Abstract
The small GTPase Rab1b is essential for endoplasmic reticulum (ER) to Golgi transport, but its exact function remains unclear. We have examined the effects of wild-type and three mutant forms of Rab1b in vivo. We show that the inactive form of Rab1b (the N121I mutant with impaired guanine nucleotide binding) blocks forward transport of cargo and induces Golgi disruption. The phenotype is analogous to that induced by brefeldin A (BFA): it causes resident Golgi proteins to relocate to the ER and induces redistribution of ER-Golgi intermediate compartment proteins to punctate structures. The COPII exit machinery seems to be functional in cells expressing the N121I mutant, but COPI is compromised, as shown by the release of β-COP into the cytosol. Our results suggest that Rab1b function influences COPI recruitment. In support of this, we show that the disruptive effects of N121I can be reversed by expressing known mediators of COPI recruitment, the GTPase ARF1 and its guanine nucleotide exchange factor GBF1. Further evidence is provided by the finding that cells expressing the active form of Rab1b (the Q67L mutant with impaired GTPase activity) are resistant to BFA. Our data suggest a novel role for Rab1b in ARF1- and GBF1-mediated COPI recruitment pathway.
INTRODUCTION
The secretory pathway in mammalian cells consists of a linear assembly of dynamic compartments. Transport and recycling between these compartments occur through generation of transport intermediates from the donor compartment and the delivery of such intermediates to the appropriate acceptor compartment. A family of Rab proteins belonging to the Ras superfamily of small GTPases has emerged as essential regulators of all stages of membrane traffic (Pfeffer, 2001; Segev, 2001b; Zerial and McBride, 2001). Rabs have been proposed to act in diverse aspects of vesicular transport, including vesicle formation, motility, docking, and fusion. However, despite considerable advances, the exact mechanism of Rab function remains unclear.
In the early secretory pathway, two isoforms of Rab1, Rab1a and Rab1b, have been shown to be required for protein transport from the endoplasmic reticulum (ER) to the cis-Golgi (Plutner et al., 1991; Tisdale et al., 1992; Nuoffer et al., 1994; Pind et al., 1994). A yeast homologue of Rab1, YPT1, is also essential for ER-to-Golgi transport (Segev et al., 1988). The cellular function of Rab1 is likely to involve temporally and spatially distinct interactions with its effectors. Two Rab1 effectors have been identified as the tethering factors p115 and GM130 (Allan et al., 2000; Moyer et al., 2001; Weide et al., 2001). Similarly, in yeast, YPT1 has been shown to interact genetically with the yeast homologue of p115, USO1 (Sapperstein et al., 1996).
Rabs cycle continuously between a GDP- and a GTP-bound form (Schimmoller et al., 1998). The GTP-bound conformation is regarded as “active” and can interact with downstream effector proteins (Segev, 2001a; Zerial and McBride, 2001). Conversion of the GTP- to the GDP-bound form is caused by GTP hydrolysis, facilitated by a GTPase-activating protein. After GTP hydrolysis and the corresponding conformational shift, Rabs interact with a GDP dissociation inhibitor (GDI) that can extract them from the membrane and support their transient existence in the cytosol. The Rab-GDI complex is then specifically recognized by a membrane Rab receptor (the identity of which is unknown) that displaces the GDI and subsequently allows the Rab to bind a new GTP in a reaction mediated by a guanine nucleotide exchange factor. Because the continuous cycling of Rabs between these states is necessary for their function, mutations that alter nucleotide loading, exchange, or hydrolysis can be used to explore the cellular role of α Rab.
To explore the function of Rab1b in vivo, we used a “dominant negative” approach shown to be useful in elucidating the functions of distinct Rabs and their effectors (Segev, 2001a; Zerial and McBride, 2001). We generated a Q67L mutant with low GTPase activity, an S22N mutant with low affinity for GTP but normal affinity for GDP (Nuoffer et al., 1994), and an N121I mutant with low affinity for both GDP and GTP (Pind et al., 1994). Although some mutants of Rab1b or the equivalent mutants of Rab1a (Q65L, S25N, N124I) have been partially characterized (Tisdale et al., 1992; Nuoffer et al., 1994; Pind et al., 1994), previous experiments examined limited number of parameters and used different experimental systems. We used the same methodology to examine the effects of all the Rab1b mutants on (1) ER-Golgi traffic, by monitoring the transport of a cargo protein; (2) Golgi and ER-Golgi intermediate compartment (ERGIC) structure, by analyzing the localization of resident Golgi and ERGIC proteins; (3) the response of Rab1b effectors, by analyzing the behavior of p115 and GM130; (4) the status of the COPII machinery, by exploring the behavior of Sec13 and Sec31; and (5) the status of the COPI machinery, by exploring the behavior of β-COP.
Our data indicate that expression of the wild-type Rab1b and the constitutively active Q67L mutant has limited effect on ER-Golgi trafficking and Golgi structure. In contrast, the inactive S22N mutant causes partial Golgi disruption, whereas the inactive N121I mutant completely disrupts Golgi structure. The N121I mutant causes brefeldin A (BFA)-like phenotype and induces the relocation of resident Golgi proteins to the ER and the redistribution of ERGIC53, GM130, and p115 to punctate structures shown in BFA-treated cells to represent ER exit sites and arrested vesicular tubular clusters (VTCs) (Ward et al., 2001). In addition, N121I causes the dissociation of β-COP from membranes, implicating Rab1b in a pathway leading to COPI recruitment. Supportive evidence for the role of Rab1b in COPI dynamics was provided by the rescue of the N121I phenotype by expression of ARF1 and GBF1, known mediators of COPI recruitment (Lippincott-Schwartz et al., 1998; Kawamoto et al., 2002). Similarly, like expression of ARF1 or GBF1, expression of the active Q67L mutant of Rab1b prevented Golgi fragmentation and β-COP dissociation in BFA-treated cells. Together, our data suggest a role for Rab1b in the GBF1/ARF1-mediated pathway for COPI recruitment. Future work will be necessary to address how Rab1b modulates the COPI recruitment machinery.
MATERIALS AND METHODS
Antibodies
Rabbit polyclonal antibodies against p115 and mouse polyclonal antibodies against GM130 have been described (Barroso et al., 1995; Nelson et al., 1998). Monoclonal anti-giantin G1/133 (Linstedt and Hauri, 1993) and monoclonal anti-ERGIC53 G1/93 (Schweizer et al., 1988) antibodies were provided by Dr. Hans-Peter Hauri (University of Basel, Basel, Switzerland). Polyclonal antibodies against Mann II (Velasco et al., 1993) were provided by Dr. Marilyn Farquhar (University of California, San Diego, CA). Rabbit polyclonal anti-Rab1b and anti-myc antibodies were purchased from Santa Cruz Biotechnology (Santa Cruz, CA). Monoclonal anti-myc antibodies were purchased from Invitrogen (Carlsbad, CA). Monoclonal anti-VSV-G (P5D4) antibodies (Roman et al., 1988) were provided by Dr. Kathryn Howell (University of Colorado, Denver, CO). Goat anti-rat and anti-mouse antibodies conjugated with Oregon Green or Texas Red-X were purchased from Molecular Probes (Eugene, OR). Antibodies against Sec13 and Sec31 (Tang et al., 2000) were provided by Dr. Bor Luen Tang (National University of Singapore, Republic of Singapore).
Generation of Constructs
The GalT-GFP construct was provided by Dr. Brian Storrie (Virginia Tech, Blacksburg, VA) (Storrie et al., 1998). ARF1 wild-type and ARF1Q71L were gifts from Dr. Julie Donaldson (National Institutes of Health, Bethesda, MD). Full-length Rab1b was cloned into the EcoRI-NotI restriction sites of the pEF6/Myc-His B 6P vector (Invitrogen, Carlsbad, CA). Full-length Rab1b was obtained by PCR using as a template a human cDNA library. The primers used for rab1b were 5′-CCGGAATTCCCATGAACCCCGAATATGAC-3′ (forward primer) and 5′-TGCCCGCGGCCGCAACAGCCACCGCCAGCG-3′ (reverse primer) to amplify full-length rab1b (sequence data available from Gen Bank under accession number NM030981). Point mutations were introduced with a Quick Change Site-Directed Mutagenesis Kit according to the manufacturer's protocol (Stratagene Corp., La Jolla, CA). All mutated Rab1 sequences were verified by sequencing. GFP-rab1b versions were subcloned from their respective rab1-myc constructs into pEGFP-vector. GBF1 was obtained from a partial human GBF1 cDNA (KIAA0248) from the Kazusa DNA Research Institute in Chiba, Japan. The missing fragment was amplified from a human lung cDNA library. The PCR product was then subcloned into the KIAA0248 clone using the internal EcoRI site at base 1124 and an engineered external XhoI site. To generate GBFmyc, wtGBF1 was amplified by PCR and subcloned into pcDNA4.0/TO/myc-his (Invitrogen, Carlsbad, CA).
