Abstract
Laminin–integrin interactions can in some settings activate the extracellular signal-regulated kinases (ERKs) but the control mechanisms are poorly understood. Herein, we studied ERK activation in response to two laminins isoforms (-1 and -10/11) in two epithelial cell lines. Both cell lines expressed β1-containing integrins and dystroglycan but lacked integrin α6β4. Antibody perturbation assays showed that both cell lines bound to laminin-10/11 via the α3β1and α6β1 integrins. Although laminin-10/11 was a stronger adhesion complex than laminin-1 for both cell lines, both laminins activated ERK in only one of the two cell lines. The ERK activation was mediated by integrin α6β1 and not by α3β1 or dystroglycan. Instead, we found that dystroglycan-binding domains of both laminin-1 and -10/11 suppressed integrin α6β1-mediated ERK activation. Moreover, the responding cell line expressed the two integrin α6 splice variants, α6A and α6B, whereas the nonresponding cell line expressed only α6B. Furthermore, ERK activation was seen in cells transfected with the integrin α6A subunit, but not in α6B-transfected cells. We conclude that laminin-1 and -10/11 share the ability to induce ERK activation, that this is regulated by integrin α6Aβ1, and suggest a novel role for dystroglycan-binding laminin domains as suppressors of this activation.
INTRODUCTION
Laminins are basement membrane components composed of heterotrimers of α, β, and γ chains (Colognato and Yurchenco, 2000). Both laminin-1 (α1β1γ1) and laminin-10/11 (α5β1γ1/α5β2γ1) seem to have important functions in embryogenesis. Laminin-1 is thought to be important for early epithelial morphogenesis in many tissues (Klein et al., 1988; Li et al., 2001, 2002), and mice lacking α5 chain die during midgestation showing multiple morphological abnormalities in several tissues and their compartments (Miner et al., 1998). Laminin α1 has a more restricted tissue distribution than the α5 laminins (Falk et al., 1999; Virtanen et al., 2000). However, both proteins are expressed in the adult stage and may thus have roles in tissue homeostasis.
Laminins bind receptors such as integrins and dystroglycan (Mercurio, 1995; Henry and Campbell, 1999), but the receptor repertoire of all known laminins is not fully clarified. Laminin-1 (α1β1γ1) binds to cells via several β1 integrins, integrin α6β4, and dystroglycan (Aumailley and Smyth, 1998). Laminin-10/11 is more adhesive than laminin-1 for several cell types (Kikkawa et al., 1998; Gu et al., 1999) and is recognized by integrin α3β1 (Kikkawa et al., 1998), integrin α6β1 (Gu et al., 1999; Tani et al., 1999), and integrin α6β4 (Kikkawa et al., 2000). Yet, binding of two epithelial cells to laminin-10/11 could not be inhibited by antibodies against α3 or α6 integrin subunits when applied singly (Ferletta and Ekblom, 1999). This could be due to complementary functions of integrins or to the presence of other receptors such as dystroglycan (Durbeej et al., 1995, 1998). Dystroglycan binds with high affinity to laminin α1 and α2 chains, mainly to the laminin globular (LG) domains (Timpl et al., 2000), but it does not bind laminin α4 (Talts et al., 2000). Recombinant α5LG domains showed some binding to endothelial dystroglycan (Shimizu et al., 1999), even though a sequence comparison revealed no typical dystroglycan binding sites in the α5 chain (Hohenester et al., 1999).
Laminin–integrin interactions can in some settings activate extracellular signal-regulated kinases (ERKs), but due to the large number of ligands and receptors, information is available only for some interactions. Laminin-5 (α3β3γ2) was reported to activate ERKs in keratinocytes through integrin α6β4, and it was proposed that α3β1 and α6β1 integrins, two major laminin receptors, do not belong to the integrins coupled to the ERK pathway (Wary et al., 1996). This view is still prevailing (Giancotti and Ruoslahti, 1999), although a few reports contradict this view. It has been shown that laminin-5 activates ERK via integrin α3β1 in keratinocytes, whereas laminin-1 was inactive (Gonzales et al., 1999). Laminin-1 can activate ERK in fibroblasts via unknown receptors (Chen et al., 1994; Fincham et al., 2000). In macrophages, intact laminin-1 does not activate ERK, whereas a shorter laminin α1 peptide does so by yet unknown receptors (Khan and Falcone, 2000). Laminin-1 can activate ERK in macrophages expressing the integrin α6A cytoplasmic splice variant but not in those expressing α6B (Wei et al., 1998). These findings suggest that ERK activation in response to laminins could be regulated at many levels.
Herein, we compared the ability of two laminin isoforms (-1 and -10/11) to activate ERK in two different epithelial cell lines. We first identified integrins α3β1 and α6β1 as the major adhesion receptors for laminin-10/11. Mouse laminin-1 was selected as a suitable control because it binds α6β1 but not α3β1 (Delwel et al., 1994). It was therefore revealing that both laminins activated ERK in only one of the two cell lines, even though both cell lines showed a similar adhesion behavior on laminin-1 and -10/11. Several assays identified integrin α6β1 as the mediator of this activation. The cell line responding by ERK activation expressed both integrin α6 splice variants but α6A more prominently, whereas the nonresponding cell line expressed only the α6B variant. These findings demonstrate a crucial role for the cytoplasmic domains of the α6 subunit in ERK activation. We also present evidence for a novel role of dystroglycan as a suppressor of integrin α6Aβ1-mediated ERK activation.
MATERIALS AND METHODS
Cell Culture
WI-26 VA4 (ATCC CCL-95.1), a human lung epithelial cell line, and WCCS–1, a human kidney epithelial cell line isolated from Wilms' tumor (Talts et al., 1993) were grown as described previously (Ferletta and Ekblom, 1999). Human erythroleukemic K562 cells transfected with integrin α6A or α6B subunits have been described previously (Delwel et al., 1993). For subculturing and for experiments, cells were harvested using 0.05% trypsin and 0.02% EDTA in phosphate-buffered saline (PBS), pH 7.4. Basic fibroblast growth factor was from Roche Diagnostics (Mannheim, Germany). Other cell culture reagents were from Invitrogen (Carlsbad, CA).
Substrates and Antibodies Used in Cell Adhesion Experiments
Mouse laminin-1 was obtained as described previously (Paulsson et al., 1987) or purchased from Invitrogen. The E8 elastase fragment of laminin-1 was prepared as described previously (Paulsson et al., 1987). Laminin-2/4 was from Invitrogen. Laminin-10/11 from human A549 cells was prepared by antibody affinity purification as described previously (Kikkawa et al., 1998). Human laminin-10/11 purified from pepsin digest of placenta by immunoaffinity chromatography by using the anti-α5 chain monoclonal antibody (mAb) 4C7 (Tiger et al., 1997; Church and Aplin; 1998; Kikkawa et al., 1998; Ferletta and Ekblom, 1999) was from Invitrogen. Recombinant laminin domains α1LG4-5, α2LG4, α2LG1-3, and α5LG4-5 were produced and purified as described previously (Talts et al., 1999; Yu and Talts, 2003). Dystroglycan from kidney and skeletal muscle was obtained by purification from isolated membranes as described previously (Ervasti et al., 1991; Ohlendieck et al., 1991; Durbeej and Campbell, 1999).
Monoclonal antibodies detecting human integrin subunits were as follows: FB12 against α1, P1E6 against α2, P1B5 against α3 (all from Chemicon International, Temecula, CA), GoH3 against α6, and M13 (BD Biosciences (San Jose, CA) or HA2/5 (BD Biosciences PharMingen, San Diego, CA) against β1. Control monoclonal isotype standards for cell adhesion assays (BD Biosciences PharMingen) were as follows: A112-2 (mouse IgG1,κ), G155-228 (mouse IgM, κ), and R35-95 (rat IgG2a, κ).
Cell Adhesion to Laminin Substrates and Inhibition Assays
Cell adhesion was performed according to Ferletta and Ekblom (1999). For cell adhesion inhibition experiments, cells were preincubated for 20 min at 37°C in suspension in the presence or absence of antibodies at 20 μg/ml and then plated on wells coated with 10 μg/ml laminin-10/11. Cell adhesion was allowed for 10 min at 37°C. Each experiment was performed in triplicate.
