Abstract
We show here that the distal regulatory region (DRR) of the mouse and human MyoD gene contains a conserved SRF binding CArG-like element. In electrophoretic mobility shift assays with myoblast nuclear extracts, this CArG sequence, although slightly divergent, bound two complexes containing, respectively, the transcription factor YY1 and SRF associated with the acetyltransferase CBP and members of C/EBP family. A single nucleotide mutation in the MyoD-CArG element suppressed binding of both SRF and YY1 complexes and abolished DRR enhancer activity in stably transfected myoblasts. This MyoD-CArG sequence is active in modulating endogeneous MyoD gene expression because microinjection of oligonucleotides corresponding to the MyoD-CArG sequence specifically and rapidly suppressed MyoD expression in myoblasts. In vivo, the expression of a transgenic construct comprising a minimal MyoD promoter fused to the DRR and β-galactosidase was induced with the same kinetics as MyoD during mouse muscle regeneration. In contrast induction of this reporter was no longer seen in regenerating muscle from transgenic mice carrying a mutated DRR-CArG. These results show that an SRF binding CArG element present in MyoD gene DRR is involved in the control of MyoD gene expression in skeletal myoblasts and in mature muscle satellite cell activation during muscle regeneration.
INTRODUCTION
The MyoD gene family, which includes MyoD, Myf-5, Myogenin, and MRF4, encode structurally related basic helix-loop-helix (bHLH) transcription factors that are essential regulators of skeletal muscle lineage determination and differentiation in vertebrates (Davis et al., 1987; Olson et al., 1990; Weintraub et al., 1991). Myogenin is required for normal biochemical and morphological differentiation of skeletal muscle, but not for commitment of cells to the myogenic lineage (Hasty et al., 1993; Nabeshima et al., 1993), and MRF4 plays a role in muscle fiber maturation (Braun and Arnold, 1995; Patapoutian et al., 1995; Rawls et al., 1998). In contrast, MyoD and Myf5 are essential for skeletal muscle lineage determination and are expressed in proliferative myoblasts, before differentiation (Braun et al., 1992; Rudnicki et al., 1992, 1993). Additional studies at postnatal stages show that a MyoD–/–mutant mouse is severely deficient in regenerative capacity after injury, indicating that MyoD plays an essential role in regulating the myogenic program of satellite cells (Megeney et al., 1996, reviewed Tajbakhsh and Cossu, 1997). Consistent with this conclusion, in many myogenic cell lines, the capacity of cells to terminally differentiate appears to be linked to the level of MyoD expression (Pinset et al., 1988; Brennan et al., 1990; reviewed Kitzmann and Fernandez, 2001). Therefore it is essential to understand the transcriptional controls regulating the MyoD gene in myoblasts in order to elucidate the molecular mechanisms by which postnatal skeletal muscle differentiation and regeneration are controlled. Myoblasts cell lines, being all derived from adult satellite cells, provide an excellent model system that mimics the regenerative process in differentiated muscle.
We and others have shown previously that the serum response factor (SRF), a DNA binding protein belonging to the MADS (MCM1, Agamous, Deficiens, SRF) box family of transcription factors (Treisman, 1992), is required for both myoblast differentiation (Vandromme et al., 1992) and MyoD gene expression in proliferating myoblasts (Gauthier-Rouvière et al., 1996; Soulez et al., 1996). Further studies revealed that SRF inactivation specifically prevented MyoD gene expression, leaving Myf-5 protein levels intact (Carnac et al., 1998). Moreover, an upstream regulator of SRF activity, the small G-protein RhoA, also specifically regulates MyoD: blocking RhoA but not Rac or CDC42 protein activity inhibits MyoD promoter activity (Carnac et al., 1998). Because our previous studies using microinjection revealed that MyoD expression was rapidly suppressed after inactivation of SRF (Gauthier-Rouvière et al., 1996; Carnac et al., 1998), we wished to determine if SRF could directly bind to MyoD regulatory sequences and regulate MyoD gene activity in proliferating myoblasts. Previous studies have shown that a 24-kbp fragment of human MyoD 5′ flanking region is sufficient to recapitulate endogenous MyoD expression during mouse muscle development (Chen and Goldhamer, 1999; Chen et al., 2001). In addition to a minimal promoter called proximal regulatory region (PRR; Tapscott et al., 1992), two muscle-specific enhancers with distinct but overlapping specificity have been characterized within MyoD flanking sequences in humans and mice. A highly conserved core enhancer sequence ∼20 kb upstream of MyoD is sufficient for early MyoD activation in somites, limb buds, and branchial arches (Goldhamer et al., 1995; Faerman et al., 1995; Kablar et al., 1998; Kucharczuk et al., 1999). However, this core enhancer is not sufficient to maintain MyoD expression in skeletal muscle, being downregulated in fetal and neonatal muscle and essentially inactive in adult muscle (Faerman et al., 1995). Five kilobases upstream of MyoD is a second enhancer, the distal regulatory region (DRR), which is unrelated in sequence to the core enhancer and exhibits largely complementary activity in transgenic mice (Tapscott et al., 1992; Asakura et al., 1995; Goldhamer et al., 1995; Kablar et al., 1998; Chen et al., 2001). DRR activity depends on myogenic b-HLH (Asakura et al., 1995) and is restricted to skeletal muscle in vivo (Kablar et al., 1997). Unlike the core enhancer, the DRR remains active in adult muscle, demonstrating a similar expression profile at this stage as the endogenous MyoD gene (Hughes et al., 1993; Chen et al., 2002). In addition, the study by Chen et al. (2002), clearly demonstrated that MyoD DRR is dispensable for MyoD expression during muscle development whereas it is essential at postnatal stages for expression in mature muscles. Because MyoD expression in mature muscle is essentially induced upon muscle regeneration and growth, the pertinent context to study a DRR-dependent regulation of MyoD appeared to be during satellite cells activation induced upon muscle regeneration.
Using satellite cell–derived myoblasts and in vivo muscle regeneration assays, we show here that an SRF-binding CArG element present in MyoD DRR enhancer plays an essential role in DRR-dependent expression of MyoD.
MATERIALS AND METHODS
Cell Culture, Stable Transfections
C2.7 mouse myoblast line was grown in DMEM supplemented with 15% fetal bovine serum (Life Technologies, Cergy Pontoise, France), 2 mM l-glutamine, 100 U/ml penicillin, and 100 μg/ml streptomycin and was maintained in a humidified incubator (37°C, 5% CO2). Stable transfections were performed with lipofectamine reagent (Life technologies, Inc.) according to the manufacturer's instructions. Stable transfectants were then grown in the proliferation medium described above.
Oligonucleotides and Electrophoretic Mobility Shift Assay
High-performance liquid chromatography–purified oligonucleotides were purchased commercially (MWG-BIOTECH, France SA) and dissolved in sterile water. Sense and antisense oligonucleotides were individually labeled with T4 polynucleotide kinase (New England Biolabs Inc., Beverley, MA). Labeled double-strand oligonucleotides were gel-purified. Nuclear extracts from proliferated C2.7 cells were prepared as previously described (Dignam et al., 1983). All extracts were aliquoted, snap-frozen in liquid nitrogen, and stored at –80°C.
For electrophoretic mobility shift assay (EMSA), 5 μg of nuclear extract was incubated for 15 min on ice in 1× Retardation mix (10 mM Tris/Cl, pH 8, 0.1 mM EDTA, 10 mM MgCl2, 2 mM DTT, 15% glycerol, 2 mg/ml bovine serum albumin [BSA]), 250 ng of single-stranded DNA, in the absence or presence of unlabeled competitor oligonucleotides (50–100×). Approximately 15,000 cpm of labeled probe was then added to the mixture and incubated at 30°C for 10 min. For supershift analysis, 0.2 μg of rabbit polyclonal antibody (SRF G20, YY1 C20, C/EBPβ δ198, and CBP C20, Santa-Cruz Biotechnology, Inc., Santa Cruz, CA) were included before probe addition and incubated with the mixture for 1 h on ice. Samples were resolved on a nondenaturing 5% polyacrylamide gels (19:1 acrylamide/bisacrylamide).
