Abstract
Eighteen (72%) of 25 badger social groups were found to excrete Salmonella enterica serovar Ried, S. enterica serovar Binza, S. enterica serovar Agama, or S. enterica serovar Lomita. Each serovar was susceptible to a panel of antimicrobials. Based on results of pulsed-field gel electrophoresis, the S. enterica serovar Agama and S. enterica serovar Binza isolates were very similar, but two clones each of S. enterica serovar Lomita and S. enterica serovar Ried were found. Badgers excreting S. enterica serovar Agama were spatially clustered.
Salmonella enterica is an important pathogen of humans and other animals. Some serovars are highly species specific; for example, S. enterica serovar Typhi is solely a human pathogen, and S. enterica serovar Gallinarum is pathogenic only for poultry. In humans, salmonellae are an important cause of food poisoning (i.e., salmonellosis) (3, 18, 23). Those salmonellae that cause salmonellosis are conveniently designated nontyphoidal salmonellae (NTS) and include S. enterica serovar Typhimurium, S. enterica serovar Enteritidis, and S. enterica serovar Choleraesuis. Although diarrheal disease is the most common manifestation of NTS infection in humans (3, 23), the NTS can also cause invasive disease, especially in children (8) and human immunodeficiency virus-infected individuals (7, 20). NTS are also an important cause of diarrhea and septicemia in a number of domestic and companion animals (10, 11, 14, 15, 19, 24, 28, 33). In many animal species, initial infection can be followed by prolonged periods of asymptomatic carriage (14, 19).
NTS have been detected in a wide range of wildlife species in the United Kingdom (29). The range of serovars reported is broad both between and within animal species. Previous reports of NTS in badgers (Meles meles) have been restricted to demonstrating the diversity of serovars detected. These included S. enterica serovar Agama and S. enterica serovar Dublin (29) as well as S. enterica serovar Agama, S. enterica serovar Bredeney, S. enterica serovar Enteritidis. S. enterica serovar Coeln, S. enterica serovar Dublin, S. enterica serovar Anatum, S. enterica serovar Bonn, S. enterica serovar Nagama, S. enterica serovar Durham, and S. enterica serovar Indiana (34).
Badgers have a very wide geographical range and occupy an array of different habitats (21). They have a diverse omnivorous diet that includes both prey items and plant material. They also scavenge around waste disposal sites and dustbins (21, 34). Thus, they are exposed to many different potential sources of NTS. It is apparent that badgers could have an important role as a potential reservoir of NTS for other animal species, including cattle. This possibility has been described on only one occasion (9), when S. enterica serovar Agama was reported as the cause of abortion in a cow. The same S. enterica serovar Agama was subsequently isolated from badger feces next to the sett on the farm. Whether and to what extent the badgers might represent a source of infection for cattle is unclear. However, the most prevalent NTS serotype affecting cattle, S. enterica serovar Typhimurium, has not been found in badgers. The present study therefore aims to establish the prevalence of NTS excretion in badgers in this area and to carry out spatial analysis to see if there were any patterns of NTS isolation.
A badger sett survey was carried out in an area of Cheshire, United Kingdom (10 km by 10 km) in the winter of 1998-1999. Sett locations were recorded on maps, and setts were classified as main or subsidiary. Main setts are distinguished from other setts by their size and extent of use. They represent the focal point of a badger social group, which is defended from adjacent social groups. Fecal samples were collected in sterile universal containers (Bibby Sterilin, Stone, United Kingdom) from latrines adjacent to main setts in a 5 km by 5 km square area in the southeast corner of the main square. There were 30 main setts in this area, and latrines were found in proximity to 25 of these. The latrines are used only by badgers living in the social group occupying the nearby main sett. Thus, bacteria recovered from feces in a given latrine represent those present in the fecal flora of an individual in that social group. The data obtained were then analyzed with regard to both social group prevalence and the spatial distribution of infection. During the survey, other environmental and habitat variables were recorded throughout the area. These data and badger sett data were all digitized by using a geographical information system package, IDR1SI 32 1 (version 132.21; Clarke Laboratories, Worcester, Mass.). This includes details of land usage such as livestock grazing pasture areas or the type of crops in arable areas.