Morphological Analysis of VSV-G Transport
Analysis was performed as described previously (Alvarez et al., 1999). Briefly, HeLa cells plated on coverslips were transfected with Rab1 constructs. After 24 h, cells were infected with VSVtsO45 at 32°C for 30 min, followed by incubation at 42°C for 3 h to accumulate VSV-G in the ER. The cells were then shifted to 32°C for different amounts of time to allow VSV-G transport to the Golgi and the plasma membrane. Transport was terminated by transferring coverslips to ice and fixing them in 3% formaldehyde/PBS for 10 min. The coverslips were then processed for double-label immunofluorescence.
Cell Culture and Immunofluorescence Microscopy
Cells grown on glass coverslips were washed three times in PBS and fixed in 3% paraformaldehyde in PBS for 10 min at room temperature. Paraformaldehyde was quenched with 10 mM ammonium chloride, and cells were permeabilized with PBS, 0.1% Triton X-100 for 7 min at room temperature. The coverslips were washed (three times, 2 min per wash) with PBS, then blocked in PBS, 0.4% fish skin gelatin, 0.2% Tween 20 for 5 min, followed by blocking in PBS, 2.5% goat serum, 0.2% Tween for 5 min. Cells were incubated with primary antibody diluted in PBS, 0.4% fish skin gelatin, 0.2% Tween 20 for 45 min at 37°C. Coverslips were washed (five times, 5 min per wash) with PBS, 0.2% Tween 20. Secondary antibodies coupled to Oregon Green or Texas Red-X were diluted in 2.5% goat serum and incubated on coverslips for 30 min at 37°C. Coverslips were washed with PBS, 0.2% Tween 20 as above and mounted on slides in 9:1 glycerol:PBS with 0.1% q-phenylenediamine. Fluorescence patterns were visualized with an Olympus IX70 epifluorescence microscope. Optical sections were captured with a CCD high-resolution camera equipped with a camera/computer interface. Images were analyzed with a power Mac using IPLab Spectrum software (Scanalytics Inc., Fairfax, VA).
RESULTS
Generation and Expression of Mutant Forms of Rab1b
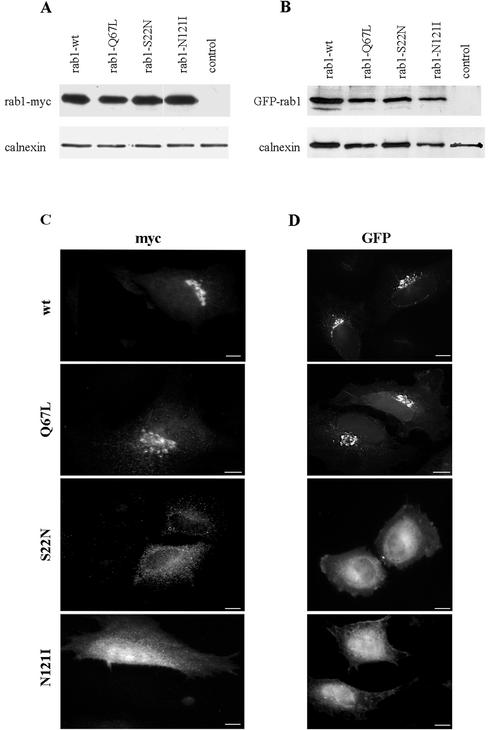
To generate reagents for our studies, a myc-his tag was added to the C terminus of wild-type and three mutant forms of Rab1b (Q67L, S22N, and N121I). Because Rab1b is prenylated on C-terminal cysteine residues (Khosravi-Far et al., 1991), and this modification is important for its membrane association, we also generated wild-type and mutant forms tagged at the N-terminus with the green fluorescent protein (GFP). As shown in Figure 1, A and B, myc- or GFP-tagged proteins of the appropriate molecular weight (∼27 and ∼55 kDa, respectively) were detected in lysates from transfected HeLa cells but not in control lysates. Approximately the same amounts of wild-type Rab1b and each mutant were detected 24 h after transfection, compared with the loading baseline provided by calnexin.
Figure 1.
Expression and localization of wild-type and mutant Rab1b. HeLa cells were transfected with myc- or GFP-tagged wild-type Rab1b, the Q67L mutant, the S22N mutant, or the N121I mutant and analyzed 24 h later by Western blotting (A and B) or immunofluorescence (C and D). Cell lysates were obtained and processed by SDS-PAGE and immunoblotted with anti-myc and anti-calnexin antibodies (A) or with anti-GFP and anti-calnexin antibodies (B). Control lanes contain lysates from untransfected cells. Levels of expression of all constructs are similar. Cells were processed for immunofluorescence by use of anti-myc antibodies (C) or GFP fluorescence (D). Wild-type Rab1b and the Q67L mutant localize to the Golgi. The S22N mutant is detected in the cytosol and also in a reticulate pattern. The N121I mutant shows diffuse cytosol staining.
The myc- and GFP-tagged proteins show analogous localization (Figure 1, C and D). The myc- and GFP-tagged wild-type and Q67L mutants localize predominantly to morphologically normal Golgi membranes. In contrast, the S22N mutants show more diffuse patterns, most likely representing cytosolic and reticular ER localization. The N121I mutants show diffuse cytosolic staining. Our results with myc- and GFP-tagged Rab1b are identical to previously reported localization of GFP-tagged Rab1a (Moyer et al., 2001b).
Effects of Mutant Forms of Rab1b on Cargo Transport
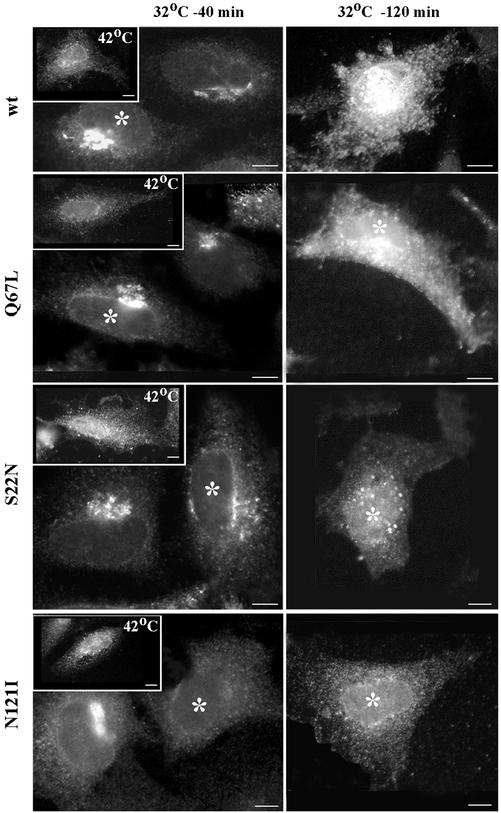
Previous studies have shown that the S22N and the N121I mutants of Rab1a cause dominant negative effects on secretory traffic (Tisdale et al., 1992; Nuoffer et al., 1994; Pind et al., 1994). To functionally characterize our Rab1b mutants, we analyzed the transport of a transmembrane glycoprotein (VSV-G) of the vesicular stomatitis virus (VSV) in HeLa cells transfected with wild-type or mutant Rab1b. The infection was performed at the nonpermissive temperature of 42°C to accumulate VSV-G in the ER (Figure 2, 42°C, insets). The cells were then shifted to the permissive temperature of 32°C for 40 min to allow VSV-G to be transported to the Golgi (Figure 2, 32°C, 40 min panels) or shifted to the permissive temperature of 32°C for 120 min to allow VSV-G to be transported to the PM (Figure 2, 32°C, 120 min panels). The localization of VSV-G was monitored by immunofluorescence. VSV-G is transported to the Golgi at 40 min and to the PM at 120 min in cells expressing wild-type Rab1b or the Q67L mutant (transfected cells in this and following figures are denoted with asterisks). These results are consistent with previous biochemical findings on Rab1a showing that VSV-G becomes endo-H resistant in cells transfected with the Q67L mutant (Tisdale et al., 1992). In cells expressing the S22N mutant, VSV-G is found in perinuclear punctate structures and does not transit to the PM, even after 120 min at the permissive temperature. This is consistent with biochemical data on Rab1a showing that expression of the S22N mutant in cells prevents endo-H resistance of VSV-G (Tisdale et al., 1992). In cells expressing the N121I mutant, VSV-G is retained in the ER, consistent with biochemical data on Rab1a showing Endo-H sensitivity of VSV-G in transfected cells (Tisdale et al., 1992; Pind et al., 1994).