Gel Filtration
A volume of 50 μl containing 500 μg of proliferating nuclear extracts were loaded on a Superose 6 gel-filtration column (Pharmacia Biotech, Orsay, France) preequilibrated with 100 mM NaCl/20 mM Tris/Cl, pH 7.4. Proteins were resolved at 4°C using an FPLC system (Pharmacia Biotech).
Microinjection
For microinjection studies, growing C2.7 cells were microinjected with wtCarG, mCarG, csCarG oligonucleotides, or buffer at two concentrations: 50 and 10 μg/ml in PBS 50% in water (v/v). In all cases, injection solutions contained inert rabbit immunoglobins (1 mg/ml) to serve subsequently in identifying injected cells. Three hours after microinjection, cells were fixed with formalin and expression of MyoD in the injected cells was analyzed by double-immunofluorescence. Cells were stained with Alexa Red–conjugated anti-rabbit antibodies (Molecular Probes) to visualize injected cells and monoclonal anti-MyoD (5.8A, PharMingen) followed by Alexa 488–conjugated anti-mouse (Molecular Probes) to probe for MyoD expression. Alternatively, as an independent control, expression of MyF5 was analyzed 3 h after injection of the CArG oligonucleotides. In that case, the injection solution contained mouse Igs and the expression of Myf5 was monitored using a rabbit polyclonal antibody directed against Myf5 (Carnac et al., 1998).
Site-directed Mutagenesis
Construct 17.11wt containing the mice DRR and PRR regions of MyoD gene fused to the β-galactosidase gene was generously provided by Dr. S.J Tapscott (Tapscott et al., 1992). Mutations of the CArG-box in the DRR of 17.11 m and 17.11cs plasmids were generated using the Quick-change mutagenesis kit (Stratagene Inc., La Jolla, CA) with the following oligonucleotides: CCCAAAAGCCAGCTCTCGATTTATAGCACCT and CCCAAAAGCCAGCTCTCCATTTATGGCACCT (and their corresponding lower strand oligonucleotides) for the mCArG and csCArG mutants, respectively. All mutations were verified by sequencing.
Transgenic Mice
Fragments from the 17.11wt and 17.11mutated constructs defined by the 5′ ApaI site and the 3′ EagI site were used for oocyte injections from B6CBA mice. Transgenic mice were identified by PCR analysis of DNA extracted from tail biopsies using the following oligonucleotides: GCGCCCATCTACACCAACGTAACC and ACGCAACTCGCCGCACATCTGAAC.
Muscle Injury
For induced regeneration studies, 8-week-old transgenic male mice (25–28 g) were anesthetized with a mix of Ketamin/Xylasin by intraperitoneal injection (0.1 ml at 1 mg/ml per 10 g weight). The tibialis anterior (TA) muscle of each mouse was exposed and carefully dissected of its overlying fascias. At the level of the proximal insertion of the TA muscle, the skin was cut 3 mm in length. The needle of a 10-μl Hamilton microsyringe was inserted near the proximal tendon and pushed down to the distal one, and 10 μl of notexin (50 μg/ml; Sigma) was injected in the TA, the needle being pulled up to deliver notexin all along the muscle. The notexin was adjusted to 50 μg/ml with physiological serum. Muscles were subsequently recovered for analysis at different times after notexin injury (0, 2, 3, 4, 5, and 7 d after myonecrotic injury). Muscle extracts, normalized for their protein content, were analyzed for β-Gal activity against ONPG and for MyoD induction by Western blot as described below.
Trichostatin A and β-Galactosidase assays
For trichostatin assays, stable transfectants (17.11wt and 17.11 m C2.7 cells) were exposed to trichostatin A (TSA, Sigma) at 50 or 100 nM for 8 and 24 h in proliferation medium (as described above). Cells were subsequently washed, harvested, and lysed in 0.25 M Tris-Cl (pH 7.8) by three cycles of freezing in dry ice and thawing at 37°C. After clearing by centrifugation, supernatants were assayed for β-galactosidase activity at 37°C (1 mg/ml ONPG, 1 mM MgCl2, 45 mM β-mercaptoethanol, 0.1 M sodium phosphate, pH 7.5). Reactions were stopped by addition of Na2CO3, and the optical density of each reaction read at 420 nm. TA muscles were snap frozen in liquid N2 before homogenization in 0.25 M Tris-Cl (pH 7.8) for β-Gal activity measurements or in nuclear suspension buffer (50 mM Tris-Cl, pH 7.5, 1% NP40, 40 mM β-glycero-phosphate, 300 mM NaCl, and antiproteases) for Western blot analyses.
Immunoprecipitation
Proliferating C2.7 cells were placed on ice and extracted with lysis buffer containing 50 mM Tris-Cl, pH 7.5, 1% w/v Nonidet P-40 (NP40), 40 mM β-glycero-phosphate, 120 mM NaCl, 0.1 sodium orthopervanadate, 1 mM phenylmethylsulfonyl fluoride (PMSF), and 1 mM benzamidine. Lysates were centrifuged for 20 min at 12,000 × g, and the SRF or C/EBP protein were immunoprecipitated from 500 μg of cell-free extracts with anti-SRF (G20) or anti-C/EBPβ (δ198). Immune complexes were precipitated using protein G-sepharose (Pharmacia) and analyzed by Western blot after washing three times in lysis buffer. Protein concentrations were determined by Bradford Assay using BSA as a standard.
Western Blot Analysis
Proteins from gel-filtration, muscle extracts, or immunoprecipitates were resolved on 10% SDS-polyacrylamide gels. After transfer to a nitrocellulose membrane (Schleicher and Schuell), Western blot analysis was performed using SRF (G20), or MyoD (C20) rabbit polyclonal antibodies (Santa Cruz), diluted 1:500 in PBS-5% nonfat powdered milk. To detect primary antibodies blots were probed with horseradish peroxidase–conjugated anti-rabbit antibodies (Amersham) at 1:5000 dilution in PBS-0.5% BSA for muscle extracts or gel-filtration and protein A/G-peroxidase (Perbio) at dilution of 1:10,000 in PBS-0.5% BSA for immunoprecipitations. Proteins were visualized using the ECL protein detection Kit (Roche Diagnostics) as described by the manufacturer.
RESULTS
Identification of an SRF-binding CArG-box in the DRR of MyoD Gene
To investigate if MyoD gene expression could be regulated by direct binding of SRF, we analyzed the regulatory regions of the MyoD gene (shown in Figure 1A) to identify potential binding sequences for SRF.
Figure 1.
Sequence alignment of the human and mouse MyoD DRR and conservation of a divergent SRE/CArG-like element. (A) Schematic diagram representing the genomic organization of MyoD locus. PRR, proximal regulatory region; DRR, distal regulatory region; CE, core enhancer. Enlarged view of the DRR with the sequence of the identified divergent CArG (MyoD-wtCArG), its position, and flanking sequences. (B) Sequence alignment of part of the mouse MyoD DRR (Tapscott et al., 1992) and the corresponding sequence from human MyoD (Chen et al., 2001). Conserved sequence binding sites are shown for SRF and known transcription factors that have been postulated to function in muscle gene regulation. (C) This sequence MyoD-wtCArG corresponds to that of the 29mer oligonucleotides used subsequently in the EMSA experiments. For comparison the sequences of consensus MyoD-csCArG and mutated MyoD-mCArG are shown underneath together with the sequences of the serum response element of the c-fos gene and the CArG of skeletal-muscle-actin.
Sequence analysis of the regulatory region of human and mouse MyoD genes showed the presence of conserved putative binding sites for transcription factors, including a potential SRF-binding sequence (CArG-box) in the DRR (Figure 1B). This element is localized 5014 base pairs upstream of the mouse gene transcription start site (Figure 1A). Its sequence (CC(A/T)6AG) diverges slightly at the 3′ end from the published SRF consensus sequence (CC(A/T)6GG) (reviewed in Shore and Sharrocks, 1995). However, this divergence is similar to that observed in the MLC1A promoter, which has been demonstrated to be transcriptionally active (Catala et al., 1995). As shown in Figure 1B, this MyoD putative CArG-box is also conserved in the human MyoD DRR enhancer region (Chen et al., 2001).
