Biosciences Clontech, Palo Alto, CA) by using BamHI and XhoI (Strom and Seabra, unpublished data). Briefly, an Eco 47III site was created within the ETSAK sequence of the Rab5 cDNA at the amino acid positions 164 and 165 by using the Quickchange mutagenesis kit (Stratagene, La Jolla, CA). A similar method was used to remove the internal Eco 47III site from the enhanced green fluorescent protein (EGFP) vector. The pEGFP-Rab5aCGC, CC, CCA, CCQNI, CVLL, CSLG and CVIM constructs were obtained by polymerase chain reaction (PCR) by using a primer 100 base pairs upstream of the multiple cloning site of pEGFPC3 and specific primers that changed the CCSN wild-type sequence. These 3′ primers introduced a SalI site downstream of the stop codon. The PCR product was digested with Eco 47III/SalI and was subcloned together with an NdeI/Eco 47III amino-terminal Rab5a fragment obtained from pEGFP-Rab5a into a pEGFPC3 vector digested with NdeI and SalI. The Rab5aSSSN and Q79L mutations were introduced into the pEGFP-Rab5a construct by using the Quickchange mutagenesis kit. The corresponding pET14b-Rab5a mutant constructs were obtained by SpeI/BamHI cloning of Rab5a cDNAs from pEGFPC3 into pET14b-Rab5a (Farnsworth et al., 1994). The myc-epitope EQKLI-SEEDL was introduced into pCMV7 (Andersson et al., 1989) (Hume and Seabra, unpublished data). Human Rab5a cDNA was subcloned into pCMV7-myc after PCR amplification to introduce KpnI and BamHI sites. The myc-Rab5aCVLL construct was obtained by introducing the CVLL sequence into pCMV7-myc-Rab5a by using the Quickchange mutagenesis kit. pEGFP-Rab27a was prepared as described previously (Hume et al., 2001). This construct served as a template to generate pEGFP-Rab27aCVLL by using the Quickchange site-directed mutagenesis kit. The myc-epitope fused with Rab27aCVLL cDNA generated by PCR was subcloned into the XhoI site of the eukaryotic expression vector pCAGGS, which contains the chicken β-actin promoter with CMV-IE enhancer, and the rabbit β-globin poly(A) signal (Barral et al., 2002). The pEGFP-CVLL and pEGFP-CVIM were obtained by replacing 12 base pairs within the stop codon region of the multiple cloning site of pEGFPC3 vector (GAT CTA GAT AAC) for the appropriate base pairs using the Quickchange site-directed mutagenesis kit (Hume and Seabra, unpublished data). Similar constructs were also donated by Mark Philips (New York University School of Medicine, New York, NY). pEGFP-Cdc42 was a gift from Alan Hall (University College, London, United Kingdom). Bovine GDIα cDNA was amplified using Pfu DNA polymerase from a pCB6 vector (a gift from J. Gruenberg, University of Geneva, Geneva, Switzerland), digested with EcoRI and XhoI, and ligated into pGEX-4T1 vector. The sequence of all inserts was confirmed by sequencing.
Recombinant Proteins
Recombinant His6-Rab5a, His6-Rab27a, and His6-KRas proteins were produced in Escherichia coli and purified by nickel Sepharose-affinity chromatography as described previously (Seabra and James, 1998). The plasmid pGEX-4T3-Rac1 was a gift from Melanie Cobb (University of Texas Southwestern Medical Center, Dallas, TX). Recombinant GST-Rac1 and GST-GDIα were expressed in BL21 cells and purified on glutathione-agarose. The recombinant protein prenyltransferases (RGGT, PGGT, and PFT) and REP were prepared by infection of Sf9 cells with recombinant baculoviruses encoding each subunit of the desired enzyme and purified by nickel Sepharose-affinity chromatography as described previously (Armstrong et al., 1995; Seabra and James, 1998). All recombinant proteins were snap frozen in small aliquots and stored at —80°C until use.
In Vitro Prenylation Assays
In vitro prenylation of His6-Rab proteins in 25-μl reaction volumes was performed as described previously (Seabra and James, 1998). For the RGGT assays, 10 μM Rab was incubated with 25 nM RGGT and 5 μM [3H]geranylgeranylpyrophosphate (GGPP) in the presence of various concentrations of REP (0–6 μM) at 37°C for 10 min. For the PGGT assays, various concentrations of the small GTPases (0–10 μM) were incubated with 20 nM PGGT and 5 μM [3H]GGPP at 37°C for 10 min. For the PFT assays, various concentrations of the small GTPases (0–10 μM) were incubated with 40 nM PFT and 1.2 μM [3H]FPP at 37°C for 15 min. In all of the prenylation experiments, the [3H]isoprenyl transferred to the small GTPases was measured by scintillation counting as the precipitable radioactivity after filtration of the reaction mixtures onto 1.2-μm glass fiber filters.
Antibodies
The monoclonal antibodies were used at the following dilutions: anti-transferrin receptor (Zymed Laboratories, South San Francisco, CA) (1:100), anti-human golgin-97 (Molecular Probes, Eugene, OR) (1:100), anti-EEA1 (Transduction Laboratories, Lexington, KY) (1: 50), anti-TRP-1 (ID Labs, Glasgow, United Kingdom) (1:200), anti-c-myc clone 9E10 (Oncogene Research Products, San Diego, CA) (1:50), and anti-Rab5a (Transduction Laboratories) (1:2000). The polyclonal antibodies used were from StressGen (Victoria, British Columbia, Canada): anti-calnexin (1:5000 for immunoblotting and 1:250 for immunofluorescence) and anti-Rab5 (1:2000 for immunoblotting). The monoclonal anti-ERGIC-53 antibody (1:100 dilution) was a gift from Hans-Peter Hauri (University of Basel, Basel, Switzerland).
Cell Culture and Transfection
HeLa and human embryonic kidney (HEK)293 cells were cultured in DMEM supplemented with 10% fetal bovine serum, 2 mM glutamine, 100 U/ml penicillin G, and 100 U/ml streptomycin at 37°C with 10% CO2. Melan-c cells were cultured in RPMI 1640 medium supplemented with 10% fetal bovine serum, 2 mM glutamine, 0.1 mM 2-mercaptoethanol, 200 nM phorbol 12-myristate 13-acetate (Calbiochem, San Diego, CA), 100 U/ml penicillin G, and 100 U/ml streptomycin at 37°C with 10% CO2. Cells used in immunofluorescence experiments were grown on 24-well plated coverslips for 24 h, transfected, and fixed after 24 h (HeLa and HEK293 cells) or 48 h (melan-c cells). Cells for subcellular fractionation were grown in 10-cm dishes, transfected, and homogenized 24 h after transfection. HeLa cells were tranfected with the liposomal transfection reagent Effectene (QIAGEN, Valenica, CA), whereas melan-c and HEK293 cells were transfected with FuGENE6 (Roche Diagnostics, Indianapolis, IN) according to manufacturers' instructions.
Subcellular Fractionation
After transfection, HeLa cells and HEK293 cells were washed with phosphate-buffered saline (PBS), scraped, transferred to 15-ml tubes, and washed once more with PBS. Cells from one dish were resuspended in 450 μl of either homogenization buffer (10 mM Tris-HCl, pH 7.5, 1 mM EDTA, 1 mM dithiothreitol, and Roche mini complete protease inhibitor cocktail) or RabGDI extraction buffer (25 mM HEPES-KOH, pH 7.2, 75 mM KOAc, 5 mM EGTA, 5 mM MgOAc, 1.8 mM CaCl2, 1 mM dithiothreitol, 1 mM GDP, and Roche mini complete protease inhibitor cocktail). Homogenization was achieved by repeated passage through a 25-gauge needle. Cell debris were removed by centrifugation at 200 × g for 5 min, and the postnuclear supernatant was subjected to ultracentrifugation at 100,000 × g by using TLA45 Beckman rotor. The resulting pellet (P100), containing the membrane fraction, was resuspended in an equivalent volume of supernatant (S100), which contained the cytosolic fraction. These fractions were either directly subjected to electrophoresis on 12.5% SDS-PAGE gels followed by immunoblotting as described previously (Barral et al., 2002) or used in subsequent RabGDI extraction assays as described below.
Triton X-114 Partition
Triton X-114 partition was performed as described previously (Seabra et al., 1995). Briefly, lysates obtained from cells transfected with the appropriate vector were adjusted to 1% Triton X-114 and placed on ice for 5 min. Samples were then incubated at 37°C for 5 min, and the phases were separated by centrifugation at 20,000 × g for 5 min. The detergent phase (lower) was removed and the aqueous phase was readjusted to 1% Triton X-114 and the above-mentioned procedure was repeated. The two detergent phase fractions were combined and the volume adjusted to equal that of the aqueous phase. SDS-sample buffer was added to both phases and samples were boiled for 5 min. Equivalent amounts of detergent and aqueous phases were loaded onto 12.5% SDS-PAGE gels, electrophoresed, blotted onto polyvinylidene difluoride membranes, and detected with anti-Rab5a monoclonal antibodies.