Badger feces (1 g) were emulsified in selenite broth (9 ml), mixed by vortexing, and incubated at 37°C for 24 h. The broth was then subcultured onto xylose-lysine decarboxylase agar (Oxoid, Ltd., Basingstoke, United Kingdom), and the plates were incubated at 37°C for 24 h. Colonies (five per plate) resembling those typical of salmonellae were subcultured onto MacConkey and blood agar plates and incubated at 37°C for 24 h. Colonies were confirmed as salmonellae by using poly-O and poly-H antisera (Murex, Dartford, United Kingdom) and standard biochemical tests (AP1 20E; BioMerieux, Basingstoke, United Kingdom). Serovar status was determined by agglutination with specific anti-H and anti-O antisera (Murex). Antimicrobial susceptibility was determined by using a controlled disk diffusion method (32). The disks used (Oxoid) contained amoxicillin (25 μg), coamoxiclav (30 μg), chloramphenicol (10 μg), gentamicin (10 μg), cefotaxime (30 μg), tetracycline (10 μg), sulfonamide (30 μg), or nalidixic acid (30 μg). Escherichia coli NCTC 10418 was used as a control. Chromosomal DNA was prepared in agarose plugs as described previously (7, 12) from an overnight culture on agar plates. Agarose plugs were then digested by SpeI (30 units per plug) according to the manufacturer's instructions (Life Technologies, Paisley, United Kingdom). Pulsed-field gel electrophoresis (PFGE) was then performed on a CHEF-DR II system (Bio-Rad Laboratories, Hercules, Calif.) on a horizontal 1% (wt/vol) agarose gel. The restriction endonuclease digest patterns were interpreted by considering the migration distances and the intensity of all visible bands and by using guidelines described previously (30).
Thirty-six isolates of S. enterica were obtained from 130 latrines around 25 setts (Table 1). The serovars isolated were S. enterica serovar Ried (15 isolates), S. enterica serovar Binza (10 isolates), S. enterica serovar Lomita (7 isolates), and S. enterica serovar Agama (4 isolates). The latrines around 7 of the 25 (28%) setts had no Salmonella spp. isolated. Five setts (20%) had two serovars isolated, and one sett had three different serovars isolated. Each isolate was fully susceptible to each of the antimicrobials tested. PFGE of macrorestricted chromosomal DNA from the different Salmonella spp. showed that each of the four serovars had their own distinctive patterns and that they were unrelated to each other (>10 bands were different in each case). Within serovars, there was much less variability. For S. enterica serovar Binza, three patterns differing by only one band each were detected. The S. enterica serovar Agama isolates produced four different patterns, but in each case they differed by less than three bands. The S. enterica serovar Ried and S. enterica serovar Lomita isolates each produced two clones differing by more than 10 bands, respectively.
TABLE 1.
