Abstract
The cyclin D1 gene encodes the regulatory subunit of a holoenzyme that phosphorylates and inactivates the retinoblastoma protein, thereby promoting cell-cycle progression. Cyclin D1 is overexpressed in hematopoetic and epithelial malignancies correlating with poor prognosis and metastasis in several cancer types. Because tumor-associated macrophages have been shown to enhance malignant progression and metastasis, and cyclin D1-deficient mice are resistant to oncogene-induced malignancies, we investigated the function of cyclin D1-/- bone marrow-derived macrophages. Cyclin D1 deficiency increased focal complex formation at the site of substratum contact, and enhanced macrophage adhesion, yielding a flattened, circular morphology with reduced membrane ruffles. Migration in response to wounding, cytokine-mediated chemotaxis, and transendothelial cell migration of cyclin D1-/- bone marrow-derived macrophages were all substantially reduced. Thus, apart from proliferative and possible motility defects in the tumor cells themselves, the reduced motility and invasiveness of cyclin D1-/- tumor-associated macrophages may contribute to the tumor resistance of these mice.
INTRODUCTION
Leukocyte motility is essential for normal development and immune function. Colony stimulating factor-1 (CSF-1) is the primary regulator of mononuclear phagocyte survival, proliferation, and differentiation (Stanley et al., 1983, 1997; Cecchini et al., 1994). Macrophage motility is also stimulated by CSF-1 (Boocock et al., 1989; Allen et al., 1997) and is associated with reorganization of the actin cytoskeleton and focal complex formation (Allen et al., 1997; Vanhaesebroeck et al., 1999). Guided migration of macrophages involves sequential protrusion of filopodia and lamellipodia at the leading edge, focal complex-dependent adhesion of the leading edge to substratum, and cytoplasmic actomyosin contraction and detachment of the trailing uropod (Sheetz et al., 1999; Jones, 2000). Coordinating sequential integration of these events, and dynamic remodeling of cellular focal complexes with the substratum are essential for sustained migration toward a chemoattractant (Jones, 2000). Because macrophages are specialized locomotory cells, the organization of their actin cytoskeleton and integrin-mediated cell-substratum contacts is distinct from that of fibroblasts. Instead of stress fibers, macrophages contain thinner F-actin cables that form upon CSF-1 stimulation and rarely arise from focal complexes (Allen et al., 1997; Pixley et al., 2001). Macrophage focal complexes contain proteins found in fibroblast focal contacts, including focal adhesion kinase (FAK), paxillin, vinculin, and β1 integrin (Allen et al., 1997), but are considerably smaller and fewer in number.
Apart from their role in development and immune function, motile macrophages may also enhance the malignant potential of tumors. They are recruited into mammary gland carcinomas (Liotta and Kohn, 2001; Kacinski, 2002) and, in the absence of such tumor-associated macrophages, metastatic progression of mammary gland tumors is profoundly reduced in Csf1op/Csf1op mice lacking CSF-1 expression (Lin et al., 2001). Furthermore, in a human malignant tumor xenograft model, CSF-1 antisense oligonucleotides suppress tumor growth (Aharinejad et al., 2002). These studies suggest that CSF-1–regulated macrophages promote neoplastic progression and metastatic spread and imply a causal link between tumor-associated macrophages and the malignant potential of breast epithelial cells.
Cyclin D1, a regulator of cell-cycle transition through G1 phase, was cloned as a CSF-1–responsive gene in murine macrophages (Matsushime et al., 1991). It belongs to a family of three closely related D-type cyclins, D1, D2, and D3, that have overlapping functions. Cyclin D1 forms a holoenzyme with a cyclin-dependent kinase (CDK), either CDK4 or CDK6 that phosphorylates the retinoblastoma gene product pRb. Overexpression of cyclin D1 promotes progression through the G1 phase of the cell cycle in cells grown on substratum (Pestell et al., 1999; Sherr and Roberts, 1999) and overexpression of cyclin D1 but not cyclin E promotes contact-independent growth (Resnitzky and Reed, 1995). Several recent studies evidence a key role for cyclin D1 in cell-cycle regulation by integrin-mediated adhesion. First, FAK induces cyclin D1 expression after integrin engagement and FAK inhibition by a dominant negative mutant inhibited both cell cycle progression and cyclin D1 expression (Zhao et al., 2001). Second, the ankyrin repeat-containing serine-threonine kinase, integrin-linked kinase (ILK), which binds the cytoplasmic domain of β1 and β3 integrin subunits, promotes contact-independent growth and directly induces cyclin D1 expression and transcription (D'Amico et al., 2000). Finally, disruption of the actin cytoskeleton by cytochalasin D inhibits cyclin D1 expression, consistent with a model in which integrin-dependent organization of the cytoskeleton plays a role in regulating cyclin D1 expression (Bohmer et al., 1996).
Dysregulated expression of cyclins and/or their CDKs can lead to aberrant cellular growth, proliferation, and tumorigenesis. The cyclin D1 gene is amplified or overexpressed in up to 50% of human breast cancers (Dickson et al., 1995; McIntosh et al., 1995), and its level of overexpression correlates with early onset of disease and risk of tumor progression and metastasis (Jares et al., 1994; Drobnjak et al., 2000). Consistent with the clinical studies implicating cyclin D1 in breast cancer, transgenic mice overexpressing cyclin D1 in the mammary gland develop mammary cancer (Wang et al., 1994), whereas mice lacking cyclin D1 are resistant to oncogene-induced tumorigenesis, including Ras-induced skin tumors (Robles et al., 1998) and ErbB2 or Ras-induced mammary tumor formation (Yu et al., 2001). Cyclin D1 is also known as the bcl-1 gene, which is commonly translocated to the Eμ enhancer of the immunoglobulin heavy chain gene, resulting in constitutive expression of cyclin D1 in B-cell lymphomas (Withers et al., 1991). Although transgenic mice expressing cyclin D1 under the transcriptional control of the Eμ enhancer rarely develop spontaneous tumor formation, they display altered lymphoid differentiation in both lineages. Eμ Myc-cyclin D1 double transgenic mice, however, rapidly develop invasive lymphoid malignancies without affecting cell cycle progression, suggesting a cooperative role for cyclin D1 in lymphomagenesis that is cell cycle independent, contributing to tumorigenesis and invasiveness (Bodrug et al., 1994; Lovec et al., 1994). Because cyclin D1 overexpression correlates with tumor metastasis, we considered a role for cyclin D1 in cellular migration. Because macrophages have been shown to enhance tumor progression and metastasis of primary mouse mammary tumors, we have analyzed primary bone marrow macrophages (BMMs) to assess the role of cyclin D1 in macrophage adhesion, motility, and guided migration.
MATERIALS AND METHODS
Mice
Mice homozygously deleted of the cyclin D1 gene (cyclin D1-/-) (Sicinski et al., 1995) on a mixed C57Bl/6x129/SvJ background were maintained as described previously (Albanese et al., 1999). Genotyping was performed on tail genomic DNA by polymerase chain reaction under the following conditions: denaturing at 96°C for 1 min, 94°C for 30 s, annealing at 58°C for 30 s, extension at 72°C 30 s for 30 cycles with a final extension at 72°C for 5 min. The cyclin D1-specific primers were as described in Sicinski et al. (1995).
Cell Culture
Day 5 BMMs from cyclin D1+/+ and cyclin D1-/- mice were prepared as described previously (Stanley, 1990) and cultured in supplemented α-modified minimal essential medium (Invitrogen, Carlsbad, CA) containing 15% fetal bovine serum (FBS) (Invitrogen) and 120 ng/ml human recombinant CSF-1 (gift of Chiron, Emeryville, CA). Human kidney 293T cells were maintained in α-modified minimal essential medium containing penicillin and streptomycin (100 mg of each/liter) and supplemented with 10% FBS.