Detection of ERK Activation by Western Blotting
ERK activation was detected from cells that had been seeded to confluence (1 × 105 cells/cm2), serum starved for 24 h, detached, and 1) plated on laminin-1 or -10/11 in the presence or absence of antibodies; 2) cultured in suspension in the presence or absence of antibodies; 3) allowed to attach in the presence or absence of recombinant laminin fragments to wells coated with laminin-receptor antibodies overnight at 4°C. For inhibition of ERK phosphorylation, cells in suspension were incubated with PD98059 for 30 min before plating onto laminin-10/11. Extraction of total cellular protein and immunoblotting were performed as described previously (Genersch et al., 2000, 2003). Antibodies were as follows: mouse monoclonal antibodies specific for ERK1, ERK2, mitogen-activated protein kinase kinase (MEK)1, and MEK2 (Transduction Laboratories, Lexington, KY), rabbit polyclonal antibodies against phospho-MEK1/2 (Ser217/221), and mouse monoclonal against phospho-ERK1/2 (T202/Y204) (New England BioLabs, Beverly, MA). An MEK-specific inhibitor of ERK phosphorylation, PD98059, was obtained from Calbiochem. Immunoblots were visualized by enhanced chemiluminescence (Amersham Biosciences, Piscataway, NJ). The degree of phosphorylation was measured quantitatively by comparing the intensities of bands of blottings made with antibodies detecting kinases irrespective of their phosphorylation status and antibodies detecting the phosphorylated forms. Quantification of data was performed using a Lumi Imager F1 (Roche Diagnostics).
Immunoprecipitation
Biotinylation of cell surface proteins by using the enhanced chemiluminescence protein biotinylation module from Amersham Biosciences was performed according to the manufacturer's instructions. Biotinylated whole cell lysates were precleared with appropriate control IgGs together with protein G plus-agarose (Santa Cruz Biotechnology, Santa Cruz, CA) and subsequently precipitated with antibodies for 2 h at 4°C, and then for 3 h after addition of protein G plus-agarose. Pellets were collected by centrifugation, washed, and boiled in 1× nonreducing Laemmli buffer. Immunoprecipitation reactions were subjected to SDS-PAGE (7.5% polyacrylamide). Electroblotting and detection of biotinylated proteins were performed as described above. The following antibodies were used: integrin β1 antibody P4C10 (Invitrogen), integrin α6 antibody GoH3, integrin β4 antibody ASC-9 (Chemicon International) or α-dystroglycan antibody IIH6.
Fluorescence-activated Cell Sorting (FACS) Analysis of Surface Integrins
Half a million of trypsinized and washed cells were resuspended in 50 μl of PBS in microtiter plates at room temperature for 10 min with GoH3 rat anti-human integrin α6 (10 μg/ml) (BD Biosciences PharMingen), or the following mouse antibodies (Chemicon International): FB12 against human integrin α1 (2 μg/ml), AK7 against α2 (10 μg/ml), ASC-6 against α3 (10 μg/ml), and ASC-9 against β4 (10 μg/ml). After 30 min at 4°C, cells were washed three times in PBS and resuspended in 50 μl of goat anti-mouse-Ig-FITC (1/50; DAKO, Glostrup, Denmark) or goat anti-rat-Ig-FITC (1/100; BD Biosciences PharMingen), and incubated in the dark for 10 min before washing and analysis on a FACScan flow cytometer (BD Biosciences). At least 10,000 cells were registered and results analyzed using CellQuest software program (BD Biosciences). Cells incubated with the secondary FITC-conjugated antibody alone were used as controls.
Reverse Transcriptase Polymerase Chain Reaction (RT-PCR)
Total RNA from WI-26 VA4 and WCCS-1 cells were isolated using RNeasy kit (QIAGEN, Valencia, CA) according to the manufacturer's instructions. RT-PCR was performed using Titan One Tube RT-PCR kit (Roche Diagnostics) according to the manufacturer's protocol. Subsequently, a touchdown PCR protocol with a final annealing temperature of 52°C was performed. To detect integrin α6A and α6B splice variants in the same reaction, the following primers were used: 5′-GACTCTTAACTGTAGCGTGA-3′ and 5′-ATCTCTCGCTCTTCTTTCCG-3′ (Tamura et al., 1991). PCR products were analyzed on a 1.2% agarose gel.
Overlay Assay
Dystroglycan isolated from rabbit skeletal muscle (Ervasti et al., 1991; Ohlendieck et al., 1991) and from rabbit kidney (Durbeej and Campbell, 1999) was separated on 3–12% SDS-PAGE gradient gels and transferred to nitrocellulose membranes. Blots were blocked in laminin binding buffer (LBB) (140 mM NaCl, 1 mM MgCl2, 1 mM CaCl2, 10 mM triethanolamine, pH 7.6) containing 5% nonfat dry milk and subsequently incubated in LBB containing 3% bovine serum albumin and 0.9 μg/ml laminin-10/11 or -1. Bound laminin-10/11 was detected using monoclonal antibodies specific for human laminin γ1 (2E8) and human laminin α5 (4C7) chains, bound laminin-1 was detected using a polyclonal antibody (317) against laminin α1 chain (Durbeej et al., 1996), followed by appropriate peroxidase-coupled secondary antibodies and developed in 4-chloro-1-naphtol and H2O2 or enhanced chemiluminescence (SuperSignal; Pierce Chemical, Rockford, IL). For inhibition of α-dystroglycan/laminin-10/11 interaction, 1 mg/ml porcine intestinal mucosa heparin (Sigma-Aldrich, St. Louis, MO) tested for its effect on laminin-1 binding to dystroglycan, was included in the LBB.
Solid Phase Assay
Laminin-1 and -10/11 were from Invitrogen. Laminin-5 was purified from the culture medium of a human gastric carcinoma cell line, MKN-45. Cells were cultured in DMEM supplemented with 2.5% fetal bovine serum and 100 ng/ml phorbol 12-myristate 13-acetate. Medium was clarified by centrifugation, supplemented with 5 mM EDTA, 50 μM NEM and 50 μM phenylmethylsulfonyl fluoride, passed over an immunoaffinity column with rabbit polyclonal antilaminin γ2 antibodies. The column was washed with 10 mM Tris-buffered saline, pH 7.5, and bound laminin-5 was eluted with 0.1 M glycine-HCl, pH 3.0, and immediately neutralized with 2 M Tris-HCl, pH 7.5. Rat Schwannoma α-dystroglycan was purified from RT4 cells. Culture supernatant was passed over a wheat germ agglutinin-Sepharose column (Amersham Biosciences) followed by purification on a laminin-1 affinity column as described previously (Matsumura et al., 1997). Chicken-α-dystroglycan was a gift from Andrea Brancaccio (Catholic University of Rome, Rome, Italy) and tested as described previously (Talts et al., 1999).
Solid phase assays were carried out with α-dystroglycan (5 μg/ml) coated onto the plastic surface of microtiter wells at 4°C. All further incubations were at room temperature. Wells were blocked for 2 h with 50 mM Tris-HCl, pH 7.4, 0.15 M NaCl, 5 mM CaCl2 (wash buffer) with 1% bovine serum albumin. Serial dilutions of laminin-1, -5, and -10/11 were added and incubated for 2 h. After washing, bound ligand was detected with specific antisera against laminin α1 chain (Klein et al., 1988), laminin α3 chain (2B10), or 4C7 mAb against laminin α5 chain (Tiger et al., 1997) diluted to give a maximal absorbance of 1.2–1.6 in enzyme-linked immunosorbent assay. After a further wash, bound antibodies were detected by horseradish peroxidase-conjugated secondary antibodies (Bio-Rad, Hercules, CA) followed by addition of 1 mg/ml 5-amino-2-hydroxybenzoic acid (Sigma-Aldrich), 0.001% H2O2. Experiments were performed both in the absence and presence of EDTA.
RESULTS
Adhesion of Epithelial Cells to Laminin-10/11 by Cooperation of Integrin α3β1 and α6β1
We previously found that laminin-10/11 is a stronger adhesive substrate than laminin-1 for two human epithelial cell lines. Northern blotting showed that both cell types expressed dystroglycan and β1 integrins, and immunofluorescence failed to detect β4 integrins but showed expression of several other integrin chains (Ferletta and Ekblom, 1999). In that study, the strong cell adhesion to laminin-10/11 (Figure 1A) could not be inhibited by antibodies against α3 or α6 integrin subunits in 60-min assays. In shorter 10-min assays, WI-26 VA4 cell attachment to laminin-10/11 could still only partially be inhibited by antibodies directed against either α3 or α6 integrin subunits (Figure 1B). No additional inhibition was seen with any of the other tested combinations of α1, α2, α3, and α6 integrin antibodies (Figure 1B). When both integrin α3 and α6 subunits were blocked, total inhibition of adhesion was achieved in 10-min assays. The combination of α3 and α6 integrin antibodies likewise completely inhibited WCCS-1 cell adhesion to laminin-10/11 (Figure 1C). Blocking the β1 integrin subunit resulted in total inhibition of WCCS-1 cell adhesion to laminin-10/11 (Figure 1C), whereas a residual adhesive activity of WI-26 VA4 cells of ∼10% was observed (Figure 1B). For both studied epithelial cell lines, either the α3β1 or the α6β1 integrin can thus mediate the initial binding to laminin-10/11.
