Abstract
Arthrobacter sp. strain PBA metabolized phenylboronic acid to phenol. The oxygen atom in phenol was shown to be derived from the atmosphere using 18O2. 1-Naphthalene-, 2-naphthalene-, 3-cyanophenyl-, 2,5-fluorophenyl-, and 3-thiophene-boronic acids were also transformed to monooxygenated products. The oxygen atom in the product was bonded to the ring carbon atom originally bearing the boronic acid substituent with all the substrates tested.
The bacterial metabolism of organoboron compounds has been poorly studied despite the knowledge that boron is required for proper biological function in microbes and plants. Boron-containing salts have long been included in bacterial growth media (29). More recently, boron has been found as a component of certain antibiotics, for example, boromycin produced by Streptomyces species (10) and the tartrolons produced by Sorangium cellulosum (14). Cyanobacteria require boron for proper formation of nitrogen-fixing heterocysts (3, 21). A molecule that mediates quorum sensing in bacteria has been isolated and shown to contain boron in a furanosyl borate diester (8). Boron has long been known to be required for the healthy growth of plants (31). Plants deficient in boron have brittle tissues, and plants grown with excess boron have highly flexible tissues (2). Recently, boron has been shown to exist as borate esters that cross-link the pectin polysaccharides in the plant cell wall (23).
While boron esters are found in nature, organic chemists typically use boron in alkyl and phenyl boranes, compounds containing direct carbon-to-boron bonds (20). Intermediate between natural-product boron compounds and the boranes are the boronic acids. Boronic acids are used in organic syntheses (5), as specific enzyme inhibitors (22), to immobilize proteins in biotechnology (30), and to sterilize insect pests (28). In the present study, phenylboronic acid was used as an enrichment substrate to identify potential bacteria that could metabolize the compound. The strategy was designed to yield bacteria capable of cleaving the C-B bond of arylboronic acids. An Arthrobacter nicotinovorans strain was obtained and shown to produce phenol in which the phenolic oxygen atom was derived from atmospheric dioxygen. Other aromatic boronic acid compounds were also oxidized to phenols. The data are consistent with an oxygenase-mediated mechanism without the occurrence of an NIH shift of the oxygen substitution.
All chemicals were purchased from Sigma-Aldrich (Milwaukee, Wis.). H218O and 18O2 were purchased from Icon Isotopes (Summit, N.J.). Enrichment studies used a minimal medium (29) containing 5 mM phenylboronic acid as the sole carbon source, added after sterilization. Fifty milliliters of the medium in a 125-ml flask was inoculated with 1 g of soil that had been previously washed with 50 mM phosphate buffer, pH 7.2. Microorganisms were cultured at 30°C in a rotary shaker at 200 rpm. After 4 days of incubation, 1:100 dilutions were made into fresh medium. Several transfers were made in this way.
Strain identification.
Two morphologically different isolates were obtained; one was characterized here. The chosen strain formed smooth colonies that turned bright yellow after several days on solid medium. The bacterium stained gram positive. Cells showed a V-shaped morphology and fragmented into spherical structures in older cultures. BiOLOG (Haywood, Calif.) test results were consistent with the assignment of the organism to the genus Arthrobacter. A 1,444-bp DNA fragment corresponding to the 16S rRNA gene was amplified by PCR with universal primers as described previously (18). The amplified 16S rRNA sequence had the strongest match, 99.5% identity, to a 16S sequence in GenBank for A. nicotinovorans (accession no. X80743). The strain isolated in this study was denoted A. nicotinovorans strain PBA.
Initial product from phenylboronic acid.