Retroviral Production and Infection
pMSCV-IRES-GFP (Persons et al., 1999) and the ecotropic, replication-defective helper virus pSV-ψ—E-MLV (Muller et al., 1991) cDNAs were gifts of Drs. A.W. Nienhuis (St. Jude Children's Research Hospital, Memphis, TN) and O.N. Witte (University of California Los Angeles, Los Angeles, CA), respectively. The coding region of mouse cyclin D1 cDNA (GenBank S78355) was inserted into the MSCV-IRES-GFP vector at the EcoRI site upstream of the IRES driving expression of GFP. MSCV retroviruses were prepared by transient cotransfection with helper virus into 293T cells, by using calcium phosphate precipitation. The retroviral supernatants were harvested 48 h after transfection (Pear et al., 1993) and filtered through a 0.45-μm filter. cyclin D1+/+ and cyclin D1-/- BMMs were incubated with fresh retroviral supernatants in the presence of 120 ng/ml CSF-1 and 4 μg/ml polybrene for 24 h, cultured for six more days, and subjected to fluorescence-activated cell sorting (FACS) (FACSVantage SE; BD Biosciences, San Jose, CA) for GFP+ cells. Sorted GFP+ cells were used for microscopic analysis of cyclin D1+/+, cyclin D1-/-, and cyclin D1-/-.MSCV-Cyclin D1-IRES-GFP (cyclin D1-/-/cycD1) BMM phenotypes. Unsorted cells (80–90% GFP+) were used for Western blot analysis of cyclin D1 expression.
Western Blotting
Forty micrograms of each cell lysate was separated by 10% SDS-PAGE and transferred to polyvinylidene dufluoride membrane. Cyclin D1 expression was assessed by Western blotting with a rabbit Ab3 anti-cyclin D1 antibody (Santa Cruz Biotechnology, Santa Cruz, CA). Equal protein loading was confirmed by blotting with anti-EF1α antibody (Edmonds et al., 1996).
Immunofluorescence Staining
Cells were seeded onto fibronectin-coated glass coverslips (BD Biosciences, Bedford, MA). When 60–70% confluent, cells were starved of CSF-1 overnight then restimulated with CSF-1 for various times before fixation and immunofluorescence staining as described previously (Pixley et al., 2001). If two primary antibodies were used, they were added sequentially, and each addition was directly followed by incubation with the respective secondary antibodies. The coverslips were mounted in ProLong antifade kit (Molecular Probes, Eugene, OR), and all samples were examined under an 1X70 inverted microscope (Olympus, Tokyo, Japan) with images recorded using a CH1 cooled charge-coupled device (CCD) camera (Photometrics, Tucson, AZ) (Bailly et al., 2000). Antibodies used included a polyclonal anti-phosphotyrosine antibody (Transduction Laboratories, Lexington, KY), a monoclonal anti-phosphotyrosine antibody (anti-PTyr; PT66; Sigma-Aldrich, St. Louis, MO), polyclonal anti-Y118 phospho-specific paxillin antibody (BioSource International, Camarillo CA), and a monoclonal anti-β-tubulin (XIV17.16; gift of Dr. Anne Johnson, Albert Einstein College of Medicine, Bronx, NY)). The expression level of CSF-1 receptor (CSF-1R) was determined by FACS analysis by using the monoclonal AFS98 antibody (Sudo et al., 1995) (a gift of Dr. S. Nishikawa).
Interference Reflection Microscopy (IRM)
IRM was performed using a 60× numerical aperture 1.4 planapo infinity-corrected objective (Nikon, Tokyo, Japan) with a Radiance 2000 confocal microscope (Bio-Rad, Hercules, CA) in reflectance mode with polarization filters at either 488 or 568 nm. Direct adherence or apposition of the cell to the substrate is imaged as black (destructive interference) and greater distance is viewed as white (direct reflection or constructive interference). Image analysis was performed on the images based on the assumption that more adherent cells would have more dark pixels per unit area spread than less adherent cells. I.P. Lab Spectrum software (Scanalytics, Fairfax, VA) was used to manually trace cells and to calculate the proportion of light versus dark pixels per cell. The total area of ventral cell surface apposition to the coverslip and the ratio of closely adherent to loosely adherent areas of the ventral surface were quantitated using NIH Image.
Adhesion Assay
Using fibronectin-coated (human fibronectin; BD Biosciences, Two Oak Park, Bedford, MA), collagen-coated (type I rat tail; Collaborative Biomedical Products, Two Oak Park, Bedford, MA), or regular 24-well plates (BD Biosciences), 5 × 104 cells were plated per well in triplicate and allowed to adhere at 37°C for various periods of time. The cells were then gently rinsed twice with warmed phosphate-buffered saline (PBS) and fixed for 7 min in 3.7% formaldehyde, and adherent cells were counted. (To control for cell number, one plate of each genotype was counted after 6 h and the actual cell count was corrected for these values) (Pixley et al., 2001). Data are expressed as the ratio of the number of adherent cells at each interval divided by the number of cells adherent at 6 h.
Phase Contrast Light Microscopy and Scanning Electron Microscopy (EM)
Cells were plated on fibronectin-coated glass coverslips and grown to 70% confluence. The cells were rinsed with PBS and either fixed for 5 min in 3.7% formaldehyde/PBS for phase contrast studies or, for scanning EM, fixed quickly with 1% osmium tetroxide/0.1 M cacodylate for 5 s at room temperature followed by 2.5% glutaraldehyde/0.1 M cacodylate fixation as described previously (Galbiati et al., 1998; Pixley et al., 2001), to prevent agonal membrane artifacts. Dehydrated cells were critical point dried by using liquid carbon dioxide in a Samdri 790 critical point drier (Tousimis Research, Rockville, MD), sputter coated with gold-palladium in a Vacuum Desk-1 sputter coater (Denton, Cherry Hill, NJ), mounted, and viewed in a JSM6400 scanning electron microscope (JEOL, Peabody, MA) by using an accelerated voltage of 10 kV for EM.
Spreading Assay
Spreading assays were done as described previously (Oh et al., 1999). Briefly, cells were starved for 18 h and then plated on 60-mm plastic tissue culture dishes in media containing 10,000 U/ml CSF-1 for the indicated time points. Dark cells were considered to be spread, and bright cells as unspread. Pictures of three independent fields were taken under the 10× objective. Experiments were done in triplicate and repeated three times.
Wound Healing
Cells were plated onto tissue culture dishes and grown to confluence before scoring with a 10-μl micropipette tip (Pixley et al., 2001). Normal medium with CSF-1 was changed immediately after scoring and daily during healing, and the wounds were photographed at intervals until they were occluded by migrating cells.
Chemotaxis
Polycarbonate filters (8.0 μm; Osmonics, Westborough, MA) were coated with collagen 1 (12.5 μg/ml final concentration; type 1 rat tail, Collaborative Biomedical Products) and a modified 48-well microchemotaxis (Boyden) chamber (Falk et al., 1980) was used to assess chemotactic activity. After overnight removal of CSF-1, cells were lifted by 0.5 mM EDTA, and 2 × 104 cells were seeded in each upper well in triplicate in the absence of CSF-1. The lower wells contained medium with either 0, 1.2, or 12 ng/ml CSF-1. The chamber was incubated for 3 h at 37°C, 5% CO2. Cells attached to the membrane were fixed with 10% neutral formaldehyde for 3 h and stained with hematoxylin. Cells were counted after washing three times with PBS and wiping off the upper surface of the membrane.
