Abstract
Cholera is a severe diarrheal disease caused by specific serogroups of Vibrio cholerae that are pathogenic to humans. The disease does not persist in a chronic state in humans or animals. The pathogen is naturally present as a free-living organism in the environment. Recently, it was suggested that egg masses of the nonbiting midge Chironomus sp. (Diptera) harbor and serve as a nutritive source for V. cholerae, thereby providing a natural reservoir for the organism. Here we report that V. cholerae O9, O1, and O139 supernatants lysed the gelatinous matrix of the chironomid egg mass and inhibited eggs from hatching. The extracellular factor responsible for the degradation of chironomid egg masses (egg mass degrading factor) was purified from V. cholerae O9 and O139 and was identified as the major secreted hemagglutinin/protease (HA/P) of V. cholerae. The substrate in the egg mass was characterized as a glycoprotein. These findings show that HA/P plays an important role in the interaction of V. cholerae and chironomid egg masses.
Cholera is a severe diarrheal disease that causes the death of many thousands of people each year and affects the lives of millions. This disease is caused by specific serogoups of Vibrio cholerae that are pathogenic to humans (22). Since 1991, the world has witnessed extension of the seventh pandemic into South America and South Africa, as well as the appearance of a previously unknown pathogenic serogroup of V. cholerae (O139) (23). The disease is not found in a chronic state in humans or animals, indicating that its natural reservoir is environmental (11).
Chironomids (Diptera; Chironomidae), the nonbiting midges, are the most widely distributed and frequently the most abundant insects in freshwater (1). Females lay egg masses on the water's edge, and each mass contains hundreds of eggs encased in a layer of gelatinous material. The presence of several thousand egg masses at one site is not unusual. In extreme cases, gelatinous layers several centimeters thick are formed (3, 18).
Recently, we suggested that chironomid egg masses are an intermediate host reservoir for V. cholerae. Chironomus egg masses collected from a waste stabilization pond settled out overnight as thousands of individual eggs, most of which did not hatch. V. cholerae O9 was isolated from the degraded egg masses. When new freshly collected egg masses were reinfected with V. cholerae O9, the egg masses were destroyed. V. cholerae grew on the chironomid egg masses as a nutritive source (4).
Hemagglutinin/protease (HA/P) is one of the main secreted proteases of V. cholerae, and it is usually associated with the stationary phase and starvation (2). HA/P was purified from V. cholerae O1 (7) and O139 strains (17), as well as from non-O1 strains (10, 16). All HA/P purified enzymes showed both hemagglutination and proteolytic activities. Honda et al. (10) compared the purified HA/P from V. cholerae O1 and non-O1 strains. They found that the proteases (or hemagglutinins) derived from V. cholerae O1 and non-O1 strains were immunologically identical, although two molecular masses (34 and 32 kDa) of the enzyme were observed in the O1 strain. Naka et al. (16) characterized the same two forms (34 and 32 kDa) of HA/P produced in V.cholerae non-O1. The N termini of the two forms were identical, suggesting that proteolytic processing in the C-terminal region of the 34-kDa HA/P resulted in the 32-kDa form. With this shift, the protease activity increased, but the hemagglutinating activity decreased (16).
Here we describe purification and properties of the factor, identified as HA/P, that causes degradation of the gelatinous matrix of the chironomid egg mass. This factor also inhibited eggs from hatching. The role that HA/P may play in the survival of V. cholerae in the environment and its possible function are discussed below.
MATERIALS AND METHODS
Bacterial strains and culturing.
V. cholerae was isolated from chironomid egg masses (4) and was later identified as serogroup O9 (E. Arakawa, personal communication). V. cholerae O139 was a kind gift from T. Ramarmurthy (National Institute of Cholera and Entric Diseases, Calcutta, India). V. cholerae O1 strain C7258 and an HA/P null mutant of this strain, 638 (2), were kind gifts from J. Benitez. V. cholerae O9 was cultured in 0.5 liter of Luria broth in a 2-liter flask with shaking (200 rpm) at 37°C for 48 h. V. cholerae O139 was cultured in 0.05 liter under the same conditions.
