Abstract
Recent studies have demonstrated that PH domains specific for PI(3,4,5)P3 accumulate at the leading edge of a number of migrating cells and that PI3Ks and PTEN associate with the membrane at the front and back, respectively, of chemotaxing Dictyostelium discoideum cells. However, the dependence of chemoattractant induced changes in PI(3,4,5)P3 on PI3K and PTEN activities have not been defined. We find that bulk PI(3,4,5)P3 levels increase transiently upon chemoattractant stimulation, and the changes are greater and more prolonged in pten– cells. PI3K activation increases within 5 s of chemoattractant addition and then declines to a low level of activity identically in wild-type and pten– cells. Reconstitution of the PI3K activation profile can be achieved by mixing membranes from stimulated pi3k1–/pi3k2– cells with cytosolic PI3Ks from unstimulated cells. These studies show that significant control of chemotaxis occurs upstream of the PI3Ks and that regulation of the PI3Ks and PTEN cooperate to shape the temporal and spatial localization of PI(3,4,5)P3.
INTRODUCTION
Chemotaxis is a fundamental cellular response and its basic mechanisms have been highly conserved in evolution. During chemotaxis, a cell senses the direction of an external gradient and responds by polarizing and migrating toward the source. This fascinating process has been implicated in lymphocyte homing, axon guidance, embryogenesis, wound healing, and in multiple inflammatory disorders, and it also plays a central role during the early stages of the developmental program of the social amoebae Dictyostelium discoideum (Parent and Devreotes, 1996; Jones, 2000; Moser and Loetscher, 2001; Carlos, 2001; Patel and Haynes, 2001; Thelen, 2001; Rubel and Cramer, 2002). Through studies in this model system and mammalian leukocytes, the signaling events of chemotaxis are being elucidated. In both systems, the response is mediated through seven transmembrane receptors coupled to heterotrimeric G-proteins (Parent and Devreotes, 1999; Rickert et al., 2000; Chung et al., 2001, Stephens et al., 2002; Weiner, 2002). Recruitment of pleckstrin homology (PH) domain containing proteins, which bind to phosphoinositides (PIs) on the membrane, is a key event downstream of G-protein activation leading to pseudopodia production.
Studies of the distributions of signaling components in chemotaxing amoeba and leukocytes have been particularly useful in delineating the mechanisms of chemotaxis. In chemotaxing cells, surface receptors are uniformly distributed on the membrane, and chemoattractant binding to the receptors mirrors the external gradient (Xiao et al., 1997; Servant et al., 1999; Ueda et al., 2001). Furthermore, the G-proteins are distributed fairly uniformly along the membrane, and their activation likely reflects the external gradient (Jin et al., 2000 and unpublished observations). However, the PH domain containing proteins bind to the membrane in sharply localized regions at the leading edges of amoebae and neutrophils (Parent et al., 1998; Meili et al., 1999; Servant et al., 2000; Funamoto et al., 2001). PH domains that bind specifically to phosphatidylinositol 3,4,5 trisphosphate (PI(3,4,5)P3) and phosphatidylinositol 3,4 bisphosphate (PI(3,4)P2), mark the front most strongly (Dormann et al., 2002; Lemmon et al., 2002).
Production and regulation of PI(3,4,5)P3 and PI(3,4)P2 require most significantly PI 3-kinases (PI3Ks) and PI 3-phoshatases (PTEN; Wymann et al., 2000; Maehama et al., 2001; Vanhaesebroeck et al., 2001). In D. discoideum, there are three PI3Ks, related to the mammalian p110 PI3K, and a single orthologue of mammalian PTEN (Zhou et al., 1995; Iijima and Devreotes, 2002). These enzymes are coordinately regulated in the opposite directions (Funamoto et al., 2002; Iijima and Devreotes, 2002). In resting cells, the PI3Ks are in the cytosol, whereas PTEN is on the membrane. With uniform stimuli, the PI3Ks transiently redistribute from the cytosol to the membrane, whereas PTEN dissociates from the membrane and enters the cytosol. Within a few minutes the enzymes return to their original configurations. In chemotaxing cells, the PI3Ks are targeted to the membrane at the front, whereas PTEN is localized on the membrane at the rear. These movements appear to play a key role in the directional response. In pi3k1–/pi3k2– cells (PI3K1 and PI3K2 have been disrupted) there is a decrease in PH domains recruited to the membrane and marking the leading edge of the chemotaxing cells (Funamoto et al., 2001). The ability of these cells to orient and move in chemotactic gradients is diminished. Similar results were obtained for neutrophils lacking PI3Kγ (Hannigan et al., 2002). In pten– cells, the association of PH domains with the membrane lasts much longer than in wild-type cells, and the region of PH domain binding and pseudopodia extension is extensively broadened suggesting that these binding sites play a central role in instructing the responses involved in chemotaxis, including actin polymerization, to occur at the cell's leading edge (Iijima and Devreotes, 2002). These observations have led to a working hypothesis for directional sensing and chemotactic orientation. Signals from uniformly distributed upstream components regulate membrane association and activation of the key enzymes of PI metabolism. Asymmetry in the response first appears as the movements of PI3Ks and PTEN to the membrane at the front and rear of the cell, respectively. The resulting local accumulation of specific PIs directs events involved in pseudopodia formation.
This is a compelling model, but the alleged changes in PI levels and enzyme activation have not been defined. The only previous report examining PI levels in D. discoideum states that there are no changes in these lipids during exposure of cells to cAMP (Zhou et al., 1998). Similarly, the lipid production in mammalian leukocytes in response to chemokines has been measured, but its relationship to PI3K activity was not determined (Traynor-Kaplan et al., 1989). Previous reports have focused on the translocation pattern of PH domains and activation of protein kinase B (PKB) as indicators of local PI increases and PI3K activation. Although these measurements are informative, they are indirect and, in fact, there is no evidence that PI3K is activated by chemoattractant. It is equally possible that changes in PIs reflected in the PH domain translocation and PKB activation are due to decreases in PTEN activity. To address these issues, we developed assays to directly measure the relative amounts of PI(3,4,5)P3 in wild-type, pi3k1–/pi3k2–, and pten– cells and to monitor the state of PI3K activation in various cells lines during chemotactic stimulation. We further devised a reconstitution assay that reproduces activation and inactivation of PI3K by chemoattractant and G-protein. Thus, for the first time the initial events leading to directional sensing can be examined in a cell free system.
MATERIALS AND METHODS
Cell Culture and Development
D. discoideum were cultured axenically in HL5 medium and developed for 5 h in development buffer (DB) as previously described (Parent et al., 1998). Cell lines used included wild-type (AX3), a PH-eGFP-His expressed in AX3 (YH7), Myr-PI3K1 and Myr-PI3K2 expressed in pi3k1–/pi3k2– (Myr-PI3K1 and Myr-PI3K2), and null mutants pten–, pi3k1–/pi3k2–, gα2-(myc2), gβ–(lw6), and yakA–.
Purification of PHCrac-eGFP-His from D. discoideum
Cells were resuspended in DB at density of 2 × 107/ml. Cells were shaken at 100 rpm for 2 h. The cells were centrifuged and resuspended in ice cold buffer A (10 mM Tris-HCl, pH 7.5, 50 mM NaCl, and 5 mM immidazole). The supernatant was obtained as described (Lilly and Devreotes, 1994) and loaded onto a Ni2+ chelating column (Novagen, Madison, WI). The column was washed with buffer A and washed again with buffer A containing 25 mM immidazole. The protein was eluted at 60, 200, 350, and 500 mM immidazole in buffer A. Then each fraction was concentrated to 1 ml using Centriplus 30 (Amicon).
