Abstract
The toxicity of Al to Desulfovibrio desulfuricans G20 was assessed over a period of 8 weeks in a modified lactate C medium buffered at four initial pHs (5.0, 6.5, 7.2, and 8.3) and treated with five levels of added Al (0, 0.01, 0.1, 1.0, and 10 mM). At pH 5, cell population densities decreased significantly and any effect of Al was negligible compared to that of the pH. At pHs 6.5 and 7.2, the cell population densities increased by 30-fold during the first few days and then remained stable for soluble-Al concentrations of <5 × 10−5 M. In treatments having total-Al concentrations of ≥1 mM, soluble-Al concentrations exceeded 5 × 10−5 M and limited cell population growth substantially and proportionally. At pH 8.3, soluble-Al concentrations were below the 5 × 10−5 M toxicity threshold and cell population density increases of 20- to 40-fold were observed. An apparent cell population response to added Al at pH 8.3 was attributed to the presence of large, spirilloidal bacteria (accounting for as much as 80% of the cells at the 10 mM added Al level). Calculations of soluble-Al speciation for the pH 6.5 and 7.2 treatments that showed Al toxicity suggested the possible presence of the Al13O4(OH)24(H2O)127+ “tridecamer” cation and an inverse correlation of the tridecamer concentration and the cell population density. Analysis by 27Al nuclear magnetic resonance spectroscopy, however, yielded no evidence of this species in freshly prepared samples or those taken 800 days after inoculation. Exclusion of the tridecamer species from the aqueous speciation calculations at pHs 6.5 and 7.2 yielded inverse correlations of the neutral Al(OH)3 and anionic Al(OH)4− monomeric species with cell population density, suggesting that one or both of these ions bear primary responsibility for the toxicity observed.
Aluminum, the most abundant metal and the third most abundant element in the earth's crust (37), finds surprisingly little use in biological systems. Although the inherent chemistry of Al certainly is a factor, the chemical conditions existing on the earth at the time the earliest organisms were developing may also have played a role (42, 43). These conditions, which include neutral pH and the absence of oxygen, were such that the solubility of Al was minimal relative to that of divalent metals such as Mg, Ca, and Fe(II). Even if availability were not limiting, however, several other chemical properties of Al strongly militate against its use in cellular metabolism (5). The inherently slow rate of inner-sphere ligand exchange for Al3+ (105 to 108 times slower than for Mg2+, Ca2+, or Fe2+ [41]) makes its complexes relatively inert. Further inertness stems from the lack of any electron transfer chemistry. Moreover, Al3+ forms complexes with greater thermodynamic stability than that of those formed by the divalent metals because of its smaller size and higher valence. Thus, the ability of Al to form strong, relatively inert complexes not only prevents its use by the cyclic feedback-regulated processes in cellular metabolism but causes it to interfere with their normal activity. A classic example is the complex of Al with ATP. This inert complex, which is nearly 107 times stronger than the Mg-ATP complex, binds to hexokinase 103 times more strongly than the Mg-ATP complex and thus shuts down glucose metabolism at very low levels of Al (24). Al also acts to accelerate the peroxidation of cell membrane lipids when Fe is also present (9, 12).
Living organisms have developed a variety of defenses against Al. These include the absence of active transport processes for trivalent ions (41); the induction of oxalate production to complex the Al extracellularly, thereby preventing its uptake (7, 14, 18), or intracellularly to prevent reaction with other cellular components (10, 11); the storage of organically complexed Al in cell vacuoles (2); the generation of extracellular alkalinity to precipitate Al(OH)3 (16, 27); and the development of enhanced divalent-cation uptake mechanisms (1, 21, 47).
Despite the general agreement that Al toxicity derives from its replacement of divalent metal complexes, chiefly Mg and Ca, in cells or cell membranes, there is less agreement about whether the effect is generic to all forms of soluble Al. Stable organic complexes of Al (e.g., oxalate or citrate) seem to mitigate the toxic effects on methanotroph activity (25) and root growth (13). However, citrate complexes also seem to be the mechanism by which soluble Al crosses the blood-brain barrier (46). With respect to inorganic forms of Al, most studies suggest that monomeric Al3+ is the actively toxic species, even though toxicity seems to peak in the slightly acidic to neutral region (pH 5 to 6.5) rather than at lower pHs, where Al3+ dominates (e.g., see reference 6). This apparent conflict is often explained in terms of increased H+ competition with Al3+ at the lower pHs preventing Al from contacting cell membranes and thus overriding the Al toxicity effect. Parker et al. (30, 31), however, showed that a metastable polymeric form of soluble Al that forms by mixing of acidic and basic waters [Al13O4(OH)24(H2O)127+] was significantly more toxic to wheat roots than an equivalent concentration of monomeric Al3+. The mode of the polymer-induced toxicity remains unclear, although it could be simply a means of delivering a large quantity of Al directly to the membrane surface, thus overwhelming the normal defenses.
Among the microorganisms, acid-tolerant yeasts and fungi seem to have the highest resistance to Al (15, 16, 27), but soil bacteria, notably Pseudomonas spp., can be Al tolerant (2, 10, 11, 19). To our knowledge, no studies of the Al tolerance of the sulfate-reducing bacteria (SRB) have been performed. Our interest in this topic stems from preliminary work (41a; J. E. Amonette, unpublished data; J. M. Suflita, D. Wong, and L. R. Krumholz, DOE-NABIR PI Workshop, abstr. LBNL-47386, p. 54, 2001) suggesting that the Al content of minerals may be one of the factors influencing the distribution of SRB in sediments. In particular, this work showed that clay minerals containing Al inhibited SRB growth whereas those with little or no Al in their structure had correspondingly little impact on SRB growth. To further investigate this phenomenon, we conducted a batch incubation experiment to assess the possible toxic effects of soluble and freshly precipitated Al on Desulfovibrio desulfuricans G20, a strain of SRB. As the soluble form of the Al changes with pH (it is predominantly cationic at pHs of <6 and anionic at pHs of >7.5, with a charge-neutral species dominating at intermediate pHs) we examined a range of pH conditions and soluble-Al concentrations.
MATERIALS AND METHODS
Organism and media.
A culture of D. desulfuricans G20 was obtained from A. L. Neal and G. G. Geesey (Montana State University). This organism contained the green fluorescent protein (Npt2CmGFP) plasmid (26) and was maintained in a modified lactate C growth medium (35) designed for metal toxicity studies.
The growth medium contained no citrate, phosphate, ascorbic acid, iron, or thioglycolate and lesser quantities of lactate (4.8 g liter−1), sulfate (1.9 g liter−1), magnesium (0.1 g liter−1), and yeast extract (50 mg liter−1) than the standard Postgate lactate C medium. New components of the growth medium included Tryptone (0.5 g liter−1) and PIPES [piperazine-N,N′-bis(2-ethanesulfonic acid); 10.9 g liter−1] as a nonreactive buffer. Although not required for this study, the expression of the plasmid was maintained by addition of chloramphenicol (35) to the growth medium. The cell cultures were maintained at pH 7.2. Inocula were prepared in growth medium that did not contain chloramphenicol.
