Abstract
Hydrogen peroxide stimulates a tyrosine kinase-dependent calcium release from intracellular stores, which is assumed to be achieved through the activation of phospholipase Cγ2 (PLCγ2) via a tyrosine phosphorylation mechanism in B cells. Here we show that H2O2 induces both tyrosine phosphorylation on PLCγ2 and the activation of phosphatidylinositol 3-kinase (PI3K) in B cells, and that the phosphatidylinositol 3-kinase inhibitor, Wortmannin, partially inhibited the H2O2-induced calcium release without affecting tyrosine phosphorylation on PLCγ2. Overexpression of human Bruton's tyrosine kinase (Btk), which was activated by H2O2, almost completely overcame the inhibition of calcium release by Wortmannin. The reversal of Wortmannin's inhibition by enhancing Btk concentration seemed unique to the H2O2-mediated effect, because Btk failed to overcome the inhibition of Wortmannin on B cell receptor-triggered calcium mobilization. Immunoblot analysis revealed that Btk formed stable complexes with several tyrosine-phosphorylated proteins, including PLCγ2, only in Btk-overexpressed cells on H2O2 stimulation. Together, our data are consistent with the notion that PIP3 and/or a high concentration of Btk target the activated PLCγ2 to its substrate site for maximal catalytic efficiency.
Calcium mobilization is a crucial event for many types of cells in response to agonists binding to their receptors. This process is achieved through the activation of phospholipase C (PLC) to catalyze the hydrolysis of phosphatidylinositol 4,5 bisphosphate (PIP2) to diacylglycerol and inositol 1,4,5-trisphosphate (IP3), which activates protein kinase C and elevates intracellular calcium ([Ca2+ ]i), respectively (1). There are three classes of known PLC isozymes, β, γ, and δ (2). PLCβ is activated by a G protein-mediated process, whereas PLCγ can be activated by a protein tyrosine kinase (PTK)-dependent pathway (3). Both receptor- (4, 5) and nonreceptor-type PTKs (6, 7) have been shown to activate PLCγ. In B cells, including chicken DT40 B cells, crosslinking of antigen receptors results in Syk- and/or (Bruton's tyrosine kinase) Btk-dependent induction of PLCγ2-mediated hydrolysis of PIP2 to IP3, which induces intracellular Ca2+ mobilization (8, 9). This sequential model is supported by Takata et al. (10), who showed that PLCγ2-deficient DT40 cells exhibit no Ca2+ mobilization after B cell receptor ligation. Tyrosine phosphorylation is critical for the activity of PLCγ, because point mutation of the specific tyrosine residues of PLCγ to phenylalanine abolishes the IP3 production mediated by the platelet-derived growth factor, epidermal growth factor, and nerve growth factor (11). Mutation of the phosphotyrosine-binding motif within the SH2 of PLCγ2 also prevents tyrosine phosphorylation of PLCγ2 and subsequent IP3 generation due to B cell receptor activation (10). However, the tyrosine phosphorylation level of PLCγ is not well correlated with IP3 production and Ca2+ mobilization in PTK-deficient cells. For example, in Btk-deficient DT40 cells, tyrosine phosphorylation of PLCγ2 is dramatically reduced but still significantly detectable on B cell receptor activation, yet IP3 production and Ca2+ mobilization are completely lost (6), suggesting that tyrosine phosphorylation is necessary but not sufficient for the full activation of PLCγ2. Furthermore, Rameth et al. demonstrated that mutated PDGF receptor, which is capable of phosphorylating PLCγ with similar efficiency as the wild-type, can stimulate an increase in [Ca2+]i, but fails to achieve full-scale Ca2+ mobilization (12).
Phosphatidylinositol 3,4,5-trisphosphate (PIP3) is a phosphorylated product of PIP2 at the D3 position catalyzed by phosphatidylinositol 3-kinase (PI3K), which is activated by ligation of a variety of receptors (13, 14). Bae et al. recently reported that PIP3 specifically activates PLCγ isozymes in vitro by interacting with their SH2 domains (3). Furthermore, the expression of an activated catalytic subunit of PI3K in COS cells resulted in an increase in IP3 formation, whereas platelet-derived growth factor-induced PLCγ activation in NIH 3T3 cells was markedly inhibited by the PI3K-specific inhibitor LY294002. Thus, receptors coupled to PI3K may activate PLCγ indirectly in the absence of PLCγ-tyrosine phosphorylation through the generation of PIP3 (3, 15). Furthermore, the PI3K inhibitors, Wortmannin or LY294002, have been shown to inhibit Fcγ receptor-dependent activation of PLCγ, and this inhibition can be restored by the preincubation with exogenous PIP3 without affecting the tyrosine phosphorylation of PLCγ2 (16). In platelet-derived growth factor signaling, Wortmannin blocks the activation of PLCγ1 by inhibiting its membrane targeting by PIP3 with no effect on tyrosine phosphorylation of PLCγ1 (17). Genetic analyses of PLCγ activation in immunoreceptor signaling reported by several groups (18–20) show that PIP3 interacts with the PH domain of the Tec family tyrosine kinases, thereby promoting their membrane targeting and activation. Tec family kinases are known to phosphorylate PLCγ and lead to its activation. In addition, Tec kinase/PIP3 complexes have been suggested to function as adaptors to bring the activated PLCγ within proximity of PIP2, independent of Tec kinase activity (6, 20).