Dot Blot Assay
The phosphoinositides (Matreya Inc. and Avanti Polar Lipids Inc.) at 0.1 μg/μl in chloroform:methanol:dH2O (1:1:0.3) were spotted (2 μl) onto a PVDF membrane. After drying, the PVDF membrane was blocked in TBS-T with 3% BSA for 1 h. The purified protein (PHCrac-eGFP-His) was added to 10 ml TBS-T, and this solution was used to incubate the PVDF membrane for 2 h at 4°C. The membrane was processed for Western blotting using anti-GFP antibody.
Inositol Headgroup Competition
The specified concentrations (10 μM, 1 μM, 100 nM, 10 nM, 1 nM, and 100 pM) of unlabeled competitor (Cal Biochem; 5 μl) were included in 50-μl samples (final volume) that contained 10 μl purified PHCrac-eGFP-His, 5 μl 10× binding buffer (250 mM Tris, pH 7.4, 1 M KCl, and 10 mM EDTA), 5 μl 5 mg/ml γ-globulin, and 20 nCi of [3H]Ins(1,3,4,5)P4 (NEN Life Science Products; 21 Ci/mmol). The samples were incubated on ice for 30 min. Protein was precipitated with 35 μl of ice cold 30% (wt/vol) polyethylene glycol (PEG) 3350. Samples were vortexed and incubated on ice for additional 10 min. Samples were centrifuged at 10,000 × g at 4°C. Pellets were washed with 1 ml of wash buffer (50 ml 1× binding buffer and 35 ml 30% PEG). Samples were centrifuged again at 10,000 × g for 5 min. The final pellet was dissolved in 1 ml of 1% SDS and counted.
PI3K Activation in Response to GTPγS and cAMP
The 5 h developed cells were treated with 20 mM caffeine for 20 min to synchronize the signaling of the cells. These cells were either treated with 100 μM GTPγS or 10 μM cAMP, and the cells were lysed through a 5-μm filter. The cell lysates were incubated with 20 μCi ATPγ32P (NEN Life Science Products; 3000 Ci/mmol) for 3 min or 30 s in response to GTPγS or cAMP stimulation, respectively. The kinase reaction was stopped by 1 ml 1N HCl. The lipids were extracted with 2 ml chloroform:methanol (1:1). The samples were centrifuged at 1000 rpm for 5 min. The lower phase was isolated and further extracted with 2 ml methanol:1N HCl(1:1). The lower phase was isolated and dried under N2 gas. The silica gel 60 TLC plate (VWR) was prerun overnight with 1.2% potassium oxalate (Sigma) in dH2O:methanol (3:2). The next day the TLC plate was dried and heat-activated in the oven (100°C) for 3 min. The dried lipid samples were resuspended in 30 μl of chloroform:methanol (2:1). Ten microliters of sample was spotted and analyzed by TLC using chloroform:acetone:methanol:acetic acid:dH2O (30:12:10:9:6) as a mobile phase. After the solvent front had reached the top of the plate, the plate was taken out the tank and dried. The Kodak film was used for autoradiograph. To calculate the specific activity of PI3K enzyme, we did the following. We excised the bands that corresponded to PI(3,4,5)P3 and counted using scintillation counter. At 5 s, the rate of PI(3,4,5)P3 production was at a peak. In a typical experiment, the input of radioactivity was 20 μCi (4.44 × 107 dpm). The PI(3,4,5)P3 produced at 5 s corresponded to 4200 dpm. We performed an experiment to estimate a 200 μl of reaction contained 2 nm of ATP. Thus PI(3,4,5)P3 produced was ∼1 pm/107 cells/min assuming 100% recovery through extraction and TLC. All the PI3K reactions in this article had similar activity range.
Adaptation Experiment
The 5 h developed cells were treated with 20 mM caffeine for 20 min. During these 20 min, the cells were either treated with buffer or with 10 μM cAMP for every 2 min. Then the cells were lysed, and the lysates were stimulated with 100 μM GTPγS. The cell lysates were incubated with ATPγ32P for 3 min as described in the previous section. The extraction of lipids follows the previous section.
Intact Cell Lipid Labeling and Extraction
The cells were developed in MES-DB buffer (20 mM MES, pH 6.5, 2 mM MgSO4, 0.2 mM CaCl2) for 5 h. Then the cells were labeled with 32P (NEN Life Science Products; 8500-9120 Ci/mmol) in which 1 mCi was added to 1 ml of cell suspension for 1 h and washed twice with MES-DB buffer. The cells were stimulated with 10 μM cAMP, and the reaction was stopped with 1 ml ice-cold 1N HCl. The lipids were extracted as previously described.
Reconstitution of PIP3 Production Using Wild-type Supernatants
The 5 h developed and caffeine-treated, wild-type and pi3k1–/pi3k2– cells were stimulated with 10 μM of cAMP. The cells were lysed through 5-μm filter. The lysates were mixed with supernatants of wild-type and pi3k1–/pi3k2– cells for 20 s. The reaction was stopped by diluting in 1 ml of ice-cold PM. For GTPγS stimulation, wild-type and pi3k1–/pi3k2– cells were lysed in the presence of 100 μM GTPγS. The lysates were incubated with supernatants of wild-type and pi3k1–/pi3k2– cells for 5 min before being stopped with 1 ml of PM. In both experiments, membrane was pelleted and blotted for anti-GFP antibody.
RESULTS
Characterization of Membrane Binding Sites for PHCrac
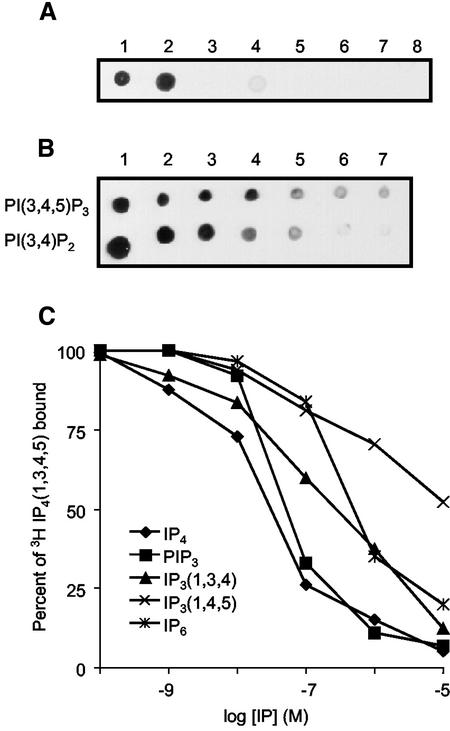
Previous reports have shown that Cytosolic regulator of adenylyl cyclase (Crac) is localized to the membrane at the leading edge of chemotaxing cells (Parent et al., 1998). Crac has a PH domain at its N-terminus, and the PH domain is necessary and sufficient to mediate membrane localization. We used PHCrac as a probe to investigate the binding sites for Crac on the plasma membrane. We expressed a PHCrac-eGFP-His fusion protein in D. discoideum and purified it using Ni2+ chelating affinity chromatography. The protein was mainly eluted in the 200–350 mM immidazole fractions; in the purified fractions it was ∼30% of the total protein (unpublished data). As shown by the dot blot assay, the purified protein bound specifically to two phosphatidyinositol lipids, PI(3,4,5)P3 and PI(3,4)P2 (Figure 1A). After serially diluting concentrations of PI(3,4,5)P3 and PI(3,4)P2, PHCrac bound to slightly lower amounts of PI(3,4,5)P3 than PI(3,4)P2 (Figure 1B). Similar results were obtained with unpurified protein in high-speed supernatant.