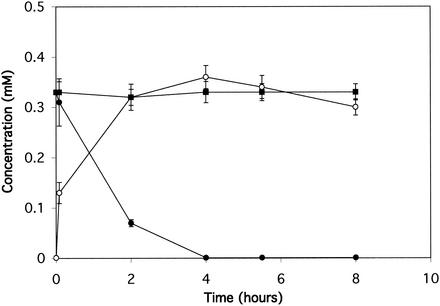
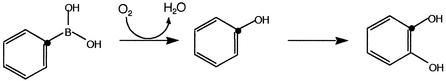
A. nicotinovorans strain PBA was grown with phenylboronic acid in the medium, harvested in exponential phase, resuspended to an optical density of 5.0 at 600 nm, and incubated with 0.35 mM phenylboronic acid. At fixed time points, the medium was analyzed for substrate and product(s) by high-pressure liquid chromatography (HPLC) using a Hewlett-Packard (San Fernando, Calif.) HP 1100 system equipped with a photodiode array detector interfaced to an HP ChemStation and fitted with an Adsorbosphere 5-μm-particle-size C18 column with dimensions of 250 by 4.6 mm (Alltech, Deerfield, Ill.). Acetonitrile was used as the mobile phase at a flow rate of 1 ml/min. The program used for separation was 5 min at 5% acetonitrile, increasing thereafter to 100% in a linear 20-min gradient. The results are shown in Fig. 1. Phenol is proposed to be the first intermediate formed during the catabolism of phenylboronic acid by A. nicotinovorans (Fig. 2). Phenylboronic acid was consumed within 4 h. The first detectable product of phenylboronic acid metabolism comigrated with standard phenol by HPLC. The identity of the product was confirmed by comigration on thin-layer chromatography, UV spectroscopy, mass spectrometry (MS), and treatment with Gibbs reagent, which gave a blue product on reaction with phenol (Table 1). Phenol was observed to form stoichiometrically and then decrease to undetectable levels (data not shown). Cells grown in rich medium also metabolized phenylboronic acid and produced phenol without a discernible lag, suggesting that the activity is not inducible.
FIG. 1.
Phenylboronic acid oxidation by resting cells of A. nicotinovorans strain PBA. •, phenylboronic acid after incubation with cells; ○, phenol in cell culture filtrates; ▪, phenylboronic acid incubated without cells in parallel cultures.
FIG. 2.
