Abstract
Biologically conjugated quantum dots (QDs) have shown great promise as multiwavelength fluorescent labels for on-chip bioassays and eukaryotic cells. However, use of these photoluminescent nanocrystals in bacteria has not previously been reported, and their large size (3 to 10 nm) makes it unclear whether they inhibit bacterial recognition of attached molecules and whether they are able to pass through bacterial cell walls. Here we describe the use of conjugated CdSe QDs for strain- and metabolism-specific microbial labeling in a wide variety of bacteria and fungi, and our analysis was geared toward using receptors for a conjugated biomolecule that are present and active on the organism's surface. While cell surface molecules, such as glycoproteins, make excellent targets for conjugated QDs, internal labeling is inconsistent and leads to large spectral shifts compared with the original fluorescence, suggesting that there is breakup or dissolution of the QDs. Transmission electron microscopy of whole mounts and thin sections confirmed that bacteria are able to extract Cd and Se from QDs in a fashion dependent upon the QD surface conjugate.
Colloidal quantum dots (QDs) are semiconductor nanocrystals whose photoluminescence emission wavelength is proportional to the size of the crystal. The emission spectra of QDs are narrow, which allows multiwavelength labeling with different sizes of QDs with little overlap. Their outer surfaces can be readily conjugated to organic molecules, resulting in a spectrum of biologically compatible labels that are all excited with a single wavelength (5, 6). Although solubilization with thiol compounds, such as mercaptoacetic acid (MAA) and mercaptoundecanoic acid, has recently been shown to yield surface coatings that are unstable in water due to photooxidation (1), here we describe conditions under which bare CdSe QDs may be used in biological applications (in this study, bare refers to CdSe nanocrystals that are not capped with any other semiconductor material or with a polymer).
A panel of QDs conjugated to molecules that label bacteria specifically according to strain, metabolism, surrounding conditions, or other factors would be extremely useful for a wide range of applications. One potential use is to study complex microbial populations, such as biofilms. Associations of microbes into biofilms result in properties that are very different from those of the individual cells, with resulting environmental, medical, and technological implications (2, 11, 15, 30).
Fluorescent probes also play an important role in the detection of small numbers of bacteria in extreme environments, most species of which cannot be cultured (13). The ability to use fluorescence detectors in situ would allow incorporation of the detectors into earth instruments, such as those used for probing Antarctica's Lake Vostok, as well as development of instruments for detection of bacteria or remains of bacteria elsewhere in the solar system (14). QDs have several advantages over organic dyes for these applications. Their broad absorption spectrum means that a single diode laser can be used to excite an entire labeling suite. Of particular interest for space applications, at least some types of QDs are radiation resistant, more so than comparable two- and three-dimensional semiconductor devices (16). Most importantly, the band structure of bare QDs means that it possible that they can be turned on and off chemically, so it is possible that they could made into zero-background labels that fluoresce only in the presence of a desired compound (26). For this reason we investigated labeling with CdSe nanocrystals that are not coated with ZnS or polymers (such coatings are present on all commercially available QDs). These nanocrystals are also smaller than coated QDs and may be able to cross cell walls and membranes more easily (J. A. Kloepfer, R. E. Mielke, and J. L. Nadeau, submitted for publication).
In this paper we describe two types of CdSe QD conjugates used to label microorganisms. The first type exhibits gram-positive specificity with wheat germ agglutinin (WGA), a lectin. Lectins are a large, structurally heterogeneous family of proteins or glycoproteins that associate selectively with mono- and oligosaccharide components of polysaccharide structures. All kingdoms of life produce specific lectins, and fluorescent conjugates of these proteins may be used to investigate polysaccharide structure and function, to selectively label microorganisms, and to track specific cell types and lineages in in vitro systems, including flow cytometry, histology, and protein blotting systems (for a review, see reference 17). WGA, which binds to sialic acid and N-acetylglucosaminyl residues, is one of the most commonly used lectins in cell biology and has many applications. For example, it adheres to gram-positive cell walls but not to gram-negative cell walls (25). It is also axonally transported in neurons (22) and labels cell nuclei (28).
The second conjugate which we describe here takes advantage of the ability of pathogenic bacteria to harvest iron from human transferrin. Bacteria cannot live within a host unless they can obtain iron, which they usually do by hijacking the host's own shuttle proteins and extracting the iron. The presence of receptors for human transferrin is highly correlated with virulence (20). Tracking these proteins with fluorescent labels thus provides not only a method of identifying pathogenic bacteria but also a method of elucidating biochemical processes involved in bacterial iron acquisition. This approach to labeling is conceptually different from that in which lectins are used. Transferrin labeling is contingent upon the bacteria being alive and metabolically active. Here we describe the ability of QD-transferrin conjugates to promote the growth of Staphylococcus aureus in iron-deprived conditions and to label the bacteria internally; comparable results were not obtained with nonpathogenic cocci.
MATERIALS AND METHODS
Unless stated otherwise, all chemicals were purchased from Sigma or Aldrich.
Synthesis and storage of QDs.
The QDs used in this study were CdSe nanocrystals synthesized by using a recently developed method in which cadmium acetate and selenium metal take the place of the usual organometallic precursors (M. S. Wong and G. D. Stucky, Abstr. Pap. Am. Chem. Soc. 221:U572, 2001). Elemental Se was incubated for at least 24 h in trioctylphosphine oxide (TOPO), generating trioctylphosphine-Se, which was then injected along with the Cd precursor into a TOPO solution at 250 to 350°C and baked for 30 s to 30 min. The resulting QDs were washed several times in methanol, in which they were insoluble, and finally suspended in dichloromethane. This resulted in CdSe particles passivated with TOPO (passivation refers to the elimination of crystal surface defects with an external coating; without passivation, fluorescence quantum yield is greatly reduced). Physical characterization of the particles was carried out by performing transmission electron microscopy with energy dispersive spectroscopy.
Solubilization.
Cap exchange with MAA was used to replace the TOPO with a water-soluble layer, which allowed the QDs to be suspended in an aqueous solution (6). Briefly, 0.48 ml of MAA was added to 2.5 ml of TOPO-capped QDs in dichloromethane and rocked gently for 2 h in the dark. After addition of 2.5 ml of phosphate-buffered saline (PBS) (pH 7.5), the solution was vigorously agitated, and the layers were then allowed to separate naturally. The pigmented layer was removed and washed two or three times in PBS by centrifugation (5 min at 18,000 × g in a microcentrifuge) with removal of the supernatant. Finally, the resulting colloid was suspended in 1 to 2 ml of PBS, placed into a microdialysis chamber (Slide-A-Lyzer 10K dialysis cassette; Pierce Chemical Company, Rockford, Ill.), and dialyzed against 2 liters of PBS for 1 h to remove the residual MAA. The resulting water-solubilized QDs were stored at room temperature in the dark, unless stated otherwise. The QDs obtained are referred to below as MAA-QDs, bare QDs, or unconjugated QDs.
