Abstract
The present study aimed at the long-term storage of rumen protozoa as living cells in liquid nitrogen. The two-step or interrupted slow freezing procedure was used to cryopreserve six of the dominant species of rumen ciliates isolated from monofaunated animals, Dasytricha ruminantium, Entodinium caudatum, Epidinium ecaudatum caudatum, Eudiplodinium maggii, Isotricha prostoma, and Polyplastron multivesiculatum. We optimized the first step in the interrupted slow freezing procedure, from the extracellular ice nucleation temperature to the holding temperature, and studied the effects of the cooling rates on survival. In addition to the nature of the cryoprotectant (dimethyl sulfoxide), the equilibration temperature and equilibration time (25°C and 5 min, respectively), and the holding time at subzero temperature (45 min) recommended previously (S. Kišidayová, J. Microbiol. Methods 22:185-192, 1995), we found that a holding temperature of −30°C, a cooling rate from extracellular ice nucleation temperature to holding temperature of between 1.2°C/min and 2.5°C/min, depending on the ciliate, and rumen juice as the freezing and thawing medium markedly improved the survival rate. Survival rates determined after 2 weeks in liquid nitrogen were 100% for Isotricha, 98% for Dasytricha, 85% for Epidinium, 79% for Polyplastron, 63% for Eudiplodinium, and 60% for Entodinium. They were not significantly modified after a period of 1 year in liquid nitrogen. Four of the five ciliate species cryopreserved for 8 months in liquid nitrogen successfully colonized the rumen when inoculated into defaunated animals. These results have made it possible to set up a bank of cryopreserved rumen protozoa.
Rumen microbial populations are characterized by a broad diversity of microorganisms, mainly bacteria, ciliate protozoa, and fungi. Protozoa represent up to 50% of the total rumen microbial biomass. They are involved particularly in the rumen digestion of cellulose (6, 7, 17, 50), starch (7, 16, 45), and proteins (40), and they contribute actively to the control of the bacterial population (19) and to the formation of the end products of ruminal fermentation (21, 44, 45). They play an important role in the biodegradation of plant toxins and mycotoxins (22, 49) and in the regulation of ruminal conditions such as pH and redox potential (1, 27, 39, 44). They have been shown to eliminate certain pathogens from the digestive tract of ruminants, protecting them from disease and so improving the food safety of edible animal products (33).
Although some rumen ciliates can usually be cultivated in vitro, it is difficult to maintain them for a long time, and most species die within a few months to a year (3, 8, 12, 13, 14, 15, 34, 35, 36). Another way to maintain rumen ciliate protozoa for long periods involves with the rumen of previously defaunated animals (18) into which a few isolated cells of single species are inoculated to obtain monofaunated animals (16, 19, 21). However, preparing and maintaining a large number of animals in a monofaunated state for a wide range of ciliate strains is difficult. A cryopreservation technique to preserve rumen ciliate protozoa for several years would thus be most useful. Cryopreservation would also facilitate the transfer of ciliates between laboratories for in vivo or in vitro digestion studies, genomic and biotechnology research, and long-term storage of living cells.
There are various methods of cell freezing. Their effectiveness depends on several variables, including the freezing medium and the type and concentration of cryoprotectant, the equilibration temperature and equilibration time during the contact between the cryoprotectant and cells, the cooling rate, the temperature at which the cells are immersed in liquid nitrogen, and the thawing medium (10, 11, 32, 42). In the two-step or interrupted slow freezing method (26, 46), an initial slow freezing period (first step), from the equilibration temperature to the holding temperature (commonly set at −20°C to −40°C) (32), is followed by maintenance of the cells at that holding temperature (second step) for a given time (holding time). The frozen cells are then immersed in liquid nitrogen and finally stored at −196°C. During the first step, the cooling rates must be strictly controlled; a low cooling rate can damage cells through a solution effect, while fast cooling favors intracellular ice formation, which is often lethal to cells (31, 41). Consequently, cooling rates must be set to optimize cell dehydration while avoiding intracellular ice formation. To date, the cryopreservation of rumen ciliates has been carried out only on a limited range of species (23, 24, 28, 29), and in most cases the survival rates have been poor.
Here we report the optimization of the first step in the interrupted slow freezing procedure, focusing on the effect of different cooling rates on the cryopreservation of dominant species of rumen ciliate protozoa. The choices of cryoprotectant, equilibration temperature, equilibration time, and holding time made here were based on previous results (23). The promising results obtained here have enabled us to begin setting up a European cryobank of rumen ciliates sampled from France, the United Kingdom, Poland, and Slovakia.
MATERIALS AND METHODS
Origin of ciliates and preparation of monofaunated sheep.
Six species of ciliates considered major species generally found in the rumen were studied: Isotricha prostoma, Dasytricha ruminantium, Entodinium caudatum, Epidinium ecaudatum caudatum, Eudiplodinium maggii, and Polyplastron multivesiculatum. About 20 cells of each ciliate species were individually sampled by means of a micropipette from mixed fauna taken from sheep or cows through a rumen fistula or at the slaughterhouse. They were introduced into the rumen of sheep previously defaunated by a technique based on rumen emptying and successive washing (18). Once the population of ciliates reached the minimum level of 103 cells/ml in the rumen, the rumen content was sampled and treated for ciliate isolation.
Rumen protozoa count in rumen content.
Samples of rumen content (500 ml) taken through the cannula of the animals were filtered through a 4-mm2 metal mesh to eliminate large plant particles. After filtrate homogenization, 0.5 ml of rumen fluid was diluted in 30 ml of Simplex buffer (47) contained in a Dolfuss cell divided up into 10 by 20 squares. Cells were fixed with 300 μl of Lugol solution for 3 min. Depending on ciliate concentration, either the entire Dolfuss cell or only 1/4 square units were counted. The trial was repeated at least three times before estimating the mean number of ciliates per milliliter of rumen content.
Sedimentation of protozoa.