Endothelial Cell Isolation and Transmigration Assay
Pulmonary endothelial cells were isolated from peripheral lung tissue of C57Bl/6 mice after digestion with type 2 collagenase (2 mg/ml; Worthington Biochemicals, Freehold, NY) for 2 h at 37°C, in Ca2+/Mg2+-free Krebs-Henseleit buffer (118 mM NaCl, 4.7 mM KCl, 1.2 mM MgSO4, 1.2 mM KH2PO4, 25 mM NaHCO3, 11 nM dextrose). Cells were counted, suspended at 1 × 107 cells/ml and mixed with magnetic beads coated with Griffona simplicifolia lectin at a ratio of 1:10 for 20 min to purify endothelial cells. Endothelial cell–bead complexes were isolated using a magnetic particle separator, and the endothelial cells were released from the beads by washing twice with 10 mM fucose in complete media (DMEM-F12, 15% FBS, 10 mM l-glutamine, 100 μg/ml endothelial cell growth supplement, 10 U/ml heparin). Endothelial cells were then grown on gelatin-coated tissue culture dishes. Endothelial cell purity was determined to be in excess of 95% by using platelet endothelial cell adhesion molecule staining, acylated low density lipoprotein uptake and cobble-stoned morphology.
For the transmigration assay, pulmonary endothelial cells, passage 3 to 5, were plated onto Transwell polycarbonate membranes (3-μm pore size; Corning Glassworks, Corning, NY) overnight. The Transwell membranes and the endothelial cell monolayers separate each well into upper and lower chambers. No endothelial cells from the confluent monolayer could be demonstrated in the lower chamber as determined by cell counts from wells receiving no macrophages. The integrity of the endothelial cell monolayers was verified using diffusion of Evans blue-stained bovine serum albumin. Intact monolayers prevented the passage of the dye from the upper to the lower chamber.
Confluent endothelial cell monolayers on inserts were transferred to a new 24-well plate with fresh medium with or without CSF-1 in lower chamber. BMMs derived from wild-type mice or cyclin D1-/- mice were added to the upper chamber, and the cells were incubated at 37°C under static conditions for 5 h. Macrophages were stained with the fluorescent dye Cell Tracker Green-AM (Molecular Probes). Cells adherent to the upper surface of the membrane were removed using a cotton applicator. The membranes were then washed extensively with PBS, fixed with 3.7% formaldehyde, and mounted on slides. Cells from three representative fields per insert were counted to quantitate macrophage transmigration. Data are from two experiments done in triplicate (mean ± SD).
RESULTS
Macrophage Morphology Is Altered by Cyclin D1 Deficiency
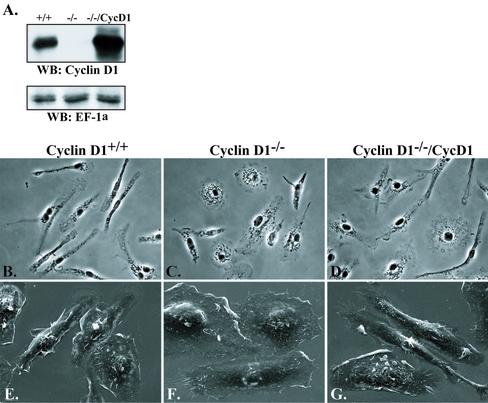
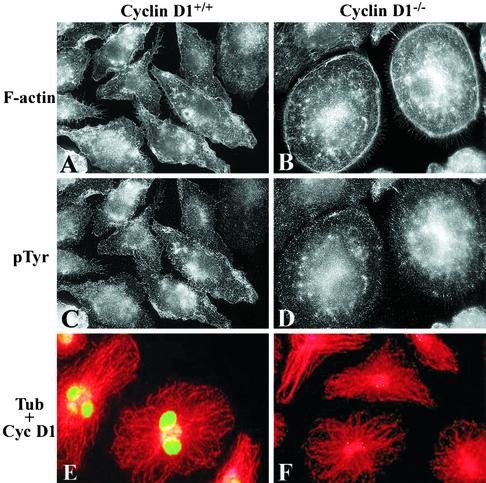
Compared with BMMs from wild-type (WT) littermate control mice, BMMs from cyclin D1-deficient mice did not express detectable cyclin D1, whereas MSCV-Cyc D1–expressing cyclin D1-/-(cyclin D1-/-/CycD1) BMMs expressed approximately fivefold greater levels (Figure 1A). Phase contrast microscopy of the cyclin D1-/- BMM indicated that, in the presence of CSF-1, they were less polarized and more spread than WT BMMs, and frequently circular in appearance, whereas cyclin D1-/-/CycD1 BMMs resembled WT cells (Figure 1, B–D). To more closely examine morphological changes such as spreading and dorsal ruffling, scanning EM was carried out and revealed that, in addition to changes in polarization and spreading, cyclin D1-/- BMMs displayed fewer dorsal ruffles than WT BMMs and reexpression of cyclin D1 reproduced WT numbers of dorsal ruffles (Figure 1, E–G). Because these differences were likely to reflect changes in the actin cytoskeleton, the distribution of F-actin was examined by tetramethylrhodamine B isothiocyanate-phalloidin staining. Although macrophages do not contain thick bundles of F-actin, fine F-actin cables that are parallel to the polarized axis of the cell or radially positioned can often be seen (Allen et al., 1997; Pixley et al., 2001). In cyclin D1-/- BMMs, however, large numbers of circumferential cortical F-actin cables were found (Figure 2, A vs. B). In contrast to the differences in F-actin staining, β-tubulin staining of the microtubule cytoskeleton revealed no differences between WT and cyclin D1-/- BMMs (Figure 2, E and F), and as expected immunofluorescence staining for cyclin D1 demonstrated the presence of nuclear cyclin D1 in the cyclin D1wt but not the cyclin D1-/- BMMs.
Figure 1.
Cyclin D1-/- BMMs display altered morphology. (A) WT, cyclin D1-/-, and cyclin D1-/-/CycD1 whole cell lysates were examined by SDS-PAGE and Western blot for Cyclin D1 and EF-1α (loading control) expression. WT (B and E), cyclin D1-/- (C and F), and cyclin D1-/-/CycD1 (D and G) BMMs were plated on fibronectin-coated coverslips and examined in the presence of CSF-1 by either phase contrast microscopy (B–D, 20×), or scanning EM (E and F, 1200×).
Figure 2.
F-actin cytoskeleton is altered and focal complexes increased in cyclin D1-/- BMMs. Cyclin D1 WT or cyclin D1-/- BMMs were plated on fibronectincoated coverslips and stimulated with CSF-1 for 30 min after over-night removal of CSF-1 (A–D) or cultured in the continuous presence of CSF-1 (E and F). Cells were stained for F-actin (A and B), phosphotyrosine (C and D), and β-tubulin and cyclin D1 (E and F), before examination by cooled CCD microscopy (A–D) or confocal microscopy (E and F) (60×).