Reactive oxygen species (ROS) have emerged as physiological mediators of cellular responses. The production of ROS has been detected in a variety of cells stimulated with cytokines (21, 22), peptide growth factor (23, 24), and agonists of receptors containing seven transmembrane spans (25). When exogenous H2O2 is applied to cells as one form of ROS, it leads to an increase in tyrosine-phosphorylated proteins that might derive from the activation of nonreceptor- and receptor-type PTKs (26–31) and/or inhibition of protein tyrosine phosphatases (32, 33). Furthermore, H2O2-stimulated Ca2+ mobilization and tyrosine phosphorylation patterns in lymphocytes are similar to those observed following antigen receptor activation (34, 35). Wienands et al. (36) also reported that the B cell receptor complex is required for oxidative stress signaling. Hydrogen peroxide-treated B cells (37, 38) have been shown to exhibit PTK-dependent IP3 generation and Ca2+ release. Comparative studies by Qin et al. (37), by using Syk- and Lyn-negative DT40 cells, revealed that Syk and Lyn regulate Ca2+ mobilization and IP3 production in B cells in response to oxidative stress, most likely through tyrosine phosphorylation of PLCγ2. In this study, we show that tyrosine phosphorylation of PLCγ2 is apparently reduced but still significantly detectable in H2O2-treated Syk-negative DT40 cells, yet IP3 production was abolished, raising the question that tyrosine phosphorylation is a primary event but not sufficient to fully activate PLCγ2. Taking into account recent progress in immunoreceptor-mediated PLCγ activation, we investigated the functions of PI3K and Bruton's tyrosine kinase (Btk), one member of the Tec family PTKs expressed in B cells, in H2O2-induced Ca2+ mobilization. Our data show that H2O2 caused the activation of PI3K and Btk. PI3K activation regulated H2O2-induced IP3 production and Ca2+ release, independent of PLCγ2 tyrosine phosphorylation. Btk overexpression overcame the inhibition of Wortmannin on H2O2-induced Ca2+ release, most likely by interacting with the activated PLCγ2 and targeting it to its substrate site to obtain maximal catalytic efficiency.
Materials and Methods
Materials.
RPMI medium 1640 and FBS were purchased from GIBCO. Protein A was from Calbiochem. Fura 2-AM was from Molecular Probes. Antiphosphotyrosine antibody (4G10), polyclonal anti-Btk antibody, and polyclonal anti-PLCγ2 antibody were from Upstate Biotechnology, PharMingen, and Santa Cruz Biotechnology, respectively. Enhanced chemiluminescence reagents were from Dupont. Assay kit for IP3 production was from Amersham, and Wortmannin was from Sigma.
Cell Culture and Harvest.
Establishment of Syk-negative DT40 and DT40 overexpressing human Btk was performed as described previously (8, 39). DT40 and DT40-derived cells were maintained in RPMI medium 1640 supplemented with 10% (vol/vol) FBS, 100 units/ml penicillin, and 100 μg/ml streptomycin in a humidified 95% air/5% CO2 atmosphere. For experiments, cells were collected by centrifugation as previously described (37). When depletion of extracellular Ca2+ was required, cells were resuspended in Ca2+-free Hanks' balanced salt solution buffer, and EGTA (final concentration 0.5 mM) was added before stimulation. Cells were stimulated by H2O2 at 37°C.
Measurement of Intracellular Ca2+.
Ca2+ mobilization was measured by using the fluorescent indicator, Fura-2, as previously described (37). The fluorometer used was a Photon Technology International QuantaMaster Model QM-1.
Measurement of IP3 Levels.
After H2O2 stimulation, IP3 in chicken B-lymphocytes was extracted by perchloric acid and measured with a highly specific D-myo-[3H]IP3 assay system (Amersham), as described by the supplier. This assay was based on the competition between unlabeled IP3 and a fixed quantity of a high-specific-activity tracer [3H]IP3 for a limited number of binding sites on a specific and sensitive bovine adrenal binding-protein preparation.
Preparation of Cell Extracts.
Stimulated cells (1 × 107 cells/ml) were lysed in ice-cold lysis buffer (5 mM EDTA/150 mM NaCl/1% Triton X-100/100 μM Na3VO4/2 mM phenylmethylsulfonyl fluoride/10 μg/ml leupeptin/50 mM Tris, pH 7.4) after a short centrifugation step. Lysates were clarified by centrifugation at 16,000 × g for 15 min at 4°C.
Immunoblot Analysis.
Cell extracts or immunoprecipitates were resolved on SDS/PAGE, transferred electrophoretically onto poly(vinylidene difluoride) membranes and then immunoblotted with the indicated antibodies. Immunoreactive proteins were visualized by enhanced chemiluminescence.
PI3K Assay.
The cell extracts from treated or untreated cells were incubated with antiphosphotyrosine antibody 4G10 for 30 min followed by further incubation with protein A-agarose for 1 h. The immunoprecipitates were washed three times with lysis buffer, twice in buffer containing 10 mM Tris⋅HCl, pH 7.5, 100 mM NaCl, and 1 mM EDTA, and once in PI3K assay buffer (20 mM Tris⋅HCl, pH 7.5/100 mM NaCl/10 mM MgCl2/0.1 mM EGTA/100 mM vanadate/20 mM ATP/200 mM adenosine). After the last wash was removed, 10 μl of sonicated PI substrate (1 mg/ml in 10 mM Hepes, pH 7.5, 3 × 20 s) was added to each sample, and the samples were incubated for 10 min on ice. Reaction was carried out at room temperature for 20 min by adding 40 ml of PI3K assay buffer containing 10 mCi of [γ32P]ATP and then quenched with 100 ml of 1 M HCl. Phospholipids were extracted once with 200 ml of CHCl3/MeOH (1:1) (8,000 rpm × 3 min using a bench-top Biofuge centrifuge (Heracus), and then the lower (organic) phase was transferred to a new microtube and extracted once with 160 ml of 1 M HCl/MeOH (1:1). The organic phase was dried under nitrogen gas and resuspended in 10 ml of CHCl3/MeOH (1:1). Phosphorylated products were resolved on oxalate-impregnated [wetted by 1.2% potassium oxalate/MeOH (1:1)] Silica 60 plates by using CHCl3/MeOH/4 M NH4OH (9:7:2) as solvent (about 2 h), and the gel was air dried for about 10 min. Autoradiogram exposure was typically for less than 3 days. Radioactive spots representing PIP3 were visualized and quantitated by using a PhosphorImager (Molecular Dynamics).