Figure 2.
Rab1b mutants block cargo transport. HeLa cells were transfected with wild-type Rab1b, the Q67L mutant, the S22N mutant, or the N121I mutant. After 24 h, cells were infected with VSVtsO45 at 42°C. After 3 h, cells were either fixed (insets), incubated at 32°C for 40 min (32°C-40 min panels), or incubated at 32°C for 120 min (32°C-120 min panels) before fixation. Cells were analyzed by immunofluorescence with anti-myc antibodies to detect transfected cells (asterisks) and with anti-VSV-G antibodies. In cells expressing wild-type Rab1b or the Q67L mutant, VSV-G is transported to the Golgi at 40 min and to the cell surface at 120 min. In cells expressing the S22N mutant, VSV-G is transported out of the ER into punctate structures at 40 min and remains in such structures at 120 min. In cells expressing the N121I mutant, VSV-G is retained in the ER at 40 min and at 120 min. Bars, 10 μm.
Effects of Mutant Forms of Rab1b on Golgi Structure
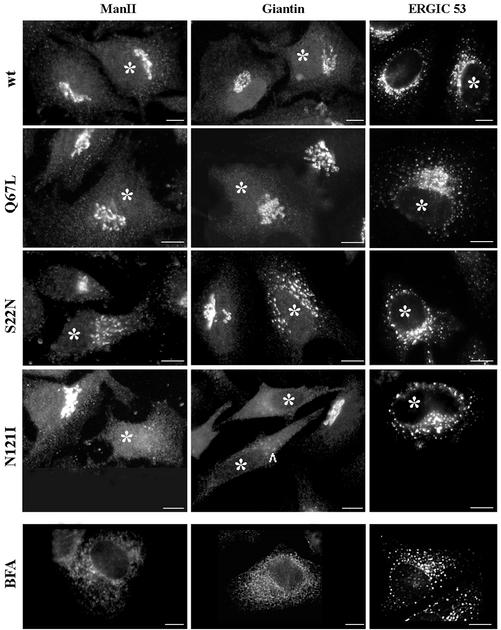
The effects of expressing wild-type or mutant Rab1b in vivo have not been reported previously. As shown in Figure 3, cells expressing the wild-type or the Q67L mutant have normal Golgi, as visualized by the localization of two resident Golgi proteins (mannosidase II and giantin), and normal ERGIC, as visualized by the distribution of ERGIC53. In contrast, transient expression of the S22N mutant leads to partial Golgi disruption and relocation of mannosidase II and giantin into perinuclear elements. Such elements seem to be concentrated in the Golgi region, but some more peripheral structures are also detected. The ERGIC53 pattern also shows partial redistribution to more peripheral elements. The Golgi is completely disrupted in cells expressing the N121I mutant, with mannosidase II and giantin disappearing from the Golgi and redistributing to the ER. The disruption seems to be progressive, and in some cells (arrowhead), punctate perinuclear structures containing giantin can be seen. An adjacent cell, presumably expressing higher levels of the mutant or for a longer period, has a completely diffuse giantin pattern. The distribution of ER-GIC53 seems to be less disturbed, although relocation of the protein from a peri-Golgi region to peripheral structures is observed. Quantitative analysis shows that the vast majority of cells expressing the S22N mutant (88% of transfected cells) or the N121I mutant (85% of transfected cells) present the described phenotypes. The disruption was observed even at moderate and low levels of expression, attesting to the strong dominant effects of these Rab1b mutants in cells.
Figure 3.
Rab1b mutants induce Golgi disruption. HeLa cells were transfected with the wild-type Rab1b, the Q67L mutant, the S22N mutant, or the N121I mutant. Cell were analyzed 24 h later by immunofluorescence with anti-myc antibodies to detect transfected cells (asterisks) and with anti-mannosidase II (anti-MannII), antigiantin, or anti-ERGIC53 antibodies (see respective panels). Wild-type Rab1b and the Q67L mutant do not affect the structure of the Golgi or ERGIC. The S22N mutant partially disrupts Golgi and induces the formation of punctate structures in the Golgi region. The N121I mutant causes complete Golgi disruption, with MannII and giantin redistributing to the ER and ERGIC53 relocating to peripheral punctate structures. The distribution of Golgi and ERGIC markers in cells expressing the N121I mutant is similar to that induced by a 30-min BFA (5 μg/ml) treatment (BFA panels). Bars, 10 μm.
The effects of the N121I mutant resembled those caused by BFA treatment, and representative images of cells treated with BFA are shown in Figure 3, BFA panels. BFA treatment causes Golgi disassembly and the relocation of resident Golgi proteins to the ER. BFA also induces relocation of ERGIC53 to punctate structures, shown by others to represent ER exit sites and immature VTCs (Ward et al., 2001).
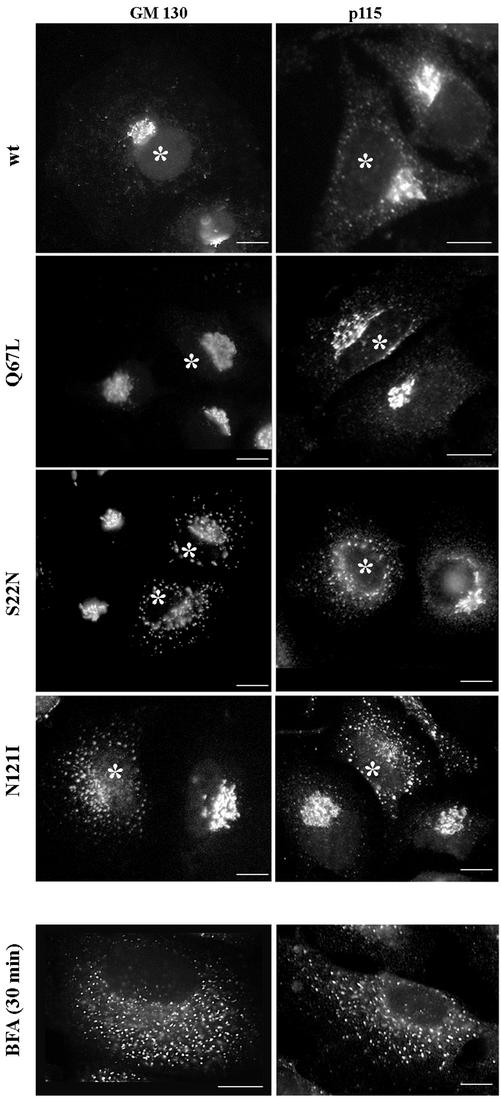
We also explored the localization of two Rab1 effectors, GM130 and p115, in cells expressing wild-type and mutant Rab1b. As shown in Figure 4, the localization of GM130 and p115 is not influenced by the expression of either the wild-type Rab1b or the Q67L mutant. In cells expressing the S22N mutant, GM130 and to a greater extent p115 are redistributed from the Golgi to punctate peripheral structures. In cells expressing the N121I mutant, GM130 and p115 localization is disrupted, and both are found in small punctate structures dispersed throughout the cell. The observed patterns are analogous to those induced by BFA treatment (Figure 4, BFA panels). BFA inhibits the activity of guanine nucleotide exchange factors (GEFs) for ARFs and prevents COPI recruitment (Donaldson et al., 1992; Helms and Rothman, 1992). Because expression of the N121I mutant causes BFA-like phenotype, we explored whether Rab1b could be involved in COPI recruitment.
Figure 4.
Rab1b mutants induce redistribution of its effectors GM130 and p115. HeLa cells were transfected with wild-type Rab1b, the Q67L mutant, the S22N mutant, or the N121I mutant and analyzed 24 h later by immunofluorescence with anti-myc antibodies to detect transfected cells (asterisks) and with either anti-GM130 or anti-p115 antibodies (see respective panels). In cells expressing wild-type Rab1b or the Q67L mutant, GM130 localizes to the Golgi, whereas p115 is found in the Golgi and in peripheral punctate structures. In cells expressing the S22N mutant, GM130 and p115 are partially localized to the Golgi but also show redistribution to peripheral punctate structures. In cells expressing the N121I mutant, both GM130 and p115 are completely redistributed to peripheral punctate structures. The redistribution of GM130 and p115 is similar to that induced by a 30-min BFA (5 μg/ml) treatment (BFA panels). Bars, 10 μm.