Figure 1.
Characterization of PHCrac binding to the PIs. (A) Purified PHCrac-GFP-His proteins were used to probe the PVDF membrane onto which eight specific phosphoinositides were spotted. They are 1) PI(3,4,5)P3, 2) PI(3,4)P2, 3) PI3P, 4) PI(4,5)P2, 5) PI4P, 6) PI, 7) PC, and 8) PS. Each spot represents 0.2 μg of lipid. (B) The purified PHCrac-GFP-His proteins were used to probe the PVDF membrane onto which seven different concentrations of PI(3,4,5)P3 and PI(3,4)P2 were spotted. They are 200, 40, 20, 10, 5, 2.5, and 2 pm (1–7, respectively). (C) The ability of various inositol phosphates and PI(3,4,5)P3 to compete with [3H]Ins(1,3,4,5)P4 for binding to PHCrac was compared using a PEG precipitation method. The concentrations of various inositol phosphates and PI(3,4,5)P3 used were plotted against percentage of [3H]Ins(1,3,4,5)P4 bound. Data represent average of three independent experiments
To examine the lipid headgroup specificity more carefully, we developed a competition assay. Four lipid headgroups, Ins(1,3,4,5)P4, Ins(1,3,4)P3, Ins(1,4,5)P3, and IP6, and PI(3,4,5)P3 were used to compete binding of [3H]Ins(1,3,4,5)P4 to PHCrac. With different concentrations of unlabeled compound ranging from 100 pM to 10 μM, each behaved as a competitive inhibitor. The concentrations that displaced 50% of bound [3H]Ins(1,3,4,5)P4 were 50, 50, and 500 nM for Ins(1,3,4,5)P4, PI(3,4,5)P3, and Ins(1,3,4)P3, respectively (Figure 1C). Ins(1,4,5)P3 and IP6 did not effectively compete [3H]Ins(1,3,4,5)P4 binding (Figure 1C).
PI(3,4,5)P3 Changes in Response to Chemoattractant Stimulation
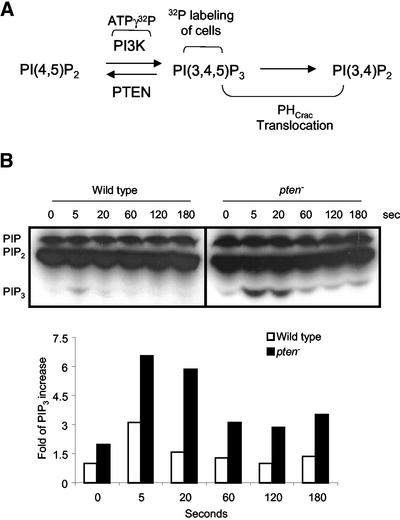
The dot blot assay indicates that PHCrac has high affinity for PI(3,4,5)P3 and PI(3,4)P2 and that its relocation to the membrane reflects changes in these PIs. We used TLC to measure changes in bulk levels of PI(3,4,5)P3 upon chemoattractant stimulation. We metabolically labeled the wild-type, pi3k1–/pi3k2–, and pten– cells with 32P and stimulated these three cell lines with 10 μM cAMP (see Figure 2A). We could not detect any changes in PI(3,4,5)P3 in pi3k1–/pi3k2– cells (unpublished data). In wild-type cells, levels of PI(3,4,5)P3 peaked within 5 s after addition of cAMP and declined after 20 s of cAMP stimulation (Figure 2B). In contrast, in pten– cells levels of PI(3,4,5)P3 peaked at 5 s, remained elevated at 20 s, and declined but remained above prestimulus levels even after 180 s of cAMP stimulation (Figure 2B).
Figure 2.
Changes in the level of PI(3,4,5)P3 in response to chemoattractant. (A) PI3K phosphorylates the 3′ position of PI(4,5)P2 to produce PI(3,4,5)P3. The in vivo and in vitro assays measure the activation of PI3K using ATPγ32P as substrate and PI(3,4,5)P3-32P as a readout or using PHCrac-GFP binding to reflect the relative amount of PI(3,4,5)P3 and PI(3,4)P2 produced. The assay that measures the relative amounts of PI(3,4,5)P3 involves labeling the intact cells with 32P. (B) The fold increase in the relative amounts of PI(3,4,5)P3 in both wild-type and pten– cells in response to cAMP. Wild-type and pten– cells were metabolically labeled with 32P for 1 h. The cells were stimulated with 10 μM cAMP, and the lipids were extracted and analyzed by TLC. Image J was used to quantify the PI(3,4,5)P3 band intensities. The intensity values of PI(3,4,5)P3 band were normalized to wild-type cells at time 0. The experiment was repeated three times.
As shown in Figure 2B, the cell labeling experiment directly measures the relative amounts of PI(3,4,5)P3. Because PHCrac is highly selective for both PI(3,4,5)P3 and PI(3,4)P2, the PHCrac translocation assay probably measures the changes in membrane levels of these two lipids (Figure 2A). Upon uniform cAMP stimulation, PHCrac associates with membrane within 5 s and dissociates from membrane within 60 s (also see Figure 6A). The PHCrac translocation profile closely tracks the increase in the level of PI(3,4,5)P3 in wild-type cells, but it does not match exactly in pten– cells. In pten– cells, the PHCrac remains associated with the membrane even after 3 min of cAMP stimulation. Yet as shown in Figure 2B, the highest levels of PI(3,4,5) P3 are sustained for only 60 s. We can offer two possible explanations for this discrepancy. First, it is possible that the combined levels of PI(3,4,5)P3 and PI(3,4)P2 are sustained at high levels for a longer time in pten– cells. Alternatively, the PHCrac-GFP binding to the membrane saturates and does not subside until PI(3,4,5)P3 decreases very substantially. Of course we cannot rule out that PHCrac may bind to something other than PI(3,4,5)P3 and PI(3,4)P2.
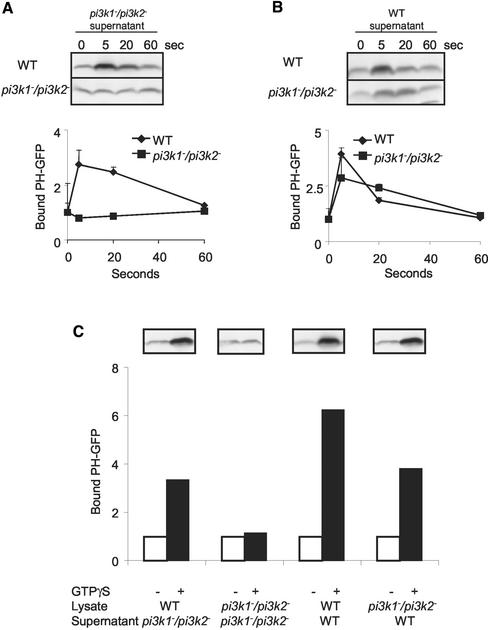
Figure 6.