Results and Discussion
In B cell receptor signaling, Syk and Btk play major roles in tyrosine phosphorylation and activation of PLCγ2, which catalyzes the hydrolysis of PIP2 to IP3 and leads to intracellular Ca2+ mobilization (8, 9, 40). Tyrosine phosphorylation of PLCγ2 has been shown to be essential in generating IP3 in response to B cell receptor activation (10). However, studies with PTK-deficient cells revealed that the level of tyrosine-phosphorylated PLCγ2 and the extent of IP3 generation and Ca2+ mobilization are not well correlated (6). It is also known that ROS have been detected in agonist-stimulated cells (21–24). Therefore, study of the H2O2-mediated Ca2+ mobilization pathway in DT40 cells should reveal mechanistic information on both receptor signaling and cellular response to oxidative stress.
Hydrogen Peroxide Stimulates an Increase in Anti-PTyr-Precipitable PI3K Activity in DT40 Cells, and This Activity Is Inhibited in Vivo by Wortmannin, a PI3K Inhibitor.
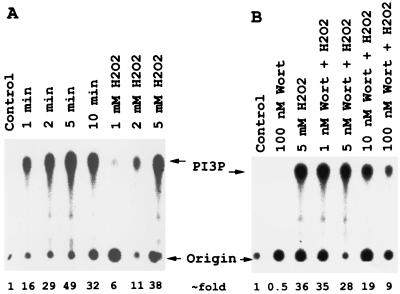
To investigate the ability of H2O2 to stimulate PI3K activity in DT40 cells, lysates from DT40 cells treated with or without H2O2 were immunoprecipitated with antiphosphotyrosine antibody, and the precipitates were analyzed for PI3K activity. As shown in Fig. 1A, treatment with H2O2 resulted in a rapid and sustained increase in PI3K activity. The PI3K activity was significantly increased after 1 min, reached a peak at 5 min, and then was sustained at a high level after 10 min. PI3K activation by H2O2 also occurred in a dose-dependent manner. One millimolar and 5 mM H2O2 stimulated a 6- and 38-fold increase in PI3K activity, respectively, after 5 min exposure (Fig. 1A). Because tyrosine phosphorylation of PI3K has not been well established (41), the activity observed is likely derived from the PI3K coprecipitated with tyrosine-phosphorylated proteins, such as CD19 or CBL adaptor protein (40). CBL is a substrate for Syk tyrosine kinase. When phosphorylated, it forms a complex with PI3K through its interaction with the SH2 domain of the p85 regulatory subunit of PI3K (40, 41). The effect of the PI3K inhibitor, Wortmannin, on PI3K activation by H2O2 in the DT40 cell system was also investigated. Wortmannin has been shown to inhibit PI3K at 1–10 nM (42). It covalently modifies Lys-802 of the catalytic subunit of the PI3K that is located at its active site (43). Fig. 1B shows that significant inhibition was observed after pretreatment with 5 nM Wortmannin. When DT40 cells were pretreated with 100 nM Wortmannin for 10 min, the H2O2-induced PI3K activity was inhibited by 75%. It should be pointed out that Wortmannin had no effect on the tyrosine phosphorylation of cellular proteins caused by H2O2 treatment (data not shown).
Figure 1.
Hydrogen peroxide induces anti-PTyr-precipitable PI3K activity in DT40 cells, and this activity is inhibited in vivo by the PI3K inhibitor, Wortmannin. (A) Time- and dose-dependent activation of PI3K by H2O2. DT40 cells were stimulated with 5 mM H2O2 for the indicated times or incubated with the indicated concentrations of H2O2 for 5 min. Lysates were precipitated with anti-PTyr and assayed for PI3K activity as described in Materials and Methods. (B) Dose response of Wortmannin inhibition of PI3K. DT40 cells were preincubated with indicated doses of Wortmannin for 10 min and then stimulated with 5 mM H2O2 for 5 min. PI3K was assayed as in A. Position of phosphatidylinositiol 3-phosphate (PI3P) is indicated.
Wortmannin Inhibits H2O2-Induced Calcium Release from Intracellular Calcium Stores.
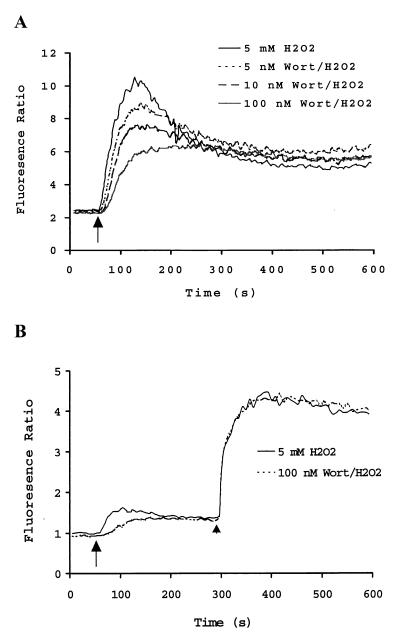
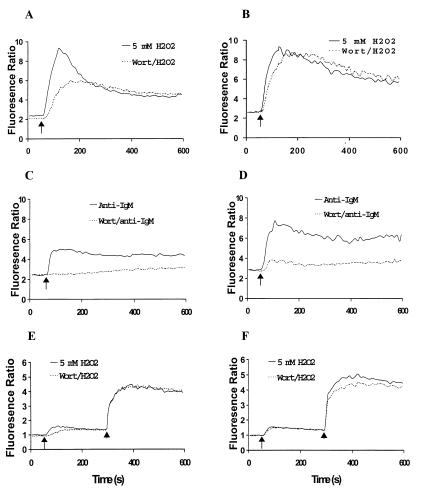
To address whether H2O2-stimulated Ca2+ mobilization is PI3K dependent, intracellular Ca2+ mobilization was monitored using a fluorescent indicator, Fura-2. A representative profile is shown in Fig. 2. Hydrogen peroxide induced a rapid and sustained increase in [Ca2+]i that was maximal within 1 min and decreased somewhat to a relatively constant level over the duration of the experiment. The rapid increase phase is likely derived from intracellular Ca2+ stores. This process is highly dependent on the activity of PLCγ2 and is regulated by a phosphorylation/dephosphorylation mechanism (1, 44). Fig. 2A shows this rapid increase phase of [Ca2+]i was apparently inhibited by 5 nM Wortmannin pretreatment. Maximal inhibition of the H2O2-induced increase in [Ca2+]i was achieved by preincubation with 100 nM Wortmannin. At this concentration, Wortmannin inhibited about 45% of the amplitude of the initial phase Ca2+ signal (Fig. 2A). These results suggested that PI3K was involved in but not absolutely required for the regulation of H2O2-induced Ca2+ mobilization.