Samples (500 ml) of rumen content were taken from monofaunated sheep before their morning meal and conveyed to the laboratory at 39°C in anaerobic conditions. The rumen content was quickly filtered through two gauze layers. To concentrate the ciliates by sedimentation, 200 ml of filtrate was transferred under CO2 to a separating funnel held in a water bath at 39°C under CO2 for 1 h to 4 h, depending on the ciliate species; the small ciliates take longer to sediment than the larger ones. The white sedimented pellet at the bottom of the separating funnel was then collected in several tubes for cryopreservation trials. To roughly estimate the number of cells to be cryopreserved, collected ciliates were quickly counted under a microscope on a Jessen numeration slide after dilution in Simplex buffer. For more accurate counting, ciliates were then fixed with formaldehyde at a final concentration of 3.7% in three test tubes. The final cell number was evaluated as the mean of the three tubes.
Equilibration of protozoa with the cryoprotectant.
Dimethyl sulfoxide (DMSO) was used as cryoprotectant in all our trials. DMSO was mixed with 1 ml of ciliate suspension (104 cells/ml minimum) to obtain final concentrations of 4, 5, 6, and 10% (vol/vol). The mixture was equilibrated at 25°C in a water bath for 5 min (equilibration time), and 0.2 ml of the mixture was placed in 2-ml Nalgene screw-cap tubes before freezing.
Freezing step.
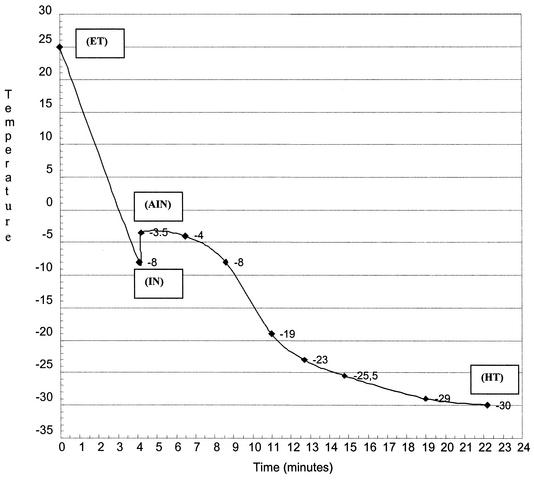
Ten Nalgene tubes containing the ciliates and the cryoprotectant were placed in a Nicool LM10 cooler (Air Liquide, Marne-la-Vallée, France), in which the cooling rate could be accurately controlled by adjusting the speed of an electric fan located above a Dewar chamber containing liquid nitrogen. A thermocouple was placed in one tube in the cooler, and the temperature was monitored on a chart recorder during freezing to record the cooling rate conditions accurately. Figure 1 gives an example of the temperature time course in the tubes during the freezing step.
FIG. 1.
Example of freezing curve 2a from an equilibration temperature (ET) of 25°C to the holding temperature (HT) of −30°C. The extracellular ice nucleation temperature (IN; −8°C) is followed by a temperature increase after extracellular ice nucleation (AIN) to −3.5°C.
Different cooling rates were tested for different ranges of temperature from the equilibration temperature to the holding temperature (around −30°C) and maintained for 45 min. At the end of the holding phase, the control tube containing the probe was thawed to test the viability of the ciliates. The other tubes were immersed in liquid nitrogen. The freezing step was repeated at least three times for each ciliate species.
Thawing step.
Frozen tubes were removed from the liquid nitrogen vessel and placed in a water bath at 39°C for 5 min. The thawed protozoa suspension was then diluted under CO2 in glass tubes containing a thawing medium made up of either a Caudatum type medium (5) supplemented with 0.04% glucose and 10% rumen juice obtained after filtration of rumen content through four layers of gauze and centrifuged at 500 × g for 15 min (Caudatum type medium C), rumen juice alone (medium RF), or rumen juice supplemented with 0.04% glucose (medium RFG). Tests were also made on freshly prepared rumen fluid or rumen fluid stored for 2 weeks at 4°C. All the media and suspensions of ciliates were handled under CO2 (see experiment 1).
Evaluation of survival rate.
The survival rate after thawing was estimated by counting the proportion of motile ciliates under a microscope. Motility was stimulated by heating the microscope slide with the thawing suspension of ciliates for 1 to 2 s above a small Bunsen flame. Counts were repeated five times per tube, and three tubes were counted for each ciliate species. Means and standard deviations were calculated for the survival rate of each ciliate species.
Inoculation of thawed ciliates into the rumen of defaunated sheep.
Trials on in vivo inoculation of cryopreserved ciliates were carried out after 8 months of cryopreservation in liquid nitrogen. One cryotube was withdrawn from the liquid nitrogen and immediately immersed in a water bath at 39°C. Five minutes later, the content of the tube was introduced into the rumen through the cannula, 2 h before the morning meal. The concentration of ciliates was checked every day from day 7.
Experiment 1: tests on cooling rate, holding temperature, and thawing medium for Entodinium caudatum.
Tests were carried out on E. caudatum with 5% DMSO as the cryoprotectant and in equilibration conditions of 25°C for 5 min, according to Kišidayová (23). Three holding temperatures were tested: −25.5°C, −30°C, and −33°C, for a constant holding time set at 45 min as previously prescribed by Kišidayová (23). Three freezing curves (curves 1, 2, and 3) were tested for each holding temperature. Details of the freezing curves are given in Tables 1, 2, and 3, respectively. The effects of three thawing media [the Caudatum type medium C, pure rumen juice RF, and rumen juice with glucose at 0.04% (wt/vol) RFG] on the viability of E. caudatum cryopreserved according to the range of temperatures described in curves 1, 2, and 3 (Tables 1, 2, and 3, respectively) were tested. These trials were repeated three times in triplicate (n = 9).
TABLE 1.
Tested cooling rates (CR) for Entodinium caudatum from the equilibration temperature (ET) to the holding temperature (HT), set at −25.5°Ca
| Curve 1 | Curve 1a
|
Curve 1b
|
|||
|---|---|---|---|---|---|
| ET → HT (°C) | CR (°C/min) | ET → HT (°C) | CR (°C/min) | ET → HT (°C) | CR (°C/min) |
| 23 → −4.4* | 8.6 | 22.5 → −6* | 11.4 | 23 → −3* | 13 |
| −1.5** → −4 | 0.6 | −3.5** → −4 | 0.6 | −2** → −4 | 0.9 |
| −4 → −8 | 2.8 | −4 → −8 | 1.4 | −4 → −8 | 2.3 |
| −8 → −19 | 4.4 | −8 → −19 | 3.1 | −8 → −19 | 13.7 |
| −19 → −23 | 2.2 | −19 → −23 | 1.4 | −19 → −23 | 6.6 |
| −23 → −25.5 | 1.9 | −23 → −25.5 | 1.0 | −23 → −25.5 | 8.3 |
The times from ET to the extracellular ice nucleation temperature (∗) were 3.2 (curve 1), 2.5 (curve 1a), and 2.0 (curve 1b) min; the times from after extracellular ice nucleation temperature (∗∗) to HT were 10.8 (curve 1), 12.6 (curve 1a), and 4.6 (curve 1b) min; and the total times from ET to HT were 14 (curve 1), 15.1 (curve 1a), and 6.6 (curve 1b) min. (see Fig. 1). ET, temperature indicated by the thermocouple in the tube containing ciliates (after the equilibration time) just before the freezing cycle.
