Abstract
Thermal inactivation of nonproteolytic Clostridium botulinum type E spores was investigated in rainbow trout and whitefish media at 75 to 93°C. Lysozyme was applied in the recovery of spores, yielding biphasic thermal destruction curves. Approximately 0.1% of the spores were permeable to lysozyme, showing an increased measured heat resistance. Decimal reduction times for the heat-resistant spore fraction in rainbow trout medium were 255, 98, and 4.2 min at 75, 85, and 93°C, respectively, and those in whitefish medium were 55 and 7.1 min at 81 and 90°C, respectively. The z values were 10.4°C in trout medium and 10.1°C in whitefish medium. Commercial hot-smoking processes employed in five Finnish fish-smoking companies provided reduction in the numbers of spores of nonproteolytic C. botulinum of less than 103. An inoculated-pack study revealed that a time-temperature combination of 42 min at 85°C (fish surface temperature) with >70% relative humidity (RH) prevented growth from 106 spores in vacuum-packaged hot-smoked rainbow trout fillets and whole whitefish stored for 5 weeks at 8°C. In Finland it is recommended that hot-smoked fish be stored at or below 3°C, further extending product safety. However, heating whitefish for 44 min at 85°C with 10% RH resulted in growth and toxicity in 5 weeks at 8°C. Moist heat thus enhanced spore thermal inactivation and is essential to an effective process. The sensory qualities of safely processed and more lightly processed whitefish were similar, while differences between the sensory qualities of safely processed and lightly processes rainbow trout were observed.
Vacuum-packaged hot-smoked fish products have frequently been reported to cause type E botulism (4-6, 25). In Northern aquatic environments the prevalence of nonproteolytic Clostridium botulinum type E is high, with heavy contamination of the Baltic Sea with spores (22). In a recent survey, 20% of the fish caught in the Baltic Sea and in Finnish freshwaters carried nonproteolytic C. botulinum type E spores. Moreover, 7% of vacuum-packaged hot-smoked rainbow trout and whitefish products marketed in Finland contained spores of this organism (23), indicating the survival of spores during the heat treatments commonly employed in industrial hot smoking.
Current guidelines developed in Europe (1, 17) include recommendations regarding the heat processing, formulation, and storage of refrigerated processed foods of extended durability (REPFEDs), including vacuum-packaged hot-smoked fish products. To reduce the risk of botulism presented by nonproteolytic C. botulinum, the recommended heat processes should reduce the probability of a single spore leading to growth and toxin production by a factor of 106. Depending on the z value used, and not taking into account the relative humidity (RH), time-temperature combinations of 90°C for 10 min, 85°C for 36 to 52 min, and 80°C for 129 to 270 min have been suggested to reduce the number of spores of nonproteolytic C. botulinum by a factor of 106 (1, 17). However, in the absence of additional controlling factors such as chill storage, these heat processes have since been shown to fail in controlling growth and toxin production from 106 spores of nonproteolytic C. botulinum types B, E, and F in meat (19, 30, 40). As the pH of fish products is near neutral and the low NaCl content of vacuum-packaged hot-smoked fish products is unlikely to contribute to control of growth and toxin production, the safety of these products relies upon a combination of heat treatment and refrigerated storage. Nonproteolytic C. botulinum has been reported to grow and produce toxin at 3.0 to 3.3°C (15, 16, 21, 42). It is thus essential that appropriate combination factors to be employed in commercial fish smoking be determined.
Lysozyme is known to facilitate the germination of heat-damaged spores of nonproteolytic C. botulinum (20), thereby increasing measured spore heat resistance. Biphasic survivor curves indicate that from 0.1 to 20% of a spore population is permeable to lysozyme and is more heat resistant than the nonpermeable fraction (37, 45). Without the addition of lysozyme to the recovery medium, decimal reduction times (D values) for spores of type E strains of 0.07 to 6.6 min at 82°C, depending on the heating medium, have been reported (7, 11, 27, 32, 41, 44, 47); with lysozyme, higher D values of 48.3, 12.6, and 3.17 min at 85, 90, and 95°C, respectively, have been determined (37).
In the 1960s, efforts towards more effective control of the risk presented by C. botulinum type E in commercial hot-smoking processes introduced a time-temperature combination in commercial hot smoking of 82.2°C for 30 min (10), although the process was subsequently shown to fail in ensuring product safety (2, 9). It was later established that this heat treatment combined with an ambient atmosphere of high RH in the smoke chamber brought about a reduction of 105 to 106 in the numbers of spores of C. botulinum type E heated in whitefish chubs (2, 35). However, lysozyme was not used in the recovery medium of heat-injured spores, and it is likely that in the presence of lysozyme more spores would have recovered following the heat treatment and a lower safety margin would have been identified. A number of other studies on the thermal destruction of spores of type E strains in fish media have been conducted without using lysozyme in the recovery medium of spores (14, 39, 40), but studies employing lysozyme have not been reported. As lysozyme is present in a number of fish species (28), it is essential to reevaluate the effect of heat processing on the survival of nonproteolytic C. botulinum spores in fish when recovered in the presence of lysozyme. This is the first time that the thermal inactivation of nonproteolytic C. botulinum in fish has been investigated with lysozyme in the recovery medium of heat-injured spores.
Fish hot smoking seems to vary in different countries, as do the heat-processing procedures and adherence to storage temperatures and shelf lives. In the United States, an in-pack pasteurization protocol in a water bath for smoked fish has been identified (14). Such a process was shown to control the growth and toxin formation from 106 nonproteolytic C. botulinum spores at 25°C. However, concerns over poor process uniformity, temperature abuse, and the growth of the more heat-resistant strains of proteolytic C. botulinum led to the recommendation that such products be labeled “Keep refrigerated. Store below 3°C.” The implementation of in-pack pasteurization procedures in the fish industry would require investment in additional equipment, such as water baths, and would change the traditional hot-smoking concept.
The aims of the present study were (i) to evaluate the lethality of pasteurization processes employed in commercial fish hot smoking in Finland with respect to C. botulinum type E; (ii) to determine the heat resistance parameters (D and z values) of C. botulinum type E strain Beluga in rainbow trout and whitefish media with lysozyme in the recovery medium; (iii) to design hot-smoking processes that will control growth and toxin production from 106 spores in rainbow trout fillets and whole whitefish during storage for 5 weeks at 8°C, employing various combinations of process time, temperature, and RH; and (iv) to investigate the effects of these processes on the sensory quality of vacuum-packaged hot-smoked rainbow trout fillets and whole whitefish products.