The experimental media were similar to the growth medium but lacked chloramphenicol and were buffered at the experimental pH with either 4-morpholineethanesulfonic acid monohydrate (MES) or 1,3-bis[tris(hydroxymethyl)methylamino]propane (BTP) in place of PIPES. After the dry ingredients were dissolved in H2O, the media were allowed to age overnight, sparged with nitrogen, and brought into an anoxic chamber, where they were adjusted to the desired pH with HCl or NaOH and distributed among the serum bottles prior to autoclaving.
Experimental design and procedure.
The experimental design was a simple factorial involving four initial pHs (5.0, 6.5, 7.2, and 8.3) and five levels of added Al (0, 0.01, 0.1, 1.0, and 10 mM), for a total of 20 treatment combinations. Each treatment combination receiving inoculum was replicated three times. A set of 20 uninoculated treatment combinations was also run as an abiotic control.
Experiments were conducted with serum bottles (Wheaton Science Products, Millville, N.J.) containing 30 ml of medium having the appropriate buffer (MES for pH 5.0 and BTP for pHs 6.5, 7.2, and 8.3) and level of Al (added as AlCl3 · 6H2O powder to the buffer medium). After addition of the experimental medium, the bottles were sealed, autoclaved, and allowed to cool. To start the experiment, 1 ml of 4- to 5-day-old inoculum (late log growth stage, 1.5 × 109 to 2.1 × 109 cells/ml) was injected through the septum with a syringe. The bottles were shaken to mix the contents thoroughly, removed from the anoxic chamber, and incubated while stationary and at room temperature (22°C) for the remainder of the experiment.
Periodically during the experiment (1, 4, 7, 14, 28, and 56 days after inoculation), small aliquots (ca. 50 μl) were taken from the serum bottles and visual counting of active bacteria was done with an optical microscope and a hemacytometer (Hausser Scientific, Horsham, Pa.). In addition to total active-cell counting, estimates of the proportion of active cells having different morphologies (e.g., vibrio versus spirilloid) were also made.
At the end of the experiment (56 days), solutions from two replicates were analyzed for selected chemical properties. Serum bottles were opened inside an anoxic chamber, and an 8-ml aliquot was analyzed immediately for soluble sulfide by a methylene blue-based colorimetric procedure available in kit form (Chemetrics, Inc., Calverton, Va.). A separate 3-ml aliquot was analyzed first for pH (Orion Ross combination electrode) and then Eh (Orion Pt electrode standardized with quinhydrone-amended pH buffers).
A third aliquot was analyzed for soluble Al and Mg after filtration (Amicon Centriplus YM-30 membrane [30,000 molecular weight cutoff, 3.6-nm nominal pore size], Millipore Corporation). Filter membranes were first soaked in deionized H2O, followed by 2% HNO3, and then centrifuged and washed thrice with deionized H2O and once with a few milliliters of the sample solution from the serum bottle. All of these supernatant solutions were discarded, and then a fresh 10 ml of sample solution was passed through the membrane. This supernatant was acidified to pH 2 with 2% HNO3 and stored in acid-washed plastic vials at 4°C until analysis by inductively coupled plasma mass spectrometry.
A 27Al nuclear magnetic resonance (NMR) spectrum was collected with a CMX Infinity spectrometer operating at 130.246 MHz. The sample (4 ml) was analyzed at room temperature in an 8-mm-diameter tetrafluoroethylene NMR tube. Chemical shifts were referenced to the resonance of 10 mM AlCl3 in 10 mM HCl or in 40 mM NaOH. The delay between scans was 0.5 s, and the radio frequency pulse width was 2.5 μs. A single scan was collected for the standards, and 128,000 scans were obtained for the experimental sample. All spectra were phase corrected.
X-ray diffraction patterns were collected for solid phases present in selected samples at the end of the experiment. Specimens were prepared as smear mounts on zero-background (i.e., off-axis single-crystal) quartz slides, and patterns were collected for both the wet and air-dried states. Diffraction data were collected with Cu-Kα radiation with a Philips X'pert MPD (PW 3040/00 type) system equipped with a curved-graphite monochromator.
Thermodynamic calculations.
Solution concentration data were speciated with the MINTEQA2 code, version 3.11 (40). The original database was augmented with values for several species and solids as listed in Table 1. Calculations were performed for the initial composition of the solution (with and without SO42− included) across a pH range of 4 to 9 to determine the solubility boundaries for Al. A second set of calculations was performed for the final solution composition. For these calculations, the concentrations of acetate and carbonate species were estimated from the measured sulfide concentrations on the basis of the following reaction stoichiometry (38):
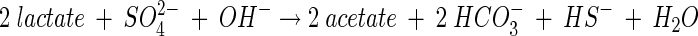 |
TABLE 1.
Additional thermodynamic data used in MINTEQA2 calculations
| Species or solid | Equilibrium quotient | Log K (25°C, I = 0) | Reference |
|---|---|---|---|
| Al-lactate2+ | ML/M · L | 2.36a | 22 |
| Al-(lactate)2+ | ML/M · L2 | 4.42a | 22 |
| Al-acetate2+ | ML/M · L | 2.22 | 23 |
| Al-(acetate)2+ | ML/M · L2 | 3.63 | 23 |
| HPIPES | HL/H · L | 6.78 | 8 |
| HMES | HL/H · L | 6.10 | 8 |
| H2BTP | H2L/H · L2 | 15.8 | 20, p. 7-9 |
| HBTP | HL/H · L | 9.0 | 20, p. 7-9 |
| Al2(OH)24+ | M2L2 · H2/M2 · H2O2 | −7.7 | 3 |
| Al3(OH)45+ | M3L4 · H4/M3 · H2O4 | −13.94 | 3 |
| Al13O4(OH)247+ | M13L28 · H32/M13 · H2O28 | −98.73 | 3 |
| Basaluminite(am)b [Al4SO4(OH)10 · 5H2O] | Solubility product | −116 | 28 |
| Basaluminite(cr)c [Al4SO4(OH)10 · 5H2O] | Solubility product | −117.7 | 28 |
| Jurbanite [Al(OH)SO4 · 5H2O] | Solubility product | −17.8 | 28 |
I = 0.6.
am, amorphous.
cr, acrystalline.
RESULTS
Cell population.
After dilution was accounted for, the initial motile-cell population density in each bottle was about 5.7 × 107 cells/ml or, with logarithmic units for easy comparison, 7.8 log cells/ml. The changes in this value in response to the pH 5 treatments (Table 2) showed a doubling of population density (+0.3 log unit) during the first day, followed by a slow decline to final levels ranging between zero and about 20% of the initial population density (6.9 to 7.1 log cells/ml). The decline was slightly more pronounced at the highest total Al concentrations (i.e., 1 and 10 mM), but in general, any effect of Al was small relative to that of the low pH. Trends in the data suggest that the bacteria would not survive over the long term at pH 5, regardless of the level of Al.