Figure 2.
Inhibition of H2O2-mediated Ca2 + mobilization in DT40 cells by the PI3K inhibitor, Wortmannin. (A) Dose-dependent inhibition of H2O2-induced Ca2+ mobilization. DT40 cells (1.5 × 106/ml) were loaded with 5 mM Fura-2 AM and stimulated with 5 mM H2O2. Shown is a representative profile of cells stimulated with H2O2 after preincubation of cells for 10 min with DMSO (vehicle alone 0.06%) or with various concentrations of Wortmannin in DMSO. (B) Inhibition of H2O2-induced Ca2+ release by Wortmannin. Fura-2 loaded DT40 cells were suspended in calcium-free Hanks' balanced salt solution buffer containing 0.5 mM EGTA and stimulated with 5 mM H2O2. After 4 min stimulation, calcium chloride was added (final concentration 1.3 mM). A representative result is shown. Arrows and arrowheads indicate the addition of H2O2 and CaCl2, respectively.
The increase in [Ca2+]i induced by H2O2 in DT40 cells consists of Ca2+ from intracellular stores and Ca2+ influx from outside (37). To address which source of H2O2-induced Ca2+ mobilization was affected by Wortmannin, DT40 cells were suspended in Ca2+-free HBSS buffer containing 0.5 mM EGTA. Wortmannin treatment significantly delayed and reduced the H2O2-mediated Ca2+ release (Fig. 2B). Replenishment of extracellular Ca2+ led to the rapid increase in [Ca2+]i that was comparable in control and Wortmannin-treated DT40 cells, suggesting that Wortmannin affects the H2O2-induced increase in [Ca2+]i mainly via the inhibition of intracellular Ca2+ release.
Wortmannin Inhibits H2O2-Induced IP3 Production Through a Mechanism Independent of Tyrosine Phosphorylation of PLCγ2.
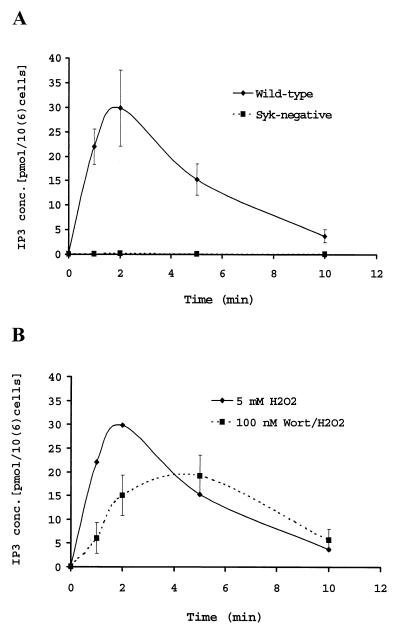
In DT40 cells, the Ca2+ release induced by H2O2 has been shown to correlate with IP3 generation (37). Therefore, the effect of Wortmannin on IP3 production was investigated. Fig. 3A shows that Syk-negative DT40 cells fail to produce IP3 in response to H2O2 treatment. These data are in accordance with the failure to observe the H2O2-induced intracellular Ca2+ release in Syk-negative cells reported by Qin et al. (37), and Syk-negative cells abolished the association of p85 PI3K with CBL, whose tyrosine phosphorylation was markedly inhibited, and anti-chicken IgM failed to stimulate Ca2+ mobilization (40). The correlation between Ca2+ release and IP3 generation is also observed with Wortmannin-treated wild-type DT40 cells. When the cells were pretreated with 100 nM Wortmannin, the H2O2-induced IP3 generation was inhibited up to about 45% (Fig. 3B), which is in good agreement with the Ca2+ release data shown in Fig. 2.
Figure 3.
Effects of Syk and Wortmannin on H2O2-induced IP3 production. (A) Complete abolishment of H2O2-induced IP3 production in Syk-negative cells. DT40 cells were stimulated with 5 mM H2O2 for the times indicated, and IP3 concentrations in the extracts were assayed as described in Materials and Methods. Shown are the mean values of three independent measurements. (B) Partial inhibition of H2O2-induced IP3 production by Wortmannin. DT40 cells were pretreated with 100 nM Wortmannin for 10 min and then stimulated with 5 mM H2O2. The data presented are the mean values of three independent measurements.