A Mutant Form of Rab1b Perturbs COPI Coat Assembly
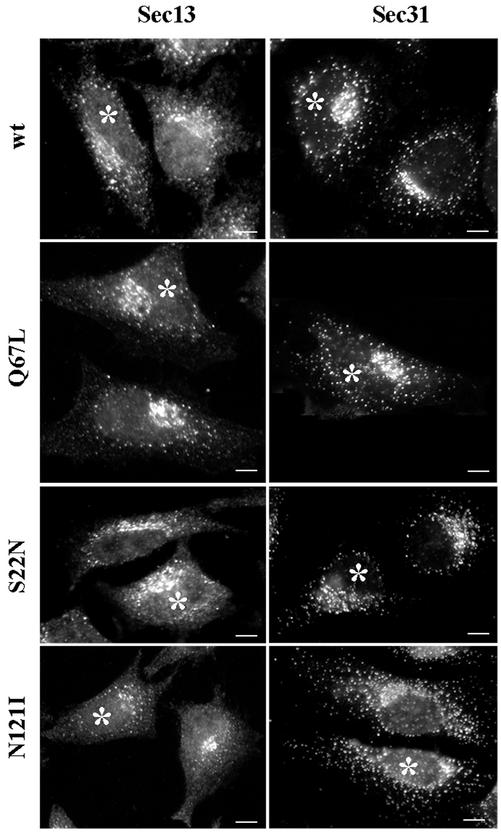
We first examined the effects of Rab1b mutants on the assembly of COPII coats, because these have been reported to be unaffected by BFA treatment (Ward et al., 2001). As shown in Figure 5, the localization of the Sec13 and the Sec31components of the COPII coat seems to be normal in cells expressing the wild-type or the Q67L mutant. Sec13 and Sec31 are present in punctate structures concentrated in the Golgi region and also in more peripheral sites. In cells expressing the S22N or the N121I mutant, the overall Sec13 and Sec31 pattern still appears normal, but with fewer peri-Golgi structures. Distribution of ER exit sites has been shown to be influenced by the Golgi (Ward et al., 2001). Significantly, even in cells expressing the N121I mutant, both Sec13 and Sec31 are efficiently recruited to ER exit sites.
Figure 5.
Rab1b mutants do not perturb COPII recruitment. HeLa cells were transfected with wild-type Rab1b, the Q67L mutant, the S22N mutant, or the N121I mutant and analyzed 24 h later by immunofluorescence with anti-myc antibodies to detect transfected cells (asterisks) and with either anti-Sec13 or anti-Sec31 antibodies (see respective panels). In cells expressing wild-type Rab1b or the Q67L mutant, COPII markers distribute in normal patterns. In cells expressing the S22N mutant or the N121I mutant, COPII markers present a relatively normal pattern but with fewer punctate structures in the Golgi region. Bars, 10 μm.
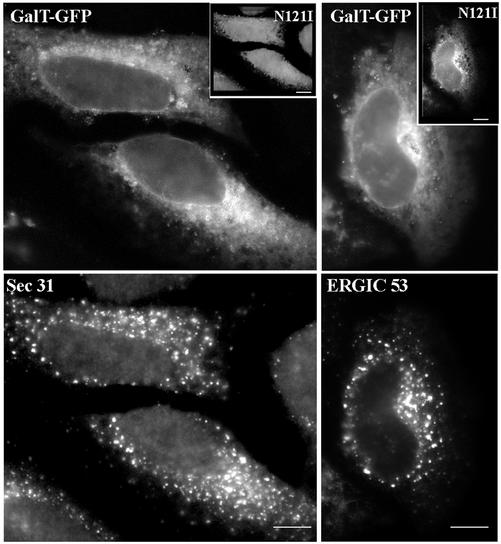
The results indicate that cells expressing the N121I mutant recruit COPII and ERGIC53 to ER exit sites and immature VTCs but are unable to sort and deliver resident Golgi proteins into those structures (compare Figures 5 and 3). To ensure that this is not because of different N121I expression levels in distinct cells, we compared the localization of Sec31 and ERGIC53 to the localization of a resident Golgi protein (galactosyl transferase, Gal-T) in the same cell. Cells were cotransfected with the N121I mutant and GFP-tagged Gal-T, and the localization of Sec31 or ERGIC53 was compared with the localization of GalT-GFP. As shown in Figure 6, in N121I-transfected cells (see insets), GalT-GFP is detected in a diffuse ER pattern. Gal-T-GFP is not recruited into ER exit sites or immature VTCs, even though Sec31 and ERGIC53 are efficiently sorted and maintained in those structures.
Figure 6.
The N121I mutant prevents sorting of a Golgi enzyme. HeLa cells were cotransfected with the N121I mutant and GFP-tagged Gal-T and analyzed 24 h later by immunofluorescence with anti-myc antibodies to detect transfected cells (insets) and with either anti-Sec31 or anti-ERGIC53 antibodies. Gal-T was detected by green fluorescence. Gal-T is localized to the ER in the same cells in which Sec31 or ERGIC53 present punctate patterns. Bars, 10 μm.
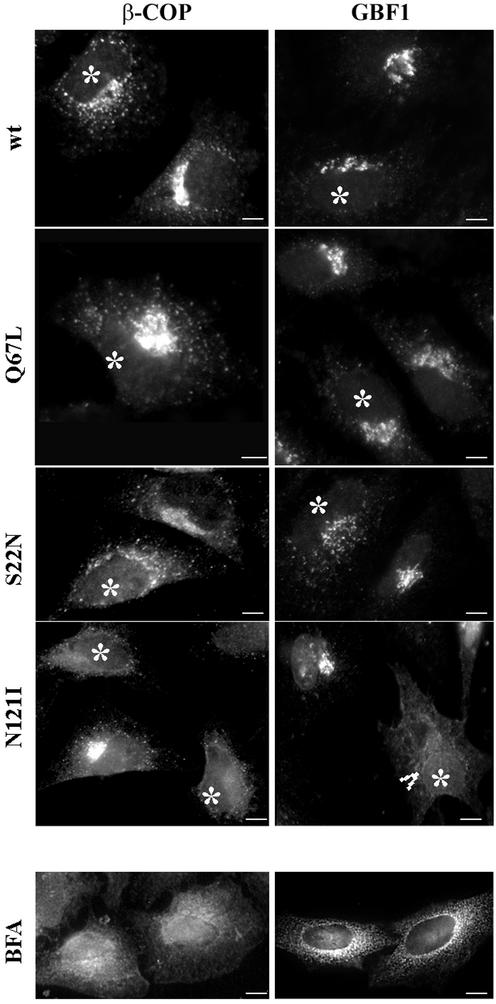
We next explored the distribution of the β-COP component of COPI in cells expressing wild-type or mutant Rab1b. As shown in Figure 7, β-COP panels, in cells expressing the wild-type or the Q67L mutant, β-COP distributes to the Golgi and to peripheral sites. The pattern is indistinguishable from that in untransfected cells. In cells expressing the S22N mutant, the β-COP pattern is more dispersed and parallels the redistribution observed for ERGIC53. A more dramatic effect is seen in cells expressing the N121I mutant, with the majority of β-COP being in a diffuse cytosolic pattern. The N121I-induced β-COP dissociation is analogous to that seen in BFA-treated cells (Figure 7, BFA panel).
Figure 7.
The N121I mutant induces β-COP dissociation and relocation of GBF1. HeLa cells were transfected with wild-type Rab1b, the Q67L mutant, the S22N mutant, or the N121I mutant (see respective panels) and analyzed 24 h later by immunofluorescence with anti-myc antibodies to detect transfected cells (asterisks) and with either anti-β-COP or anti-GBF1 antibodies. In cells expressing wild-type Rab1b or the Q67L mutant, β-COP and GBF1 distribute in normal patterns. In cells expressing the S22N mutant, β-COP and GBF1 present a more punctate peri-Golgi pattern. In cells expressing the N121I mutant, β-COP and GBF1 are not associated with a distinguishable compartment and appear diffuse. GBF1 is detected in the nuclear membrane (arrowhead), indicating ER localization. The redistribution of β-COP and GBF1 is similar to that induced by a 30-min BFA (5 μg/ml) treatment (BFA panels). Bars, 10 μm.
Because β-COP recruitment requires membrane-associated ARF, and GBF1 has been shown to recruit ARFs to membranes (Kawamoto et al., 2002), we examined the distribution of GBF1 in cells expressing the wild-type Rab1b and the various mutants. As shown in Figure 7, GBF1 panels, in cells expressing the wild-type or the Q67L mutant (transfected cells denoted by asterisks), GBF1 distributes to the Golgi and to peripheral sites. The pattern is indistinguishable from those in neighboring untransfected cells. In cells expressing the S22N mutant, GBF1 localization is altered, with GBF1 relocating to punctate structures concentrated in the Golgi region. A dramatic redistribution of GBF1 is seen in cells expressing the N121I mutant. Although the distribution of GBF1 appears diffuse, the nuclear membrane is labeled (arrowhead), indicative of ER localization. The N121I-induced GBF1 pattern is analogous to that seen in BFA-treated cells (Figure 7, BFA panel), in which GBF1 relocates to the ER.