The MyoD CArG-box Binds SRF-containing Complexes, But with Reduced Affinity When Compared with the c-fos SRE
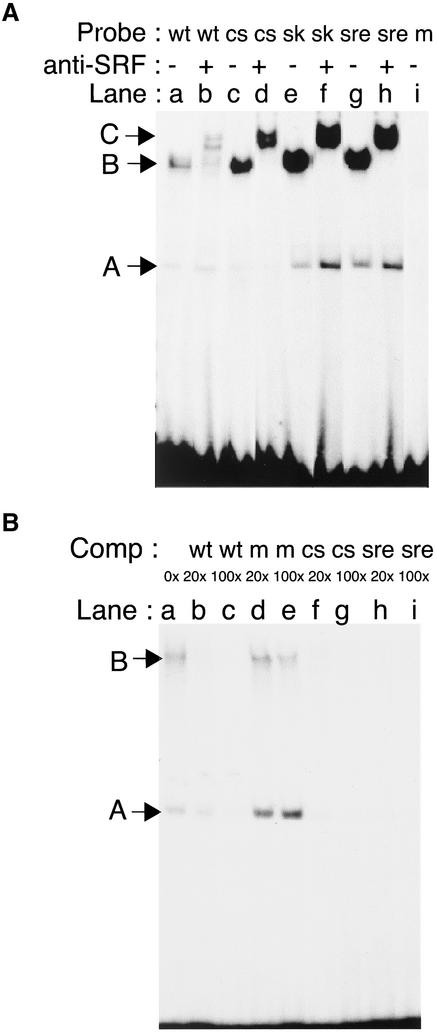
To test the capacity of this CArG-box to bind nuclear factors using EMSA, several specific probes were designed spanning this region (Figure 1C). In the presence of nuclear extracts from growing C2.7 myoblasts, the MyoDCArG-box formed two complexes, a major slow-migrating complex (complex B) and a minor faster migrating complex (complex A; Figure 2A, lane a). To compare this binding with that of other well-known SRF-binding sequences, we performed similar experiments with the CArG box from skeletal muscle actin and the c-fos SRE sequence. Two complexes of similar mobility but higher intensity for complex B were observed with these probes (Figure 2A, lanes a, e, and g). These results suggest that MyoDCArG-box formed the same major complexes (B) but with lower affinity than canonical SRF binding sequences. Complex formation is specific because a mutated CArG probe (CG(A/T)6AG) containing a C to G change in 5′ (Figure 1) abolished binding to either complex A or B (Figure 2A, lane i). Supershift analysis using a specific anti-SRF antibody showed that SRF was present in complex B but not complex A (supershifted complex C, Figure 2A, lanes b, d, f, and h). Further supershift experiments using anti-YY1 antibody revealed that complex A contained the transcription factor YY1 as shown below (Figure 3C, lanes a and b).
Figure 2.
MyoD DRR enhancer contains an SRE/CArG-like element that binds SRF-containing complexes. (A) MyoD wild-type (wt), MyoD consensus (cs), skeletal actin (sk), c-fos SRE (sre), and MyoD mutated (m) oligonucleotides shown in A were radiolabeled and used as probes in electrophoretic mobility shift assays using nuclear extracts from growing C2.7 myoblasts. Rabbit polyclonal antibodies raised against SRF were added or not before the DNA binding reaction. Two complexes, A and B, and anti-SRF supershifted (complex C) are indicated by arrowheads. (B) The wt oligonucleotide was used as probe in gel-shift assay in the presence of increasing excess of unlabeled oligonucleotides containing either wild-type (wt), mutated (m), consensus (cs) MyoD-CArGs or SRE motif. Complexes A and B are indicated by arrowheads. 0× to 200× refer to fold excess over labeled probe.
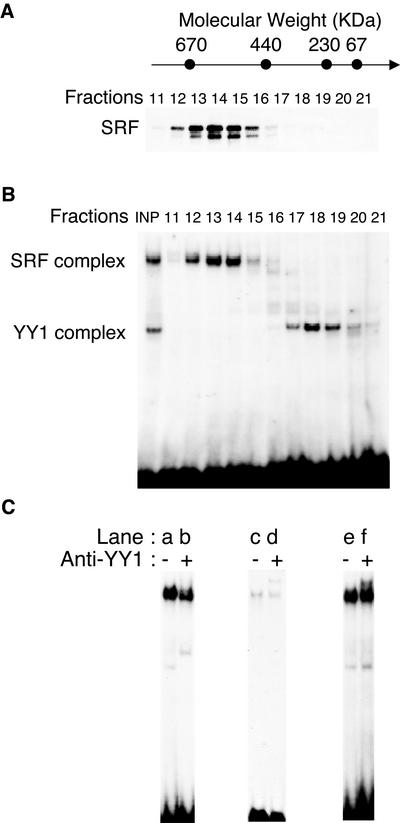
Figure 3.
MyoD CArG element binds high-molecular-weight complexes containing SRF associated with CBP acetyltransferase and members of the C/EBP family. (A and B) Nuclear extracts from proliferating C2.7 cells were separated by gel filtration on a Superose 6 column. The relative elution position of the calibration markers, thyroglobulin (669 kDa), ferritin (440 kDa), catalase (232 kDa), aldolase (158 kDa), and BSA (67 kDa) is schematized at the top. Gel-filtration was carried out at 4°C using a FPLC system. (A) Fractions (500 μl) were collected, concentrated, separated by SDS-PAGE, and analyzed by Western blot using a polyclonal anti-SRF antibody. (B) Aliquots from indicated fractions were used in electrophoretic mobility shift assay with the MyoD-wtCArG probe. Although SRF complexes appear in high-molecular-weight fractions, the YY1 complex is present in low-molecular-weight fractions. (C) To analyze protein contents of the SRF/CArG-complex, electrophoretic mobility shift assays were performed using proliferated myoblasts nuclear extracts with wtCArG probe in presence of anti-YY1, anti-C/EBP, and anti-CBP antibodies.
To further examine the affinity of both complexes for the CArG-box motif, we performed competitive EMSA analysis. As shown in Figure 2B, complexes formed with SRF and YY1 were abolished when MyoD-wtCArG but not mutated MyoD-mCArG oligonucleotides were added as competitors (Figure 2B, lanes a–e). In addition, both complexes A and B formed with MyoD-wtCArG were more efficiently competed when SRE (Figure 2B, lanes h and i) or skeletal muscle actin-CArG-box oligonucleotides were used (unpublished data). These observations further confirm that the MyoD-CArG-box bound SRF with lower affinity than SRE or skeletal muscle actin-CArG elements.
The lower affinity of the MyoDCArG-box for the SRF-containing complex B most likely resulted from the single nucleotide change in the decanucleotide core of MyoDCArG that differentiates it from the SRF consensus sequence. To test this, we used the mutated oligonucleotide MyoD-csCArG (CC(A/T)6GG), which contained an A to G transition thus creating a consensus CArG (Figure 1B). EMSA using this MyoD-csCArG in the presence of myoblast nuclear extracts formed two complexes with the same mobility as complexes A and B, but of greater intensity, similar to that obtained with SRE or Sk-actin CArG probes (Figure 2A, lanes c and d). Competition assays using this consensus MyoD-csCArG oligonucleotide abolished both complexes with the same affinity as an SRE oligonucleotide (Figure 2B, lanes f and g). Thus, the single nucleotide divergence in the MyoDCArG-box present in DRR is associated with a lower binding affinity for SRF.
These data show that the DRR enhancer of MyoD contains a noncanonical CArG-box that is able to bind SRF and YY1 protein complexes when incubated in presence of myoblast extracts. The SRF containing complex binds MyoDCArG-box with a weaker affinity than a canonical nonmuscle CArG, such as the c-fos SRE-CArG-box and this weaker affinity is uniquely due to the single nucleotide divergence at the 3′ end of MyoDCArG-box.
The CArG-box of MyoD Interacts with SRF in High-Molecular-Weight Complexes
It has been long known and documented that transcriptional activation via SRF does not involve any change in protein expression or level but rather changes in associated factors (and phosphorylation state; reviewed Treisman, 1992, Treisman et al., 1998). SRF associates with DNA in the form of homodimers interacting with other accessory regulatory proteins that appear to potentiate its transcriptional activity (Belaguli et al., 1997; Montaner et al., 1999; Belaguli et al., 2000; Gineitis and Treisman, 2001; Wang et al., 2001). We therefore examined the SRF-containing complexes from C2.7 myoblasts.