Immunofluorescence and Confocal Microscopy
After transfection with the various EGFP-Rab constructs, the cells were washed in PBS and permeabilized for 5 min in K-PIPES buffer containing 0.05% saponin. Cells were then fixed with 3% paraformaldehyde for 15 min, washed three times with phosphate-buffered saline, and mounted onto coverslips. When antibody detection was applied, the cells were further incubated for 15 min in phosphate-buffered saline containing 0.5% bovine serum albumin and 0.05% saponin. The subsequent immunodetection steps were performed in this solution. The cells were incubated with the primary antibody for 30 min and washed three times, followed by incubation of the secondary antibody conjugated to tetramethylrhodamine B isothiocyanate and washed as described above. The coverslips were mounted in ImmunoFluor medium (ICN, Basingstoke, Hants, United Kingdom) and the fluorescence was visualized using a DM-IRBE confocal microscope (Leica, Wetzlar, Germany). Images were processed using TCS-NT software (Leica) associated with the microscope and Adobe Photoshop 4.0 software. All images presented are single sections in the z-plane and are representative of at least 80% of the transfected cells in the coverslip. The derivation, culture, and light microscopic analysis of primary melanocytes was described previously (Barral et al., 2002).
Drug Treatment and Temperature Shift Experiments
For treatment with brefeldin-A cells were seeded in 24-well plates, transfected, and incubated 24 h later with 10 μg/ml brefeldin-A for 1 h before fixation. Cells were then processed for immunofluorescence as described above. GGTI-298, provided by Said Sebti (University of South Florida) (McGuire et al., 1996), is a competitive CAAX peptidomimetic inhibitor specific for PGGT. For inhibition studies, HeLa or HEK293 cells were seeded in six- or 24-well plates and transfected 24 h later. The inhibitor was added 4 h after transfection at a final concentration of 15 μM followed by a 20-h incubation, permeabilization, and fixation as described above. Temperature block experiments were performed at 20°C to prevent exit of secretory proteins from the Golgi apparatus. HeLa cells were seeded in 24-well plates and grown overnight as described above. The following day, cells were transfected and 4 h later they were placed at 20°C for 3 h in media supplemented with 20 mM HEPES buffer (catalog no. 15630-056; Invitrogen, Carlsbad, CA). Some cells were further incubated at 37°C for 1 h and all cells permeabilized and fixed as described above.
GDI Extraction Assays
The P100 fraction obtained by subcellular fractionation as described above from a 10-cm dish was resuspended in 500 μl of RabGDI extraction buffer, recentrifuged at 100,000 × g, and resuspended in 150 μl of GDI extraction buffer. GST-RabGDIα (0–1.25 μg) was incubated at 37°C for 30 min with P100 (5 μl) in RabGDI extraction buffer in a final volume of 25 μl and reactions were centrifuged at 100,000 × g for 30 min. The resulting supernatants were removed and subjected to electrophoresis on 12.5% SDS-PAGE gels followed by immunoblotting by using anti-Rab5a monoclonal antibodies.
Generation of Transgenic Mice and Genetic Crosses
All mice described were maintained and propagated under UK Home Office Project License 70/5071 at the Central Biomedical Services of Imperial College. Transgenic mouse lines were generated as described previously (Barral et al., 2002). Briefly, a 3.4-kb SpeI-BamHI fragment containing PCAG/mycRab27a/β-globin was isolated and microinjected into the pronuclei of one-cell stage embryos from a C57BL/6JxCBA background collected from superovulated female mice. Microinjected eggs were transferred at two-cell stage into the oviducts of pseudopregnant recipient females. Mice born were identified for incorporation of the transgene by PCR by using genomic DNA obtained from mouse-tail biopsy and the oligonucleotides JR119 (5′-ATGGAACAAAAACTCATCTCAGAAGAGG) and JR14 (5′-AGTTGAACTTCCCATCAGTGTACTGGTA) corresponding to the myc-tag and the Rab27a coding sequence, respectively.
RESULTS
In Vitro Prenylation of Rab5a Wild Type and Mutants
To determine the influence of geranylgeranylation in the targeting of Rab proteins, we chose Rab5a as a model. Using site-directed mutagenesis, we substituted the Rab5a carboxyl-terminal geranylgeranylation motif (CCSN) for di-cysteine motifs present in other Rab proteins, CC (Rab1a), CGC (Rab27a), CCQNI (Rab11a), CCA (Rab20), as well as mono-cysteine motifs CSLG (Rab13) and CVLL (Rab8a). Rab5aCVLL is expected to also serve as substrate for PGGT (Casey and Seabra, 1996; Wilson et al., 1998). Additionally, we have generated a Rab5a mutant that contains the K-Ras farnesylation motif CVIM and a nonprenylatable mutant, Rab5aSSSN, where both carboxyl-terminal cysteines were replaced by serine residues.
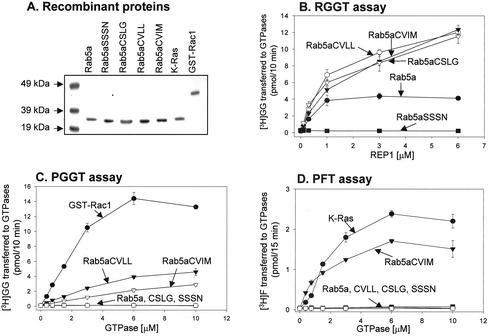
We purified the recombinant Rab5a mutant proteins from E. coli, which lack the prenylation machinery, and used them as substrates in established in vitro prenylation reactions (Figure 1A). Recombinant Rab proteins except Rab5aSSSN are efficiently prenylated by RGGT in vitro, as expected (Figure 1B). Mono-cysteine Rab5a mutants exhibited higher Vmax (our unpublished data), in agreement with recent data suggesting that transfer of the first geranylgeranyl group to Rab7 is 4 times faster than the transfer of the second geranylgeranyl group to Rab7-GG (Thoma et al., 2001c). We subjected the same set of proteins to in vitro prenylation by the CAAX prenyltransferases PFT and PGGT, together with a known substrate for each enzyme, Rac1 for PGGT and K-Ras for PFT. Rab5aCVLL and Rab5aCVIM undergo geranylgeranylation by PGGT but are poor substrates in vitro (Figure 1C). The fact that Rab5a CVLL is a poor substrate for PGGT is in agreement with data from Wilson and coworkers who have shown that Rab8, a Rab with an intrinsic CVLL motif, is a poor substrate for PGGT compared with Cdc42Hs, a natural substrate for this enzyme (Wilson et al., 1998). Unlike PFT, PGGT may recognize structural elements in addition to the carboxyl-terminal CAAX motif (Yokoyama and Gelb, 2000). As expected Rab5aCVIM, but not Rab5aCVLL, is modified by PFT (Figure 1D). Conversely, neither Rab5aCCSN nor Rab5aCSLG are substrates for PGGT (Figure 1C) or PFT (Figure 1D). These results, summarized in Table 1, suggest that the mutations resulted in the expected prenylation behavior in vitro.
Figure 1.
In vitro prenylation of recombinant Rab5a proteins by protein prenyl transferases. (A) Approximately 2 μg of each recombinant small GTPase was subjected to SDS-PAGE and visualized by staining with Coomassie-Blue. (B) RGGT assay. Each reaction contained 25 nM RGGT, 5 μM [3H]GGPP (3000 dpm/pmol), 10 μM either Rab5aCCSN (•), Rab5aCSLG (•), Rab5aCVLL (○), Rab5aCVIM (•), or Rab5aSSSN (▪), and the indicated concentrations of REP. (C) PGGT assay. Each reaction contained 20 nM PGGT, 5 μM [3H]GGPP (3000 dpm/pmol), and the indicated concentrations of Rac1 (•), Rab5aCCSN (○), Rab5aCSLG (▪), Rab5aCVLL (•), Rab5aCVIM (•), or Rab5aSSSN (□). (D) PFT assay. Each reaction contained 40 nM recombinant PFT, 1.2 μM [3H]FPP (11,275 dpm/pmol), and the indicated concentrations of K-Ras (•), Rab5aCCSN (○), Rab5aCSLG (▪), Rab5aCVLL (•), Rab5aCVIM (•), or Rab5aSSSN (□). After 37°C incubation for 10 min (RGGT and PGGT assays) or 15 min (PFT assay), the amount of [3H]GGPP or [3H]FPP transferred to each protein was determined as described under MATERIALS AND METHODS. Each value is the average of triplicate determinations.
Table 1.
Protein prenyl transferase specificities of Rab5a wild-type and mutants in in vitro prehylation assays
| Substrate | RGGT | PGGT | PFT |
|---|---|---|---|
| Rab5a | + | - | - |
| Rab5aCSLG | + | - | - |
| Rab5aCVLL | + | + | - |
| Rab5aCVIM | + | + | + |
| Rab5aSSSN | - | - | - |
Di-cysteine Rab5a Mutants Are Correctly Targeted to Early Endosomes and Are Functional
We constructed mammalian expression vectors expressing the same Rab5a mutants fused to the carboxy terminus of EGFP. After transient transfection in cultured HeLa cells, we performed subcellular fractionation of cellular lysates. As predicted, all mono- and di-cysteine fusion proteins were efficiently prenylated as determined by membrane association (Supplemental Figure 1) and Triton X-114 partition (Supplemental Figure 2, Figure 2). Nevertheless, all transfections resulted in some degree of soluble proteins, which probably resulted from the saturation of the cellular prenylation machinery and of increased association with RabGDI.