Salmonella serovars isolated from badger setts
| Sett no. | No. of latrines sampled | No. positive for S. enterica serovar:
|
|||
|---|---|---|---|---|---|
| Ried | Binza | Agama | Lomita | ||
| 1 | 5 | 3 | 1 | 0 | 0 |
| 2 | 8 | 0 | 4 | 0 | 0 |
| 3 | 4 | 0 | 0 | 0 | 1 |
| 4 | 3 | 0 | 0 | 0 | 0 |
| 5 | 2 | 0 | 1 | 0 | 0 |
| 6 | 9 | 0 | 0 | 0 | 0 |
| 7 | 1 | 1 | 0 | 0 | 0 |
| 8 | 2 | 0 | 0 | 0 | 0 |
| 9 | 7 | 3 | 0 | 1 | 0 |
| 10 | 5 | 0 | 2 | 0 | 0 |
| 11 | 4 | 0 | 0 | 0 | 0 |
| 12 | 14 | 0 | 0 | 0 | 1 |
| 13 | 3 | 1 | 0 | 0 | 0 |
| 14 | 3 | 1 | 0 | 1 | 0 |
| 15 | 3 | 0 | 0 | 1 | 0 |
| 16 | 8 | 1 | 0 | 1 | 1 |
| 17 | 15 | 2 | 0 | 0 | 1 |
| 18 | 3 | 0 | 0 | 0 | 0 |
| 19 | 3 | 0 | 0 | 1 | 0 |
| 20 | 4 | 3 | 0 | 0 | 0 |
| 21 | 5 | 0 | 1 | 1 | 0 |
| 22 | 3 | 0 | 1 | 0 | 0 |
| 23 | 2 | 0 | 0 | 1 | 0 |
| 24 | 7 | 0 | 0 | 0 | 0 |
| 25 | 7 | 0 | 0 | 0 | 0 |
| Total | 130 | 15 | 10 | 7 | 4 |
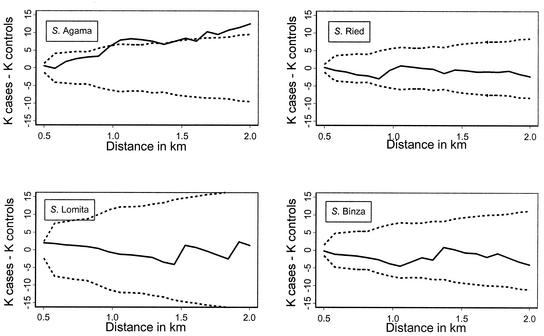
Spatial clustering of serotypes was investigated by using K functions (25). The distribution of positive setts was compared with the distribution of negative setts for each serotype by using the difference between the two K functions (for details of this approach, see reference 2). The difference between the two K functions was plotted against distance (h), along with upper and lower simulation envelopes. For this analysis, the simulation envelopes were widened to allow for multiple testing by using a Bonferroni correction. If the difference between the K functions fell above the upper envelopes, this indicated that the positive setts were significantly more clustered than the negative setts (P was <0.05, allowing for multiple testing). The relationship between the number of samples taken and the probability of a sett being positive was examined by using logistic-regression analysis.
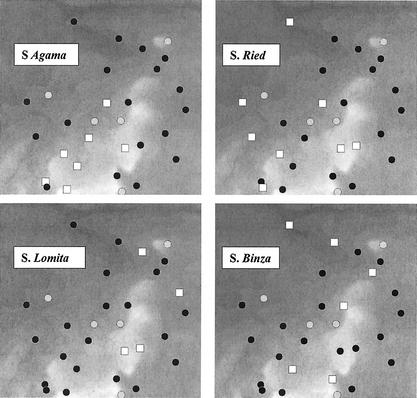
There was some clustering of setts from which S. enterica serovar Agama was isolated, which was not apparent for the other three serovars (Fig. 1). In areas with no spatial clustering, the K function always falls within the 95% simulation envelopes, which was the case for the isolates of S. enterica serovar Lomita, S. enterica serovar Binza, and S. enterica serovar Ried (Fig. 2). In contrast, S. enterica serovar Agama showed evidence of spatial clustering. The probability of a sett being positive was not significantly related to the number of samples taken (P > 0.1).
FIG. 1.
The spatial distribution of Salmonella spp. isolates in the badger setts. The black circles show the location of negative setts, the white squares represent positive setts, and the gray circles are unsampled setts. The background represents altitude ranging from 26 (dark gray) to 222 (light gray) meters above sea level.
FIG. 2.
K-function analysis of cases (positive) and controls (negative setts). The solid line represents the difference between the two K functions, and the dashed lines are the 95% simulation envelopes (adjusted for multiple testing).