Reconstitution of PIP3 production with wild-type supernatant. (A) Wild-type and pi3k1–/pi3k2– cells were stimulated with 10 μM cAMP. At specific time points, aliquots of cells were lysed and incubated with pi3k1–/pi3k2– supernatant containing PHCrac-eGFP. The incubation allowed the PI(3,4,5)P3 production, which was measured by PHCrac-eGFP bound to the membrane. The membrane fraction was isolated and blotted for PHCrac-eGFP using GFP antibody. The data were normalized to that at time 0. (B) Amount of PHCrac-eGFP that becomes associated with membrane fractions followed chemoattractant stimulation as described in (A) except the supernatant was wild-type supernatant containing PHCrac-eGFP. (C) The cell lysates of wild-type and pi3k1–/pi3k2– were stimulated with GTPγS. The cell lysates were incubated with supernatant as indicated in the panel. Image J was used to quantify the band intensities of PHCrac-eGFP. The GTPγS treated samples were normalized to those of w/o GTPγS treatment. All the graphs in this figure are the average of two independent experiments.
Activation of PI3K in Response to Chemoattractant and GTPγS Stimulation
The steady state level of PI(3,4,5)P3 results from the balance between PI3K and PTEN activities. We developed two assays to measure the activation state of PI3K, reflecting chemoattractant regulation. The in vivo assay measures the state of PI3K activation in brief intervals during stimulation of cells with cAMP. The in vitro assay measures PI3K activation in cell lysates treated with GTPγS. The nonhydrolyzable analogue of GTP can directly activate trimeric G proteins bypassing the need for chemoattractant receptors.
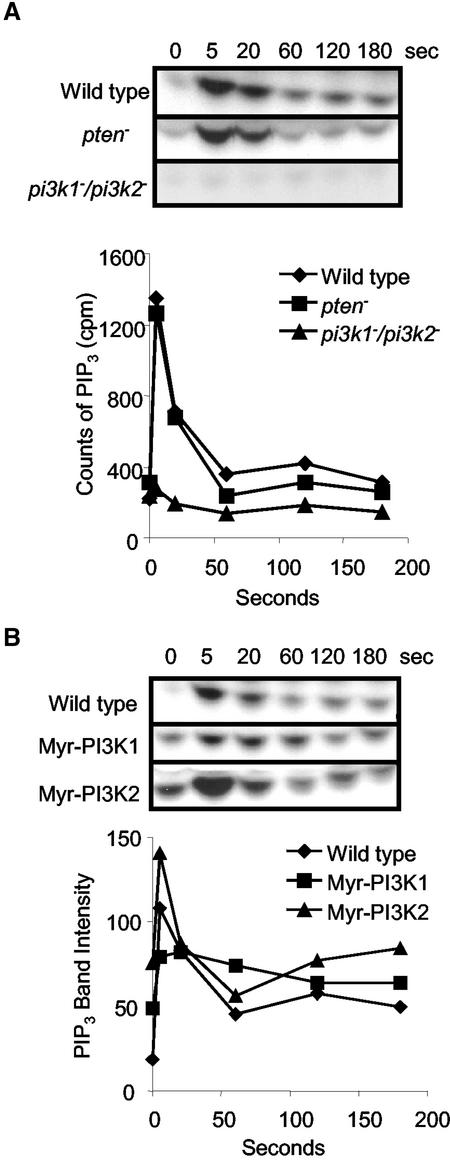
To determine whether the pattern of the PI3K activation during chemoattractant stimulation parallels its translocation to the membrane, we used the in vivo assay. The wild-type, pi3k1–/pi3k2–, and pten– cells were persistently stimulated with uniform cAMP. At times during stimulation, the cells were lysed and the state of PI3K activation was monitored with a 30-s incubation with ATPγ32P followed by measurement of PI(3,4,5)P3-32P. This experiment differs from that described above where cells were labeled with 32P because here only newly synthesized lipids will incorporate the label. In wild-type cells, the rate of PI(3,4,5)P3-32P synthesis peaked within 5 s and started to decline within 20 s (Figure 3A). Significantly, the kinetics of PI3K activation was essentially identical in pten– cells and in wild-type cells, suggesting that the products of PI3K do not influence its activation (Figure 3A). In addition, the phosphatase activity of PTEN is not a significant factor in the assay. In pi3k1–/pi3k2– cells, the activation of PI3K was greatly reduced but not completely abolished: its peak level was ∼4% of that wild-type and pten– cells (Figure 3A). The residual activation in pi3k1–/pi3k2– cells is likely due to another PI3K, such as PI3k3.
Figure 3.
PI3K activation elicited by chemoattractant stimulation. (A) PI3K activation of wild-type, pten– and pi3k1–/pi3k2– cells elicited by cAMP. The cells were lysed at specific time points after cAMP stimulation. The cell lysates were incubated with ATPγ32P for 30 s, and lipids were extracted and analyzed by TLC. The bands corresponding to PI(3,4,5)P3 were cut from plate and counted in scintillation vials. The Y-axis is cpm generated in 30 s, which represents the rate of activation. The graph represents average of three independent experiments. (B) PI3K activation of wild-type, Myr-PIK1, and Myr-PIk2 cells after cAMP stimulation. The cells were processed as described in Figure 3A. The Y-axis represents values of the band intensities of PI(3,4,5)P3 quantified using Image J. The graph represents average of two independent experiments
Because the PI3K activation mirrors the translocation pattern of the kinases, we examined the role of membrane recruitment in the activation. We expressed N-terminal myristol tagged PI3Ks that were targeted to the membrane. In wild-type cells, the basal activities of PI3K were minimal and showed a transient activation upon cAMP stimulation (Figure 3, A and B). Similarly, when wild-type PI3K2 was transformed into pi3k1–/pi3k2– cells, the basal level of PI3K activity was not elevated (unpublished data). However, when myristolated PI3K1 and PI3K2 (Myr-PI3K1 and Myr-PI3K2) were introduced into pi3k1–/pi3k2– cells individually, the prestimulus levels of PI3K activities were significantly elevated in both cells lines (Figure 3B). Upon cAMP stimulation, there was a further activation of PI3K in both cell lines. In Myr-PIK1 cells, the increase was twofold, and the activation decayed very slowly. For Myr-PI3K2 cells, the peak PI3K activation at 5 s was higher than in wild-type cells, and the profile of Myr-PI3K2 activation was more similar to that of wild-type cells. These results indicate that this membrane localization of PI3Ks alone can increase the basal activities of the enzymes, but there may be additional activation during stimulation.