Protein conjugation.
Solubilized QDs were conjugated to molecules with a free primary amine by using the activator 1-ethyl-3-(3-dimethylaminopropyl) carbodiimide hydrochloride (EDC). Each 1-ml reaction mixture in PBS contained 1 to 2 mg of EDC, 0.025 to 1 mg of protein, and 200 μl of the solubilized QD solution with an optical density of 0.1 at the excitation peak (the approximate concentration was 1 μM, assuming a particle diameter of 3.5 nm and an extinction coefficient of 105 cm−1 M−1 [23]). Conjugation was performed for 2 h in the dark, unless otherwise stated, and unbound conjugate was removed by dialysis or centrifugation and washing in distilled H2O. Formation of amide bond linkages was confirmed by Fourier transform infrared spectroscopy (27). To avoid confounding variables, conjugates used for bacterial labeling experiments were added to the cells within at most 3 to 4 h after preparation, unless stated otherwise.
Microorganism strains.
All organisms used were wild-type strains purchased from the American Type Culture Collection, Manassas, Va.; all media were Difco media (BD Biosciences, Franklin Lakes, N.J.). The incubation temperatures were 37°C for Escherichia coli ATCC 25922, Pseudomonas aeruginosa ATCC 10145U, Staphylococcus warneri ATCC 17917, Staphylococcus saprophyticus ATCC 15305, Staphylococcus epidermidis ATCC 12228, and Staphylococcus aureus ATCC 29213; 30°C for Bacillus subtilis ATCC 9372,Bacillus megaterium ATCC 14581,Micrococcus luteus ATCC 14581, and Schizosaccharomyces pombe ATCC 2476; and room temperature for Penicillium chrysogenum ATCC 9783. Most bacteria were grown as clonal cultures in Luria-Bertani broth; the only exception was S.aureus, which was grown in brain heart infusion medium. S. pombe and P. chrysogenum were grown in malt yeast extract.
Labeling of bacteria with QDs and controls.
Bacteria were grown in recommended media and under recommended growth conditions until they reached the exponential growth phase. Approximately 100 μl of bacterial suspension was then mixed with approximately 650 μl of 2× growth medium and approximately 250 μl of QD conjugate in distilled H2O. Controls were prepared by adding each of the following to a preparation containing 100 μl of bacterial suspension and 650 μl of growth medium: 250 μl of distilled H2O; 250 μl of unconjugated QDs; approximately 0.25 mg of protein per ml in H2O; and approximately 2 mg of EDC in H2O. Each 1-ml reaction mixture was incubated for 10 min to 8 h (incubation times were chosen empirically; 10 to 20 min was sufficient for external labeling, whereas 3 to 5 h was ideal for visualizing internalization, as discussed below). The cells were then pelleted, washed at least twice in H2O or 1% (wt/vol) aqueous NaCl, resuspended, and prepared for imaging. Unused labeled cells were stored at 4°C in the dark for up to 18 months to track QD fluorescence changes with time and cell degradation.
Epifluorescence microscopy.
Fluorescence microscopy was used to examine the extent, specificity, and cellular localization of QD labeling. Bacteria were flame fixed and examined unstained by using a Nikon Eclipse E600 upright microscope with a Nikon PlanFluor 100 × lens (N.A. = 1.30) (stains, such as Gram stains, create undesirable autofluorescence). Epifluorescence images were obtained with a modified UV-DAPI (4′,6′-diamidino-2-phenylindole) filter cube set (excitation wavelength, 330 to 380 nm; dichroic wavelength, 400 nm; emission, 435 long pass). There appeared to be no difference between bacteria that were flame fixed on a microscope slide and samples that were observed wet by using a coverslip.
Spectrophotometry.
Emission spectra were used to corroborate and quantify epifluorescence images and to detect features outside the visible range. Spectra were collected with a Hitachi F-4500 fluorescence spectrophotometer illuminated by a 100-W Xe lamp. Spectra were typically taken at a scanning rate of 1,200 nm/min with 5-nm excitation and emission slits and a 950-V photomultiplier tube voltage. Efforts were made to keep the samples dilute, so that their optical densities at 400 nm were ≤0.05. When a higher optical density was necessary in the case of weakly fluorescent samples, the fluorescence measurements were treated qualitatively. Absorbance spectra were recorded with a Hewlett-Packard 8453 UV-visible spectrophotometer.
Transmission electron microscopy sectioning and imaging.
Unstained whole mounts were prepared by depositing 10 to 20 μl of fresh (collected within 24 h) bacteria in distilled H2O onto carbon-coated Cu grids. After 2 min, the excess solvent was wicked away with filter paper, which left behind cells deposited on the grid surface. After the grids were allowed to air dry, they were washed two or three times in distilled H2O to remove any traces of buffer. Thin sections were prepared by using freshly incubated bacteria in culture medium. Samples were fixed overnight at 4°C in 2.5 to 3% (vol/vol) glutaraldehyde and washed three times in distilled H2O; one-half of the samples were stained by postfixing in osmium tetroxide and staining with uranyl acetate, while the other half were left unstained. Both stained and unstained specimens were then dehydrated in ethanol and acetone before they were embedded in EPON resin (4). An Akashi EM-002B microscope operating at 100 kV was used for microscopy and also for energy dispersive X-ray spectroscopy of unstained whole mounts. The area sampled by the Oxford spectrum analyzer was approximately 8.8 nm. The acquisition rates were maintained at 10 to 20% dead time with 60 s of live time at 83,000×. The electron beam was defocused at the condenser lens to maintain the counting rate below 1 kHz and a live time efficiency of >95%.
RESULTS
Photoluminescence of CdSe QDs in aqueous solution.
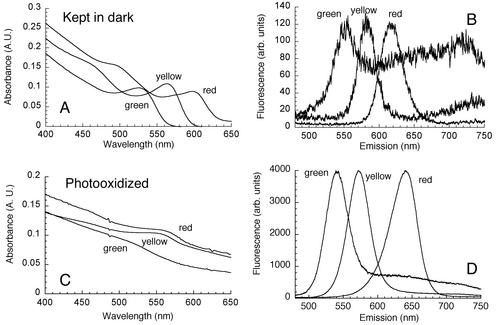
It has recently been reported that MAA-conjugated QDs in aqueous solution are unstable under photooxidation conditions (1). Oxidation is visibly detected by precipitation of the QDs from the solution. This is usually accompanied by broadening of the first exciton transition in the absorption spectrum and a large increase in photoluminescence as the surface is passivated with SeO2 (7). We delayed the oxidation process by preparing and storing solubilized samples in the dark. These samples remained highly soluble for 2 to 3 months, and any QDs that precipitated were discarded.