MATERIALS AND METHODS
Process evaluation in five Finnish fish processing plants.
The hot-smoking processes employed in five Finnish small-scale fish processing plants in the production of rainbow trout fillets were evaluated with respect to their lethality for spores of nonproteolytic C. botulinum type E (Table 1). The temperature at the fish surface and the internal temperature of the smoke chamber were monitored (DP-158; Envic, Turku, Finland) at 3-min intervals with temperature probes (Ellab A/S, Roedovre, Denmark). The fish surface temperatures were measured at a 1-mm depth of the fish. The smoke chamber temperature and RH were measured at the central part of the smoke chamber. The data on fish surface temperatures were used to calculate the inactivation ratio (P/t) for each process time. The equation used was P/t = 10([T − Tref]/z) (8), where P is the pasteurization time describing the equivalent process time (minutes) at the reference temperature Tref (85°C) and t is the process time (minutes) at the actual temperature T (degrees Celsius). A z value of 7°C (17) was used.
TABLE 1.
Commercial hot-smoking processes employed in five fish-smoking plants in the production of vacuum-packaged hot-smoked rainbow trout in Finland, evaluated for safety with respect to the thermal destruction of nonproteolytic C. botulinum
| Company | Pasteurization time (min)a | Actual fish surface temp (°C)b | RH (%)b | Possible to control RHc |
|---|---|---|---|---|
| A | 6.0 | 81.0 (80.3-82.0) | NDd | Yes |
| B | 5.5 | 82.8 (82.2-83.4) | 80 (77-83) | Yes |
| C | 0.03 | 63.6 (63.1-64.2) | 60 (61-63) | No |
| D | 0.02 | 62.3 (61.8-62.8) | 60 (58-60) | Yes |
| E | 0.01 | 56.3 (55.4-57.7) | 60 (58-59) | Yes |
Assuming a Tref of 85°C and a z value of 7°C (16).
Mean value (approximation of RH to the nearest 5%) measured during the most effective 10-min period at the end of the heat process (the range is given in parentheses).
Yes, the RH inside the smoking chamber is controllable electrically; no, the RH is not controllable electrically.
ND, not determined.
C. botulinum type E spores.
The nonproteolytic C. botulinum type E strain Beluga E was used in thermal death time measurements, while a mixture of three strains (Beluga E, 211 E, and 250 E) was used in the inoculated-pack studies. The origins and sources of the strains have been described previously (29). Spores were produced, enumerated, and checked for toxin production as described previously (12). Washed spores were suspended in sterile distilled water and stored at 4°C until use.
Thermal death time of C. botulinum type E in model fish medium. (i) Preparation of fish medium.
An anaerobic fish medium was prepared by a method described previously (38), with modifications. The medium contained 250 g of skinless rainbow trout (Oncorhyncus mykiss) or skinless whitefish (Coregonus lavaretus) bought from a local fishery, 10 g of glucose (BDH Laboratory Supplies, Poole, Dorset, United Kingdom), 10 g of NaCl (Merck KGaA, Darmstadt, Germany), 10 g of starch (Merck KGaA), and distilled water to 1,000 g. In brief, the fish was chopped for 30 s and suspended with an equal volume of distilled water. The suspension was heated in a water bath at 100°C for 10 min and blended (Stomacher 400; GWB Ltd., Espoo, Finland) for a further 60 s. After addition of NaCl, glucose, and starch, the container was weighed and the suspension was cooked for 15 min with continuous stirring. Evaporation was compensated for by addition of sterile water to give 1,000 g, and the medium was cooled to 50°C. The pH of the medium was adjusted to 6.4 with HCl, and 5-ml aliquots of the medium were dispensed into metal-capped glass tubes. The tubes were autoclaved at 121°C for 15 min and stored in anaerobic jars with a gas generation kit (AnaeroGen; Oxoid, Basingstoke, Hampshire, United Kingdom) at 4°C until use.
(ii) Inoculation, thermal processing, and cooling of fish medium.
Glass tubes containing 5 ml of fish medium were each inoculated with spores of C. botulinum type E strain Beluga. Two noninoculated tubes for each heating temperature served as negative controls. Three noninoculated tubes were used for temperature monitoring at 30- to 120-s intervals with probes (Ellab A/S) calibrated against a certified mercury-in-glass thermometer. The temperature was measured to the nearest 0.5°C. All tubes were placed in a hot water bath set at a target temperature of 75 to 93°C, and the experiment was started as soon as the core temperatures of the noninoculated tubes reached the target temperature. Depending on the target temperature, this took approximately 1 to 2 h. To avoid the lethal effect of the times to reach the target temperatures, 10 tubes at a time were inoculated with 106 spores of strain Beluga, and timing was started immediately. The final heating times varied from 0.5 to 450 min. On the basis of the temperature data obtained for the noninoculated temperature tubes at the target temperature and collected by a data logger (Envic DP-158), the equivalent times at the target temperatures were calculated by using the formula 10([T − Tref]/z), where T is the mean of the actual temperatures measured in the three temperature tubes and Tref is the target temperature (75 to 93°C). A z value of 7°C was used (17). All experiments were conducted twice.
Two or three replicate tubes were removed from the water bath at each sampling time. The tubes were placed in a deep ice-water bath with vigorous shaking to ensure rapid cooling, in order to eliminate spore destruction during the temperature fall. The tubes were kept in the ice bath until culturing and enumeration.
(iii) Culturing and enumeration of C. botulinum.
Two replicate tubes (referred to below as culture tubes) for every heating time were cultured in anaerobic tryptose-peptone-glucose-yeast extract broth (Difco, Detroit, Mich.) containing hen egg white lysozyme (625 IU/ml; Sigma Chemical Co., St. Louis, Mo.) by gently pouring 10 ml of broth on top of the fish medium. The tubes were incubated in an anaerobic cabinet with an internal atmosphere of 85% N2, 10% CO2, and 5% H2 (MK III; Don Whitley Scientific Ltd., Shipley, United Kingdom) at 30°C for 90 days and observed for turbidity and gas production daily for 2 weeks and once a week for the following 10 weeks. In addition to the culture tubes, at every second or third sampling time the spores in one tube were enumerated by a three-tube most-probable-number (MPN) technique immediately after the heat treatments. When the C. botulinum count was below the detection limit, an MPN estimate of 100.18 spores was used, provided that growth was observed in the corresponding culture tubes. If growth was not observed in either the culture tubes or the MPN tubes, the quantity was considered to be less than one spore.