Degradation activity.
Culture supernatants were obtained by centrifugation (4,000 × g for 30 min) and were filtered through a 0.45-μm-pore-size filter (Corning, Corning, N.Y.), which resulted in a clear supernatant. Degradation activity was determined by using freshly collected or ethanol (70%)-rinsed chironomid egg masses. A reaction was considered positive when the egg mass structure disintegrated and a large excess of free eggs was found separated from the egg mass compared with the control. For a more quantitative analysis, samples were serially diluted, and activity was considered positive at a given time when ca. 50% of the eggs were separated from an egg mass. The titer was defined as the reciprocal of the highest dilution that digested the egg mass after 12 h of incubation.
Egg hatching bioassay.
Freshly collected egg masses were rinsed five times in sterile tap water. Fifty-microliters of the HA/P fraction (in 20 mM phosphate-buffered saline [PBS], pH 7.5) was added to one egg mass in 1 ml (total volume) of sterile tap water. In the control, only PBS was added. Each treatment was repeated five times. The tubes were incubated for 48 h (25°C), which was the time needed for egg hatching in the control.
Purification of the degrading factor.
Cell-free culture supernatants were obtained by centrifugation (4,000 × g, 30 min) and filtration through a 0.45-μm-pore-size membrane. The supernatant was fractionated with ammonium sulfate. The material that was insoluble in 70% ammonium sulfate was suspended in 50 mM Tris-HCl buffer (pH 8.0) and was concentrated by ultrafiltration through a 12-kDa-cutoff membrane (Intersep;Fugisep). The concentrated sample was applied to a Pharmacia Superose 2 column (gel filtration) connected to a fast protein liquid chromatographic system. Isocratic elution was performed with 50 mM Tris-HCl buffer (pH 8.0) containing 100 mM NaCl. Protein was detected with a UV detector (280 nm). Eluted samples containing protein were applied to a sodium dodecyl sulfate (SDS)-polyacrylamide gel electrophoresis (PAGE) gel and stained with Coomassie brilliant blue. The bands in the gel were analyzed by in-gel proteolysis, as described below.
In-gel proteolysis and mass spectrometry analysis.
The stained protein bands in the gel were cut out with a razor blade, and the proteins in the gel were reduced with 10 mM dithiothreitol and modified with 100 mM iodoacetamide in 10 mM ammonium bicarbonate. The gel pieces were treated with 50% acetonitrile in 10 mM ammonium bicarbonate to remove the stain from the proteins, and this was followed by drying of the gel pieces. The dried gel pieces were rehydrated with 10 mM NH4HCO3 (ammonium bicabonate) containing about 0.1 mg of trypsin per sample. The gel pieces were incubated overnight at 37°C, and the resulting peptides were recovered with 60% acetonitrile-0.1% trifluorocetate.
The peptides were deposited on a metal target as cocrystals with cyano-4-hydroxycinnamic acid (Aldrich). A mass spectrometry analysis was performed by using matrix-assisted laser desorption ionization—time of flight mass spectrometry (2E; Micromass, Manchester, United Kingdom) in the positive ion mode. The mass spectrometry data were compared to the results of simulated proteolysis of the proteins in the Genpept database by using the Masslynx software (Micromass).
Protein concentration determination.
Protein concentration was determined with a protein assay kit (Bio-Rad Laboratories, Richmond, Calif.). Bovine serum albumin (BSA) was used as the standard.
SDS-PAGE was carried out as described previously (15). The sample, which was denatured by boiling for 3 min in 2% SDS and 5% β-mercaptoethanol, was electrophoresed on a 12 or 15% polyacrylamide gel at a constant voltage of 140 mA for 1 h. After this, the gel was visualized by Coomassie brilliant blue staining (15) or silver staining (24).
Western blotting (immunoblotting).