Unoxidized CdSe QDs that were solubilized and dissolved in PBS appeared to be orange to dark red, with an absorption peak at 700 to 500 nm depending on the size of the QDs (Fig. 1A) (21). Correspondingly, the emission spectra had narrow fluorescence peaks in the green to red range when the QDs were excited with near-UV light (350 to 400 nm), with a typical half-width at half-maximum of 33 ± 6 nm (Fig. 1B). Lower energy emission from surface trap states was also observed, particularly for the green-fluorescing QDs shown in Fig. 1B. On average, freshly solubilized QDs were only weakly fluorescent, which was probably a result of quenching by the attached MAA (1). Fluorescence remained weak when the QDs were stored in the dark; it was enhanced up to 100-fold by photooxidation, and this was accompanied by an approximately 10-nm blue shift regardless of the initial color of the QDs (Fig. 1C and D) with precipitation. CdSe QDs were highly sensitive to pH and appeared to decompose in acidic solutions (pH < 5.0); most of our measurements were obtained at a neutral pH (pH 7.0 ± 1.0). No dependence on solution ionic strength was observed.
FIG. 1.
CdSe QD photoluminescence in physiological saline. (A) Visible absorbance spectra for three independent preparations of bare CdSe QDs, solubilized with MAA and dissolved in PBS. The QDs were labeled on the basis of their fluorescence emission (green, yellow, and red). (B) Emission spectra of the samples in panel A, showing both the visible peaks and the near-infrared trap emission. Excitation was at 400 nm. (C) After 2 to 24 h of exposure to room light, the absorbance peak was markedly flattened. (D) Changes in the absorbance spectrum were accompanied by dramatic increases in emission. The values are the values for samples from panel A diluted 10-fold. There was also a 10-nm emission blue shift for each color. A.U., absorbance units; arb. units, arbitrary units.
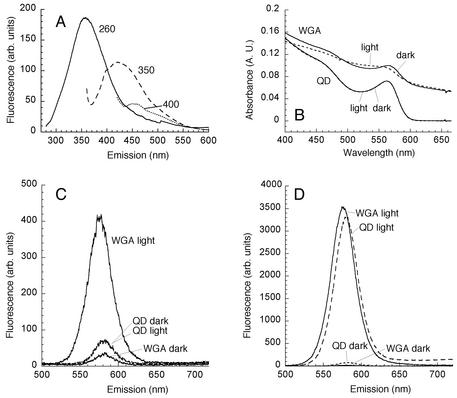
The visible peaks of CdSe QDs were clearly distinct from bacterial autofluorescence, which was maximally excited by deep UV and which occurred as a broad curve around 350 nm, visualized as blue in all species (Fig. 2A). In in situ labeling experiments with soil or rocks, autofluorescence was also observed from minerals. Like bacterial autofluorescence, it was mostly concentrated in the UV to blue emission range. Bacterial autofluorescence was only partially excited with near-UV light (Fig. 2A). When preparations were excited with deep UV, signals were seen in the range from 280 to 350 nm; these signals were produced by the presence of proteins and medium ingredients (data not shown) (fluorescence peak of tryptophan, ∼350 nm; fluorescence peak of transferrin, ∼330 nm). Deep UV light also excited the cross-linking reagent EDC, which produced a peak at ∼290 nm. Not only did these signals obscure the QD signal, but resonance energy transfer may have occurred between QDs and a conjugated protein when preparations were excited at or near the protein absorbance peak at 280 nm (18). For these reasons, visible or infrared imaging of biological specimens is preferred, and the excitation wavelength for spectra that was chosen for all spectrofluorimetry experiments was 400 nm.
FIG. 2.
Conjugation of QDs to a lectin via amide bonds created useful biological labels. (A) S. aureus emission spectra at different excitation wavelengths, including 260 nm (solid line), 300 nm (dashed line), and 400 nm (dotted line). The spectra are characteristic of the spectra of a wide range of bacteria and microscopic fungi. (B) QDs conjugated to WGA for 2 h under room light (WGA light) or in the dark (WGA dark). This amount of light exposure had no effect on bare QDs (QD light and QD dark), but the WGA conjugates showed increased absorption with broadening of the exciton peak, which was more pronounced in the light than in the dark. (C) While the dark-conjugated QDs showed a small decrease in emission, the QDs conjugated under room light exhibited a more-than-fourfold increase. (D) After 24 h under room light, both the bare QDs and the WGA-conjugated QDs exhibited a large increase in emission. This was due to photooxidation and was accompanied by irreversible precipitation of the QDs. A.U., absorbance units; arb. units, arbitrary units.
Unconjugated QDs adhere to some bacterial strains.
Both bacteria and the yeast S. pombe were incubated with bare CdSe QDs (i.e., nanocrystals coated only with MAA), and no internalization was observed (in spore-forming species, both vegetative cells and spores were tested). However, in some cases there was external labeling. Bacteria were incubated with MAA-coated QDs for 10 min to 5 h, and then the solution was centrifuged for 1 min at 14,000 rpm. When the bacteria were externally labeled, the bacterial pellet was the color of the QDs; when there was no external labeling, the QDs remained in solution and the bacterial pellet was white. Maximum binding occurred by the 10-min time point. Table 1 summarizes the results when MAA-coated QDs were used as external labels. S. aureus and S. epidermidis were readily labeled by bare QDs, whereas S. saprophyticus,S. warneri, and E. coli were not. There appeared to be no distinction between gram-positive and gram-negative cells.
TABLE 1.
Abilities of unconjugated and conjugated QDs to label microorganisms
| Microorganism | Labeling witha:
|
|
|---|---|---|
| QD-MAAb | QD-WGA conjuate | |
| E. coli | − | − |
| B. subtilis | − | + |
| B. megaterium | − | NDc |
| S. epidermidis | + | ++ |
| S. aureus | + | ++ |
| S. saprophyticus | − | ++ |
| S. warneri | − | ++ |
| M. luteus | ND | ++ |
| P. aeruginosa | ND | − |
| S. pombe | − | − |
| P. chrysogenum | + | + |
−, no adherence of QDs to cells; +, some adherence of QDs to cells and some outlining of cells with QDs; ++, excellent external labeling and adherence to pellet.
MAA cap, unconjugated.
ND, not done.
WGA conjugation preserves binding specificity.
WGA and other lectins reduced the solubility of MAA-coated QDs. Their presence free in solution or conjugated to the QDs resulted in formation of aggregates and pelleting of the QDs upon centrifugation for 1 min at 3,000 rpm unless precautions were taken to maximize solubility. Practices that improved the solubility of conjugates included use of fresh MAA for solubilization of the QDs, use of low levels of protein and carbodiimide (1 mg of EDC and 0.025 mg of lectin in a 1-ml reaction mixture), and blocking of the carboxyl groups of the lectin by reaction with Tris buffer (10). It was also possible to eliminate the EDC altogether and allow the lectin to adhere to the surface of the QDs by electrostatic forces during a 1- to 2-h incubation; in this case, more protein had to be used (0.1 to 0.25 mg/1-ml reaction mixture). Aggregates did not show specific labeling; after conjugation, the solution was centrifuged at bacterial pelleting speeds, and the insoluble fragments were discarded.