Extracts from C2 myoblasts were fractionated on a FPLC Superose 6 gel filtration column. Different fractions (from 1–24) were analyzed by Western blot using antibodies against SRF. As shown in Figure 3A in myoblast cell extracts, SRF eluted in fractions 12–16 that correspond to protein complexes of 500–700 kDa.
Because the mass of SRF is 67 kDa and it is associated as an homodimer (Treisman, 1992), our data suggest that in myoblasts, SRF interacts with a number of other cofactors. The fractions containing SRF were subsequently examined in EMSA and results (Figure 3B) show that SRF-containing complex B eluted as high-molecular-weight fractions, whereas YY1-containing complex A eluted at a molecular weight of 100–300 kDa.
We therefore examined the protein content of these high-molecular-mass complexes. Members of C/EBP family transcription factors and the acetyltransferases CBP/P300 have been identified with SRF before, in transcriptionally active complexes (Ramirez et al., 1997; Montaner et al., 1999; Qiu and Li, 2002). Supershift assays performed with antibodies specific to YY1, CBP, or C/EBP (Figure 3C) show that members of the C/EBP family (lanes c and d) as well as the acetyltransferase CBP (lanes e and f) are present together with SRF in complex B. In addition, we found that C/EBP coimmunoprecipitated with SRF from myoblast extracts, further supporting that this cofactor contributes to SRF activity at the MyoD-CArG (unpublished data).
Endogeneous MyoD Expression Is Specifically and Rapidly Suppressed after Microinjection of MyoD-CArG Competing Oligonucleotides.
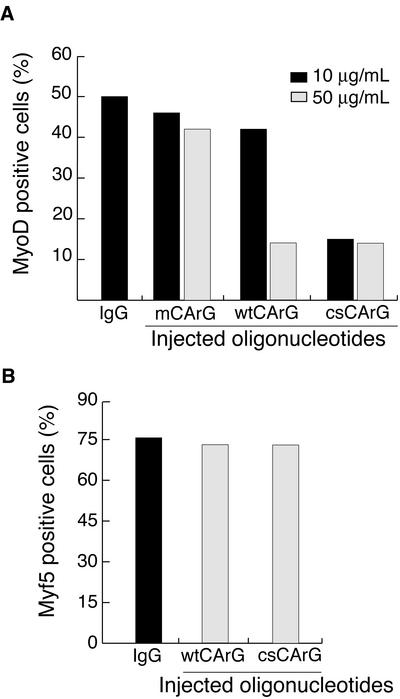
To examine the consequence on endogeneous MyoD expression of performing in vivo competition assays with microinjected oligonucleotides (Lamb et al., 1996), we made use of the fact that both protein and mRNA for MyoD are short lived (<2 h), and therefore the direct effects of the injected oligos could be examined rapidly. Wild-type, mutated or consensus oligonucleotides corresponding to MyoDCArG box sequences shown in Figure 1C were injected in growing myoblasts and cells were fixed after 3 h, a time considered to be sufficient to allow for MyoD protein and RNA turnover. Approximately 50–60% of cells express MyoD in growing myoblasts. Microinjection of IgG marker alone (control) or mutated MyoD-mCArG oligonucleotides had little or no effect on MyoD expression (50–60% of microinjected cells expressed MyoD both in control microinjected cells and in surrounding uninjected cells; Figure 4). When wtCArG oligonucleotides were used at a high concentration (50 μg/ml), we observed a significant decrease of the number of MyoD-positive cells, with fewer than 15% of injected cells expressing MyoD (70% inhibition; Figure 4A). A similar degree of inhibition was also observed when MyoD-csCArG oligonucleotides were microinjected. However, at a lower concentration (10 μg/ml instead of 50 μg/ml), only the MyoD-csCArG, and not the MyoD-wtCArG, still inhibited MyoD expression (Figure 4A). These results confirm the lower affinity of SRF for the wild-type MyoD-wtCArG compared with the canonical MyoD-csCArG sequence as observed in EMSA experiments. As an additional control, to test whether the injected CArG oligos would affect an SRF-independent target gene, we have performed the same microinjection experiment, instead assaying for the effects on Myf5 expression. Like MyoD, Myf5 is expressed in growing myoblasts and has a similarly short half-life. However, we have shown before that its expression, unlike that of MyoD, is not dependent on SRF activity (Carnac et al., 1998). As shown in Figure 4B, neither MyoD-wtCArG nor MyoD-csCArG microinjection had any effect on Myf5 expression although they were used at 50 μg/ml, a concentration sufficient to inhibit MyoD expression with both oligos. These results support that inhibition of SRF binding to MyoD-CArG by competition with MyoD-CArG-box oligonucleotides specifically blocked MyoD gene expression in proliferating myoblasts and that the MyoD DRR-CarG sequence is active in modulating endogenous MyoD expression in myoblasts.
Figure 4.
Microinjection of a CArG sequence inhibits MyoD gene expression in proliferating myoblasts. Proliferating C2.7 myoblasts were microinjected with oligonucleotides corresponding to wt, m, and cs CArG sequences as represented in Figure 1C, at the indicated concentrations. Cells were fixed 3 h after injection and expression of either MyoD or Myf5 was analyzed in microinjected cells by double immunofluoresence staining as described in MATERIALS AND METHODS. Control represents injection of inert Igs marker alone, it resulted in the same percentage of cells expressing MyoD or Myf5 as in surrounding uninjected cells. Values above the bars represent the number of cells injected in a given experiment. These data were reproduced three times.
The CArG-box Present in MyoD DRR Enhancer Is Required for Transcriptional Activation of MyoD in Myoblasts
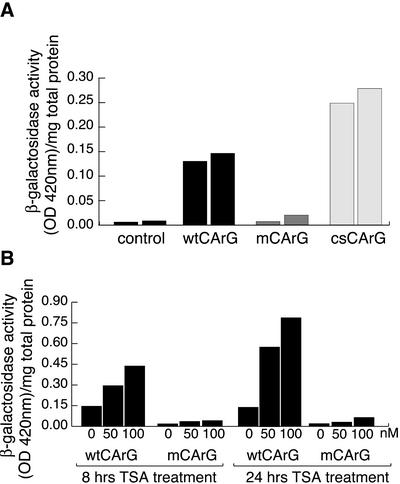
Previous results from stable transfections into C2.7 myoblasts and in transgenic mice demonstrated that a chimeric construct (17.11wt), containing the 720 base pairs of the DRR fused to the PRR followed by β-gal, was expressed specifically in muscle cells in vivo and in myoblast cell lines but not in 10T1/2 fibroblast cells (Tapscott et al., 1992). To test if MyoD-CArG-box was required for the specific activation of the MyoD gene in adult skeletal myoblasts, we separately introduced two mutations in the DRR-PRR-β-galactosidase fusion construct. The first mutation, a C to G substitution at position 5′ is the same as that shown in Figure 1A, resulting in the complete abolition of complexes A and B (Figure 1B, lane i). The second single nucleotide mutation transforms a MyoD-wtCArG into the consensus MyoD-csCArG and strongly increased the binding of the SRF-containing complex but not that of the YY1-containing complex (Figure 1B, lane c). Each construct 17.11wt (wtCArG), 17.11 m (mCArG), and 17.11c (csCArG) was stably transfected into C2.7 cells, and enhancer activity was measured in growing myoblasts. Consistent with the data obtained by EMSA, results presented in Figure 5A show a mutation that suppressed the binding of both complexes A and B to the MyoDCArG (MyoD-mCArG) abolished MyoD DRR enhancer activity in C2.7 cells. In contrast, a mutation reverting divergent MyoDCArG-box to the consensus CArG sequence (MyoD-cs-CArG) resulted in a twofold increase in enhancer activity (Figure 5A). In addition, to probe whether the presence of CBP found in a complex with SRF in myoblasts nuclear extracts (Figure 3C) plays a functional role in SRF activity, we have tested if treatment with the histone deacetylase inhibitor, trichostatin A (TSA), resulted in enhanced activation of the stably transfected DRR-driven reporter constructs in myoblasts. As shown Figure 5B, TSA treatment induced a clear increase in β-Gal reporter activity that was specific for a CArG-dependent activation because no such effect was seen in myoblasts carrying a DRR construct with a mutated CArG (Figure 5B). These data show that a single base mutation in MyoD-CArG sequence produced major effects on the expression of the reporter placed downstream of MyoD enhancer, thus demonstrating that this CArG element must play an important role in the control of MyoD expression by the DRR enhancer.