Proposed metabolic pathway for catabolism of phenylboronic acid by A. nicotinovorans strain PBA. Dots on the rings indicate conservation of position in substituents.
TABLE 1.
Substrate specificity and product identification
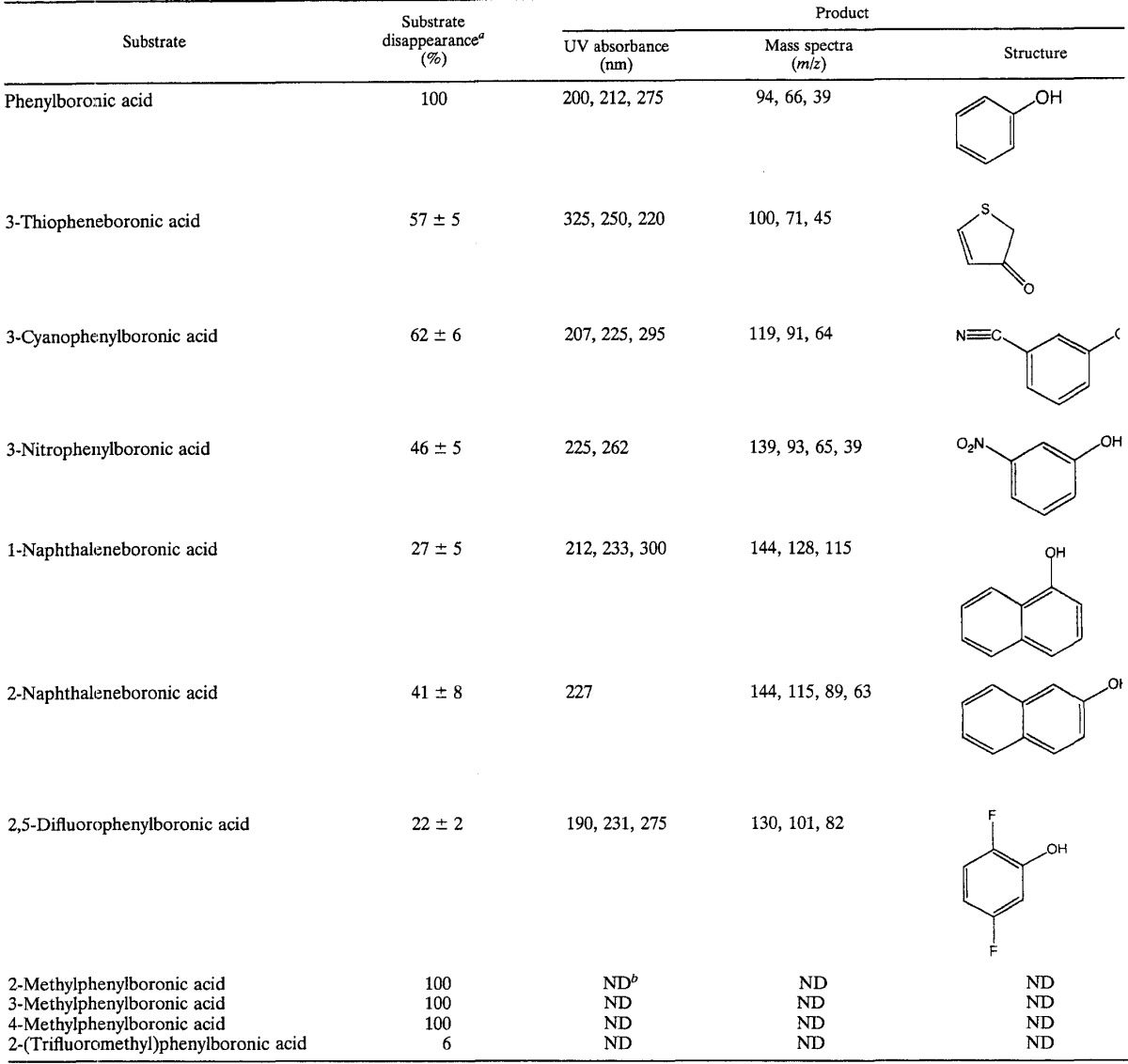
All substrates were added at 0.3 mM initial concentration.
ND, not determined.
Source of oxygen in phenol.
To obtain insight into the enzyme mechanism, the source of oxygen in the phenol intermediate was determined. The oxygen atom could come from water or from atmospheric dioxygen. This was tested in separate experiments using cell incubation mixtures containing phenylboronic acid and either H218O or 18O2. Harvested cells were resuspended in 50 mM phosphate buffer, pH 7.2, to a final optical density at 600 nm of 5. For the H218O experiment, the buffer contained 32% H218O. For the 18O2 experiments, the same cell and buffer solution was incubated in a 10-ml septum-sealed vial. The cell suspension was flushed with nitrogen gas and replaced with 18O2. Phenol was extracted during the time in which it appeared stoichiometrically in the culture medium (4 h) by acidifying the medium to pH 2.0 with phosphoric acid, adding 20% (wt/vol) NaCl, extracting twice with an equal volume of ethyl acetate, and drying the extract with anhydrous sodium sulfate. Extracts were concentrated to 100 μl and analyzed by gas chromatography-MS (GC/MS) on a Varian (Walnut Creek, Calif.) Saturn GC/MS fitted with a 30-m DB-5 MS column. Authentic phenol containing the naturally occurring oxygen isotope 16O showed a parent ion of 94 atomic mass units. When cells were incubated with phenylboronic acid and [18O]water, the phenol product also showed a parent ion of 94 atomic mass units with no discernible peak at 96 atomic mass units. When cells were incubated with phenylboronic acid and [18O]dioxygen in the gas phase, the parent ion of the product was increased by 2 atomic mass units, consistent with the incorporation of one atom of 18O into the biological phenol product.