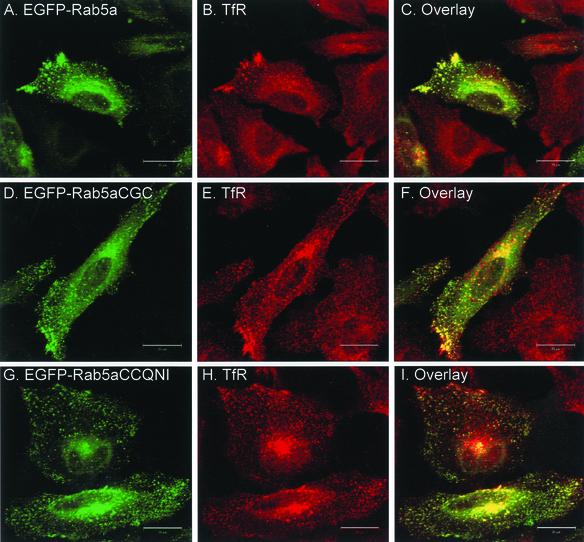
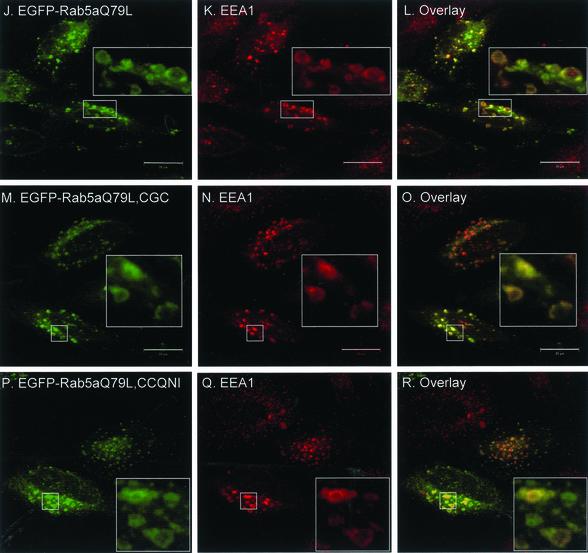
Figure 2.
Transient overexpression of EGFP-Rab5a wild-type and double-cysteine mutant proteins in HeLa cells. HeLa cells were transfected with EGFP-Rab5a (A–C), EGFP-Rab5aCGC (D–F), EGFP-Rab5aCCQNI (G–I), or with the respective constitutively active constructs, EGFP-Rab5aQ79L (J–L), EGFP-Rab5aQ79L, CGC (M–O) and EGFP-Rab5aQ79L, CCQNI (P–R). After 24 h, cells were permeabilized, fixed, and immunostained with an anti-TfR antibody (A–I) or with anti-EEA1 antibody (Panels J-R) as described under MATERIALS AND METHODS. The panels on the right show the merged fluorescent signals. Bar, 20 μm.
Next, we asked whether the Rab5 mutants would correctly target to early endosomes. To address this issue, we performed intracellular localization studies by immunofluorescence on HeLa cells transiently transfected with the EGFP-Rab5a constructs. The cells were costained either for transferrin receptor (TfR), an endosome marker, or with early endosome autoantigen-1 (EEA1), a Rab5 effector protein (Simonsen et al., 1998). EGFP-Rab5a wild-type (Rab5aCCSN) labels punctate cytoplasmic structures peripherally distributed throughout the cell as well as the juxtanuclear region as shown in Figure 2A, for a cell that is representative of the population of transfected cells. These structures colocalize with TfR (Figure 2, A–C) and EEA1 (our unpublished data), as documented previously (Bucci et al., 1992; Mu et al., 1995; Stenmark et al., 1996). The EGFP-Rab5aSSSN protein is present in the nucleus and diffusely throughout the cytoplasm, as observed when EGFP alone is expressed (our unpublished data). The di-cysteine EGFP-Rab5 constructs exhibit a pattern very similar to the EGFP-Rab5a wild-type protein, showing extensive colocalization with TfR (Figure 2, D–I).
To demonstrate that the EGFP-Rab5a di-cysteine proteins are functional in addition to being properly localized, we made use of the Q79L mutation in Rab5a that locks the protein in the active, GTP-bound state. This constitutively active Rab5a mutant induces the formation of enlarged endosomes, consistent with its role in homotypic endosome fusion (Bucci et al., 1992; Stenmark et al., 1994). Transient overexpression of EGFP-Rab5aQ79L causes the redistribution of endosomes from small punctate structures in the cytoplasm to enlarged vesicles in the juxtanuclear region (Figure 2, J–L). The majority of EGFP-Rab5a colocalizes with its effector protein EEA1 in these enlarged endosomes as expected, although we have also occasionally observed some vesicular Rab5a staining absent of EEA1 (Figure 2L). When the Q79L mutation was introduced in two of the di-cysteine constructs, EGFP-Rab5aCGC and EGFP-Rab5aCCQNI, we could also observe the formation of enlarged early endosomes, suggesting that these proteins are both correctly targeted and functional (Figure 2, M–R).
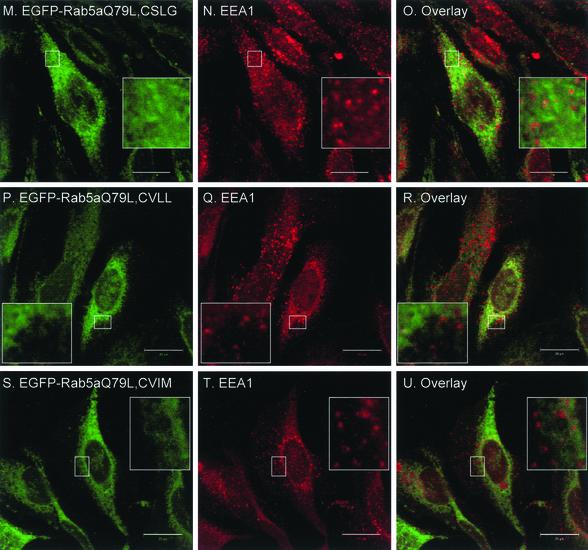
Mono-cysteine Rab5a Mutants Are Mistargeted and Nonfunctional
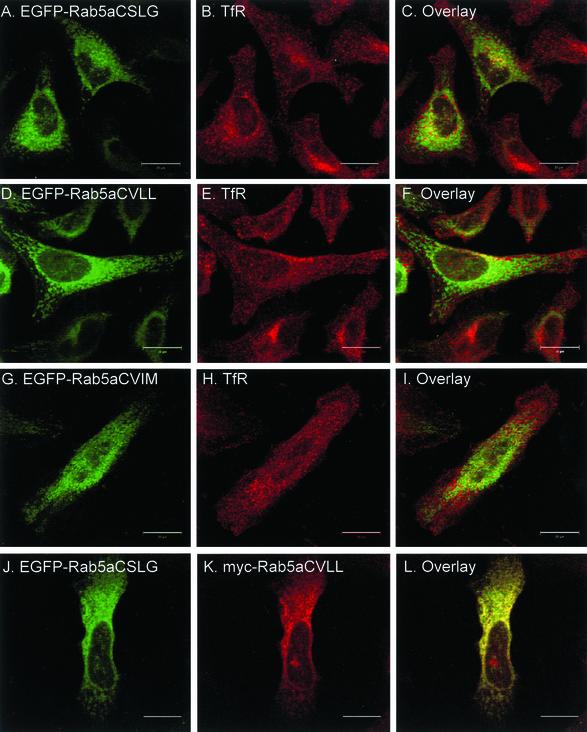
Having established that di-cysteine Rab5a constructs are correctly targeted to endosomes, we then proceeded to analyze the intracellular localization of the mono-cysteine Rab5a mutants. We transiently expressed the various EGFP-Rab5a mutants in HeLa cells and costained for TfR. Surprisingly, we observed that all three mono-cysteine Rab5a mutants displayed a diffuse pattern with staining of the nuclear envelope and some accumulation in the perinuclear region (Figure 3, A, D, and G) rather than the characteristic peripheral punctate appearance of wild-type Rab5a (Figure 2A). Labeling of TfR in the same cells revealed a clear separation of signals, suggesting that the mono-cysteine Rab mutants were mistargeted (Figure 3, A–I). We then introduced the Q79L mutation in the context of the prenylation motif mutations and transiently expressed the mutant proteins as EGFP-fusion proteins in HeLa cells. We observed that Rab5aQ79L mono-cysteine mutants did not induce the formation of enlarged endosomes nor colocalize with EEA1 (Figure 3, M–U). Therefore, the prenylation-induced mistargeting of Rab5a seemed to have prevented its interaction with EEA1. Furthermore, the similarity of the staining pattern suggested that all mono-cysteine Rab5a mutants seemed to be mistargeted to the same intracellular compartment. This suggestion was confirmed when a myc-tagged version of Rab5aCVLL was coexpressed with EGFP-Rab5a, EGFP-Rab5aCSLG, or EGFP-Rab5aCVIM. As expected, myc-Rab5aCVLL did not show colocalization with the small peripheral punctate structures labeled by EGFP-Rab5a (our unpublished data) but instead showed a nearly complete overlapping pattern with EGFP-Rab5aCSLG (Figure 3, J–L) or EGFP-Rab5aCVIM (our unpublished data). Together, these results suggest that the mono-cysteine Rab5a mutants are not functional and not correctly targeted to early endosomes.
Figure 3.
Transient overexpression of EGFP-Rab5a single-cysteine mutant proteins in HeLa cells. HeLa cells were transfected with EGFP-Rab5aCSLG (A–C), EGFP-Rab5aCVLL (D–F), EGFP-Rab5aCVIM (G–I); with the respective constitutively active constructs, EGFP-Rab5aQ79L, CSLG (M–O), EGFP-Rab5aQ79L, CVLL (P–R), and EGFP-Rab5aQ79L, CVIM (S–U), or cotransfected with EGFP-Rab5a and myc-Rab5aCVLL (our unpublished data) as well as EGFP-Rab5aCSLG and myc-Rab5aCVLL (J–L). After 24 h, cells were permeabilized, fixed, and immunostained with an anti-TfR, anti-EEA1, and anti-myc antibodies (middle) as indicated and as described under MATERIALS AND METHODS. The panels on the right show the merged fluorescent signals. Bar, 20 μm.