TABLE 2.
Tested cooling rates (CR) for Entodinium caudatum from the equilibration temperature (ET) to the holding temperature (HT), set at −30°Ca
| Curve 2 | Curve 2a
|
Curve 2b
|
|||
|---|---|---|---|---|---|
| ET → HT (°C) | CR (°C/min) | ET → HT (°C) | CR (°C/min) | ET → HT (°C) | CR (°C/min) |
| 25 → −6.5* | 10.1 | 25 → −8* | 8.2 | 22.5 → −7* | 11.8 |
| −2.5** → −4 | 0.6 | −3.5** → −4 | 0.2 | −1.5** → −4 | 1.1 |
| −4 → −8 | 2.8 | −4 → −8 | 1.9 | −4 → −8 | 3.0 |
| −8 → −19 | 4.4 | −8 → −19 | 4.7 | −8 → −19 | 9.1 |
| −19 → −23 | 2.1 | −19 → −23 | 2.3 | −19 → −23 | 6.6 |
| −23 → −25.5 | 1.9 | −23 → −25.5 | 1.2 | −23 → −25.5 | 4.1 |
| −25.5 → −29 | 2.0 | −25.5 → −29 | 0.8 | −25.5 → −29 | 1.9 |
| −29 → −30 | 1.4 | −29 → −30 | 0.3 | −29 → −30 | 0.3 |
The times from ET to extracellular ice nucleation temperature (*) were 3.0 (curve 2), 4.1 (curve 2a), and 2.5 (curve 2b) min; the times from after extracellular ice nucleation temperature (**) to HT were 12.0 (curve 2), 17.6 (curve 2a), and 10.6 (curve 2b) min; and the total times from ET to HT were 15 (curve 2), 21.7 (curve 2a), and 13.1 (curve 2b) min. (see Fig. 1). ET, temperature indicated by the thermocouple in the tube containing ciliates (after the equilibration time) just before the freezing cycle.
TABLE 3.
Tested cooling rates (CR) for Entodinium caudatum from the equilibration temperature (ET) to the holding temperature (HT), set at −33°Ca
| Curve 3 | Curve 3a
|
Curve 3b
|
|||
|---|---|---|---|---|---|
| ET → HT (°C) | CR (°C/min) | ET → HT (°C) | CR (°C/min) | ET → HT (°C) | CR (°C/min) |
| 25 → −6* | 12.4 | 26 → −5* | 6.8 | 26 → −5.5* | 13 |
| −2.5** → −4 | 0.6 | −3** → −4 | 0.4 | −2.5** → −4 | 0.7 |
| −4 → −8 | 2.6 | −4 → −8 | 1.0 | −4 → −8 | 7.8 |
| −8 → −19 | 4.2 | −8 → −19 | 5.2 | −8 → −19 | 7.7 |
| −19 → −23 | 2.1 | −19 → −23 | 3.0 | −19 → −23 | 7.8 |
| −23 → −25.5 | 1.7 | −23 → −25.5 | 2.1 | −23 → −25.5 | 3.6 |
| −25.5 → −29 | 2.5 | −25.5 → −29 | 1.0 | −25.5 → −29 | 3.9 |
| −29 → −30.5 | 1.8 | −29 → −30.5 | 0.8 | −29 → −30.5 | 2.3 |
| −30.5 → −33 | 1.7 | −30.5 → −33 | 0.7 | −30.5 → −33 | 2.1 |
Times from ET to extracellular ice nucleation temperature (∗) were 3.2 (curve 3), 2.5 (curve 3a), and 2.0 (curve 3b) min; the times from after extracellular ice nucleation temperature (∗∗) to HT were 10.8 (curve 3), 12.6 (curve 3a), and 4.6 (curve 3b) min; and the total times from ET to HT were 14 (curve 3), 15.1 (curve 3a), and 6.6 (curve 3b) min; (see Fig. 1). ET, temperature indicated by the thermocouple in the tube containing ciliates (after the equilibration time) just before the freezing cycle.
Experiment 2: modification of cooling rates used in experiment 1 on survival rate of E. caudatum.
The influence of changing the cooling rates used in experiment 1 was checked by reducing them (curves 1a, 2a, and 3a in Tables 1, 2, and 3, respectively) and increasing them (curves 1b, 2b, and 3b in Tables 1, 2, and 3, respectively). These trials were repeated three times in triplicate (n = 9) (Tables 4 and 5).
TABLE 4.
Effect of thawing medium and holding temperature on survival rates of Entodinium caudatum after 48 h in liquid nitrogen (cryopreserved cell number, 906,510 ± 47,763 per ml; cryoprotectant, 5% DMSO; n = 9)a
| Curve | Holding temp (°C) | Mean survival (%) ± SD
|
||||
|---|---|---|---|---|---|---|
| Medium C | Medium RF
|
Preserved RF
|
||||
| RF | RFG | RF | RFG | |||
| 1 | −25 | 3.6 ± 1.0a* | 6.7 ± 0.6b* | 10.0 ± 0.9c* | 1.2 ± 0.1d* | 3.9 ± 0.1a* |
| 2 | −30 | 6.4 ± 1.6a* | 30.3 ± 4.5b** | 32.2 ± 4.9b** | 2.6 ± 0.5a* | 11.0 ± 1.5a** |
| 3 | −33 | 5.7 ± 0.4a* | 24.1 ± 4.2b** | 23.8 ± 3.1b** | 3.1 ± 0.8ac* | 9.6 ± 3.6ad** |
Values with different letters within a line, are significantly different; within a column, values with different numbers of asterisks are significantly different (P < 0.05). Curves 1, 2, and 3 are described in Tables 1, 2, and 3, respectively. C, Coleman buffer; RF, rumen fluid; RFG, rumen fluid plus 0.04% glucose. RF was preserved for a 2-week period at 4°C.