Alternative substrates.
The substrate specificity for aromatic boronic acid oxygenation was tested by using the same conditions previously described for phenylboronic acid and an incubation time of 26 h (Table 1). The products of the reaction were confirmed by UV spectroscopy and MS. All compounds tested were substrates except for 2-(trifluoromethyl)phenylboronic acid, which showed no disappearance within the limits of experimental error. Phenylboronic acid and the isomeric methylphenylboronic acids were completely consumed. 3-Substituted boronic acids containing nitro or cyano groups were also substrates, as well as 2,5-difluorophenylboronic acid, but they were incompletely consumed during the 26-h incubation. The bicyclic aromatic compounds 1- and 2-naphthaleneboronic acid were also incompletely oxidized. With 3-thiopheneboronic acid, the reaction mixtures turned yellow. This is consistent with the initial production of the 3-hydroxy-substituted product followed by partial equilibrium isomerization to the conjugated keto isomer shown in Table 1 (6, 7, 13).
Positions of oxygen substitution in the products.
In some oxygenase-catalyzed reactions, the position of the oxygen atom in the product can differ from the position of the substituent it replaces via a mechanism known as the NIH shift (12, 17, 26). Mass spectral analysis of the product would not readily reveal this. In this context, HPLC experiments were conducted with starting substrates in which all three isomeric phenol products could be cleanly separated by HPLC and the positional isomerism could be further discerned by UV-visible spectroscopy. A C18 column was used to analyze the products from 1- and 2-naphthaleneboronic acid. The phenolic products from 3-cyanophenylboronic acid and 3-nitrophenylboronic acid were analyzed with a polyethylene glycol 5-μm-particle-size column, 250 by 4.6 mm, from Supelco (Bellefonte, Pa.). The product obtained with 3-nitrophenylboronic acid had the same retention time as standard 3-nitrophenol, and there was no evidence for the formation of minor 2- and 4-nitrophenol isomers. UV-visible spectroscopy confirmed the identification of the product as 3-nitrophenol. Similarly, 1-naphthaleneboronic acid gave rise to 1-naphthol and 2-naphthaleneboronic acid gave rise to only 2-naphthol. 3-Cyanophenylboronic acid yielded only 3-cyanophenol. These data show that the ring position bearing substituents before and after the reaction is conserved (Fig. 2).
Evidence against a nonenzymatic mechanism of arylboronic acid oxidation.
Arthrobacter strains are known to produce a number of oxidases that generate hydrogen peroxide (1, 9, 15), and the chemical reaction of hydrogen peroxide with arylboronic acids to produce phenols is well known (32, 33). In this context, experiments were conducted to ascertain whether or not biologically generated hydrogen peroxide might be reacting directly with arylboronic acids. First, cells of A. nicotinovorans PBA were incubated with 3-thiopheneboronic acid in the presence of 1 to 10 U (micromoles per minute) of bovine liver catalase (Sigma, St. Louis, Mo.) activity. Hydrogen peroxide is substantially permeable across bacterial membranes and thus could diffuse into the medium to react with arylboronic acid substrates (27). However, the oxidation of 3-thiopheneboronic acid to 3-hydroxythiophene by intact cells was not inhibited. Next, cell extracts were prepared from A. nicotinovorans PBA cells grown on phenylboronic acid. The catalase activity of the cell extract was measured as described previously (25) and found to be 10 U per mg of protein. Ten units of catalase was shown to strongly inhibit the in vitro chemical reaction of hydrogen peroxide and 3-thiopheneboronic acid at concentrations of hydrogen peroxide that completely consume the substrate within 2 h. These data indicate that freely diffusible hydrogen peroxide is very unlikely to be responsible for the arylboronic acid oxidation reactions observed with A. nicotinovorans strain PBA. However, efforts to measure cell-free oxygenase activity in protein extracts from A. nicotinovorans, with 3-thiopheneboronic acid as the substrate and NADH or NADPH as reductants, were unsuccessful. In total, these data mitigate against a reaction of free hydrogen peroxide with arylboronic acids and are consistent with a direct enzymatic mechanism of biological arylboronic acid oxidation.