Figure 1.
Adhesion of WI-26 VA4 and WCCS-1 cells to laminin isoforms and inhibition of cell adhesion to laminin-10/11 by antiintegrin antibodies. (A) WI-26 VA4 (rectangles) and WCCS-1 (circles) cell adhesion to multiwell plates coated with different concentrations of laminin-1 (open symbols) or -10/11 (closed symbols) was measured after 1 h by using a colorimetric reaction. Adhesion of WI-26 VA4 cells (B) or WCCS-1 cells (C) to laminin-10/11 in the presence or absence of integrin antibodies. In B and C, plates were coated with laminin-10/11 at 10 μg/ml and cells were then incubated in the presence or absence of 20 μg/ml either anti-α1 integrin antibody (FB12), anti-α2 (P1E6), anti-α3 (P1B5), anti-α6 (GoH3), or anti-β1 (Mab13), or combinations of antibodies as indicated in the figure. Cell adhesion was measured after 10 min. Adhesion of control cells in the absence of antibodies was set as 100%, and values reported in the bar graph express the percentage of adherent cells in presence of antibodies. Each point represents the mean of triplicate wells (± SD). The experiments were repeated multiple times with similar results for A, B, and C.
Laminin-1 and -10/11 Induce Mitogen-activated Protein Kinase Signaling in One of the Cell Lines
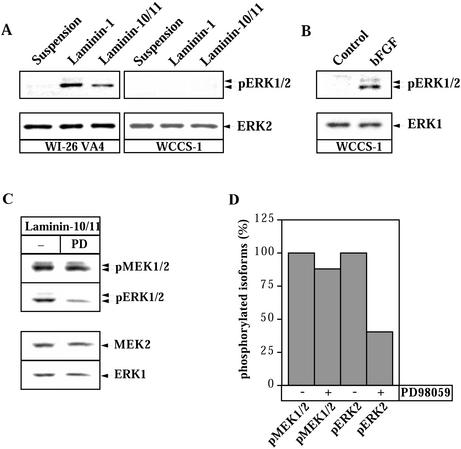
Whereas human laminin-1 binds both integrin α3β1 and α6β1 (Virtanen et al., 2000), mouse laminin-1 binds α6β1 but not α3β1 (Delwel et al., 1994). We therefore compared ERK activation initiated by cell binding to mouse laminin-1 and human laminin-10/11. Serum-starved epithelial cells were detached and either kept in suspension or plated on dishes coated with laminin-1. No ERK activation was seen when cells were seeded on plastic. In WI-26 VA4 cells, adhesion to both laminin-1 and laminin-10/11 strongly increased the amount of phosphorylated isoforms of ERK1/2 compared with control cells in suspension (Figure 2A), indirectly suggesting involvement of integrin α6β1 in ERK signaling in this cell type. In contrast, adhesion of WCCS-1 cells to either laminin did not result in detectable phosphorylation of ERK1/2, although WCCS-1 cells readily responded by ERK activation to basic fibroblast growth factor (Figure 2B). Hence, variability of the ERK activation was not due to different laminin isoforms tested, but rather due to differential ability of cell types to respond to the ligands. Furthermore, firm WCCS-1 cell adhesion to laminin-10/11, mediated by α6 and α3 integrins, was not sufficient to activate ERK.
Figure 2.
ERK phosphorylation in WI-26 VA4 and WCCS-1 cells attached to laminin-1 or laminin-10/11. (A). Serum-starved cells were detached and kept in suspension or cultured for 60 min on plates coated with laminin-1 or -10/11 at 10 μg/ml as indicated in the figure. Total cellular protein extracts (20 μg) were subjected to Western blot analysis by using antibodies specific for the phosphorylated isoforms of ERK1/2. To evaluate loading efficiency, membranes were stripped and reprobed with antibodies recognizing ERK2 irrespective of its phosphorylation status. The positions of phospho-ERK1/2 and ERK2 are indicated. The same results were seen in more than three experiments for both cell lines. (B) WCCS-1 cells were cultured in medium containing 10% fetal calf serum for 48 h. ERK activation was then measured as in A from cells cultured for an additional 5 min in the presence or absence of basic fibroblast growth factor at 5 nM. (C) Serum-starved cells were detached and then cultured with or without the MEK-specific inhibitor PD98059 (50 μM, PD) for 30 min in suspension and then for 30 min on wells coated with laminin-10/11 at 10 μg/ml. The presence of kinases and their phosphorylated forms was detected by immunoblotting as described above. The positions of phospho-MEK1/2, phospho-ERK1/2, MEK2, and ERK1 are indicated. (D) Quantitative measurements of the phosphorylation status of the kinases. Phosphorylation of MEK1/2 and ERK1/2 in nontreated control cells attached to laminin-10/11 was set as 100%. The same results were seen in three independent experiments.
In the classical mitogen-activated protein kinase signaling pathway, ERK1/2 is phosphorylated through MEK1/2. PD98059, a specific inhibitor for MEK1/2, significantly prevented ERK phosphorylation in response to laminin-10/11 compared with noninhibited control cells (Figure 2, C and D), suggesting that phosphorylation of ERK in WI-26 VA4 cells plated on laminins is achieved through activity of MEK1/2. Time-course experiments showed significant activation of MEK at 40 min on both substrates. Activation peaked at 60 min and was still detectable at 120 min. Activation of ERK showed similar kinetics (Figure 3, A and B).
Figure 3.
ERK1/2 and MEK1/2 phosphorylation in WI-26 VA4 cells attached to laminin-1 or laminin-10/11. (A) Serum-starved cells were detached and seeded onto wells coated with laminin-1 or laminin-10/11 at 10 μg/ml. Each lane was loaded with 20 μg of total protein extracted at indicated time points and subsequently analyzed for the presence of ERK1/2, MEK1/2, and their phosphorylated forms by immunoblotting. (B) Quantitative measurements of the phosphorylation status of the kinases. Duplicate experiments gave the same results. (C) Serum-starved WI-26 VA4 cells were cultured for 80 min in the presence or absence of α3 or α6 integrin antibodies at 10 μg/ml, first for 20 min in suspension, and then for 60 min attached to wells coated with laminin-1 or -10/11. The presence of ERK1 and phosphorylated forms of ERK1/2 and MEK1/2 was measured by immunoblotting. During the 60-min attachment period, the presence of α3 and α6 antibodies does not influence the number of attached cells (Ferletta and Ekblom, 1999). Cells kept in suspension without antibodies served as an additional control. The positions of phospho-MEK1/2, phospho-ERK1/2, and ERK1 are indicated. The same results were seen in two experiments.
Integrin α6β1 Is Responsible for Mitogen-activated Protein Kinase Activation
To define receptors involved in ERK activation, we first examined the phosphorylation status of MEK1/2 and ERK1/2 of WI-26 VA4 cells bound to laminin-1 or -10/11 in the presence of α6 or α3 integrin antibodies. Significant but not complete inhibition of MEK and ERK phosphorylation was observed in the presence of α6 antibodies but not in the presence of α3 antibodies (Figure 3C). The incomplete inhibition of signaling by α6 antibodies was most likely due to the ability of these antibodies to induce ERK activation, but more weakly than the natural ligands. As a stringent test of this possibility, we analyzed the ability of antibodies to induce ERK activation in cells kept in suspension. Of the tested antibodies, only integrin α6 antibodies increased phosphorylation of MEK1/2 and ERK1/2 (Figure 4A). Several experiments suggested that natural ligands induce a stronger ERK activation than antibodies, possibly due to ligation of several receptor types. Yet, the IIH6 α-dystroglycan antibody decreased ERK activation mediated by cell attachment to plates coated with the α6 antibody, with maximal reduction at 10 μg/ml (Figure 4B). In contrast, IIH6 slightly increased ERK activation at 100 μg/ml, and at 200 μg/ml a two- to threefold increase was noted (our unpublished data). ERK activation by attachment to laminin-10/11 was also decreased by 10 μg/ml IIH6, and increased by 100 μg/ml IIH6 (our unpublished data). IIH6 is an IgM and might at high concentrations cause significant cross-linking of receptors or have unspecific effects. The influence of 10 μg/ml anti-dystroglycan antibodies on cells and on integrin α6-mediated ERK activation suggested that natural dystroglycan ligands might decrease integrin-mediated signaling pathways. However, these data must be cautiously interpreted, because high concentrations of IIH6 caused a reverse effect. Furthermore, in these experiments exposure times were longer than in experiments with natural ligands, revealing some residual ERK activation also in cells in suspension.