For Western blotting (immunoblotting) (21), the proteins separated by SDS-PAGE were transferred to a nitrocellulose membrane (Sartorius, Tokyo, Japan) by using a constant current of 200 mA for 1 h at room temperature in 20 mM Tris-150 mM glycine-20% methanol (pH 8.3). After blocking for 1 h with 1% BSA, the membrane was incubated for 45 min at room temperature with anti-HA/P chicken antibody diluted with PBS containing 1% BSA. The nonspecifically bound antibody was removed by washing the membrane with PBS containing 1% BSA and 0.5% Tween 20. The antibody-treated membrane was then incubated with peroxidase-conjugated anti-chicken immunoglobulin G antibody. After incubation at room temperature for 45 min, the enzyme activity of the bound peroxidase was measured in the presence of 4-methoxy-1-naphthol and H2O2.
Assay of proteolytic activities.
The proteolytic activities of the supernatant and the purified fractions were assayed by a modification of the method of Secades and Guijarro (19). Briefly, azocasein (Sigma) was used as the substrate. A 120-μl aliquot of a diluted sample was added to 480 μl of azocasein (1%, wt/vol) in reaction buffer (Tris-HCl buffer containing MgCl2 [final concentration, 5 mM]). The mixture was incubated at 30°C for 30 min. The reaction was terminated by adding 600 μl of 10% (vol/wt) trichloroacetic acid, and the mixture was left for 30 min on ice; this was followed by centrifugation at 15,000 × g at 4°C for 10 min. An 800-μl aliquot of the supernatant was neutralized by adding 200 μl of 1.8 N NaOH, and the absorbance at 420 nm (A420) was determined. One unit of enzyme activity was defined as the amount of enzyme that resulted in an increase of 0.01 A420 unit in 30 min at 30°C.
Hemagglutination assay.
The method used for the hemagglutination assay was adapted from the method of Jones et al. (12). A serial dilution of 50 μl of the hemagglutinin preparation in 20 mM PBS (pH 7.5) was mixed with 50 μl of freshly prepared chicken erythrocytes (2%) in the wells of a 96-well polystyrene U-bottom microtiter plate (Greiner, Nurtingen, Germany). The mixture was incubated at room temperature for 45 min, and the hemagglutination was monitored visually; 1 hemagglutination unit was defined as the reciprocal of the highest dilution of the sample that caused visible agglutination of the erythrocytes.
Inactivation of egg mass degradation.
Inactivation of egg mass degradation by V. cholerae O9 purified supernatant was assayed by examining egg mass degradation activity under the following conditions: in the presence of the metal chelator EDTA, in the presence of the serine protease inhibitor phenylmethylsulfonyl fluoride, and after incubation at 50, 60, 70, and 80°C for 15 min. The residual egg mass degradation, proteolytic, and hemagglutination activities were compared with those of an untreated sample having the same protein content. The controls included a preparation containing enzyme only (positive control) and buffers without enzyme (negative controls). The final reaction volume was 0.2 ml. The reactions were performed in 1.5-ml microtubes or in a 96-well microplate (Costar, Corning, N.Y.).
Glycoprotein-specific staining.
Glycoprotein staining was done with the acid-Schiff reagent (ASR) (Sigma-Aldrich Chemie, Deisenhofen, Germany) by following the manufacturer's instructions. This reagent is specific for glycoproteins and proteoglycans.
RESULTS
A chironomid egg mass was exposed to the supernatant of either an overnight culture of an environmental isolate of V. cholerae (V. cholerae O9) or to the supernatant of a clinical isolate of V. cholerae (V. cholerae O139). The egg mass structure was degraded, and eggs were freed from the matrix. Most of the eggs did not hatch (Fig. 1). Thus, the factor that causes the degradation of chironomid egg masses (egg mass degrading factor [EMDF]) was present in the supernatant. Members of other serogroups of V. cholerae (serogroups O2, O10, O12, O23, O39, O62, O70, O124, O128, and O184) were isolated from chironomid egg masses. The supernatants of these strains showed the same egg mass degradation activity that the supernatant of V. cholerae O9 showed.