Focal Complexes Are Increased in Cyclin D1-deficient Macrophages
The flattened, unpolarized phenotype of cyclin D1-/- BMMs suggested that they might be more adherent than WT BMMs. Macrophage focal complexes are usually small in size and number (Allen et al., 1997; Pixley et al., 2001) but are increased when macrophages become more adherent (Pixley et al., 2001). Although they are more difficult to detect in primary BMMs than macrophage cell lines, phosphotyrosine immunofluorescence staining of WT and cyclin D1-/- BMMs shows more phosphotyrosine-rich focal complexes in cyclin D1-deficient BMMs than WT BMMs (Figure 2, C and D). Paxillin is a focal adhesion protein (Turner, 2000). On tyrosine phosphorylation, phospho-paxillin has been shown to translocate to focal contacts and dorsal ruffles in normal mammary epithelial cells (Nakamura et al., 2000). Further immunofluorescence imaging of the BMM focal complexes was carried out using a phospho-specific Y118 anti-paxillin antibody, because phosphoY118 paxillin is localized almost exclusively to focal complexes in macrophages. After 30 min of CSF-1 treatment, when macrophage focal complexes are most pronounced, striking differences could be seen in cyclin D1-/- BMM focal complexes compared with those of WT cells. Cyclin D1-deficient BMMs displayed large numbers of circumferential focal complexes, whereas focal complexes in WT macrophages remained difficult to identify (Figure 3). CSF-1 receptor expression, as measured by FACS analysis, was similar in the cyclin D1-/- and WT BMMs, indicating that the increase in focal complex tyrosine phosphorylation and phosphorylated paxillin was not attributable to an increase in cell surface CSF-1 receptor expression (our unpublished data).
Figure 3.
Incorporation of phosphoY118 paxillin into focal complexes is increased in cyclin D1-/- BMMs. Cyclin D1 WT or cyclin D1-/- BMMs were plated on fibronectincoated coverslips and, after overnight removal of CSF-1, stimulated with CSF-1 for the times indicated. Cells were stained for phosphoY118 paxillin and examined by cooled CCD microscopy (60×).
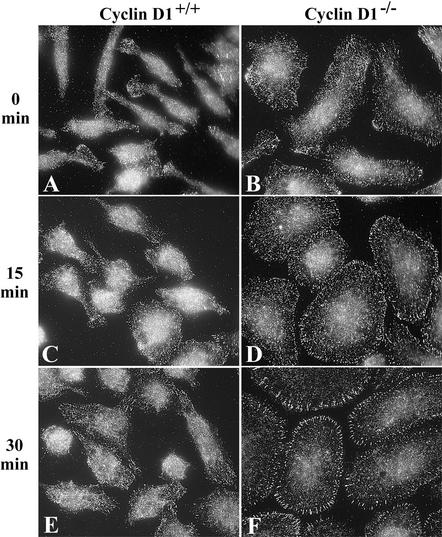
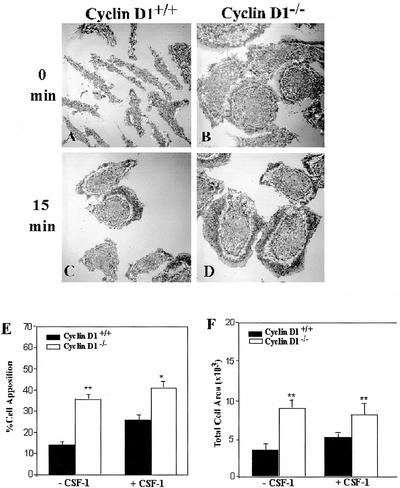
Spreading and Adhesion Are Increased in Cyclin D1-deficient Macrophages
Increased focal complex number and paxillin tyrosine phosphorylation are associated with increased adhesion in macrophages (Pixley et al., 2001). As an indirect measure of macrophage adhesion, IRM was used to determine appositional proximity. IRM, which displays areas of close contact with the substratum as dark gray and regions less closely apposed as white, has been widely used to measure adhesion in a variety of cell types (Zand and Albrecht-Buehler, 1989; Bailly et al., 1998). In cycling WT, cyclin D1-/-, and cyclin D1-/-/CycD1 BMM, IRM analysis of cell spreading revealed that the WT and cyclin D1-/-/CycD1 cells were similarly spread, whereas cyclin D1-/- BMM displayed significantly larger footprints (our unpublished data), consistent with their flattened phenotype (Figure 1F). Further IRM analysis was carried out on WT and cyclin D1-/- BMMs that had been starved of CSF-1 overnight followed by CSF-1 stimulation for 0 or 15 min. In the absence of CSF-1, cyclin D1-/- BMMs were significantly more adherent than WT BMMs (Figure 4, A, B, and E). Because CSF-1 stimulation promotes macrophage spreading and adhesion (Boocock et al., 1989; Pixley et al., 2001), the effect of CSF-1 on BMM apposition was determined. In contrast to WT BMMs, the CSF-1-induced increase in spreading and close apposition was not apparent in cyclin D1-/- BMMs (Figure 4, A–F). Indeed, cyclin D1-/- BMMs seemed to be constitutively well spread and closely adherent
Figure 4.
Cyclin D1-/- BMMs display increased apposition and constitutive spreading. (A–D) WT and cyclin D1-/- BMMs were plated on fibronectin-coated coverslips, and after overnight removal of CSF-1, stimulated with CSF-1 for the times indicated. Cells were fixed and examined by IRM (60×). (E) Quantitation, by pixel intensity analysis, of closely apposed areas (dark) (mean ± SEM). (F) Quantitation of total ventral surface area of attached cells (mean ± SEM).
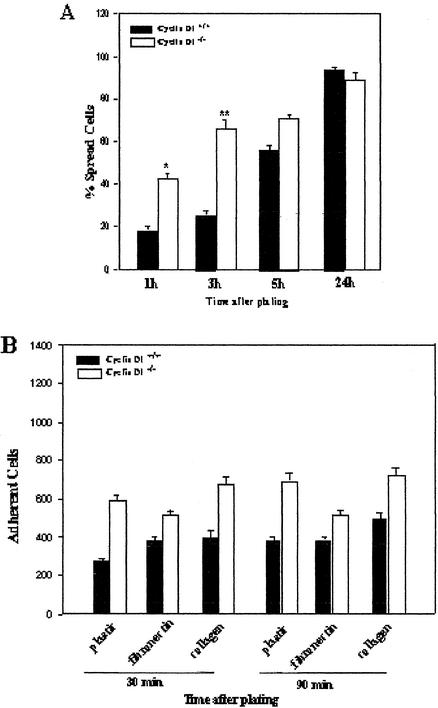
To further investigate the effect of cyclin D1 deficiency on macrophage spreading and adhesion, the ability of WT and cyclin D1-/- BMMs to spread after replating was measured. Cyclin D1-/- BMMs spread significantly more rapidly than WT BMMs at 1 and 3 h, as judged by the ratio of dark to bright cells under phase contrast (Figure 5A). This difference had decreased by 5 h and, after 24 h, almost all cells were spread. Thus, cyclin D1-deficient macrophages spread more rapidly than control cells. As a more direct measure of cell adhesion, the ability of replated WT and cyclin D1-/- BMMs to maintain adhesion on tissue culture plastic, fibronectin, or collagen in the presence of a disrupting force was assessed. Cyclin D1-deficient macrophages were more adherent than WT BMMs on all surfaces at all time points investigated (Figure 5B).
Figure 5.
Cyclin D1-deficient BMMs spread more rapidly and are more adherent than WT BMMs. (A). Wild-type and cyclin D1-/- BMMs were assessed for early spreading in which dark cells were considered to be spread, and bright cells as unspread. The mean ± SEM of the number of spread cells are shown at each time point. (B). BMMs of each genotype were detached and replated on fibronectincoated, collagen-coated, or regular culture dishes. The number of adherent cells was assessed at 30 and 90 min.
These studies demonstrate that cyclin D1 deficiency leads to increased macrophage spreading and adhesion in response to both CSF-1– and integrin-mediated stimulation.
