Abstract
Phagocytosis in macrophages is thought to involve insertion of cytoplasmic vesicles at sites of membrane expansion before particle ingestion (“focal” exocytosis). Capacitance (Cm) measurements of cell surface area were biphasic, with an initial rise indicative of exocytosis followed by a fall upon phagocytosis. Unlike other types of regulated exocytosis, the Cm rise was insensitive to intracellular Ca2+, but was inhibited by guanosine 5′-O-(2-thio)diphosphate. Particle uptake, but not Cm rise, was affected by phosphatidylinositol 3-kinase inhibitors. Inhibition of actin polymerization eliminated the Cm rise, suggesting possible coordination between actin polymerization and focal exocytosis. Introduction of anti-pan-dynamin IgG blocked Cm changes, suggesting that dynamin controls focal exocytosis and thereby phagocytosis. Similarly, recombinant glutathione S-transferase•amphiphysin-SH3 domain, but not a mutated form that cannot bind to dynamin, inhibited both focal exocytosis and phagocytosis. Immunochemical analysis of endogenous dynamin distribution in macrophages revealed a substantial particulate pool, some of which localized to a presumptive endosomal compartment. Expression of enhanced green fluorescent protein•dynamin-2 showed a motile dynamin pool, a fraction of which migrated toward and within the phagosomal cup. These results suggest that dynamin is involved in the production and/or movement of vesicles from an intracellular organelle to the cell surface to support membrane expansion around the engulfed particle.
INTRODUCTION
Phagocytosis is a specialized form of endocytosis used by hematopoietic and other cells to internalize large extracellular particles into the cytoplasm (for reviews, see Aderem and Underhill, 1999; Greenberg, 1999). This process has been extensively studied in macrophages and involves complex signaling mechanisms initiated by engagement and clustering of cell surface Fcγ or complement C3 receptors (Kwiatkowska and Sobota, 1999; Greenberg, 2001). Internalization after engagement of these two types of receptor is strikingly different; in the case of Fcγ-mediated phagocytosis pseudopodial, extensions reach out from the cell surface to envelop the particle, whereas in C3-mediated phagocytosis particles seem to “sink in” to the cell (Kaplan, 1977). One hallmark of Fcγ-mediated phagocytosis is the central role played by actin polymerization in driving the extension of the phagocytic “cup” around the ingested particle (May and Machesky, 2001). Coincident with this event is the postulated insertion of new membrane at the sites of pseudopodial extension, presumably to accommodate the growing actin filaments (for review, see Booth et al., 2001). This process has been named “focal exocytosis” (Bajno et al., 2000), a terminology we retain herein to distinguish it from other secretory mechanisms in phagocytes (Di et al., 2002).
Many proteins participate in phagocytosis, several of which are involved in signaling cascades from the Fcγ receptor, or in the regulation of actin assembly. Recently, the large GTPase dynamin-2 was implicated in phagocytosis (Gold et al., 1999). Introduction of dominant-negative dynamin into macrophages blocked particle internalization and overexpressed dynamin was found to localize to phagocytic cups. Dynamins constitute a family of proteins thought to be involved in fission reactions at various membranes, including both rapid endocytosis and receptor-mediated endocytosis at the plasma membrane, as well as transport vesicle formation in the trans-Golgi network and possibly at endosomal membranes (for review, see McNiven et al., 2000). Yet other functions have been attributed to this protein, including a role in actin organization at podosomes as well as in the actin “comets” that may power organelle and bacterial motion around the cytoplasm (Orth et al., 2002) and as an element in signal transduction cascades (Fish et al., 2000). A natural supposition would be that dynamin might regulate the final pinch-off reaction that leads to internalization of the phagosome in stimulated macrophages. However, the point of blockade with dynamin-2 dominant-negative mutants in macrophages seems to be before the completion of the phagocytic cup (Gold et al., 1999) (interestingly, similar dynamin-1 dominant negatives were recently reported to be inactive in the same assay; Tse et al., 2002). Potentially, dynamin might modulate either focal exocytosis or the organization of actin filaments during the early stages of particle engulfment, or it could possibly act as a coordinating molecule regulating both aspects of pseudopodial extension.
We recently used the electrophysiological technique of membrane capacitance measurement to analyze exocytosis and endocytosis events in macrophages (Holevinsky and Nelson, 1998; Di et al., 2001, 2002). Phagocytosis can be accurately captured in real-time by using this method, making it suitable for kinetic analysis of processes contributing to particle uptake. Indeed, unitary step decreases in capacitance are related to the size of the ingested particles, and the technique can also be used to analyze the rate and magnitude of secretion in these cells (Di et al., 2001, 2002). An interesting feature of these experiments was the occurrence of a rapid rise in Cm just before the decreases characteristic of membrane internalization (Holevinsky and Nelson, 1998). We interpreted this increase as insertion of vesicular material into the surface membrane, most likely by exocytosis. Such an observation of exocytosis accompanying a phagocytic stimulus may be the electrophysiological signature of the focal exocytosis phenomenon alluded to above. Other techniques also reveal an increase in cell surface area that correlates with pseudopodial extension in phagocytosing macrophages (Cox et al., 1999; Bajno et al., 2000). Apart from its excellent temporal resolution, the whole-cell patch-clamp technique has the added advantage that the intracellular milieu can be precisely controlled via the patch pipette. For example, it is easy to set the level of free cytoplasmic Ca2+ ([Ca2+]i) by the use of buffers in the pipette, and other small mediators can be added and deleted at will. Antagonism of specific proteins by using antibodies and peptides can also be readily achieved. Using this approach, we have addressed whether focal exocytosis is a Ca2+- and GTP-dependent process and whether it is dependent on actin polymerization in this study. To evaluate the role of dynamin in this process, we introduced affinity-purified IgGs and other reagents directly into macrophages. We find that dynamin antagonism blocks exocytosis and subsequent phagocytosis, suggesting that the protein is likely involved in the generation or insertion of exocytotic vesicles needed for the elaboration of the phagocytic cup. In complementary studies, we show that dynamin-2 localizes to an intracellular presumptive endosomal compartment and becomes transiently associated with the incipient phagosome in a manner consistent with local signaling from successively occupied Fcγ receptors.
MATERIALS AND METHODS
Materials
Primary rat alveolar macrophages were obtained by lung lavage as described previously (Holevinsky and Nelson, 1998); cells were collected and washed in Tris-buffered saline then plated onto coverslips in DMEM/10% fetal bovine serum. After 30 min, wells were washed twice with medium to reduce contaminating cells (largely erythrocytes); adherent cells were used within 3 d of isolation. The mouse J774 and mouse RAW264.7 macrophage cell lines were propagated as described previously (Di et al., 2001). Plasmids encoding fusion proteins of glutathione S-transferase (GST)•amphiphysin (amph)-I SH3 domain and its mutant variant (Grabs et al., 1997) were obtained from Dr. P. De Camilli (Yale University, New Haven, CT), expressed in bacteria and purified by glutathione affinity chromatography. We confirmed in binding studies that the wild-type domain could bind dynamin-2, whereas the mutant domain was inactive in this regard (our unpublished data). Anti-pan-dynamin antibodies were prepared in rabbits to a conserved sequence in the G-domain by QBC (Hopkinton, MA), the IgGs were affinity purified by sequential protein A-agarose and peptide affinity column chromatography. All proteins were centrifugally dialyzed against internal pipette solution before introduction into cells. Other reagents were obtained from the following sources: wortmannin (LC Labs, Woburn, MA), herbimycin A (Invitrogen, Carlsbad, CA), LY294002 (BIOMOL Research Laboratories, Plymouth Meeting, PA), cytochalasin D, guanosine 5′-O-(2-thio)diphosphate (GDPβS) (Sigma-Aldrich, St. Louis, MO), and latrunculin A (Calbiochem, San Diego, CA). A C-terminal truncated recombinant version of Rho-GDI was obtained from Dr. Z. Derewenda (Department of Molecular Physiology and Biological Physics, University of Virginia, Charlottesville, VA). Human aggregated IgG (HAIGG) was prepared as described previously (Di et al., 2001).