Metabolism of phenol.
To identify potential further metabolites of phenylboronic acid oxidation, phenol was incubated with A. nicotinovorans strain PBA. Culture supernatants were extracted and derivatized with N,O-bis-trismethylsilyl trifluoroacetamide, and the resulting material was subjected to GC/MS. A compound with a GC retention time of 12.6 min was observed. This was the same as the retention time of authentic catechol that was derivatized in the same manner. MS was also consistent with an identification of the product as a silylated catechol (m/z, 254, 239, 151, 73, 45). The oxidation of phenol to catechol (Fig. 2) has many biological precedents (16, 24).
Conclusions.
The present study begins to define organoboronic acid metabolism, a previously little-studied aspect of microbial metabolism. The success of the enrichments, yielding more than one pure culture from the initial soil surveyed, suggests that this metabolic capability is not rare. The observations that the first detectable product is phenol and that the origin of the oxygen atom is diatomic oxygen indicate why bacteria with the targeted metabolic activity were readily obtained. Oxygenases are widespread in soil microorganisms and are widely known to displace different substituents from alicyclic and aromatic rings to generate hydroxylated intermediates (4, 11, 34; L. B. M. Ellis and L. P. Wackett, The University of Minnesota Biocatalysis/Biodegradation Database, http://umbbd.ahc.umn.edu/, 22 December 2002). For example, aromatic rings containing fluorine, chlorine, bromine, cyano, nitro, amino, sulfonate, and carboxylate substituents are oxygenated to produce hydroxylated products with displacement of the original substituent (12, 17, 34).
There is precedent for biological oxygenation of the nonaromatic compound 2-methylcyclohexylboronic acid (19). The flavoprotein monooxygenase cyclohexanone monooxygenase displaced a boronic acid substituent from a chiral carbon to yield a product alcohol with same absolute configuration as the substrate. These data were interpreted as being consistent with oxygen attack at the boron atom and rearrangement of the intermediate to produce a boronate ester, which subsequently underwent hydrolysis to the alcohol. Currently, studies are being conducted to identify the gene(s) involved in microbial phenylboronic acid metabolism to further define the enzyme catalyzing the transformation.
Acknowledgments
We thank Gil Johnson for help with GC/MS analysis, Jack Richman for help with NMR, and Jeff Osborne and Charlotte Rosendahl for helpful discussions.
This work was supported under grant ER63268-1018305-0007173 from the Office of Science's Office of Biological and Environmental Research, U.S. Department of Energy.
REFERENCES
- 1.Basran, J., N. Bhanji, A. Basran, D. Nietlispach, S. Mistry, R. Meskys, and N. S. Scrutton. 2002. Mechanistic aspects of the covalent flavoprotein dimethylglycine oxidase of Arthrobacter globiformis studied by stopped-flow spectrophotometry. Biochemistry 41:4733-4743. [DOI] [PubMed] [Google Scholar]