Cyclin D1-/- Macrophages Display Decreased Motility
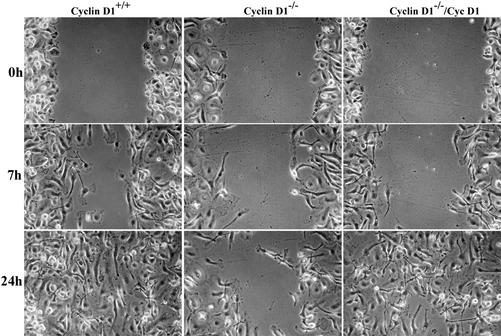
Because increased adhesion and loss of polarity is associated with decreased motility, WT and cyclin D1-/- BMM were subjected to a wound healing assay. WT BMMs migrated into the wound and closed it within 24 h, whereas cyclin D1-deficient BMMs were significantly less motile (Figure 6A). Motility of cyclin D1-/-/CycD1 BMMs approximated the motility of WT cells. Overnight time-lapse videomicroscopy was also performed to assess wound closure at 10-min intervals in WT and cyclin D1-/- BMMs. The mean rate of closure, measured as uncovered area, was delayed in the cyclin D1-deficient BMMs (Figure 6B, side-by-side-b.mov). Increased expression of a protein tyrosine phosphatase, PTPϕ, has been shown to reduce adhesion and increase motility in macrophages by disrupting focal complex formation (Pixley et al., 2001). Consistent with this, the levels of PTPϕ in cyclin D1-/- BMMs were reduced to ∼50% of WT levels (our unpublished data).
Figure 6.
Cyclin D1-deficient BMMs display decreased motility. (A). WT, cyclin D1-/- and cyclin D1-/-/CycD1. BMMs were grown to confluence on tissue culture plastic and the monolayers wounded with a P10 pipette tip. The cultures were fed with CSF-1–containing media and photographed as the wound healed (10×). (B). Time-lapse videomicroscopy performed >16 h of WT and cyclin D1-/- BMMs.
Cyclin D1-deficient Macrophages Are Defective in Both Chemotaxis and Transmigration to CSF-1
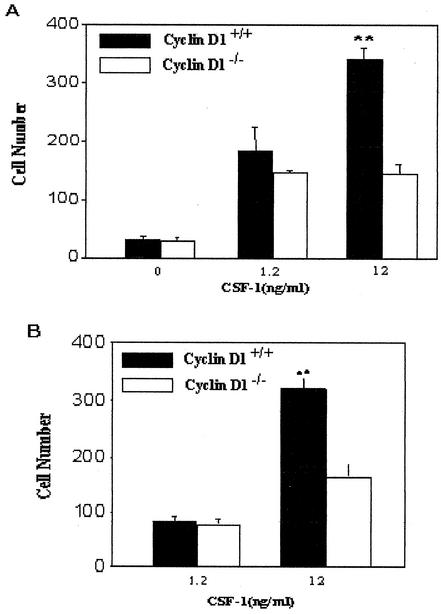
A modified Boyden chamber assay was carried out to determine whether the chemotactic response to CSF-1 was also altered in cyclin D1-/- BMMs. The chemotactic responses of WT and cyclin D1-/- BMMs were equivalent at low concentrations of CSF-1 (Figure 7A). However, a further increase in the response at higher concentrations was only observed for the WT cells (Figure 7A), indicating a role for cyclin D1 in CSF-1–induced macrophage chemotaxis. Because chemotaxis assays are not influenced by proliferative rates these findings further support the conclusion that cyclin D1 affects migration independently of a cell-cycle function.
Figure 7.
Chemotaxis and trans-endothelial cell migration to CSF-1 is defective in cyclin D1-/- bone marrow macrophages. (A). A Boyden microchemotaxis chamber was used to assess guided chemotaxis to CSF-1 with WT and cyclin D1-/- BMMs. (B). Transendothelial migration of BMMs was assessed using a pulmonary endothelial monolayer. Wild-type and cyclin D1-deficient BMMs were exposed to CSF-1 at the concentrations indicated.
The increased adhesion and decreased motility of cyclin D1-/- macrophages suggest that their ability to transmigrate through an endothelial cell barrier would also be diminished. WT and cyclin D1-deficient BMMs were exposed to different concentrations of CSF-1, and migration across a pulmonary primary endothelial cell layer was assessed. Transmigration of cyclin D1-/- BMMs to high CSF-1 concentrations was significantly reduced compared with WT BMMs. As for the chemotactic response, the transmigration defect was concentration dependent (Figure 7B).
DISCUSSION
A growing literature indicates important roles for cyclin D1 in diverse physiological and pathological processes. Cyclin D1 regulates normal cell cycle progression and differentiation (Sherr, 1996; Pestell et al., 1999; Sherr and Roberts, 1999) (reviewed in Fu et al., 2002), and its overexpression is associated with a variety of neoplasias, including centrocytic lymphoma and breast cancer (Bodrug et al., 1994; Weinstat-Saslow et al., 1995; Donnellan and Chetty, 1998). In addition to its key role in cell cycle regulation and differentiation, overexpression of cyclin D1 is associated with a cell cycle-independent increase in tumor invasiveness and metastasis (Bodrug et al., 1994; Jares et al., 1994; Lovec et al., 1994). Delineating these additional functions of cyclin D1 may contribute to a more fundamental understanding of the pathogenesis and progression of tumors in which cyclin D1 is overexpressed. In the present study, we have shown that cyclin D1-deficient macrophages have an altered morphology, increased adhesion, and decreased motility and chemotaxis toward CSF-1.
Cyclin D1 deficiency confers a dramatic morphological phenotype that overrides the significant CSF-1–regulated morphological changes observed in WT macrophages (Figure 1) (Boocock et al., 1989; Pixley et al., 2001). Cyclin D1-/- macrophages are constitutively well spread and attached (Figure 4), and their attachment is mediated via increased numbers of circumferentially arrayed focal complexes rich in phosphoY118 paxillin (Figure 3). The circumferential arrangement of these adhesion sites is associated with a closely aligned distribution of multiple cortical F-actin cables (Figure 2). On replating in the presence of CSF-1, Cyclin D1-/- BMMs also demonstrate an ability to adhere more firmly and to spread more rapidly (Figure 5). This increased adherence is consistent with their reduced motility in the wound-healing assay (Figure 6) and reduced chemotaxis toward CSF-1 through membrane and endothelial cell barriers (Figure 7). Reexpression of cyclin D1 in cyclin D1-/- BMMs leads to reversion of the morphological, adhesion, and motility changes to produce cells that resemble WT BMMs, indicating that the phenotype is a consequence of the loss of cyclin D1.
Macrophages are highly motile and respond to a variety of different adhesion and motility signals through complex signaling pathways, including G protein-coupled receptor pathways, receptor tyrosine kinase pathways, and integrin-mediated signaling. Macrophage motility is enhanced by the Src family tyrosine kinases and pathways that activate FAK or Pyk2 (Lowell and Berton, 1999; Jones, 2000). Activation of the CSF-1R and integrins results in the sequential tyrosine phosphorylation and translocation of several proteins to the focal complexes, including paxillin and FAK (Allen et al., 1997), leading to increased focal complex formation and adhesion (Richardson et al., 1997; Hagel et al., 2002), followed by cellular polarization, disengagement from the substratum, and directed migration (Jones, 2000). These morphological changes result from CSF-1– and integrin-induced activation of the Rho GTPases, Rac, Rho, and Cdc42, which stimulate actin polymerization and are required for CSF-1–induced migration (Allen et al., 1998; Vanhaesebroeck et al., 1999; Jones, 2000). In addition, the protein tyrosine phosphatase, PTPϕ, disrupts macrophage focal complexes by dephosphorylating paxillin, thereby reducing its incorporation into nascent focal complexes (Pixley et al., 2001). In a physiological context, monocyte/macrophage transmigration through endothelial cells is the initial step in the recruitment of macrophages into areas of tissue damage/infection, as well as for transmigration into tumors. After adhering to the surface of the endothelial cells, the migrating cell must penetrate the endothelial junction and undergo cytoskeletal reorganization. In the case of melanoma cells, the penetration of the endothelial junction is initiated by pseudopodia (Ballestrem et al., 2000). Transendothelial cell migration requires multiple adhesive interactions with the endothelial cells and correlates with the metastatic potential of tumor cells (Ridley, 2001; Voura et al., 1998, 2001). The finding that cyclin D1 deficiency retards transendothelial cell migration supports a model in which cyclin D1 may contribute directly to the metastatic phenotype.