Specific labeling could be achieved with conjugates prepared under both dark and light conditions. Conjugation of solubilized QDs to WGA for 2 h had moderate effects on the absorption and emission spectra when the solution was kept in the dark. Overall absorption was increased, and the solution was visibly cloudier, but the position of the emission peak and half-width at half-maximum were not affected when QDs were excited at 400 nm. Exposure to light during the conjugation period resulted in a dramatic increase (more than 25-fold) in emission and significant changes in the absorption spectrum, with marked broadening and shift of the first exciton peak (Fig. 2B and C). The mechanisms behind these effects are still being determined. Aggregation and precipitation occurred in light-exposed conjugates after several hours, and an additional large increase in emission indicated that there was photooxidation (Fig. 2D). After this occurred, the QDs could not be used for labeling bacteria.
Gram-positive specificity was preserved both with QD-conjugated WGA and with WGA bound electrostatically to QDs (no EDC). Labeling was detectable grossly, microscopically, and spectroscopically after 10 to 20 min of incubation with live or dead bacteria. When gram-positive bacteria incubated with QD-WGA conjugates were pelleted, the orange-red QD conjugate remained an integral part of the pellet, and the supernatant was colorless. When the pellet was washed with clean water and resuspended, the solution was the color of the QDs, and vigorous pipetting could not separate the QDs from the bacteria. In contrast, the gram-negative bacterial pellet was white, and most of the QDs remained in solution. Upon washing, the pigment was removed, and the resuspended bacteria appeared to be white (data not shown).
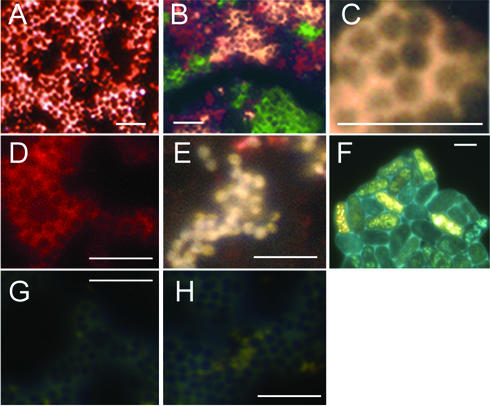
As determined by optical microscopy, QD-WGA-labeled gram-positive bacteria exhibited fluorescence all around their outer surfaces (Fig. 3A to C). No labeling was seen on gram-negative bacteria. P. chrysogenum exhibited patchy labeling due to chitin binding; no labeling was seen with the chitin-free organism S. pombe (data not shown) (3). Multiple labeling of separate populations of bacteria with different colors of QD-WGA conjugates allowed cultures to be mixed and monitored separately; at least three colors could be used simultaneously with excellent visual separation (Fig. 3B).
FIG. 3.
Microscopic appearance of QD-labeled organisms: micrographs of stained and unstained bacteria obtained by epifluorescence microscopy. Magnification for all images, ×1,000 (scale bars = 5 μm). (A) M. luteus incubated with red QD-WGA conjugates (conjugated under room light) for 30 min. The labeling was extremely bright, saturating the camera gain in many places (the image was deliberately overexposed for comparison with panel D). (B) Triple labeling with red, yellow, and green QD-WGA conjugates. S. aureus cultures were labeled separately for 30 min and then mixed. (C) Close-up of a section of panel B, showing uniform external labeling by yellow QD-WGA conjugates. (D) S. epidermidis incubated with red QD-holotransferrin conjugates for 3 h at 30°C. External labeling is apparent, but there was no internal loading. The gain and exposure time were identical to those in panel A, demonstrating the decreased brightness of these conjugates compared with the brightness of the light-exposed lectins. (E) S.aureus incubated with red QD-holotransferrin conjugates for 3 h at 37°C and then stored for 2 weeks in the dark at 4°C. The labeling permeated the entire bacterial cell, often obscuring autofluorescence. The QDs outside the cell retained their original color, whereas those inside the bacteria were significantly blue shifted. Similar labeling was seen when the QD-transferrin conjugates were allowed to photooxidize before incubation (data not shown). (F) S. pombe incubated with green QD-holotransferrin conjugates for 3 h at 30°C. Rapid and intense internal labeling was apparent, and no QDs were visible in the medium outside the cells. Similar observations were made with P. chrysogenum (data not shown). (G and H) S. epidermidis (G) and S. aureus (H) after 3 h of incubation with QD-holotransferrin conjugates and a 10-fold excess of unlabeled holotransferrin. Most QDs did not adhere to the cells and were removed from the pellet by washing; there were a few QDs unassociated with cells in the medium.
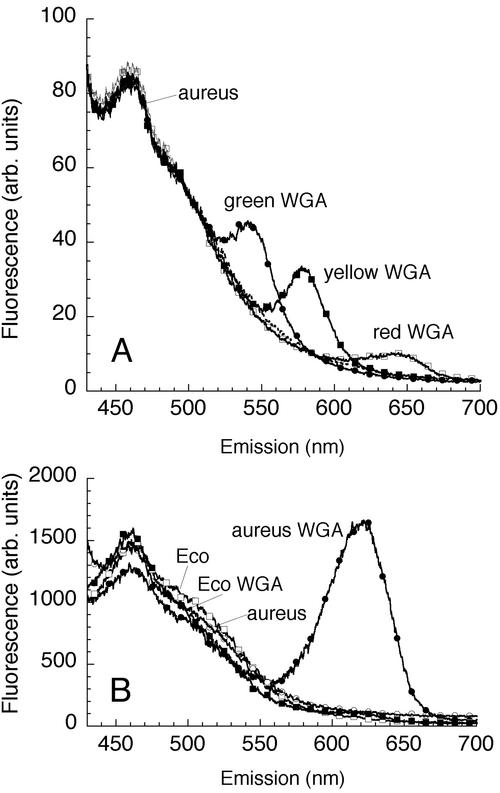
Spectrofluorimetry of washed pellets confirmed that there was no change in gram-negative autofluorescence during incubation with QD-WGA conjugates and that there was a large peak in the spectrum of gram-positive bacteria; the peaks of green, yellow, and red QDs exhibited almost no overlap (Fig. 4A). Conjugates allowed to sit under room light for several days exhibited increased fluorescence with a blue shift, which is consistent with photooxidation of the QDs, although the specific signal remained (Fig. 4B).
FIG. 4.
Spectroscopic signal of specific QD-WGA conjugate labeling. (A) QD-WGA conjugates specifically labeled the gram-positive bacterium S. aureus (aureus). At least three colors were readily distinguishable. Bacterial cultures were labeled separately for 30 min and then mixed. (B) After 2 weeks of incubation at 4°C under room light, the spectrum of red QD-WGA conjugates bound to S. aureus showed the typical enhancement and blue shift that indicated photooxidation; however, lack of binding to the gram-negative bacterium E. coli (Eco) was retained. arb. units, arbitrary units.