- 2.Blevins, D., and K. Lukaszewski. 1994. Proposed physiologic functions of boron in plants pertinent to animal and human metabolism. Environ. Health Perspect. 7(Suppl.):31-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bonilla, I., M. Garcia-Gonzalez, and P. Mateo. 1990. Boron requirement in cyanobacteria. Plant Physiol. 94:1554-1560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Branchard, B. P., and C. T. Walsh. 1985. Functional group diversity in enzymatic oxygenation reactions catalyzed by bacterial flavin-containing cyclohexanone monooxygenase. J. Am. Chem. Soc. 107:2153-2161. [Google Scholar]
- 5.Brown, H. C., P. K. Jadhav, and C. Disai. 1982. Direct chiral synthesis of boronic acids and esters of high optical purity via asymmetric hydroboration displacement. J. Am. Chem. Soc. 104:4303-4304. [Google Scholar]
- 6.Camici, L., A. Ricci, and M. Taddei. 1986. Heterocyclic silyl enol ether chemistry: synthesis and reactivity of trimethylsiloxyfuran and 3-trimethylsiloxythiophene. Tetrahedron Lett. 27:5155-5158. [Google Scholar]
- 7.Capon, B., and F.-C. Kwok. 1986. The tautomerism of hydroxy derivatives of five-membered oxygen, nitrogen and sulfur heterocycles. Tetrahedron Lett. 27:3275-3278. [Google Scholar]
- 8.Chen, X., S. Schauder, N. Potier, A. Van Dorsselaer, I. Pelczer, B. Bassler, and F. Hughson,. 2002. Structural identification of a bacterial quorum-sensing signal containing boron. Nature 415:545-549. [DOI] [PubMed] [Google Scholar]
- 9.Choi, Y. H., R. Matsuzaki, T. Fukui, E. Shimizu, T. Yorifuji, H. Sato, Y. Ozaki, and K. Tanizawa. 1995. Copper/topa quinone-containing histamine oxidase from Arthrobacter globiformis. Molecular cloning and sequencing, overproduction of precursor enzyme, and generation of topa quinone cofactor. J. Biol. Chem. 270:4712-4720. [DOI] [PubMed] [Google Scholar]
- 10.Dunitz, J., D. Hawley, D. Miklos, D. White, Y. Berlin, R. Marusic, and V. Prelog. 1971. Structure of boromycin. Helv. Chim. Acta 54:1709-1713. [DOI] [PubMed] [Google Scholar]
- 11.Harayama, S., M. Kok, and E. Neidle. 1992. Functional and evolutionary relationships amongst diverse oxygenases. Annu. Rev. Microbiol. 46:565-601. [DOI] [PubMed] [Google Scholar]
- 12.Hillas, P., and P. Fitzpatrick. 1996. A mechanism for hydroxylation by tyrosine hydroxylase based on partitioning of substituted phenylalanines. Biochemistry 35:6969-6975. [DOI] [PubMed] [Google Scholar]
- 13.Hunter, G. A., and H. McNab. 1990. Simple 3-hydroxythiophenes [thiophen-3(2H)-ones]. J. Chem. Soc. Chem. Commun. 1990:375-376.
- 14.Irschik, H., D. Schummer, K. Gerth, G. Hofle, and H. Reichenbach. 1995. The tartrolons, new boron-containing antibiotics from a myxobacterium, Sorangium cellulosum. J. Antibiot. (Tokyo) 48:26-30. [DOI] [PubMed] [Google Scholar]
- 15.Juda, G. A., J. A. Bollinger, and D. M. Dooley. 2001. Construction, overexpression, and purification of Arthrobacter globiformis amine oxidase-Strep-tag II fusion protein. Protein Expr. Purif. 22:455-461. [DOI] [PubMed] [Google Scholar]
- 16.Kasak, L., R. Horak, A. Nurk, K. Talvik, and M. Kivisaar. 1993. Regulation of the catechol 1,2-dioxygenase- and phenol monooxygenase-encoding pheBA operon in Pseudomonas putida PaW85. J. Bacteriol. 175:8038-8042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Koerts, J., A. Soffers, J. Vervoort, A. De Jager, and I. Rietjens. 1998. Occurrence of the NIH shift upon the cytochrome P450-catalyzed in vivo and in vitro aromatic ring hydroxylation of fluorobenzenes. Chem. Res. Toxicol. 11:503-512. [DOI] [PubMed] [Google Scholar]
- 18.Lane, D. 1991. Nucleic acid techniques in bacterial systematics, p. 115-175. John Wiley & Sons, Ltd., New York, N.Y.