Electrophysiology
Our patch-clamp capacitance methods for macrophages were as described previously (Di et al., 2001, 2002) and are only summarized herein. Whole-cell capacitance recordings were obtained using an EPC-9/2 computer-controlled patch-clamp amplifier running PULSE software (HEKA Electronik, Lambrecht, Germany). The EPC-9 includes a built-in data acquisition interface (ITC-16; InstruTECH, Port Washington, NY). The software package controlled stimulus delivery and data acquisition for the lock-in amplifier in the “sine + dc” mode as described by Gillis (2000). The temporal resolution of the capacitance data was 40 ms/point using a 1-kHz, 20-mV sine wave. The holding potential in the capacitance experiments was –10 mV. Standard recording solutions were 80 mM K aspartate, 40 mM KCl, 2 mM MgCl2, and 10 mM HEPES (pH 7.2) in the pipette with Mg•ATP (1 mM) and Mg•GTP (0.3 mM) added as needed; [Ca2+]i was buffered with EGTA to different values as before (Di et al., 2001). The standard bath solution contained 100 mM NaCl, 50 mM KCl, 2 mM MgCl2, 2 mM CaCl2 and 10 Na•HEPES (pH 7.4). All recordings shown herein were obtained at 37°C unless indicated in the text. The temporal resolution of the fluorescence data was 20 ms/point. Data was acquired using a Pentium3 PC and analyzed off-line by using the integrated graphics package IGOR Pro (WaveMetrics, Lake Oswego, OR). All quantitation of stimulus-induced changes in membrane capacitance were determined as the difference between the steady-state attained after a 10-min equilibration period and the subsequent peak of increase or decrease after stimulation. Superoxide release was determined electrochemically using the EPC-9/2, which allows simultaneous amperometric current and capacitance measurements in single voltage-clamped cells (Di et al., 2002). Insulated polypropylene 5-μm-diameter carbon fibers (Dagan, Minneapolis, MN) were directly connected to the headstage of the amplifier. The electrodes were placed within 1 μm of the surface of the J774 cells used for these assays and oxidation current was determined with 120 mV applied to the fiber. Total amperometric charge was calculated using the automated event detection program events written for IGOR by Segura et al. (2000).
Immunocytochemistry
Alveolar macrophages, J774 or RAW 267.4 cells were plated onto coverslips and cultured overnight before fixation in 4% paraformaldehyde in Hanks' balanced salt solution for 20 min at room temperature. Cells were blocked and permeabilized in PBS containing 0.25% fish skin gelatin plus 0.01% saponin with 0.2% NaN3. To visualize dynamin-2 and various markers, fixed cells were exposed to primary IgGs as follows: dynamin-2 polyclonal antibody (pAb) (1:1000; Oncogene Research, Cambridge, MA) or MC-63 anti-pandynamin pAb (0.5 μg/ml; kind gift of Mark McNiven, Mayo Institute); TGN-38 monoclonal antibody (mAb) (1:100; Transduction Laboratories, Lexington, KY); giantin mAb (1:100; kind gift of Adam Linstedt, Carnegie Mellon University, Pittsburgh, PA); EEA-1 mAb (1:100; Transduction Laboratories); and clathrin heavy chain mAb X-22 (1:100; Affinity Bioreagents, Golden, CO). In general, overnight incubation in primary antibody at 4°C was used; Alexa 488-coupled goat anti-rabbit secondary antibodies (Molecular Probes, Eugene, OR) were used to visualize dynamin, whereas rhodamine- or Alexa 633-coupled secondaries were used to visualize other antigens (1-h incubation at room temperature). To label the endosomal system, we incubated cells at 37°C for various times in either Cy5-transferrin (50 μg/ml; Jackson Immunoresearch Laboratories, West Grove, PA) or lysine-fixable rhodamine-dextran (1 mg/ml; Mr 70,000; Molecular Probes), taken up by receptor-mediated and fluid phase endocytosis, respectively. In some experiments, fixed and permeabilized cells were treated with rhodamine-phalloidin (165 nM; Molecular Probes) to visualize polymerized actin. For bead uptake experiments, cells were exposed to either unlabeled or rhodamine-labeled latex beads (Spherotech, Libertyville, IL) that were IgG-coated, or opsonized zymosan particles (Molecular Probes). Cells were examined in an IX70 Fluoview confocal microscope (Olympus, Tokyo, Japan); images were captured and digitized with Fluoview software then processed using Image J software (National Institutes of Health) and Adobe Photoshop.
Preparation and Expression of GFP•Dynamin-2
A plasmid encoding rat dynamin-2 (bb form) was obtained from Drs. P. Okamoto and R.B. Vallee (University of Massachusetts Medical School, Worcester, MA; Okamoto et al., 1997). The coding sequence was excised and inserted in-frame with the sequence for enhanced green fluorescent protein (EGFP) at the N terminus by using the pEGFP-C2 vector (BD Biosciences Clontech, Palo Alto, CA). The sequence of the resultant plasmid pEGFP-N-dynamin-2 was verified and functional expression was tested by transient transfection into human embryonic kidney 293 cells. A protein of the appropriate size (∼140 kDa) was detected by immunoblotting (our unpublished data). RAW 264.7 cells were transiently transfected with the same vector by using Superfect (QIAGEN, Santa Clara, CA; 5-h incubation), resulting in ∼25% transfection efficiency as judged by counting fluorescent cells 24–48 h later. At this time, coverslips containing transfected cells were exposed to unlabeled opsonized latex beads (0.8 or 3 μm in diameter) or zymosan particles and allowed to internalize for periods of 10 min to 1 h. Coverslips were fixed and counterstained with rhodamine-phalloidin then analyzed by confocal microscopy as described above.
EGFP-dynamin-2 K44A was prepared by point mutation of the appropriate base in pEGFP-N-dynamin-2 by using the Quick-Change system (Stratagene, La Jolla, CA). The product was verified by sequencing and expressed in RAW 264.7 cells exactly as described above for the wild-type vector. Cells were incubated 24 h for expression and then subjected to patch-clamp capacitance recording as described above, after location of expressing cells by fluorescence, or to bead uptake followed by microscopic analysis.
Online Supplemental Material
Several videos and confocal image stacks showing details of dynamin localization in macrophages are included in supplemental material accessible via the MBC Web site.
RESULTS
Three Types of Macrophage Exhibit a Rise in Cm before Phagocytosis
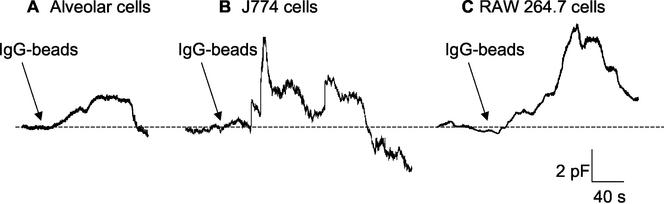
Capacitance measurements reliably track cell surface area and provide accurate kinetic details of both exo- and endocytosis in several systems (Thomas et al., 1994; Artalejo et al., 1995). Our previous experiments showed that macrophages exhibit a rapid rise in Cm before the fall in surface area concomitant with particle ingestion (Holevinsky and Nelson, 1998). This can be interpreted as a cycle of exocytosis followed by pinch-off of the phagosome, as illustrated in Figure 1. To evaluate the generality of this pattern, we examined the phenomenon in three types of macrophage in response to IgG-bead challenge. As shown in Figure 2, rat primary alveolar macrophages as well as the murine J774.1 and RAW 264.7 cell lines all exhibited rapid rises in Cm (peaking in <2 min) that preceded bead internalization, reflected as a stepwise decrease in Cm. It is likely that some overlap between focal exocytosis and phagocytosis occurs in such recordings, as also exists in neuroendocrine secretion (Artalejo et al., 1995), resulting in some attenuation of the Cm rise. The time course of this event is in keeping with the kinetics of phagocytosis determined morphologically. For example, in the synchronous phagocytosis of prebound erythrocytes investigated by Diakanova et al. (2002) in bone marrow macrophages, formation of the phagocytic cup occurred within 10–20 s after stimulation. The increase in cell surface area represents a significant enlargement of up to ∼30% in total cell surface at peak (for baseline values, see legend to Figure 2). These results suggest that exocytosis generally precedes phagocytosis in macrophages and may contribute to the formation of the phagocytic cup.