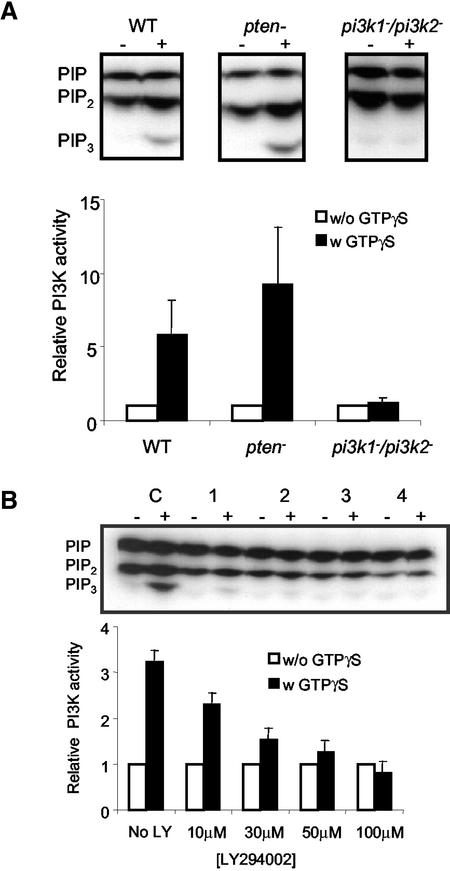
The PI3Ks were also directly activated in cell lysates treated with GTPγS. Using the in vitro assay, lysates of wild-type, pi3k1–/pi3k2–, and pten– cells were incubated with GTPγS and ATPγ32P, and the lipids were extracted and analyzed by TLC. As shown in Figure 4A, the intensities of PI(3,4,5)P3 bands, nearly undetectable in unstimulated cells, were greatly increased in both wild-type and pten– lysates during GTPγS treatment. The fold of PI3K activation by GTPγS was about six- and eightfold in wild-type and pten– cells, respectively. In pi3k1–/pi3k2– cells, the PI3K activation by GTPγS was ∼4% of that of wild type. We pretreated the wild-type cells with different concentrations of LY294002, a PI3K inhibitor, to determine whether the treatment mimics the effect observed in pi3k1–/pi3k2– cells (Figure 4B). With 10 μM LY294002, the intensity of PI(3,4,5)P3 band with GTPγS stimulation was significantly reduced, and at 100 μM there was essentially no increase over the basal level. The intensity of PIP2 band containing both PI(4,5)P2 and PI(3,4)P2 was also reduced with increasing concentrations of LY294002. Although the TLC cannot separate PI(3,4)P2 from PI(4,5)P2, we speculate that a reduced amount of PI(3,4)P2 contributed to the weaker intensity (Zhou et al., 1998).
Figure 4.
Activation of PI3K in response to GTPγS in various cell lines and the effect of LY294002 on PI3K activation. (A) PI3K activation of wild-type, pten– and pi3k1–/pi3k2– cells. The cell lysates of these three different cell lines were treated with GTPγS, and the cell lysates were incubated with ATPγ32P for 3 min. The lipids were extracted and were analyzed by TLC plate. Image J was used to measure the intensities of the PI(3,4,5)P3 bands. The data of activated level (w GTPγS) were normalized to the basal level (w/o GTPγS). The graph represents the average of three independent experiments. (B) The effect of different concentrations of LY294002 on PI3K activation in wild-type cells. Wild-type cell lysates were either incubated with no LY294002 (C) or with 10, 30, 50, and 100 μM of LY294002, respectively (1–4), in the presence or absence of GTPγS. The graph represents the average of two independent experiments
Dependence of PI3K Activation on Gβγ Complex, Gα2, and YakA
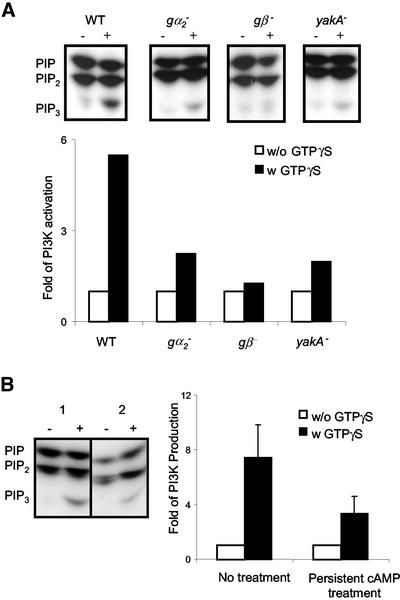
We next examined the upstream signaling components required for the activation of PI3K. In D. discoideum, the trimeric G proteins that coupled to cAMP receptors (cAR1s) are comprised of Gα2 and a unique Gβγ complex. YakA is a member of Dyrk (dual specificity Yak-related kinases) family. Its position in the G-protein–mediated signaling pathway remains to be defined, but it was suggested that YakA lies downstream of G-protein but upstream of PHCrac translocation (van Es et al., 2001). We measured PI3K activation in wild-type, gα2–, gβ–, and yakA– cells. We also included piaA– (pianissimo) cells, which specifically lack adenyly cyclase activation as an additional control (Chen et al., 1997). In the in vivo assay with cAMP, the wild-type and piaA– cells responded normally, whereas all of the other cells failed to give a response. In the in vitro assay with GTPγS, PI3K activation in gα2-cells was reduced 60% compared with that in wild-type cells (Figure 5A). In gβ– cells, GTPγS-mediated PI3K activation was absent. Levels of Gα2 and Gβ are unaffected by disruption of their counterparts (Janetopoulos et al., 2001 and unpublished observations). In yakA– cells the reduction in PI3K activation was not complete as in gβ– cells, but the fold was greatly reduced and was lower than that in gα2– null cells (Figure 5A). Functional levels of receptor/G-protein interaction and PI3K activity are normal in yakA– cells (van Es et al., 2001 and unpublished data). These results demonstrate that signal that triggers the activation of PI3K acts through Gβγ complex and that Gα2 and YakA also contribute to the activation of PI3K.
Figure 5.
PI3K activation in different mutant cell lines and adaptation of PI3K. (A) PI3K activation in gα2–, gβ–, and yakA– null cells. Cell lysates of wild-type, gα2–, gβ–, and yakA– cells were stimulated with GTPγS and processed as described in Figure 4A. The experiment was repeated twice. (B) Adapted wild-type cells show a reduced PI3K activation. Wild-type cells were either treated with buffer (1) or with 10 μM cAMP every 2 min for 20 min (2). The untreated and persistently treated cells were lysed and stimulated with GTPγS. TLC was processed with Image J as described in Figure 4A. The graph represents three independent experiments.
PI3K Activation Is Reduced during Adaptation
Prolonged stimulation of wild-type cells with cAMP induces adaptation, a phenomenon in which cells, once having responded, cannot respond further to the existing stimulus unless a higher concentration is given. Activation of adenylyl and guanylyl cyclase, actin polymerization, and shape changes all display this behavior (Parent and Devreotes, 1996). FRET (fluorescence resonance energy transfer) studies of Gα2 demonstrate that the α2 and βγ subunits remain dissociated as long as the stimulus persists (Janetopoulos et al., 2001). Like the other physiological responses, the recruitment of PHCrac to the plasma membrane adapts during the persistent treatment with cAMP (Lilly and Devreotes, 1995). These observations suggest that adaptation does not require the G-protein subunits to reassociate but occurs upstream of PI(3,4,5)P3. We next examined whether activation of PI3K by GTPγS was affected during adaptation. The in vitro assay was performed on lysates of wild-type cells treated with either buffer or with persistent cAMP. With no cAMP pretreatment, GTPγS elicited an eightfold increase in PI3K activation (Figure 5B). However, in cells that had been pretreated with 10 μM cAMP, GTPγS elicited only a threefold increase in PI3K activation, a 65% decrease in the response (Figure 5B). These data demonstrate that the capacity to activate PI3K adapts during persistent stimulation.