Growing evidence supports a role for chemokines in tumor progression and metastasis (Murphy, 2001). Elevated expression of CSF-1 and c-fms (CSF-1R) is associated with poor prognosis in several epithelial cancers, including breast, uterus, ovary, and prostate (Kacinski, 2002). CSF-1, probably via macrophage recruitment and regulation, plays a role in the progression and metastasis of mammary carcinoma in transgenic mice induced by mammary gland-targeted Polyoma middle t antigen. Mice containing a recessive null mutation in the CSF-1 gene (CSF-1OP) are resistant to the induction of mammary tumors and lack the mammary gland macrophage infiltration observed in the CSF-1 wild-type littermate controls (Lin et al., 2001). Furthermore, treatment with CSF-1 antisense oligonucleotides of mice bearing transplanted metastatic tumors inhibits tumor growth and angiogenesis and increased survival (Aharinejad et al., 2002). Consistent with the important role of macrophages in the onset and progression of mammary tumorigenesis (Coussens and Werb, 2001), the reduced migration of macrophages derived from the mammary tumor-resistant cyclin D1-/- mice (Yu et al., 2001), suggests that this macrophage motility defect may contribute to the tumor resistance in these mice. It will be of importance to determine whether cyclin D1 abundance contributes to chemokine induced migration in other cell types. Our studies in cyclin D1-/- mouse embryo fibroblasts and endothelial cells also demonstrated reduced migration (Pestell and Neumeister, unpublished data), suggesting the migratory function observed in BMMs may be a more general function of cyclin D1.
What are the implications of these studies for understanding the role of cyclin D1 in oncogenesis? First, because cyclin D1 reduced adhesion to cellular surfaces and enhanced guided cell motility and migration of primary BMMs, this function of cyclin D1 may be relevant to hematopoetic malignancies. Cyclin D1 is also known as the bcl-1 gene, which is commonly translocated to the Eμ enhancer of the immunoglobulin heavy chain gene. The cyclin D1 translocation results in constitutive expression in B-cell lymphomas (Withers et al., 1991). Transgenic mice expressing cyclin D1 under the transcriptional control of the Eμ enhancer showed altered lymphoid differentiation but rarely develop spontaneous tumors; however, Eμ Myc-cyclin D1 double transgenic mice developed invasive lymphoid malignancies without affecting the cell cycle (Bodrug et al., 1994; Lovec et al., 1994). Thus, the current studies suggest that cyclin D1 overexpression in hemopoietic malignancies could contribute to the invasiveness and/or metastatic phenotype, independently of effects on proliferation, by regulating cellular adhesiveness. Second, because cyclin D1 overexpression correlates with invasive and metastatic phenotype in several cell types (Jares et al., 1994; Drobnjak et al., 2000), and cyclin D1 enhances migration in several distinct cell types, the abundance of cyclin D1 may contribute directly to cellular metastasis and invasiveness through the effects on cellular adhesion we describe. Third, as dynamic adhesion to the cellular substratum contributes through integrin engagement to aberrant cellular survival, and cyclin D1 abundance regulates the dynamics of cellular adhesion, cyclin D1 may also contribute to cellular growth properties through regulating cellular substratum interactions. Integrin engagement and perhaps the resulting mechanical force generated, contribute to growth signaling pathways by activating members of the Rho family and thereby to cell survival pathways, including extracellular signal-regulated kinase, phosphati-dylinositol-3 kinase, and Akt (Parise et al., 2000). Cyclin D1-deficient cells exhibit increases apoptosis on several different substrata (Albanese et al., 1999). The dissection of the independent contribution of cyclin D1 to survival via its effects on adhesion versus effects on driving oncogenic growth and thereby decreasing adhesion may be challenging, because several oncogenes that induce cyclin D1 also decrease adhesion. For example, epithelial cells transformed by oncogenic Rac, which induces cyclin D1 (Westwick et al., 1997), acquire a mesenchymal phenotype with decreased cell-cell adhesion and stress fibers (Zhong et al., 1997; Zondag et al., 2000).
Acknowledgments
We thank Dr. P. Sicinski for cyclin D1-/- mice and helpful discussion. This work was supported in part by grants from National Institutes of Health (R01CA70897, R01CA86072, and R01CA75503 to R.G.P.; R01CA26504 and R01CA32551 to E.R.S.; and 1K08CA097348 to F.J.P), the Susan G. Komen Breast Cancer Foundation, and the Department of Defense (to R.G.P.). R.G.P. was a recipient of the Weil Caulier Irma T. Hirschl Career Scientist award and was the Diane Belfer Faculty Scholar in Cancer Research. P.N. was supported by an Erwin Schroedinger Fellowship of the Fonds zur Foerderung Oesterreichischer Forschung.
Article published online ahead of print. Mol. Biol. Cell 10.1091/mbc.02-07-0102. Article and publication date are at www.molbiolcell.org/cgi/doi/10.1091/mbc.02-07-0102.
On line version of this article contains video material. Online version is available at www.molbiolcell.org.