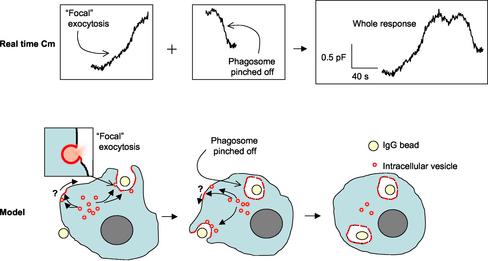
Figure 1.
Focal exocytosis and phagocytosis as visualized by capacitance recordings. (upper panel) Sample continuous real-time Cm recordings from a J774 macrophage exposed to IgG-coated beads. Left, exocytotic (Cm rise) and endocytotic (Cm fall) phases have been separated to correspond to the individual panels in the model shown in the lower panel. Focal exocytosis is depicted as fusion of small intracellular vesicles (pink circles with red membrane) with the surface membrane at the sites of bead (yellow spheres) internalization, eliciting a rise in Cm due to the incorporation of vesicular membrane into the cell surface. Phagosome pinch-off then results in a decrease in Cm due to internalization of surface membrane. A second bead then becomes the target of focal exocytosis resulting in a repetition of the cycle.
Figure 2.
Rapid capacitance increase (focal exocytosis) precedes phagocytosis in three different types of macrophage. Primary rat alveolar macrophages (A), or cells from J774 (B) or RAW 264.7 (C) cell lines were whole-cell clamped and continuous Cm measurements made as described in MATERIALS AND METHODS. At the arrow IgG-coated latex beads (0.8 μm in diameter) were added. Average baseline Cm was 6.3 pF (A), 17.2 pF (B), and 14.7 pF (C).
Exocytosis before Phagocytosis Is Ca2+ Independent but Is Blocked by GDPβS
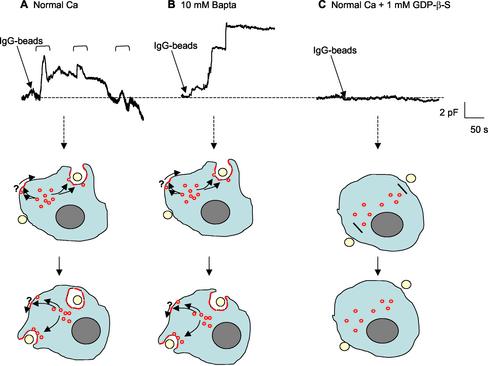
Exocytosis in several systems is regulated by [Ca2+]i and in some hematopoietic cells also by GTP (sometimes in concert with [Ca2+]i). We, therefore, tested whether focal exocytosis was dependent on either of these two effectors. As shown in Figure 3, A and B, a rapid increase in Cm was observed when cells contained [Ca2+]i close to “normal” values (40 nM in the patch pipette, rising to ∼300 nM on IgG stimulation) or when [Ca2+]i is reduced to vanishingly low levels by using the chelator BAPTA (10 mM; [Ca2+]i <1 nM) (Di et al., 2001). A striking difference between the two conditions was that in normal [Ca2+]i phagocytosis occurred, as reflected in the decrease in Cm, whereas in low [Ca2+]i phagocytosis was blocked. Global secretion in macrophages has been shown to require GTP and to be stimulated by guanosine 5′-O-(3-thio)triphosphate and inhibited by GDPβS (Di et al., 2002). To determine whether focal exocytosis is dependent on G proteins, we introduced GDPβS intracellularly before stimulation with IgG-coated beads. GDPβS completely blocked the initial Cm rise in normal [Ca2+]i, suggesting that G proteins are essential to focal exocytosis (Figure 3C). We did not use GTPγS in this study because it induces secretion of other intracellular vesicles that would contaminate measurements of focal exocytosis (Di et al., 2001).
Figure 3.
Focal exocytosis is not dependent on [Ca2+]i but is inhibited by GDPβS. Top, continuous Cm recordings from J774.1 macrophages whole-cell clamped with normal [Ca2+]i (A), 10 mM BAPTA (B) or 1 mM GDPβS (C) in the patch pipette solution. Note that in A, IgG-bead stimulation leads to an increase in [Ca2+]i to ∼300 nM, as previously reported, whereas BAPTA-buffered cells do not show this increase (Di et al., 2001). Bottom, cartoons depicting events likely to underlie Cm changes in top panels. In A, focal exocytosis (Cm increase) precedes particle engulfment (Cm decrease and this cycle can be repeated (see brackets above trace). In B, focal exocytosis is not affected but phagocytosis is blocked. In C, both events are blocked.
Focal Exocytosis Is Blocked by Compromising Tyrosine Phosphorylation or Actin Polymerization but Not by Phosphatidylinositol 3 (PI3) Kinase Inhibition
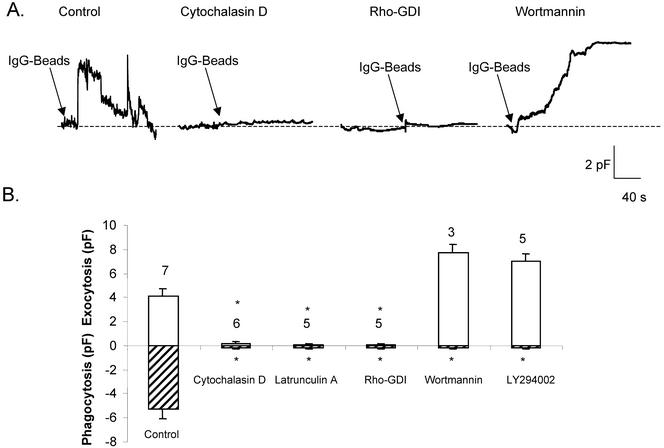
Signaling from Fcγ receptors requires the participation of various cytoplasmic tyrosine kinases including Src-family members and Syk (Greenberg, 1999, 2001). Phagocytic cups accumulate phosphotyrosine immunoreactivity and blockade of tyrosine phosphorylation inhibits phagocytosis, but it is not known whether tyrosine phosphorylation is required for focal exocytosis. In one study, it was shown that pseudopod extension in fibroblasts transfected with various Fcγ receptors did not require the presence of tyrosine activation motifs in the cytoplasmic tail (Lowry et al., 1998). However, we found that preincubation of J774 cells with the inhibitor herbimycin A, a potent blocker of several tyrosine kinases and phagocytosis, inhibited both the initial rise and subsequent fall in Cm, suggesting that tyrosine phosphorylation is required for focal exocytosis (our unpublished data). Phagocytosis is crucially dependent on actin polymerization, initiated by the activation of Rho-family proteins, mediated in part through Fcγ-mediated tyrosine phosphorylation of the Rho-GEF Vav (Caron and Hall, 1998). Although actin assembly has been thought to provide structural integrity to the growing pseudopod, it has not been determined whether it is also involved in focal exocytosis. We blocked actin polymerization by using three different approaches: cytochalasin D (caps barbed ends), latrunculin A (binds actin monomers), and introduction of the Rho-family inhibitor Rho-GDI (blocks all Rho protein actions) via the patch pipette into the cell interior. All three strategies totally prevented the typical Cm changes associated with IgG-bead addition (Figure 4).
Figure 4.
Focal exocytosis is not affected by inhibitors of PI3 kinase but is blocked by actin polymerization antagonists. (A) Continuous Cm recordings from J774 macrophages incubated under control conditions or in the presence of cytochalasin D (0.5 μg/ml), latrunculin A (50 nM), Rho-GDI (10 μg/ml), or wortmannin (100 nM). All agents were preincubated with cells for 30 min before recording. In the case of Rho-GDI, 5–10 min was allowed to elapse to permit intracellular dialysis of the protein via the patch pipette before bead challenge. (B) Summary of mean peak Cm values (± SEM) obtained from cells incubated with actin polymerization antagonists or inhibitors of PI3 kinase (50 μM LY294002). Focal exocytosis (above baseline) and phagocytosis (below line) are shown; number of cells per condition indicated above bars. (Apparent “enhancement” of exocytosis by wortmannin and LY294002 is likely due to absence of contaminating phagocytosis, rather than to any genuine stimulatory effect.)