Rab27aCVLL Does Not Associate with Melanosomes and Is Not Functional
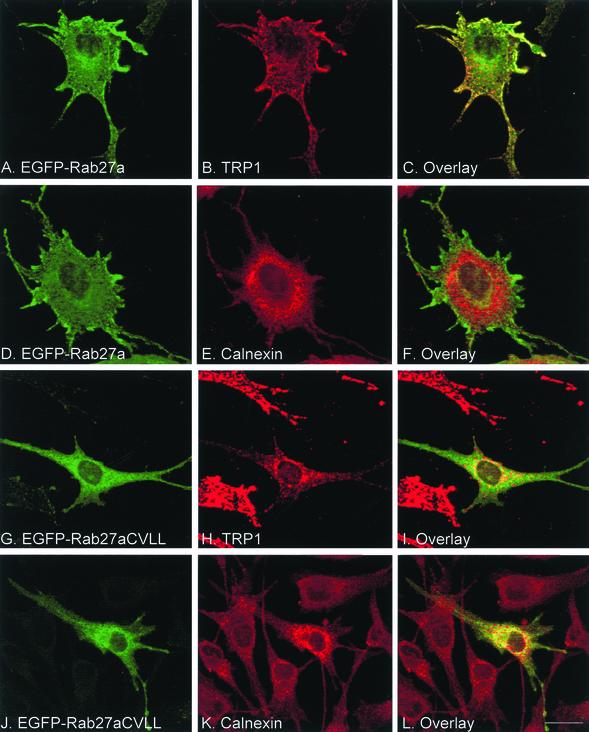
To rule out the possibility that Rab5a is the only Rab that requires two cysteines for proper targeting, we performed similar experiments on Rab27a, a protein that associates specifically with melanosomes (Marks and Seabra, 2001). We replaced the ACGC sequence at the carboxyl terminus of Rab27a for CVLL by site-directed mutagenesis and compared the in vitro prenylation properties and intracellular targeting of wild-type and mutant proteins. As expected, both recombinant His-Rab27a wild type and His-Rab27aCVLL are prenylated by REP/RGGT in vitro, but only His-Rab27aCVLL is geranylgeranylated by PGGT (Supplemental Figure 3, Figure 3). Next, EGFP-Rab27a and Rab27aCVLL constructs were expressed in melan-c cells, melanocytes derived from albino C57BL/6 mice. As expected, EGFP-Rab27a associates with the limiting membrane of melanosomes as deduced from comparisons of the staining patterns of Rab27a and TRP1, a melanosome marker (Figure 4, A–C). Conversely, Rab27aCVLL does not seem to associate with melanosomes (Figure 4, G–I), rather its signal overlaps significantly with that of calnexin, suggesting that the protein associates with ER (Figure 4, J–L, compare with D–F). These results support the Rab5a studies, further suggesting that mono-cysteine prenylation motifs have a mistargeting effect on Rab proteins.
Figure 4.
Transient overexpression of EGFP-Rab27a wild-type and mutant in cultured melanocytes. melan-c cells were transfected with EGFP-Rab27a (A–F) or EGFP-Rab27aCVLL (G–L). After 48 h, cells were permeabilized, fixed, and immunostained with either an anti-TRP1 antibody (B and H) or with an anti-calnexin antibody (E and K) as described under MATERIALS AND METHODS. The panels on the right show the merged fluorescent signals. Bar, 20 μm.
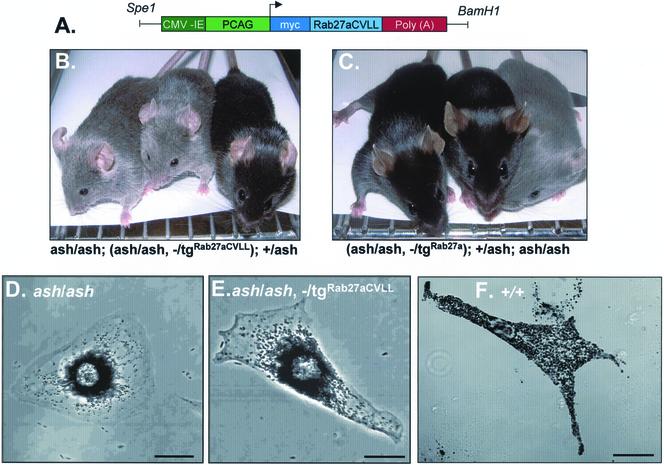
To test the functionality of Rab27aCVLL, we decided to pursue a transgenic approach taking advantage of a Rab27a null mouse strain, ashen (Rab27aash). Ashen mice have hypopigmentation as a result of deficient melanosome transport within skin melanocytes (Marks and Seabra, 2001). We have recently shown that the ashen coat color phenotype can be rescued by crossing with a transgenic mouse line ubiquitously expressing Rab27a wild type (Figure 5C) (Barral et al., 2002). When we generated transgenic lines expressing Rab27aCVLL on an ashen background, we observed little if any coat color correction (Figure 5B). These results were confirmed at the cellular level. Primary cultures of melanocytes derived from ash/ash, —/tgRab27aCVLL mice revealed clustering of melanosomes in the perinuclear area (Figure 5D), as observed in ash/ash melanocytes (Figure 5E) but not in ash/ash, —/tgRab27a melanocytes (Figure 5F) (Barral et al., 2002). Furthermore, transfection of an ashen melanocyte cell line with EGFP-Rab27aCVLL did not result in the redistribution of melanosomes to the cell periphery (our unpublished data), in contrast with the movement rescue effect observed upon transfection of EGFP-Rab27a (Barral et al., 2002). These results suggest that Rab27aCVLL is not functional in vivo.
Figure 5.
Transgenic expression of Rab27a and Rab27aCVLL in ashen mice. (A) Organization of transgenic construct encoding Rab27aCVLL under the control of β-actin promoter. The PCAG/mycRab27/β-globin construct contains the CMV enhancer (dark green) and the chicken β-actin promoter sequence (light green) upstream of a myc-tag (dark blue) in frame with Rab27a cDNA (light blue), followed by the rabbit β-globin poly(A) sequence (red). (B and C) Photography of representative mice for a Rab27aCVLL rescue experiment. Littermates resulting from a cross between a homozygous ashen mouse with a heterozygous transgenic mouse were genotyped as described under MATERIALS AND METHODS. In B from left to right are shown an ashen homozygous mouse that does not carry the transgene (ash/ash), an ashen homozygous mouse expressing transgenic Rab27aCVLL (ash/ash, —/tgRab27aCVLL), and a heterozygous control mouse (+/ash). In C from left to right are shown an ashen homozygous mouse expressing transgenic Rab27a (ash/ash, —/tgRab27a), a heterozygous control mouse (+/ash), and ashen homozygous mouse that does not carry the transgene (ash/ash). (D–F) Primary melanocytes from littermate ash/ash (D), ash/ash, —/tgRab27CVLL (E), or wild type +/+ (F) were cultured and subjected to phase contrast light microscopy as described under MATERIALS AND METHODS. Bars, 20 μm.