Transferrin conjugation preserves protein function and increases solubility.
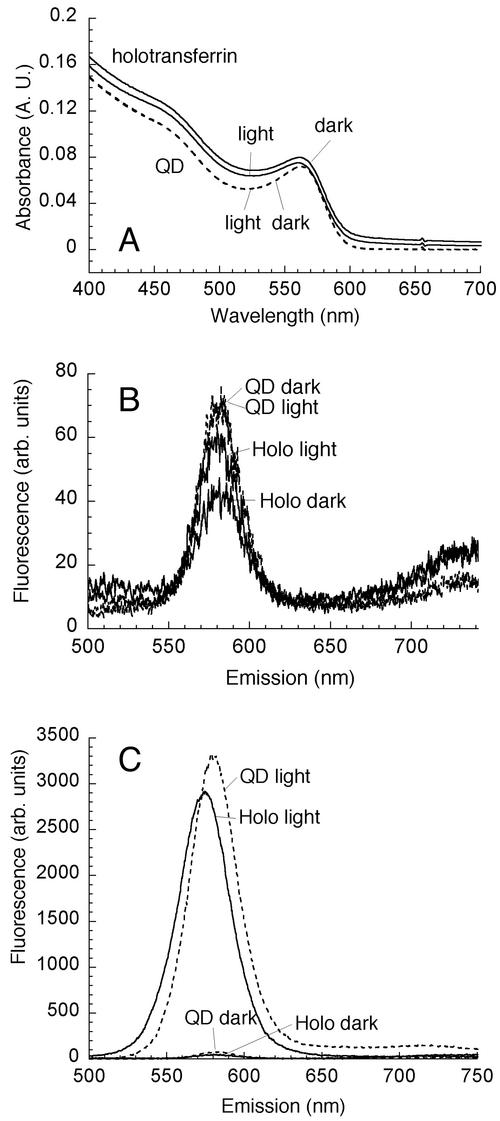
As with conjugation of QDs to lectins, conjugating QDs to human transferrin over several hours resulted in only small spectral changes when the procedure was performed in the dark. However, in marked contrast to the results obtained with WGA, exposure of the solution to room light during conjugation had very little effect (Fig. 5A and B). The peak wavelength and the half-width at half-maximum were not affected, and the quantum yield was changed by no more than 50% in either direction. The results shown here are the results for iron-bound holotransferrin, but the results obtained with iron-free apotransferrin were similar (data not shown).
FIG. 5.
Conjugation of QDs to transferrin created stable, soluble labels. (A) Holotransferrin conjugation in either dark (holotransferrin dark) or light (holotransferrin light) conditions resulted in little change in the first exciton absorbance peak compared with control orange QDs (QD). (B) There was correspondingly little change in emission, and the light-exposed conjugates (Holo light) were nearly indistinguishable from the controls (QD light and QD dark). A 40% decrease in emission was seen upon conjugation in the dark (Holo dark). The emission peak at 590 nm was not affected. (C) After 3 days, QDs kept in the dark had not changed compared with the baseline data, while the fluorescence emission of QDs exposed to room light had increased more than 40-fold. MAA-solubilized QDs (QD light) exhibited a 10-nm blue shift compared with the baseline data, with peak at 580 nm, and holotransferrin conjugates (Holo light) were blue shifted an additional 10 nm, to 570 nm. A.U., absorbance units; arb. units, arbitrary units.
Aggregation and precipitation did not occur with these conjugates; in fact, transferrin-QD conjugates were more soluble than bare MAA-solubilized QDs under room light and were not precipitated by centrifugation at 14,000 rpm in a microcentrifuge. Conjugates have been stored for more than 1 year in the dark without precipitation. However, when the conjugates were exposed to light, large increases in fluorescence were eventually seen after incubation for 4 weeks or more at 4°C, indicating that QD photooxidation occurred (Fig. 5C). Despite this, the conjugates remained soluble for several weeks and could be used to label cells.
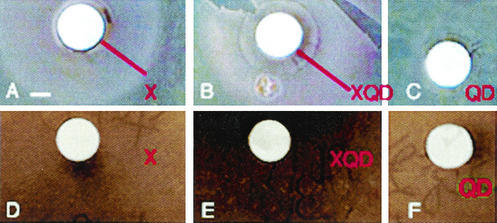
Transferrin remained functional after conjugation with QDs, as shown by several tests. First, the ability of QD-transferrin conjugates to promote growth in iron-deprived conditions correlated with the presence of receptors for the protein on the bacterium (Table 2). Visible internal labeling was also seen only in bacteria that are able to use transferrin-bound iron, although external labeling may have occurred in other species, particularly S. epidermidis, but not in S. warneri, S. saprophyticus, or M. luteus (Fig. 3D to F). Binding was eliminated by blocking receptors with a 10-fold excess of unlabeled transferrin (Fig. 3G and H). Second, transferrin-conjugated QDs acted just like unbound transferrin as a fungistatic agent (24); mixed cultures of Penicillium and Micrococcus sp. or Staphylococcus sp. exhibited a progressive loss of fungal cells over a period of 3 days, and there was no effect on the bacteria (data not shown). Finally, transferrin receptor-positive bacteria in iron-deprived conditions were rescued equally well by holotransferrin and QD-holotransferrin conjugates (Fig. 6) (the zone of growth was 3 mm for transferrin and for QD-transferrin conjugates in S. aureus disk assays, as determined with three independent preparations).
TABLE 2.
Abilities of human transferrin (iron loaded) and QD-conjugated transferrin to support growth of bacteria in iron-deprived media and correlation with the presence of transferrin receptors and with fluorescent labelinga
| Organism | Pathogen | Receptor | Plate assay zone (mm) with transferrin-QDb | Fluorescent labelingc |
|---|---|---|---|---|
| E. coli | No | No | 0/0 | − |
| B. subtilis | No | No | 0/0 | − |
| S. epidermidis | Sometimes | Sometimesd | 0/0 | + (external) |
| S. aureus | Yes | Yes | 3 ± 1/3 ± 1e | ++ (internal and external) |
| S. pombe | No | (Endocytosis) | NDf | ++ (internal) |
Zone of growth around a standard disk on an agar plate containing growth medium and 1 mM bipyridyl (value for disks soaked in a solution containing 2 mg of holotransferrin per ml/value for disks soaked in a solution containing QD transferrin conjugates).
−, absent; +, present; ++, very strong.
Data from reference 19.
Means ± standard deviations for three assays.
ND, not done.
FIG. 6.