TABLE 2.
Motile-cell counts obtained with a hemacytometer
| Treatment (pH, Al concn [mM]) | Mean motile cell count (log no. of cells/ml)a
|
||||||
|---|---|---|---|---|---|---|---|
| Day 1 | Day 4 | Day 7 | Day 14 | Day 28 | Day 56 | Treatment mean | |
| 5.0, 0 | 8.1 | 7.8 | 7.4 | 7.4b | 7.5 | 7.1c | 7.7d |
| 5.0, 0.01 | 8.0c | 7.8d | 7.5d | 7.5d | 7.4b | 7.2c | 7.7c |
| 5.0, 0.1 | 8.1 | 7.9 | 7.4c | 7.2a | 7.5b | 7.0c | 7.7c |
| 5.0, 1 | 8.1 | 7.6 | 7.4 | 7.5d | 7.6b | —e | 7.6d |
| 5.0, 10 | 8.1 | 7.6d | 7.4 | 7.2 | 7.3b | — | 7.6c |
| 6.5, 0 | 7.8d | 9.4 | 9.6 | 9.6 | 9.4 | 9.4 | 9.4 |
| 6.5, 0.01 | 7.9 | 9.4c | 9.6 | 9.5 | 9.4 | 9.3 | 9.4 |
| 6.5, 0.1 | 7.9 | 9.4c | 9.6 | 9.5 | 9.3 | 9.3 | 9.3 |
| 6.5, 1 | 7.9 | 7.9d | 7.7 | 8.4c | 8.1 | 8.3d | 8.1d |
| 6.5, 10 | 7.7d | 7.9d | 7.7d | 7.7c | 7.8 | 8.0 | 7.8d |
| 7.2, 0 | 8.2b | 9.6 | 9.6 | 9.4 | 9.3d | 9.3 | 9.4 |
| 7.2, 0.01 | 8.3d | 9.6 | 9.5 | 9.5 | 9.4 | 9.4 | 9.4 |
| 7.2, 0.1 | 8.3b | 9.5 | 9.6 | 9.4 | 9.3 | 9.3d | 9.4 |
| 7.2, 1 | 8.0d | 7.9c | 8.3 | 9.2b | 9.2 | 9.0 | 8.9d |
| 7.2, 10 | 7.8c | 7.9 | 8.3c | 8.0d | 7.5c | — | 7.9c |
| 8.3, 0 | 7.6d | 9.5 | 9.5c | 9.4 | 9.4 | 9.3 | 9.3 |
| 8.3, 0.01 | 7.9d | 9.4 | 9.3 | 9.3 | 9.3c | 9.3d | 9.2d |
| 8.3, 0.1 | 8.0 | 9.4 | 9.3d | 9.3c | 9.4d | 9.3 | 9.3 |
| 8.3, 1 | 8.2 | 8.4 | 8.7 | 9.4 | 9.2 | 9.1 | 9.0 |
| 8.3, 10 | 8.0 | 8.4 | 8.7 | 9.0 | 9.1 | 8.6c | 8.8 |
Means of three replicates are shown. The relative error of the mean is <20% unless otherwise indicated.
Relative error, >50% of reported value.
Relative error, 30 to 50% of reported value.
Relative error, 20 to 30% of reported value.
—, below detection limit for cells (6.88 log cells/ml).
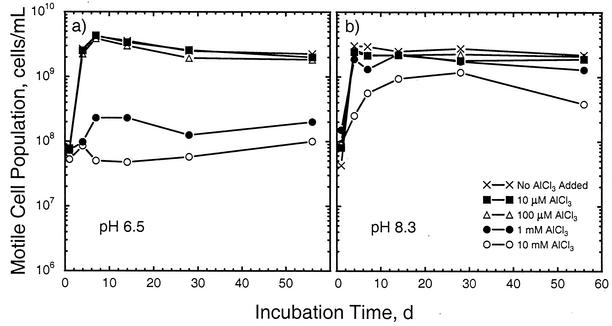
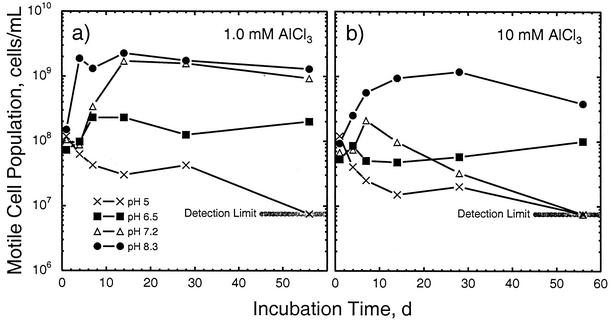
At higher pHs, substantial population growth was seen in most of the treatments and a strong effect of added Al was observed (Table 2). For example, at pH 6.5 and low levels of Al, the cell population density increased by nearly 2 orders of magnitude in the first week before stabilizing at a level about 40-fold higher than the initial level (Fig. 1, left). However, the amount of growth at a total-Al level of 1 mM was much less, and essentially no growth was seen at the 10 mM Al level. The results obtained at pH 7.2 essentially mirrored those obtained at pH 6.5 with low levels of Al. At the 1 mM Al level, less inhibition of growth was seen at pH 7.2 than at pH 6.5 (Table 2; Fig. 2, left). At 10 mM Al, however, the population density declined after the first week and was at the detection limit by the end of the experiment (Table 2; Fig. 2, right). At pH 8.3, growth was observed at all Al levels (Fig. 1, right). At the 10 mM Al level, however, the rate of growth and the maximum cell population density were less than at the other pH 8.3 Al levels, and a decline in population density was observed after 28 days. Thus, at pHs of ≥6.5, the deleterious effect of Al on cell population density was essentially confined to total concentrations of ≥1 mM (Fig. 1). At a given level of Al, however, the cell population density was generally higher at higher pHs (Fig. 2).
FIG. 1.
Changes in motile-cell population densities in response to total-Al levels ranging from 0 to 10 mM at initial pHs of 6.5 (a) and 8.3 (b). d, days.
FIG. 2.
Changes in motile-cell population densities in response to initial pH levels ranging from 5 to 8.3 at total-Al levels of 1 (a) and 10 (b) mM. d, days.
Solution chemistry.