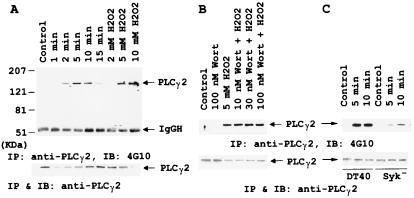
PLCγ2 is the only isoform of PLCγ expressed in DT40 cells (10), and tyrosine phosphorylation is known to activate the PLCγ (2). Fig. 4 shows the differential effects of Wortmannin and Syk on the H2O2-induced tyrosine phosphorylation of PLCγ2. Anti-PLCγ2 immunoprecipitates were electrophoresed and immunoblotted with antiphosphotyrosine mAb. Apparent induction of tyrosine phosphorylation on PLCγ2 was observed at 2 min after 5 mM H2O2 was added, reached a peak at 5 min, and then declined after 10 min (Fig. 4A). Tyrosine phosphorylation levels of PLCγ2 also depended on H2O2 concentrations. The concentration dependence data were obtained after 5 min incubation with the indicated concentrations of H2O2. To our surprise, up to 100 nM of Wortmannin did not alter the level of tyrosine phosphorylation of PLCγ2 (Fig. 4B), indicating that PI3K does not regulate the catalytic efficiency of PLCγ2 by varying its extent of tyrosine phosphorylation. This finding is in agreement with the reports that Wortmannin inhibits PLCγ activity without affecting its tyrosine phosphorylation level (16, 17). Gratacap et al. (16) showed that Fcγ receptor-dependent activation of PCLγ2 is inhibited by Wortmannin and this inhibition is independent of its tyrosine phosphorylation. Furthermore, Wortmannin blocks the platelet-derived growth factor-dependent activation of PLCγ1 by inhibiting its membrane targeting mediated by PIP3, with no effect on the tyrosine phosphorylation of PLCγ1 (17). However, Scharenberg et al. (20) showed that with Btk- and p110-Btk-overexpressed B cells, pretreatment with Wortmannin caused an inhibition of PLCγ2 tyrosine phosphorylation induced by Fab′2 rabbit anti-mouse IgG. This observation may be unique with the Btk-overexpressed B cells (see below). Nevertheless, our data and those reported by Falasca et al. (17) are in accordance with the notion that PI3K exerts its effect on PLCγ2 by generating PIP3, which interacts with the tyrosine-phosphorylated PLCγ2 and targets the enzyme to its substrate site for maximizing its catalytic efficiency. This notion is supported by the facts that (i) PIP3 has been shown to interact with the PH domain (17) and the tandem SH2 domains (3, 45) of PLCγ, and (ii) PLCγ1 has been shown to translocate to the membrane fraction on PDGF (17) and IgM (40) treatment of L6, COS, IMR33, and B cells, respectively. In addition, the tyrosine phosphorylation level of PLCγ2 was reduced by 70% in Syk-negative DT40 cells (Fig. 4C), a mutant fails to generate IP3 and mobilize intracellular Ca2+. This suggests that, when tyrosine phosphorylation of PLCγ2 is minimal, activation of PI3K is necessary to observe the PLCγ2-catalyzed hydrolysis of PIP2. Together, these observations indicated that Wortmannin regulated the activity of PLCγ2 through PI3K independent of its tyrosine phosphorylation.
Figure 4.
Differential effects of Wortmannin and Syk on H2O2-induced tyrosine phosphorylation of PLCγ2. (A) Tyrosine phosphorylation of PLCγ2 mediated by H2O2 in time- and dose-dependent manners. Anti-PLCγ2 immunoprecipitates obtained from DT40 cells stimulated with H2O2 were immunoblotted with anti-PTyr (Top) or anti-PLCγ2 (Bottom). IP, immunoprecipitation; IB, immunoblot. (B) No effect of Wortmannin on H2O2-induced PLCγ2 tyrosine phosphorylation. DT40 cells were pretreated with the indicated concentrations of Wortmannin and then stimulated with 5 mM H2O2 for 10 min. Anti-PLCγ2 immunoprecipitates were immunoblotted with anti-PTyr (Top) or anti-PLC2 (Bottom). (C) Mediation of H2O2-induced PLCγ2 tyrosine phosphorylation by Syk. Anti-PLCγ2 immunoprecipitates from DT40 cells or Syk-negative DT40 cells stimulated with H2O2 were immunoblotted with anti-PTyr (Top) or anti-PLCγ2 (Bottom).
Hydrogen Peroxide Activates Btk and Btk Overexpression Enhances H2O2-Induced Tyrosine Phosphorylation.
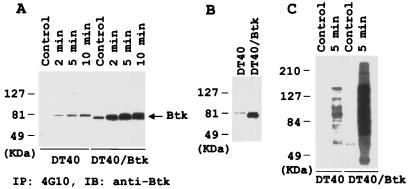
Tec family kinases, which contain N-terminal PH followed by SH3 and SH2 domains, have been implicated in B-cell antigen receptor (BCR)-mediated signaling. Among the Tec kinases, Btk has been recognized as a critical transducer in this process (6, 20, 21, 46, 47), as indicated by the naturally occurring Btk loss-of-function mutation in human S-linked agammaglobulinemia and murine X-linked immunodeficiency syndromes (48, 49). In DT40 cells, activation of BCR leads to tyrosine phosphorylation of Btk, which is well correlated with its tyrosine kinase activity (46, 47). Therefore, we monitored Btk activation in DT40 cells by immunoblotting the antiphosphotyrosine immunoprecipitates with anti-Btk. Significant tyrosine phosphorylation of Btk was detected after incubating cells with 5 mM H2O2 for 2 min and reached a peak at 5 min (Fig. 5A). This indicates that H2O2 activates Btk. Overexpression of human Btk in DT40 cells (Fig. 5B Left) resulted in a detectable increase in Btk tyrosine phosphorylation, and H2O2 stimulation strongly enhanced this phosphorylation (Fig. 5A). A pronounced induction of tyrosine phosphorylation of whole proteins in Btk overexpressing cells was also observed on H2O2 stimulation (Fig. 5C).
Figure 5.
Activation of Btk by H2O2 in DT40 cells. (A) Tyrosine phosphorylation of Btk after 5 mM H2O2 stimulation. Anti-PTyr immunoprecipitates from DT40 or DT40 cells overexpressing human Btk were immunoblotted with anti-Btk antibody. The observed signal in DT40 cells represents the endogenous chicken Btk, whereas that in DT40/Btk is from the overexpressed human Btk. (B) Expression level of endogenous and transfected Btk protein detected by anti-Btk immunoblotting. (C) Enhanced tyrosine phosphorylation of whole cell proteins in Btk expressing cells after 5 mM H2O2 stimulation. Extracts from 2 × 105 cells were run on 8% SDS/PAGE and immunoblotted with anti-PTyr.
