Abstract
The nucleic acid stain SYBR Green I was evaluated for use with solid-phase laser cytometry to obtain total bacterial cell counts from several water sources with small bacterial numbers. Results were obtained within 30 min and exceeded or equaled counts on R2A agar plates incubated for 14 days at room temperature.
Sensitive, rapid detection methods for determining total bacterial cell numbers in water are needed for many applications, including production of pharmaceuticals, manufacture of computer chips, and analysis of potable supplies for space flight. Traditional culture methods determine only culturable cells in the sample and require 1 to 2 weeks of incubation at 20 to 30°C for optimal enumeration of environmental samples (4).
Epifluorescent microscopy can be used to rapidly estimate total cell numbers in water samples by using dyes, such as 4′,6-diamidino-2-phenylindole dihydrochloride, acridine orange, and SYBR Green I (SG), that bind to nucleic acids (2, 3, 6, 10). For accurate estimates by epifluorescent microscopy there must be at least 105 cells evenly distributed on the filter, so when a sample contains small numbers of cells, extremely large quantities of liquid must be filtered (J. T. Lisle, A. Willse, B. H. Pyle, M. Hamilton, and G. A. McFeters, Abstr. 99th Gen. Meet. Am. Soc. Microbiol. 1999, abstr. Q-203, p. 572, 1999).
Flow cytometry can be used to count SG-stained and other fluorescently stained bacterial cells in water (1, 7, 8, 12). Although cells can be enumerated at a lower concentration than that required by epifluorescent microscopy, cell numbers must still be greater than 100 bacteria per ml to obtain reliable counts in a short time. Additionally, the presence of autofluorescent particles and debris stained by nonspecific binding of the fluorescent dye will cause erroneous results, limiting the use of flow cytometry in evaluating environmental samples (5, 12).
The ScanRDI (Chemunex, Paris, France) is a solid-phase laser scanner that is able to scan the entire surface of a 25-mm-diameter filter in 3 min by using a 488-nm laser (4, 9, 11, 13). Detectors record emitted fluorescence, and computer software screens all the fluorescent events by using several discriminant parameters, displays the number of events determined to be cells, and maps the location of each particle. The operator then transfers the filter to an epifluorescence microscope, and the ScanRDI system directs the microscope stage to each fluorescent particle, allowing the operator to confirm the discrimination of bacterial cells and detritus. This system allows the detection and verification of a single bacterial cell on a filter.
ChemChrome V6 (AES-Chemunex, Inc., Princeton, N.J.), a proprietary reagent developed for use with the ScanRDI, is a reducible fluorescein-labeled substrate which, when degraded by esterase activity, releases free fluorescein inside the cell. Cells that are detected by this green fluorescence are reported in the viable cell count due to esterase activity (13).
The fluorescent characteristics of stains such as 4′,6-diamidino-2-phenylindole dihydrochloride and acridine orange are incompatible with the ScanRDI system. It was determined that cells stained with SG are detectable by the ScanRDI by using modified discriminant settings. SG and ChemChrome can be used in parallel to enumerate total and viable bacteria, respectively. Viable counts enumerated by using ChemChrome and total counts determined by SG staining were compared to R2A agar plate counts.
Three different water sources were used to determine the effectiveness of SG staining for use with the ScanRDI system: ultrapure laboratory water (Milli-Q; Millipore), tap water, and diluted stream water containing a natural multispecies community. The efficacy of SG staining on fixed versus unfixed samples was determined. Milli-Q water that was filtered through a 0.2-μm-pore-sized filter and then autoclaved was used as a negative control.
Plate counts on water samples containing less than 30 CFU/ml were filtered in triplicate through sterile filters (0.2 μm pore size, 47 mm diameter; catalog no. GSWP 047 S0; Millipore) in volumes sufficient to give 20 to 200 CFU/filter. The filters were placed on R2A agar containing 0.002% triphenyl tetrazolium chloride. Water samples with more than 30 CFU/ml were plated directly on R2A medium. Colonies were enumerated after 14 days of incubation at ambient room temperature (ca. 22°C).
Detection of bacteria with esterase activity was performed on appropriate aliquots of the water according to the manufacturer's protocol by using ChemChrome V6. Cells were enumerated on a ScanRDI by using the discriminant settings supplied by AES-Chemunex.
For SG staining the water sample was filtered through a black polycarbonate membrane, and then an overlay of dye was applied to the filter. After incubation the stain was removed by vacuum and the membranes were dried and stored in the dark at 4°C prior to enumeration. Using results of preliminary experiments, all SG staining was done in the dark at a final concentration of 100× in water for 5 min at room temperature.
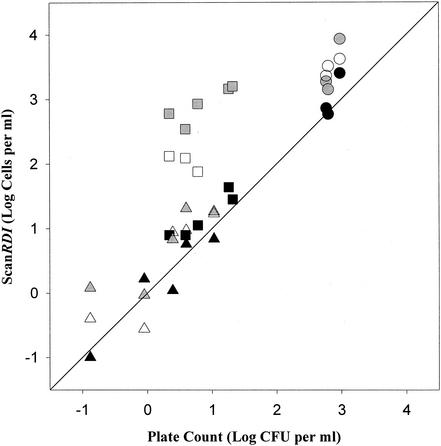
As shown in Fig. 1, results from the analysis of Milli-Q water were similar to plate count results for both esterase activity and total numbers. Reflecting the effects of chlorination, esterase counts for tap water were statistically equivalent to plate counts, and total cell numbers determined by SG staining without formalin fixation were more than a log higher. Formalin fixation reduced the number of stained cells detected in tap water, but the numbers were still nearly 35 times the numbers determined by plate counts. The numbers of bacterial cells from surface water samples were 1.59 times the plate count when determined by esterase activity and were 4.77 times the plate count when determined by total cell staining with SG. Little difference was observed in surface water results due to formalin fixation.