Expression of ARF1 or GBF1 Rescues β-COP Dissociation Induced by a Mutant Form of Rab1b
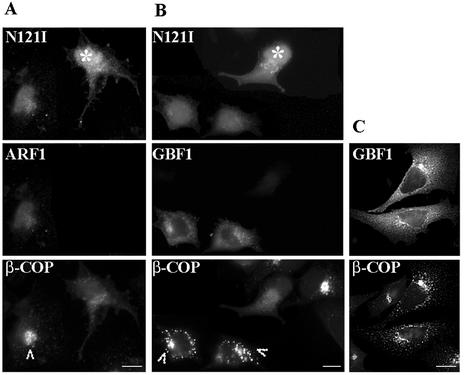
Golgi disruption and β-COP dissociation induced by the N121I mutant are analogous to those induced by BFA. BFA inhibits the activity of a GEF for ARFs and prevents ARF activation (Donaldson et al., 1992; Helms and Rothman, 1992). To determine whether Rab1b-catalyzed events might participate in COPI recruitment, we explored the possibility that the N121I mutant causes its effects through an inhibitory effect on the ARF1-mediated COPI recruitment pathway. If that were the case, than overexpression of ARF1 should rescue the N121I-induced β-COP dissociation. To test this, we cotransfected the N121I mutant with wild-type ARF1. Expression of ARF1 has no detectable effect on Golgi structure or β-COP localization (our unpublished results) (Teal et al., 1994). As shown in Figure 8A, a cell cotransfected with N121I and ARF1 (arrowhead) shows β-COP in a membrane-associated Golgi-like pattern. In contrast, a cell transfected only with the N121I mutant (asterisk) shows diffuse distribution of β-COP.
Figure 8.
Expression of ARF1 or GBF1 rescues β-COP disassembly induced by the N121I mutant. HeLa cells were cotransfected with GFP-tagged N121I mutant and HA-tagged wild-type ARF1 (A) or with GFP-tagged N121I mutant and myc-tagged GBF1 (B). After 24 h, cells were analyzed by immunofluorescence with anti-β-COP antibodies and with either anti-HA or anti-myc antibodies. GFP-N121I was detected by green fluorescence. In cotransfected cells (arrowheads), β-COP is membrane associated. In cells transfected only with N121I (asterisks), β-COP is cytosolic. In C, cells were transfected only with myc-tagged GBF1. Overexpression of GBF1 causes partial Golgi fragmentation. Bars, 10 μm.
The Sec7-family member GBF1 is an exchange factor for ARF1 in vivo (Kawamoto et al., 2002). Because expression of ARF1 rescues the N121I phenotype, we explored whether GBF1 could also reverse the N121I effect. We cotransfected cells with the N121I mutant and wild-type GBF1. As shown in Figure 8B, in cells cotransfected with the N121I mutant and GBF1 (arrowheads), β-COP is retained on membranes. β-COP is associated with the Golgi and with punctate peri-Golgi structures. This pattern is analogous to that in cells expressing only GBF1 (Figure 8C). The peri-Golgi structures are most likely caused by a GBF1-mediated increase in COPI recruitment to membranes. In cells expressing only the N121I mutant (asterisk), β-COP is diffusely distributed in the cytosol. It seems that both ARF1 and GBF1 can antagonize the effect of the N121I mutant and maintain β-COP association with membranes.
Expression of the Active Mutant of Rab1b Confers BFA Resistance to Cells
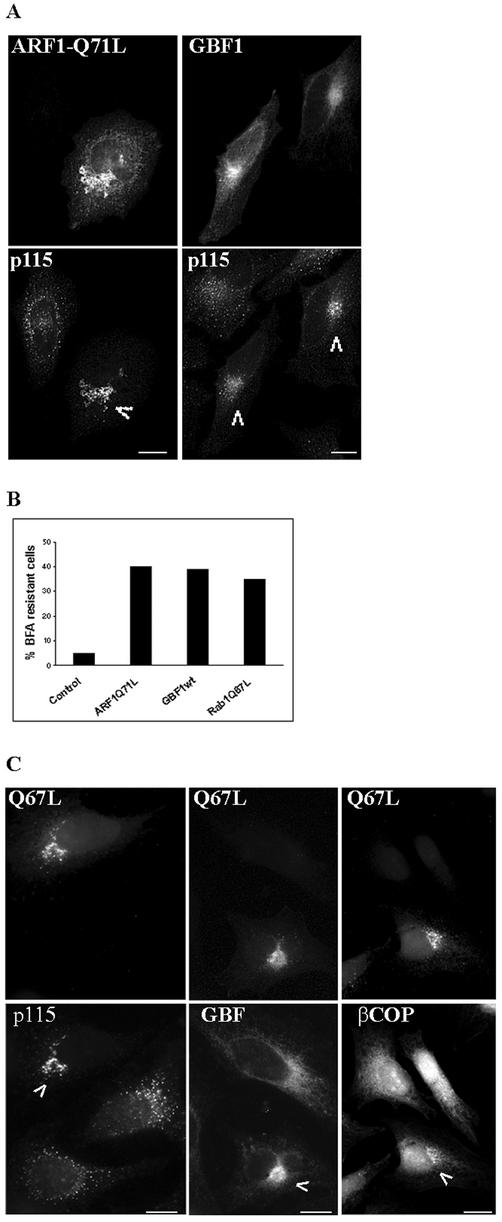
Previous studies have documented that expression of proteins known to be involved in COPI recruitment, such as the active form of ARF1 and GBF1, confers BFA resistance to transfected cells (Teal et al., 1994; Claude et al., 1999; Kawamoto et al., 2002; Zhao et al., 2002). In agreement, we show that the expression of the active Q71L mutant of ARF1 prevents Golgi redistribution in BFA-treated cells (Figure 9A). As shown in that figure, a cell transfected with ARF1-Q71L shows normal Golgi localization of p115 (arrowhead). An adjacent untransfected cell shows the typical BFA-induced p115 pattern (compare to p115 localization in Figure 4, BFA panel). Similarly, expression of GBF1 confers BFA resistance (Figure 9A), in agreement with previous reports (Claude et al., 1999; Kawamoto et al., 2002; Zhao et al., 2002). Cells expressing GBF1 show Golgi localization of p115 (arrowheads), whereas an untransfected cell shows a dispersed p115 pattern. Quantification of the BFA resistance of transfected cells (defined as Golgi pattern of p115) shows that ∼4% of cells transfected with a control plasmid encoding GFP are BFA resistant, whereas ∼41% of cells transfected with the ARF1-Q71L plasmid and ∼39% of cells transfected with the GBF1 plasmid are BFA resistant (Figure 9B).
Figure 9.
Rab1-Q67L-transfected cells are BFA resistant. HeLa cells were transfected with HA-tagged ARF1-Q71L myc-tagged GBF1, or GFP-tagged Rab1b-Q67L. After 24 h, cells were treated with BFA (30 min; 5 μg/ml) and analyzed by immunofluorescence with either anti-HA or anti-myc antibodies to detect transfected cells and with anti-p115, anti-GBF1, or anti-β-COP antibodies to detect the Golgi. GFP-Rab1b-Q67L was detected by green fluorescence. Cells transfected with either ARF1-Q71L or GBF1 are BFA resistant, as shown by a Golgi pattern of p115 (arrowheads in A). The percentage of cells transfected with a control plasmid (encoding GFP), ARF1Q71L, GBF1, or Rab1-Q67L that are BFA resistant was quantified and is plotted as a bar graph (B). Bars represent the average of two independent experiments using p115 as a Golgi marker (50 transfected cells were counted in each experiment). Cells transfected with Rab1b-Q67L are BFA resistant, as shown by Golgi pattern of p115 and GBF1 and partial membrane association of β-COP (arrowheads in C). Bars, 10 μm.