Btk Overexpression Overcomes Wortmannin Inhibition of H2O2-Induced Ca2+ Release.
Comparative studies were carried out to reveal differences between DT40 and Btk overexpressed DT40 cells in response to H2O2- and anti-IgM-induced Ca2+ mobilization. Fig. 6 A and B show that the Wortmannin-inhibited Ca2+ increase because of the addition of 5 mM H2O2 observed with DT40 cells was almost overcome by Btk overexpression. However, the Wortmannin-inhibited Ca2+ increase by B cell receptor crosslinking was not overcome by Btk overexpression (Fig. 6 C and D). Unlike the externally added H2O2-mediated Ca2+ mobilization system, overexpressed Btk leads to an enhancement in the amplitude of the Ca2+ release induced by anti-IgM in the presence or absence of Wortmannin (Fig. 6D). This observation is in agreement with the report that increased dosage of Btk resulted in enhancing extracellular Ca2+ influx after BCR activation (18). In the absence of extracellular Ca2+, Wortmannin inhibited the initial Ca2+ release mediated by H2O2 and, again, this inhibition was restored by Btk overexpression (Fig. 6 E and F).
Figure 6.
Btk expression overcomes Wortmannin inhibition on H2O2- but not B cell receptor-induced Ca2 + mobilization. Fura-2-loaded DT40 (A, C, and E) and DT40 overexpressing Btk (B, D, and F) were washed and then suspended in HBSS buffer (A–D) or calcium-free Hanks' balanced salt solution buffer containing EGTA (E and F). Cells were preincubated with DMSO vehicle (0.06%) or 100 nM Wortmannin in DMSO for 10 min and then stimulated with 5 mM H2O2 or 2 μg/ml of α-IgM. Arrows and arrowheads indicate the start of stimulation or the addition of calcium chloride (final concentration 1.3 mM), respectively.
Physical Association of PLCγ2 with Btk and Enhancement of PLCγ2 Tyrosine Phosphorylation After H2O2 Stimulation.
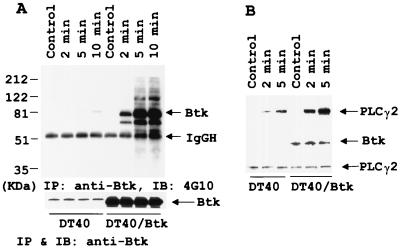
To investigate the mechanisms by which Btk overcomes the Wortmannin inhibition of the H2O2-induced Ca2+ release, we examined its effect on PLCγ2 phosphorylation and complex formation. Immunoblot analysis of anti-Btk immunoprecipitates with antiphosphotyrosine antibody demonstrated that several tyrosine-phosphorylated proteins that have apparent approximate molecular masses ranging from 60–200 kDa were coimmunoprecipitated with Btk only in H2O2-treated overexpressing Btk cells (Fig. 7A). The identities of these tyrosine phosphorylated proteins remain to be worked out. To determine whether PLCγ2 was one of these proteins, we immunoblotted the anti-PLCγ2 immunoprecipitates with antiphosphotyrosine antibody (Fig. 7B Top), anti-Btk (Fig. 7B Middle), and anti-PLCγ2 (Fig. 7B Bottom). The data show that Btk protein was detectable in anti-PLCγ2 immunoprecipitates only in DT40 cells overexpressing Btk, suggesting the association of Btk with PLCγ2. Hydrogen peroxide stimulation did not further enhance the association of Btk with PLCγ2, which existed only in the Btk overexpressed cells. However, tyrosine phosphorylation of PLCγ2 was significantly enhanced in Btk-overexpressing cells after H2O2 stimulation.
Figure 7.
Enhanced complex formation and tyrosine phosphorylation of PLCγ2 in DT40 cells overexpressing Btk after 5 mM H2O2 stimulation. (A) Coprecipitation of several tyrosine-phosphorylated proteins with anti-Btk in Btk-expressing cells. Anti-Btk immunoprecipitates from DT40 or DT40 overexpressing Btk were immunoblotted with anti-PTyr (Top) or with anti-Btk (Bottom). (B) Association of PLCγ2 with Btk and enhancement of PLCγ2 tyrosine phosphorylation in Btk-overexpressing cells. Anti-PLCγ2 immunoprecipitates from DT40 or DT40 overexpressing Btk were immunoblotted with anti-PTyr (Top), anti-Btk (Middle), or anti-PLCγ2 (Bottom).
Together, these observations suggest that the high quantity of Btk, which will overcome the relatively low binding affinity between certain proteins and their ligands, might function as an adaptor to form a complex between PLCγ2, Btk, PIP3 and/or PIP2 to enhance both the activation of PLCγ2 catalyzed by Btk and the catalytic efficiency of the activated PLCγ2 because of the close proximity with its substrate. Consistent with this proposed mechanism, wild-type or kinase-dead Btk overexpression has been shown to protect PIP3 from degradation by endogenous inositol phosphatase (21), and overexpression of kinase-dead Btk in Btk-negative DT40 cells resulted in partial restoration of Ca2+ signaling (6). Therefore, the overexpressed Btk that functions as an adaptor can overcome the inhibition of H2O2-induced intracellular Ca2+ release caused by Wortmannin. In addition, the fact that overexpressed Btk overcomes the Wortmannin inhibition much more effectively when the signal was induced by H2O2 than by anti-IgM suggests that more extensive tyrosine phosphorylation of proteins is needed to make Btk an effective adaptor. However, currently we cannot exclude other pathways that involve Btk to achieve this effect.