Another prominent response to the occupation of Fcγ receptors is the activation of PI3 kinase (Ninomiya et al., 1994). Agreement exists that interference with PI3 kinase activity blocks phagocytosis, but there is debate about the phase of phagocytosis that is affected, with some claiming an effect on an early stage, whereas others posit an effect at a stage close to phagosome closure (Araki et al., 1996; Cox et al., 1999). To investigate this question in our paradigm of focal exocytosis, we treated cells with the PI3 kinase antagonists wortmannin and LY294002. Both were found to leave increases in Cm untouched but to block the subsequent decline (Figure 4). These results suggest that PI3 kinase is not involved in the early stage of vesicular production and fusion, but may well be required for later steps that result in scission of the phagosome from the cell surface.
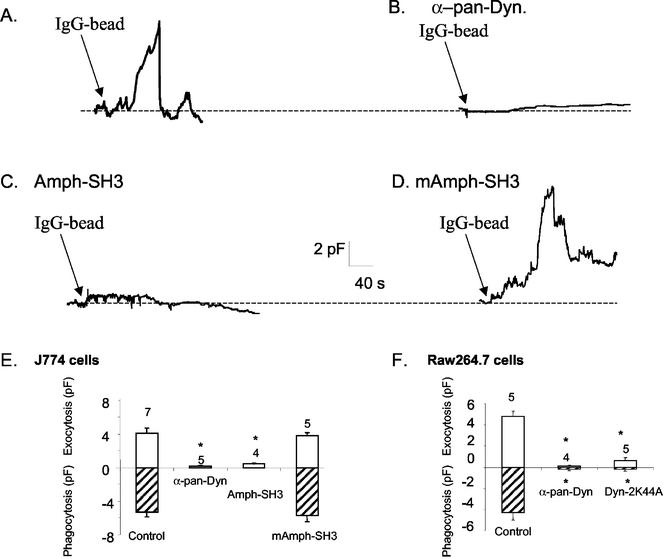
Focal Exocytosis Is Blocked by Anti-Dynamin-2 IgGs and Amphiphysin-Src Homology 3 (SH3) Domain, but Not by a Mutant Amphiphysin-SH3 Domain
Having demonstrated that the capacitance technique provides considerable insight into the initial stages of phagocytosis, we proceeded to investigate the role of the large GTPase dynamin. To determine whether the protein is involved in focal exocytosis, we introduced affinity-purified anti-pandynamin IgGs into J774 macrophages and allowed ∼10 min for equilibration before stimulation with IgG beads (Figure 5B). We have shown previously that a similar strategy abolishes rapid endocytosis in chromaffin cells (Artalejo et al., 1995). Both the early increase in Cm, as well as the later decrease representing phagocytosis, were blocked by this procedure; preimmune IgG was inactive, suggesting that the effect was specific (our unpublished data). The antibody itself had no discernible influence on baseline capacitance in cells not exposed to beads, over periods comparable with those used in the present study. This suggests that dynamin inhibition in the short term does not affect some ongoing constitutive endocytotic process that might contribute to subsequent phagocytosis in macrophages. Dynamin has been reported to interact with the protein amphiphysin in receptor-mediated endocytosis, and an isoform of amphiphysin-II is present in macrophages and is involved in phagocytosis (Gold et al., 2000). Amphiphysin links to the proline-rich C terminus of dynamin via its SH3 domain, and GST•amph-SH3 domain constructs can block receptor-mediated endocytosis in fibroblasts and neurons (Shupliakov et al., 1997; Wigge et al., 1997). Introduction of GST•amph-SH3 protein into macrophages blocked focal exocytosis and phagocytosis in a manner similar to anti-dynamin IgG (Figure 5, C and E). Interaction with dynamin has been localized to a key sequence of six amino acids in amph-SH3; mutation of two of these residues ablates binding to dynamin and destroys the dominant negative behavior of the domain in vivo (Grabs et al., 1997). Introduction of this mutated domain into macrophages had no effect on IgG-bead–induced exocytosis or phagocytosis (Figure 5, D and E), indicating that the effects of the wild-type domain are specific. Finally, we tested the effects of expressing a mutant dynamin that acts as a dominant-negative in other systems (van der Bliek et al., 1993). Previous studies showed that ectopic expression of dominant-negative dynamin-2 in phagocytes inhibited particle internalization, probably at a stage before final closure of the phagocytic cup (Gold et al., 1999). In agreement with this study, we found that overexpression of EGFP-dynamin-2 K44A in RAW 264.7 macrophages blocked Cm changes characteristic of focal exocytosis and phagocytosis (Figure 5F) and internalization of fluorescent latex beads (our unpublished data). These studies place the effects of dynamin antagonism clearly at an early stage in the phagocytic process, rather than simply in final pinch-off of the internalized particle.
Figure 5.
Interfering with dynamin function blocks focal exocytosis and phagocytosis. Continuous Cm recordings from J774.1 macrophages internally dialyzed with control (A), anti-pan dynamin IgG (100 μg/ml) (B), amphiphysin-SH3 domain (amph-SH3; 100 μg/ml) (C), or mutant amphiphysin-SH3 domain (mAmph-SH3; 100 μg/ml) (D). Proteins were allowed to dialyze into the cell (10 min) before addition of IgG beads at arrows. (E) Summary of mean peak Cm values (± SEM) for conditions shown in A–D plus recordings from cells transfected with EGFP-dynaminK44A; focal exocytosis (above baseline) and phagocytosis (below line) are shown; number of cells per condition indicated above bars. (F) Summary of results with RAW264.7 macrophages as in E except that cells were either treated intracellularly with anti-dynamin IgG or transfected with EGFP-dynamin-2 K44A. In the latter experiments, transfected cells were identified by fluorescence 24 h after treatment, and patch-clamp Cm analysis was carried out on these cells. Cells transfected with wild-type EGFP-dynamin-2 exhibited normal focal exocytosis and phagocytosis in bead uptake assays (our unpublished data).
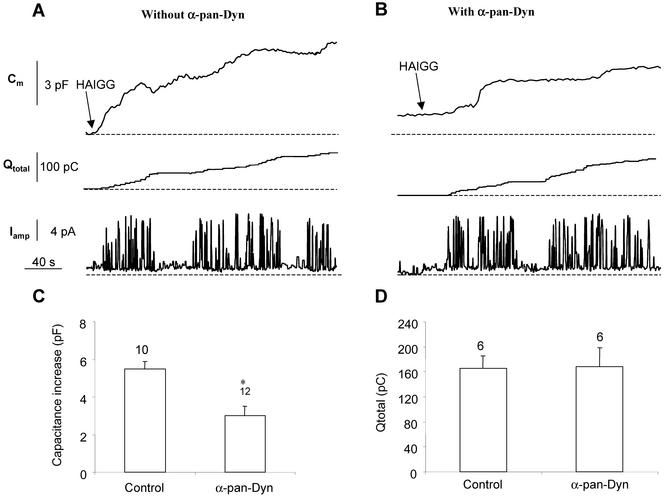
To further assure the specificity of these effects on focal exocytosis, we studied the influence of anti-dynamin IgGs on other types of exocytosis in macrophages. Recently, we have shown that phagosomes in cells loaded with opsonized latex beads could be induced to undergo secretion upon a second stimulation by HAIGG (Di et al., 2002). This type of secretion can be observed electrophysiologically as a stepwise increase in Cm after HAIGG challenge of cells preloaded with IgG beads, as well as a quantal release of superoxide anions into the extracellular space that can be detected by amperometry (Di et al., 2002) (Figure 6A). Introduction of anti-dynamin IgG (Figure 6B) or GST-amphiphysin-SH3 (our unpublished data) into bead-loaded cells significantly reduced, but did not abolish, exocytosis under these conditions. In this situation, exocytosis is likely a mixture of rapid focal exocytosis (<2 min) and slower release of granular material, including phagosomes (>2 min). Anti-dynamin IgG seemed to block the first phase while leaving the second phase intact as revealed by time-to-peak analysis (Figure 6, legend and C). That this interpretation is likely correct was supported by the fact that the IgG had no effect on superoxide release, which we previously showed is due to phagosomal secretion (Figure 6, A and B, bottom; D).
Figure 6.