Mono-cysteine Rab Proteins Are Targeted to Endoplasmic Reticulum
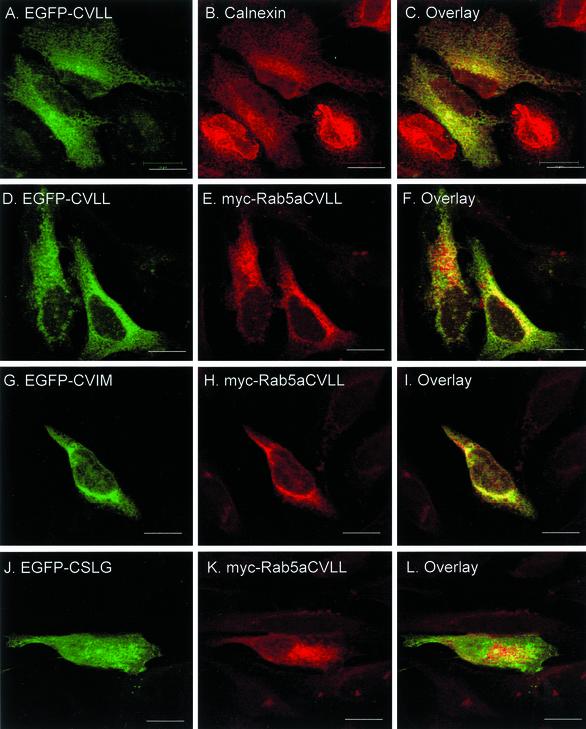
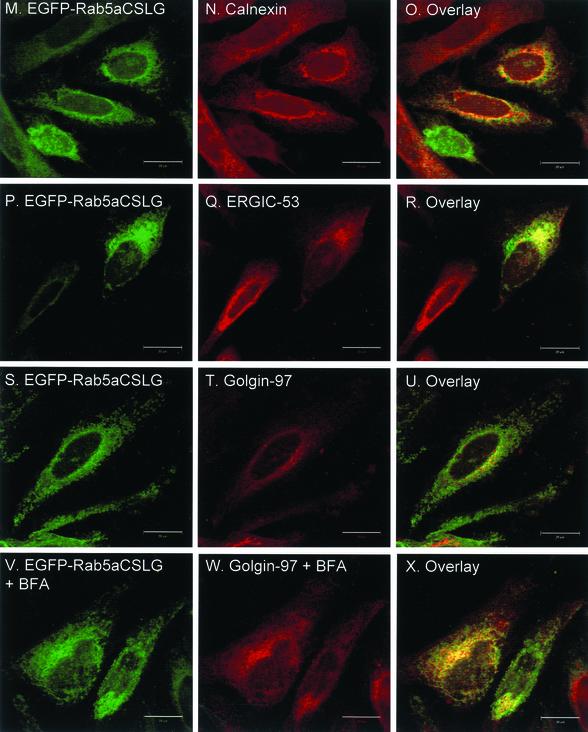
Recent studies on targeting of CAAX-containing proteins revealed that the initial membrane-insertion event occurs in the ER and that reporter-CAAX chimeric constructs such as EGFP-CVLL or EGFP-CVIM associate with and are retained within ER membranes (Choy et al., 1999). Indeed, we confirmed that transfected EGFP-CVLL showed extensive colocalization with calnexin, an ER marker (Figure 6, A–C). When myc-tagged Rab5aCVLL was transiently coexpressed with EGFP-CVLL or EGFP-CVIM in HeLa cells, we observed a good degree of colocalization (Figure 6, D–F and G–I). Conversely, transient overexpression of an EGFP-CSLG construct, which does not conform to the CAAX motif, exhibits a nuclear and diffuse cytoplasmic staining that is typical of soluble proteins (Figure 6, J–L). This distribution contrasts with the pattern exhibited by EGFP-Rab5aCSLG (Figure 3,Figure 3), suggesting that the former is unable to undergo prenylation, whereas the latter undergoes geranylgeranylation via the REP/RGGT pathway. To further study the subcellular localization of the mono-cysteine Rab mutants, we stained cells transfected with EGFP-Rab5aCSLG with known organellar markers. We used the early secretory pathway markers, calnexin (ER), ERGIC-53 (intermediate compartment, IC) and Golgin-97 (Golgi apparatus). We observed a significant overlap of signals between EGFP-Rab5aCSLG and calnexin (Figure 6, M–O), and to a lesser extent ERGIC-53 (Figure 6, P–R) and Golgin-97 (Figure 6, S–U). Finally, we treated EGFP-Rab5aCSLG–transfected cells with the fungal metabolite brefeldin-A, a reagent that causes a dramatic disorganization of the Golgi apparatus with Golgi markers redistributing to ER membranes (Lippincott-Schwartz et al., 1989). In treated cells, the distribution of Golgin-97 became diffuse, an appearance consistent with its association with the ER (Figure 6W), whereas the EGFP-Rab5aCSLG pattern did not change (Figure 6V). Together, these studies suggest that mono-cysteine Rab mutants associate with ER membranes.
Figure 6.
Intracellular localization of mono-cysteine Rab5a mutant proteins after transient transfection of HeLa cells. HeLa cells were transfected with either EGFP-CVLL (A–C), EGFP-CVLL and myc-Rab5aCVLL (D–F), EGFP-CVIM, and myc-Rab5aCVLL (G–I), EGFP-CSLG and myc-Rab5aCVLL (J–L), or EGFP-CSLG (M–X). After 24 h, cells were washed, permeabilized, fixed, and immunostained with either an anti-calnexin antibody (A–C and M–O), an anti-myc antibody (D–L), an anti-ERGIC-53 antibody (P–R), or an anti-Golgin-97 antibody (S–X) as described under MATERIALS AND METHODS. The cells depicted in V–X were treated for 1 h with brefeldin-A as described under MATERIALS AND METHODS. The panels on the right show the merged fluorescent signals. Bar, 20 μm.
Rab Proteins Are Recruited to Membranes via a CAAX-independent Mechanism
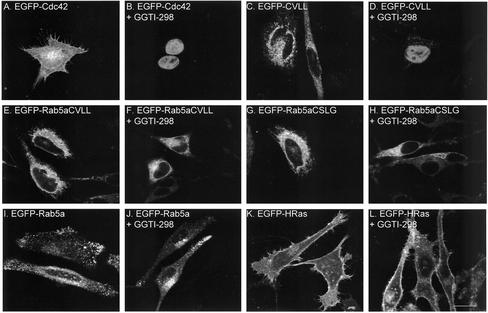
The presence of the mono-cysteine Rab mutants in the ER raised the possibility that they were targeted there via a CAAX-dependent mechanism. To address this issue, we transfected cells with several EGFP-fusion constructs in the presence or absence of a highly specific and effective PGGT inhibitor, GGTI-298 (McGuire et al., 1996). EGFP-Cdc42 was used as a positive control because it is a natural substrate for PGGT. The cellular distribution of EGFP-Cdc42 and EGFP-CVLL changed dramatically upon addition of GGTI-298 (Figure 7, A–D). In the absence of inhibitor, EGFP-Cdc42 exhibited a Golgi/plasma membrane pattern (Figure 7A) as expected (Michaelson et al., 2001) and EGFP-CVLL exhibited an ER pattern (Figure 7C; see also Figure 6). In the presence of inhibitor, both proteins were observed in the nucleus in permeabilized cells (Figure 7, B and D) or in diffuse cytoplasmic and nuclear pattern in nonpermeabilized cells (our unpublished data). A similar pattern is observed when EGFP alone and other soluble proteins are expressed, suggesting that the mentioned EGFP constructs have become soluble. Conversely, the ER-like pattern of expression of EGFP-Rab5aCVLL, EGFP-Rab5aCSLG, EGFP-Rab5a wild type or EGFP-HRas did not change in the presence of GGTI-298 (Figure 7). Furthermore, treatment of GGTI-298 did not affect the proportion of membrane-association or degree of prenylation as determined by Triton X-114 partition of Rab5aCVLL, EGFP-Rab5aCSLG, and EGFP-Rab5a (Supplemental Figure 2, Figure 2). These results are consistent with the in vitro prenylation experiments (for EGFP-Rab5aCSLG and wild type) and suggest that these proteins do not depend on PGGT-mediated geranylgeranylation for membrane association. These results are especially significant for EGFP-Rab5aCVLL because it is a potential substrate for PGGT, although we cannot exclude the possibility that the presence of GGTI-298 shifts prenylation of Rab5aCVLL from PGGT to RGGT. Together, these results suggest that wild-type and mono-cysteine Rab5a mutants are delivered to membranes via a REP/RGGT-dependent mechanism.
Figure 7.
Effect of the PGGT inhibitor GGTI-298 on membrane targeting of small GTPases. HeLa cells were transiently transfected with EGFP-Cdc42 (A and B), EGFP-CVLL (C and D), EGFP-Rab5aCVLL (E and F), EGFP-Rab5aCSLG (G and H), EGFP-Rab5a (I and J), or EGFP-HRas (K and L) for 4 h, followed by incubation for 20 h in the absence or presence of 15 μM GGTI-298 as indicated. Cells were then permeabilized, fixed and visualized as described under MATERIALS AND METHODS. Bar, 20 μm.
Intracellular Trafficking of Rabs Is Distinct from CAAX-containing Proteins
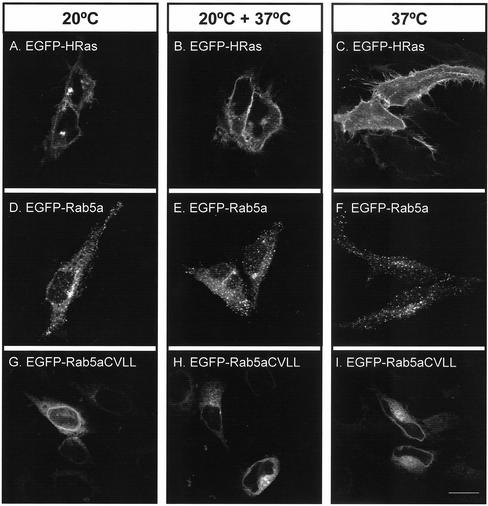
We also addressed the possibility that Rab proteins are first delivered to the ER and then passively follow the secretory pathway until they reach their target organelle, as demonstrated for H-Ras and N-Ras (Choy et al., 1999; Apolloni et al., 2000). In this model, the presence of the mono-cysteine Rab mutants in the ER could be due to a failure to progress through the secretory pathway. To test this hypothesis, we used a temperature shift experiment. Incubating cultured cells at 20°C blocks protein exit from the Golgi apparatus, thus resulting in massive accumulation of secretory proteins in this organelle (Matlin and Simons, 1983; Stinchcombe et al., 1995). We confirmed this finding with EGFP-HRas, which accumulated in the Golgi apparatus after 3 h at 20°C (Figure 8A). Shifting the cells back to 37°C led to the expression of EGFP-HRas at the plasma membrane (Figure 8B). The temperature shift to 20°C did not affect the localization of EGFP-Rab5aCVLL to the ER (Figure 8, G–I) or that of EGFP-Rab5a to early endosomes (Figure 8, D–F). These results provide for the first time evidence that wild-type Rab proteins do not follow the same pathway as H-Ras and other CAAX-containing GTPases and thus argue against the idea that the ER represents the initial point of membrane insertion of Rabs.
Figure 8.