In essence, in DT40 cells our results indicate that H2O2 causes the activation of Btk, which phosphorylates and activates PLCγ2, and of PI3K, which generates PIP3. The interaction of PIP3 with the activated PLCγ2 targets the enzyme to its substrate site to achieve its maximal catalytic efficiency. In addition, Btk, when overexpressed in cells, can facilitate the translocation of the activated PLCγ2 to its substrate site through its ability to function as an adaptor likely via its PH and SH2 domains under global protein tyrosine phosphorylation conditions.
Acknowledgments
We thank Jeffrey V. Ravetch of Rockefeller University, New York, and Tomohiro Kurosaki of Kansai Medical University, Moriguchi, Japan, for providing the DT40 overexpressing human Btk and Syk-negative DT40 cells, respectively.
Abbreviations
- Btk
Bruton's tyrosine kinase
- PI3K
phosphatidylinositol 3-kinase
- PLC
phospholipase C
- PIP2
phosphatidylinositol 4,5-bisphosphate
- PIP3
phosphatidylinositol 3,4,5-trisphosphate
- IP3
inositol 1,4,5-trisphosphate
- PTK
protein tyrosine kinase
- BCR
B-cell antigen receptor
Footnotes
Article published online before print: Proc. Natl. Acad. Sci. USA, 10.1073/pnas.130198197.
Article and publication date are at www.pnas.org/cgi/doi/10.1073/pnas.130198197
References
- 1.Berridge M J. Nature (London) 1993;361:315–325. doi: 10.1038/361315a0. [DOI] [PubMed] [Google Scholar]
- 2.Rhee S G, Bae S G. J Biol Chem. 1997;272:15045–15048. doi: 10.1074/jbc.272.24.15045. [DOI] [PubMed] [Google Scholar]
- 3.Bae Y S, Cantley L G, Chen C S, Kim S R, Kwon K S, Rhee S G. J Biol Chem. 1998;273:4465–4469. doi: 10.1074/jbc.273.8.4465. [DOI] [PubMed] [Google Scholar]
- 4.Schlessinger J, Mohammadi M, Margolis B, Ullrich A. Cold Spring Harbor Symp Quant Biol. 1992;57:67–74. doi: 10.1101/sqb.1992.057.01.009. [DOI] [PubMed] [Google Scholar]
- 5.Carpenter G. FASEB J. 1992;6:3283–3289. doi: 10.1096/fasebj.6.14.1385243. [DOI] [PubMed] [Google Scholar]
- 6.Takata M, Kurosaki T. J Exp Med. 1996;184:31–40. doi: 10.1084/jem.184.1.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dibirdik I, Kristupaitis D, Kurosaki T, Tuel-Ahlgren L, Chu A, Pond D, Tuong D, Luben R, Uckun F M. J Biol Chem. 1998;273:4035–4039. doi: 10.1074/jbc.273.7.4035. [DOI] [PubMed] [Google Scholar]
- 8.Takata M, Sabe H, Hata A, Inazu T, Homma Y, Nukada T, Yamamura H, Kurosaki T. EMBO J. 1994;13:1341–1349. doi: 10.1002/j.1460-2075.1994.tb06387.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kurosaki T, Kurosaki M. J Biol Chem. 1997;272:15595–15598. doi: 10.1074/jbc.272.25.15595. [DOI] [PubMed] [Google Scholar]
- 10.Takata M, Homma Y, Kurosaki T. J Exp Med. 1995;182:907–914. doi: 10.1084/jem.182.4.907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Noh D Y, Shin S H, Rhee S D. Biochim Biophys Acta. 1995;1424:99–114. doi: 10.1016/0304-419x(95)00006-0. [DOI] [PubMed] [Google Scholar]
- 12.Rameh L E, Rhee S G, Spokes K, Kazlauskas A, Cantley L C, Cantley L G. J Biol Chem. 1998;273:23750–23757. doi: 10.1074/jbc.273.37.23750. [DOI] [PubMed] [Google Scholar]
- 13.Carpenter C L, Cantley L C. Curr Opin Cell Biol. 1996;8:153–158. doi: 10.1016/s0955-0674(96)80060-3. [DOI] [PubMed] [Google Scholar]
- 14.Vanhaesebroeck B, Leevers S J, Panayotou G, Waterfield M D. Trends Biochem Sci. 1997;22:267–272. doi: 10.1016/s0968-0004(97)01061-x. [DOI] [PubMed] [Google Scholar]
- 15.Sekiya F, Bae Y S, Rhee S G. Chem Phys Lipids. 1999;98:3–11. doi: 10.1016/s0009-3084(99)00013-4. [DOI] [PubMed] [Google Scholar]
- 16.Gratacap M, Payrastre B, Viala C, Mauco G, Plantavid M, Chap H. J Biol Chem. 1998;273:24314–24321. doi: 10.1074/jbc.273.38.24314. [DOI] [PubMed] [Google Scholar]
- 17.Falasca M, Logan S K, Lehto V P, Baccante G, Lemmon M A, Schlessinger J. EMBO J. 1998;17:414–422. doi: 10.1093/emboj/17.2.414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fluckiger A C, Li Z, Kato R M, Wahl M I, Ochs H D, Longnecker R, Kinet J P, Witte O N. EMBO J. 1998;17:1973–1985. doi: 10.1093/emboj/17.7.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Liu K Q, Bunnell S C, Gurmiak C B, Berg L J. J Exp Med. 1998;187:1721–1727. doi: 10.1084/jem.187.10.1721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Scharenberg A M, El-Hillal O, Fruman D A, Beitz L O, Li Z, Lin S, Gout I, Cantley L C, Rawling D J, Kinet J P. EMBO J. 1998;17:1961–1972. doi: 10.1093/emboj/17.7.1961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ohta M, Shilbanuma M, Kuroki K, Nose K. J Cell Biol. 1994;126:1079–1088. doi: 10.1083/jcb.126.4.1079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Thannickal V J, Fanburg B J. J Biol Chem. 1995;270:30334–30338. doi: 10.1074/jbc.270.51.30334. [DOI] [PubMed] [Google Scholar]