Anti-dynamin antibodies do not inhibit secretion of phagosomes. (A and B) J774.1 macrophages were preloaded with IgG-coated latex beads for 30 min at 37°C. Bead-loaded cells were selected for analysis and were further stimulated at room temperature with heat-aggregated IgG (HAIGG, at arrows), previously shown by us to stimulate phagosomal secretion (Di et al., 2002). Top, Cm changes in control cells (A) and cells loaded with anti-dynamin IgG (B). Note that in the control cells the existence of a rapid rise in Cm followed by a more steady stepwise increase, whereas in cells cytoplasmically dialyzed with anti-dynamin IgG (100 μg/ml), the initial rapid phase is reduced, whereas the subsequent steps are retained. Middle, cumulative amperometric charge recorded with an extracellular carbon fiber electrode calibrated to detect superoxide radicals. Bottom, sample of amperometric current traces showing quantal nature of superoxide release. (C) Summary of Cm rise in sample cells treated as in A and B. Values are means ± SEM; numbers of cells above bars. (D) Summary of total amperometric charge released from cells treated as in A and B. Values are means ± SEM; number of cells above bars.
Intracellular Localization of Endogenous Dynamin-2 in Macrophages
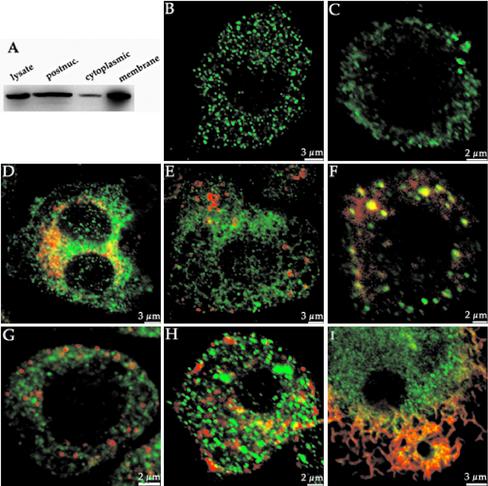
If dynamin is involved in focal exocytosis then it may well localize to and be a marker for those intracellular membranes that provide vesicular material for pseudopodial extension in macrophages. Immunoblotting studies confirmed that macrophages express only dynamin-2 (our unpublished data; cf. Gold et al., 1999), and subcellular fractionation showed that dynamin is localized for the most part to a postnuclear particulate fraction in J774 macrophages (Figure 7A). Confocal microscopy revealed that dynamin-2 staining in J774 or primary alveolar macrophages is punctate and throughout the cell (Figure 7, B and C). These results resemble the distribution of dynamin-2 seen by others in epithelial cells (Cao et al., 1998; Nicoziani et al., 2000). Only a minor pool of dynamin seemed to colocalize with peripheral polymerized actin ruffles in resting cells (Figure 1, supplemental data). Some dynamin was found in the trans-Golgi network, prominently labeled in some other cell types (Cao et al., 1998), as evidenced by colocalization with TGN-38 (Figure 7D), but dynamin did not overlap with the cis-Golgi marker giantin (Figure 2, supplemental data). Dynamin-2 also showed minimal overlap with clathrin at the plasma membrane (our unpublished data) or with the early endosome marker EEA1 (Figure 7E). To ascertain whether dynamin was located in part to an endosomal compartment, we allowed macrophages to internalize dye-labeled transferrin or dextran. Significant overlap was found between the dextran and dynamin-2 signals (Figures 3, supplemental data; and 7F). Little dynamin-2 was found associated with transferrin at early times (1 min) after internalization (Figure 7G), but at later times (10 min) when transferrin should have permeated the entire endosomal system, we found significant overlap of the two signals, especially evident toward the middle of the cell in confocal sections (Figures 4, supplemental data; and 7H). These results suggest that dynamin-2 associates with a part of the endosomal network in macrophages, as is true in other cells (Nicoziani et al., 2000; van Dam and Stoorvogel, 2002). We then localized dynamin-2 in cells ingesting fluorescent IgG-coated beads. In favorable circumstances, we were able to observe proximity between the dynamin-2 signal and the periphery of the bead, marked by an accumulation of polymerized actin. Nevertheless, most of the dynamin remained associated with punctate cytoplasmic structures under these conditions (Figure 7I).
Figure 7.
Dynamin localizes to a presumptive intracellular vesicular compartment in macrophages. (A) Immunoblot of dynamin-2 in J774 macrophages. Cells were homogenized in a hypotonic buffer, and the postnuclear fraction was separated into total particulate and cytosolic fractions. Proteins (50 μg/lane) were separated by SDS-7.5% PAGE, transferred to nitrocellulose, and then probed with antidynamin-2 pAb followed by enhanced chemiluminescence detection. Only the Mr ∼100,000 region of the blot is shown. (B) Staining pattern of dynamin-2 in resting J774 macrophages. Cells were plated onto coverslips and analyzed immunocytochemically 24 h later. Stacks of confocal images were collected using a 100× objective, and a single section through the midpoint of the cell is shown. (C) Staining pattern of dynamin-2 in primary alveolar macrophages. Treatment as in B. (D) Partial dynamin-2 colocalization with TGN-38. Merged image of dynamin-2 (green) and TGN-38 (red). Overlap exists in the juxtanuclear region of the trans-Golgi network marked by TGN38. (E) Dynamin-2 does not colocalize with early endosomes. Merged image of J774 cells stained with antidynamin-2 (green) and anti-EEA1 (red). (F) Dynamin-2 partially colocalizes with internalized dextran. J774 macrophages were incubated in rhodamine-dextran (Mr ∼70, 000; 1 mg/ml) for 1.25 h to label the endosomal system. Cells were then fixed and counterstained with anti-dynamin-2 pAb followed by Alexa 488-coupled secondary IgG (see further images from this cell in supplemental data, Figure 3). (G and H) Dynamin-2 partially colocalizes with internalized transferrin at later times after internalization. J774 macrophages were allowed to internalize Cy5-transferrin (red) for 1 or 10 min and then fixed and counterstained with anti-dynamin-2 pAb, followed by Alexa 488-coupled secondary IgG (green). Note absence of overlap at early time (G) but some overlap toward the center of the cell at the later time point (H) (see further images from this cell in supplemental data, Figure 4). (I) Dynamin-2 distribution in macrophages internalizing unlabeled opsonized latex beads. J774 cells were exposed to 3-μm latex beads for 20 min, and then fixed and counterstained with anti-dynamin-2 pAb/Alexa 488 sary followed by rhodamine-phalloidin to visualize polyactin. Merged image is shown with bead (lower right) surrounded by an extensive network of polyactin with some colocalized dynamin (yellow pixels). Note that the bulk of the remaining punctate dynamin-2 staining resides in the unengaged part of the cell.
Dynamin-2 Migrates toward Sites of Particle Internalization and Transiently Associates with the Phagocytic Cup
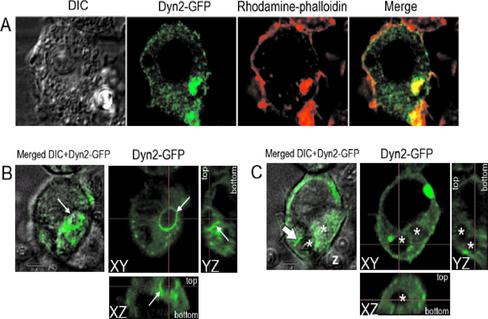
To visualize the kinetic behavior of dynamin-2 during phagocytosis, we expressed an EGFP-tagged version of dynamin-2 in RAW macrophages and studied the disposition of the protein after IgG-bead challenge. Overexpression of this construct, even to high levels, did not seem to compromise particle internalization in these cells. As shown in selected fixed images (Figure 8A) accumulation of dynamin-2 around the bead periphery could be seen, colocalizing to some extent with polymerized actin, visualized with rhodamine-phalloidin. Comparison of this result with that found in untransfected cells (Figure 7I) suggested that the ectopic version of the protein behaved quite similarly to endogenous dynamin-2. Time-lapse studies showed that the punctate dynamin-2–containing structures were motile and transiently associated with large membrane ruffles (supplemental data, Movie 1). Moreover, when cells were challenged with opsonized beads or zymosan, EGFP-dynamin-2 transiently accumulated in the phagocytic cup (Figure 8B; supplemental data, Movie 2); sequential rounds of dynamin recruitment could be seen in cells ingesting more than one particle (supplemental data, Movie 4 and corresponding intensity profiles in Movie 5). These results suggest that a pool of dynamin-2 is mobilized near internalizing beads. That this is likely a transitory event during the formation of the phagosome is illustrated in Figure 8C, where two internalized zymosan particles are seen to lack significant peripheral EGFP-dynamin-2 accumulation (see also three-dimensional reconstruction in supplemental data, Movie 6). Close examination of dynamin behavior during the internalization process revealed an intensity pattern that seemed to progress around the captured particle (supplemental data, Movies 2, 4, and 5). Moreover, strands of fluorescent material apparently “peel” away from the cup after internalization (supplemental data, Movie 3), suggesting that dynamin is dissociating from the base of the phagosome while still being recruited to the tip. These results are consistent with an active role for dynamin throughout particle internalization and support the “zipper” model of signaling during Fcγ-mediated phagocytosis (see DISCUSSION).