Key Regulator Event in Chemotaxis Lies Upstream of PI3Ks
We next addressed whether these regulatory events acted directly on PI3K or whether there are other regulators upstream of PI3Ks that predetermine the activation and inhibition. In the latter instance, the PI3Ks may merely transmit and amplify these upstream signals. We devised a reconstitution assays to distinguish these possibilities. Wild-type or pi3k1–/pi3k2– cells were stimulated with 10 μM cAMP and lysed at specific time points as described in the previous assays. However, in the reconstitution assays, cell lysates were mixed with supernatants of pi3k1–/pi3k2– or wild-type cells. The supernatants also contained PHCrac-eGFP, which bound to PI(3,4,5)P3 and PI(3,4)P2 produced during a brief incubation of the mixed lysates and supernatants. Thus, we are using PHCrac-eGFP bound to membrane as a readout for the production of PI(3,4,5)P3 and PI(3,4)P2. Figure 6A shows that for wild-type cells, the capacity of mixed cell lysates and supernatants to produce PI(3,4,5)P3 varied with the time after stimulation with chemoattractant. Lysates taken at 5 s after stimulation were extremely active, whereas lysates at 60 s were much less active. In the lysates of pi3k1–/pi3k2– cells, there was no PI(3,4,5)P3 production. These results indicate that PI3Ks are required for PI(3,4,5)P3 production. However when the supernatant mixed into the assay was from wild-type cells, the capacity of the pi3k1–/pi3k2– cell lysates to produce PI(3,4,5)P3 was restored (Figure 6B). Similar results were also obtained in assays where lysates were treated with GTPγS (Figure 6C). The lysates of wild-type cells treated with GTPγS produced PI(3,4,5)P3 regardless the source of supernatant that was mixed into the assay. However, lysates of pi3k1–/pi3k2– cells treated with GTPγS only produced PI(3,4,5)P3 when supplied with wild-type supernatant. These results demonstrate that an unknown regulator is transient activated upon cAMP stimulation even in the absence of PI3Ks. Addition of PI3Ks in the supernatant transmits and propagates this signal.
DISCUSSION
All our observations point to the critical role of PI3K regulation in chemotactic signaling. There is a change in the bulk levels of PI(3,4,5)P3 in response to chemoattractant stimulation. The PI3Ks are rapidly activated via chemoattractant receptors or guanine nucleotides, and their activation is diminished in adapted cells. The activation profile of the PI3Ks is dependent on the G-protein βγ complex but independent of PTEN, suggesting that PI(3,4,5)P3 and PI(3,4)P2 do not markedly affect regulation. Membrane recruitment of PI3Ks plays a partial role in activating the enzymes, and there may be additional activation of membrane bound enzymes. Finally, the temporal regulation of the PI3Ks can be reproduced in a cell free system. With this reconstitution assay, we show that the binding/activation sites for PI3Ks are generated in the absence of the enzymes themselves.
Our data are the first demonstration that chemoattractant stimulation elicits an increase in PI(3,4,5)P3 levels in D. discoideum. The rise is transient in wild-type cells, whereas it is higher and more prolonged in pten– cells. The sustained high levels of PI(3,4,5)P3 likely contribute to the dramatic chemotactic defects observed in pten– cells. Consistent with this, the defects in pten– cells can be ameliorated by LY294002. A low dose of LY294002 reduces the prolonged PH domain association with membrane and restricts the localization of these proteins to the leading edge (Chen, personal communication). In pi3k1–/pi3k2– cells, the changes in PI(3,4,5)P3 levels in response to stimulus were below our limits of detection. This is consistent with the observation that PH domains weakly redistribute to membrane and are not localized at leading edge of chemotaxing pi3k1–/pi3k2– cells (Funamoto et al., 2001). Thus the level of PI(3,4,5)P3, a result of a balance between PI3Ks and PTEN activity, is critical for a cell to sense direction properly. Our methods using TLC cannot resolve PI(4,5)P2 and PI(3,4)P2 so we have not yet examined the changes in the level of PI(3,4)P2. It would be interesting to measure the time course of this PI and learn whether the changes of PI(3,4)P2 are primarily due to the breakdown of PI(3,4,5)P3.
Our results are the first evidence that the PI3Ks are activated upon chemoattractant stimulation. The rise in PI(3,4,5)P3 levels elicited by chemoattractant can be in principle due to activation of PI3Ks, inhibition of PTEN, or combination of both mechanisms. The in vivo assay using PI(3,4,5)P3-32P as a readout rules out the possibility that the rise in the level of PI(3,4,5)P3 is solely due to the inhibition of PTEN with no regulation on PI3Ks. We observed a highly regulated pattern of PI3K activity, similar to that of wild-type cells, even in pten– cells. Because the assay relies on endogenous substrate and ATPγ32P to form PI(3,4,5)P3-32P, it theoretically could report other activities besides PI3Ks. However, because most of the activity is absent in the pi3k1–/pi3k2– cells, it is likely that the assay is specific for PI3Ks that act on PI(4,5)P2. There also appears to be a PI3K activity that uses PI(4)P as substrate in vitro because LY294002 decreases the amount of combined PI(4,5)P2 and PI(3,4)P2. However, this activity does not appear to be influenced by chemoattrantant receptor or G-protein activation.
The finding that PI3K activation is independent of PTEN has important consequences for feedback regulation. Recent studies using a cationic lipid shuttling system to deliver exogenous PI(3,4,5)P3 to neutrophils suggested that PI(3,4,5)P3 cooperates with endogenous PI3Ks (and Rho GT-Pase) in a positive feedback loop that is necessary to establish polarity in response to chemoattractant (Niggli, 2000; Weiner et al., 2002). Our results in D. discoideum rule out feedback from PI(3,4,5)P3 to PI3K. Even though the levels of PI(3,4,5)P3 are higher and sustained in pten– cells, the time course of PI3K activation is essentially identical with that in wild-type cells: peaking at 5 s and already declining by 20 s. Thus the decline in PI3K activation appears to be independent of PI levels.
The PI3K activity is critically dependent on the localization of the enzymes, but the membrane targeting may not be the sole mechanism of activation. In wild-type cells activation of PI3Ks mirrors their redistribution from cystosol to the membrane in response to chemoattractant stimulation. Myristoylation targets the enzymes constitutively to the membrane and raises the basal level of activation, indicating that membrane association is sufficient for activation. Thus close proximity with substrate PI(4,5)P2 apparently can lead to increased activity. However, additional mechanisms are required to further activate the enzyme upon chemoattractant stimulation because both Myr-PI3K1 and Myr-PI3K2 can be further activated by addition of chemoattractant. These additional mechanisms might simply be the recruitment of an additional myristoylated PI3Ks in the cytosol. Alternatively, the membrane tethered PI3Ks can be further activated.
Our studies focus attention on the upstream components that recruit and activate the PI3Ks and PTEN. Although it has been reported that Ras can activate PI3Ks, it is not required for their membrane recruitment (Funamoto et al., 2002). The mammalian PI3Kγ is activated by βγ subunits of G-protein in vitro, and evidence suggests that chemotactic signaling does proceed through the βγ complex (Hirsch et al., 2000; Li et al., 2000; Sasaki et al., 2000). However Gβγ complex cannot be the sole upstream signaling component because the distribution and activation of Gβ in a chemotaxing cell are not as polarized as that of PI3Ks (Jin et al., 2000). Furthermore, the activation of the G-protein is persistent, whereas that of PI3K is transient (Janetopoulos et al., 2001). The binding sites for PTEN are not well understood, although PTEN has a conserved N-terminal PI(4,5)P2 binding motif and its deletion renders the enzyme totally cytosolic (Iijima and Devreotes, 2002). This suggests that binding site for PTEN is PI(4,5)P2, raising an interesting possibility. As reported previously, the enzymes PI3K and PTEN are reciprocally regulated. It is intriguing to speculate based on the observation by Weiner et al. (2002) that PI(3,4,5)P3 activates PI3K and that the binding site for PI3K is PI(3,4,5)P3. If this were the case, during chemoattractant stimulation the binding site for PTEN would be converted to that for PI3Ks, and the binding of the two enzymes would be mutually exclusive. However, as noted in D. discoideum the creation of binding sites for PI3Ks is unaffected by perturbations which lower or elevate the level of PI(3,4,5)P3. Thus this idea needs to be further studied.