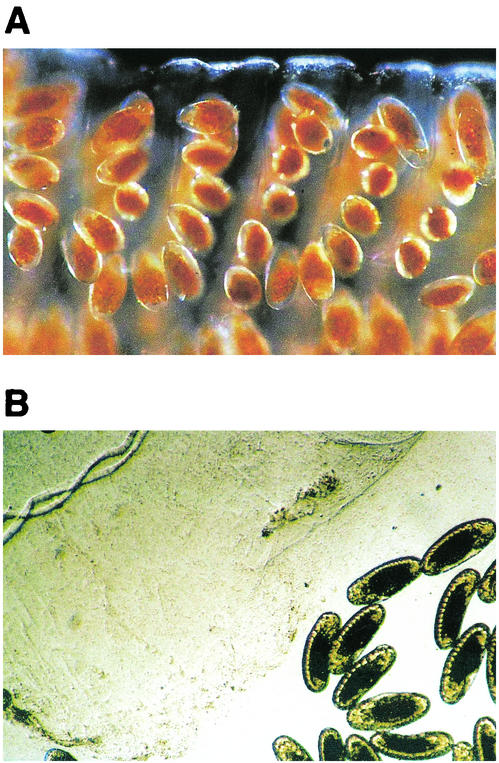
FIG. 1.
(A) Fresh collected chironomid egg mass. The eggs are embedded in a gelatinous material. The eggs are in a chain, which is folded into loops that form a spiral. (B) Egg mass incubated for 2 h with the supernatant of a 48-h culture of V. cholerae O9. The eggs were freed from the degraded gelatinous matrix. Original magnifications, ×40 (egg mass size, ca. 5 by 20 mm).
In order to identify the EMDF, the supernatant of a V. cholerae O9 culture was fractionated by ammonium sulfate precipitation. The 0 to 70% ammonium sulfate precipitate was dissolved in Tris-HCl buffer (50 mM Tris-HCl, 100 mM NaCl; pH 8.0) and concentrated by ultrafiltration (12-kDa cutoff). The concentrated sample was subjected to gel filtration on a Sepharose 12 column. All fractions were tested for EMDF activity. The most active fractions were analyzed to determine their specific activities (Table 1). SDS-PAGE of the fractions having the highest egg mass degradation specific activities resulted in a dominant band at 34 to 32 kDa. This protein band was characterized by in-gel proteolysis and mass spectrometry analysis (performed at the The Smoler Protein Center, The Technion, Haifa, Israel), and it was identified as HA/P of V. cholerae.
TABLE 1.
Purification of the EMDF produced by V. cholerae O9
| Step | Vol (ml) | Total protein (mg) | Total activity (U)a | Sp act (U/mg)a | Purification (fold) | Yield (%) |
|---|---|---|---|---|---|---|
| Supernatant | 330 | 20.8 | 1,650 | 79 | 1.0 | 100 |
| Ammonium sulfate | 6.0 | 17.8 | 960 | 54 | 0.7 | 58 |
| Ultrafiltration | 2.0 | 3.3 | 1,280 | 384 | 4.8 | 78 |
| Fraction 44 (FPLC)b | 0.3 | 0.048 | 320 | 6,709 | 84.6 | 19 |
One unit of activity was defined as the amount of EMDF that resulted in disintegration of the egg mass structure after 12 h of incubation at 37°C.
FPLC, fast protein liquid chromatography.
All fractions were also tested for prevention of egg mass hatching. There was overlap in the egg mass degradation and hatching prevention activities. Hatching was completely prevented by the fractions having the highest egg mass degradation specific activities (350 larvae hatched in the control after 48 h of incubation).
To confirm that the degradation activity was derived from HA/P, the proteolytic and hemagglutination activities of all the fractions were determined. All the fractions with EMDF activity exhibited hemagglutination and proteolytic activities as well (Tables 1 and 2), and the specific activities of the different fractions varied in parallel. Similar results were obtained when EMDF was purified from V. cholerae O139 (data not shown). We noted that the specific activities of EMDF, hemagglutinin, and protease in the step involving ammonium sulfate (Tables 1 and 2) were lower than expected. We attributed this to interference by ammonium sulfate. Thermal inactivation showed that at temperatures above 60°C the egg mass degradation activity, as well as the proteolytic and hemagglutination activities, were virtually abolished. EMDF activity after incubation with EDTA (at a final concentration of 1 mM) was undetectable, while incubation with phenylmethylsulfonyl fluoride (at concentrations as high as 10 mM) had no effect.