References
- Aharinejad, S., Abraham, D., Paulus, P., Abri, H., Hofmann, M., Grosschmidt, K., Schafer, R., Stanley, E.R., and Hofbauer, R. (2002). CSF-1 anti-sense treatment suppresses growth of human tumor xenografts in mice. Cancer Res. 62, 5317–5324. [PubMed] [Google Scholar]
- Albanese, C., et al. (1999). Activation of the cyclin D1 gene by the E1A-associated protein p300 through AP-1 inhibits cellular apoptosis. J. Biol. Chem. 274, 34186–34195. [DOI] [PubMed] [Google Scholar]
- Allen, W.E., Jones, G.E., Pollard, J.W., and Ridley, A.J. (1997). Rho, Rac and Cdc42 regulate actin organization and cell adhesion in macrophages. J. Cell Sci. 110, 707–720. [DOI] [PubMed] [Google Scholar]
- Allen, W.E., Zicha, D., Ridley, A.J., and Jones, G.E. (1998). A role for cdc42 in macrophage chemotaxis. J. Cell. Biol. 141, 1147–1157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bailly, M., Wyckoff, J., Boumedienne, B., Hammerman, M., Sylvestre, V., Cammer, M., Pestell, R.G., and Segall, J.E. (2000). Distribution of the EGF receptor in chemotaxing adenocarcinoma cells. Mol. Biol. Cell 11, 3873–3883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bailly, M., Yan, L., Whitesides, G.M., Condeelis, J.S., and Segall, J.E. (1998). Regulation of protrusion shape and adhesion to the substratum during chemotactic responses of mammalian carcinoma cells. Exp. Cell Res. 241, 285–299. [DOI] [PubMed] [Google Scholar]
- Ballestrem, C., Wehrle-Haller, B., Hinz, B., and Imhof, B.A. (2000). Actin-dependent lamellipodia formation and microtubule-dependent tail retraction control-directed cell migration. Mol. Biol. Cell 11, 2999–3012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bodrug, S.E., Warner, B.J., Bath, M.L., Lindeman, G.J., Harris, A.W., and Adams, J.M. (1994). Cyclin D1 transgene impedes lymphocyte maturation and collaborates in lymphomagenesis with the myc gene. EMBO J. 13, 2124–2130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bohmer, R.M., Scharf, E., and Assoian, R.K. (1996). Cytoskeletal integrity is required throughout the mitogen stimulation phase of the cell cycle and mediates the anchorage dependent expression of cyclin D1. Mol. Biol. Cell 7, 101–111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boocock,, C.A., Jones, G.E., Stanley, E.R., and Pollard, J.W. (1989). Colony stimulating factor-1 induces rapid behavioral responses in the mouse macrophage cell lines, Bac1. J. Cell Sci. 93, 447–456. [DOI] [PubMed] [Google Scholar]
- Cecchini, M.G., Dominguez, M.G., Mocci, S., Wetterwald, A., Felix, R., Fleisch, H., Chisholm, O., Pollard, J.W., Hofstetter, W., and Stanley, E.R. (1994). Role of colony stimulating factor-1 in the establishment and regulation of tissue macrophages during postnatal development of the mouse. Development 120, 1335–1372. [DOI] [PubMed] [Google Scholar]
- Coussens, L.M., and Werb, Z. (2001). Inflammatory cells and cancer: think different! J. Exp. Med. 193, 23–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D'Amico, M., et al. (2000). The integrin-linked kinase regulates the cyclin D1 gene through glycogen synthase kinase 3β and CREB-dependent pathways. J. Biol. Chem. 275, 32649–32657. [DOI] [PubMed] [Google Scholar]
- Dickson, C., Fantl, V., Gillett, C., Brookes, S., Bartek, J., Smith, R., Fisher, C., Barnes, D., and Peters, G. (1995). Amplification of chromosome band 11q13 and a role for cyclin D1 in human breast cancer. Cancer Lett. 90, 43–50. [DOI] [PubMed] [Google Scholar]
- Donnellan, R., and Chetty, R. (1998). Cyclin D1 and human neoplasia. Mol. Pathol. 51, 1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drobnjak, M., Osman, I., Scher, H.I., Fazzari, M., and Cordon-Cardo, C. (2000). Overexpression of cyclin D1 is associated with metastatic prostate cancer to bone. Clin. Cancer Res. 6, 1891–1895. [PubMed] [Google Scholar]
- Edmonds, B.T., Wyckoff, J., Yeung, Y.-G., Wang, Y., Stanley, E.R., Jones, J., Segall, J., and Condeelis, J. (1996). Elongation factor-1α is an overexpressed actin binding protein in metastatic rat mammary adenocarcinoma J. Cell Sci. 109, 2705–2714. [DOI] [PubMed] [Google Scholar]
- Falk, W., Goodwin, R.H., and Leonard, E.J. (1980). A 48-well micro chemotaxis assembly for rapid and accurate measurement of leukocyte migration. J. Immunol. Methods 33, 239–247. [DOI] [PubMed] [Google Scholar]
- Fu, M., Wang, C., Wang, J., Zafonte, B., Lisanti, M.P., and Pestell, R.G. (2002). Acetylation in hormone signaling and the cell-cycle. Cytokine Growth Factor Rev. 13, 259–276. [DOI] [PubMed] [Google Scholar]
- Galbiati, F., Volonte, D., Engelman, J.A., Watanabe, G., Burk, R., Pestell, R.G., and Lisanti, M.P. (1998). Targeted down-regulation of caveolin-1 is sufficient to drive cell transformation and hyperactivate the p42/44 MAP kinase cascade. EMBO J. 17, 6633–6648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagel, M., George, E.L., Kim, A., Tamimi, R., Opitz, S.L., Turner, C.E., Imamoto, A., and Thomas, S.M. (2002). The adaptor protein paxillin is essential for normal development in the mouse and is a critical transducer of fibronectin signaling. Mol. Cell. Biol. 22, 901–915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jares, P., Fernandez, P.L., Campo, E., Nadal, E., Bosch, F., Aiza, G., Nayach, I., Traserra, J., and Cardesa, A. (1994). PRAD-1/cyclin D1 gene amplification correlates with messenger RNA overexpression and tumor progression in human laryngeal carcinomas. Cancer Res. 79, 4813–4817. [PubMed] [Google Scholar]
- Jones, G.E. (2000). Cellular signaling in macrophage migration and chemotaxis. J. Leukoc. Biol. 68, 593–602. [PubMed] [Google Scholar]
- Kacinski, B. (2002). Expression of CSF-1 and its receptor CSF-1R in non-hematopoietic neoplasms. Cancer Treat Res. 107, 285–292. [DOI] [PubMed] [Google Scholar]
- Lin, E.Y., Nguyen, A.V., Russel, R.G., and Pollard, J.W. (2001). Colony-stimulating factor-1 promotes progression of mammary tumors to malignancy. J. Exp. Med. 193, 727–739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liotta, L.A., and Kohn, E.C. (2001). The microenvironment of the tumor-host interface. Nature 411, 375–379. [DOI] [PubMed] [Google Scholar]
- Lovec, H., Grzeschiczek, A., Kowalski, M.B., and Moroy, T. (1994). Cyclin D1/bcl-1 cooperates with myc genes in the generation of B-cell lymphoma in transgenic mice. EMBO J. 13, 3487–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowell, C.A., and Berton, G. (1999). Integrin signal transduction in myeloid leukocytes. J. Leukoc. Biol. 65, 313–320. [DOI] [PubMed] [Google Scholar]
- Matsushime, H., Roussel, M.F., Ashmun, R.A., and Sherr, C.J. (1991). Colony-stimulating factor 1 regulates novel cyclins during the G1 phase of the cell cycle. Cell 65, 701–713. [DOI] [PubMed] [Google Scholar]
- McIntosh, G.G., Anderson, J.J., Milton, I., Steward, M., Parr, A.H., Thomas, M.D., Henry, J.A., Angus, B., Lennard, T.W., and Horne, C.H. (1995). Determination of the prognostic value of cyclin D1 overexpression in breast cancer. Oncogene 11, 885–891. [PubMed] [Google Scholar]
- Muller, A.J., Young, J.C., Pendergast, A-M., Pondel, M., Landau, N.R., Littman, D.R., and Witte, O.N. (1991). BCR first exon sequences specifically activate the BCR/ABL tyrosine kinase oncogene of Philadelphia chromosome-positive human leukemias. Mol. Cell. Biol. 11, 1785–1792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy, P.M. (2001). Chemolines and the molecular basis of cancer metastasis. N. Engl. J. Med 345, 833–835. [DOI] [PubMed] [Google Scholar]