(iv) Determination of D and z values and proportion of heat-resistant spores.
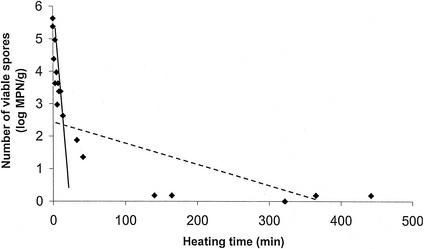
As the thermal destruction curves were biphasic, two regression lines were fitted to the data points: one was fitted to the log-linear phase of the reduction curve describing the spore fraction nonpermeable to lysozyme, and the other was fitted to the tailed phase of the curve describing the fraction permeable to lysozyme. The D values of each fraction at each temperature were calculated from the best-fit line by linear regression (Statistix version 1.0; Analytical Software, Tallahassee, Fla.). The percentage of heat-resistant spores at each temperature was determined as a ratio of the mean spore count at the intercept of the two regression lines. D values were then plotted on thermal death time (TD) curves, and z values for the lysozyme-permeable spore fractions in trout and whitefish were determined.
Inoculated-pack study. (i) Fish samples.
Rainbow trout fillets with skin and whole split whitefish were bought from a local fishery, where the trout were beheaded and filleted and the whole whitefish were gutted. These forms of the two fish are most frequently used to produce vacuum-packaged hot-smoked fish in Finland. The final sample size was typically 500 to 600 g (trout, 530 ± 75 g; whitefish, 600 ± 270 g). The average pH of trout was 6.6 (range, 6.4 to 6.9), and that of whitefish was 7.0 (range, 6.7 to 7.6). The NaCl concentration in both fish species was <0.5% (wt/vol).
(ii) Inoculation with C. botulinum type E spores.
The fish were placed on aluminum foil trays, with the skin surface of the rainbow trout facing down, and inoculated with approximately 106 spores per kg, which were evenly spread on the skinless side of the trout fillets and in the abdominal cavity of the whole whitefish. The spore mixture consisted of equal concentrations of spores of C. botulinum type E strains Beluga E, 211 E, and 250 E, with a final inoculation volume of 0.5 ml per sample.
(iii) Thermal processing of fish samples.
The fish samples on the foil trays were laid on metal racks that were placed in an electrically controlled and heated smoke chamber equipped with an external smoke generator (Vemag; Kerres GmbH, Sulzbach/Murr, Germany). The temperature data were monitored and pasteurization values were calculated as described above. The reference temperature of 85°C corresponded to the mean actual temperatures measured on the fish surface layer during most of the heat processes (Table 2). The z values of 10.4°C for trout and 10.1°C for whitefish were used as determined in the model fish media in this study (Table 3). In addition to the temperature data, the internal RH of the smoke chamber was measured with a humidity probe (Delta Ohm, AMX 510; Envic).
TABLE 2.
Heat treatments employed in processing of vacuum-packaged hot-smoked rainbow trout fillets and whole whitefish samples
| Study(ies)a | Fish | Processb | Pasteurization time (min)c | Actual fish surface temp (°C)d | RH (%)d |
|---|---|---|---|---|---|
| I | Rainbow trout | RI | 1.5 | 66.5 (65.3-70.3) | 60 (55-61) |
| I | Rainbow trout | RII | 25 | 82.4 (81.5-83.6) | 25 (24-27) |
| I | Rainbow trout | RIII | 26 | 82.9 (81.5-84.2) | 70 (56-85) |
| I | Rainbow trout | RIV | 34 | 84.0 (80.9-88.3) | 75 (67-82) |
| I | Whitefish | WI | 1.5 | 67.3 (66.3-70.7) | 50 (47-56) |
| I, S | Whitefish | WII | 42 | 84.2 (83.2-85.8) | 80 (80-86) |
| I | Whitefish | WIII | 44 | 83.9 (80.3-87.5) | 10 (11-13) |
| I, S | Whitefish | WIV | 62 | 86.3 (84.9-87.4) | 80 (65-89) |
| S | Both species | RWI | 0.3 | 64.9 (62.5-66.5) | 50 |
| S | Both species | RWII | 202 | 92.5 (88.9-95.0) | 80 |
I, inoculated-pack study; S, sensory evaluation study.
R, rainbow trout; W, whitefish; RW, both fish species.
Assuming a Tref of 85°C and z values of 10.4 and 10.1°C for trout and whitefish, respectively, as determined in this study.
Mean value (approximation of RH to the nearest 5%) measured during the most effective 30-min period at the end of the heat process (the range is given in parentheses).
TABLE 3.
D and z values of C. botulinum type E strain Beluga in rainbow trout and whitefish media heated at 75 to 93°C
| Heating medium |
D values (min) for the following temp (°C) and fractiona:
|
z value (°C)b | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| 75
|
81
|
85
|
90
|
93
|
|||||||
| NR | R | NR | R | NR | R | NR | R | NR | R | ||
| Rainbow trout | 4.6 | 255 | NDc | ND | 2.0 | 98 | ND | ND | 0.4 | 4.2 | 10.4 |
| Whitefish | ND | ND | 1.9 | 55 | ND | ND | 1.0 | 7.1 | ND | ND | 10.1 |
NR, non-heat resistant fraction; R, heat resistant fraction.
z values were determined for the heat-resistant fraction.
ND, not determined.
The heat processes RI to RIV were used for rainbow trout, WI to WIV were used for whitefish, and RWI and RWII were used for both fish species (Table 2). The processes varied in temperatures, times, and percent RHs (Table 2). The processes RWI and RWII represented excessive under- and overprocessing, respectively, and were used only in the sensory analysis.
(iv) Packaging and storage of fish samples.
After thermal processing and cooling at room temperature for 2 h, the samples on foil trays were vacuum packaged (Multivac A 300/16 1986; Multivac Verpackungsmaschinen, Wolfertschwenden, Germany) in polyamide-polyethylene pouches (Wipak Oy, Nastola, Finland) with an oxygen permeability of 31 cm3/m2/24 h (23°C, 50% RH) and a water vapor permeability of 1.6 g/m2/24 h (38°C, 90% RH). The samples were stored at 8°C for 5 weeks, i.e., 2 weeks beyond the typical commercial shelf life for vacuum-packaged fish products in Finland.