TABLE 5.
Effect of freezing rate and holding temperature on survival of Entodinium caudatum in RFG after 48 h in liquid nitrogen (cryoprotectant, 5% DMSO; n = 9)a
| Holding temp (°C) | Curve | Mean survival (%) ± SD | No. of cryopreserved cells/ml |
|---|---|---|---|
| −25 | 1a | 41.5 ± 1.8* | 1,017,060 ± 36,105 |
| 1b | 4.5 ± 1.0** | ||
| −30 | 2a | 58.8 ± 2.2*** | 1,046,540 ± 43,382 |
| 2b | 6.3 ± 0.9** | ||
| −33 | 3a | 62.0 ± 3.9*** | 906,510 ± 47,763 |
| 3b | 6.2 ± 1.8** |
Experiment 3: tests on concentration of cryoprotectant applied to E. caudatum.
Four concentrations of DMSO (4, 5, 6, and 10%) added to the tubes containing the cells of ciliates to be frozen were tested at the different holding temperatures, −25.5°C, −30°C, and −33°C, and with the cooling rates described in curves 1a, 2a, and 3a (Tables 1, 2, and 3). The trials were repeated three times in triplicate (n = 9).
Experiment 4: tests on cooling rates and cryoprotectant concentrations applied to I. prostoma, D. ruminantium, E. ecaudatum caudatum, P. multivesiculatum, and E. maggii.
Freezing curves (curves 1a, 2a, and 3a) previously described for E. caudatum (Tables 1, 2, and 3) were applied to all these species. From the results obtained in experiment 3, the holding temperature was set at −30°C and maintained for 45 min. The four concentrations of DMSO (4, 5, 6, and 10%) were tested again on each of the ciliate species. The trials were repeated three times in triplicate (n = 9)
Statistical analysis.
Data for survival rates were analyzed by one-way analysis of variance with the software Analyzed-it for Microsoft Excel (Microsoft, Leeds, United Kingdom). Differences in means were statistically tested with the Tukey test. The level of significance was set at P < 0.05.
RESULTS
Experiments 1 and 2: effect of cooling rate, holding temperature, and thawing medium for E. caudatum.
Several cooling rates during the period between equilibration temperature and extracellular ice formation in the range from 2.1°C/min to 17.5°C/min were tested. The optimal mean cooling rate for this period was between 7°C/min and 10°C/min. However, the increase noted in the survival rate did not reach the significance threshold. Accordingly, we focused mainly on the temperature range between the extracellular ice nucleation temperature and the holding temperature (Fig. 1).
Survival rates of cryopreserved E. caudatum were significantly higher (P < 0.05) when the thawing medium RF was used rather than the Caudatum type medium C (Table 4). Thawing medium RF stored at 4°C for 2 weeks impaired the survival of thawed cells, but the addition of glucose to RF stimulated cell viability. The observed survival rates of E. caudatum (Table 4) obtained with the freezing conditions described in curves 1, 2, and 3 (Tables 1, 2, and 3) were low. Slowing down the cooling rates (curves 1a, 2a, and 3a in Tables 1, 2, and 3) in this temperature range significantly (P < 0.05) improved the survival rate of E. caudatum, while increasing the cooling rates (curves 1b, 2b, and 3b in Tables 1, 2, and 3) lowered the survival rate of the ciliates (Table 5). Also, the holding temperatures −30°C and −33°C gave better survival rates (P < 0.05) than −25°C. No significant difference (P > 0.05) was noted between −30°C and −33°C as the holding temperature.
Experiment 3: effect of cryoprotectant concentration.
DMSO at concentrations of 5% and 6% gave the best survival rates for E. caudatum (Table 6). No significant difference (P > 0.05) in survival rate was noted between the two DMSO concentrations. At the three holding temperatures tested, the survival rates were maximum when the holding temperature was −30°C or −33°C.
TABLE 6.
Effect of cryoprotectant concentration and holding temperature on survival of Entodinium caudatum in RFG after 48 h in liquid nitrogen (n = 9)a
| Holding temp (°C) | DMSO (%) | Mean survival (%) ± SD | No. of cryopreserved cells/ml |
|---|---|---|---|
| −25 | 4 | 31.7 ± 6.8a | 899,140 ± 10,422 |
| 5 | 44.6 ± 5.7ab | ||
| 6 | 49.8 ± 9.2b | ||
| 10 | 20.3 ± 2.5a | ||
| −30 | 4 | 38.1 ± 6.1a | 818,070 ± 18,052 |
| 5 | 56.4 ± 7.5b | ||
| 6 | 60.4 ± 4.5b | ||
| 10 | 10.0 ± 1.8c | ||
| −33 | 4 | 33.3 ± 6.6a | 847,550 ± 20,845 |
| 5 | 58.6 ± 11.1b | ||
| 6 | 69.5 ± 8.4b | ||
| 10 | 7.8 ± 2.6c |
Experiment 4: determination of optimal cooling rate for cryopreservation of I. prostoma, D. ruminantium, E. ecaudatum caudatum, P. multivesiculatum, and E. maggii.
Optimal cooling rates were determined from the three freezing curves tested for each of the species tested (Tables 7 and 8). Optimal conditions varied according to the species. I. prostoma and D. ruminantium were the least sensitive to the freezing conditions, followed by Epidinium ecaudatum caudatum. For Dasytricha and Epidinium, although 5% DMSO seemed to be the optimal concentration, no significant difference (P > 0.05) was noted between the survival rates at 5, 6, and 10% DMSO. E. maggii was the most sensitive to experimental conditions during the freezing step. For this ciliate species, 3% instead of 4% DMSO as the cryoprotectant increased its survival rate from 54% to 63%, but the difference was not significant (P > 0.05). DMSO at 10% was toxic to P. multivesiculatum and E. maggii; only 5% of cells from the two species survived in those conditions.
TABLE 7.