Figure 4.
ERK activation mediated by antibodies in WI-26 VA4 cells. (A) Serum-starved WI-26 VA4 cells were detached and incubated in suspension in serum-free medium in the absence or presence of α3 integrin antibody (anti-α3), α6 integrin antibody (anti-α6), or anti-dystroglycan antibody (anti-α-DG) at 10 μg/ml, as indicated. The presence of ERK1 and phosphorylated forms of ERK1/2 and MEK1/2 was detected by immunblotting. (B) Influence of α-dystroglycan antibodies on ERK activation induced by cell attachment to the integrin α6 antibody. Serum-starved WI-26 VA4 cells were preincubated in suspension for 20 min in the presence of different concentrations of antibodies against α-dystroglycan as indicated, and then seeded on wells coated with antibodies against integrin α6 (10 μg/ml) and allowed to attach for 1 h. The phosphorylated isoforms were analyzed as described in text. The lower panels show quantitative measurements of the phosphorylation status. The positions of phospho-MEK1/2, phospho-ERK1/2, and ERK1 are indicated. The experiments were repeated multiple times and all gave similar results.
We also compared ERK activation in cells allowed to attach to wells coated with anti-α6, anti-α3, or anti-dystroglycan antibodies. ERK activation was increased only in cells attached to the α6 antibodies (Figure 5, A and B). Inspection of cultures suggested that an equal number of cells attached to each of these three antibody-coated wells, whereas cells did not attach to wells coated with their respective control antibodies. Interestingly, the cells attached to wells coated with IIH6 dystroglycan antibody but remained rounded and did not spread. Quantification revealed equal binding to wells coated with α3 or α6 antibodies, but it was not possible to count cells bound to the IIH6 antibody. During the more harsh washing procedures required for quantification of cell adhesion compared with the assays for ERK activation, essentially all cells bound to IIH6-coated wells detached (Figure 5C).
Figure 5.
Comparison of ERK activation induced by attachment of WI-26 VA4 cells to wells precoated with integrin α3, α6, or dystroglycan antibodies. (A) ERK activation in cells allowed to attach for 1 h to wells that had been precoated overnight at 4°C with the indicated antibody at 10 μg/ml. Activation was determined by immunoblotting of protein extracts from cells. The positions of phospho-ERK1/2, and ERK1 are indicated. (B) Quantitative measurements of the phosphorylation status. The same results were obtained in three experiments. (C) Quantification of WI-26 VA4 cell adhesion to antibodies. Cells were allowed to attach for 60 min to multiwell plates that were coated overnight at 4°C with 20 μg/ml P1B5 mouse IgG antibody against integrin α3 (anti-α3), rat IgG antibody GoH3 against integrin α6 (anti-α6), or mouse IgM antibody IIH6 against α-dystroglycan (anti-α-DG), antibody against β1 integrin (anti-β1), or appropriate control antibodies. After stringent washings, the amount of bound cells was measured using a colorimetric reaction. Each point represents the mean of triplicate wells (± SD).
To further test the possibility that dystroglycan ligands counteract integrin α6-mediated ERK activation, we tested recombinant laminin fragments with highly varying affinities to dystroglycan. Recombinant laminin α1LG4–5, which binds with high affinity to dystroglycan (Talts et al., 1999) but does not bind any known integrins, reduced ERK activation mediated by the α6 integrin antibody. Inhibition was observed at 11 nM α1LG4–5, with no further inhibition at 100-fold higher concentrations (Figure 6A). In agreement with the hypothesis that integrin α6 ligation increases and dystroglycan ligation decreases ERK activation, recombinant α2LG1-3 decreased α6 antibody-mediated ERK activation less efficiently than α1LG4–5 (Figure 6A). Although recombinant α2LG1-3 binds dystroglycan with higher affinity than α1LG4-5, it also binds integrins (Talts et al., 1999; Talts and Timpl, 1999). Finally, recombinant α2LG4, which has no dystroglycan-binding activity (Talts et al., 1999), slightly increased α6 integrin antibody-mediated ERK activation. This is shown herein for 40-fold higher concentrations than those of α1LG4–5 sufficient to reduce ERK activation (Figure 6A). The influence of purified recombinant proteins is thus not due to unspecific toxic effects.
Figure 6.
Modulation of ERK signaling by recombinant laminin LG domains. The presence of ERK1 and phosphorylated forms of ERK1/2 and MEK1/2 was measured by immunoblotting. The quantification of results is shown below the gels. (A) WI-26 VA4 cells were cultured for 80 min in the presence or absence of recombinant laminin fragments, first preincubated in suspension for 20 min, and then allowed to attach for 60 min to wells precoated overnight with integrin α6 antibodies (10 μg/ml). Cells kept in solution served as control. Similar results were seen in three experiments. (B) ERK activation after 30 min in response to attachment of WI-26 VA4 cells to integrin α6 antibodies, laminin-1, -10/11, or E8 fragment of laminin-1 at a concentration of 10 μg/ml. The same results were seen in two experiments. (C) The cells were preincubated in the presence of soluble recombinant laminin LG modules as indicated and then allowed to attach to the E8 fragment of laminin-1 (10 μg/ml). The ERK activation was detected after 30 min of attachment. Similar results were seen in three experiments.
The E8 fragment of laminin-1 binds integrin α6 but lacks the dystroglycan binding site of E3 (Delwel et al., 1994). Binding of cells to E8 was used to test whether signaling through dystroglycan has an effect on ERK activation resulting from an interaction of α6β1 with laminin-1. Cells adhered avidly within 30 min to E8, but detached during subsequent 30 min of culture. Cell attachment to E8 at 30 min activated ERK (Figure 6B). Recombinant α1LG4-5 at 100 nM significantly decreased ERK activation mediated by cell attachment to E8, whereas 100 nM α2LG4 and α5LG4-5 failed to do so in these 30-min assays (Figure 6C). To test whether α5LG4-5 nevertheless has activity, cells were plated on laminin-10/11 for 60 min in the presence or absence of the recombinant proteins. In these assays, α5LG4-5 very significantly reduced ERK activation (Figure 7).
Figure 7.
Influence of recombinant laminin LG modules on laminin-10/11 mediated ERK activation. WI-26 VA4 cells were pretreated with the indicated LG modules as described in Figure 6. The cells were then allowed to attach on laminin-10/11 for 1 h in the presence or absence of the indicated LG modules. The presence of ERK1 and phosphorylated pERK1/2 is indicated and quantitative measurements of the phosphorylation status are shown in the lower panels. Two of the three experiments with similar results are shown.
Expression of Integrin Subunits and Dystroglycan by WI-26 VA4 and WCCS-1 Cells
To distinguish whether laminin-induced ERK activation was due to α6β1 or α6β4 integrins in WI-26 VA4 cells, protein complexes precipitated with antibodies against β1, β4, or α6 integrin subunits were analyzed by SDS-PAGE. Due to alternative splicing or proteolysis, β4 can exist as variants with different molecular masses, but none of these were precipitated with antibodies against β4 subunits. In contrast, four bands were detected in β1 immunoprecipitates (Figure 8A). Based on the known molecular masses, the 180-kDa protein was identified as α1 integrin, the broad 160- to 150-kDa protein complex as a likely mixture of α2, α3, and α6 integrins, and the 120-kDa protein as β1 integrin. The identity of the 90-kDa protein remained unclear. Immunoprecipitation with α6 antibodies revealed two proteins typical for the α6 and β1 subunits, but no apparent β4 subunits (Figure 8A). One major α-dystroglycan form of ∼120 kDa was detected in WI-26 VA4 cells (Figure 8A).
Figure 8.
Detection of cell surface receptors expressed by WI-26 VA4 and WCCS-1 cells. (A) Immunoprecipitation of integrins and dystroglycan from WI-26 VA4 cells. Whole cell lysates of surface labeled cells were precipitated with antibodies to β1, α6, β4 integrin subunits, or α-dystroglycan, as indicated on top. Precipitates were separated by SDS-PAGE under nonreducing conditions. Biotinylated proteins were detected as described under MATERIALS AND METHODS. The known migration patterns of some integrin subunits are indicated at left. The positions of molecular weight markers and the major band precipitated with the α-dystroglycan antibody IIH6 are indicated at right. (B) Surface expression levels of some integrin subunits on WI-26 VA4 and WCCS-1 cells, shown by FACS profiles. Cells were stained with antibodies to integrin α1, α2, α3, α6, or β4 integrin subunits before analysis with flow cytometry (black profiles). The negative control (white profiles) shows background levels of staining with the secondary antibody alone. Duplicate experiments gave the same results. (C) Detection of the mRNA for the two splice variants of α6-integrin with different cytoplasmic domains in WI-26VA4 and WCCS-1 cells. Total RNA from WI-26 VA4 and WCCS-1 cells was extracted and subjected to RT-PCR analysis. A primer pair that detects both the 550-base pair amplicon corresponding to variant α6A and the 420-base pair variant corresponding to α6B was used. A control PCR reaction was performed in the absence of templates. PCR products were analyzed by agarose gel electrophoresis. The positions of three marker bands as well as of the α6A- And α6B-amplicons are indicated.