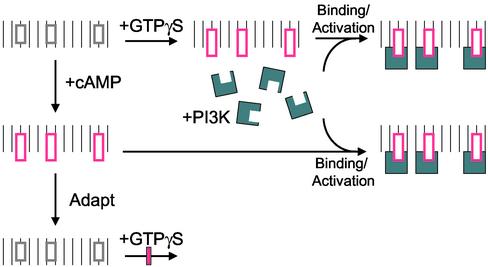
Figure 7 schematically summarizes the new findings of the mechanism of activation of PI3K by chemoattractant receptor and G-protein. In the resting cells, there are few binding/activation sites on the membrane for PI3Ks, and PI3Ks are in the cytosol. Upon activation of cells with cAMP or cell lysates with GTPγS, binding/activation sites for PI3Ks rapidly appear on the inner face of the membrane. The sites recruit and activate PI3Ks. More importantly, the creation of binding/activation sites can take place in the absence of PI3Ks, and cytosolic PI3Ks from unstimulated cells can be supplied to bind to the activated membrane to restore the production of PI(3,4,5)P3. Because the binding sites are created so transiently, we anticipate the sites to be a modification of an existing protein by conformational change or by phosphorylation/dephosphorylation. Persistent cAMP treatment leads to adaptation, resulting in the loss of binding/activation sites and a diminished capacity of GTPγS to generate these sites.
Figure 7.
The schematic diagram demonstrating the new mechanisms of PI3K activation. At resting state, the sites (gray rectangle) on the membrane are incapable of recruiting and activating PI3Ks (blue symbol) in the cytosol. After stimulation of cells with cAMP or cell lysates with GTPγS, the binding/activation sites (pink rectangle) appear on the membrane, which recruit and activate PI3Ks (blue symbol). However with persistent treatment of chemoattractant, these sites decline. The further activation of adapted cell by GTPγS cannot generate these binding/activation sites.
Our data suggest that models for directional sensing should focus on the binding sites for PI3K and PTEN. One model suggests that sensing depends on the balance between excitation and inhibition processes (Parent and Devreotes, 1999; Levchenko and Iglesias, 2002). Our results suggest that this balance controls the binding/activation sites for PI3Ks. When a cell is exposed to a uniform increase in chemoattractant, the binding/activation sites for PI3Ks rapidly increase and then gradually decrease. The binding sites of PTEN behave oppositely to those of PI3Ks. When a gradient is applied, the sites for PI3Ks form at the cell front while those for PTEN form at the back. In wild-type cells, the net effect of the coordinated movements of these two enzymes generates transient increase in the membrane levels of PI(3,4,5)P3 upon uniform stimulation and leads to a large increase in PI(3,4,5)P3 at the front of chemotaxing cells. In pten– cells, the PI3K activation is not affected, but elevated lipids are sustained, causing PH domains to associate with membrane longer. In chemotaxing pten– cells, the binding/activation sites are generated and PI3Ks move normally to the anterior. However directional sensing is impaired because there is wider front with PI(3,4,5)P3 accumulation, presumably because PI(3,4,5)P3 diffusing further from its source at the front.
Acknowledgments
We thank members of PND lab for reading the manuscript. This work was supported by National Institutes of Health Grants GM34933 and 29007 to P.N.D.
Article published online ahead of print. Mol. Biol. Cell 10.1091/mbc.E02-10-0703. Article and publication date are at www.molbiolcell.org/cgi/doi/10.1091/mbc.E02-10-0703.
References
- Carlos, T.M. (2001). Leukocyte recruitment at sites of tumor: dissonant orchestration. J. Leukoc. Biol. 70, 171–184. [PubMed] [Google Scholar]
- Chen, M.Y., Long, Y., and Devreotes, P.N. (1997). A novel cytosolic regulator, Pianissimo, is required for chemoattractant receptor and G protein-mediated activation of the 12 transmembrane domain adenylyl cyclase in Dictyostelium. Genes Dev. 11, 3218–3231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung, C.Y., Funamoto, S., and Firtel, R.A. (2001). Signaling pathways controlling cell polarity and chemotaxis. Trends Biochem Sci. 26, 557–566. [DOI] [PubMed] [Google Scholar]
- Dormann, D., Weijer, G., Parent, C., Devreotes, P., and Weijer, C. (2002). Visualizing PI3 kinase-mediated cell-cell signaling during Dictyostelium development. Curr. Biol. 12, 1178–1188. [DOI] [PubMed] [Google Scholar]
- Funamato, S., Milan, K., Meili, R., and Firtel, R. (2001). Role of phosphatidylinositol 3′ kinase and a downstream pleckstrin homology domain-containing protein in controlling chemotaxis in Dictyostelium. J. Cell Biol. 153, 795–810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Funamoto, S., Meili, R., Lee, S., Parry, L., and Firtel, R.A. (2002). Spatial, and temporal regulation of 3-phosphoinositides by PI 3-kinase, and PTEN mediates chemotaxis. Cell 109, 611–623. [DOI] [PubMed] [Google Scholar]
- Hannigan, M., Zhan, L., Li, Z., Ai, Y., Wu, D., and Huang, C.K. (2002). Neutrophils lacking phosphoinositide 3-kinase gamma show loss of directionality during N-formyl-Met-Leu-Phe-induced chemotaxis. Proc. Natl. Acad. Sci. USA 99, 3603–3608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirsch, E. et al. (2000). Central role for G protein-coupled phosphoinositide 3-kinase gamma in inflammation. Science 287, 1049–1053. [DOI] [PubMed] [Google Scholar]
- Iijima, M., and Devreotes, P.N. (2002). Tumor suppressor PTEN mediates sensing of chemoattractant gradients. Cell 109, 599–610. [DOI] [PubMed] [Google Scholar]
- Janetopoulos, C., Jin, T., and Devreotes, P.N. (2001). Receptor mediated activation of heterotrimeric G-proteins in living cells. Science 291, 2408–2411. [DOI] [PubMed] [Google Scholar]
- Jin, T., Zhang, N., Long, Y., Parent, C.A., and Devreotes, P.N. (2000). Localization of the G protein βγ complex in living cells during chemotaxis. Science 287, 1034–1036. [DOI] [PubMed] [Google Scholar]
- Jones, G.E. Cellular signaling in macrophage migration and chemotaxis. (2000). J. Leukoc. Biol. 68, 593–602. [PubMed] [Google Scholar]
- Lemmon, M.A. et al. (2002). Pleckstrin homology domains and cytoskeleton. FEBS Lett. 513, 71–76. [DOI] [PubMed] [Google Scholar]