Optimal cooling rates (CR) from equilibration temperature (ET) to holding temperature (HT) and survival rates of rumen ciliates after 2 weeks in liquid nitrogen (n = 9)a
| I. prostoma (44,880 ± 3,966) |
D. ruminantium (278,666 ± 27,438)
|
E. ecaudatum caudatum (83,325 ± 7,855)
|
P. multivesiculatum (12,920 ± 1,074)
|
E. maggii (97,502 ± 4,050)
|
|||||
|---|---|---|---|---|---|---|---|---|---|
| ET → HT (°C) | CR (°C/min) | ET → HT (°C) | CR (°C/min) | ET → HT (°C) | CR (°C/min) | ET → HT (°C) | CR (°C/min) | ET → HT (°C) | CR (°C/min) |
| 25 → −4.5* | 9.5 | 24 → −6* | 8.5 | 23.5 → −7.5* | 8.3 | 25 → −5* | 9.3 | 23 → −7* | 9.3 |
| −1.5** → −4 | 1.0 | −2.5** → −4 | 0.4 | −3.5** → −4 | 0.2 | −3** → −4 | 0.3 | −3** → −4 | 0.5 |
| −4 → −8 | 2.1 | −4 → −8 | 2.2 | −4 → −8 | 1.5 | −4 → −8 | 2.3 | −4 → −8 | 1.9 |
| −8 → −19 | 5 | −8 → −19 | 5.7 | −8 → −19 | 5 | −8 → −19 | 5.7 | −8 → −19 | 5.5 |
| −19 → −23 | 3.1 | −19 → −23 | 4 | −19 → −23 | 2.3 | −19 → −23 | 3.3 | −19 → −23 | 4 |
| −23 → −25.5 | 0.9 | −23 → −25.5 | 2.5 | −23 → −25.5 | 0.9 | −23 → −25.5 | 1.8 | −23 → −25.5 | 2.5 |
| −25.5 → −29 | 0.7 | −25.5 → −29 | 0.9 | −25.5 → −29 | 0.6 | −25.5 → −29 | 0.9 | −25.5 → −29 | 1.6 |
| −29 → −30 | 0.4 | −29 → −30 | 0.4 | −29 → −30 | 0.2 | −29 → −30 | 0.3 | −29 → −30 | 0.6 |
The times from ET to extracellular ice nucleation temperature (∗) were 3.1, 3.5, 3.7, 3.2, and 3.2 min, respectively; the times from after extracellular ice nucleation temperature (∗∗) to HT were 18.2, 15.5, 21.2, 16.1, and 11.7 min, respectively. The number of cryopreserved cells is shown for each species.
TABLE 8.
Effect of DMSO concentration on survivala
| DMSO concn (%) | Mean survival (%) ± SDb
|
||||
|---|---|---|---|---|---|
| I. prostoma | D. rumi- nantium | E. ecaudatum caudatum | P. multivesi- culatum | E. maggii | |
| 3 | NDc | ND | ND | ND | 63 ± 5.7a |
| 4 | 100a | 72.6 ± 13.0a | 68.4 ± 0.3a | 78.9 ± 7.3a | 54 ± 6.8a,b |
| 5 | 100a | 96.8 ± 5.6b | 84.8 ± 0.3b | 68.7 ± 8.1ab | 45.3 ± 7.6b |
| 6 | 100a | 98.3 ± 2.7b | 78.0 ± 2.5b | 60.4 ± 8.8b | 46.0 ± 3.8b |
| 10 | 100a | 88.5 ± 4.0ab | 80.0 ± 5.5b | 5.03 ± 1.8c | 5.1 ± 3.1c |
See Table 7.
Values with different letters are significantly different (P <0.05).
ND, not determined.
Inoculation of cryopreserved ciliates into the rumen of defaunated sheep.
Thawed ciliates belonging to the species I. prostoma, D. ruminantium, E. maggii, and E. ecaudatum caudatum generally proliferated 2 weeks after being inoculated and stabilized in the rumen at a mean concentration of between 103/ml and 104/ml (Table 9). This means that the conditions used to cryopreserve the ciliates allowed them to grow in the rumen and produce a normal monofaunated population. However, for as yet unexplained reasons, P. multivesiculatum failed to grow in the rumen of sheep. This may be a consequence of cell degeneration due to the fact that the strain was isolated 30 years ago from only few cells (five to six cells) and was cultivated in vivo in several monofaunated sheep for this long period. In support of this explanation, we note that Polyplastron multivesiculatum definitely disappeared from the rumen of monofaunated animals from which the cryopreserved ciliates had been initially sampled. Also, some recent experiments indicate that the ability of ciliates to grow in the rumen is markedly lowered when they originate from a few cells grown in vitro (20).
TABLE 9.
Number of cryopreserved cells, survival after 8 months of preservation in liquid nitrogen, and number of cells inoculated into the rumen of defaunated sheep
| Ciliate species | No. of cryopreserved cells/ml | Survival (%) | No. of cells inoculated | Implan- tationa |
|---|---|---|---|---|
| D. ruminantium | 98,175 ± 6,059 | 90 | 17,671 | + |
| E. ecaudatum caudatum | 206,635 ± 8,043 | 80 | 33,061 | + |
| E. maggii | 23,833 ± 674 | 60 | 2,860 | + |
| I. prostoma | 52,360 ± 1,322 | 100 | 10,472 | + |
| P. multivesiculatum | 11,440 ± 2,335 | 70 | 1,601 | − |
+, successful implantation; −, unsuccessful implantation.
DISCUSSION
Rumen ciliates need specific environmental conditions to survive. For example, oxygen must be absent and the temperature must be maintained at 39°C (47). Such constraints explain why it is so difficult to preserve the ciliates in in vitro cultures and in deep-freeze conditions.
The cryoprotectant acts at the cell membrane level by limiting the effect of cell dehydration during freezing (43), lowering the freezing point of extra- and intracellular biological liquids (4, 31, 43) and promoting vitrification rather than intracellular ice crystal formation. Preliminary tests carried out by us showed that all the species of ciliates used here, treated with concentrations of DMSO of between 1% and 10% and equilibrated at 25°C for 5 min, had low sensitivities to the potential toxicity of DMSO. Also, the concentrations used in previous experiments on an Entodinium sp. (23, 24) ranged between 3% and 6%. It has been shown that both a low DMSO content (1% to 2%) and a high DMSO content (7% to 8%) have damaging effects on cryopreserved ciliates (23, 24). Furthermore, several cryopreservation studies carried out on other eukaryotic cells (Toxoplasma gondii, Trichomonas, and Entamoeba spp.) showed a high recovery when 10% DMSO was used (2, 37). Accordingly, concentrations of 4%, 5%, 6%, and 10% DMSO were tested here.