As in the cell population density results, changes in pH occurred during the experiment in response to both the amount of Al added and the initial pH values (Table 3). As one might expect from the acidic nature of AlCl3, the greater the amount of Al added, the lower the final pH within a given pH treatment. Regardless of the level of added Al, pH increases were observed during the course of the experiment in the pH 5, 6.5, and 7.2 treatments, and pH decreases were observed in the pH 8.3 treatment. The greatest change in pH (+0.7 pH unit) was seen in the pH 6.5 treatment with no added Al. Interpolation of data between pHs 7.2 and 8.3 suggests that the pH at which no change would occur (i.e., the equilibrium pH at which bacteria are active) would be about 7.7 to 7.8 for all of the treatments except 10 mM Al, at which an equilibrium value of about 7.3 would apply.
TABLE 3.
Chemical properties of solutions at end of experimenta
| Treatment (pH, Al concn [mM]) | pH | Platinum electrode potentialb (mV) | Soluble sulfide (mol/liter) | Soluble Al (mol/liter)
|
Soluble Mg (mol/liter) bacteria presentd | |
|---|---|---|---|---|---|---|
| Bacteria present | Sterile controlc | |||||
| 5.0, 0 | 5.31 | 5 | 1.8 × 10−5 | —e | — | 4.6 × 10−3 |
| 5.0, 0.01 | 5.30 | −64 | 2.9 × 10−5 | 4.1 × 10−5 | 1.8 × 10−5 | 4.5 × 10−3 |
| 5.0, 0.1 | 5.26 | 48 | —f | 1.4 × 10−4 | 1.0 × 10−4 | 4.5 × 10−3 |
| 5.0, 1 | 5.29 | −39 | 1.5 × 10−5 | 1.2 × 10−3 | 1.1 × 10−3 | 4.5 × 10−3 |
| 5.0, 10 | 5.11 | −23 | 1.5 × 10−5 | 8.9 × 10−3 | 9.7 × 10−3 | 4.2 × 10−3 |
| 6.5, 0 | 7.23 | −357 | 2.9 × 10−3 | — | — | 4.4 × 10−3 |
| 6.5, 0.01 | 7.21 | −362 | 2.9 × 10−3 | 1.1 × 10−5 | 1.6 × 10−5 | 4.5 × 10−3 |
| 6.5, 0.1 | 7.19 | −356 | 2.9 × 10−3 | 4.6 × 10−5 | 8.0 × 10−5 | 4.5 × 10−3 |
| 6.5, 1 | 6.70 | −283 | 6.0 × 10−5 | 4.8 × 10−4 | 4.9 × 10−4 | 4.5 × 10−3 |
| 6.5, 10 | 6.74 | −245 | 4.1 × 10−5 | 1.2 × 10−3 | 1.5 × 10−3 | 4.6 × 10−3 |
| 7.2, 0 | 7.47 | −355 | 3.0 × 10−3 | — | — | 4.6 × 10−3 |
| 7.2, 0.01 | 7.43 | −378 | 2.2 × 10−3 | — | 1.7 × 10−5 | 4.6 × 10−3 |
| 7.2, 0.1 | 7.38 | −380 | 3.0 × 10−3 | 4.4 × 10−5 | 7.3 × 10−5 | 4.6 × 10−3 |
| 7.2, 1 | 7.39 | −388 | 2.9 × 10−3 | 1.5 × 10−4 | 2.5 × 10−4 | 4.6 × 10−3 |
| 7.2, 10 | 7.27 | −349 | 2.6 × 10−4 | 2.2 × 10−4 | 2.7 × 10−4 | 4.6 × 10−3 |
| 8.3, 0 | 8.08 | −371 | 2.2 × 10−3 | — | — | 4.6 × 10−3 |
| 8.3, 0.01 | 8.04 | −383 | 2.6 × 10−3 | 8.0 × 10−6 | 1.6 × 10−5 | 4.5 × 10−3 |
| 8.3, 0.1 | 8.02 | −391 | 2.9 × 10−3 | 3.8 × 10−5 | 8.3 × 10−5 | 4.6 × 10−3 |
| 8.3, 1 | 8.00 | −397 | 2.3 × 10−3 | 4.7 × 10−6 | 8.4 × 10−5 | 4.5 × 10−3 |
| 8.3, 10 | 7.93 | −399 | 1.9 × 10−3 | — | 6.3 × 10−5 | 4.4 × 10−3 |
| 8.3, 10i | NDg | ND | ND | ND | 6.2 × 10−6h | ND |
The experiment lasted 56 days. Means of two replicates are shown.
Relative to standard hydrogen electrode.
Single replicate.
Third replicate only, sampled 800 days after start of experiment.
Below detection limit for aluminum (1.9 × 10−6 mol/liter).
Below detection limit for sulfide (1.5 × 10−6 mol/liter).
ND, not determined.
Freshly prepared sample.
No lactate present.
Measured values for the reduction potential and the soluble-sulfide concentrations of the solutions at the end of the experiment (Table 3) show slightly reducing conditions in the pH 5 treatments and highly reducing conditions at the other three pHs. Very little sulfide was generated at pH 5, in contrast to the higher pHs. Some evidence of an Al effect on sulfide concentration and reduction potential was seen in the treatments at pH 6.5 and 7.2 with 1 and 10 mM total Al.
Soluble-Al concentrations ranged from those expected on the basis of complete dissolution of the amount added to as low as the analytical detection limit of 1.9 × 10−6 M (Table 3). In general, only the pH 5 treatments and the sterile controls at 0.01 mM Al yielded dissolved-Al concentrations comparable to the added Al concentrations. At pHs of >5, some degree of Al precipitation occurred, and the degree of precipitation was correlated with the pH. For example, at pH 8.3 with 10 mM total Al added, the soluble-Al concentration in the inoculated sample at the end of the experiment was below the limit of detection. Also, at pHs of ≥6.5, the soluble-Al concentrations in the inoculated treatments were consistently less than those in the corresponding sterile control.
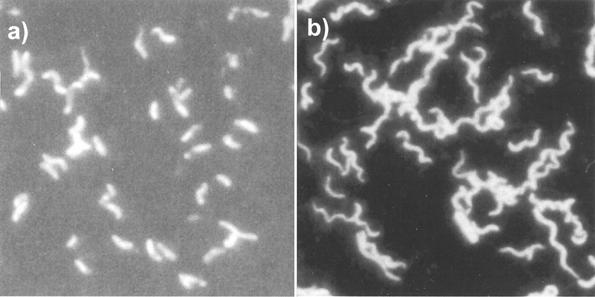
Cell morphology.
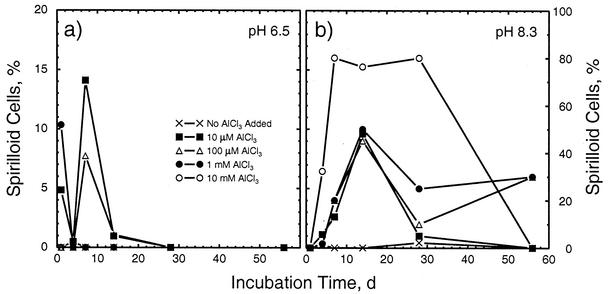
As noted by Postgate (33, 34), Desulfovibrio spp. change morphology in response to environmental conditions. In this experiment, we observed two morphologies, vibrio (i.e., slightly curved rod) and spirilloid (Fig. 3). In general, the vibrio form dominated. The spirilloid form, however, was increasingly seen under higher-pH conditions and was the dominant morphology in the pH 8.3, 10 mM Al treatment during a major portion of the experiment (Fig. 4). At pH 8.3 during the first 28 days, the fraction of spirilloid bacteria generally increased with the total Al concentration (Fig. 4, right).