(v) Sampling procedures.
The MPN count of C. botulinum, the presence of botulinum toxin, and the pH of each product were determined in four equally treated samples at 1, 3, and 5 weeks after the thermal processing. The NaCl concentration was measured in six fresh trout fillets and six fresh whitefish.
(vi) Detection of C. botulinum and botulinum toxin.
Viable counts of C. botulinum type E were quantified as described previously (30), with slight modifications. In brief, 20 1-g pieces from a depth of not more than 1 mm beneath the surface layer of each trout fillet or whitefish were transferred to tubes containing 10 ml of tryptose-peptone-glucose-yeast extract broth and incubated in an anaerobic cabinet (MK III; Don Whitley Scientific Ltd.) at 30°C for 3 days, followed by overnight culturing under various conditions. Cells from 1 ml of each overnight culture were washed with Tris-HCl (0.01 M)-EDTA (0.001 M) buffer at 37°C for 1 h and suspended in 1 ml of distilled water. One microliter of the suspension was heated at 99°C for 10 min and used as a template in a PCR with primers specific to the botulinum neurotoxin type E gene (43), DynaZyme DNA polymerase (Finnzymes, Espoo, Finland), and a 96-well thermal cycler (MJ Research, Watertown, Mass.). The sizes of the amplified PCR products were determined in agarose gels by comparison with standard DNA fragments (DNA molecular weight marker VI; Boehringer, Mannheim, Germany). Quantification was done by the one-dilution MPN technique with Thomas' approximation (46) for 80 1-g tubes. When all tubes were negative in the PCR analysis, the viable count was determined as less than half of the estimate for a sample yielding one positive tube out of 80, corresponding to an MPN of <100.8 CFU/kg. In such cases, a count of 100.8 CFU/kg was used in calculating the mean viable count. The relationship between the C. botulinum count, the pasteurization values, and the RH related to the process was evaluated by linear regression analysis (Statistix). For samples that were positive by PCR, the original fish products were then tested for the presence of botulinum neurotoxin by the mouse bioassay (33).
(vii) Determination of pH and NaCl concentration.
The pH of each sample, homogenized with distilled water (1:1 [wt/vol]) was determined by using a digital microprocessor pH 537 measuring device (Wissenschaflich-Technische Werkstätten, Weilheim, Germany). The water phase NaCl content was measured by the method of the International Organization for Standardization (24).
(viii) Sensory evaluation.
The sensory quality of uninoculated vacuum-packaged rainbow trout fillets and whole whitefish, both processed by heat processes WII, WIV, RWI, and RWII (Table 2), was analyzed by a trained 12-member panel using a profiling method (26). The samples were stored at 3°C and evaluated 3 days after processing. Prior to the evaluation, the samples were stored at room temperature for 2 h. For each of the four heat treatments, approximately 40-g composite samples of fish from two separate packages were presented to the panelists in a randomized order. The evaluations were carried out in four sessions, in each of which samples processed by the four heat treatments were offered side by side. Trout and whitefish were assessed in separate sessions, and the assessments were performed in duplicate.
The sensory profile applied to describe the sensory quality of the fish products consisted of intensities of flavor and odor (0, weak; 10, strong), juiciness (0, dry; 10, juicy), firmness (0, soft; 10, firm), and degree of cooking (0, undercooked; 5, cooked to the correct degree; 10, overcooked). The intensities of the attributes in both species were rated on 11-point category scales, and the means of the attributes were calculated. The statistical significances of the differences between samples treated by the four heat processes were tested by using two-way analysis of variance (Statistix), taking into account the process (four levels) and replicate tests (two levels).
RESULTS
Process evaluation in five Finnish fish-smoking plants.
The parameters of the hot-smoking processes employed for rainbow trout by five Finnish smoked fish companies are shown in Table 1.
Thermal death time of C. botulinum type E in model fish media: D and z values and proportion of heat-resistant spores of Beluga E.
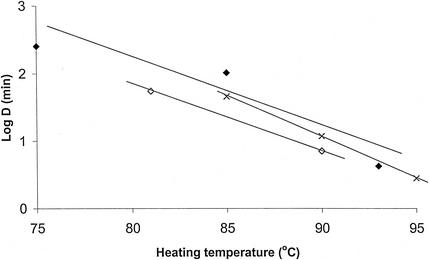
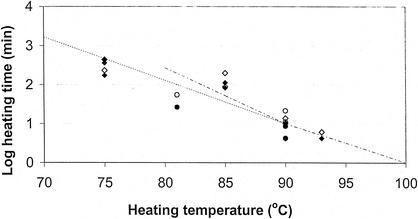
Biphasic thermal destruction curves were obtained at each temperature in both fish species (an example is given in Fig. 1). The z values of 10.4°C in rainbow trout and 10.1°C in whitefish were obtained from the TD curves (Fig. 2; Table 3). Approximately 0.1% of spores were permeable to lysozyme and thus were in the heat-resistant fraction. The total heating times required to prevent growth from 106 spores within 90 days at 30°C were 149 min at 85°C or 6.2 min at 93°C in rainbow trout medium and 55 min at 81°C or 8.6 min at 90°C in whitefish medium (Fig. 3).
FIG. 1.
Thermal destruction of spores of C. botulinum type E strain Beluga in rainbow trout medium heated at 75°C for 450 min. Solid line, best fit of the spore fraction nonpermeable to lysozyme (non-heat-resistant fraction); dashed line, best fit of the spore fraction permeable to lysozyme (heat-resistant fraction).
FIG. 2.
Comparison of D values obtained for C. botulinum type E strain Beluga spores (heat-resistant [permeable] fraction) in rainbow trout and whitefish media heated at 75 to 95°C. ⧫, rainbow trout medium (this study); ◊, whitefish medium (this study); ×, phosphate buffer (37).
FIG. 3.
Heating times at 75 to 93°C required to prevent growth from 106 spores of C. botulinum type E strain Beluga in rainbow trout and whitefish media. ⧫, growth observed in rainbow trout medium within 90 days at 30°C, ◊, no growth observed in rainbow trout medium; •, growth observed in whitefish medium within 90 days at 30°C, ○, no growth observed in whitefish medium; - - - -, 6D heat treatments (i.e., heat treatments proposed to eliminate 106 nonproteolytic spores) recommended by the Advisory Committee on the Microbiological Safety of Foods (1); - - - - - -, 6D heat treatments recommended by the European Chilled Food Federation (17).