As DMSO toxicity was assessed only for the 5-min equilibration time at 25°C, the toxic effect observed after thawing experiments at 10% DMSO may be due to a continuous action of DMSO on cells during the freezing step. A concentration of 5% DMSO, which was optimal for most of the ciliates tested (Tables 6, 7, and 8), is close to the concentration used for other eukaryotic cells, 5% for Plasmodium berghei (38) and algae (9) and 8% for Tetrahymena thermophila (4). As the toxicity of DMSO to cells increased at high temperatures (48), this cryoprotectant should be used at room temperature or at 4°C and the equilibration time should be minimized. This supports the good survival rates obtained when the equilibration temperature and equilibration time were 25°C and 5 min, respectively (26).
With the Nicool LM10 apparatus, it was possible to control and repeat the optimized conditions of cooling during the first step. It was noted that the cooling rate during the period following extracellular ice formation until the holding temperature, ranging from −5°C to −25°C in our experiment, strongly influenced the survival rates of the rumen ciliates. Cooling rates in this temperature range were set by taking into account data from previous experiments carried out on E. caudatum (23, 24) and on mouse cells, red blood cells, and yeast cells (25, 30, 31). The formation of intracellular ice crystals can damage the cells. This effect occurred at a cooling rate of 4°C/min or above (mouse embryos) and at 5°C/min or above (ovarian cells), but never at 1°C/min or below (ovarian cells) and was minimized at the cooling rate of 2°C/min or below (mouse embryos) (25). For this reason, to avoid the adverse effects of low and high cooling rates, we decided to use cooling rates close to 5°C/min in each temperature range and near 2°C/min on average for the whole freezing period between extracellular ice formation to the holding temperature in our first trials (curves 1, 2, and 3 in Tables 1, 2, and 3). Thus, we observed that the freezing conditions could be improved to obtain better survival rates (curves 1a, 2a, and 3a in Tables 1, 2, and 3), and so an optimal cooling protocol was drawn up for each ciliate species (Tables 5, 7, and 8). The optimal cooling rates, averaged from extracellular ice formation to the holding phase, were 1.5°C/min, 1.7°C/min, 1.5°C/min, 1.2°C/min, 1.6°C/min, and 2.3°C/min for I. prostoma, D. ruminantium, E. caudatum, E. caudatum caudatum, P. multivesiculatum, and E. maggii, respectively.
The optimal holding temperature was clearly established at −30°C or −33°C for all the ciliates tested. A significant decrease in survival rate was noted when the holding temperature was −25°C. This may be due to incomplete dehydration of the cells when this holding temperature was reached, giving rise to ice crystal formation inside the cells when immersed in liquid nitrogen. In a complementary experiment, we observed that a decrease in the holding phase temperature to −36°C had no beneficial effect on or even reduced the survival rate of rumen ciliates.
In a preliminary experiment, we found that ciliates sampled from the rumen before feeding the host animals were less stressed by cryopreservation than ciliates sampled after feeding (unpublished data). Therefore, the nutritional state of ciliates affects the stability of cells during the cryopreservation procedure. Thus, sampling of cells for cryopreservation was carried out before the morning meal of the animal donors.
Rumen juice is the natural medium for ciliates. This can explain why its use in the freezing and thawing steps improves the viability of cells. Addition of glucose at a low concentration (0.04%, wt/vol) to rumen juice just after the thawing step enhanced the vitality of the thawed ciliates. Glucose is probably involved in the regulation of osmotic pressure between the outside and inside of the cell membrane. Also, it can be utilized as an energy substrate by the stressed cells.
For the first time, E. maggii, I. prostoma, and D. ruminantium have been cryopreserved with good survival rates. Between 90 and 100% of the two holotrichs were found alive after cryopreservation, while only 54% and 63% of E. maggii cells survived when cryopreserved with 4% and 3% DMSO, respectively. The two-step freezing technique was applied for the first time to P. multivesiculatum and E. ecaudatum caudatum. By this technique, the survival rate of P. multivesiculatum was improved (80% versus 50%) compared with the single-step method (24).
Inspections after 2 weeks, 4 weeks, 8 months, and 1 year of preservation of ciliates in liquid nitrogen indicated that their survival rates were maintained for a long period. As indicated in Table 10, the survival rates of E. caudatum and E. maggii were low throughout the preservation time in liquid nitrogen. Thus, more precise conditions have to be determined for these species in the future. The growth of ciliates in the rumen of defaunated sheep after cryopreservation evidenced their capacity to totally recover their basic metabolic functions for ATP production. About 100 tubes each of the six species I. prostoma, D. ruminantium, E. ecaudatum caudatum, P. multivesiculatum, E. maggii, and E. caudatum are currently preserved in liquid nitrogen under the conditions described in this paper.
TABLE 10.
Survival rates of rumen ciliates after 2 weeks and 4, 8, and 12 months in liquid nitrogena
| Family | Species | Mean survival (%) ± SDb
|
|||
|---|---|---|---|---|---|
| 2 wk | 4 mo | 8 mo | 12 mo | ||
| Diplodiniinae | E. maggii | 63.6 ± 5.6a | 62.5 ± 6.6a | 60.4 ± 3.5a | 59.0 ± 10.5a |
| P. multivesiculatum | 78.9 ± 7.3b | 74.3 ± 5.3b | 70.4 ± 6.2b | 80.4 ± 4.3b | |
| Entodiniinae | E. caudatum | 58.8 ± 2.2c | 56.7 ± 12.7c | 46.2 ± 8.1c | 50.8 ± 7.7c |
| Isotrichidae | D. ruminantium | 96.8 ± 5.6d | 90.9 ± 7.4d | 90.1 ± 2.6d | 94.0 ± 5.1d |
| I. prostoma | 100e | 100e | 100e | 100e | |
| Ophryoscolescinae | E. ecaudatum caudatum | 84.8 ± 0.3f | 78.7 ± 7.8f | 82.2 ± 5.1f | 83.7 ± 4.2f |
DMSO was used at 5% for Entodinium, Isotricha, Dasytricha, Epidinium, 3% for Eudiplodinium, and 4% for Polyplastron (n = 9). Within each ciliate species, values with different letters were significantly different (P < 0.05).