TABLE 2.
Hemagglutination and proteolytic activities of the purified EMDF produced by V. cholerae O9
| Step | Hemagglutination activity
|
Proteolytic activity
|
||||
|---|---|---|---|---|---|---|
| Sp act (U/mg)a | Purification (fold) | Yield (%) | Sp act (U/mg)b | Purification (fold) | Yield (%) | |
| Supernatant | 254 | 1.0 | 100 | 143 | 1.0 | 100 |
| Ammonium sulfate | 171 | 1.7 | 58 | 173 | 1.2 | 104 |
| Ultrafiltration | 770 | 4.0 | 48 | 360 | 2.5 | 40 |
| Fraction 44 (FPLC)c | 2,680 | 10.6 | 2 | 1,358 | 9.5 | 4 |
One unit of activity was defined as the amount of EMDF that resulted in an increase in A420 of 0.01 in 30 min during incubation at 30°C.
One unit of activity was defined as the amount of EMDF that resulted in visible agglutination of erythrocytes.
FPLC, fast protein liquid chromatography.
Further evidence of the role of HA/P in egg mass degradation was obtained by comparing the supernatants of a V. cholerae O1 strain and an HA/P null mutant of this organism. Egg mass degradation activity was evident only in the O1 parental strain supernatant (Fig. 2). The supernatants of the parental strain and the HA/P null mutant were each incubated with an egg mass for 10 and 120 min at 37°C (Fig. 3). All of the V. cholerae supernatants contained the same protein concentration. An egg mass without a supernatant and supernatants without egg masses incubated under the same conditions were used as controls (Fig. 3, lanes 1, 2, and 6). After incubation the samples were centrifuged for 1 min, and the upper fluid was loaded onto SDS-PAGE gels. The gels were stained either with ASR (Fig. 3a) or with silver stain (Fig. 3b). The presence of HA/P in the parental strain and its absence from the HA/P null mutant were demonstrated by Western blotting (Fig. 3c). Some of the egg mass degradation products were visualized by ASR staining (Fig. 3a, lane 4). The apparent molecular masses of these products were approximately 200 kDa or more. These products were obtained only from the supernatant of the parental strain and were not obtained from the supernatant of the HA/P null mutant.
FIG. 2.
Egg mass degradation bioassay in a 96-well microplate. An egg mass incubated at 37°C with V. cholerae O1 supernatant for 2 h is shown on the left, and an egg mass incubated at 37°C with V. cholerae O1 HA/P null mutant 638 supernatant for 2 h is shown on the right.
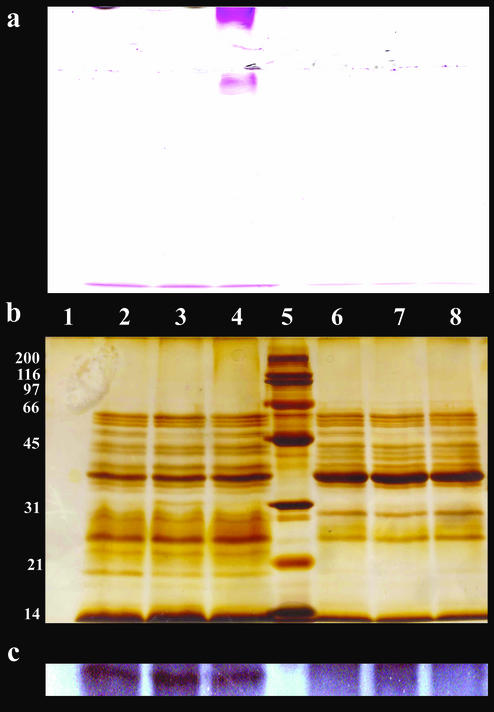
FIG. 3.