Inoculated-pack study. (i) PCR and toxin analyses.
In the vacuum-packaged rainbow trout samples, process RIV alone completely eliminated C. botulinum type E, with no organism or toxin detected during the 5-week storage at 8°C (Table 4). Trout samples processed by RIII were also negative for organisms at weeks 1 and 3, but at week 5 one sample out of four contained a slightly elevated viable count. Of the samples processed by RI and RII, 13 out of 24 samples contained viable C. botulinum type E, with higher counts at weeks 1 and 3 than at week 5. None of the trout samples contained toxin (Table 4).
TABLE 4.
PCR detection of and toxin production by C. botulinum type E in vacuum-packaged hot-smoked rainbow trout fillets and whole whitefish stored at 8°C for 1 to 5 weeks
| Fish species | Process | PCR detection and toxin production by C. botulinum type Ea at:
|
||
|---|---|---|---|---|
| 1 wk | 3 wk | 5 wk | ||
| Rainbow trout | RI | 4/4 (2.0) | 3/4 (2.4) | 2/4 (1.4) |
| RII | 2/4 (2.0) | 1/4 (1.4) | 1/4 (1.1) | |
| RIII | 0/4 (<0.8) | 0/4 (<0.8) | 1/4 (1.1) | |
| RIV | 0/4 (<0.8) | 0/4 (<0.8) | 0/4 (<0.8) | |
| Whitefish | WI | 3/4 (2.6) | 4/4 (3.8) | 3/4 (3.2), T |
| WII | 0/4 (<0.8) | 0/4 (<0.8) | 0/4 (<0.8) | |
| WIII | 2/4 (1.6) | 4/4 (4.3) | 4/4 (3.0), T | |
| WIV | 0/4 (<0.8) | 0/4 (<0.8) | 0/4 (<0.8) | |
Number of positive samples/number of all samples tested (the MPN estimate [log10 CFU/kilogram] for the C. botulinum type E count is given in parentheses). T, botulinum toxin was detected in three (process WI) or two (process WIII) samples. All others were negative for type E toxin.
The whitefish samples produced by processes WII and WIV were all negative for C. botulinum type E and its toxin, whereas the majority of the samples processed by WI and WIII contained approximately 103 to 104 CFU/kg at weeks 3 and 5 (Table 4). Three samples processed by WI and two samples processed by WIII were toxic at week 5 (Table 4).
A higher RH significantly enhanced the thermal destruction of C. botulinum type E spores in both fish species (P < 0.01). One (2%) out of the 48 samples processed at an RH of >70% contained viable organisms, while 19 out of 24 samples (79%) processed at an RH of 50 to 60% and 14 out of 24 samples (58%) processed at an RH of <30% contained viable organisms (Table 4).
Based on the above data, heat processes RIV, WII, and WIV were considered to control the growth and toxin production from spores of nonproteolytic C. botulinum type E by a factor of 106.
(ii) Sensory evaluation.
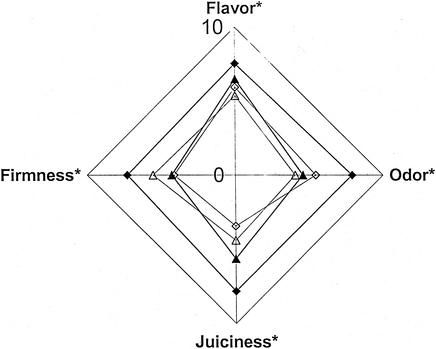
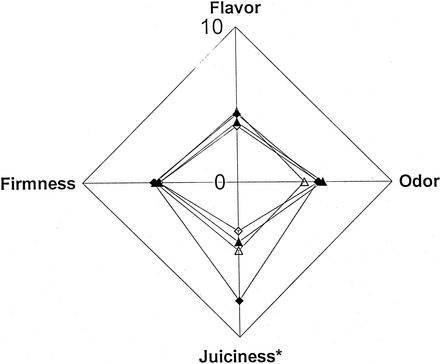
Limited variability was observed between replicate tests, and thus only the effect of the process on the sensory profiles of each fish species is presented. The samples treated by processes RWI and RWII, representing under- and overprocessed products, respectively, differed both from each other and from samples processed by WII and WIV, representing safe products (Fig. 4 and 5). In rainbow trout, the intensities of the attributes in samples produced by RWI were higher (P < 0.01) than those of the other products (Fig. 4). Although differences in the degree of cooking were observed, all trout products included in the sensory evaluation were perceived to be within 2 points of each other (mean intensity ratings of between 4.9 and 6.8), and none were perceived to be overcooked. In the whitefish, differences between products were minor; only juiciness was higher in samples produced by RWI than in the other products (P < 0.01) (Fig. 5). Like the trout, all whitefish products were considered to be appropriately cooked, with mean intensity ratings varying between 4.3 and 5.9.
FIG. 4.
Sensory profiles of vacuum-packed hot-smoked rainbow trout samples processed by heat treatments RWI ⧫, WII ▴, WIV ▵, and RWII ◊ (Table 2) and stored at 3°C for 3 days. Asterisks indicate that differences between products were significant (P < 0.01).
FIG. 5.
Sensory profiles of vacuum-packed hot-smoked whitefish samples processed by heat treatments RWI ⧫, WII ▴, WIV ▵, and RWII ◊ (Table 2) and stored at 3°C for 3 days. The asterisk indicates that differences between products were significant (P < 0.01).
DISCUSSION
Biphasic thermal destruction curves for spores of nonproteolytic C. botulinum type E heated in fish media were observed with lysozyme in the recovery medium of the heat-damaged spores. At 75 to 93°C the heat-resistant spore fraction showed D values of 4.2 to 255 min, whereas those of the nonresistant spores were 0.4 to 4.6 min at the same temperatures. The D values measured in trout were generally longer than those measured in whitefish, possibly because of a higher fat content in the trout or a higher lysozyme concentration in the muscle. Tests in phosphate buffers have revealed similar D values for spores of type E strains that are permeable to lysozyme, i.e., 24.2 to 48.3 min at 85°C (36, 37), 5.0 to 13.5 min at 90°C (3, 37), 3.8 min at 93.3°C (3), and 2.8 min at 95°C (37), as opposed to 0.07 to 6.6 min at 82°C without lysozyme (7, 11, 27, 32, 44, 47). The heat-resistant spore fraction was estimated to be on the order of 0.1%, while in phosphate buffer percentages of 0.1 to 1.0% and sometimes up to 20% have been reported (37). The presence of a heat-resistant spore fraction may therefore greatly complicate the safe processing of REPFEDs, potentially substantially extending the required process times. Therefore, the significance of employing lysozyme in the recovery of spores in inoculated-pack studies should be stressed.