Figure 5.
Point mutation of MyoD-CArG element abolishes MyoD PRR-DRR construct activity. C2.7 cells were stably transfected with 17.11wt and 17.11mut constructs containing either wild-type CArG or mutated CArG as shown in Figure 1. 4A: Cells were exposed to 50 nM or 100 nM trichostatin A for the indicated time before harvesting for β-galactosidase activity measurement as described in MATERIALS AND METHODS. 4B: DRR enhancer activity was measured in growing myoblasts stably transfected with the wild-type, mutated or consensus CArG-containing DRR reporter. Shown in both A and B are β-gal activity measurements from two series of experiments carried out with a whole population of stably transfected C2.7 cells. Control represents basal β-galactosidase activity in extracts from nontransfected C2.7.
The CArG Element Present in MyoD DRR Is Essential for the Induction of MyoD Expression during Muscle Regeneration
We have shown (Figure 5) that in the myogenic cell line C2.7, the expression of a MyoD-DRR–driven β-gal reporter (made and described previously by Tapscott et al., 1992) is modulated by point mutations in the CArG element present in this DRR. Most, if not all myogenic cell lines are derived from adult skeletal muscle satellite cells and a recent report has shown that the activity of DRR is dispensable during embryogenesis but essential at postnatal stages in mature muscle when the core enhancer cannot substitute for its role (Chen et al., 2002). Therefore, we examined if the DRR enhancer and CArG element we identified are specifically implicated in MyoD expression in the context of its induced expression in vivo, i.e., upon activation of satellite cells when muscle regeneration is induced. We generated transgenic mice carrying the PRR-DRR-β-galactosidase chimeric constructs 17.11wt (with wild-type CArG sequence) or 17.11 m (with mutated CArG sequence) used in Figure 5.
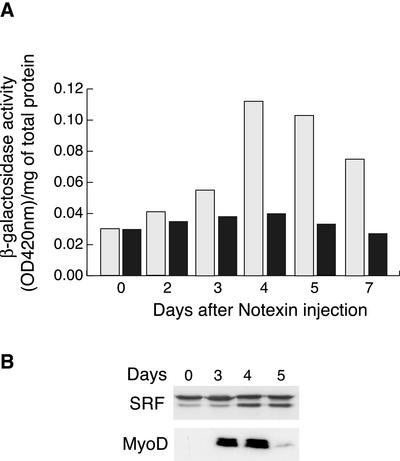
Muscle regeneration can be artificially induced by injecting the snake venom notexin, which causes muscle fiber degeneration but does not affect the satellite cells. Once the lesion is induced, satellite cells are activated, proliferate, and finally differentiate, thereby effecting repair and regeneration (Harris and Montgomery, 1975; Lefaucheur and Sebille, 1995). Notexin was injected in the TA muscle of transgenic mice and the muscle was subsequently dissected at different times thereafter. After normalizing for total protein content, β-galactosidase assays were performed to examine MyoD-DRR promoter activity, followed by Western blotting for MyoD and SRF expression. The results are presented in Figure 6. At day 0, we did not detect any β-Gal transgene activity and this was correlated with an absence of MyoD expression, because MyoD is not significantly expressed either in mature muscle fibers or in quiescent satellite cells. At day 2, when the muscle is entirely degenerated with a major inflammatory response, still no β-galactosidase activity nor MyoD protein could be detected. By days 3 and 4 after injury, a clear increase in transgene activity was seen in regenerating muscle from mice carrying wild-type wtCArG-box DRR, whereas no significant increase in transgene activity was detected in muscle from mice carrying a mutated mCArG in the DRR (Figure 6A). However, in both wtCArG (unpublished data) and mutated CArG transgenic mice muscles, there was a clear induction of endogenous MyoD gene expression, which corresponds to the induction of satellite cell proliferation (Figure 6B). The difference observed between the slow reduction of β-galactosidase activity (in muscle from wt-CarG transgenic mice) and the sharp drop in MyoD expression after day 4 is most likely due to the higher stability of β-galactosidase (at least 15 h) compared with MyoD protein (45 min).
Figure 6.
Activation of MyoD DRR during muscle regeneration requires MyoD-CArG element. To study DRR function during muscle regeneration, we generated transgenic mice carrying either 17.11wt or 17.11mut transgene (DRR-PRR-β-galactosidase) and carried out regeneration experiments. Muscle regeneration was induced in the tibialis anterior (TA) after fiber destruction with the snake toxin, notexin, as described in MATERIALS AND METHODS. At different times thereafter, injured TA muscles were analyzed for the activity of the MyoD DRR reporter activity by β-galactosidase activity (A) and for the expression of SRF and MyoD by Western blotting (B). (A) β-Galactosidase activity normalized per milligram protein at different days after muscle injury from mice carrying the wild-type (clear boxes) or mutated (dark boxes) MyoD-DRR reporter gene. The results shown are the average from two to three mice from independent experiments for each time point. (B) Western blot analysis of SRF (top panel) and MyoD (bottom panel) endogenous protein levels in injured TA muscle from 17.11m transgenic mice at different time after notexin injection.
In contrast to this induced expression of MyoD, SRF gene is already expressed at day 0, before injury, and its expression does not vary during the regeneration process, which is in agreement with the fact that in muscle cells we have shown the need for SRF activity without changes in protein level (Vandromme et al., 1992).
These results show that the transgene 17.11wt exhibit largely similar induction of expression as the endogenous MyoD gene during muscle regeneration, whereas the CArG-mutated version of the transgene 17.11 m does not. As the minimal promoter PRR does not exhibit any activity by itself in transgenic studies (Tapscott et al., 1992), we can conclude that the DRR is sufficient to induce MyoD gene expression in vivo during satellite cell activation linked to muscle regeneration. Therefore these data shown that the CArG element present in MyoD-DRR promoter plays an essential role in vivo in the regulation of MyoD expression during muscle regeneration.
DISCUSSION
We have used a number of complementary approaches to analyze the potential role of a CArG-like sequence present in the distal regulatory promoter region of MyoD. Using specific oligonucleotide sequences related to this MyoD DRR-CArG sequence in EMSA and microinjection experiments, we show here that this MyoD DRR-CArG element is a site involved in the modulation of MyoD expression by the DRR enhancer. This was further confirmed by mutational analysis of a DRR-driven β-Gal construct activity both in stably transfected myoblasts and in transgenic mice.
MyoD DRR Promoter and Its CArG Element Play a Unique Role in the Control of MyoD Induction during Muscle Regeneration
From previous work on the two MyoD enhancers (the DRR at –5 kb and the core enhancer at –20 kb), the picture emerging is that the core enhancer is required for MyoD expression during embryogenesis (Goldhamer et al., 1995; Kablar et al., 1998; Kucharczuk et al., 1999) and inactive after birth (Faerman et al., 1995). In contrast, the DRR is not essential for expression of MyoD during development and it is required at postnatal stages in mature muscle (Chen et al., 2002). In addition, although the two myogenic factors MyoD and Myf5 expressed in myoblasts and involved in muscle lineage determination can compensate for each other in muscle development (Rudnicki et al., 1992, 1993), at postnatal stages, MyoD is required for muscle regeneration (Megeney et al., 1996). Indeed, MyoD expression in mature muscle is mostly induced in satellite cells activated into proliferation during muscle regeneration; therefore it seemed pertinent to examine MyoD modulation by the DRR in the context of growing myoblasts and in vivo–induced regeneration.
In agreement with these observations and hypotheses, using a DRR-containing reporter construct in transgenic mice, we show that this construct was sufficient to mimic the in vivo kinetics of MyoD expression upon induction of muscle regeneration by notexin injury. We show in addition that a single nucleotide mutation in MyoD-CArG, impaired SRF binding and abolished MyoD-DRR activity, both in stably transfected myoblasts and in regenerating muscle.