(a and b) SDS-PAGE gel stained with ASR (a) and silver stain (b). (c) Western blot analysis with anti-HA/P antibody. After SDS-PAGE, the samples were transferred onto a nitrocellulose membrane, and HA/P was detected with the antibody against HA/P from V. cholerae O9. Lane 1, egg mass control; lanes 2 to 4, V. cholerae O1 supernatant incubated at 37°C (lane 2, supernatant control without egg mass; lane 3, supernatant with egg mass incubated for 10 min; lane 4, supernatant with egg mass incubated for 120 min); lane 5, broad-range size markers (200, 116, 97, 66, 45, 31, 21, and 14 kDa; Bio-Rad); lanes 6 to 8, V. cholerae O1 HA/P null mutant 638 supernatant incubated at 37°C (lane 6, supernatant control without egg mass; lane 7, supernatant with egg mass incubated for 10 min; lane 8, supernatant with egg mass incubated for 120 min). All V. cholerae supernatant samples contained 20 μg of protein.
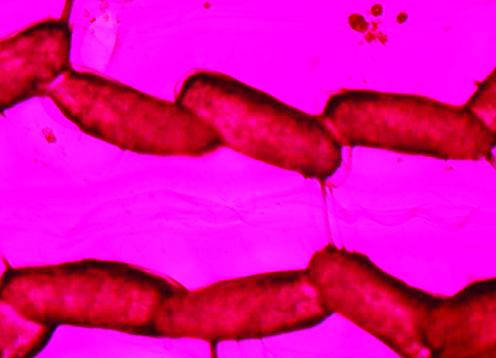
Coomassie brilliant blue staining and silver staining (protein stains) did not stain the gelatinous matrix. In contrast, ASR staining of the whole egg mass was quick and strong (Fig. 4), indicating that there were sugar components in the intact egg mass.
FIG. 4.
Chironomid egg mass stained with ASR (egg size, ca. 70 by 250 μm).
DISCUSSION
Recently, findings which indicated that chironomid egg masses may serve as an intermediate host reservoir for V. cholerae were described (4). In that study it was found that V. cholerae O9 degraded chironomid egg masses and prevented hatching of the eggs. Other serogroups of V. cholerae isolated from egg masses exhibited the same activities. In the present study, an environmental isolate of V. cholerae O9 and a clinical isolate of V. cholerae O139 were studied, as were a V. cholerae O1 strain and an HA/P null mutant of this organism. It was found that the activities mentioned above are associated with the bacterial supernatant. EMDF was purified from the supernatant and was identified by mass spectrometry as the previously described V. cholerae HA/P (9, 10, 16). It was found that purified EMDF releases chironomid eggs from the gelatinous matrix and prevents them from hatching. A comparison of the V. cholerae O1 strain and its HA/P null mutant showed that the mutant could not degrade egg masses, demonstrating the role of HA/P in egg mass degradation (Fig. 2 and 3).
Altogether, we found four different functional activities in the purified EMDF, including the previously described hemagglutination and protease activities and two new activities, degradation of the glycoprotein matrix of the egg mass and prevention of the hatching of chironomid eggs. The classical biological activities of HA/P (hemagglutination and proteolytic activities) (Table 1 and 2) were expressed as a distinct activity with the egg masses. The egg mass gelatinous matrix was found to consist of glycoproteins. In this context, the proteolytic activity of HA/P is more properly referred to as a glycoproteolytic activity that might be connected to HA/P's hemagglutination capabilities. We found that the biological activity of HA/P from V. cholerae O9 is indistinguishable from the biological activity of V. cholerae O139 or O1. These data are consistent with previously described data for classical HA/P activities (i.e., protease and hemagglutination activities) (10, 17). The gene encoding HA/P has been cloned (9), and the predicted amino acid sequence is a 65-kDa protein. The protein is processed into a secreted 46-kDa protein. In the absence of protease inhibitors, the 46-kDa protein is further processed to a 32-kDa protein, which is the form usually isolated (13).
HA/P has been considered a virulence factor of V. cholerae O1 and O139 and of V. cholerae non-O1 pathogenic strains (17). In vitro studies have demonstrated that HA/P cleaves a number of macromolecules, including ovomucin and fibronectin, which are found on the surfaces of intestinal epithelial cells, as well as lactoferrin, which is found in the intestinal lumen (7). It has been suggested, therefore, that HA/P plays a role in promoting the detachment of V. cholerae from intestinal colonization sites, thereby facilitating its dissemination. However, experiments with an infant rabbit model indicated that HA/P is not a virulence factor (8). Kimsey and Waldor (14) suggested that HA/P protects V. cholerae from infection by CTX phage (a bacteriophage known as cholera toxin phage, which encodes the cholera toxin and a large pathogenicity island), thereby limiting the emergence of new pathogenic strains of V. cholerae.