Holotransferrin and QD-holotransferrin conjugates promoted the growth of S. aureus but not S. epidermidis in disk assays. Plates contained ordinary growth media with 1 mM bipyridyl as an iron chelator. Scale bar = 1 mm. (A) S. aureus plus holotransferrin (X), showing darker ring of enhanced growth; (B) S. aureus plus QD-holotransferrin conjugates (XQD), showing a ring of the same (even slightly larger) size; (C) S. aureus plus unconjugated QDs (QD), showing no growth enhacement; (D) S. epidermidis plus holotransferrin, showing no enhancement; (E) S. epidermidis plus QD-holotransferrin conjugates, showing no enhancement; (F) S. epidermidis plus unconjugated QDs, showing no enhancement.
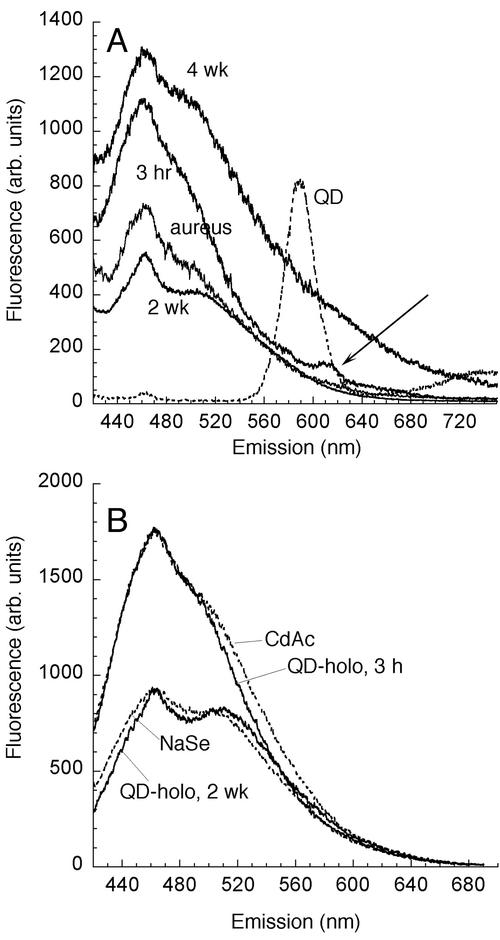
Because the labeling occurred with the processing of transferrin by the bacteria, labeling did not occur in minutes, as it did with lectins. In S. aureus cultures in the exponential growth phase, visible internal labeling began to be apparent only after 1 h, was maximal at approximately 3 to 4 h, and decreased thereafter as the bacteria multiplied and overwhelmed the signal. The label decreased almost to the point of invisibility by 7 h. Spectrofluorimetry of washed pellets yielded a small peak that was ∼20 nm red shifted from the peak of the original QDs (Fig. 7A); its relative strength was 1.0 at 1 h, 4.5 ± 0.2 at 3 h, and 0.6 ± 0.3 at 7 h, as determined with five independent preparations.
FIG. 7.
QDs were processed and broken down by S. aureus. (A) Emission spectra of S. aureus alone (aureus); S. aureus incubated for 3 h with freshly prepared (unoxidized) QD-holotransferrin conjugates (3 hr); and S. aureus incubated for 3 h with unoxidized conjugates and allowed to sit at 4°C under room light for 2 weeks (2 wk) or 4 weeks (4 wk). There was a large peak immediately apparent at ∼460 nm, and a feature appeared over time at ∼520 nm. A small peak at ∼615 nm was seen right after incubation (arrow), but it disappeared with time. Incubation with oxidized QD-holotransferrin conjugates led to a signal much like the 2-week spectrum (data not shown). The spectrum obtained at 4 weeks suggested that the cells were beginning to break up, as it was similar to the spectrum obtained with cells killed by heat shock or freezing and thawing (data not shown). (B) Comparison of spectra of S. aureus incubated with QD-holotransferrin conjugates (QD-holo) with spectra of S. aureus incubated with heavy metal salts (1 mM, 3 h). NaSe, sodium selenide; CdAc, cadmium acetate; arb. units, arbitrary units.
Due to the weak fluorescence of the QD-transferrin conjugates, the internal labeling strength in both dark and light conditions was low compared with the QD-WGA conjugate labeling strength. It was often observed best after photobleaching of autofluorescence or through a filter that did not excite bacterial autofluorescence (e.g., a fluorescein isothiocyanate filter set). The concentration of iron in the solution had no effect on transferrin labeling in S. aureus (as determined with more than 10 preparations in iron-depleted brain heart infusion medium compared with labeling in normal brain heart infusion medium).
Observation of the bacterial samples after 2 weeks of storage of the washed pellets at 4°C in the dark often revealed a striking increase in fluorescence, accompanied by a spectral blue shift of 40 ± 5 nm. Because the medium contained only distilled H2O, this did not represent new uptake but instead represented spectral changes in QDs that were taken up during the initial 3-h incubation period. These changes are typical of QD photooxidation, but in this case the oxidation was occurring only within the bacterial cells, not in the surrounding medium. Control QD-transferrin conjugates stored under room light, as well as the QDs that remained external to the bacteria in the washed samples, exhibited a smaller change in the emission wavelength during the same 2-week period (Fig. 3E).
In aged QD-transferrin conjugates that showed signs of photooxidation (but without loss of solubility), intense cell labeling was observed after the 3- to 5-h incubation period (Fig. 3E). In one case, the conjugates had been stored under room light for 18 months. Table 3 summarizes the preparation and storage conditions that led to useful labeling for both lectin- and transferrin-QD conjugates. In contrast, fungi showed rapid, intense internal labeling with QD-transferrin conjugates (Fig. 3F). As with S. aureus, labeling was independent of iron in the medium.
TABLE 3.
Characteristics of QDs and incubation conditions that yielded specific microbial labeling
| Prepn | Labeling aftera:
|
||
|---|---|---|---|
| 10 min | 3 h | 3 h + 2 wk at 4°C | |
| QD-WGA conjugates | + | + | ++ |
| QD-transferrin conjugates | 0 | +/− | + |
| Oxidized QD-WGA conjugates | 0 | 0 | 0 |
| Oxidized QD-transferrin conjugates | 0 | + | + |
QD-transferrin conjugtes oxidize much more slowly and do not precipitate; they are useful for labeling even in the oxidized state. +, bright labeling, easily visible with a strong spectrographic signal; ++, extremely bright labeling; 0, no labeling; +/−, weak or occasional labeling.
Internalization of Cd and Se occurs, but internalization of a measurable number of whole QDs does not occur.