Studies to date have suggested that SRF activity is likely to be regulated at posttranslational levels but neither through changes in SRF expression nor through its ability to bind DNA. In agreement with this, we have observed, using the same gel-filtration experiments as shown in Figure 3, that in G0-quiescent myoblasts extracts, SRF is present in low-molecular-weight complexes from 100–200 kDa.(whereas in growing myoblasts it is found in high-molecular-weight complexes ranging from 500 to 700 kDa). These complexes in G0 myoblasts can bind CArG DNA (as assayed by EMSA) and most likely correspond to SRF homodimers, without any associated cofactors (L'honore, A., Carnac, G., Fernandez, A., unpublished observations). Modulation of these complexes at the G0-G1 transition is known to be linked to changes in SRF phosphorylation, triggered by such diverse stimuli as growth factors and cytoskeletal actin polymerization (Marais et al., 1992; Treisman, 1995; Sotiropoulos et al., 1999).
In synchronized growing myoblasts, we have shown that whereas MyoD protein is absent in quiescent-G0 cells, its expression is induced within 3–4 h after myoblasts entry into the cell cycle (Kitzmann et al., 1998). These results are also corroborated in satellite cells using isolated muscle fibers: Beauchamp et al. have shown MyoD expression to be induced within6hin satellite cells after fiber isolation and serum activation (Figure 5 in Beauchamp et al., 2000). These results further support that myoblast cell lines, being all derived from adult satellite cells, provide a reliable model system that mimics the regenerative process in mature muscle.
From these data, a picture emerge whereby in vivo activation of satellite cells upon induced regeneration, like in vitro G0 to G1 entry of myoblasts, is accompanied by an SRF-dependent induction of MyoD. Our data show that the low-affinity CArG-box present in MyoD-DRR plays a key role in this process.
SRF Binding in Association with High-Molecular-Weight Complexes Is Responsible for the Transcriptional Activity of the MyoD CArG-box in Myoblasts
Using EMSA, we showed that MyoD-CArG-box bound two protein complexes from myoblast nuclear extracts. To probe the potential function of this CArG element in the control of MyoD expression, we examined the effect of two point mutations of MyoD-CArG sequence in a reporter construct. The first mutation, which disrupted the CArG sequence and abolished binding of SRF and YY1-containing complexes, resulted in inactivation of the MyoD enhancer. In contrast, a mutation that enhanced SRF binding without affecting YY1 interaction, induced a major increase in MyoD enhancer activity. Although these results strongly support that SRF and not YY1 binding to the CArG element is responsible for transcriptional activation, it remains interesting to determine the functionality of YY1 binding because recent reports described a functional interference between SRF and YY1 in the regulation of smooth-muscle promoters (Itoh et al., 2001; Strobeck et al., 2001).
Binding of SRF as a dimer produces only a weak transcriptional activation per se, and previous studies have shown that SRF can efficiently activate transcription only after association with a number of cofactors (Montaner et al., 1999; Belaguli et al., 2000; Gineitis and Treisman, 2001; Wang et al., 2001). In this model, SRF is viewed as an essential core element for the assembly of a multiprotein complex that may be unique to any given promoter. For example, activity of the CArG element in cardiac genes requires the cooperative interplay between SRF and a newly identified specific factor, myocardin (Wang et al., 2001). Here, we show that SRF is bound to MyoD-CArG-box associated with high-molecular-weight complexes. Members of the C/EBP family of transcription factors as well as the acetyltransferases CBP and P300 can interact with SRF and behave as “accessory proteins” in the modulation of the transcriptional activity of SRF by RhoA (Montaner et al., 1999). Because SRF-dependent regulation of myogenesis and MyoD implies an upstream control by the small GTPase RhoA (Carnac et al., 1998; Wei et al., 1998), we investigated whether CBP and C/EBP family members were interacting with SRF on the MyoDCArG-box. We show by supershift analysis that members of the C/EBP family and the acetyltransferase CBP are present with SRF in the complexes formed on the MyoD-CArG sequence, and we observed by coimmunoprecipitation that C/EBP interact with SRF in myoblasts nuclear extracts (unpublished data). In addition, using TSA to inhibit histone deacetylases, we found that the activity of DRR increased in a manner dependent on an intact CArG sequence, upon TSA treatment of stably transfected myoblasts. This result favors that CBP plays a functional role in the CArG-dependent activation of MyoD via the DRR and strongly supports that SRF binding to MyoD-CArG is accompanied by transcriptional activation. Together, these data show that SRF binding to MyoD-CArG element modulates the transcriptional activity of MyoD DRR enhancer in myoblasts. These results extend our previous observations that inactivation of SRF through microinjection of dominant negative forms of SRF proteins rapidly abolished MyoD expression (Gauthier-Rouvière et al., 1996) by showing that this effect is likely mediated by the direct binding of SRF-containing high-molecular-weight complexes to a CArG element present in MyoD gene.
Is the Low SRF Affinity MyoD-CArG-box Involved in the Muscle-specific Expression of MyoD Gene?
EMSA analysis showed that MyoDCArG-box binds SRF-containing complexes (B) with a weaker affinity when compared with the c-fos SRE or other muscle canonical CArG sequences. In addition, a single nucleotide mutation in the 3′ end of MyoDCArG (A to G, in csCArG) confirmed that the divergence in 3′ end of the decanucleotide is responsible for this lower affinity. This conclusion was reinforced and extended in living cells by microinjection experiments. Indeed, MyoD-csCArG oligonucleotides presenting a high affinity for SRF have a higher capacity to inhibit MyoD expression than wild-type muscle CArG oligonucleotide, which binds SRF with lower affinity. Previous studies suggested a correlation between low-affinity DNA binding of SRF and muscle activity. In comparison to the c-fos promoter, which contains a single high-affinity binding site for SRF, many muscle-specific genes including MLC1A (Catala et al., 1995), cardiac α-actin (Sartorelli et al., 1990), SM α-actin (Mack et al., 2000), SM-MHC (Itoh et al., 2001), and SM-22α, contain CArG elements that bind SRF with relatively lower affinity.
Replacement of the most proximal CArG-box in the skeletal α-actin promoter with the c-fos SRE resulted in constitutive expression in transfected nonmuscle cells (Santoro and Walsh, 1991). One possible explanation is that weaker CArG elements might offer an additional level of control through mechanisms that influence SRF binding: CArG-boxes with relatively high affinities for SRF are rapidly overloaded by low levels of SRF in a wide range of cell types, whereas muscle-specific CArG-boxes, which exhibit reduced affinity for SRF, can only bind SRF when it is present at higher levels, which appears to be the case for muscle cells (Belaguli et al., 1997). Another possible function of the divergence in 3′ of MyoD-wtCArG-box could be that the mutation G to A would create a new binding site for other transcription factors in addition to SRF. This or these factors could be muscle specific and thus bring tissue specificity to MyoD gene expression. In support of this hypothesis, Latinkic et al. (2002) have recently shown that differential affinities for SRF play an important role in sensing SRF levels in cardiac vs. skeletal muscle expression of cardiac actin. In the same line of evidence, Chang et al. (2001) have shown that multimerized CArG-box transgenes with different flanking sequences can direct distinct development-specific expression patterns during mouse embryogenesis. We show here that a single nucleotide change in MyoD-CArG drastically affected both its in vitro binding properties as well as the specific activity of a corresponding CArG-containing DRR enhancer in myoblasts and in vivo in regenerating muscle.
Together, our observations prove that the CArG element present in MyoD-DRR must play an essential role in the control of MyoD gene expression and represent to our knowledge the first demonstration of a functional transcriptional element in MyoD DRR enhancer sequence.
Acknowledgments
We thank Dr. S.J. Tapscott (Fred Hutchinson Cancer Research Center, Washington, DC) for MyoD promoter plasmid constructs. We are grateful to Celine Franckhauser for technical help with the FPLC, and Drs. M.C. Romey and Y. Vassetsky for helpful discussions. This work was supported by grants from Association Française contre les Myopathies (AFM; to A.F.), Association pour la Recherche contre le Cancer (ARC 4459 to N.J.L.), and a fellowship from the Ministère de l'Education Nationale, de l'Enseignement Supérieur et de la Recherche (AC) to A.L.
Article published online ahead of print. Mol. Biol. Cell 10.1091/mbc.E02-07-0451. Article and publication date are at www.molbiolcell.org/cgi/doi/10.1091/mbc.E02-07-0451.