- Levchenko, A., and Iglesias, P.A. (2002). Models of eukaryotic gradient sensing: application to chemotaxis of amoebae and neutrophils. Biophys. J. 82, 50–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li, Z., Jiang, H., Xie, W., Zhang, Z., Smrcka, A.V., and Wu, D. (2000). Roles of PLC-β2 and–β3 and PI3Kγ fin chemoattractant-mediated signal transduction. Science 287, 1046–1049. [DOI] [PubMed] [Google Scholar]
- Lilly, P.J., and Devreotes, P.N. (1994). Identification of CRAC, a cytosolic regulator required for guanine nucleotide stimulation of adenylyl cyclase in Dictyostelium. J. Biol. Chem. 269, 14123–9. [PubMed] [Google Scholar]
- Lilly, P.J., and Devreotes, P.N. (1995). Chemoattractant and GTPγS-mediated stimulation of adenylyl cyclase in Dictyostelium requires translocation of CRAC to membranes. J. Cell Biol. 129, 1659–16665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maehama, T., Taylor, G.S., and Dixon, J.E. (2001). PTEN and myotubularin: novel phosphoinositide phosphatases. Annu. Rev. Biochem. 70, 247–79. [DOI] [PubMed] [Google Scholar]
- Meili, R., Ellsworth, C., Lee, S., Reddy, T.B., Ma, H., and Firtell, R.A. (1999). Chemoattractant-mediated transient activation and membrane localization of Akt/PKB is required for efficient chemotaxis to cAMP in Dictyostelium. EMBO J. 18, 2092–2105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moser, B., and Loetscher, P. (2001). Lymphocyte traffic control by chemokines. Nat. Immunol. 2, 123–128. [DOI] [PubMed] [Google Scholar]
- Niggli, V. (2000). A membrane-permeant ester of phosphatidylinositol 3,4, 5-trisphosphate (PIP(3)) is an activator of human neutrophil migration. FEBS Lett. 473, 217–221. [DOI] [PubMed] [Google Scholar]
- Parent, C., and Devreotes, P.N. (1996). Molecular genetics of signal transduction in Dictyostelium. Annu. Rev. Biochem. 65, 411–440. [DOI] [PubMed] [Google Scholar]
- Parent, C., and Devreotes, P.N. (1999). A cell's sense of direction. Science 284, 765–770. [DOI] [PubMed] [Google Scholar]
- Parent, C., Blacklock, B., Froelich, W., Murphy, D., and Devreotes, P.N. (1998). G protein signaling events are activated at the leading edge of chemotactic cells. Cell 95, 81–91. [DOI] [PubMed] [Google Scholar]
- Patel, D.D., and Haynes, B.F. (2001). Leukocyte homing to synovium. Curr. Dir. Autoimmun. 3, 133–167. [DOI] [PubMed] [Google Scholar]
- Rickert, P., Weiner, O.D., Wang, F., Bourne, H.R., and Servant, G. (2000). Leukocytes navigate by compass: roles of PI3Kγ and its lipid products. Trends Cell Biol. 10, 466–473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubel, E.W., and Cramer, K.S. (2002). Choosing axonal real estate: location, location, location. J. Comp .Neurol. 448, 1–5. [DOI] [PubMed] [Google Scholar]
- Sasaki, T. et al. (2000). Function of PI3Kγ in thymocyte development, T cell activation, and neutrophil migration. Science 287, 1046–1049. [DOI] [PubMed] [Google Scholar]
- Servant, G., Weiner, O.D., Herzmark, P., Balla, T., Sedat, J.W., and Bourne, H.R. (2000). Polarization of chemoattractant receptor signaling during neutrophil chemotaxis. Science 287, 1037–1040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Servant, G., Weiner, O.D., Neptune, E.R., Sedat, J.W., and Bourne, H.R. (1999). Dynamics of a chemoattractant receptor in living neutrophils during chemotaxis. Mol. Biol. Cell. 10, 1163–1178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stephens, L., Ellson, C., and Hawkins, P. (2002). Roles of PI3Ks in leukocyte chemotaxis and phagocytosis. Curr. Opin. Cell Biol. 14, 203–213. [DOI] [PubMed] [Google Scholar]
- Thelen, M. (2001). Dancing to the tune of chemokines. Nat. Immunol. 2, 129–134. [DOI] [PubMed] [Google Scholar]
- Traynor-Kaplan, A.E., Thompson, B.L., Harris, A.L., Taylor, P., Omann, G.M., and Sklar, L.A. (1989). Transient increase in phosphatidylinositol 3,4-bisphosphate and phosphatidylinositol trisphosphate during activation of human neutrophils. J. Biol. Chem. 264, 15668–73. [PubMed] [Google Scholar]
- Ueda, M., Sako, Y., Tanaka, T., Devreotes, P., and Yanagida, T. (2001). Single-molecule analysis of chemotactic signaling in Dictyostelium cells. Science 294, 864–867. [DOI] [PubMed] [Google Scholar]
- van Es, S., Weening, K.E., and Devreotes, P.N. (2001). The protein kinase YakA regulates G-protein-linked signaling responses during growth, and development of Dictyostelium. J. Biol. Chem. 276, 30761–30765. [DOI] [PubMed] [Google Scholar]
- Vanhaesebroeck, B., Leevers, S.J., Ahmadi, K., Timms, J., Katso, R., Driscoll, P.C., Woscholski, R., Parker, P.J., and Waterfield, M.D. (2001). Synthesis, and function of 3-phosphorylated inositol lipids. Annu. Rev. Biochem. 70, 535–602. [DOI] [PubMed] [Google Scholar]
- Weiner, O.D. (2002). Regulation of cell polarity during eukaryotic chemotaxis: the chemotactic compass. Curr. Opin. Cell Biol. 14, 196–202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiner, O.D., Neilsen, P.O., Prestwich, G.D., Kirschner, M.W., Cantley, L.C., and Bourne, H.R. (2002). A PtdInsP(3)- and Rho GTPase-mediated positive feedback loop regulates neutrophil polarity. Nat. Cell Biol. 4, 509–513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wymann, M.P., Sozzani, S., Altruda, F., Mantovani, A., and Hirsch, E. (2000). Lipids on the move: phosphoinositide 3-kinases in leukocyte function. Immunol. Today 21, 260–264. [DOI] [PubMed] [Google Scholar]
- Xiao, Z., Zhang, N., Murphy, D.B., and Devreotes, P.N. (1997). Dynamic distribution of chemoattractant receptors in living cells during chemotaxis and persistent stimulation. J. Cell Biol. 139, 365–374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou, K., Pandol, S., Bokoch, G., and Traynor-Kaplan, A.E. (1998). DisruptionofDictyosteliumPI3Kgenesreduces[32P]phosphatidylinositol 3,4 bisphosphate and [32P]phosphatidylinositol triphosphate levels, alters F-actin distribution and impairs pinocytosis. J. Cell Sci. 111, 283–294. [DOI] [PubMed] [Google Scholar]
- Zhou, K., Takegawa, K., Emr, S.D., and Firtel, R.A. (1995). A phosphatidylinositol (PI) kinase gene family in Dictyostelium discoideum: biological roles of putative mammalian p110 and yeast Vsp34p PI 3-kinase homologs during growth and development. Mol. Cell. Biol. 15, 5645–5656. [DOI] [PMC free article] [PubMed] [Google Scholar]