- 19.Latham, J., and C. Walsh. 1986. Retention of configuration in oxidation of a chiral boronic acid by the flavoenzyme cyclohexanone oxygenase. J. Chem. Soc. Chem. Commun. 1986:527-528. [Google Scholar]
- 20.March, J. 1997. Advanced organic chemistry. Reactions, mechanisms, and structure, 2nd ed., p. 17-22. McGraw-Hill, Inc., New York, N.Y.
- 21.Mateo, P., I. Bonilla, E. Fernandez-Valiente, and E. Sanchez-Maeso. 1986. Essentiality of boron for dinitrogen fixation in Anabaena sp. PCC 7119. Plant Physiol. 81:430-433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Matteson, D. S., and K. M. Sadler. 1981. (R)-1-acetamide-2-phenylethaneboronic acid. A specific transition-state inhibitor for chymotrypsin. J. Am. Chem. Soc. 103:5241-5242. [Google Scholar]
- 23.O'Neill, M., S. Eberhard, P. Albersheim, and A. Darvill. 2001. Requirement of borate cross-linking of cell wall rhamnogalacturonan II for Arabidopsis growth. Science 294:846-849. [DOI] [PubMed] [Google Scholar]
- 24.Powlowski, J., and V. Shingler. 1994. Genetics and biochemistry of phenol degradation by Pseudomonas sp. CF600. Biodegradation 5:219-236. [DOI] [PubMed] [Google Scholar]
- 25.Ramana, K. V., and K. K. Kohli. 1999. Effect of coadministration of antituberculous drugs on the hepatic drug metabolizing enzymes and oxidative stress in the mouse. Indian J. Pharmacol. 31:299-305. [Google Scholar]
- 26.Sakurai, H., E. Hatayama, K. Fujitani, and H. Kato. 1982. Occurrence of aromatic methyl migration (NIH-shift) during oxidation of p-methylanisole by hemin-thioester complex as a cytochrome P-450 model. Biochem. Biophys. Res. Commun. 108:1649-1654. [DOI] [PubMed] [Google Scholar]
- 27.Seaver, L. C., and J. A. Imlay. 2001. Hydrogen peroxide fluxes and compartmentalization inside growing Escherichia coli. J. Bacteriol. 183:7182-7189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Settepani, J. A., J. B. Stokes, and A. B. Borkovec. 1970. Insect chemosterilants. 8. Boron compounds. J. Med. Chem. 13:128-131. [DOI] [PubMed] [Google Scholar]
- 29.Stanier, R. Y., N. J. Palleroni, and M. Duodoroff. 1966. The aerobic pseudomonads: a taxonomic study. J. Gen. Microbiol. 43:159-271. [DOI] [PubMed] [Google Scholar]
- 30.Stolowitz, M. L., C. Ahlem, K. A. Hughes, R. J. Kaiser, E. A. Kesicki, G. Li, K. P. Lund, S. M. Torkelson, and J. P. Wiley. 2001. Phenylboronic acid-salicylhydroxamic acid bioconjugates. 1. A novel boronic acid complex for protein immobilization. Bioconjug. Chem. 12:229-239. [DOI] [PubMed] [Google Scholar]
- 31.Thatcher, R. 1934. A proposed classification of the chemical elements with respect to their function in plant nutrition. Science 79:463-466. [DOI] [PubMed] [Google Scholar]
- 32.Ueda, M., A. Saitoh, and N. Miyaura. 2002. Asymmetric hydrogenation of 1-phenylethenylboronic acid and esters for the synthesis of chiral organoboron compounds. J. Organometallic Chem. 642:145-147. [Google Scholar]
- 33.Webb, K., and D. Levy. 1995. A facile oxidation of boronic acids and boronic esters. Tetrahedron Lett. 36:5117-5118. [Google Scholar]
- 34.Xun, L., E. Topp, and C. S. Orser. 1992. Diverse substrate range of a Flavobacterium pentachlorophenol hydroxylase and reaction stoichiometries. J. Bacteriol. 174:2898-2902. [DOI] [PMC free article] [PubMed] [Google Scholar]