References
- Asakura, A., Lyons, G.E., and Tapscott, S.J. (1995). The regulation of MyoD gene expression: conserved elements mediate expression in embryonic axial muscle. Dev. Biol. 171(2), 386–398. [DOI] [PubMed] [Google Scholar]
- Beauchamp, J.R., Heslop, L., Yu, D.S., Tajbakhsh, S., Kelly, R.G., Wernig, A., Buckingham, M.E., Partridge, T.A., Zammit, P.S. (2000). Expression of C.D34, and Myf5 defines the majority of quiescent adult skeletal muscle satellite cells. J. Cell. Biol. 151(6), 1221–1234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belaguli, N.S., Schildmeyer, L.S., and Schwartz, R.J. (1997). Organization and myogenic restricted expression of the murine serum response factor gene. A role for autoregulation. J. Biol. Chem. 272(29), 18222–18231. [DOI] [PubMed] [Google Scholar]
- Belaguli, N.S., Sepulveda, J.L., Nigam, V., Charron, F., Nemer, M., and Schwartz, R.J. (2000). Cardiac tissue enriched factors serum response factor, and GATA-4 are mutual coregulators. Mol. Cell. Biol. 20(20), 7550–7558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braun, T., Rudnicki, M.A., Arnold, H.H., and Jaenisch, R. (1992). Targeted inactivation of the muscle regulatory gene Myf-5 results in abnormal rib development and perinatal death. Cell 71(3), 369–382. [DOI] [PubMed] [Google Scholar]
- Braun, T., and Arnold, H.H. (1995). Inactivation of Myf-6 and Myf-5 genes in mice leads to alterations in skeletal muscle development. EMBO J. 14(6), 1176–1186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brennan, T.J., Edmondson, D.G., and Olson, E.N. (1990). Aberrant regulation of MyoD1 contributes to the partially defective myogenic phenotype of BC3H1 cells. J. Cell Biol. 110(4), 929–937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carnac, G., Primig, M., Kitzmann, M., Chafey, P., Tuil, D., Lamb, N., and Fernandez, A. (1998). RhoA GTPase and serum response factor control selectively the expression of MyoD without affecting Myf5 in mouse myoblasts. Mol. Biol. Cell 9(7), 1891–1902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Catala, F., Wanner, R., Barton, P., Cohen, A., Wright, W., and Buckingham, M. (1995). A skeletal muscle-specific enhancer regulated by factors binding to E and CArG boxes is present in the promoter of the mouse myosin light-chain 1A gene. Mol. Cell. Biol. 15(8), 4585–4596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang, P.S., Li, L., McAnally, J., and Olson, E.N. (2001). Muscle specificity encoded by specific serum response factor-binding sites. J. Biol. Chem. 276(20), 17206–17212. [DOI] [PubMed] [Google Scholar]
- Chen, J.C., and Goldhamer, D.J. (1999). Transcriptional mechanisms regulating MyoD expression in the mouse. Cell Tissue Res. 296(1), 213–219. [DOI] [PubMed] [Google Scholar]
- Chen, J.C., Love, C.M., and Goldhamer, D.J. (2001). Two upstream enhancers collaborate to regulate the spatial patterning, and timing of MyoD transcription during mouse development. Dev. Dyn. 221(3), 274–288. [DOI] [PubMed] [Google Scholar]
- Chen, J.C., Ramachandran, R., and Goldhamer, D.J. (2002). Essential, and redundant functions of the MyoD distal regulatory region revealed by targeted mutagenesis. Dev. Biol. 245(1), 213–223. [DOI] [PubMed] [Google Scholar]
- Davis, R.L., Weintraub, H., and Lassar, A.B. (1987). Expression of a single transfected cDNA converts fibroblasts to myoblasts. Cell 51(6), 987–1000. [DOI] [PubMed] [Google Scholar]
- Dignam, J.D., Lebovitz, R.M., and Roeder, R.G. (1983). Accurate transcription initiation by RNA polymerase II in a soluble extract from isolated mammalian nuclei. Nucleic Acids Res. 11(5), 1475–1489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faerman, A., Goldhamer, D.J., Puzis, R., Emerson, C.P., Jr., and Shani, M. (1995). The distal human myoD enhancer sequences direct unique muscle-specific patterns of lacZ expression during mouse development. Dev. Biol. 171(1), 27–38. [DOI] [PubMed] [Google Scholar]
- Gauthier-Rouviere, C., Vandromme, M., Tuil, D., Lautredou, N., Morris, M., Soulez, M., Kahn, A., Fernandez, A., and Lamb, N. (1996). Expression and activity of serum response factor is required for expression of the muscle-determining factor MyoD in both dividing and differentiating mouse C2C12 myoblasts. Mol. Biol. Cell 7(5), 719–729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gineitis, D., and Treisman, R. (2001). Differential usage of signal transduction pathways defines two types of serum response factor target gene. J. Biol. Chem. 276(27), 24531–24539. [DOI] [PubMed] [Google Scholar]
- Goldhamer, D.J., Brunk, B.P., Faerman, A., King, A., Shani, M., and Emerson, C.P., Jr. (1995). Embryonic activation of the myoD gene is regulated by a highly conserved distal control element. Development 121(3), 637–649. [DOI] [PubMed] [Google Scholar]
- Harris, J.B., and Montgomery, A. (1975). Some mechanical and electrical properties of distal hind limb muscles of genetically dystrophic mice (C57BL/6Jdy2j/dy2j). Exp. Neurol. 48(3 Pt 1), 569–58. [DOI] [PubMed] [Google Scholar]
- Hasty, P., Bradley, A., Morris, J.H., Edmondson, D.G., Venuti, J.M., Olson, E.N., and Klein, W.H. (1993). Muscle deficiency and neonatal death in mice with a targeted mutation in the myogenin gene. Nature 364(6437), 501–506. [DOI] [PubMed] [Google Scholar]
- Hughes, S.M., Taylor, J.M., Tapscott, S.J., Gurley, C.M., Carter, W.J., and Peterson, C.A. (1993). Selective accumulation of MyoD and myogenin mRNAs in fast and slow adult skeletal muscle is controlled by innervation and hormones. Development 118(4), 1137–1147. [DOI] [PubMed] [Google Scholar]
- Itoh, S., Katoh, Y., Konishi, H., Takaya, N., Kimura, T., Periasamy, M., and Yamaguchi, H. (2001). Nitric oxide regulates smooth-muscle-specific myosin heavy chain gene expression at the transcriptional level-possible role of SRF, and YY1 through CArG element. J. Mol. Cell. Cardiol. 33(1), 95–107. [DOI] [PubMed] [Google Scholar]
- Kablar, B., Krastel, K., Ying, C., Asakura, A., Tapscott, S.J., and Rudnicki, M.A. (1997). MyoD and Myf-5 differentially regulate the development of limb versus trunk skeletal muscle. Development 124(23), 4729–4738. [DOI] [PubMed] [Google Scholar]
- Kablar, B., Asakura, A., Krastel, K., Ying, C., May, L.L., Goldhamer, D.J., and Rudnicki, M.A. (1998). MyoD and Myf-5 define the specification of musculature of distinct embryonic origin. Biochem. Cell. Biol. 76(6), 1079–1091. [PubMed] [Google Scholar]
- Kitzmann, M., Carnac, G., Vandromme, M., Primig, M., Lamb, N.J., and Fernandez, A. (1998). The muscle regulatory factors MyoD and myf-5 undergo distinct cell cycle-specific expression in muscle cells. J. Cell Biol. 142(6), 1447–1459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitzmann, M., and Fernandez, A. (2001). Crosstalk between cell cycle regulators, and the myogenic factor MyoD in skeletal myoblasts. Cell. Mol. Life Sci. 58(4), 571–579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kucharczuk, K.L., Love, C.M., Dougherty, N.M., and Goldhamer, D.J. (1999). Fine-scale transgenic mapping of the MyoD core enhancer: MyoD is regulated by distinct but overlapping mechanisms in myotomal and non-myotomal muscle lineages. Development 126(9), 1957–1965. [DOI] [PubMed] [Google Scholar]