Effect of temperature shift on membrane targeting of small GTPases. HeLa cells were transfected with either EGFP-HRas (A–C), EGFP-Rab5a (D–F), or EGFP-Rab5aCVLL (G–I) for 4 h and subjected to one of the indicated treatments: 3 h at 20°C (A, D, and G); 3 h at 20°C followed by 1 h at 37°C (B, E, and H); or 3 h at 37°C (C, F, and I). Subsequently, cells were permeabilized, fixed, and visualized as described under MATERIALS AND METHODS. Bar, 20 μm.
Mono-cysteine Rab Proteins Are Able to Interact with GDI
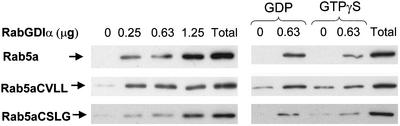
The results mentioned above support the current model that Rabs are directly targeted to their final destination, but they do not exclude the possibility that Rabs are initially targeted to the ER by the REP/RGGT pathway and then retrieved by RabGDI, which mediates specific organelle targeting. In this model, the presence of the mono-cysteine Rab mutants in the ER could be due to a failure of RabGDI to retrieve the mono-geranylgeranylated Rabs from ER membranes. To test this hypothesis, membrane fractions obtained from transiently transfected cells containing approximately equivalent levels of Rab5a wild-type and mono-cysteine mutant proteins were incubated with RabGDIá and then recentrifuged to separate soluble and membrane-associated proteins. The presence of EGFP-Rab5a in the soluble fraction suggested membrane extraction by RabGDIα. Indeed, increasing concentrations of RabGDIα resulted in increasing amounts of Rab5a in the soluble fraction (Figure 9, left). Furthermore, Rab5a extraction was promoted by incubation with GDP and prevented by GTPγS (Figure 9, right). We observed no differences between wild-type and mutant Rab5aCVLL or Rab5aCSLG in their ability to be extracted from membranes by RabGDI (Figure 9). Furthermore, cooverexpression of RabGDIα with the EGFP-Rab5a mutants did not lead to endosome targeting of these mutants (our unpublished data). These results suggest that the ER mistargeting of mono-cysteine Rab5a mutants is not due to loss of retrieval by RabGDI.
Figure 9.
GDI extraction of Rab5a wild-type and mono-cysteine mutants. HEK293 cells transiently transfected with EGFP-Rab5a, EGFP-Rab5aCVLL, or EGFP-Rab5aCSLG were homogenized, fractionated into membrane and soluble fractions, and the membrane fraction (denoted Total) was subjected to extraction with the indicated concentrations of GST-GDIα in the presence or absence of GDP or GTPγS as indicated, followed by SDS-PAGE as described under MATERIALS AND METHODS. The EGFP-Rab5a proteins were visualized by immunoblotting with an anti-Rab5a antibody. Calnexin was used as a loading control (our unpublished data).
DISCUSSION
The present work suggests the following insights into the mechanisms regulating the membrane-association of Rab GTPases: 1) di-geranylgeranylation is required for targeting and function of Rab5a and Rab27a to endosomes and melanosomes, respectively, because mono-geranylgeranylated mutants are mistargeted to ER membranes and are unable to function; 2) mono- and di-geranylgeranylated Rabs are preferentially processed by the REP/RGGT pathway; 3) the mechanism of membrane association of Rabs is distinct from CAAX-containing Ras and Rho proteins; and 4) the ER represents a default location for Rabs that have lost targeting information.
Rab proteins exhibit a variety of mono- and di-cysteine prenylation motifs (Pereira-Leal and Seabra, 2000, 2001). We decided to use Rab5a and Rab27a as models to study the influence of those prenylation motifs on the membrane targeting of Rab GTPases. Replacement of the di-cysteine carboxyl-terminal prenylation motif in Rab5a by di-cysteine motifs present in other Rabs, such as CC, CCA, CGC, and CCQNI, had no effect on targeting to early endosomes. Moreover, these constructs seemed to be functioning properly because the introduction of the GTPase-deficient Q79L mutation onto the prenylation motif mutants resulted in the formation of enlarged endosomes as observed for EGFP-Rab5aQ79L. One of these di-cysteine motifs (CGC) serves also as a methylation signal. Unfortunately, our results do not shed any new light into the role of Rab methylation, because Rab5aCGC seemed to be correctly targeted to early endosomes at steady state. Furthermore, targeting of Rabs susceptible to be methylated is not affected in cells that lack Icmt activity (Bergo et al., 2001) (Gomes and Seabra, unpublished observations).
When the di-cysteine carboxyl-terminal motifs of both EGFP-Rab5a and EGFP-Rab27a were changed to mono-cysteine motifs, the transiently expressed proteins were mistargeted and seemed to associate with ER membranes irrespective of the motif itself (CSLG, CVLL, or CVIM). Furthermore, the mutants were inactive as determined in cultured cells for Rab5a and in vivo for Rab27a, presumably as a direct consequence of the mistargeting. Previous work showed that a Rab1b protein engineered to be prenylated by both RGGT and PGGT (Rab1bCLLL) is correctly targeted to ER/Golgi membranes and is functional (Overmeyer et al., 2001). Although apparently contradictory to the present results, these observations could be explained by the fact that the target membrane for the wild-type Rab1b protein and the location for mistargeted mono-geranylgeranylated mutants is coincidentally the same one, i.e., the ER. The use of post-Golgi Rabs in this study may have helped in the identification of mistargeted proteins given the different cellular distributions of the ER versus endosomes or melanosomes.
Despite a decade of exciting research into the function of Rab proteins, little is known about the mechanisms regulating their specific targeting to distinct organelles. One model proposes that newly synthesized and geranylgeranylated Rabs are delivered by REP to their specific target membrane (Alexandrov et al., 1994; Wilson et al., 1996; Seabra, 1998; Pfeffer, 2001). It is also possible that REP delivers the newly geranylgeranylated Rab directly to RabGDI in the cytosol, which then mediates specific targeting of Rabs (Sanford et al., 1995). Another possibility is that REP delivers Rabs nonspecifically to ER membranes, which serves as the initial membrane insertion site. In this scenario, Rabs reach their final destination by flowing passively via vesicular transport, as proposed for transmembrane proteins and for the related Ras and Rho CAAX-containing proteins, or alternatively RabGDI extracts ER-associated Rabs and delivers them specifically to their target membranes.
The unexpected observation that Rab mono-cysteine mutants were mistargeted to ER membranes provided us with a new tool to investigate this topic. The present results further suggest that Rab prenylation and targeting are distinct from the CAAX-containing proteins. First, we established that the CSLG motif present in Rab13 is not recognized by the two known CAAX-prenyl transferases, suggesting that the REP/RGGT pathway is the only possible route to geranylgeranylation and membrane-association for at least one mono-prenylated Rab. Second, we demonstrated that a specific PGGT inhibitor, GGTI-298, has no effect on the membrane association of the mono-geranylgeranylated Rabs. This result is consistent with a previous study suggesting that PGGT inhibitors do not affect prenylation and membrane association of the mono-geranylgeranylated wild-type Rab, Rab8a (Wilson et al., 1998). Third, we subjected cells to a 20°C block to prevent exit of proteins from the Golgi apparatus and showed that the incubation did not affect the membrane location of either wild-type di-GG-Rab5a to endosomes or mutant mono-GG-Rab5a to the ER. Our results are most consistent with a direct pathway for the specific targeting of Rabs, although we cannot distinguish at present whether targeting is mediated by REP or RabGDI. Future work will be necessary to clarify these issues.
Why are the mono-GG-Rab mutants unable to stably associate with their target membranes and instead associate with the ER? One possibility is that the ER serves as the initial entry site for newly prenylated Rabs (see above) and that specific targeting is dependent on RabGDI-mediated ER extraction and correct delivery. Our results are inconsistent with this hypothesis, because we show that RabGDIα is able to extract both mono- and di-geranylgeranylated Rabs, in agreement with a previous report (Figure 9) (Ullrich et al., 1993). Nevertheless, there remains the possibility that additional factors required for extraction in vivo, such as the putative Rab recycling factor, require di-geranylgeranylated Rabs (Luan et al., 1999). Another possibility is that membrane targeting of Rabs is partially regulated by the lipid environment, such that mono-GG-Rabs exhibit lower affinity or are unstable in the target membranes. A recent report from Gruenberg and coworkers suggests that changes in the biophysical properties of membranes seem to influence Rab activity because increases in cholesterol content of late endosomes results in a stabilization of Rab7 at the membrane (Lebrand et al., 2002). The mechanism underlying the cholesterol effect is not understood, it could be promoting the activation of Rab7 or preventing its cytosol extraction by RabGDI, or both. Nevertheless, lipid environment is unlikely to be the sole targeting factor because mono-prenylated Rho proteins, such as RhoD associate with endosomes (Murphy et al., 1996; Michaelson et al., 2001). A third possibility is that REP forms a tight complex with the mono-cysteine Rab5a and Rab27a mutants preventing their specific release into their target organelle (Shen and Seabra, 1996). However, this does not explain why the mono-GG-Rabs are able to associate with ER membranes. A further possibility is that mistargeting to the ER could be a result of methylation after membrane insertion (Dai et al., 1998). However, as mentioned above, the lack of Icmt activity does not affect the targeting of Rabs susceptible to be methylated (Bergo et al., 2001). Irrespective of the precise mechanism, our results suggest that the ER is the default location of prenylated proteins when they do not possess a clear targeting motif.
The evidence suggesting that the number of geranylgeranyl groups influences the membrane targeting of Rab proteins raises the question why do a few Rabs (such as Rab8a and Rab13) only possess a single prenylatable cysteine. Future studies on the mechanisms underlying REP-mediated targeting of Rab proteins should clarify this and the other issues raised by the present work.