In previous work with vacuum-packaged smoked salmon, a heat treatment (total time in the water bath) at 85°C for 85 min was required to prevent growth from 106 spores of nonproteolytic C. botulinum type E at 25°C for 21 days (14), while heating at 89°C for 65 min was required to control toxin formation from 106 spores of nonproteolytic C. botulinum type B in crabmeat at 27°C for more than 150 days (40), and in tests with crab analogs (2.1% [wt/vol] NaCl) a heat process at 85°C for 15 to 27 min controlled toxin formation from 3 × 105 spores of nonproteolytic C. botulinum type E at 10°C for more than 120 days (39). The high measured heat resistance observed in salmon and crabmeat may be due to the presence of lysozyme or an enzyme with similar properties. Lysozyme may be naturally present in fresh fish and other seafood (28). To interpret results from inoculated-pack studies, it is essential that related determinations of spore heat resistance include tests with lysozyme in the recovery medium. This is especially relevant for foods, such as fish, that are known to contain lysozyme or enzymes with similar activity.
The z values of approximately 10°C obtained in the temperature range of 75 to 93°C are on the order of those reported for spores of type E strains in crabmeat and menhaden surimi heated at 70 to 85°C (31, 41). Lower z values of around 6 to 7°C have been reported for other seafoods, in fish (7, 11, 32) and in phosphate buffers (34, 37). Such z values have been recommended for use in calculating pasteurization values related to heat processing in the production of REPFEDs at temperatures below 90°C (1, 17). However, this study showed that the heating medium greatly affects relative spore heat resistance, and the heat resistance parameters should be determined for each type of heating medium.
In the inoculation study, heat processes RI and WI (1.5 min at 85°C, RH of 50 to 60%) were relevant to the commercial heat treatments currently employed in hot-smoking practices in Finland. Based on heat resistance data obtained in the present study, heat treatments RI and WI would achieve an approximately 10-fold reduction in numbers of spores of nonproteolytic C. botulinum, leaving a great number of spores in the product after heating. It is not surprising, therefore, that processes WI and RI caused little destruction of C. botulinum type E spores. Moreover, following process WI, there were high viable counts and subsequent toxin production in whitefish at 8°C, an inappropriately high storage temperature frequently measured at the retail and consumer levels in Europe (18).
Processes RIV (34 min at 85°C) and WII and WIV (42 to 44 min at 85°C) ensured product safety with respect to the foodborne botulism hazard in the inoculated-pack study. A feature common to all three heat treatments was a high RH, suggesting that a high RH significantly enhances spore thermal destruction in both fish species. This supports the earlier findings on moist heat eliminating more spores than dry heat (2, 35). Furthermore, in whitefish, WII, with an RH of 80%, resulted in no growth or toxin production during the whole storage period, while the similar heat process WIII, with an RH of 10%, resulted in grossly elevated viable counts and toxigenesis at week 5. In trout, processes RIII and RIV (26 to 34 min at 85°C) combined with an RH of >70% yielded no growth at week 3, with process RIII resulting in a minor elevation in the viable count but not in toxigenesis at week 5. The effect of moist heat is thus notable and in combination with increased heating would markedly enhance the thermal processes currently employed in the fish industry. These observations are relevant to the safety of vacuum-packaged hot-smoked fish products, as the control of RH in most smoked fish plants in Finland is technologically possible without investment in additional equipment.
In the trout medium, heating at 85°C for 149 min was required to control growth from 106 spores within 90 days at 30°C, while in trout fillets, heating at 85°C for 34 min was sufficient to prevent growth from 106 spores within 35 days at 8°C. In whitefish, on the other hand, similar processes were required to control growth in both tests, i.e., 34 min in whitefish medium (interpolated from the thermal destruction data) and 42 min in whole products. Possible explanations for this inconsistency include the following: (i) product shape, heat, and smoke transfer may have been more effective in the fillets than in the whole fish, and (ii) the whole whitefish may also have provided the heat-damaged spores with better conditions for germination and growth than the trout fillets, in which all surfaces were in contact with the plastic film. Smoke has been reported to lower the NaCl concentration required to inhibit C. botulinum type E in vacuum-packaged hot-smoked whitefish stored at 10°C for 42 days (13). In the present study, in order to simulate the industrial processes as closely as possible, smoke was used for both fish species, and the smoke may have penetrated the trout fillets more effectively than the whole whitefish, thus inhibiting germination and growth from spores more effectively in trout than in whitefish. The smoke may also have potentiated the thermal destruction of spores in trout fillets better than in whole whitefish. Moreover, in a recent paper on the detection of C. botulinum by PCR combined with enrichment, hot-smoked whitefish was reported to support growth of strain Beluga; the optimal enrichment time for whitefish was 1 day, while those for beef and feces were 3 and 5 days, respectively (29). This may suggest the presence of appropriate growth-supporting factors for nonproteolytic C. botulinum type E in whitefish, but further investigation with other growth media, including different fish species, is warranted.
The sensory evaluation revealed that the safety of vacuum-packaged hot-smoked whitefish can be ensured without an impact on sensory quality. Compared to underprocessed products, only the juiciness of whitefish was reduced by the safe heat processes WII and WIV. The degree of cooking of all trout and whitefish products was rated as close to optimal. In trout the differences between the products were somewhat greater, and the underprocessed trout (RWI) were perceived to differ from the other products by greater intensity of aroma, flavor, juiciness, and firmness. These findings may indicate that a change in consumer preferences towards safer processing of trout is required. Otherwise, the applicability of these processes in the production of vacuum-packaged hot-smoked rainbow trout may be limited, and alternative control strategies such as restriction of shelf life should be considered.