- 23.Sundaresan M, Yu Z X, Ferrans V, Irani K, Finkel T. Science. 1995;270:296–299. doi: 10.1126/science.270.5234.296. [DOI] [PubMed] [Google Scholar]
- 24.Bae Y S, Kang S W, Seo M S, Baines I C, Tekle E, Chock P B, Rhee S G. J Biol Chem. 1997;272:217–221. [PubMed] [Google Scholar]
- 25.Griendling K K, Minieri C A, Ollerenshaw J D, Alexander R W. Circ Res. 1994;72:1141–1148. doi: 10.1161/01.res.74.6.1141. [DOI] [PubMed] [Google Scholar]
- 26.Simon A R, Rai U, Fanburg B L, Cochran B H. Am J Physiol. 1998;275:C1640–1652. doi: 10.1152/ajpcell.1998.275.6.C1640. [DOI] [PubMed] [Google Scholar]
- 27.Sonoda Y, Watanabe S, Matsumoto Y, Aizu-Yokota E, Kasahara T. J Biol Chem. 1999;274:10566–10570. doi: 10.1074/jbc.274.15.10566. [DOI] [PubMed] [Google Scholar]
- 28.Griffith C E, Zhang W, Wange R L. J Biol Chem. 1998;273:10771–10776. doi: 10.1074/jbc.273.17.10771. [DOI] [PubMed] [Google Scholar]
- 29.Hardwick J S, Sefton B M. Proc Natl Acad Sci USA. 1995;92:4527–4531. doi: 10.1073/pnas.92.10.4527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Knebel A, Rahmsdorf H J, Ullrich A, Herrlich P. EMBO J. 1996;15:5314–5325. [PMC free article] [PubMed] [Google Scholar]
- 31.Rao G N. Oncogene. 1996;13:713–719. [PubMed] [Google Scholar]
- 32.Lee S R, Kwon K S, Kim S R, Rhee S G. J Biol Chem. 1998;273:15366–15372. doi: 10.1074/jbc.273.25.15366. [DOI] [PubMed] [Google Scholar]
- 33.Barrett W C, DeGnore J P, Keng Y F, Zhang Z Y, Yim M B, Chock P B. J Biol Chem. 1999;274:34543–34546. doi: 10.1074/jbc.274.49.34543. [DOI] [PubMed] [Google Scholar]
- 34.Schieven G L, Kirihara J M, Burg D L, Geahlen R L, Ledbetter J A. J Biol Chem. 1993;268:16688–16692. [PubMed] [Google Scholar]
- 35.Schieven G L, Mittler R S, Nadler S G, Kirihara J M, Bolen J B, Kanner S B, Ledbetter J A. J Biol Chem. 1994;269:20718–20726. [PubMed] [Google Scholar]
- 36.Wienands J, Larbolette O, Reth M. Proc Natl Acad Sci USA. 1996;93:7865–7870. doi: 10.1073/pnas.93.15.7865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Qin S, Inazu T, Takata M, Kurosaki T, Homma Y, Yamamura H. Eur J Biochem. 1996;236:443–449. doi: 10.1111/j.1432-1033.1996.00443.x. [DOI] [PubMed] [Google Scholar]
- 38.Schieven G L, Kirihara J M, Myers D E, Ledbetter J A, Uckun F M. Blood. 1993;82:1212–1220. [PubMed] [Google Scholar]
- 39.Bolland S, Pearse R N, Kurosaki T, Ravetch J V. Immunity. 1998;8:509–516. doi: 10.1016/s1074-7613(00)80555-5. [DOI] [PubMed] [Google Scholar]
- 40.Beitz L O, Fruman D A, Kurosaki T, Cantley L C, Scharenberg A M. J Biol Chem. 1999;274:32662–32666. doi: 10.1074/jbc.274.46.32662. [DOI] [PubMed] [Google Scholar]
- 41.Fruman D A, Meyers R E, Cantley L C. Annu Rev Biochem. 1998;67:481–507. doi: 10.1146/annurev.biochem.67.1.481. [DOI] [PubMed] [Google Scholar]
- 42.Arcaro A, Wymann M P. Biochem J. 1993;296:2297–2301. doi: 10.1042/bj2960297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wymann M P, Bulgarelli-Leva G, Zvelebil M J, Pirola L, Vanhaesebroeck B, Waterfield M D, Panayotou G. Mol Cell Biol. 1996;16:1722–1733. doi: 10.1128/mcb.16.4.1722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zhu D M, Tekle E, Chock P B, Huang C Y. Biochem. 1996;35:7214–7223. doi: 10.1021/bi952471h. [DOI] [PubMed] [Google Scholar]
- 45.DeBell K E, Stoica B A, Veri M C, Baldassarre A D, Miscia S, Graham L J, Rellahan B L, Ishiai M, Kurosaki T, Bonvini E. Mol Cell Biol. 1999;19:7388–7398. doi: 10.1128/mcb.19.11.7388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Nisitani S, Kato R M, Rawlings D J, Witte O N, Wahl M I. Proc Natl Acad Sci USA. 1999;96:2221–2226. doi: 10.1073/pnas.96.5.2221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Buhl A M, Cambier J C. J Immunol. 1999;162:4438–4446. [PubMed] [Google Scholar]
- 48.Satterthwaite A B, Li Z, Witte O N. Semin Immunol. 1998;10:309–316. doi: 10.1006/smim.1998.0123. [DOI] [PubMed] [Google Scholar]
- 49.Thomas J D, Sideras P, Smith C I E, Vorechovsky I, Chapman V, Paul W E. Science. 1993;261:355–358. doi: 10.1126/science.8332900. [DOI] [PubMed] [Google Scholar]