To test whether expression of the active form of Rab1b also confers BFA resistance, we transfected cells with the Q67L mutant and then treated them with BFA. As shown in Figure 9C, cells expressing the Q67L mutant are BFA resistant, as shown by Golgi localization of the Q67L protein and of p115 (arrowhead). Two adjacent untransfected cells show typical BFA-induced p115 pattern. Quantification of the BFA resistance of transfected cells (defined as Golgi pattern of p115) indicates that ∼35% of cells transfected with the Q67L mutant are resistant to BFA (Figure 9B). Expression of the Q67L mutant also prevents GBF1 relocation to the ER in BFA-treated cells (Figure 9C). A cell expressing the Q67L mutant shows Golgi localization of GBF1 (arrowhead), whereas an adjacent untransfected cell shows redistribution of GBF1 to the ER. Quantification of the BFA resistance of transfected cells (defined as Golgi pattern of GBF1) indicates that ∼35% of cells transfected with the Q67L mutant are resistant to BFA (our unpublished results). The level of BFA resistance conferred by the Rab1b-Q67L mutant is analogous to that conferred by ARF1-Q71L (∼41%) or by GBF1 (∼39%). Expression of wild-type Rab1 also prevents p115 relocation in BFA-treated cells; quantification of the BFA resistance of transfected cells (defined as Golgi pattern of p115) indicates that ∼30% of cells transfected with wild-type Rab1 are resistant to BFA (our unpublished results).
Expression of the Q67L mutant partially antagonizes BFA-induced release of β-COP, and a cell expressing the Q67L mutant shows membrane-associated β-COP in a Golgi region (arrowhead), in addition to a more diffuse cellular staining (Figure 9C). Adjacent untransfected cells show exclusively diffuse localization of β-COP. Quantification of the persistence of membrane-associated β-COP in transfected cells (defined as Golgi pattern of β-COP) indicates that ∼15% of cells transfected with either the Q67L mutant or GBF1 show BFA-resistant β-COP localization (our unpublished results).
DISCUSSION
Here, we provide a comprehensive analysis of Rab1b function in cells by examining the effects of mutant forms of Rab1b on (1) ER-Golgi traffic, (2) Golgi and ERGIC structure, (3) the response of Rab1b effectors, (4) the status of the COPII machinery, and (5) the status of the COPI machinery. In our studies, we use Rab1b constructs tagged at the C terminus with myc or at the N-terminus with GFP. Rabs are posttranslationally prenylated (by addition of a 20-carbon geranylgeranyl to the C-terminal GGCC motif) (Farnsworth et al., 1994), and prenylation has been shown to be required for membrane association of the yeast homologue of rab1a, YPT1 (Newman et al., 1992). We did not formally analyze whether our C-terminally myc-tagged constructs are prenylated. However, because the myc-tagged wild-type Rab1b associates efficiently with membranes, we assume that the myc tag does not prevent prenylation. It has been shown that Rabs containing the -CCXXX motif are prenylated (Pereira-Leal and Seabra, 2000), suggesting that addition of C-terminal amino acids might not prevent prenylation. Furthermore, we show that the myc-tagged constructs behave identically to constructs tagged at the N-terminus with GFP and induce the same phenotypes.
Effects of Rab1b Mutants
Q67L The Q67L mutant localizes to peripheral VTCs and to the Golgi in a pattern indistinguishable from that of a wild-type protein. Expression of the Q67L mutant has no detectable effect on any of the tested parameters, in agreement with previously published data (Tisdale et al., 1992). Although the Q67L mutant is expected to have reduced intrinsic GTPase activity in vitro, it is likely to interact with a GAP that would promote GTP hydrolysis to normal levels in vivo. The lack of visible enlargement of VTCs or the Golgi in transfected cells differs from the effects observed in the endosomal system, in which the expression of GTP-restricted Rab5 increases the rate of internalization and leads to the formation of enlarged endosomes (Barbieri et al., 1996; Seachrist et al., 2001).
S22N The S22N mutant presents a diffuse cellular staining without a clear compartmental staining. In some cells, a faint nuclear and reticular pattern can be discerned, suggesting that S22N might associate with the ER. The partially cytosolic localization of this mutant is consistent with its preferred GDP status and GDI-mediated extraction from membranes (Alexandrov et al., 1994). Expression of the S22N mutant leads to inhibition in transport and the arrest of cargo VSV-G in fragmented perinuclear Golgi structures. This is consistent with biochemical data showing that cargo VSV-G remains endo-H sensitive in cells expressing the analogous Rab1a mutant (Tisdale et al., 1992). Cells transfected with S22N show Golgi disruption and relocation of Golgi proteins to perinuclear fragments. It is likely that those are analogous to Golgi elements induced in cells by microinjection of the S25N Rab1a mutant and shown by electron microscopic analysis to resemble polarized Golgi ministacks formed in the presence of nocodazole (Wilson et al., 1994). The COPII and COPI machinery is not noticeably perturbed in transfected cells. It is likely that the S22N mutant mediates its effect by competing with the endogenous Rab1b for binding to cellular accessory proteins (possibly GDI or GEF) but does not act as an irreversible inhibitor, because its effects can be rescued by overexpression of the wild-type protein (Nuoffer et al., 1994).
N121I The N121I mutant localizes in a diffuse cellular pattern without associating with a clearly defined compartment. Expression of N121I in cells blocks the exit of cargo VSV-G from the ER and causes the complete disassembly of the Golgi as monitored by the disappearance of mannosidase II, giantin, GOS28 (not shown), and gal-T from the Golgi region and their redistribution to the ER. This result is completely reproducible when using either myc-tagged or GFP-tagged N121I mutant, and the redistribution of four different proteins argues against a protein-specific phenotype. The collapse of the Golgi is most easily explained by a block in the anterograde pathway and continuing retrograde recycling, in agreement with the established role of Rab1 in the forward traffic (Plutner et al., 1991; Tisdale et al., 1992). Our findings differ from published data that show arrest in VSV-G transport at the level of VTCs in cells expressing the N121I mutant (Tisdale et al., 1992) or in semi-intact cells supplemented with N121I (Pind et al., 1994), and the relocation of Golgi proteins into perinuclear structures in cells microinjected with the N121I mutant (Wilson et al., 1994). The rationale for the differences in reported results and our data is currently unclear. However, we provide further experimental support for the disruptive effects of N121I by showing that it causes dissociation of β-COP from membranes. The phenotype we describe is analogous to that observed in cells treated with BFA (Klausner et al., 1992) or expressing the inactive mutant of ARF1 (Dascher and Balch, 1994). In each case, β-COP is not associated with membranes, there is no sorting of cargo and Golgi proteins into VTCs, and ER-Golgi traffic is inhibited. Recruitment of COPI is required for the maturation of COPII-differentiated ER exit sites into transport-competent VTCs, and this process seems to be inhibited by N121I. The significantly more severe phenotype induced by N121I versus S22N can be explained by the finding that N121I is expected to act as an irreversible inhibitor in vivo, because its effects cannot be suppressed by overexpression of wild-type Rab1b (Pind et al., 1994).
Rab1b and Its Effectors
GM130 has been identified as a Rab1a and Rab1b effector (Moyer et al., 2001a) (Weide et al., 2001), whereas p115 has been identified as a Rab1a effector (Allan et al., 2000). Rab1a shares 92% identity with Rab1b (Touchot et al., 1987), and we detected p115 interaction with the active Q67L mutant of Rab1b in a yeast dihybrid system (our unpublished results). Surprisingly, expression of Q67L had a limited effect on GM130 and p115 distribution, but expression of S22N, and to an even larger extent, expression of N121I, significantly influenced GM130 and p115 localization. The S22N mutant caused partial relocation of both proteins from the Golgi to peripheral punctate structures, whereas the N121I mutant caused complete redistribution into a BFA-like punctate pattern. Significantly, both proteins remained membrane associated. It has been suggested that the active form of Rab1 is required for the recruitment of p115 to membranes in an in vitro COPII budding assay (Allan et al., 2000), but our in vivo results indicate that p115 can associate with membranes independently of Rab1 activity. Similarly, the Rab3 effector rabphilin3A also associates with membranes in a manner independent of active Rab3 (Shirataki et al., 1994). Although it seems that p115 recruitment to membranes is mediated by other protein(s), subsequent p115 sorting or function might be regulated by the active Rab1.
Rab1b and COPI Recruitment
The ability of the N121I mutant to induce β-COP dissociation from membranes suggests that Rab1b participates in events leading to COPI recruitment. A link between Rabs and COPI is not unexpected, because the active form of Rab2 promotes β-COP recruitment to membranes (Tisdale and Jackson, 1998), and a relationship between Rabs and ARFs has been shown genetically in yeast (Segev, 2001b). The Rab1b yeast homologue YPT1 has been shown to interact genetically with GEA2, a guanine nucleotide exchange factor for ARF1/2 (Jones et al., 1999). Furthermore, there is synthetic lethality between YPT1 and ARF1, suggesting a functional link between these GTPase families. In agreement, we document a functional relationship between Rab1b and ARF1 by showing that (1) β-COP dissociation induced by N121I can be reversed by overexpression of ARF1; (2) overexpression of GBF1, a mammalian exchange factor for ARF, is also able to reverse β-COP dissociation induced by N121I; and (3) overexpression of the active Q67L mutant reverses β-COP dissociation induced by BFA. The combined data suggest that the Rab and ARF families of GTPases interact in a regulatory cascade mediating COPI recruitment.