Figure 8.
EGFP-dynamin migrates toward internalizing particles and partially colocalizes with actin around phagocytic cups. (A) RAW 264.7 cells were transfected with EGFP-dynamin-2 (wild-type) and analyzed 48 h later. Cultures were then challenged with unlabeled IgG-coated latex beads (3 μm), fixed at various times, and imaged by confocal microscopy. Some coverslips were counterstained with rhodamine-phalloidin to visualize polymerized actin. From left to right, differential interference contrast image of cell ingesting bead; EGFP-dynamin-2 (note lack of accumulation around nucleus toward top of cell but strong staining around bead at lower right); polyactin distribution; merge of two central images showing colocalization of dynamin and actin (yellow pixels) around bead, but relatively little in rest of cell. (B) Formation of a dynamin ring around internalizing particles. Cells were transfected as in A except that analysis was conducted 24 h later. Cultures were challenged with opsonized zymosan particles and visualized in the confocal microscope over the ensuing hour. Figure represents a single frame viewed from three angles, from a series of images collected as a time lapse in supplemental data (Movie 2). Note ring of intense GFP fluorescence around particle (arrows). (C) Lack of association of dynamin with internalized particles. Cells were from the same batch as those visualized in B, except that the focal plane was adjusted to focus on ingested particles (asterisks). Note that no accumulation of dynamin around the internalized particles is apparent. The very bright region is a presumed EGFP-dynamin-2 aggregate that did not seem to influence or participate in particle uptake in this cell. A three-dimensional reconstruction of this cell is shown in supplementary data as Movie 6.
DISCUSSION
Many previous observations suggested that the cell surface undergoes extensive remodeling during phagocytosis in macrophages (for review, see Booth et al., 2001). Although the membrane trafficking events contributing to pseudopodial extension in Fcγ-mediated phagocytosis have come under increased scrutiny, no definitive conclusions have been reached. Local insertion of internally derived vesicles might be required for surface membrane expansion to accommodate the developing phagocytic cup. In the present work, we show that such addition of membrane to the macrophage cell surface can be appraised in real time by using capacitance measurements. This technique has several advantages over other methods, having particularly high resolution in the time domain along with the ability to follow the entire cycle of exocytosis-endocytosis with a single recording. Other methods, like dye-labeling with FM1-43, for example (Cox et al., 1999; Bajno et al., 2000), do not reveal the complex kinetics of both focal exocytosis and phagocytosis. Our results support the contention that rapid plasma membrane expansion, seen as a rise in Cm, is an essential prelude to particle internalization. All three macrophage types surveyed displayed the increase, and maneuvers that blocked the Cm rise also ablated the subsequent Cm fall characteristic of particle internalization. At present, it is not possible to state with certainty whether the increase in Cm represents the fusion of many small vesicles or lesser numbers of larger structures with the plasma membrane. In most cases, we observed a fairly smooth increase in Cm with little indication of step-like events, whereas in some instances spike-like increases in Cm were seen that might be indicative of sudden merging of larger cytoplasmic structures with the cell surface. A recent study showed that elements of the endoplasmic reticulum could fuse with macrophage plasma membrane at the site of enveloping phagosomes, resulting in deposition of endoplasmic reticulum markers in the phagosomal membrane (Gagnon et al., 2002). The occasional recording of sudden large jumps in Cm (see Figure 1B in particular) might reflect such an event and is worthy of further analysis. It is interesting to speculate on how many vesicles would be needed to give rise to the magnitude of signals we commonly see (1–4 pF). If vesicular dimensions were comparable with synaptic vesicles (∼50 nm; ∼70 aF) from 30,000 to 120,000 would be needed, but only 250-1000 vesicles of a size similar to chromaffin granules (∼300 nm; 2.5 fF) would suffice. Evidently, the former number is prodigious, whereas the latter seems manageable. It is likely that the truth lies somewhere in between. Cm measurements also do not indicate the location of vesicular insertion in the plasma membrane. It will be necessary to use various membrane- or compartment-specific labeling techniques to approach this question at the morphological level.
Using the Cm assay, we have analyzed the requirements for focal exocytosis in macrophages and come to some novel conclusions that cast light on the regulation of this phenomenon. Unlike many other types of regulated exocytosis, elevated cytoplasmic Ca2+ is apparently not required for focal exocytosis. However, cells did not phagocytose when [Ca2+]i was strongly buffered, suggesting that some aspect of particle internalization is [Ca2+]i dependent. A [Ca2+]i requirement for phagocytosis has been controversial and may be cell and stimulus dependent (Greenberg, 1999). Although there is little doubt that ligation of Fcγ receptors leads to a rise in [Ca2+]i flux, some have reported that phagocytosis can continue even when [Ca2+]i is buffered to extremely low levels (McNeil et al., 1986; but see Edberg et al., 1995). Our results suggest that particle pinch-off is blocked (or delayed) when [Ca2+]i is buffered to <10–9 M. Thus, our data delimit the participation of Ca2+ to a late stage in phagocytosis. These results also clearly differentiate macrophages from neutrophils, where elevated [Ca2+]i is apparently required for fusion of intracellular granules with the invaginating phagosome (Tapper et al., 2002). In contrast to the results with Ca2+ chelators, blockade of G proteins with the antagonist GDPβS eliminated both focal exocytosis and subsequent phagocytosis. Because vesicular trafficking requires participation of diverse G proteins, this is not inherently surprising; indeed, several G proteins could be involved in focal exocytosis, including Rab or Rho-family GTPases and dynamin-2.
Two other characteristics of focal exocytosis were less expected: insensitivity to PI3 kinase blockade and necessity for actin polymerization. PI3 kinases are activated after Fcγ receptor stimulation, probably by recruitment to the tyrosine kinase Syk (Greenberg, 2001). Activation is essential for phagocytosis because antagonism of these enzymes inhibits particle uptake. Type I PI3 kinase, which generates primarily phosphatidylinositol-3,4,5-trisphosphate is activated early in the phagocytic cascade and the lipid product of this enzyme has been localized to phagocytic cups before and including their closure (Vieira et al., 2001). In contrast, elevated type III PI3 kinase activity, generating primarily phosphatidylinositol 3-phosphate, occurs later and may be involved in phagosome maturation (Ellson et al., 2001; Vieira et al., 2001). The precise role of type I PI3 kinase and phosphatidylinosiotl-3,4,5-trisphosphate before phagosome pinch-off is not clear. Some claim the lipid may only be essential for final engulfment (Araki et al., 1996; Gagnon et al., 2002), whereas others postulate an earlier role, possibly in focal exocytosis (Cox et al., 1999). Our results suggest that PI3 kinase inhibition does not affect focal exocytosis per se, but does impair particle internalization. The fact that PI3 kinase inhibition did not affect focal exocytosis while dynamin-2 antagonism did, suggests that PI3 kinase and its lipid products act downstream rather than upstream of dynamin-2 in phagocytosis.
Actin polymerization is also essential for phagocytosis. There is convincing evidence that components of the actin assembly network (e.g., N-WASP and Arp2/3) are localized to developing phagosomes and that interference with these proteins compromises particle uptake (May and Machesky, 2001). Members of the Rho family of small GTPases likely control actin polymerization via N-WASP and Arp 2/3. Both Rac and Cdc42 are activated during Fcγ signaling, probably due to the enhancement of various guanine nucleotide exchange factors (e.g., the Rac1 guanine nucleotide exchange factor Vav is stimulated by tyrosine phosphorylation; Caron and Hall, 1998). We found that blockade of Rho family members by using intracellular application of Rho-GDI, or antagonism of actin polymerization by using drugs, unexpectedly ablated focal exocytosis. Whether the effect occurs at the level of vesicle production, transport, or insertion is not known, but the results suggest that there must be some coordination between membrane insertion and actin polymerization during pseudopodial extension.