- Lamb, N.J.C., Gauthier-Rouviere, C., and Fernandez, A. (1996). Microinjection strategies for the study of mitogenic signaling in mammalian cells. Front. Biosci. 1, d19–d29. [DOI] [PubMed] [Google Scholar]
- Latinkic, B.V., Cooper, B., Towers, N., Sparrow, D., Kotecha, S., and Mohun, T.J. (2002). Distinct enhancers regulate skeletal, and cardiac muscle-specific expression programs of the cardiac α-actin gene in xenopus embryons. Dev. Biol. 245, 57–70. [DOI] [PubMed] [Google Scholar]
- Lefaucheur, J.P., and Sebille, A. (1995). The cellular events of injured muscle regeneration depend on the nature of the injury. Neuromuscul. Disord. 5(6), 501–509. [DOI] [PubMed] [Google Scholar]
- Mack, C.P., Thompson, M.M., Lawrenz-Smith, S., and Owens, G.K. (2000). Smooth muscle alpha-actin CArG elements coordinate formation of a smooth muscle cell-selective, serum response factor-containing activation complex. Circ. Res. 86(2), 221–232. [DOI] [PubMed] [Google Scholar]
- Marais, R.M., Hsuan, J.J., McGuigan, C., Wynne, J., and Treisman, R. (1992). Casein kinase II phosphorylation increases the rate of serum response factor-binding site exchange. EMBO J. 11(1), 97–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Megeney, L.A., Kablar, B., Garrett, K., Anderson, J.E., and Rudnicki, M.A. (1996). MyoD is required for myogenic stem cell function in adult skeletal muscle. Genes Dev. 10(10), 1173–1183. [DOI] [PubMed] [Google Scholar]
- Montaner, S., Perona, R., Saniger, L., and Lacal, J.C. (1999). Activation of serum response factor by RhoA is mediated by the nuclear factor-kappaB and C/EBP transcription factors. J. Biol. Chem. 274(13), 8506–8515. [DOI] [PubMed] [Google Scholar]
- Nabeshima, Y., Hanaoka, K., Hayasaka, M., Esumi, E., Li, S., Nonaka, I., and Nabeshima, Y. (1993). Myogenin gene disruption results in perinatal lethality because of severe muscle defect. Nature 364(6437), 532–535. [DOI] [PubMed] [Google Scholar]
- Olson, E. et al. (1990). Myogenin is in an evolutionarily conserved linkage group on human chromosome 1q31–q41 and unlinked to other mapped muscle regulatory factor genes. Genomics 3, 427–434. [DOI] [PubMed] [Google Scholar]
- Patapoutian, A., Yoon, J.K., Miner, J.H., Wang, S., Stark, K., and Wold, B. (1995). Disruption of the mouse MRF4 gene identifies multiple waves of myogenesis in the myotome. Development 121(10), 3347–3358. [DOI] [PubMed] [Google Scholar]
- Pinset, C., Montarras, D., Chenevert, J., Minty, A., Barton, P., Laurent, C., and Gros, F. (1988). Control of myogenesis in the mouse myogenic C2 cell line by medium composition and by insulin: characterization of permissive and inducible C2 myoblasts. Differentiation 38(1), 28–34. [DOI] [PubMed] [Google Scholar]
- Qiu, P., and Li, L. (2002). Histone acetylation, and recruitment of serum responsive factor, and CREB-binding protein onto SM22 promoter during SM22 gene expression. Circ. Res. 90(8), 858–865. [DOI] [PubMed] [Google Scholar]
- Ramirez, S., Ait-Si-Ali, S., Robin, P., Trouche, D., and Harel-Bellan, A. (1997). The CREB-binding protein (CBP) cooperates with the serum response factor for transactivation of the c-fos serum response element. J. Biol. Chem. 272(49), 31016–31021. [DOI] [PubMed] [Google Scholar]
- Rawls, A., Valdez, M.R., Zhang, W., Richardson, J., Klein, W.H., and Olson, E.N. (1998). Overlapping functions of the myogenic bHLH genes MRF4 and MyoD revealed in double mutant mice. Development 125(13), 2349–2358. [DOI] [PubMed] [Google Scholar]
- Rudnicki, M.A., Braun, T., Hinuma, S., and Jaenisch, R. (1992). Inactivation of MyoD in mice leads to up-regulation of the myogenic HLH gene Myf-5 and results in apparently normal muscle development. Cell 71(3), 383–390. [DOI] [PubMed] [Google Scholar]
- Rudnicki, M.A., Schnegelsberg, P.N., Stead, R.H., Braun, T., Arnold, H.H., and Jaenisch, R. (1993). MyoD or Myf-5 is required for the formation of skeletal muscle. Cell 75(7), 1351–1359. [DOI] [PubMed] [Google Scholar]
- Santoro, I.M., and Walsh, K. (1991). Natural and synthetic DNA elements with the CArG motif differ in expression and proteinbinding properties. Mol. Cell. Biol. 11(12), 6296–6305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sartorelli, V., Webster, K.A., and Kedes, L. (1990). Muscle-specific expression of the cardiac alpha-actin gene requires MyoD1, CArG-box binding factor, and Sp1. Genes Dev. 4(10), 1811–1822. [DOI] [PubMed] [Google Scholar]
- Shore, P., and Sharrocks, A.D. (1995). The MADS-box family of transcription factors. Eur. J. Biochem. 229(1), 1–13. [DOI] [PubMed] [Google Scholar]
- Sotiropoulos, A., Gineitis, D., Copeland, J., and Treisman, R. (1999). Signal-regulated activation of serum response factor is mediated by changes in actin dynamics. Cell 98, 159–169. [DOI] [PubMed] [Google Scholar]
- Soulez, M., Rouviere, C.G., Chafey, P., Hentzen, D., Vandromme, M., Lautredou, N., Lamb, N., Kahn, A., and Tuil, D. (1996). Growth and differentiation of C2 myogenic cells are dependent on serum response factor. Mol. Cell. Biol. 16(11), 6065–6074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strobeck, M., Kim. S., Zhang. J.C., Clendenin, C., Du, K.L., and Parmacek, M.S. (2001). Binding of serum response factor to CArG box sequences is necessary but not sufficient to restrict gene expression to arterial smooth muscle cells. J. Biol. Chem. 276(19), 16418–16424. [DOI] [PubMed] [Google Scholar]
- Tajbakhsh, S., and Cossu, G. (1997). Establishing myogenic identity during somitogenesis. Curr. Opin. Genet. Dev. 7(5), 634–641. [DOI] [PubMed] [Google Scholar]
- Tapscott, S.J., Lassar, A.B., and Weintraub, H. (1992). A novel myoblast enhancer element mediates MyoD transcription. Mol. Cell. Biol. 12(11), 4994–5003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Treisman, R. (1992). The serum response element. Trends Biochem. Sci. 17, 423–426. [DOI] [PubMed] [Google Scholar]
- Treisman, R. (1995). Journey to the surface of the cell: Fos regulation and the SRE. EMBO J. 14, 4905–4913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Treisman, R., Alberts, A.S., and Sakai, E. (1998). Regulation of SRF activity by Rho family GTPases. Cold Spring Harb. Symp. Quant. Biol. 63, 643–651. [DOI] [PubMed] [Google Scholar]
- Vandromme, M., Gauthier-Rouviere, C., Carnac, G., Lamb, N., and Fernandez, A. (1992). Serum response factor p67SRF is expressed and required during myogenic differentiation of both mouse C2 and rat L6 muscle cell lines. J. Cell Biol. 118(6), 1489–1500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, D., Chang, P.S., Wang, Z., Sutherland, L., Richardson, J.A., Small, E., Krieg, P.A., and Olson, E.N. (2001). Activation of cardiac gene expression by myocardin, a transcriptional cofactor for serum response factor. Cell 105(7), 851–862. [DOI] [PubMed] [Google Scholar]
- Wei, L., Zhou, W., Croissant, J.D., Johansen, F.E., Prywes, R., Balasubramanyam, A., and Schwartz, R.J. (1998). RhoA signaling via serum response factor plays an obligatory role in myogenic differentiation. J. Biol. Chem. 273(46), 30287–30294. [DOI] [PubMed] [Google Scholar]
- Weintraub, H. et al. (1991). The myoD gene family: nodal point during specification of the muscle cell lineage. Science 251(4995), 761–766. [DOI] [PubMed] [Google Scholar]