FIG. 3.
Photomicrographs of acridine orange-stained D. desulfuricans G20 showing predominantly vibrio morphology (a) and spirilloid morphology (b).
FIG. 4.
Proportions of bacterial cells exhibiting spirilloid morphology in response to total-Al levels ranging from 0 to 10 mM at initial pHs of 6.5 (a) and 8.3 (b). d, days.
DISCUSSION
Effects of pH.
The initial pHs of the solutions before autoclaving averaged within 0.02 ± 0.014 pH unit of the nominal treatment values. As the solutions containing Al had been aged overnight before pH adjustment, any changes due to autoclaving were minimal. Thus, the final pH values (Table 3) represent changes due to the activity of the organism. Further support for the biotic influence on pH is that the changes observed are in the opposite direction than would be expected if precipitation (i.e., aging) of Al(OH)3(s) were driving the process. That is, conversion of the soluble cationic Al species prevalent at pHs of <7 to the solid hydroxide would generate acidity, whereas conversion of the anionic Al(OH)4− species prevalent at pH 8.3 would generate alkalinity.
Toxicity of soluble Al.
Examination of the mean motile-cell counts for the pH 5 treatments (Table 2) shows only slight evidence of Al toxicity. The overwhelming effect in these five treatments is that of a low pH, a result not unexpected from the reported pH tolerance range of SRB (i.e., from pHs of <5 to 9.5 [reference 33, p. 87]). Consequently, these treatments will be ignored for the remainder of the discussion of Al toxicity.
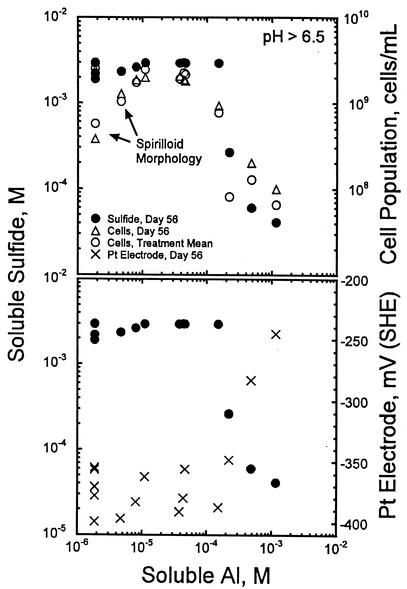
At the higher pHs, comparison of the cell population density, soluble-sulfide, and Pt electrode data with the soluble-Al data suggest that the threshold concentration for soluble-Al toxicity is near 5 × 10−5 M Al. When soluble-Al concentrations exceed this level, the cell population density and sulfide concentration decrease significantly (Fig. 5, top) and the Pt electrode potential increases (Fig. 5, bottom). At soluble-Al concentrations below the toxicity threshold, the sulfide and Pt electrode data are generally stable. However, the motile-cell population density data diverge into two groups, one that remains at the plateau level (ca. 3 × 109 cells/ml) and one that decreases with decreasing soluble-Al content. The values in the second group are associated with the 1 and 10 mM Al, pH 8.3, treatments.
FIG. 5.
Changes in the measured concentration of dissolved sulfide, motile-cell population density, and platinum electrode potential for pH 6.5, 7.2, and 8.3 treatments at different measured soluble-Al concentrations.
During the first 28 days, significant fractions of the bacteria in the second group exhibited spirilloid morphology (Fig. 4), suggesting that the low cell population density data at pH 8.3 for much of the experiment likely reflect the presence of fewer larger cells rather than a decrease in biomass. The decline in cell population density after 28 days in the 10 mM Al, pH 8.3, treatment, however, likely stems from exhaustion of the lactate supply. Although lactate concentrations were not measured, indirect evidence of this comes from a comparison of the soluble-Al concentrations at 56 days for inoculated and uninoculated controls. When bacteria were absent and lactate was present, soluble-Al concentrations of about 6 × 10−5 M were measured for the 10 mM Al, pH 8.3, treatment (Table 3). When both bacteria and lactate were absent, soluble-Al concentrations were an order of magnitude lower (i.e., about 6 × 10−6 M; Table 3). Complexation of Al by lactate maintains the soluble-Al concentration at a level 10-fold higher than that when lactate is absent. Consumption of lactate by SRB, therefore, would lower the soluble-Al concentration, as observed in the 56-day inoculated samples (Table 3). The same effect was seen for the 1 mM Al, pH 8.3, treatment, where the soluble-Al concentrations in the 56-day uninoculated and inoculated samples were 8.4 × 10−5 and 5 × 10−6 M, respectively (Table 3). Only the 10 and 1 mM Al, pH 8.3, treatments demonstrated such strong differences between inoculated and uninoculated samples.
Development of spirilloid morphology generally occurs as cultures age or in response to a specific biochemical stress, such as high levels of antibiotics or sulfite, or low levels of Mg2+ (34). Severe stress apparently does not result in morphological changes. For example, only a few spirilloid bacteria were seen in one sampling in one of the pH 5 treatments, where stress was clearly severe. In contrast, maximal numbers of spirilloid bacteria were seen during the first 28 days in the pH 8.3 treatments containing added Al. No antibiotics were present, sulfite accumulation was not expected (particularly during the first 7 to 14 days, when spirilloid maxima were reached), and analyses of the 56-day solutions for Mg (including an analysis of the third replicate sampled 28 months after initiation of the experiment; Table 3) showed no change in concentration from the original values. In the absence of added Al, at most 2% of the bacteria at pH 8.3 were spirilloid, whereas as much as 80% were spirilloid in the 10 mM Al treatment. During the last 28 days of the experiment, the likely exhaustion of the lactate supply in the 10 mM Al treatment would have placed severe stress on the SRB population, thereby causing both a decline in the total number of bacteria and complete reversion to the vibrio morphology.
In contrast to the treatments at pH 8.3, a direct toxic effect of Al was seen at the 1 and 10 mM Al levels for the pH 6.5 and 7.2 treatments. In three of these four treatments, no spirilloid bacteria were seen after the first day. In the 1 mM Al, pH 7.2, treatment, which showed the least response to Al toxicity of the four treatments, moderate levels (20 to 30%) of spirilloid bacteria were seen after the first 2 weeks of incubation. As in the pH 5 treatments, where severe pH stress was applied, it seems that D. desulfuricans under severe Al stress does not exhibit the spirilloid morphology. This morphology apparently indicates an intermediate state of stress where biomass growth occurs but cell division is inhibited.