In areas where infection is endemic, cholera epidemics occur in a regular seasonal pattern. It is not clear what determines the seasonal appearance of epidemic V. cholerae strains and cholera outbreaks. It has been suggested that during interepidemic periods toxigenic V. cholerae exists in an unexplained ecological association with aquatic organisms, possibly in a viable but nonculturable form (5, 6). The secreted HA/P of V. cholerae can support free-living survival of the organism in the environment. The results presented here support previous findings regarding the possible role of chironomid egg masses as an environmental reservoir of this pathogen (4).
A BLAST search revealed high degrees of homology between the V. cholerae HA/P mature form and Vibrio proteolyticus neutral protease (91%), Vibrio anguillarum metalloprotease (88%), Vibrio vulnificus metalloprotease (88%), Aeromonas punctata protease (76%), Aeromonas hydrophila metalloprotease (75%), and Pseudomonas aeruginosa elastase (75%). Toma et al. (20) demonstrated an immunologic relatedness of V. cholerae HAIP and Aeromonas caviae metalloprotease.
Benitez et al. (2) showed that V. cholerae HA/P is induced by nutrient limitation and is strongly repressed by glucose (2). This observation can explain the importance of HA/P for V. cholerae in the environment with regard to utilization of egg masses. Our results imply that HA/P may have a general role in enabling the bacteria to survive in the environment.
Acknowledgments
Malka Halpern and Hanan Gancz contributed equally to this work.
We thank J. Benitez for providing V. cholerae O1 strains C2738 and 638. We thank Y. Shoaham, B. Podbilewicz, and A. Admon for their helpful advice.
This research was supported by the Israel Water Research Institute and by the Technion Otto Meyerhof Center for Biotechnology, established by the Minerva Foundation, Germany.
REFERENCES
- 1.Armitage, P., P. S. Cranston, and L. C. V. Pinder (ed.). 1995. The Chironomidae: the biology and ecology of non-biting midges. Chapman & Hall, London, United Kingdom.
- 2.Benitez, J. A., A. J. Silva, and R. A. Finkelstein. 2001. Environmental signals controlling production of hemagglutinin/protease in Vibrio cholerae. Infect. Immun. 69:6549-6553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Broza, M., M. Halpern, and M. Inbar. 2000. Nonbiting midges (Diptera; Chironomidae) in waste stabilization ponds; an intensifying nuisance in Israel. Water Sci. Technol. 42:71-74. [Google Scholar]
- 4.Broza, M., and M. Halpern. 2001. Chironomid egg masses and Vibrio cholerae. Nature 412:40. [DOI] [PubMed] [Google Scholar]
- 5.Colwell, R. R., and A. Huq. 1994. Vibrios in the environment: viable but not culturable Vibrio cholerae, p. 117-133. In I. K. Wachsmuth, P. A. Blake, and Ø. Olsvik (ed.), Vibrio cholerae and cholera: molecular to global perspective. ASM Press, Washington, D.C.