Fluorescence-activated cell sorting showed that all analyzed α chains (α1, α2, α3, and α6) were expressed on the surface of WI-26 VA4 cells and that WCCS-1 expressed only the α3 and α6 subunits (Figure 8B). Of note, both cell types expressed similar levels of integrin α6. Fluorescence-activated cell sorting confirmed that neither cell type expressed β4 subunits (Figure 8B). The failure of WCCS-1 cell adhesion to laminins to activate ERK is thus neither due to low cell surface expression of α6, nor its association with the β4 subunit. The data also show that integrin α6-mediated ERK activation in WI-26 VA4 cells is mediated by α6β1 rather than α6β4. To explain why laminins induce ERK activation in one but not in the other cell line, it was of interest to study the expression of the integrin α6A and α6B splice variants, which have different cytoplasmic tails. Consistent with the results that forced expression of integrin subunit α6A in macrophages confers laminin-1 the ability to induce ERK activation (Wei et al., 1998), we found that the responding cell line WI-26 VA4 expressed both splice variants but α6A more prominently, whereas the nonresponding cell line WCCS-1 only expressed the α6B variant (Figure 8C).
To further substantiate these findings, we analyzed laminin-10/11–mediated ERK activation in human erythroleukemic K562 cells, transfected either with the α6A or α6B variants. Integrin activation increases α6-dependent attachment of these cells to laminin-1 (Delwel et al., 1993), but activation is not required for binding to laminin-10/11 (Delwel et al., 1994; Kikkawa et al., 2000). Experiments with K562 cells and laminin-10/11 were therefore carried out without integrin activation. Whereas untransfected K562 cells bind poorly to laminin-1 or -10/11 (Gu et al., 2003), the K562 α6A or α6B variants bound equally well to laminin-10/11 or to integrin α6 antibodies (Figure 9A). Selective MEK/ERK activation was seen in the K562α6A cells bound to laminin-10/11 (Figure 9, B–D) or to the integrin α6 antibody (our unpublished data).
Figure 9.
ERK and MEK activation in K562 cells expressing integrin α6A or α6B variants. (A) Cell adhesion at 1 h to the indicated laminin isoforms, integrin α6 antibodies or to control antibodies (20 μg/ml) was measured colorimetrically. Each point represents the mean of triplicate wells (± SD). The same results were obtained in two different experiments. (B) K562α6A and K562α6B cells were attached to wells precoated with laminin-10/11 (10 μg/ml) for 1 h. The presence of kinases and their phosphorylation status was examined by immunoblotting as described previously. Quantitative measurements of the phosphorylation status of MEK (C) and ERK (D). Phosphorylation of MEK1/2 and ERK1/2 in cells kept in suspension were set as 100%. Triplicate experiments gave the same results.
Laminin-10/11 as a Ligand for α-Dystroglycan
Because both the known α-dystroglycan ligand α1LG4-5 and the corresponding laminin-10/11 fragment α5LG4-5 reduced integrin α6Aβ1-induced ERK activation, it was of interest to test whether intact laminin-10/11 from adult tissues is a ligand for dystroglycan. Skeletal muscle and kidney α-dystroglycan were therefore separated in SDS-PAGE and incubated with purified laminin-10/11. Analysis of bound laminin-10/11 by antibodies specific for laminin γ1 and α5 chains revealed that α-dystroglycan is able to bind laminin-10/11 (Figure 10A). Binding of laminin-1 to α-dystroglycan was inhibited by heparin as expected (Gee et al., 1993; Pall et al., 1996), but the interaction of laminin-10/11 with α-dystroglycan was heparin insensitive (Figure 10B).
Figure 10.
Binding of laminin-10/11 to α-dystroglycan. (A) Purified α-dystroglycan from skeletal muscle and kidney was subjected to 3–12% gradient SDS-PAGE, blotted onto nitrocellulose membranes, and subsequently incubated in the presence (left lane) or absence (right lane) of purified laminin-10/11. Bound laminin chains were detected by antibodies specific for laminin α5 and γ1 chains (mAbs 4C7 and 2E8) together with appropriate secondary horseradish peroxidase-coupled antibodies. Position of α-dystroglycan/laminin-10/11 complex is indicated. (B) Purified α-dystroglycan from rabbit skeletal muscle was subjected to 3–12% gradient SDS-PAGE, blotted onto nitrocellulose membranes, and subsequently incubated with purified laminin-1 or laminin-10/11 in the presence or absence of 1 mg/ml heparin as indicated in the figure. Bound laminin-1 was detected by a polyclonal antibody against laminin α1 chain (polyclonal antibody 317), and bound laminin-10/11 was detected by antibodies specific for laminin α5 and γ1 chains (mAbs 4C7 and 2E8, respectively). Positions of α-dystroglycan/laminin-1 and α-dystroglycan/laminin-10/11 complexes are indicated. (C) Rat Schwannoma α-dystroglycan binds to laminin-1 and -10/11, but not to laminin-5, as detected by solid phase assays. Absorbance was monitored at 490 nm as a function of whole laminin concentration. Binding of soluble laminin-1 (•), -10/11 (▾), or laminin-5 (♦) to α-dystroglycan adsorbed to plates was measured as described under MATERIALS AND METHODS. Experiments were also performed in the presence of 10 mM EDTA with soluble laminin-1 (○) or -10/11 (▿). Data are shown as semilogarithmic titration curves. The same results were obtained in three experiments.
Quantitative differences in laminin-1, -5, and -10/11 binding to α-dystroglycan from rat Schwannoma cells were tested in a solid phase binding assay. Laminin-1 showed a distinct binding profile with ∼30 nM laminin-1 required for half-maximal binding (Figure 10C). Laminin-5 did not bind at any of the concentrations tested (maximum 400 nM). Laminin-10/11 showed distinct binding. However, although recombinant α5LG4-5 efficiently reduced laminin-mediated ERK activation, binding was weak compared with the laminin-1/dystroglycan interaction. Due to the low binding, half-maximal binding could not be measured exactly. Very similar binding profiles were obtained with chicken kidney α-dystroglycan as the immobilized ligand (our unpublished data). Dystroglycan binding to both laminin-1 and laminin-10/11 was completely inhibited by EDTA, showing a dependence on divalent cations for both interactions (Figure 10C).
DISCUSSION
Cell adhesion to laminins does not invariably activate the ERK pathway, but the control mechanisms are poorly understood. The α6β1 integrin is a common adhesion receptor for many laminins and its α chain can be alternatively spliced to generate the α6A and α6B cytoplasmic domain variants. We found that laminin-1 and -10/11 activated ERK only in cells expressing α6Aβ1. The integrin α3β1 was a potent adhesion receptor for laminin-10/11 for both cell lines, but it could not be linked to ERK activation. We also provide evidence for a novel role of dystroglycan as a suppressor of integrin-mediated ERK activation. The recombinant laminin-1 fragment α1LG4–5, known to bind with high affinity to dystroglycan, as well as dystroglycan antibodies at low concentrations, suppressed ERK activation mediated by the integrin α6 antibody. A similar inhibition of laminin-10/11-mediated ERK activation was seen with a recombinant laminin α5 fragment, which also binds dystroglycan.
ERK Activation by Laminin-1 and -10/11
Both tested cell lines expressed the laminin receptors, integrins α3β1 and α6β1, and dystroglycan. Also, both cell lines bound laminin-10/11 via the α3β1 and α6β1 integrins. We previously showed that both cell lines bound more efficiently to laminin-10/11 than to laminin-1 (Ferletta and Ekblom, 1999). An obvious possibility was that the more adhesive substrate activates ERK more profoundly. However, regardless of the substrate tested, only one of the cell lines responded by increased phosphorylation of MEK1/2 and ERK1/2. The observed prolonged ERK activation is typically initiated by cell–matrix interactions and is distinct from the strong but more transient activation by many growth factors (Aplin et al., 1998). Sustained rather than a transient ERK phosphorylation may be necessary for ERK-mediated changes in gene expression (McCawley et al., 1999; Zeigler et al., 1999; Genersch et al., 2000).