Aluminum speciation calculations.
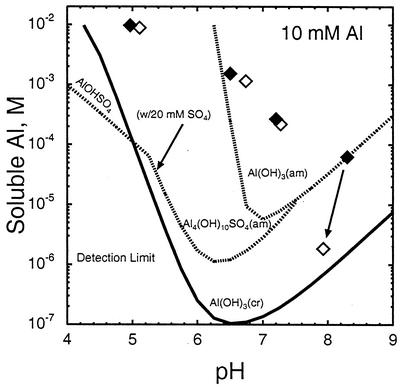
Calculations of the solubility of possible solid phases in equilibrium with the final solution composition show that at all of the pHs involving 10 mM Al, the solution was supersaturated with respect to crystalline Al(OH)3 (Fig. 6). At pHs of ≤7.2, supersaturation with respect to the amorphous Al hydroxysulfate phases jurbanite (AlOHSO4 · 5H2O) and basaluminite [Al4SO4(OH)10 · 5H2O] was also seen. X-ray diffraction patterns of the solids, in both the moist and air-dried states, yielded very broad peaks with no evidence of crystallinity (data not shown). Although the solutions were in apparent equilibrium with amorphous Al(OH)3 for the sterile pH 8.3 treatment and both pH 6.5 treatments, the trends suggested by the pH 7.2 data suggest that this may have been coincidental. As the soluble-Mg data showed no evidence of the formation of an Al-Mg hydroxy compound, the solid phase most likely consisted of an amorphous Al-hydroxy gel whose crystallization was frustrated by the slow ligand exchange rate and by inclusions of minor amounts of sulfate and lactate.
FIG. 6.
Solubility of Al after 56 days in inoculated (open squares) and uninoculated (filled squares) 10 mM treatments. Solubilities of possible solid phases, i.e., amorphous and crystalline gibbsite [Al(OH)3], jurbanite [Al(OH)SO4 · 5H2O], and amorphous basaluminite [Al4(OH)10SO4 · 5H2O], in equilibrium with the 56-day solution composition are also shown. Hydroxysulfate phases assume a 20 mM SO4 concentration. am, amorphous; cr, crystalline.
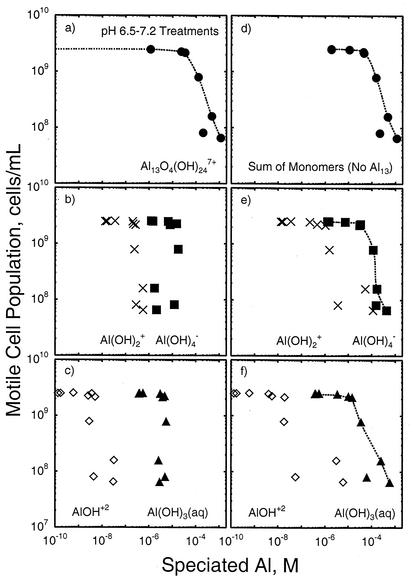
An important question relates to whether a specific form of soluble Al causes the toxic effect or whether the toxicity is generic to all forms of soluble Al. To answer this question, we performed thermodynamic speciation calculations for the pH 6.5 and 7.2 treatments with the measured and inferred concentrations of soluble Al and other solution constituents present at the end of the experiment. Two sets of calculations were performed. In the first, both monomeric and polymeric Al species were considered possible, including the Al13O4(OH)247+ “tridecamer” cation. In the second set, the tridecamer cation was excluded from the database, leaving only monomeric and organically complexed forms of Al in the speciation matrix. The concentration of each species was then plotted against the motile-cell population density data (as done earlier for total soluble Al) to determine whether any one species appeared to be responsible for the toxic effect.
The results of these speciation calculations showed that when the tridecamer cation was considered, it completely dominated the Al toxicity effect (Fig. 7, left column). No other Al species exceeded the critical 5 × 10−5 M concentration threshold, showed such a wide range of concentrations (24 orders of magnitude!), or exhibited a pattern that suggests a direct link to the toxicity. Indeed, the concentration of the tridecamer was essentially negligible until a total Al concentration of 10−5 M was exceeded. When the tridecamer was excluded from the calculation (Fig. 7, right column), the Al(OH)3(aq) and Al(OH)4− species both seemed to contribute to the toxicity effect, although the neutral species seemed to conform more closely to the cell population density trend observed. A toxic effect of the neutral species would be reasonable given that most cell defenses are devoted to combating ionic species. The sum of the monomers, as expected, yielded the same correlation with toxicity as seen for total Al and the tridecamer cation. In both sets of calculations, the maximum concentrations of the Al3+ ion and the organic complexes of Al were at least 4 orders of magnitude lower than that of the most concentrated Al species (data not shown) and did not correlate well with the cell concentration trends, suggesting that they made little or no contribution to the toxic effect at these pH levels.
FIG. 7.
Relationship between measured motile-cell population density and possible soluble-Al species in the pH 6.5 and 7.2 treatments predicted by thermodynamic calculations that include (a to c) or exclude (d to f) the Al13O4(OH)247+ tridecamer cation.
The chemistry of the tridecamer ion, and even its existence in natural systems, has been the subject of considerable debate (4). The ion forms during acid-base reactions involving Al solutions and is metastable to crystalline forms of Al(OH)3. It consists of an AlO4 tetrahedron at its center that is surrounded by 12 edge-sharing Al(OH)2(H2O) octahedra and is about 1.3 nm in size. Because of its size and the fact that it carries a formal charge of +7, the tridecamer does not fit the standard Debye-Hückel ionic model or any other model used to estimate ion activity. This, coupled with its metastability, suggests that its activity cannot be rigorously described by thermodynamic speciation algorithms. Furthermore, the yield of the cation relative to that of crystalline Al(OH)3 varies substantially with the conditions of synthesis (32). The presence of SO42− during the Al neutralization reaction can inhibit formation of the tridecamer in unbuffered systems (17), presumably by interfering with the formation of the Al(OH)4− anion central to the tridecamer. It is unknown whether a similar effect of SO42− would prevail in systems buffered at a higher pH, as in this study. On the other hand, binding of organic acids, such as lactate, to the tridecamer ion can alter its formal charge (39) and, presumably, its stability. Consequently, the speciation results shown in Fig. 7 (left) must be considered speculative and used to indicate the possible presence of the tridecamer rather than its absolute concentration.
In the laboratory, where solutions having known tridecamer concentrations can be prepared, the tridecamer ion has been shown to be significantly more toxic than other forms of Al to wheat plants (through inhibition of root growth [31, 36]), an aquatic alga (Chlorella pyrenoidosa [29]), and a bacterium (Rhizobium trifolii [44, 45]). A good argument can be made for its toxicity to fish, either directly or as a precursor to precipitates that form on the gill surface under conditions in which acidic and basic waters mix (references 4 [p. 156] and 6).