A plausible model for Rab1b function is that it might act to recruit or activate GBF1, which in turn activates ARF, which then recruits COPI and allows COPII/COPI exchange on VTCs. This model fits with the BFA-like phenotype induced by N121I, because BFA has been shown to inhibit GEF activity, thus preventing ARF activation and COPI recruitment. The model is also consistent with our findings that Rab1b acts in the COPI pathway upstream of ARF1 and GBF1 and that the block induced by N121I precedes formation of VTCs. Previous work has shown that COPII/COPI exchange is necessary for formation of VTCs (Pepperkok et al., 1993; Peter et al., 1993) and that the process seems to involve at least two stages. The initial differentiation of the ER membrane into ER exit sites occurs through the action of the COPII machinery, and the subsequent maturation to VTCs requires recruitment of the COPI coat. In the absence of active Rab1b, ER exit sites form, as shown by the localization of COPII components and the sorting of ERGIC53, GM130, and p115, but such structures do not differentiate into VTCs, as shown by the retention of cargo and Golgi proteins in the ER. That Rab1b might participate in exit from the ER is also strongly suggested by studies in Drosophila expressing the N124I mutant under heat-shock promotor, in which expression of the mutant protein caused extensive ER swelling in addition to the disruption of the Golgi and block in ER-to-Golgi transport of rhodopsin (Satoh et al., 1997). Interestingly, expression of N124I also caused the accumulation of clusters of small vesicles (<150 nm) close to the ER, perhaps indicating that COPI function is required for their differentiation into VTCs.
Our results significantly extend previous studies examining the effects of mutant rab1b on Golgi structure and ER-Golgi transport in vivo and in vitro and provide support for a novel function of rab1b in COPI coat assembly. Future studies are necessary to elucidate the exact mechanism of rab1b function, to identify all its compartment-specific effectors, and to define all the compartments and traffic steps at which it acts.
Acknowledgments
We thank Dr. Hans-Peter Hauri (University of Basel, Basel, Switzerland), Dr. Marilyn Farquhar (University of California, San Diego, CA), Dr. Kathryn Howell (University of Colorado, Denver, CO), and Dr. Bor Luen Tang (National University of Singapore, Republic of Singapore) for providing antibodies. We are grateful to Dr. Brian Storrie (Virginia Tech, Blacksburg, VA) for providing GalT-GFP constructs and to Dr. Marisa Colombo (University of Cuyo, Mendoza, Argentina) for subcloning Rab1myc constructs into pEGFP vector and for reading this manuscript. We also thank A. Tousson (University of Alabama at Birmingham, AL) for expert assistance with immunofluorescence microscopy. This work was supported by grant GM-508358 from the National Institutes of Health (to E.S.) and a postdoctoral fellowship (9920146X) from the American Heart Association (to C.A.).
Article published online ahead of print. Mol. Biol. Cell 10.1091/mbc.E02-09-0625. Article and publication date are at www.molbiolcell.org/cgi/doi/10.1091/mbc.E02-09-0625.
Abbreviations used: ER, endoplasmic reticulum; VTC, vesicular tubular clusters; BFA, brefeldin A; GFP, green fluorescent protein, GDI, GDP-dissociation inhibitor; ERGIC, ER-Golgi intermediate compartment; VSV-G, vesicular stomatitis virus glycoprotein.
References
- Alexandrov K, Horiuchi H, Steele-Mortimer O, Seabra MC, Zerial M. (1994). Rab escort protein-1 is a multifunctional protein that accompanies newly prenylated rab proteins to their target membranes. EMBO J 13, 5262–5273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allan, B.B., Moyer, B.D., and Balch, W.E. (2000). Rab1 recruitment of p115 into a cis-SNARE complex: programming budding COPII vesicles for fusion. Science 289, 444–448. [DOI] [PubMed] [Google Scholar]
- Alvarez, C., Fujita, H., Hubbard, A., and Sztul, E. (1999). ER to Golgi transport: requirement for p115 at a pre-Golgi VTC stage. J. Cell Biol. 147, 1205–1222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barbieri, M.A., Li, G., Mayorga, L.S., and Stahl, P.D. (1996). Characterization of Rab5:Q79L-stimulated endosome fusion. Arch. Biochem. Biophys. 326, 64–72. [DOI] [PubMed] [Google Scholar]
- Barroso, M., Nelson, D.S., and Sztul, E. (1995). Transcytosis-associated protein (TAP)/p115 is a general fusion factor required for binding of vesicles to acceptor membranes. Proc. Natl. Acad. Sci. USA 92, 527–531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Claude, A., Zhao, B.P., Kuziemsky, C.E., Dahan, S., Berger, S.J., Yan, J.P., Armold, A.D., Sullivan, E.M., and Melancon, P. (1999). GBF1: a novel Golgi-associated BFA-resistant guanine nucleotide exchange factor that displays specificity for ADP-ribosylation factor 5. J. Cell Biol. 146, 71–84. [PMC free article] [PubMed] [Google Scholar]
- Dascher, C., and Balch, W.E. (1994). Dominant inhibitory mutants of ARF1 block endoplasmic reticulum to Golgi transport and trigger disassembly of the Golgi apparatus. J. Biol. Chem. 269, 1437–1448. [PubMed] [Google Scholar]
- Donaldson, J.G., Finazzi, D., and Klausner, R.D. (1992). Brefeldin A inhibits Golgi membrane-catalyzed exchange of guanine nucleotide onto ARF protein. Nature 360, 350–352. [DOI] [PubMed] [Google Scholar]
- Farnsworth, C.C., Seabra, M.C., Ericsson, L.H., Gelb, M.H., and Glomset, J.A. (1994). Rab geranylgeranyl transferase catalyzes the geranylgeranylation of adjacent cysteines in the small GTPases Rab1A, Rab3A, and Rab5A. Proc. Natl. Acad. Sci. USA 91, 11963–11967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helms, J.B., and Rothman, J.E. (1992). Inhibition by brefeldin A of a Golgi membrane enzyme that catalyzes exchange of guanine nucleotide bound to ARF. Nature 360, 352–354. [DOI] [PubMed] [Google Scholar]
- Jones, S., Jedd, G., Kahn, R.A., Franzusoff, A., Bartolini, F., and Segev, N. (1999). Genetic interactions in yeast between YPT GTPases and ARF guanine nucleotide exchangers. Genetics 152, 1543–1556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawamoto, K., Yoshida, Y., Tamaki, H., Torii, S., Shinotsuka, C., Yamashina, S., and Nakayama, K. (2002). GBF1, a guanine nucleotide exchange factor for ADP-ribosylation factors, is localized to the cis-Golgi and involved in membrane association of the COPI coat. Traffic 3, 483–495. [DOI] [PubMed] [Google Scholar]
- Khosravi-Far, R., Lutz, R.J., Cox, A.D., Conroy, L., Bourne, J.R., Sinensky, M., Balch, W.E., Buss, J.E., and Der, C.J. (1991). Isoprenoid modification of rab proteins terminating in CC or CXC motifs. Proc. Natl. Acad. Sci. USA 88, 6264–6268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klausner, R.D., Donaldson, J.G., and Lippincott-Schwartz, J. (1992). Brefeldin A: insights into the control of membrane traffic and organelle structure. J. Cell Biol. 116, 1071–1080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linstedt, A.D., and Hauri, H.P. (1993). Giantin, a novel conserved Golgi membrane protein containing a cytoplasmic domain of at least 350 kDa. Mol. Biol. Cell 4, 679–693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lippincott-Schwartz, J., Cole, N.B., and Donaldson, J.G. (1998). Building a secretory apparatus: role of ARF1/COPI in Golgi biogenesis and maintenance. Histochem. Cell Biol. 109, 449–462. [DOI] [PubMed] [Google Scholar]
- Moyer, B.D., Allan, B.B., and Balch, W.E. (2001a). Rab1 interaction with a GM130 effector complex regulates COPII vesicle cis-Golgi tethering. Traffic 2, 268–276. [DOI] [PubMed] [Google Scholar]
- Moyer, B.D., Matteson, J., and Balch, W.E. (2001b). Expression of wild-type and mutant green fluorescent protein-Rab1 for fluorescence microscopy analysis. Methods Enzymol. 329, 6–14. [DOI] [PubMed] [Google Scholar]