Supplementary Material
Acknowledgments
We thank Tony Magee for critical reading of the manuscript; Molly Strom and José Leal for useful suggestions, advice with molecular biology techniques, and the construction of some vectors used in this study; Ross Anders for microinjections to generate transgenic mice; and Alan Hall, Melanie Cobb, Hans Peter Hauri, Jean Gruenberg, Mark Philips, and Said Sebti for gifts of reagents. This work was supported by a Wellcome Trust Program Grant. A.Q.G. and D.C.B. were supported by Ph.D. studentships PRAXIS XXI from Fundação para a Ciência e a Tecnologia, Portugal.
Article published online ahead of print. Mol. Biol. Cell 10.1091/mbc.E02-10-0639. Article and publication date are at www.molbiolcell.org/cgi/doi/10.1091/mbc.E02-10-0639.
Online version of this article contains supplemental figure materials. Online version available at http://www.molbiolcell.org.
References
- Alexandrov, K., Horiuchi, H., Steele-Mortimer, O., Seabra, M.C., and Zerial, M. (1994). Rab escort protein-1 is a multifunctional protein that accompanies newly prenylated rab proteins to their target membranes. EMBO J. 13, 5262–5273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anant, J.S., Desnoyers, L., Machius, M., Demeler, B., Hansen, J.C., Westover, K.D., Deisenhofer, J., and Seabra, M.C. (1998). Mechanism of Rab geranylgeranylation: formation of the catalytic ternary complex. Biochemistry 37, 12559–12568. [DOI] [PubMed] [Google Scholar]
- Andersson, S., Davis, D.L., Dahlback, H., Jornvall, H., and Russell, D.W. (1989). Cloning, structure, and expression of the mitochondrial cytochrome P-450 sterol 26-hydroxylase, a bile acid biosynthetic enzyme. J. Biol. Chem. 264, 8222–8229. [PubMed] [Google Scholar]
- Andres, D.A., Seabra, M.C., Brown, M.S., Armstrong, S.A., Smeland, T.E., Cremers, F.P., and Goldstein, J.L. (1993). cDNA cloning of component A of Rab geranylgeranyl transferase and demonstration of its role as a Rab escort protein. Cell 73, 1091–1099. [DOI] [PubMed] [Google Scholar]
- Apolloni, A., Prior, I.A., Lindsay, M., Parton, R.G., and Hancock, J.F. (2000). H-ras but not K-ras traffics to the plasma membrane through the exocytic pathway. Mol. Cell Biol. 20, 2475–2487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armstrong, S.A., Brown, M.S., Goldstein, J.L., and Seabra, M.C. (1995). Preparation of recombinant Rab geranylgeranyltransferase and Rab escort proteins. Methods Enzymol. 257, 30–41. [DOI] [PubMed] [Google Scholar]
- Barral, D.C., Ramalho, J.S., Anders, R., Hume, A.N., Knapton, H.J., Tolmachova, T., Collinson, L.M., Goulding, D., Authi, K.S., and Seabra, M.C. (2002). Functional redundancy of Rab27 proteins and the pathogenesis of Griscelli syndrome. J. Clin. Investig. 110, 247–257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergo, M.O., Leung, G.K., Ambroziak, P., Otto, J.C., Casey, P.J., Gomes, A.Q., Seabra, M.C., and Young, S.G. (2001). Isoprenylcysteine carboxyl methyltransferase deficiency in mice. J. Biol. Chem. 276, 5841–5845. [DOI] [PubMed] [Google Scholar]
- Bucci, C., Parton, R.G., Mather, I.H., Stunnenberg, H., Simons, K., Hoflack, B., and Zerial, M. (1992). The small GTPase rab5 functions as a regulatory factor in the early endocytic pathway. Cell 70, 715–728. [DOI] [PubMed] [Google Scholar]
- Casey, P.J., and Seabra, M.C. (1996). Protein prenyltransferases. J. Biol. Chem. 271, 5289–5292. [DOI] [PubMed] [Google Scholar]
- Choy, E., Chiu, V.K., Silletti, J., Feoktistov, M., Morimoto, T., Michaelson, D., Ivanov, I.E., and Philips, M.R. (1999). Endomembrane trafficking of ras: the CAAX motif targets proteins to the ER and Golgi. Cell 98, 69–80. [DOI] [PubMed] [Google Scholar]
- Christoforidis, S., McBride, H.M., Burgoyne, R.D., and Zerial, M. (1999). The Rab5 effector EEA1 is a core component of endosome docking. Nature 397, 621–625. [DOI] [PubMed] [Google Scholar]
- Dai, Q., Choy, E., Chiu, V., Romano, J., Slivka, S.R., Steitz, S.A., Michaelis, S., and Philips, M.R. (1998). Mammalian prenylcysteine carboxyl methyltransferase is in the endoplasmic reticulum. J. Biol. Chem. 273, 15030–15034. [DOI] [PubMed] [Google Scholar]
- Farnsworth, C.C., Seabra, M.C., Ericsson, L.H., Gelb, M.H., and Glomset, J.A. (1994). Rab geranylgeranyl transferase catalyzes the geranylgeranylation of adjacent cysteines in the small GTPases Rab1A, Rab3A, and Rab5A. Proc. Natl. Acad. Sci. USA 91, 11963–11967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukuda, M., Kuroda, T.S., and Mikoshiba, K. (2002). Slac2-a/melanophilin, the missing link between Rab27 and myosin Va: implications of a tripartite protein complex for melanosome transport. J. Biol. Chem. 277, 12432–12436. [DOI] [PubMed] [Google Scholar]
- Giannakouros, T., Newman, C.M., Craighead, M.W., Armstrong, J., and Magee, A.I. (1993). Post-translational processing of Schizosaccharomyces pombe YPT5 protein. In vitro and in vivo analysis of processing mutants. J. Biol. Chem. 268, 24467–24474. [PubMed] [Google Scholar]
- Glomset, J.A., and Farnsworth, C.C. (1994). Role of protein modification reactions in programming interactions between ras-related GTPases and cell membranes. Annu. Rev. Cell Biol. 10, 181–205. [DOI] [PubMed] [Google Scholar]
- Haddad, E.K., Wu, X., Hammer, J. A., 3rd, and Henkart, P.A. (2001). Defective granule exocytosis in Rab27a-deficient lymphocytes from Ashen mice. J. Cell Biol. 152, 835–842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hancock, J.F., Cadwallader, K., Paterson, H., and Marshall, C.J. (1991). A CAAX or a CAAL motif and a second signal are sufficient for plasma membrane targeting of ras proteins. EMBO J. 10, 4033–4039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hume, A.N., Collinson, L.M., Hopkins, C.R., Strom, M., Barral, D.C., Bossi, G., Griffiths, G.M., and Seabra, M.C. (2002). The leaden gene product is required with Rab27a to recruit myosin Va to melanosomes in melanocytes. Traffic 3, 193–202. [DOI] [PubMed] [Google Scholar]
- Hume, A.N., Collinson, L.M., Rapak, A., Gomes, A.Q., Hopkins, C.R., and Seabra, M.C. (2001). Rab27a regulates the peripheral distribution of melanosomes in melanocytes. J. Cell Biol. 152, 795–808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lebrand, C., Corti, M., Goodson, H., Cosson, P., Cavalli, V., Mayran, N., Faure, J., and Gruenberg, J. (2002). Late endosome motility depends on lipids via the small GTPase Rab7. EMBO J. 21, 1289–1300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li, G., and Stahl, P.D. (1993). Post-translational processing and membrane association of the two early endosome-associated rab GTP-binding proteins (rab4 and rab5). Arch. Biochem. Biophys. 304, 471–478. [DOI] [PubMed] [Google Scholar]
- Lippincott-Schwartz, J., Yuan, L.C., Bonifacino, J.S., and Klausner, R.D. (1989). Rapid redistribution of Golgi proteins into the ER in cells treated with brefeldin A: evidence for membrane cycling from Golgi to ER. Cell 56, 801–813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luan, P., Balch, W.E., Emr, S.D., and Burd, C.G. (1999). Molecular dissection of guanine nucleotide dissociation inhibitor function in vivo. Rab-independent binding to membranes and role of Rab recycling factors. J. Biol. Chem. 274, 14806–14817. [DOI] [PubMed] [Google Scholar]
- Marks, M.S., and Seabra, M.C. (2001). The melanosome: membrane dynamics in black and white. Nat. Rev. Mol. Cell. Biol. 2, 738–748. [DOI] [PubMed] [Google Scholar]
- Matesic, L.E., Yip, R., Reuss, A.E., Swing, D.A., O'Sullivan, T.N., Fletcher, C.F., Copeland, N.G., and Jenkins, N.A. (2001). Mutations in Mlph, encoding a member of the Rab effector family, cause the melanosome transport defects observed in leaden mice. Proc. Natl. Acad. Sci. USA 98, 10238–10243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matlin, K.S., and Simons, K. (1983). Reduced temperature prevents transfer of a membrane glycoprotein to the cell surface but does not prevent terminal glycosylation. Cell 34, 233–243. [DOI] [PubMed] [Google Scholar]