To verify the possible presence or absence of the tridecamer ion in this experiment, we attempted to analyze a freshly prepared sample (pH 6.5 with 10 mM Al added) by solution 27Al NMR spectroscopy. The only signal obtained was a very weak, broad peak near 15 ppm that was difficult to distinguish from the instrumental background. No evidence of a discrete tridecamer ion (a sharp line near 63 ppm) was seen. The presence of SO42− (17) could have prevented the formation of the tridecamer ion. In addition, Thomas et al. (39) noted that high lactate-Al ratios (>3:1) prevent formation of the tridecamer ion as a result of strong complexing of monomeric Al by lactate and the slow kinetics of ligand exchange. As the initial lactate-Al ratio in our experiment was 4:1, this factor could also have prevented tridecamer ion formation.
We concluded, therefore, that Al is toxic to D. desulfuricans G20 (and, presumably, other SRB) over a limited neutral-pH range and when its aqueous solubility exceeds 5 × 10−5 M. Both the neutral Al species [Al(OH)3(aq)] and the monomeric anion [Al(OH)4−] species are likely involved in the toxic effect. Although speciation calculations suggest that the polymeric tridecamer cation could account entirely for the toxic effects seen, no direct evidence of this species was found under our experimental conditions. No evidence of the toxicity of monomeric Al cations was seen either, although such toxicity would be overshadowed by the general pH-induced toxicity seen at the pHs at which these cations are the dominant species. Soluble Al at concentrations of <5 × 10−5 M puts moderate stress on SRB, causing a shift in morphology from vibrio to spirilloid that is particularly pronounced at pHs of >8. The vibrio morphology dominates under normal growth conditions and when severe nutritional or pH-induced stress occurs, whereas the spirilloid morphology is indicative of moderate stress conditions induced by nutritional deficiency or soluble Al.
Acknowledgments
We thank Tom W. Wietsma for the Al and Mg analyses by inductively coupled plasma mass spectrometry, Sarah D. Burton and Herman M. Cho for the 27Al NMR analyses, and two anonymous reviewers for constructive comments.
This research was supported by the Natural and Accelerated Bioremediation Research (NABIR) program, Office of Biological and Environmental Research (OBER), U.S. Department of Energy (DOE), and was performed at the W. R. Wiley Environmental Molecular Sciences Laboratory, a national scientific user facility sponsored by OBER and located at the Pacific Northwest National Laboratory (PNNL). The PNNL is operated for the DOE by the Battelle Memorial Institute under contract DE-AC06-76RL0 1830.
REFERENCES
- 1.Andersson, M. 1988. Toxicity and tolerance of aluminium in vascular plant. Water Air Soil Pollut. 39:439-462. [Google Scholar]
- 2.Appana, V. D., and M. St. Pierre. 1996. Aluminum elicits exocellular phosphatidylethanolamine production in Pseudomonas fluorescens. Appl. Environ. Microbiol. 62:2778-2782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Baes, C. F., Jr., and R. E. Mesmer. 1976. The hydrolysis of cations. John Wiley & Sons, Inc., New York, N.Y.
- 4.Bertsch, P. M., and D. R. Parker. 1996. Aqueous polynuclear aluminum species, p. 117-168. In G. Sposito (ed.), The environmental chemistry of aluminum, 2nd ed. Lewis Publishers, Boca Raton, Fla.
- 5.Exley, C., and J. D. Birchall. 1992. The cellular toxicity of aluminum. J. Theor. Biol. 159:83-98. [DOI] [PubMed] [Google Scholar]
- 6.Gensemer, R. W., and R. C. Playle. 1999. The bioavailability and toxicity of aluminum in aquatic environments. Crit. Rev. Environ. Sci. Technol. 29:315-450. [Google Scholar]
- 7.Ginting, S., B. B. Johnson, and S. Wilkens. 1998. Alleviation of aluminium phytotoxicity on soybean growth by organic anions in nutrient solutions. Aust. J. Plant Physiol. 25:901-908. [Google Scholar]
- 8.Good, N. E., G. D. Winget, W. Winter, T. Connelly, S. Izawa, and R. M. M. Singh. 1966. Hydrogen ion buffers for biological research. Biochemistry 5:467-477. [DOI] [PubMed] [Google Scholar]
- 9.Gutteridge, J. M. C., G. J. Quinlan, I. Clark, and B. Halliwall. 1985. Aluminium salts accelerate peroxidation of membrane lipids stimulated by iron salts. Biochim. Biophys. Acta 835:441-447. [DOI] [PubMed] [Google Scholar]
- 10.Hamel, R., and V. D. Appanna. 2001. Modulation of TCA cycle enzymes and aluminum stress in Pseudomonas fluorescens. J. Inorg. Biochem. 87:1-8. [DOI] [PubMed] [Google Scholar]
- 11.Hamel, R., R. Levasseur, and V. D. Appanna. 1999. Oxalic acid production and aluminum tolerance in Pseudomonas fluorescens. J. Inorg. Biochem. 76:99-104. [DOI] [PubMed] [Google Scholar]
- 12.Hino, T., C. Kiyoka, and H. Sakurai. 1997. Aluminum enhances Fe2+-induced lipid peroxidation. J. Inorg. Biochem. 67:375. [Google Scholar]
- 13.Hue, N. V., G. R. Craddock, and F. Adams. 1986. Effect of organic acids on aluminum toxicity in subsoils. Soil Sci. Soc. Am. J. 50:28-34. [Google Scholar]
- 14.Jorge, R. A., and P. Arruda. 1997. Aluminum-induced organic acids exudation by roots of an aluminum-tolerant tropical maize. Phytochemistry 45:675-681. [Google Scholar]
- 15.Kanazawa, S., and T. Kunito. 1996. Preparation of pH 3.0 agar plate, enumeration of acid-tolerant, and Al-resistant microorganisms in acid soils. Soil Sci. Plant Nutr. 42:165-173. [Google Scholar]
- 16.Kawai, F., D. Zhang, and M. Sugimoto. 2000. Isolation and characterization of acid- and Al-tolerant microorganisms. FEMS Microbiol. Lett. 189:143-147. [DOI] [PubMed] [Google Scholar]
- 17.Kerven, G. L., P. L. Larsen, and F. P. C. Blarney. 1995. Detrimental sulfate effects on formation of Al-13 tridecameric polycation in synthetic soil solutions. Soil Sci. Soc. Am. J. 59:765-771. [Google Scholar]
- 18.Kochian, L. V. 1995. Cellular mechanisms of aluminium toxicity and resistance in plants. Annu. Rev. Plant Physiol. Plant Mol. Biol. 46:2237-2260. [Google Scholar]
- 19.Konishi, S., I. Souta, J. Takahashi, M. Ohmoto, and S. Kaneko. 1994. Isolation and characteristics of acid- and aluminum-tolerant bacterium. Biosci. Biotech. Biochem. 58:1960-1963. [Google Scholar]
- 20.Lide, D. R. (ed.). 2000. CRC handbook of chemistry and physics, 81st ed. CRC Press, Inc., Boca Raton, Fla.