- Nakamura, K., Yano, H., Uchida, H., Hashimoto, S., Schaefer, E., and Sabe, H. (2000). Tyrosine phosphrylation of paxillin α is involved in temporospatial regulation of paxillin-containing focal adhesion formation and F-actin organization in motile cells. J. Biol. Chem. 275, 27155–27164. [DOI] [PubMed] [Google Scholar]
- Oh, E.S., Gu, H., Saxton, T.M., Timms, J.F., Hausdorff, S., Frevert, E.U., Kahn, B.B., Pawson, T., Neel, B.G., and Thomas, S.M. (1999). Regulation of early events in integrin signaling by protein tyrosine phosphatase SHP-2. Mol. Cell. Biol. 19, 3205–3215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parise, L.V., Lee, J., and Juliano, R.L. (2000). New aspects of integrin signaling in cancer. Semin. Cancer Biol. 10, 407–14. [DOI] [PubMed] [Google Scholar]
- Pear, W.S., Nolan, G.P., Scott, M.L., and Baltimore, D. (1993). Production of high-titer helper-free retroviruses by transient transfection. Proc. Natl. Acad. Sci. USA 90, 8392–8396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Persons, D.A., Allay, J.A., Allay, E.R., Ashmun, R.A., Orlic, D., Jane, S.M., Cunningham, J.M., and Nienhuis, A.W. (1999). Enforced expression of the GATA-2 transcription factor blocks normal hematopoiesis. Blood 93, 488–499. [PubMed] [Google Scholar]
- Pestell, R.G., Albanese, C., Reutens, A.T., Segall, J.E., Lee, R.J., and Arnold, A. (1999). The cyclins and cyclin-dependent kinase inhibitors in hormonal regulation of proliferation and differentiation. Endocr. Rev. 20, 501–534. [DOI] [PubMed] [Google Scholar]
- Pixley, F., Lee, P.S., Condeelis, J.S., and Stanley, E.R. (2001). Protein tyrosine phosphotase ϕ regulates paxilin tyrosine phosphorylation and mediates colony-stimulating factor 1-induced morphological changed in macrophages. Mol. Cell. Biol. 21, 1795–1809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Resnitzky, D., and Reed, S.I. (1995). Different roles for cyclins D1 and E in regulation of the G1-to-S transition. Mol. Cell. Biol. 15, 3463–3469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richardson, A., Malik, R.K., Hildebrand, J.D., and Parsons, J.T. (1997). Inhibition of cell spreading by expression of the C-terminal domain of focal adhesion kinase (FAK) is rescued by coexpression of Src or catalytically inactive FAK: a role for paxillin tyrosine phosphorylation. Mol. Cell. Biol. 17, 6906–6914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ridley, A. (2001). Rho proteins, PI 3-kinases, and monocyte/macrophage motility. FEBS Lett. 498, 168–171. [DOI] [PubMed] [Google Scholar]
- Robles, A.I., et al. (1998). Reduced skin tumor development in cyclin D1-deficient mice highlights the oncogenic ras pathway in vivo. Genes Dev. 12, 2469–2474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheetz, M.P., Felsenfeld, D., Galbraith, C.G., and Choquet, D. (1999). Cell migration as a five-step cycle. Biochem. Sec. Symp. 65, 233–243. [PubMed] [Google Scholar]
- Sherr, C.J. (1996). Cancer cell cycles. Science 274, 1672–1677. [DOI] [PubMed] [Google Scholar]
- Sherr, C.J., and Roberts, J.M. (1999). CDK inhibitors: positive and negative regulators of G1-phase progression. Genes Dev. 13, 1501–1512. [DOI] [PubMed] [Google Scholar]
- Sicinski, P., et al. (1995). Cyclin D1 provides a link between development and oncogenesis in the retina and breast. Cell 82, 621–630. [DOI] [PubMed] [Google Scholar]
- Stanley, E.R. (1990). Murine bone marrow-derived macrophages. Methods Mol. Biol. 75, 301–304. [DOI] [PubMed] [Google Scholar]
- Stanley, E.R., Guilbert, L.J., Tushinski, R.J., and Bartelmez, S.H. (1983). CSF-1-α mononuclear phagocytic lineage-specific hemopoietic growth factor. J. Cell Biol. 21, 151–159. [DOI] [PubMed] [Google Scholar]
- Stanley, E.R., Berg, K.J., Einstein, D.B., Lee, P.S.W., Pixley, F.J., Wang, Y., and Yeung, Y.G. (1997). Biology and action of colony-stimulating factor-1. Mol. Reprod. Dev. 46, 4–10. [DOI] [PubMed] [Google Scholar]
- Sudo, T., Nishikawa, S., Ogawa, M., Kataoka, H., Ohno, N., Izawa, A., Hayashi, S.I., and Nishikawa, S.I. (1995). Functional hierarchy of c-kit and c-fms in intramarrow production. Oncogene 11, 2469–2476. [PubMed] [Google Scholar]
- Turner, C.E. (2000). Paxillin and focal adhesion signaling. Nat. Cell. Biol. 2, 231–236. [DOI] [PubMed] [Google Scholar]
- Vanhaesebroeck, B., Jones, G., Allen, W., Zicha, D., Hooshmand-Rad, R., Sawyer, C., Wells, C., Waterfield, M., and Ridley, A. (1999). Distinct PI(3)Ks mediate mitogenic signaling and cell migration in macrophages. Nat. Cell Biol. 1, 69–71. [DOI] [PubMed] [Google Scholar]
- Voura, E.B., Ramjeesingh, R.A., Montgomery, A.M., and Sui, C.H. (2001). Involvement of integrin α(v)β(3) and cell adhesion molecule L1 in transendothelial migration of melanoma cells. Mol. Biol. Cell 12, 2699–2710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voura, E.B., Sandig, M., and Siu, C.H. (1998). Cell-cell interactions during transendothelial migration of tumor cells. Microsc. Res. Tech. 43, 265–275. [DOI] [PubMed] [Google Scholar]
- Wang, T.C., Cardiff, R.D., Zukerberg, L., Lees, E., Arnold, A., and Schmidt, E.V. (1994). Mammary hyperplasia and carcinoma in MMTV-cyclin D1 transgenic mice. Nature 369, 669–671. [DOI] [PubMed] [Google Scholar]
- Weinstat-Saslow, D., Merino, M.J., Manrow, R.E., Lawrence, J.A., Bluth, R.F., Wittenbel, K.D., Simpson, J.F., Page, D.L., and Steeg, P.S. (1995). Overexpression of cyclin D mRNA distinguishes invasive and in situ breast carcinomas from non-malignant lesions. Nat. Med. 1, 1257–1260. [DOI] [PubMed] [Google Scholar]
- Westwick, J.K., Lambert, Q.T., Clark, G.J., Symons, M., Van Aelst, L., Pestell, R.G., and Der, C.J. (1997). Rac regulation of transformation, gene expression and actin organization by multiple, PAK-independent pathways. Mol. Cell. Biol. 17, 1324–1335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Withers, D.A., Harvey, R.C., Faust, J.B., Melnyk, O., Carey, K., and Meeker, T.C. (1991). Characterization of a candidate bcl-1 gene. Mol. Cell. Biol. 11, 4846–4853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu, Q., Geng, Y., and Sicinski, P. (2001). Specific protection against breast cancers by cyclin D1 ablation. Nature 411, 1017–1021. [DOI] [PubMed] [Google Scholar]
- Zand, M., and Albrecht-Buehler, G. (1989). Long-term observation of cultured cells by interference-reflection microscopy: near-infrared illumination and Y-contrast image processing. Cell Motil. Cytoskeleton 13, 94–103. [DOI] [PubMed] [Google Scholar]
- Zhao, J., Pestell, R.G., and Guan, J.-L. (2001). Transcriptional activation of Cyclin D1 promoter by Fak contributes to cell cycle progression. Mol. Biol. Cell 12, 4066–4077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhong, C., Kinch, M.S., and Burridge, K. (1997). Rho-stimulated contractility contributes to the fibroblastic phenotype of Ras-transformed epithelial cells. Mol. Biol. Cell 8, 2329–2344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zondag, G.C.M., Evers, E.E., ten Klooster, J.P., Janssen, L., van der Kammen, R.A., and Collard, J.C. (2000). Oncogenic Ras downregulates Rac activity, which leads to increased Rho activity, and epithelial mesenchymal transition. J. Cell Biol. 149, 775–781. [DOI] [PMC free article] [PubMed] [Google Scholar]