Values with different letters are significantly different (P < 0.05).
This first European cryobank has been set up as part of a program (European Rumen Ciliate Culture Collection) involving scientists from five different European countries. Frozen samples are now available for European scientists who wish to use them for research.
Acknowledgments
This work was supported by European Union Infrastructure grant QLRI-CT-2000-01455.
REFERENCES
- 1.Blackburn, T. H., and P. N. Hobson. 1960. The degradation of protein in the rumen of the sheep and the redistribution of the protein nitrogen after feeding. Br. J. Nutr. 14:445.
- 2.Bos, H. J., P. Mas Bakal, A. A., Van den Eijk, N. in't Veld, E. G. Boorsma, and H. J. Van der Kaay. 1978. Cryopreservation of parasitic protozoa. Acta Leiden. 46:17-30. [PubMed] [Google Scholar]
- 3.Broad, T. E., and R. M. C. Dawson. 1976. Role of choline in the nutrition of the rumen protozoon Entodinium caudatum. J. Gen. Microbiol. 92:391-397. [DOI] [PubMed] [Google Scholar]
- 4.Cassidy-Hanley, D., H. R. Smith, and P. J. Bruns. 1995. A simple efficient technique for freezing Tetrahymena thermophila. J. Eukaryot. Microbiol. 42:510-515. [DOI] [PubMed] [Google Scholar]
- 5.Coleman, G. S. 1978. Rumen entodiniomorphid protozoa, p. 39-54. In A. E. Taylor and J. R. Baker (ed.), Methods of cultivating parasites in vitro. Academic Press, London, England.
- 6.Coleman, G. S. 1983. The cellulolytic activity of thirteen species of rumen entodiniomorphid protozoa. J. Protozool. 30:36A.
- 7.Coleman, G. S. 1992. The rate of uptake and metabolism of starch grains and cellulose particles by Entodinium species, Eudiplodinium maggii, some other entodiniomorphid protozoa and natural protozoa populations taken from the ovine rumen. J. Appl. Bacteriol. 73:507-513. [DOI] [PubMed] [Google Scholar]
- 8.Coleman, G. S., and D. J. Reynolds. 1982. The effects of sterols and haemin on the growth of the rumen ciliate Ophryoscolex caudatus and some other entodiniomorphid protozoa. J. Appl. Bacteriol. 52:129-134. [Google Scholar]
- 9.Day, J. G., and M. M. Deville. 1995. Cryopreservation of algae. Methods Mol. Biol. 38:81-89. [DOI] [PubMed] [Google Scholar]
- 10.Eriksson, B. M., J. M. Vazquez, E. A. Martinez, J. Roca, X. Lucas, and H. Rodriguez-Martinez. 2001. Effects of holding time during cooling and of type of package on plasma membrane integrity, motility and in vitro oocyte penetration ability of frozen-thawed boar spermatozoa. Theriogenology 55:1593-1605. [DOI] [PubMed] [Google Scholar]
- 11.Farrant, J., C. A. Walter, L. Heather, and L. E. McGann. 1977. Use of two-step cooling procedure to examine factors influencing cell survival following freezing and thawing. Cryobiology 14:273-286. [DOI] [PubMed] [Google Scholar]
- 12.Hino, T., M. Kametaka, and M. Kandatsu. 1973. The cultivation of rumen oligotrich protozoa. I. Factors influencing the life of Entodinia. J. Gen. Appl. Microbiol. 19:305-315. [Google Scholar]
- 13.Hino, T., M. Kametaka, and M. Kandatsu. 1973. The cultivation of rumen oligotrich protozoa. II. Growth of Entodinia in vitro. J. Gen. Appl. Microbiol. 19:325-337. [Google Scholar]
- 14.Hino, T., M. Kametaka, and M. Kandatsu. 1973. The cultivation of rumen oligotrich protozoa. III. White clover factors which stimulate the growth of Entodinia. J. Gen. Appl. Microbiol. 19:397-413. [Google Scholar]
- 15.Hungate, R. E. 1966. The rumen and its microbes, p 91-147. Academic Press, New York, N.Y.
- 16.Ivan, M., L. Neil, R. Forster, R. Alimon, L. M. Rode, and T. Entz. 2000. Effects of Isotricha, Dasytricha, Entodinium, and total fauna on fermentation and duodenal flow in wethers fed different diets. J. Dairy Sci. 83:776-787. [DOI] [PubMed] [Google Scholar]
- 17.Jouany, J. P., and J. Senaud. 1979. Role of rumen protozoa in the digestion of food cellulolytic materials. Ann. Rech. Vet. 10:261-263. [PubMed] [Google Scholar]
- 18.Jouany, J. P., and J. Senaud. 1979. Défaunation du rumen de mouton. Ann. Biol. Anim. Biochem. Biophys. 19:619-624. [Google Scholar]
- 19.Jouany, J. P., and K. Ushida. 1999. The role of protozoa in feed digestion. Asian-Aust. J. Anim. Sci. 12:113-128. [Google Scholar]
- 20.Jouany, J. P., E. Nsabimana, S. Kišidayová, T. Michalowski, and D. Macheboeuf. 2002. Why cannot some species of protozoa grow in the rumen? Reprod. Nutr. Dev. 42(Suppl. 1):S79. [Google Scholar]
- 21.Jouany, J. P., J. Senaud, S. Toillon, M. Ben Salah, J. Bohatier, and G. Prensier. 1995. Effect of ruminal inoculation of Isotricha alone or a mixed B-type fauna in a defaunated rumen on the digestion of a hay-maize diet (70:30) in sheep. Reprod. Nutr. Dev. 35:11-25. [DOI] [PubMed] [Google Scholar]
- 22.Kiessling, K. H., H. Petterson, K. Sandholm, and M. Olsen. 1984. Metabolism of aflatoxin, ochratoxin, zealarenone, and three trichothecenes by intact fluid, rumen protozoa, and rumen bacteria. Appl. Environ. Microbiol. 47:1070-1073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kišidayová, S. 1995. Two-step freezing of the rumen ciliate protozoon Entodinium caudatum. J. Microbiol. Methods 22:185-192. [Google Scholar]