- 21.Macdiarmid, C. S., and R. C. Gardner. 1998. Overexpression of the Saccharomyces cerevisiae magnesium transport system confers resistance to aluminum ion. J. Biol. Chem. 273:1727-1732. [DOI] [PubMed] [Google Scholar]
- 22.Marklund, E., S. Sjoberg, and L.-O. Ohman. 1986. Equilibrium and structural studies of silicon(IV) and aluminium(III) in aqueous solution. 14:. Speciation and equilibria in the aluminium(III)-lactic acid-OH system. Acta Chem. Scand. Ser. A 40:367-373. [Google Scholar]
- 23.Martell, A. E., and R. M. Smith. 1977. Critical stability constants, vol. 3: other organic ligands. Plenum Press, New York, N.Y.
- 24.Martin, R. B. 1986. The chemistry of aluminum as related to biology and medicine. Clin. Chem. 32:1797-1806. [PubMed] [Google Scholar]
- 25.Nanba, K., and G. M. King. 2000. Response of atmospheric methane consumption by Maine forest soils to exogenous aluminum salts. Appl. Environ. Microbiol. 66:3674-3679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Neal, A. L., S. Techkarnjanaruk, A. Dohnalkova, D. McCready, B. M. Peyton, and G. G. Geesey. 2001. Iron sulfides and sulfur species produced at hematite surfaces in the presence of sulfate-reducing bacteria. Geochim. Cosmochim. Acta 65:223-235. [Google Scholar]
- 27.Nguyen, V. A. T., K. Senoo, T. Mishima, and M. Hisamatsu. 2001. Multiple tolerance of Rhodotorula glutinis R-1 to acid, aluminum ion and manganese ion, and its unusual ability of neutralizing acidic medium. J. Biosci. Bioeng. 92:366-371. [DOI] [PubMed] [Google Scholar]
- 28.Nordstrom, D. K. 1982. The effect of sulfate on aluminum concentrations in natural waters: some stability relations in the system Al2O3-SO3-H2O at 298 K. Geochim. Cosmochim. Acta 46:681-692. [Google Scholar]
- 29.Parent, L., and P. G. C. Campbell. 1994. Aluminum bioavailability to the green alga Chlorella pyrenoidosa in acidified synthetic soft water. Environ. Toxicol. Chem. 13:587-598. [Google Scholar]
- 30.Parker, D. R., T. B. Kinraide, and L. W. Zelazny. 1988. Aluminum speciation and phytotoxicity in dilute hydroxy-aluminum solutions. Soil Sci. Soc. Am. J. 52:67-75. [Google Scholar]
- 31.Parker, D. R., T. B. Kinraide, and L. W. Zelazny. 1989. On the phytotoxicity of polynuclear hydroxy-aluminum complexes. Soil Sci. Soc. Am. J. 53:789-796. [Google Scholar]
- 32.Parker, D. R., and P. M. Bertsch. 1992. Formation of the “Al13” tridecameric polycation under diverse synthesis conditions. Environ. Sci. Technol. 26:914-921. [Google Scholar]
- 33.Postgate, J. R. 1979. The sulphate-reducing bacteria. Cambridge University Press, Cambridge, United Kingdom.
- 34.Postgate, J. R. 1984. Genus Desulfovibrio Kluyver and van Niel 1936, 397AL, p. 666-672. In N. L. Krieg and J. G. Holt (ed.) Bergey's manual of systematic bacteriology, vol. 1. The Williams & Wilkins Co., Baltimore, Md.
- 35.Sani, R. K., G. G. Geesey, and B. M. Peyton. 2001. Assessment of lead toxicity to Desulfovibrio desulfuricans G20: influence of components of lactate C medium. Adv. Environ. Res. 5:269-276. [Google Scholar]
- 36.Shann, J. R., and P. M. Bertsch. 1993. Differential cultivar response to polynuclear hydroxo-aluminum complexes. Soil Sci. Soc. Am. J. 57:116-120. [Google Scholar]
- 37.Taylor, S. R., and S. M. McLennan. 1985. The continental crust: its composition and evolution. Blackwell, Oxford, England.
- 38.Thauer, R. K., K. Jungerman, and K. Decker. 1977. Energy conservation in chemotrophic anaerobic bacteria. Bacteriol. Rev. 41:100-180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Thomas, F., A. Masion, J. Y. Bottero, J. Rouiller, F. Montigny, and F. Genevrier. 1993. Aluminum(III) speciation with hydroxy carboxylic acids: 27Al NMR study. Environ. Sci. Technol. 27:2511-2516. [Google Scholar]
- 40.U.S. Environmental Protection Agency. 1991. Equilibrium metal speciation model (MINTEQA2), version 3.11. Center for Exposure Assessment Modeling, U. S. Environmental Protection Agency, Athens, Ga.
- 41.Williams, R. J. P. 1999. What is wrong with aluminium? The J. D. Birchall memorial lecture. J. Inorg. Biochem. 76:81-88. [DOI] [PubMed] [Google Scholar]
- 41a.Wong, D., J. M. Suflita, J. P. McKinley, and L. R. Krumholz. Impact of clay minerals on sulfate-reducing activity in aquifers. Microb. Ecol., in press. [DOI] [PubMed]
- 42.Wood, J. M. 1984. Microbial strategies in resistance to metal ion toxicity, p. 333-351. In H. Sigel (ed.) Metal ions in biological systems, vol. 18: circulation of metals in the environment. Marcel Dekker, Inc., New York, N.Y.
- 43.Wood, J. M. 1985. Effects of acidification on the mobility of metals and metalloids: an overview. Environ. Health Perspect. 63:115-119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wood, W., and J. E. Cooper. 1984. Aluminium toxicity and multiplication of Rhizobium trifolii in a defined growth medium. Soil Biol. Biochem. 16:571-576. [Google Scholar]
- 45.Wood, W., and J. E. Cooper. 1988. Acidity, aluminium and multiplication of Rhizobium trifolii: possible mechanisms of aluminium toxicity. Soil Biol. Biochem. 20:95-99. [Google Scholar]
- 46.Yokel, R. A., D. D. Allen, and D. C. Ackley. 1999. The distribution of aluminum into and out of the brain. J. Inorg. Biochem. 76:127-132. [DOI] [PubMed] [Google Scholar]
- 47.Zel, J., and L. Bevc. 1993. Effects of aluminium on mineral content of mycorrhizal fungi in vitro. Water Air Soil Pollut. 71:271-279. [Google Scholar]