All results consistently defined integrin α6β1 as the mediator of ERK activation in WI-26 VA4 cells. Mouse laminin-1, a ligand of integrin α6β1 but not α3β1, stimulated ERK phosphorylation in these cells as efficiently as laminin-10/11. The ability of WI-26 VA4 cells to remain fully attached to laminin-10/11 when confronted with either the α6 or α3 antibody singly in 60-min assays (Ferletta and Ekblom, 1999) was used to more directly dissect the role of these two integrin subunits for ERK activation. In this assay, α6 but not α3 antibodies decreased laminin-mediated ERK activation. This inhibition was incomplete, suggesting that α6 antibodies themselves cause some stimulation of the MEK/ERK pathway. Indeed, in the absence of extracellular ligands, the α6 antibody activated ERK both in cells kept in solution and in cells allowed to attach to α6 antibodies. No such stimulation was observed with antibodies against integrin α3 or dystroglycan.
WCCS-1 cells adhered to both laminins were shown to use the same receptors as the WI-26 VA4 cells for adhesion to laminin-10/11, expressed the same amount of integrin α6 on the cell surface as WI-26 VA4 cells, could respond to growth factor activation by ERK activation, but neither laminin isoform activated ERK in WCCS-1 cells. The integrin α6 subunit can be alternative spliced to generate α6A and α6B cytoplasmic domain variants (de Melker and Sonnenberg, 1999). The responding cell line WI-26 VA4 expressed both splice variants but α6A more prominently, whereas the nonresponding cell line WCCS-1 only expressed the α6B variant. Similar findings have been reported for laminin-1 in macrophages forced to express these variants (Wei et al., 1998) and was herein demonstrated with laminin-10/11 and K562 cells forced to express either integrin α6A or α6B. It may thus be a general rule that alternative splicing of the cytoplasmic domains of α6 can determine whether the integrin α6β1 can activate ERK, regardless of the type of extracellular ligands. The cytoplasmic domains of the α6A and α6B variants are almost completely different (de Melker and Sonnenberg, 1999) so they should have distinct intracellular functions.
The integrin α3β1 could not be linked to activation of the MEK/ERK pathway in either of the tested cell lines, in agreement with the proposals of Wary et al. (1996). However, this integrin may activate ERK in some settings (Gonzales et al., 1999). It should be noted that we did not test the influence of laminin-5 or antibodies against one of its chains, as was done by Gonzales et al. (1999). It is possible that only some ligands for α3β1 integrin can activate ERK or that the α3A and α3B cytoplasmic splice variants differ in their signaling capacity. These possibilities should be analyzed further with cells of defined expression of such variants (DiPersio et al., 2001). Modest ERK activation and more prominent AKT activation in response to cell attachment to laminin-10/11 was recently reported (Gu et al., 2002) for cells entirely dependent on integrin α3β1 for cell binding to laminin-10/11, but the receptor responsible for ERK activation was not identified. The current data showing a prominent role of α6A in laminin-mediated ERK activation is supported by recent findings in human ECV304 cells. These cells use α3β1 as the major and α6β1 as a minor laminin-10/11 adhesion receptor, yet ERK is activated strongly by α6 antibodies and less efficiently by α3 antibodies (Genersch et al., 2003).
Activated ERK has many targets, ranging from transcription factors to diverse cytoplasmic components (Aplin et al., 1998). In fibroblasts, ERK activated by fibronectin or laminin-1 becomes localized both to the nucleus and focal adhesion complexes (Miyamoto et al., 1995; Fincham et al., 2000). Our findings of distinct roles for α3 and α6 integrin subunits in postadhesion intracellular signaling cascades are interesting in view of reports that α3 and α6 integrin subunits stimulate the formation of different types of focal adhesion complexes in fibroblasts (Dogic et al., 1998) and that α3β1 regulates cytoskeletal assembly as an inhibitor of other integrins in keratinocytes (Hodivala-Dilke et al., 1998). One future issue is therefore whether integrin α6Aβ1 activated ERK is recruited to focal adhesion macroaggregates in fibroblasts (Laplantine et al., 2000) or to discrete focal adhesion complexes in epithelial cells.
Suppression of Integrin-mediated ERK Activation by Dystroglycan
Recently, Chen et al. (2001) hypothesized that the presence of coreceptors might be necessary for integrin α6β1-mediated ERK activation. Herein, we demonstrate suppression of this activation by a coreceptor. The dystroglycan antibody IIH6 suppressed integrin α6Aβ1-induced ERK activation in WI-26 VA4 cells. A similar decrease was obtained by recombinant laminin fragment α1LG4-5, which binds dystroglycan with high affinity but lacks integrin-binding sites (Talts et al. 1999). Recombinant laminin fragments with capacity to bind both dystroglycan and integrin α6β1 (Talts et al., 1999) were not as efficient inhibitors of ERK activation as the dystroglycan-specific α1LG4-5 module. In this context, it is noteworthy that Cyr61, a small nonlaminin ligand for α6β1 presumably not binding dystroglycan, causes a strong and even more sustained ERK activation than laminins in fibroblasts (Chen et al., 2001).
Dystroglycan Binding to Laminin-10/11
Laminin α1 or α2 LG domains bind dystroglycan with high affinity in a strictly calcium-dependent manner, but lack of similar calcium-binding sites in LG domains of α3, α4, and α5 chains suggest that these should bind poorly to dystroglycan (Hohenester et al., 1999; Timpl et al., 2000). Nevertheless, binding of dystroglycan from an endothelial cell line to recombinant fragment α5LG1-5 has been reported, and α5LG1-5 could even displace laminin-1 attachment to dystroglycan (Shimizu et al., 1999). These domains were produced in bacteria so they might lack important posttranslational modifications. Herein, we demonstrate that dystroglycan from several sources can bind intact laminin-10/11 in overlay and solid phase assays.
Solid phase assays demonstrated no binding of the α3-containing laminin-5 to dystroglycan, as predicted by Timpl et al. (2000). However, some binding to the α5-containing laminin-10/11 was noted, but the binding was weak. Binding of laminin-10/11 could be abolished by EDTA, suggesting divalent cation dependence. Overlay assays also demonstrated binding of laminin-10/11 to dystroglycan isolated both from muscle and a tissue rich in epithelium (kidney). Binding of laminin α1LG4 to dystroglycan can be blocked by heparin (Talts et al., 1999), and a heparin-sensitive cell binding site was recently mapped to mouse α5LG4 (Nielsen et al., 2000). Yet, laminin-10/11 binding to dystroglycan in overlay assays was not perturbed by heparin, suggesting that heparin and dystroglycan binding requires distinct sites. Heparin-insensitive binding to dystroglycan has been shown also for laminin-2/4 (Pall et al., 1996; Talts et al., 1999).
The quantitative binding studies, showing a clear hierarchy among laminin isoforms for α-dystroglycan binding are in reasonable agreement both with structural predictions (Hohenester et al., 1999; Timpl et al., 2000) and the report that α5LG1-5 can interact with dystroglycan (Shimizu et al., 1999). Measured binding affinities in cell free assays of some integrins to laminins are also rather low, although these interactions are of obvious biological importance. For instance, integrin α3β1 had a low binding activity of >600 nM for laminin-5 in conditions reflecting those found in tissues, and bound laminin-10/11 even less efficiently (Eble et al., 1998). Recombinant α5LG4-5 was recently shown to contain the dystroglycan-binding site in another study (Yu and Talts, 2003) and was in the present study shown to be a potent inhibitor of laminin-10/11–mediated ERK activation. This was evident in 60-min assays, but not in 30-min assays carried out with laminin E8 as the substratum. The differences may be explained by the low affinity of laminin-10/11 modules to dystroglycan, or other unknown differences in the binding mechanisms. The finding is notable considering the low affinity of the interaction, but strongly supports the view that the dystroglycan-binding domains of laminins can suppress ERK activation. Hence, the recognition of laminin-10/11 by α-dystroglycan might play a significant role in the modulation of signaling cascades initiated by laminins and integrins.
Acknowledgments
We thank Dr. T. Olofsson (Department of Medicine, Lund University, Lund, Sweden) for the help with FACS analyses. This work was supported by a postdoctoral stipend from Wenner-Gren Foundation to Y.K., and a postdoctoral stipend to M.D. and the fellowship program (to P.E. and K.P.C.) of the Swedish Foundation for International Cooperation in Research and Higher Education, the Swedish Cancer Society, the Swedish Natural Science Research Council, Barncancerfonden, and Crafoordska Stfitelsen. K.P.C. is an Investigator of the Howard Hughes Medical Institute.
Article published online ahead of print. Mol. Biol. Cell 10.1091/mbc.E03-01-0852. Article and publication date are at www.molbiolcell.org/cgi/doi/10.1091/mbc.E03-01-0852.