- 24.Kišidayová, S. 1996. The cryopreservation of some large ciliate entodiniomorphid protozoa taken from the rumen. Lett. Appl. Microbiol. 23:389-392. [DOI] [PubMed] [Google Scholar]
- 25.Leibo, S. P., J. McGrath, and E. G. Cravalho. 1978. Microscopic observation of intracellular ice formation in mouse ova as a function of cooling rate. Cryobiology 15:257-271. [DOI] [PubMed] [Google Scholar]
- 26.Liu, J., E. J. Woods, Y. Agca, E. S. Critser, and J. K. Critser. 2000. Cryobiology of rat embryo. II. A theoretical model of the development of interrupted slow-freezing procedures. Biol. Reprod. 63:1303-1312. [DOI] [PubMed] [Google Scholar]
- 27.Mackie, R. I., F. M. C. Gilchrist, A. M. Robberts, P. E. Hannah, and H. M. Schwartz. 1978. Microbiological and chemical changes in the rumen during the stepwise adaptation of sheep to high concentrate diets. J. Agric. Sci. 90:241. [Google Scholar]
- 28.Marcin, A., F. Gyulai, J. Varady, and M. Sorokova. 1989. A simple technique for cryopreservation of the rumen protozoa Entodinium simplex. Cryo-Letters 10:89-104. [Google Scholar]
- 29.Marcin, A., S. Kišidayová, J. Fejes, J. Zelenak, and V. Kmet. 1992. A simple technique for cryopreservation of rumen protozoon Entodinium caudatum. Cryo-Letters 13:175-182. [Google Scholar]
- 30.Mazur, P. 1977. The role of intracellular freezing in the death of cells cooled at supra optimal rates. Cryobiology 14:251-272. [DOI] [PubMed] [Google Scholar]
- 31.Mazur, P. 1984. Freezing of living cells: mechanisms and implications. Am. J. Physiol. 247:125-142. [DOI] [PubMed] [Google Scholar]
- 32.McGann, L. E., and J. Farrant. 1976. Survival of tissue culture cells frozen by a two-step procedure to −196°C. I. Holding temperature and time. Cryobiology 13:261-268. [DOI] [PubMed] [Google Scholar]
- 33.McIntosh, F. M., K. N. Stanley, C. S. Stewart, and C. J. Newbold. 2002. The role of protozoa in the survival of E. coli, verocytotoxin encoding bacteriophage and their VTEC lysogens in the rumen. Reprod. Nutr. Dev. 42(Suppl. 1):S34. [Google Scholar]
- 34.Michalowski, T., P. Szczepkowski, and P. Muszynski. 1986. The nutritive factors affecting the cultivation of the rumen ciliate Diploplastron affine in vitro. Acta Protozool. 25:419-426. [Google Scholar]
- 35.Muszynski, P., T. Michalowski, and P. Szczepkowski. 1985. The influence of protein on the number of rumen ciliates Entodinium caudatum and Diplodinium affine in vitro. Acta Protozool. 24:307-317. [Google Scholar]
- 36.Onodera, R., and C. Henderson. 1980. Growth factors of bacterial origin for the culture of the rumen oligotrich protozoon Entodinium caudatum. J. Appl. Bacteriol. 48:125-134. [Google Scholar]
- 37.Popescu, C., and D. Steriu. 1993. Long term cryopreservation of Toxoplasma gondii. Cryo-Letters 14:381-386. [Google Scholar]
- 38.Popescu, C., D. Steriu, and C. David. 1995. Effect of cryoprotective additives and cryopreservation protocol on Plasmodium berghei. Cryo-Letters 16:353-358. [Google Scholar]
- 39.Russell, J. B., W. M. Sharp, and R. L. Baldwin. 1979. The effect of pH on maximum bacterial growth rate and its possible role as a determinant of bacterial competition in the rumen. J. Anim. Sci. 48:251. [DOI] [PubMed] [Google Scholar]
- 40.Shinchi, S., and M. Abe. 1987. Species of rumen ciliate protozoa which produce extracellular protease and influence bacteria on its activity. Jpn J. Zootechnol. Sci. 58:72-79. [Google Scholar]
- 41.Shirakashi, R., and I. Tanasawa. 1998. Method of designing pre-freezing protocol in cryopreservation of biological materials. Ann. N. Y. Acad. Sci. 858:175-182. [DOI] [PubMed] [Google Scholar]
- 42.Sukhato, P., S. Thongsodseang, A. Utha, and N. Songsasen. 2001. Effects of cooling and warming conditions on post-thawed motility and fertility of cryopreserved buffalo spermatozoa. Anim. Reprod. Sci. 67:67-77. [DOI] [PubMed] [Google Scholar]
- 43.Tanasawa, I. 1998. Things we do not know about cryopreservation of biological organs. Ann. N. Y. Acad. Sci. 858:227-234. [DOI] [PubMed] [Google Scholar]
- 44.Ushida, K., and J. P. Jouany. 1996. Methane production associated with rumen ciliated protozoa and its effect on protozoa activity. Lett. Appl. Microbiol. 23:129-132. [DOI] [PubMed] [Google Scholar]
- 45.Veira, D. M., M. Ivan, and P. Y. Jui. 1983. Rumen ciliate protozoa: effects on digestion in the stomach of sheep. J. Dairy Sci. 66:1015-1022. [DOI] [PubMed] [Google Scholar]
- 46.Viveiros, A. T. M., E. J. Lock, H. Woelders, and J. Komen. 2001. Influence of cooling rates and plunging temperatures in an interrupted slow-freezing procedure for semen of the African catfish, Clarias gariepinus. Cryobiology 43:276-287. [DOI] [PubMed] [Google Scholar]
- 47.Williams, A. G., and G. S. Coleman. 1992. The rumen protozoa. Springer-Verlag, New York, N.Y.
- 48.Wolfe, J., and G. Bryant. 2001. Cellular cryobiology: thermodynamic and mechanical effects. Int. J. Refrig. 24:438-450. [Google Scholar]
- 49.Yiannikouris, A., and J. P. Jouany. 2002. Mycotoxins in feeds and their fate in animals: a review. Anim. Res. 51:81-99. [Google Scholar]
- 50.Yoder, R. D., A. Trenkle, and W. Burroughs. 1966. Influence of rumen protozoa and bacteria upon cellulose digestion in vitro. J. Anim. Sci. 25:609-612. [DOI] [PubMed] [Google Scholar]