In conclusion, based on the present D values in fish medium, the thermal processes used in commercial hot-smoking plants in Finland appeared to achieve less than a 103 reduction in the numbers of spores of nonproteolytic C. botulinum type E. Avoidance of foodborne botulism would therefore seem to depend on a low prevalence of spores in the product or control factors such as refrigerated storage. In the present study, moist heat treatments at 85°C for 34 and 42 min provided a safety factor of 106 with respect to spores of nonproteolytic C. botulinum type E for rainbow trout fillets and whole whitefish, respectively, stored at 8°C for 5 weeks. However, the safety margin for vacuum-packaged hot-smoked fish products is highly dependent on appropriate refrigerated storage and will be further increased by storage below 3°C, as is currently recommended in Finland.
Acknowledgments
We are indebted to Kirsi Ristkari, Jouni Hirvonen, and Maria Stark for their excellent technical assistance and to Eija Hyytiä-Trees, Centers for Disease Control and Prevention, Atlanta, Ga., for her contribution to designing the inoculated-pack study. We thank Tuija Lyijynen, VTT Biotechnology, for her contribution to the sensory analysis.
This study was supported by the Finnish Ministry of Agriculture and Forestry, the Finnish fish industry, The Finnish Veterinary Foundation, the Walter Ehrström Foundation, and a competitive strategic grant from the BBSRC.
REFERENCES
- 1.Advisory Committee on the Microbiological Safety of Foods. 1992. Report on vacuum packaging and associated processes. Her Majesty's Stationery Office, London, United Kingdom.
- 2.Alderman, G. G., G. J. King, and H. Sugiyama. 1972. Factors in survival of Clostridium botulinum type E spores through the fish smoking process. J. Milk Food Technol. 35:163-166. [Google Scholar]
- 3.Alderton, G., J. K Chen, and K. A. Ito. 1974. Effect of lysozyme on the recovery of heated Clostridium botulinum spores. Appl. Microbiol. 27:613-615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Anonymous. 1964. Botulism outbreak from smoked whitefish. Food Technol. 18:71. [Google Scholar]
- 5.Anonymous. 1991. Botulism. Epid-aktuellt 14:9. [Google Scholar]
- 6.Anonymous. 1998. Fallbericht: Botulismus nach dem Verzehr von geräucherten Lachsforellen. Epidemiol. Bull. 4:20. [Google Scholar]
- 7.Bohrer, C. W., C. B. Denny, and M. G. Yao. 1973. Thermal destruction of type E Clostridium botulinum. Final report on RF 4603. National Canners Association Research Foundation, Washington, D.C.
- 8.Brown, K. L. 1993. Principles of heat preservation, p. 33. In J. A. G. Rees and J. Bettison (ed.), Processing and packaging of heat preserved foods. Blackie Academic & Professional, London, United Kingdom.
- 9.Christiansen, L. N., J. Defner, E. M. Foster, and H. Sugiyama. 1968. Survival and outgrowth of Clostridium botulinum type E spores in smoked fish. Appl. Microbiol. 16:133-137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.City of Milwaukee. 1964. Smoked fish and smoked fish products. Ordinance no. 735, pt. 1, sec. 70-55 through 70-71 of the Milwaukee Code. City of Milwaukee, Milwaukee, Wis.
- 11.Crisley, F. D., J. T. Peeler, R. Angellotti, and H. E. Hall. 1968. Thermal resistance of spores of 5 strains of Clostridium botulinum type E in ground whitefish chubs. J. Food Sci. 33:411-416. [Google Scholar]
- 12.Doyle, M. P. 1991. Evaluating the potential risk from extended-shelf-life refrigerated foods by Clostridium botulinum inoculation studies. Food Technol. 45:154-156. [Google Scholar]
- 13.Eklund, M. W. 1993. Control in fishery products, p. 224-225. In A. H. W. Hauschild, and K. L. Dodds (ed.), Clostridium botulinum. Ecology and control in foods. Marcel Dekker, Inc., New York, N.Y.
- 14.Eklund, M. W., M. E. Peterson, R. Paranjpye, and G. A. Pelroy. 1988. Feasibility of a heat-pasteurization process for the inactivation of nonproteolytic Clostridium botulinum types B and E in vacuum-packaged, hot-process (smoked) fish. J. Food Prot. 51:720-726. [DOI] [PubMed] [Google Scholar]
- 15.Eklund, M. W., D. I. Wieler, and F. T. Poysky. 1967. Outgrowth and toxin production of nonproteolytic type B Clostridium botulinum at 3.3 to 5.6°C. J. Bacteriol. 93:1461-1462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Eklund, M. W., F. T. Poysky, and D. I. Wieler. 1967. Characteristics of Clostridium botulinum type F isolated from the Pacific Coast of the United States. Appl. Microbiol. 15:1316-1323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.European Chilled Food Federation. 1996. Guidelines for the hygienic manufacture of chilled foods. European Chilled Food Federation, London, United Kingdom.
- 18.Evans, J. 1998. Consumer perceptions and practice in the handling of chilled foods, p. 1-24. In S. Ghazala (ed.), Sous vide and cook-chill processing for the food industry. Aspen Publishers, Inc., Gaithersburg, Md.
- 19.Fernandez, P. S., and M. W. Peck. 1999. A predictive model that describes the effect of prolonged heating at 70 to 90°C and subsequent incubation at refrigeration temperatures on growth from spores and toxigenesis by nonproteolytic Clostridium botulinum in the presence of lysozyme. Appl. Environ. Microbiol. 65:3449-3457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gould, G. W. 1984. Injury and repair mechanisms in bacterial spores, p. 199. In M. H. E Andrew and A. D. Russell (ed.), The revival of injured microbes, Academic Press, London, United Kingdom.
- 21.Graham, A. F., D. R. Mason, F. J. Maxwell, and M. W. Peck. 1997. Effect of pH and NaCl on growth from spores of nonproteolytic Clostridium botulinum at chill temperatures. Lett. Appl. Microbiol. 24:95-100. [DOI] [PubMed] [Google Scholar]
- 22.Hielm, S., E. Hyytiä, A.-B. Andersin, and H. Korkeala. 1998. A high prevalence of Clostridium botulinum type E in Finnish freshwater and Baltic Sea sediment samples. J. Appl. Microbiol. 84:133-137. [DOI] [PubMed] [Google Scholar]
- 23.Hyytiä, E., S. Hielm, and H. Korkeala. 1998. Prevalence of Clostridium botulinum type E in Finnish fish and fishery products. Epidemiol. Infect. 120:245-250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.International Organization for Standardization. 1989. Water quality. Determination of chloride. Silver nitrate titration with chromate indicator (Mohr's method). ISO 9297. International Organization for Standardization, Geneva, Switzerland.