- 6.Colwell, R. R., and A. Huq. 1999. Global microbial ecology: biogeography and diversity of Vibrios as a model. J. Appl. Microbiol. Symp. Suppl. 85:134S-137S. [DOI] [PubMed]
- 7.Finkelstein, R. A., M. Bosman-Finkelstein, and P. Holt. 1983. Vibrio cholerae hemaglutinin/lectin/protease hydrolyzes fibronectin and ovomucin. Proc. Natl. Acad. Sci.USA 80:1092-1095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Finkelstein, R. A., M. Bosman-Finkelstein, Y. Chang, and C. C. Hass. 1992. Vibrio cholerae hemaglutinin/protease, colonial variation, virulance, and detachment. Infect. Immun. 60:472-478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hase, C. C., and R. A. Finkelstein. 1991. Cloning and nucleotide sequence of the Vibrio cholerae hemaglutinin/protease (HA/protease) gene and construction of an HA/protease-negative strain. J. Bacteriol. 173:3311-3317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Honda, T., K. Lertpocasombat, A. Hata, T. Miwatani, and R. A. Finkelstein. 1989. Purification and characterization of a protease produced by Vibrio cholerae non-O1 and comparison with a protease of V. cholerae O1. Infect. Immun 57:2799-2803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Islam, M. S., B. S. Drasar, and R. B. Sack. 1996. Ecology of Vibrio cholerae: role of aquatic flora and fauna, p. 187-227. In B. S. Drasar and B. D. Forrest (ed.), Cholera and the ecology of Vibrio cholerae. Chapman & Hall, London, United Kingdom.
- 12.Jones, A. C., R. P. Logan, S. Foynes, A. Cockayne, B. W. Wren, and C. W. Penn. 1997. A flagellar sheath protein of Helicobacter pylori is identical to HpaA, a putative N-acetylneuraminyllactose-binding hemagglutinin, but is not an adhesin for AGS cells. J. Bacteriol. 179:5643-5647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kaper, J. B., J. G. Glenn Morris, and M. M. Levine. 1995. Cholera. Clin. Microbiol. Rev. 8:48-86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kimsey, H. H., and M. K. Waldor. 1998. Vibrio cholerae hemagglutinin/protease inactivates CTXf. Infect. Immun. 66:4025-4029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Laemmli, U. K. 1970. Cleavage of structural proteins during the assembly of bacteriophage T4. Nature 227:680-685. [DOI] [PubMed] [Google Scholar]
- 16.Naka, A., K. Yamamoto, T. Miwatani, and T. Honda. 1992. Characterization of two forms of hemagglutinin/protease produced by Vibrio cholerae non-O1. FEMS Microbiol. Lett. 77:197-200. [DOI] [PubMed] [Google Scholar]
- 17.Naka, A., K. Yamamoto, M. J. Albert, and T. Honda. 1995. Vibrio cholerae O139 produces a protease which is indistinguishable from the haemagglutinin/protease of Vibrio cholerae O1 and non-O1. Immunol. Med. Microbiol. 11:87-90. [DOI] [PubMed] [Google Scholar]
- 18.Nolte, U. 1993. Egg masses of Chironomidae (Diptera). A review, including new observations and a preliminary key. Entomol. Scand. Suppl. 43:5-75. [Google Scholar]
- 19.Secades, P., and J. A. Guijarro. 1999. Purification and characterization of an extracellular protease from the fish pathogen Yersinia ruckeri and effect of culture conditions on production. Appl. Environ. Microbiol. 65:3969-3975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Toma, C. A., Y. B. Ichinose, and M. A. Iwanaga. 1999. Purification and characterization of an Aeromonas caviae metalloprotease that is related to the Vibrio cholerae hemagglutinin/protease. FEMS Microbiol. Lett. 170:237-242. [DOI] [PubMed] [Google Scholar]
- 21.Towbin, H., T. Staehelin, and J. Gordon. 1979. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc. Natl. Acad.Sci. USA 76:4350-4354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wachsmuth, K., Ø. Olsvik, G. M. Evins, and T. Popovic. 1994. Molecular epidemiology of cholera, p. 357-370. In I. K. Wachsmuth, P. A. Blake, and Ø Olsvik (ed.), Vibrio cholerae and cholera: molecular to global perspective. ASM Press, Washington, D.C.
- 23.World Health Organization. 2001. Cholera 2000. Wkly. Epidemiol. Rec. 76:233-240. [PubMed] [Google Scholar]
- 24.Yan, J. X., R. Wait, T. Berkelman, R. A. Harry, J. A. Westbrook, C. H. Wheeler, and M. J. Dunn. 2000. A modified silver staining protocol for visualization of proteins compatible with matrix-assisted laser desorption/ionization and electrospray ionization-mass spectrometry. Electrophoresis 21:3666-3672. [DOI] [PubMed] [Google Scholar]