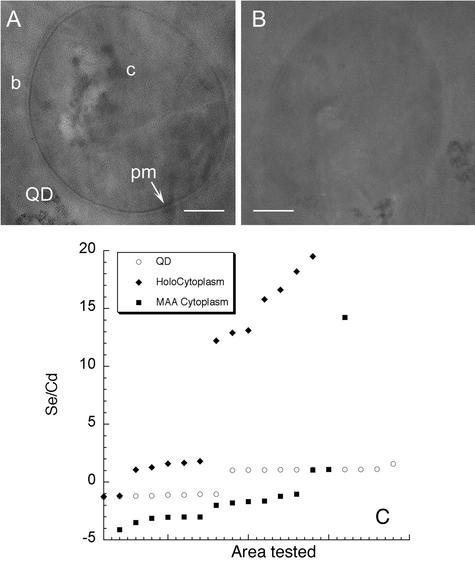
Most of the fluorescent signal of S. aureus incubated with QD-transferrin conjugates was not a narrow peak corresponding to the original QDs but rather was a broad peak significantly blue shifted from the peak of either the original or oxidized QDs (Fig. 7A). The spectrum of this peak did not vary with the emission spectrum of the QDs (data not shown) (the QDs tested were green [peak at 560 nm], yellow [peak at 590 nm], and red [peak at 620 nm] QDs). In fact, the spectrum was similar to the spectrum seen when S. aureus was incubated with Cd and Se salts instead of nanocrystals (Fig. 7B). Immediately after incubation with QD-holotransferrin conjugates, the peak corresponded to Cd, and Se seemed to increase over time (Fig. 7). Transmission electron microscopy of thin sections of labeled bacteria suggested that the nanocrystals were fragmented, leading to a significant concentration of Se inside the cytoplasm even after 3 h. Such an Se concentration was not seen in S. aureus incubated with unconjugated QDs (Fig. 8).
FIG. 8.
Transmission electron microscopy revealed Se uptake in cells incubated with QD-holotransferrin conjugates. Scale bars = 100 nm. (A) S. aureus cell after 3 h of incubation with QD-holotransferrin conjugates. Twelve cells from thin sections were used for analysis, and two areas within each cell were selected for energy dispersive spectroscopy of the cytoplasmic area (c), plasma membrane-cell wall (pm), background (b), and external QDs (QD). (B) Control cell, incubated for 3 h with MAA-QDs, showing less electron density around the cell perimeter and in the cytoplasm. (C) Of the 24 cytoplasmic areas tested, 12 in cells incubated with QD-holotransferrin conjugates (♦) showed a significant increase in the amount of Se compared with the amount in the original QDs (○). Only 1 of 24 MAA-QD spots showed such an increase (▪) (P < 0.001 for comparisons of cells incubated with QD-holotransferrin conjugates with original QDs and with cells incubated with MAA-QDs). Values less than zero indicate that the amount of Cd was less than the amount of Se. There was no statistically significant difference between the Cd concentrations in cells incubated with MAA-QDs and cells incubated with QD-holotransferrin conjugates or between the Cd concentrations in any cell area other than the cytoplasm.
DISCUSSION
The utility of colloidal QDs has been demonstrated previously for labeling of mammalian cells (29) and live slime molds (12) and in multicolor arrays (9). However, this is the first use of fluorescent nanocrystals for bacterial labeling. External labeling is a straightforward application whose advantages over organic dyes include narrow emission spectra, radiation hardness, and a single excitation wavelength. In this study, external labeling was demonstrated with both MAA- and WGA-coated bare CdSe QDs. The techniques described here should be equally applicable to CdSe/ZnS core shell QDs and other types of fluorescent nanocrystals.
WGA-QD conjugates retain their specificity for gram-positive cell walls, but weak solubility shortens the useful life span of the conjugates. Recent work implicates the numerous disulfides that are characteristic of the lectin family of proteins in these processes (1). It is also possible that there are strong hydrophobic and/or ionic interactions between some proteins and MAA-coated QDs, similar to what is observed with colloidal gold-labeled proteins (10). Our results suggest that chemical conjugation of WGA to MAA-QDs via EDC is not necessary.
Unlike WGA-conjugated QDs, transferrin-conjugated QDs are highly soluble and remain highly soluble for many months. Also, their interactions with microorganisms are noticeably different from those of WGA-conjugated QDs. Because bacteria do not endocytose but rely on transporters to shuttle nutrients across their cell walls, it was not known whether QDs that are 3 to 10 nm in diameter can enter a cell. As determined by optical microscopy, QDs conjugated to human transferrin showed clear internal labeling in both fungi (S. pombe, P. chrysogenum) and human pathogens (S. aureus), and there was no internal labeling of nonpathogenic staphylococci and micrococci; however, labeling of bacteria never appeared to be as quick or as bright as labeling of fungi, and the results have not been fully interpreted. Electron microscopy confirmed that incubation with transferrin-conjugated QDs leads to Cd and Se enrichment in the cytoplasm of S. aureus, but it is not known which enzymes are involved in the process, precisely how the fragments correspond to the fluorescence observed, or whether any nanocrystals enter the cell without being broken up.
The ability of the conjugates to inhibit fungal growth and their ability to rescue S. aureus from iron-deprived conditions indicate that the protein's function was not compromised by its attachment to the nanocrystal. This labeling technique may be useful for other iron-binding proteins and siderophores, and there are several different potential uses. First, the ability to utilize human transferrin indicates that a strain is pathogenic, and this may be used as a rapid test for invasive staphylococci. Second, the amount of transferrin uptake may vary with the level of iron in the medium for some strains (8), although it did not for S. aureus; this may be used in real-time studies of iron concentrations in complex microbial communities. Third, other iron-binding proteins and siderophores may be conjugated to QDs, allowing study of uptake and trafficking of these molecules.
Further work should elucidate the processes occurring within the bacterial cells that lead to QD spectral shifts and fluorescence enhancement. The photostability issue raised in previous work (1) has primarily been addressed by rigorously preparing and synthesizing QD conjugates under little or no room light. We chose uncapped QDs because of their susceptibility to environmental conditions and electron transfer, and the development of protocols for consistent handling of these particles should allow construction of more complicated probes, such as probes that respond to redox potential, pH, and other conditions. Work continues in our lab on optimizing both capped and uncapped QDs for microbiological applications.
Acknowledgments
This study was based upon work supported by the National Aeronautics and Space Administration under contract 1238316 issued through the NAI Office.
We thank Susanne Douglas for critical reading of the manuscript.
REFERENCES
- 1.Aldana, J., Y. A. Wang, and X. G. Peng. 2001. Photochemical instability of CdSe nanocrystals coated by hydrophilic thiols. J. Am. Chem. Soc. 123:8844-8850. [DOI] [PubMed] [Google Scholar]
- 2.Attaway, H., C. H. Gooding, and M. G. Schmidt. 2002. Comparison of microporous and nonporous membrane bioreactor systems for the treatment of BTEX in vapor streams. J. Ind. Microbiol. Biotechnol. 28:245-251. [DOI] [PubMed] [Google Scholar]
- 3.Barkai-Golan, R., D. Mirelman, and N. Sharon. 1978. Studies on growth inhibition by lectins of penicillia and aspergilli. Arch. Microbiol. 116:119-121. [DOI] [PubMed] [Google Scholar]
- 4.Beveridge, T. J., T. J. Popkin, and R. M. Cole. 1993. Electron microscopy, p. 42-71. In P. Gerhardt, R. G. E. Murray, W. A. Wood, and N. R. Krieg (ed.), Methods for general and molecular bacteriology. American Society for Microbiology, Washington, D.C.