Abbreviations used: ERK, extracellular signal-regulated kinase; MEK, mitogen-activated protein kinase kinase; LG, laminin globular module.
References
- Aplin, A.E., Howe, A., Alahari, S.K., and Juliano, R.L. (1998). Signal transduction and signal modulation by cell adhesion receptors: the role of integrins, cadherins, immunoglobulin-cell adhesion molecules, and selectins. Pharmacol. Rev. 50, 197–263. [PubMed] [Google Scholar]
- Aumailley, M., and Smyth, N. (1998). The role of laminins in basement membrane function. J. Anat. 193, 1–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen, C.C., Chen, N., and Lau, L.F. (2001). The angiogenic factor Cyr61 and connective tissue factor induce adhesive signaling in primary human fibroblasts. J. Biol. Chem. 276, 10443–10452. [DOI] [PubMed] [Google Scholar]
- Chen, Q., Kinch, M.S., Lin, T.H., Burridge, K., and Juliano, R.L. (1994). Integrin-mediated cell adhesion activates mitogen-activated protein kinases. J. Biol. Chem. 269, 26602–26605. [PubMed] [Google Scholar]
- Church, H.J., and Aplin, J.D. (1998). BeWo choriocarcinoma cells produce laminin 10. Biochem. J. 332, 491–498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colognato, H., and Yurchenco, P.D. (2000). Form and function: the laminin family of heterotrimers. Dev. Dyn. 218, 213–234. [DOI] [PubMed] [Google Scholar]
- Delwel, G.O., de Melker, A.A., Hogervorst, F., Jaspars, L.H., Fles, D.L., Kuikman, I., Lindblom, M., Paulsson, M., Timpl, R., and Sonnenberg, A. (1994). Distinct and overlapping ligand specificities of the α6Aβ1 and α6Bβ1 integrins: recognition of laminin isoforms. Mol. Biol. Cell 5, 203–215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delwel, G.O., Hogervorst, F., Kuikman, I., Paulsson, M., Timpl, R., and Sonnenberg, A. (1993). Expression and function of the cytoplasmic variants of the integrin α6 subunit in transfected K562 cells. Activation-dependent adhesion and interaction with isoforms of laminin. J. Biol. Chem. 268, 25865–25875. [PubMed] [Google Scholar]
- de Melker, A.A., and Sonnenberg, A. (1999). Integrins: alternative splicing as a mechanism to regulate ligand binding and integrin signaling events. Bioessays 6, 499–509. [DOI] [PubMed] [Google Scholar]
- DiPersio, C.M., Trevithick, J.E., and Hynes, R.O. (2001). Functional comparison of the α3A and α3B cytoplasmic domain variants of the chicken α3 integrin subunit. Exp. Cell Res. 268, 45–60. [DOI] [PubMed] [Google Scholar]
- Dogic, D., Rousselle, P., and Aumailley, M. (1998). Cell adhesion to laminin 1 or 5 induces isoform-specific clustering of integrins and other focal adhesion components. J. Cell Sci. 111, 793–802. [DOI] [PubMed] [Google Scholar]
- Durbeej, M., and Campbell, K.P. (1999). Biochemical characterization of the epithelial dystroglycan complex. J. Biol. Chem. 274, 26609–26616. [DOI] [PubMed] [Google Scholar]
- Durbeej, M., et al. (1996). Expression of laminin α1, α5 and β2 chains during embryogenesis of the kidney and vasculature. Matrix Biol. 15, 397–413. [DOI] [PubMed] [Google Scholar]
- Durbeej, M., Henry, M.D., Ferletta, M., Campbell, K.P., and Ekblom, P. (1998). Distribution of dystroglycan in normal adult mouse tissues. J. Histochem. Cytochem. 46, 449–457. [DOI] [PubMed] [Google Scholar]
- Durbeej, M., Larsson, E., Ibraghimov-Beskrovnaya, O., Roberds, S.L., Campbell, K.P., and Ekblom, P. (1995). Non-muscle α-dystroglycan is involved in epithelial development. J. Cell Biol. 130, 79–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eble, J.A., Wucherpfennig, K.W., Gauthier, L., Dersch, P., Krukonis, E., Isberg, R.R., and Hemler, M.E. (1998). Recombinant soluble human α3β1 integrin: purification, processing, regulation, and specific binding to laminin-5 and invasin in mutually exclusive manner. Biochemistry 37, 10945–10955. [DOI] [PubMed] [Google Scholar]
- Ervasti, J.M., Kahl, S.D., and Campbell, K.P. (1991). Purification of dystrophin from skeletal muscle. J. Biol. Chem. 266, 9161–9165. [PubMed] [Google Scholar]
- Falk, M., Ferletta, M., Forsberg, E., and Ekblom, P. (1999). Restricted distribution of laminin α1 chain in normal adult mouse tissues. Matrix Biol. 18, 557–568. [DOI] [PubMed] [Google Scholar]
- Ferletta, M., and Ekblom, P. (1999). Identification of laminin-10/11 as a strong cell adhesive complex for a normal and a malignant human epithelial cell line. J. Cell Sci. 112, 1–10. [DOI] [PubMed] [Google Scholar]
- Fincham, V.J., James, M., Frame, M.C., and Winder, S.J. (2000). Active ERK/MAP kinase is targeted to newly forming cell-matrix adhesions by integrin engagement, and v-Src. EMBO J. 19, 2911–2923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gee, S.H., Blacher, R.W., Douville, P.J., Provost, P.R., Yurchenco, P.D., and Carbonetto, S. (1993). Laminin-binding protein 120 from brain is closely related to the dystrophin-associated glycoprotein, dystroglycan, and binds with high affinity to the major heparin binding domain of laminin. J. Biol. Chem. 268, 14972–14980. [PubMed] [Google Scholar]
- Genersch, E., Ferletta, M., Virtanen, I., Haller, H., and Ekblom, P. (2003). Integrin αvβ3 binding to human α5-laminins facilitates FGF-2 and VEGF induced proliferation of human ECV304 carcinoma cells. Eur. J. Cell Biol. 82, 105–117. [DOI] [PubMed] [Google Scholar]
- Genersch, E., Hayess, K., Neuenfeld, Y., and Haller, H. (2000). Sustained ERK phosphorylation is necessary but not sufficient for MMP-9 regulation in endothelial cells: involvement of Ras-dependent and -independent pathways. J. Cell Sci. 113, 4319–4330. [DOI] [PubMed] [Google Scholar]
- Giancotti, F.G., and Ruoslahti, E. (1999). Integrin signaling. Science 285, 1028–1032. [DOI] [PubMed] [Google Scholar]
- Gonzales, M., Haan, K., Baker, S.E., Fitchmun, M., Todorov, I., Weitzman, S., and Jones, J.C.R. (1999). A cell signal pathway involving laminin-5, α3β1 integrin, and mitogen-activated protein kinase can regulate epithelial cell proliferation. Mol. Biol. Cell 10, 259–270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu, J., Fujibayashi, A., Yamada, K.M., and Sekiguchi, K. (2002). Laminin-10/11 and fibronectin differentially prevent apoptosis induced by serum removal via phosphatidylinositol 3-kinase/Akt- and MEK1/ERK-dependent pathways J. Biol. Chem. 277, 19922–19928. [DOI] [PubMed] [Google Scholar]
- Gu, Y., Kortesmaa, J., Tryggvason, K., Persson, J., Ekblom, P., Jacobsen, S.-E., and Ekblom, M. (2003). Laminin isoform specific promotion of adhesion and migration of human bone marrow progenitor cells. Blood 101, 877–885. [DOI] [PubMed] [Google Scholar]
- Gu, Y., Sorokin, L., Durbeej, M., Hjalt, T., Jönsson, J.-I., and Ekblom, M. (1999). Characterization of bone marrow laminins and identification of α5-containing laminins as adhesive proteins for multipotent hematopoietic FDCP-Mix cells. Blood 93, 2533–2542. [PubMed] [Google Scholar]
- Henry, M.D., and Campbell, K.P. (1999). Dystroglycan inside and out. Curr. Opin. Cell Biol. 11, 602–607. [DOI] [PubMed] [Google Scholar]
- Hodivala-Dilke, K.M., DiPersio, C.M., Kreidberg, J.A., and Hynes, R.O. (1998). Novel roles for α3β1 integrin as a regulator of cytoskeletal assembly and as a trans-dominant inhibitor of integrin receptor function in mouse keratinocytes. J. Cell Biol. 142, 1357–1369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hohenester, E., Tisi, D., Talts, J.F., and Timpl, R. (1999). The crystal structure of a laminin G-like module reveals the molecular basis of α-dystroglycan binding to laminins, perlecan, and agrin. Mol. Cell 4, 783–792. [DOI] [PubMed] [Google Scholar]