- McGuire, T.F., Qian, Y., Vogt, A., Hamilton, A.D., and Sebti, S.M. (1996). Platelet-derived growth factor receptor tyrosine phosphorylation requires protein geranylgeranylation but not farnesylation. J. Biol. Chem. 271, 27402–27407. [DOI] [PubMed] [Google Scholar]
- Michaelson, D., Silletti, J., Murphy, G., D'Eustachio, P., Rush, M., and Philips, M.R. (2001). Differential localization of Rho GTPases in live cells: regulation by hypervariable regions and RhoGDI binding. J. Cell Biol. 152, 111–126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mu, F.T., Callaghan, J.M., Steele-Mortimer, O., Stenmark, H., Parton, R.G., Campbell, P.L., McCluskey, J., Yeo, J.P., Tock, E.P., and Toh, B.H. (1995). EEA1, an early endosome-associated protein. EEA1 is a conserved α-helical peripheral membrane protein flanked by cysteine “fingers” and contains a calmodulin-binding IQ motif. J. Biol. Chem. 270, 13503–13511. [DOI] [PubMed] [Google Scholar]
- Murphy, C., Saffrich, R., Grummt, M., Gournier, H., Rybin, V., Rubino, M., Auvinen, P., Lutcke, A., Parton, R.G., and Zerial, M. (1996). Endosome dynamics regulated by a Rho protein. Nature 384, 427–432. [DOI] [PubMed] [Google Scholar]
- Newman, C.M., Giannakouros, T., Hancock, J.F., Fawell, E.H., Armstrong, J., and Magee, A.I. (1992). Post-translational processing of Schizosaccharomyces pombe YPT proteins. J. Biol. Chem. 267, 11329–11336. [PubMed] [Google Scholar]
- Overmeyer, J.H., Wilson, A.L., and Maltese, W.A. (2001). Membrane targeting of a Rab GTPase that fails to associate with Rab escort protein (REP) or guanine nucleotide dissociation inhibitor (GDI). J. Biol. Chem. 276, 20379–20386. [DOI] [PubMed] [Google Scholar]
- Pereira-Leal, J.B., and Seabra, M.C. (2000). The mammalian Rab family of small GTPases: definition of family and subfamily sequence motifs suggests a mechanism for functional specificity in the Ras superfamily. J. Mol. Biol. 301, 1077–1087. [DOI] [PubMed] [Google Scholar]
- Pereira-Leal, J.B., and Seabra, M.C. (2001). Evolution of the Rab family of small GTP-binding proteins. J. Mol. Biol. 313, 889–901. [DOI] [PubMed] [Google Scholar]
- Pfeffer, S.R. (2001). Rab GTPases: specifying and deciphering organelle identity and function. Trends Cell Biol. 11, 487–491. [DOI] [PubMed] [Google Scholar]
- Provance, D.W., James, T.L., and Mercer, J.A. (2002). Melanophilin, the product of the leaden locus, is required for targeting of myosin-Va to melanosomes. Traffic 3, 124–132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanford, J.C., Yu, J., Pan, J.Y., and Wessling-Resnick, M. (1995). GDP dissociation inhibitor serves as a cytosolic acceptor for newly synthesized and prenylated Rab5. J. Biol. Chem. 270, 26904–26909. [DOI] [PubMed] [Google Scholar]
- Schmidt, W.K., Tam, A., Fujimura-Kamada, K., and Michaelis, S. (1998). Endoplasmic reticulum membrane localization of Rce1p and Ste24p, yeast proteases involved in carboxyl-terminal CAAX protein processing and amino-terminal a-factor cleavage. Proc. Natl. Acad. Sci. USA 95, 11175–11180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seabra, M.C. (1998). Membrane association and targeting of prenylated Ras-like GTPases. Cell Signal 10, 167–172. [DOI] [PubMed] [Google Scholar]
- Seabra, M.C. (2000). Biochemistry of Rab geranylgeranyl transferase. In: Lipid Modifications of Proteins, vol. XXI, eds. F. Tamanoi and D. Sigman, New York: Academic Press, 131–154. [Google Scholar]
- Seabra, M.C., Ho, Y.K., and Anant, J.S. (1995). Deficient geranylgeranylation of Ram/Rab27 in choroideremia. J. Biol. Chem. 270, 24420–24427. [DOI] [PubMed] [Google Scholar]
- Seabra, M.C., and James, G.L. (1998). Prenylation assays for small GTPases. Methods Mol. Biol. 84, 251–260. [DOI] [PubMed] [Google Scholar]
- Seabra, M.C., Mules, E.H., and Hume, A.N. (2002). Rab GTPases, intracellular traffic, and disease. Trends Mol. Med. 8, 23–30. [DOI] [PubMed] [Google Scholar]
- Shen, F., and Seabra, M.C. (1996). Mechanism of digeranylgeranylation of Rab proteins. Formation of a complex between monogeranylgeranyl-Rab and Rab escort protein. J. Biol. Chem. 271, 3692–3698. [DOI] [PubMed] [Google Scholar]
- Simonsen, A., Lippe, R., Christoforidis, S., Gaullier, J.M., Brech, A., Callaghan, J., Toh, B.H., Murphy, C., Zerial, M., and Stenmark, H. (1998). EEA1 links PI(3)K function to Rab5 regulation of endosome fusion. Nature 394, 494–498. [DOI] [PubMed] [Google Scholar]
- Smeland, T.E., Seabra, M.C., Goldstein, J.L., and Brown, M.S. (1994). Geranylgeranylated Rab proteins terminating in Cys-Ala-Cys, but not Cys-Cys, are carboxyl-methylated by bovine brain membranes in vitro. Proc. Natl. Acad. Sci. USA 91, 10712–10716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stenmark, H., Aasland, R., Toh, B.H., and D'Arrigo, A. (1996). Endosomal localization of the autoantigen EEA1 is mediated by a zinc-binding FYVE finger. J. Biol. Chem. 271, 24048–24054. [DOI] [PubMed] [Google Scholar]
- Stenmark, H., Parton, R.G., Steele-Mortimer, O., Lutcke, A., Gruenberg, J., and Zerial, M. (1994). Inhibition of rab5 GTPase activity stimulates membrane fusion in endocytosis. EMBO J. 13, 1287–1296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stinchcombe, J.C., Barral, D.C., Mules, E.H., Booth, S., Hume, A.N., Machesky, L.M., Seabra, M.C., and Griffiths, G.M. (2001). Rab27a is required for regulated secretion in cytotoxic T lymphocytes. J. Cell Biol. 152, 825–834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stinchcombe, J.C., Nomoto, H., Cutler, D.F., and Hopkins, C.R. (1995). Anterograde and retrograde traffic between the rough endoplasmic reticulum and the Golgi complex. J. Cell Biol. 131, 1387–1401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strom, M., Hume, A.N., Tarafder, A.K., Barkagianni, E., and Seabra, M.C. (2002). A family of Rab27-binding proteins: melanophilin links Rab27a and myosin Va function in melanosome transport. J. Biol. Chem. 277, 25423–25430. [DOI] [PubMed] [Google Scholar]
- Tall, G.G., Barbieri, M.A., Stahl, P.D., and Horazdovsky, B.F. (2001). Ras-activated endocytosis is mediated by the Rab5 guanine nucleotide exchange activity of RIN1. Dev. Cell 1, 73–82. [DOI] [PubMed] [Google Scholar]
- Thoma, N.H., Iakovenko, A., Goody, R.S., and Alexandrov, K. (2001a). Phosphoisoprenoids modulate association of Rab geranylgeranyltransferase with REP-1. J. Biol. Chem. 276, 48637–48643. [DOI] [PubMed] [Google Scholar]
- Thoma, N.H., Iakovenko, A., Kalinin, A., Waldmann, H., Goody, R.S., and Alexandrov, K. (2001b). Allosteric regulation of substrate binding and product release in geranylgeranyltransferase type II. Biochemistry 40, 268–274. [DOI] [PubMed] [Google Scholar]
- Thoma, N.H., Niculae, A., Goody, R.S., and Alexandrov, K. (2001c). Double prenylation by RabGGTase can proceed without dissociation of the mono-prenylated intermediate. J. Biol. Chem. 276, 48631–48636. [DOI] [PubMed] [Google Scholar]
- Ullrich, O., Stenmark, H., Alexandrov, K., Huber, L.A., Kaibuchi, K., Sasaki, T., Takai, Y., and Zerial, M. (1993). Rab GDP dissociation inhibitor as a general regulator for the membrane association of rab proteins. J. Biol. Chem. 268, 18143–18150. [PubMed] [Google Scholar]
- Wilson, A.L., Erdman, R.A., Castellano, F., and Maltese, W.A. (1998). Prenylation of Rab8 GTPase by type I and type II geranylgeranyl transferases. Biochem. J. 333, 497–504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson, A.L., Erdman, R.A., and Maltese, W.A. (1996). Association of Rab1B with GDP-dissociation inhibitor (GDI) is required for recycling but not initial membrane targeting of the Rab protein. J. Biol. Chem. 271, 10932–10940. [DOI] [PubMed] [Google Scholar]
- Wu, X., Wang, F., Rao, K., Sellers, J.R., and Hammer, J.A., 3rd. (2002). Rab27a is an essential component of melanosome receptor for myosin Va. Mol. Biol. Cell 13, 1735–1749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yokoyama, K., and Gelb, M.H. (2000). Protein geranylgeranyltransferase type I. In: Lipid Modifications of Proteins, vol. XXI, eds. F. Tamanoi and D. Sigman, New York: Academic Press, 106–130. [Google Scholar]
- Zerial, M., and McBride, H. (2001). Rab proteins as membrane organizers. Nat. Rev. Mol. Cell. Biol. 2, 107–117. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.