- Nelson, D.S., Alvarez, C., Gao, Y.S., Garcia-Mata, R., Fialkowski, E., and Sztul, E. (1998). The membrane transport factor TAP/p115 cycles between the Golgi and earlier secretory compartments and contains distinct domains required for its localization and function. J. Cell Biol. 143, 319–331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newman, C.M., Giannakouros, T., Hancock, J.F., Fawell, E.H., Armstrong, J., and Magee, A.I. (1992). Post-translational processing of Schizosaccharomyces pombe YPT proteins. J. Biol. Chem. 267, 11329–11336. [PubMed] [Google Scholar]
- Nuoffer, C., Davidson, H.W., Matteson, J., Meinkoth, J., and Balch, W.E. (1994). A GDP-bound of rab1 inhibits protein export from the endoplasmic reticulum and transport between Golgi compartments. J. Cell Biol. 125, 225–237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pepperkok, R., Scheel, J., Horstmann, H., Hauri, H.P., Griffiths, G., and Kreis, T.E. (1993). Beta-COP is essential for biosynthetic membrane transport from the endoplasmic reticulum to the Golgi complex in vivo. Cell 74, 71–82. [DOI] [PubMed] [Google Scholar]
- Pereira-Leal, J.B., and Seabra, M.C. (2000). The mammalian Rab family of small GTPases: definition of family and subfamily sequence motifs suggests a mechanism for functional specificity in the Ras superfamily. J. Mol. Biol. 301, 1077–1087. [DOI] [PubMed] [Google Scholar]
- Peter, F., Plutner, H., Zhu, H., Kreis, T.E., and Balch, W.E. (1993). Beta-COP is essential for transport of protein from the endoplasmic reticulum to the Golgi in vitro. J. Cell Biol. 122, 1155–1167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfeffer, S.R. (2001). Rab GTPases: specifying and deciphering organelle identity and function. Trends Cell Biol. 11, 487–491. [DOI] [PubMed] [Google Scholar]
- Pind, S.N., Nuoffer, C., McCaffery, J.M., Plutner, H., Davidson, H.W., Farquhar, M.G., and Balch, W.E. (1994). Rab1 and Ca2+ are required for the fusion of carrier vesicles mediating endoplasmic reticulum to Golgi transport. J. Cell Biol. 125, 239–252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plutner, H., Cox, A.D., Pind, S., Khosravi-Far, R., Bourne, J.R., Schwaninger, R., Der, C.J., and Balch, W.E. (1991). Rab1b regulates vesicular transport between the endoplasmic reticulum and successive Golgi compartments. J. Cell Biol. 115, 31–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roman, L.M., Scharm, A., and Howell, K.E. (1988). Immunoselection of stably transformed MDCK cells expressing the vesicular stomatitis virus G-protein at the basolateral surface. Exp. Cell Res. 175, 376–387. [DOI] [PubMed] [Google Scholar]
- Sapperstein, S.K., Lupashin, V.V., Schmitt, H.D., and Waters, M.G. (1996). Assembly of the ER to Golgi SNARE complex requires Uso1p. J. Cell Biol. 132, 755–767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Satoh, A., Tokunaga, F., Kawamura, S., and Ozaki, K. (1997). In situ inhibition of vesicle transport and protein processing in the dominant negative Rab1 mutant of Drosophila. J. Cell Sci. 110, 2943–2953. [DOI] [PubMed] [Google Scholar]
- Schimmoller, F., Simon, I., and Pfeffer, S.R. (1998). Rab GTPases, directors of vesicle docking. J. Biol. Chem. 273, 22161–22164. [DOI] [PubMed] [Google Scholar]
- Schweizer, A., Fransen, J.A., Bachi, T., Ginsel, L., and Hauri, H.P. (1988). Identification, by a monoclonal antibody, of a 53-kD protein associated with a tubulo-vesicular compartment at the cis-side of the Golgi apparatus. J. Cell Biol. 107, 1643–1653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seachrist, J.L., Laporte, S.A., Dale, L.B., Babwah, A.V., Caron, M.G., Anborgh, P.H., and Ferguson, S.S.G. (2001). Rab5 association with the angiotensin II type 1A receptor promotes Rab5 GTP binding and vesicular fusion. J. Biol. Chem. 277, 679–685. [DOI] [PubMed] [Google Scholar]
- Segev, N., Mulholland, J., and Botstein, D. (1988). The yeast GTP-binding YPT1 protein and a mammalian counterpart are associated with the secretion machinery. Cell 52, 915–924. [DOI] [PubMed] [Google Scholar]
- Segev, N. (2001a). YPT and Rab GTPases: insight into functions through novel interactions. Curr. Opin. Cell Biol. 13, 500–511. [DOI] [PubMed] [Google Scholar]
- Segev, N. (2001b). YPT/Rab GTPases: regulators of protein trafficking. Sci STKE, RE11. [DOI] [PubMed]
- Shirataki, H., Yamamoto, T., Hagi, S., Miura, H., Oishi, H., Jin-no, Y., Senbonmatsu, T., and Takai, Y. (1994). Rabphilin-3A is associated with synaptic vesicles through a vesicle protein in a manner independent of Rab3A. J. Biol. Chem. 269, 32717–32720. [PubMed] [Google Scholar]
- Storrie, B., White, J., Rottger, S., Stelzer, E.H., Suganuma, T., and Nilsson, T. (1998). Recycling of Golgi-resident glycosyltransferases through the ER reveals a novel pathway and provides an explanation for nocodazole-induced Golgi scattering. J. Cell Biol. 143, 1505–1521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang, B.L., Zhang, T., Low, D.Y., Wong, E.T., Horstmann, H., and Hong, W. (2000). Mammalian homologues of yeast sec31p. An ubiquitously expressed form is localized to endoplasmic reticulum (ER) exit sites and is essential for ER-Golgi transport. J. Biol. Chem. 275, 13597–13604. [DOI] [PubMed] [Google Scholar]
- Teal, S.B., Hsu, V.W., Peters, P.J., Klausner, R.D., and Donaldson, J.G. (1994). An activating mutation in ARF1 stabilizes coatomer binding to Golgi membranes. J. Biol. Chem. 269, 3135–3138. [PubMed] [Google Scholar]
- Tisdale, E.J., Bourne, J.R., Khosravi-Far, R., Der, C.J., and Balch, W.E. (1992). GTP-binding mutants of rab1 and rab2 are potent inhibitors of vesicular transport from the endoplasmic reticulum to the Golgi complex. J. Cell Biol. 119, 749–761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tisdale, E.J., and Jackson, M.R. (1998). Rab2 protein enhances coatomer recruitment to pre-Golgi intermediates. J. Biol. Chem. 273, 17269–17277. [DOI] [PubMed] [Google Scholar]
- Touchot, N., Chardin, P., and Tavitian, A. (1987). Four additional members of the ras gene superfamily isolated by an oligonucleotide strategy: molecular cloning of YPT-related cDNAs from a rat brain library. Proc. Natl. Acad. Sci. USA 84, 8210–8214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Velasco, A., Hendricks, L., Moremen, K.W., Tulsiani, D.R., Touster, O., and Farquhar, M.G. (1993). Cell type-dependent variations in the subcellular distribution of alpha-mannosidase I and II. J. Cell Biol. 122, 39–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward, T.H., Polishchuk, R.S., Caplan, S., Hirschberg, K., and Lippincott-Schwartz, J. (2001). Maintenance of Golgi structure and function depends on the integrity of ER export. J. Cell Biol. 155, 557–570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weide, T., Bayer, M., Koster, M., Siebrasse, J.P., Peters, R., and Barnekow, A. (2001). The Golgi matrix protein GM130: a specific interacting partner of the small GTPase rab1b. EMBO Rep. 2, 336–341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson, B.S., Nuoffer, C., Meinkoth, J.L., McCaffery, M., Feramisco, J.R., Balch, W.E., and Farquhar, M.G. (1994). A Rab1 mutant affecting guanine nucleotide exchange promotes disassembly of the Golgi apparatus. J. Cell Biol. 125, 557–571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zerial, M., and McBride, H. (2001). Rab proteins as membrane organizers. Nat. Rev. Mol. Cell Biol. 2, 107–117. [DOI] [PubMed] [Google Scholar]
- Zhao, X., Lasell, T.K., and Melancon, P. (2002). Localization of large ADP-ribosylation factor-guanine nucleotide exchange factors to different Golgi compartments: evidence for distinct functions in protein traffic. Mol. Biol. Cell 13, 119–133. [DOI] [PMC free article] [PubMed] [Google Scholar]