- 5.Bruchez, M., Jr., M. Moronne, P. Gin, S. Weiss, and A. P. Alivisatos. 1998. Semiconductor nanocrystals as fluorescent biological labels. Science 281:2013-2016. [DOI] [PubMed] [Google Scholar]
- 6.Chan, W. C., and S. Nie. 1998. Quantum dot bioconjugates for ultrasensitive nonisotopic detection. Science 281:2016-2018. [DOI] [PubMed] [Google Scholar]
- 7.Dabbousi, B. O., J. Rodriguez Viejo, F. V. Mikulec, J. R. Heine, H. Mattoussi, R. Ober, K. F. Jensen, and M. G. Bawendi. 1997. (CdSe)ZnS core-shell quantum dots: synthesis and characterization of a size series of highly luminescent nanocrystallites. J. Phys. Chem. B 101:9463-9475. [Google Scholar]
- 8.Evans, R. W., and J. S. Oakhill. 2002. Transferrin-mediated iron acquisition by pathogenic Neisseria. Biochem. Soc. Trans. 30:705-707. [DOI] [PubMed] [Google Scholar]
- 9.Han, M., X. Gao, J. Z. Su, and S. Nie. 2001. Quantum-dot-tagged microbeads for multiplexed optical coding of biomolecules. Nat. Biotechnol. 19:631-635. [DOI] [PubMed] [Google Scholar]
- 10.Hermanson, G. T. 1996. Bioconjugate techniques. Academic Press, San Diego, Calif.
- 11.Hogan, D. A., and R. Kolter. 2002. Pseudomonas-Candida interactions: an ecological role for virulence factors. Science 296:2229-2232. [DOI] [PubMed] [Google Scholar]
- 12.Jaiswal, J. K., H. Mattoussi, J. M. Mauro, and S. M. Simon. 2003. Long-term multiple color imaging of live cells using quantum dot bioconjugates. Nat. Biotechnol. 21:47-51. [DOI] [PubMed] [Google Scholar]
- 13.Karner, M. B., E. F. DeLong, and D. M. Karl. 2001. Archaeal dominance in the mesopelagic zone of the Pacific Ocean. Nature 409:507-510. [DOI] [PubMed] [Google Scholar]
- 14.Kawasaki, Y. 1994. Development of detection system of extraterrestrial microorganisms. Biol. Sci. Space 8:103-113. [DOI] [PubMed]
- 15.Kuchma, S. L., and G. A. O'Toole. 2000. Surface-induced and biofilm-induced changes in gene expression. Curr. Opin. Biotechnol. 11:429-433. [DOI] [PubMed] [Google Scholar]
- 16.Leon, R. 2000. Changes in luminescence emission induced by proton irradiation: InGaAs/GaAs quantum wells and quantum dots. Appl. Phys. Lett. 76:2074-2076. [Google Scholar]
- 17.Lis, H., and N. Sharon. 1998. Lectins: carbohydrate-specific proteins that mediate cellular recognition. Chem. Rev. 98:637-674. [DOI] [PubMed] [Google Scholar]
- 18.Mamedova, N. N., N. A. Kotov, A. L. Rogach, and J. Studer. 2001. Albumin-CdTe nanoparticle bioconjugates: preparation, structure, and interunit energy transfer with antenna effect. Nano Lett. 1:281-286. [Google Scholar]
- 19.Matinaho, S., L. von Bonsdorff, A. Rouhiainen, M. Lonnroth, and J. Parkkinen. 2001. Dependence of Staphylococcus epidermidis on non-transferrin-bound iron for growth. FEMS Microbiol. Lett. 196:177-182. [DOI] [PubMed] [Google Scholar]
- 20.Modun, B., J. Morrissey, and P. Williams. 2000. The staphylococcal transferrin receptor: a glycolytic enzyme with novel functions. Trends Microbiol. 8:231-237. [DOI] [PubMed] [Google Scholar]
- 21.Murray, C. B., D. J. Norris, and M. G. Bawendi. 1993. Synthesis and characterization of nearly monodisperse Cde (E = S, Se, Te) semiconductor nanocrystallites. J. Am. Chem. Soc. 115:8706-8715. [Google Scholar]
- 22.Saleem, K. S., J. M. Pauls, M. Augath, T. Trinath, B. A. Prause, T. Hashikawa, and N. K. Logothetis. 2002. Magnetic resonance imaging of neuronal connections in the macaque monkey. Neuron 34:685-700. [DOI] [PubMed] [Google Scholar]
- 23.Schmelz, O., A. Mews, T. Basche, A. Herrmann, and K. Mullen. 2001. Supramolecular complexes from CdSe nanocrystals and organic fluorophors. Langmuir 17:2861-2865. [Google Scholar]
- 24.Shiraishi, A., and T. Arai. 1979. Antifungal activity of transferrin. Sabouraudia 17:79-83. [DOI] [PubMed] [Google Scholar]
- 25.Sizemore, R. K., J. J. Caldwell, and A. S. Kendrick. 1990. Alternate Gram staining technique using a fluorescent lectin. Appl. Environ. Microbiol. 56:2245-2247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tran, P. T., E. R. Goldman, G. P. Anderson, J. M. Mauro, and H. Mattoussi. 2002. Use of luminescent CdSe-ZnS nanocrystal bioconjugates in quantum dot-based nanosensors. Phys. Status Solid. B Basic Res. 229:427-432. [Google Scholar]
- 27.Wang, Y. A., J. J. Li, H. Y. Chen, and X. G. Peng. 2002. Stabilization of inorganic nanocrystals by organic dendrons. J. Am. Chem. Soc. 124:2293-2298. [DOI] [PubMed] [Google Scholar]
- 28.Wilson, G. L., B. S. Dean, G. Wang, and D. A. Dean. 1999. Nuclear import of plasmid DNA in digitonin-permeabilized cells requires both cytoplasmic factors and specific DNA sequences. J. Biol. Chem. 274:22025-22032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wu, X., H. Liu, J. Liu, K. N. Haley, J. A. Treadway, J. P. Larson, N. Ge, F. Peale, and M. P. Bruchez. 2003. Immunofluorescent labeling of cancer marker Her2 and other cellular targets with semiconductor quantum dots. Nat. Biotechnol. 21:41-46. [DOI] [PubMed] [Google Scholar]
- 30.Zhu, J., M. B. Miller, R. E. Vance, M. Dziejman, B. L. Bassler, and J. J. Mekalanos. 2002. Quorum-sensing regulators control virulence gene expression in Vibrio cholerae. Proc. Natl. Acad. Sci. USA 99:3129-3134. [DOI] [PMC free article] [PubMed] [Google Scholar]