Antagonism of dynamin-2 function by using three independent approaches also abolished the Cm rise after opsonized particle challenge. This result complements previous findings that dominant-negative dynamin-2 overexpression in macrophages interfered with particle uptake (Gold et al., 1999) and intimates that the locus of the effect is at an early stage in the process. Immunocytochemical analysis of endogenous dynamin-2 in macrophages revealed punctate structures, possibly of an endosomal nature. These compartments could be labeled in part by rhodamine-dextran, which enters by fluid-phase endocytosis and is thought to mark the entire endosomal system, and to a lesser extent by labeled transferrin. Current evidence supports a model in which focal exocytosis involves an endosomal compartment, and there is ample evidence that fusion of endosomes with internalized phagosomes plays an important role in phagosomal maturation (Vieira et al., 2002). Phagocytosis seems to involve soluble N-ethylmaleimide-sensitive factor attachment protein receptor (SNARE)-dependent fusion processes, as demonstrated by its partial inhibition on microinjection of v-SNARE degrading protease toxins (Hackam et al., 1998), and appearance of the v-SNARE vesicle-associated membrane protein-3 on early phagosomal membranes (Bajno et al., 2000). Endosomes whose normal function may be to recycle cell surface components such as the transferrin receptor have been implicated in focal exocytosis, and components such as Rab11, that regulate recycling from this compartment, may be involved (Cox et al., 2000). In some cells dynamin-2 is involved in the production of vesicles from this pool and has been localized at the electron microscope level to tubular recycling endosomes (van Dam and Stoorvogel, 2002). Thus, an attractive explanation for the effects of antagonizing dynamin-2 on focal exocytosis is prevention of vesicle budding from such a compartment.
It proved difficult to find association between endogenous dynamin-2 and particle uptake sites in fixed cells, thus the possibility of a transient association was investigated using overexpressed EGFP-dynamin-2. In transfected cells, mobilization of dynamin-2 toward the incipient phagocytic cup was apparent and time-lapse studies revealed that the dynamin-2 signal completely encircled the internalizing particle during engulfment. During this process, the intensity of the dynamin signal followed the production of the phagocytic cup, with the highest intensity ultimately occurring at the distal end or tip, whereas the proximal end or base became fainter. On completion of phagocytosis, EGFP-dynamin-2 rapidly fell away from the phagosomal surface and totally internalized particles showed no evidence of dynamin enrichment. Such a cycle is compatible with the zipper model of Fcγ-mediated phagocytosis (for review, see Swanson and Baer, 1995), in which signaling from successively occupied receptors induces local recruitment of relevant proteins and structures in a progressive manner as the particle becomes surrounded. An intriguing possibility is that dynamin-2 serves to coordinate vesicle production with actin assembly processes, possibly using its fission and actin-binding properties in a sequential manner, as has been proposed for receptor-mediated endocytosis (Qualmann et al., 2000). Recent studies with total internal reflectance microscopy revealed a close association between coated vesicle fission by dynamin at the plasma membrane and assembly of actin, possibly as part of a force-generating system powering movement of coated vesicles into the cytoplasm (Merrifield et al., 2002). To understand the role of dynamin in macrophages, it will be important to identify dynamin-2 interacting species. Several proteins, including amphiphysin, have been postulated to be dynamin partners in regulating receptor-mediated endocytosis, in addition to the lipid messenger phosphatidylinositol bisphosphate (PIP2). An amphiphysin-II isoform has been found in macrophages and overexpression of a truncated version, acting as a dominant negative, led to a phenotype much like mutant dynamin expression in these cells (Gold et al., 2000). Localized changes in PIP2 have been reported to occur in the vicinity of the phagocytic cup (Botelho et al., 2000). We have recently found that, aside from amphiphysin, macrophages express syndapin II and cortactin (our unpublished data), both putative dynamin partners in regulating the actin cytoskeleton. It will be interesting to determine whether either of these proteins or PIP2 interacts with macrophage dynamin-2 in vivo.
Supplementary Material
Article published online ahead of print. Mol. Biol. Cell 10.1091/mbc.E02-09-0626. Article and publication date are at www.molbiolcell.org/cgi/doi/10.1091/mbc.E02-09-0626.
Online version of this article contains video material for some figures. Online version available at www.molbiolcell.org.
References
- Aderem, A., and Underhill, D.M. (1999). Mechanisms of phagocytosis in macrophages. Annu. Rev. Immunol. 17, 593–623. [DOI] [PubMed] [Google Scholar]
- Araki, N., Johnson, M.T., and Swanson, J.A. (1996). A role for phosphoinositide 3-kinase in the completion of macropinocytosis and phagocytosis by macrophages. J. Cell Biol. 135, 1249–1260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Artalejo, C.R., Henley, J.R., McNiven, M.A., and Palfrey, H.C. (1995). Rapid endocytosis coupled to exocytosis in adrenal chromaffin cells involves Ca2+, GTP, and dynamin but not clathrin. Proc. Natl. Acad. Sci. USA 92, 8328–8332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bajno, L., Peng, X.-R., Schreiber, A., Moore, H.-P., Trimble, W., and Grinstein, S. (2000). Focal exocytosis of VAMP3-containing vesicles at sites of phagosome formation. J. Cell Biol. 149, 697–705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Booth, J.W., Trimble, W.S., and Grinstein, S. (2001). Membrane dynamics in phagocytosis. Semin. Immunol. 13, 357–364. [DOI] [PubMed] [Google Scholar]
- Botelho, R.J., Teruel, M., Dierckman, R., Anderson, R., Wells, A., York, J.D., Meyer, T., and Grinstein, S. (2000). Localized biphasic changes in phosphatidylinositol-4,5-bisphosphate at sites of phagocytosis. J. Cell Biol. 151, 1353–1368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao, H., Garcia, F., and McNiven, M.A. (1998). Differential distribution of dynamin isoforms in mammalian cells. Mol. Biol. Cell 9, 2595–2609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caron, E., and Hall, A. (1998). Identification of two distinct mechanisms of phagocytosis controlled by different Rho GTPases. Science 282, 1717–1721. [DOI] [PubMed] [Google Scholar]
- Cox, D., Lee, D.J., Dale, B.M., Calafat, J., and Greenberg, S. (2000). A Rab11-containing rapidly recycling compartment in macrophages that promotes phagocytosis. Proc. Natl. Acad. Sci. USA 97, 680–685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox, D., Tseng, C.C., Bjekic, G., and Greenberg, S. (1999). A requirement for phosphatidylinositol 3-kinase in pseudopod extension. J. Biol. Chem. 274, 1240–1247. [DOI] [PubMed] [Google Scholar]
- Di, A., Krupa, B., Bindokas, V.P., Chen, Y., Brown, M.E., Palfrey, H.C., Naren, A.P., Kirk, K.L., and Nelson, D.J. (2002). Quantal release of free radicals during exocytosis of phagosomes. Nat. Cell Biol. 4, 279–285. [DOI] [PubMed] [Google Scholar]
- Di, A., Krupa, B., and Nelson, D.J. (2001). Calcium-G protein interactions in the regulation of macrophage secretion. J. Biol. Chem. 276, 37124–37132. [DOI] [PubMed] [Google Scholar]
- Diakonova, M., Bokoch, G., and Swanson, J.A. (2002). Dynamics of cytoskeletal proteins during Fcγ receptor-mediated phagocytosis in macrophages. Mol. Biol. Cell 13, 402–411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edberg, J., Lin, C.-T., Lau, D., Unkeless, J., and Kimberly, R. (1995). The Ca2+ dependence of human Fcγ receptor-initiated phagocytosis. J. Biol. Chem. 270, 22301–22307. [DOI] [PubMed] [Google Scholar]
- Ellson, C.D., Anderson, K.E., Morgan, G., Chilvers, E.R., Lipp, P., Stephens, L.R., and Hawkins, P.T. (2001). Phosphatidylinositol 3-phosphate is generated in phagosomal membranes. Curr. Biol. 11, 1631–1635. [DOI] [PubMed] [Google Scholar]
- Fish, K.N., Schmid, S.L., and Damke, H. (2000). Evidence that dynamin-2 functions as a signal-transducing GTPase. J. Cell Biol. 150, 145–154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gagnon, E., Duclos, S., Rondeau, C., Chevet, E., Cameron, P.H., Steele-Mortimer, O., Paiement, J., Bergeron, J.J., and Desjardins, M. (2002). Endoplasmic reticulum-mediated phagocytosis is a mechanism of entry into macrophages. Cell 110, 119–131. [DOI] [PubMed] [Google Scholar]
- Gillis, K. (2000). Admittance-based measurement of membrane capacitance using the EPC-9 patch-clamp amplifier. Pfluegers Arch. Eur. J. Physiol. 439, 655–664. [DOI] [PubMed] [Google Scholar]
- Gold, E.S., Morrissette, N.S., Underhill, D.M., Guo, J., Bassetti, M., and Aderem, A. (2000). Amphiphysin IIm, a novel amphiphysin II isoform, is required for macrophage phagocytosis. Immunity 12, 285–292. [DOI] [PubMed] [Google Scholar]
- Gold, E.S., Underhill, D.M., Morrissette, N.S., Guo, J., McNiven, M.A., and Aderem, A. (1999). Dynamin 2 is required for phagocytosis in macrophages. J. Exp. Med. 190, 1849–1856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grabs, D., Slepnev, V.I., Songyang, Z., David, C., Lynch, M., Cantley, L.C., and De Camilli, P. (1997). The SH3 domain of amphiphysin binds the proline-rich domain of dynamin at a single site that defines a new SH3 binding consensus sequence. J. Biol. Chem. 272, 13419–13425. [DOI] [PubMed] [Google Scholar]
- Greenberg, S. (1999). Biology of phagocytosis. In: Inflammation: Basic Principles and Clinical Correlates, ed. J.I. Gallin and R. Snyderman, Philadelphia: Lippincott, 681–701.