- Khan, K.M., and Falcone, D.J. (2000). Selective activation of MAPK (erk1/2) by laminin-1 peptide α1:Ser (2091)-Arg(2108) regulates macrophage degradative phenotype. J. Biol. Chem. 275, 4492–4498. [DOI] [PubMed] [Google Scholar]
- Kikkawa, Y., Sanzen, N., Fujiwara, H., Sonnenberg, A., and Sekiguchi, K. (2000). Integrin binding specificity of laminin-10/11: laminin-10/11 are recognized by α3β1, α6β1, and α3β4 integrins. J. Cell Sci. 113, 869–876. [DOI] [PubMed] [Google Scholar]
- Kikkawa, Y., Sanzen, N., and Sekiguchi, K. (1998). Isolation and characterization of laminin-10/11 secreted by human lung carcinoma cells. J. Biol. Chem. 273, 15854–15859. [DOI] [PubMed] [Google Scholar]
- Klein, G.M., Langegger, M., Timpl, R., and Ekblom, P. (1988). Role of laminin A chain in the development of epithelial cell polarity. Cell 55, 331–341. [DOI] [PubMed] [Google Scholar]
- Laplantine, E., Vallar, L., Mann, K., Kieffer, N., and Aumailley, M. (2000). Interaction between the cytodomains of the α3β1 integrin subunits regulates remodelling of adhesion complexes on laminin. J. Cell Sci. 113, 1167–1176. [DOI] [PubMed] [Google Scholar]
- Li, S., Harrison, D., Carbonetto, S., Fässler, R., Smyth, N., Edgar, D., and Yurchenco, P.D. (2002). Matrix assembly, regulation, and survival functions of laminin and its receptors in embryonic stem cell differentiation. J. Cell Biol. 157, 1279–1290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li, X., Chen, Y., Schéele, S., Arman, E., Haffner-Krauz, R., Ekblom, P., and Lonai, P. (2001). Fibroblast growth factor signaling and basement membrane assembly are connected during epithelial morphogenesis of the embryoid body. J. Cell Biol. 153, 811–822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsumura, K., et al. (1997). A role of dystroglycan in Schwannoma cell adhesion to laminin. J. Biol. Chem. 272, 13904–13910. [DOI] [PubMed] [Google Scholar]
- McCawley, L.J., Li, S., Wattenberg, E.V., and Hudson, L.G. (1999). Sustained activation of the mitogen-activated protein kinase pathway. A mechanism underlying receptor tyrosine specificity for matrix-metalloproteinase-9 induction and cell migration. J. Biol. Chem. 274, 4347–4353. [DOI] [PubMed] [Google Scholar]
- Mercurio, A.M. (1995). Laminin-receptors- achieving specificity through cooperation. Trends Cell Biol. 5, 419–423. [DOI] [PubMed] [Google Scholar]
- Miner, J.H., Cunningham, J., and Sanes, J.R. (1998). Roles for laminin in embryogenesis: exencephaly, syndactyly, and placentopathy in mice lacking the laminin α5 chain. J. Cell Biol. 143, 1713–1723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyamoto, S., Teramoto, H., Coso, O.A., Gutkind, J.S., Burbelo, P.D., Akiyama, S.K., and Yamada, K.M. (1995). Integrin function: molecular hierarchies of cytoskeletal and signaling molecules. J. Cell Biol. 131, 791–805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nielsen, P.K., Gho, Y.G., Hoffmann, M.P., Watanabe, H., Makino, M., Nomizu, M., and Yamada, Y. (2000). Identification of a major heparin and cell binding site in the LG4 module of the laminin α5 chain. J. Biol. Chem. 275, 14517–14523. [DOI] [PubMed] [Google Scholar]
- Ohlendieck, K., Ervasti, J.M., Snook, J.B., and Campbell, K.P. (1991). Dystrophin-glycoprotein complex is highly enriched in isolated skeletal muscle sarcolemma. J. Cell Biol. 112, 135–148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pall, E.A., Bolton, K.M., and Ervasti, J.M. (1996). Differential heparin inhibition of skeletal muscle α-dystroglycan binding to laminins. J. Biol. Chem. 271, 3817–3821. [DOI] [PubMed] [Google Scholar]
- Paulsson, M., Aumailley, M., Deutzmann, R., Timpl, R., Beck, K., and Engel, J. (1987). Laminin-nidogen complex. Extraction with chelating agents and structural characterization. Eur. J. Biochem. 166, 11–19. [DOI] [PubMed] [Google Scholar]
- Shimizu, H., Hosokawa, H., Ninomiya, H., Miner, J.H., and Masaki, T. (1999). Adhesion of cultured bovine aortic endothelial cells to laminin-1 mediated by dystroglycan. J. Biol. Chem. 274, 11995–20000. [DOI] [PubMed] [Google Scholar]
- Talts, J.F., Andac, Z., Göhring, W., Brancaccio, A., and Timpl, R. (1999). Binding of the G domains of laminin α1 and α2 chains and perlecan to heparin, sulfatides, α-dystroglycan, and several extracellular matrix proteins. EMBO J. 18, 863–870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talts, J.F., Aufderheide, E., Sorokin, L., Ocklind, G., Mattson, R., and Ekblom, P. (1993). Induction of mouse tenascin expression by a human sarcomatoid Wilms' tumor cell line growing in nude mice. Int. J. Cancer 54, 868–874. [DOI] [PubMed] [Google Scholar]
- Talts, J.F., Sasaki, T., Miosge, N., Goehring, W., Mann, K., Mayne, R., and Timpl, R. (2000). Structural and functional analysis of the recombinant G domains of the laminin α4 chain and its proteolytic processing in tissues. J. Biol. Chem. 275, 35192–35199. [DOI] [PubMed] [Google Scholar]
- Talts, J.F., and Timpl, R. (1999). Mutation of a basic sequence in the laminin α2LG3 module leads to a lack of proteolytic processing and has different effects on β1 integrin-mediated cell adhesion and α-dystroglycan binding. FEBS Lett. 458, 319–323. [DOI] [PubMed] [Google Scholar]
- Tamura, R.N., Cooper, H.M., Collo, G., and Quaranta, V. (1991). Cell type-specific integrin variants with alternative α chain cytoplasmic domains. Proc. Natl. Acad. Sci. USA 88, 10183–10187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tani, T., Lehto, V.-P., and Virtanen, I. (1999). Expression of laminin 1 and 10 in carcinoma cells and comparison of their roles in cell adhesion. Exp. Cell Res. 248, 115–121. [DOI] [PubMed] [Google Scholar]
- Tiger, C.F., Champliaud, M.F., Pedrosa-Domellof, F., Thornell, L.E., Ekblom, P., and Gullberg, D. (1997). Presence of laminin α5 chain and lack of laminin α1 chain during human muscle development and in muscular dystrophies. J. Biol. Chem. 272, 28590–28595. [DOI] [PubMed] [Google Scholar]
- Timpl, R., Tisi, D., Talts, J.F., Andac, Z., Sasaki, T., and Hohenester, E. (2000). Structure and function of laminin LG modules. Matrix Biol. 19, 309–317. [DOI] [PubMed] [Google Scholar]
- Virtanen, I., Gullberg, D., Rissanen, J., Kivilaakso, E., Kiviluoto, T., Laitinen, L.A., Lehto, V.-P., and Ekblom, P. (2000). Laminin α1-chain shows a restricted distribution in epithelial basement membranes of fetal and adult human tissues. Exp. Cell Res. 257, 298–309. [DOI] [PubMed] [Google Scholar]
- Wary, K.K., Mainiero, F., Isakoff, S.J., Marcantonio, E.E., and Giancotti, F.G. (1996). The adaptor protein Shc couples a class of integrins to the control of cell cycle progression. Cell 87, 733–743. [DOI] [PubMed] [Google Scholar]
- Wei, J., Shaw, L.M., and Mercurio, A.M. (1998). Regulation of mitogen-activated protein kinase activation by the cytoplasmic domain of the α6 integrin subunit. J. Biol. Chem. 273, 5903–5907. [DOI] [PubMed] [Google Scholar]
- Yu, H., and Talts, J.F. (2003). β1 integrin and α-dystroglycan binding sites are localized to different LG modules within the laminin α5G domain. Biochem. J. 371, 289–299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeigler, M.E., Chi, Y., Schmidt, T., and Varani, J. (1999). Role of ERK and JNK pathways in regulating cell motility and matrix metalloproteinase 9 production in growth factor-stimulated human epidermal keratinocytes. J. Cell. Physiol. 180, 271–284. [DOI] [PubMed] [Google Scholar]