- 25.Korkeala, H., G. Stengel, E. Hyytiä, B. Vogelsang, A. Bohl, H. Wihlman, P. Pakkala, and S. Hielm. 1998. Type E botulism associated with vacuum-packaged hot-smoked whitefish. Int. J. Food Microbiol. 43:1-5. [DOI] [PubMed] [Google Scholar]
- 26.Lawless, H. T., and H. Heymann. 1998. Descriptive analysis, p. 341-378. In H. T. Lawless and H. Heymann (ed.), Sensory evaluation of food. Principles and practices. Chapman & Hall, New York, N.Y.
- 27.Licciardello, J. J. 1983. Botulism and heat processed seafoods. Mar. Fish. Rev. 45:1-7. [Google Scholar]
- 28.Lie, Ø., Ø. Evensen, A. Sørensen, and E. Frøysadal. 1989. Study on lysozyme activity in some fish species. Dis. Aquat. Org. 6:1-5. [Google Scholar]
- 29.Lindström, M., R. Keto, A. Markkula, M. Nevas, S. Hielm, and H. Korkeala. 2001. Multiplex PCR assay for detection and identification of Clostridium botulinum types A, B, E, and F in food and fecal material. Appl. Environ. Microbiol. 67:5694-5699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lindström, M., M. Mokkila, E. Skyttä, E. Hyytiä-Trees, L. Lähteenmäki, S. Hielm, R. Ahvenainen, and H. Korkeala. 2001. Inhibition of growth of nonproteolytic Clostridium botulinum type B in sous vide cooked meat products is achieved by using thermal processing but not nisin. J. Food Prot. 64:838-844. [DOI] [PubMed] [Google Scholar]
- 31.Lynt, R. K., D. A. Kautter, and H. M. Solomon. 1983. Effect of delayed germination by heat-damaged spores on estimates of heat-resistance of Clostridium botulinum types E and F. J. Food Sci. 48:226-229. [Google Scholar]
- 32.Lynt, R. K., H. M. Solomon, J. R. Lilly, and D. A. Kautter. 1977. Thermal death time of Clostridium botulinum type E in meat of the blue crab. J. Food Sci. 42:1022-1025. [DOI] [PubMed] [Google Scholar]
- 33.Nordic Committee on Food Analysis. 1991. Botulinum toxin. Detection in foods, blood, and other test materials. Method no. 79, 2nd ed. Nordic Committee on Food Analysis, Espoo, Finland.
- 34.Ohye, D. F., and W. J. Scott. 1957. Studies on the physiology of Clostridium botulinum type E. Austr. J. Biol. Sci. 10:85-94. [Google Scholar]
- 35.Pace, P. J., E. R. Krumbiegel, and H. J. Wisniewski. 1972. Interrelationship of heat and relative humidity in the destruction of Clostridium botulinum type E spores on whitefish chubs. Appl. Microbiol. 23:750-757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Peck, M. W., D. A. Fairbairn, and B. M. Lund. 1992. Factors affecting growth from heat-treated spores of non-proteolytic Clostridium botulinum. Lett. Appl. Microbiol. 15:152-155. [DOI] [PubMed] [Google Scholar]
- 37.Peck, M. W., D. A. Fairbairn, and B. M. Lund. 1993. Heat-resistance of spores of non-proteolytic Clostridium botulinum estimated on medium containing lysozyme. Lett. Appl. Microbiol. 16:126-131. [Google Scholar]
- 38.Peck, M. W., B. M. Lund, D. A. Fairbairn, A. S. Kaspersson, and P. C. Undeland. 1995. Effect of heat treatment on survival of, and growth from, spores of nonproteolytic Clostridium botulinum at refrigeration temperatures. Appl. Environ. Microbiol. 61:1780-1785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Peterson, M., R. N. Paranjpye, F. T. Poysky, G. A. Pelroy, and M. W. Eklund. 2002. Control of nonproteolytic Clostridium botulinum types B and E in crab analogs by combinations of heat pasteurization and water phase salt. J. Food Prot. 65:130-139. [DOI] [PubMed] [Google Scholar]
- 40.Peterson, M. E., G. A. Pelroy, F. T. Poysky, R. N. Paranjpye, F. M. Dong, G. M. Pigott, and M. W. Eklund. 1997. Heat-pasteurization process for inactivation of nonproteolytic types of Clostridium botulinum in picked Dungeness crabmeat. J. Food Prot. 60:928-934. [DOI] [PubMed] [Google Scholar]
- 41.Rhodehamel, E. J., H. M. Solomon, T. Lilly, Jr., D. A. Kautter, and J. T. Peeler. 1991. Incidence and heat resistance of Clostridium botulinum type E spores in menhaden surimi. J. Food Sci. 56:1562-1563,1592.
- 42.Schmidt, C. F., R. V. Lechowich, and J. F. Folinazzo. 1961. Growth and toxin production by type E Clostridium botulinum below 40°F. J. Food Sci. 26:626-630. [Google Scholar]
- 43.Sciacchitano, C. J., and I. N. Hirschfield. 1996. Molecular detection of Clostridium botulinum type E neurotoxin gene in smoked fish by polymerase chain reaction and capillary electrophoresis. J. AOAC Int. 79:861-865. [PubMed] [Google Scholar]
- 44.Scott, V. N., and D. T. Bernard. 1982. Heat resistance of spores of non-proteolytic type B Clostridium botulinum. J. Food Prot. 45:909-912. [DOI] [PubMed] [Google Scholar]
- 45.Smelt, J. P. P. M. 1980. Heat resistance of Clostridium botulinum in acid ingredients and its signification for the safety of chilled foods. Doctoral thesis. University of Utrecht, Utrecht, The Netherlands.
- 46.Thomas, H. A. 1942. Bacterial densities from fermentation tube tests. J. Am. Water Works Assoc. 34:572-576. [Google Scholar]
- 47.Tui-Jyi, C., and L. T. Kuang. 1992. Thermal resistance of spores from five type E Clostridium botulinum strains in eastern oyster homogenates. J. Food Prot. 55:18-22. [DOI] [PubMed] [Google Scholar]