FIG. 1.
Comparison of enumeration of bacterial cells from surface water, tap water, and Milli-Q water by the ScanRDI by using SG and ChemChrome staining to enumeration of bacterial cells by plate counts on R2A agar (n = 2 to 3). Symbols: black circles, esterase surface water; gray circles, SYBR Green surface water; open circles, SYBR Green surface water after formalin fixation; black squares, esterase tap water; gray squares, SYBR Green tap water; open squares, SYBR Green tap water after formalin fixation; black triangles, esterase Milli-Q; gray triangles, SYBR Green Milli-Q; open triangles, SYBR Green Milli-Q after formalin fixation. The diagonal line is the line of equality.
Staining with SG, when used in conjunction with the ScanRDI, can provide rapid analysis of total bacterial cell numbers from water samples with a lower detection limit of 1 cell per filter. The lower threshold for surface waters and other samples depends on the volume that can be filtered. If water samples are relatively free of extraneous particles, high volumes of water can be filtered, reducing the detection limit to the limit of filterability. It is vital that reagents are filtered before use in order to remove all bacterial cells, even those rendered inactive by other sterilization methods, and that negative controls be done to determine that all cells have been removed from stains and reagents. The entire staining, filtration, enumeration, and verification procedure can be completed in less than 30 min. Use of SG for total cell counts, in parallel with ChemChrome V6 for viable cell counts, followed by solid-phase laser cytometry for enumeration permits the rapid, sensitive detection of very small numbers of total and viable bacteria in water. These techniques are applicable for use in situations such as space flight, pharmaceutical production, and industrial applications where the timeliness of obtaining bacterial counts is a prime consideration.
Acknowledgments
We are indebted to Gordon A. McFeters for his past leadership and continuing support. We thank John T. Lisle for help with studies that led to this research and Margaret A. Juergensmeyer for supporting experiments.
Funding from NASA (NAG 9-1082 and NCC 2-1143) is gratefully acknowledged.
REFERENCES
- 1.Barbesti, S., S. Citterio, M. Labra, M. D. Baroni, M. G. Neri, and S. Sgorbati. 2000. Two- and three-color fluorescence flow cytometric analysis of immunoidentified viable bacteria. Cytometry 40:214-218. [PubMed] [Google Scholar]
- 2.Daley, R. J. 1979. Direct epifluorescence enumeration of native aquatic bacteria: uses, limitations, and comparative accuracy, p. 29-45. In J. W. Costeron and R. R. Colwell (ed.), Native aquatic bacteria: enumeration, activity and ecology. ASTM STP 605. American Society for Testing and Materials, Philadelphia, Pa.
- 3.Grigorova, R., and J. R. Norris (ed.). 1990. Methods in microbiology, p. 8-13, vol. 22. Academic Press, San Diego, Calif.
- 4.Jones, D. L., M. A. Brailsford, and J.-L. Drocourt. 1999. Solid-phase, laser-scanning cytometry: a new two-hour method for the enumeration of microorganisms in pharmaceutical water. Pharmacop. Forum 25:7626-7645. [Google Scholar]
- 5.Joux, F., and P. Lebaron. 2000. Use of fluorescent probes to assess physiological functions of bacteria at single-cell level. Microbes Infect. 2:1523-1525. [DOI] [PubMed] [Google Scholar]
- 6.Kepner, R. L., Jr., and J. R. Pratt. 1994. Use of fluorochromes for direct enumeration of total bacteria in environmental samples: past and present. Microbiol. Rev. 58:603-615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.LeBaron, P., N. Parthuisot, and P. Catala. 1998. Comparison of blue nucleic acid dyes for flow cytometric enumeration of bacteria in aquatic systems. Appl. Environ. Microbiol. 64:1725-1730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Marie, D., F. Partensky, S. Jacquet, and D. Vaulot. 1997. Enumeration and cell cycle analysis of natural populations of marine picoplankton by flow cytometry using the nucleic acid stain SYBR Green I. Appl. Environ. Microbiol. 63:186-193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mignon-Godefroy, K., J. G. Guillet, and C. Butor. 1997. Solid phase cytometry for rare events. Cytometry 27:336-344. [PubMed] [Google Scholar]
- 10.Noble, R. T., and J. A. Fuhrman. 1998. Use of SYBR Green I for rapid epifluorescence counts of marine viruses and bacteria. Aquat. Microb. Ecol. 14:113-116. [Google Scholar]
- 11.Pyle, B. H., S. C. Broadaway, and G. A. McFeters. 1999. Sensitive detection of Escherichia coli O157:H7 in food and water by immunomagnetic separation and solid-phase laser cytometry. Appl. Environ. Microbiol. 65:1966-1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Vives-Rego, J., P. Lebaron, and G. Nebe-von Caron. 2000. Current and future applications of flow cytometry in aquatic microbiology. FEMS Microbiol. Rev. 24:429-448. [DOI] [PubMed] [Google Scholar]
- 13.Wallner, G., D. Tillmann, K. Haberer, P. Cornet, and J.-L. Drocourt. 1997. The ChemScan system: a new method for rapid microbiological testing of water. Eur. J. Parenter. Sci. 2:123-126.