- Greenberg, S. (2001). Diversity in phagocytic signaling. J. Cell Sci. 114, 1039–1040. [DOI] [PubMed] [Google Scholar]
- Hackam, D., Rotstein, O., Sjolin, C., Schreiber, A., Trimble, W., and Grinstein, S. (1998). v-SNARE-dependent secretion is required for phagocytosis. Proc. Natl. Acad. Sci. USA 95, 11691–11696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holevinsky, K.O., and Nelson, D.J. (1998). Membrane capacitance changes associated with particle uptake during phagocytosis in macrophages. Biophys. J. 75, 2577–2586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaplan, G. (1977). Differences in the mode of phagocytosis with Fc and C3 receptors in macrophages. Scand. J. Immunol. 6, 797–807. [DOI] [PubMed] [Google Scholar]
- Kwiatkowska, K., and Sobota, A. (1999). Signaling pathways in phagocytosis. Bioessays 21, 422–431. [DOI] [PubMed] [Google Scholar]
- Lowry, M.B., Duchemin, A.M., Robinson, J.M., and Anderson, C.L. (1998). Functional separation of pseudopod extension and particle internalization during Fcγ receptor-mediated phagocytosis. J. Exp. Med. 187, 161–176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- May, R.C., and Machesky, L.M. (2001). Phagocytosis and the actin cytoskeleton. J. Cell Sci. 114, 1061–1077. [DOI] [PubMed] [Google Scholar]
- McNeil, P., Swanson, J., Wright, S., Silverstein, S., and Taylor, D. (1986). Fc-receptor-mediated phagocytosis occurs in macrophages without an increase in average [Ca++]i. J. Cell Biol. 1986, 1586–1592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNiven, M.A., Cao, H., Pitts, K.R., and Yoon, Y. (2000). The dynamin family of mechanoenzymes: pinching in new places. Trends Biochem. Sci. 25, 115–120. [DOI] [PubMed] [Google Scholar]
- Merrifield, C.J., Feldman, M.E., Wan, L., and Almers, W. (2002). Imaging actin and dynamin recruitment during invagination of single clathrin-coated pits. Nat. Cell. Biol. 4, 691–698. [DOI] [PubMed] [Google Scholar]
- Nicoziani, P., Vilhardt, F., Llorente, A., Hilout, L., Courtoy, P.J., Sandvig, K., and van Deurs, B. (2000). Role for dynamin in late endosome dynamics and trafficking of the cation-independent mannose 6-phosphate receptor. Mol. Biol. Cell 11, 481–495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ninomiya, N., Hazeki, K., Fukui, Y., Seya, T., Okada, T., Hazeki, O., and Ui, M. (1994). Involvement of phosphatidylinositol 3-kinase in Fcγ receptor signaling. J. Biol. Chem. 269, 22732–22737. [PubMed] [Google Scholar]
- Okamoto, P.M., Herskovits, J.S., and Vallee, R.B. (1997). Role of the basic, proline-rich region of dynamin in Src homology 3 domain binding and endocytosis. J. Biol. Chem. 272, 11629–11635. [DOI] [PubMed] [Google Scholar]
- Orth, J.D., Krueger, E.W., Cao, H., and McNiven, M.A. (2002). The large GTPase dynamin regulates actin comet formation and movement in living cells. Proc. Natl. Acad. Sci. USA 99, 167–172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qualmann, B., Kessels, M.M., and Kelly, R.B. (2000). Molecular links between endocytosis and the actin cytoskeleton. J. Cell Biol. 150, F111–116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Segura, F., Brioso, M.A., Gomez, J.F., Machado, J.D., and Borges, R. (2000). Automatic analysis for amperometrical recordings of exocytosis. J. Neurosci. Methods 103, 151–156. [DOI] [PubMed] [Google Scholar]
- Shupliakov, O., Low, P., Grabs, D., Gad, H., Chen, H., David, C., Takei, K., De Camilli, P., and Brodin, L. (1997). Synaptic vesicle endocytosis impaired by disruption of dynamin-SH3 domain interactions. Science 276, 259–263. [DOI] [PubMed] [Google Scholar]
- Swanson, J.A., and Baer, S.C. (1995). Phagocytosis by zippers and triggers. Trends cell Biol. 5, 89–93. [DOI] [PubMed] [Google Scholar]
- Tapper, H., Furuya, W., and Grinstein, S. (2002). Localized exocytosis of primary (lysosomal) granules during phagocytosis: role of Ca2+-dependent tyrosine phosphorylation and microtubules. J. Immunol. 168, 5287–5296. [DOI] [PubMed] [Google Scholar]
- Thomas, P., Lee, A.K., Wong, J.G., and Almers, W. (1994). A triggered mechanism retrieves membrane in seconds after Ca2+-stimulated exocytosis in single pituitary cells. J. Cell Biol. 124, 667–675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tse, S.M., Furuya, W., Gold, E., Schreiber, A.D., Sandvig, K., Inman, R.D., and Grinstein, S. (2003). Differential role of actin, clathrin and dynamin in Fcγ receptor-mediated endocytosis and phagocytosis. J. Biol. Chem. 278, 3331–3338. [DOI] [PubMed] [Google Scholar]
- van Dam, E.M., and Stoorvogel, W. (2002). Dynamin-dependent transferrin receptor recycling by endosome-derived clathrin-coated vesicles. Mol. Biol. Cell 13, 169–182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Bliek, A.M., Redelmeier, T.E., Damke, H., Tisdale, E.J., Meyerowitz, E.M., and Schmid, S.L. (1993). Mutations in human dynamin block an intermediate stage in coated vesicle formation. J. Cell Biol. 122, 553–563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vieira, O.V., Botelho, R.J., and Grinstein, S. (2002). Phagosome maturation: aging gracefully. Biochem. J. 366, 689–704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vieira, O.V., Botelho, R.J., Rameh, L., Brachmann, S.M., Matsuo, T., Davidson, H.W., Schreiber, A., Backer, J.M., Cantley, L.C., and Grinstein,S.(2001).Distinct roles of class I and class III phosphatidylinositol 3-kinases in phagosome formation and maturation. J. Cell Biol. 155, 19–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wigge, P., Vallis, Y., and McMahon, H.T. (1997). Inhibition of receptor-mediated endocytosis by the amphiphysin SH3 domain. Curr. Biol. 7, 554–560. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.