Many different NTS have been isolated from wild animals and birds. Such wildlife species have been considered reservoirs of salmonellae that can then infect domestic animals and humans. By making a systematic collection from different badger social groups, we have demonstrated both a high prevalence of NTS (72% of groups) and unusual serovars (S. enterica serovar Ried, S. enterica serovar Binza, and S. enterica serovar Lomita). In a previous study of 585 badger carcasses and fecal samples, S. enterica serovar Agama was isolated from 31 (5.3%) samples from the southwest United Kingdom, with nine other serovars being detected in much smaller numbers (34). S. enterica serovar Agama was also found in an earlier United Kingdom study (29), which has led to the suggestion that badgers may be a reservoir host for S. enterica serovar Agama—and there is circumstantial evidence linking badgers to infection in cattle (9). PFGE analysis of our S. enterica serovar Agama isolates indicated that they all appeared to be related. Spatial analysis showed that the S. enterica serovar Agama isolates clustered together—unlike the other serovars. This indicates that S. enterica serovar Agama infection may be spatially constrained rather than colonizing badgers through out the area, and this scenario argues against S. enterica serovar Agama being badger specific. S. enterica serovar Agama was originally isolated from and named for the rainbow lizard (Agama agama) in West Africa but has also been isolated from the African great cane rat (Thryonomys swinderianus) as well as from dogs in Nigeria (4, 22, 26). S. enterica serovar Agama is an uncommon cause of human salmonellosis (1, 17).
S. enterica serovar Ried was the most prevalent serovar, being found in 8 of 25 (36%) setts sampled. However, PFGE of macrorestricted chromosomal DNA indicated that there were two distinct clones (differing by >10 bands). Spatial analysis indicated that there was no significant clustering of isolates. S. enterica serovar Ried is an extremely rare cause of infection in humans and animals. S. enterica serovar Binza was next in order of prevalence, being isolated from 6 (24%) of 25 social groups. Again, the isolates were all similar by PFGE and showed no evidence of spatial clustering. S. enterica serovar Binza seems to be particularly associated with infection in poultry but is rarely found in human infections (6). The badgers in the study area shared habitat and food sources with a large population of reared game birds. These birds may have been the primary source of S. enterica serovar Binza infection. S. enterica serovar Lomita was the least common serovar (occurring in 16% of the social groups). There was evidence of two different clones of S. enterica serovar Lomita by PFGE but not of spatial clustering. S. enterica serovar Lomita has been detected in poultry in India and, in experimental infection, was able to establish colonization in the rumen of a sheep (13, 27). There is one case of human spondylodiscitis reported in the literature (5).
How the badgers have acquired the NTS, whether they are persistent excreters, and whether they can transfer the bacteria to other species is unclear. However, badgers do have a varied omnivorous diet and could, for example, have acquired S. enterica serovar Lomita or S. enterica serovar Binza from scavenging food around poultry or game bird farms. It is noteworthy that some of the badgers were also excreting vancomycin-resistant strains of Enterococcus faecium (16), a result which in the United Kingdom and other parts of Europe has been associated with feeding poultry with avoparcin, the growth promoter. Finally, none of the NTS isolated were resistant to any of the eight antimicrobial agents tested. NTS isolated from cattle or humans in the United Kingdom have shown an inexorable rise in resistance to commonly used antimicrobials (10, 18, 31). This, taken with our serovar findings, suggests that there is little movement of NTS between badgers and cattle in the area or vice versa.
Acknowledgments
C.A.H. and N.P.F. gratefully acknowledge financial support from the Wellcome Trust and from DEFRA (United Kingdom).
REFERENCES
- 1.Appas, J., L. Kieffer, and D. Sigwalt. 1966. A case of salmonellosis due to S. agama. Arch. Fr. Pediatr. 23:1197-1200. [PubMed] [Google Scholar]
- 2.Bailey, T. C., and A. C. Gatrell. 1995. Interactive spatial data analysis. Longman, Harlow, United Kingdom.
- 3.Baird-Parker, A. C. 1990. Foodborne salmonellosis. Lancet 336:1231-1235. [DOI] [PubMed] [Google Scholar]
- 4.Chah, K. F., and S. I. Oboegbulem. 1999. Enterotoxigenicity of salmonellae isolated from dogs in Nigeria. Res. Vet. Sci. 67:99-101. [DOI] [PubMed] [Google Scholar]
- 5.Chevalier, X., P. Brugieres, and B. Larget-Piet. 1993. Salmonella lomita spondylodiscitis. Br. J. Rheumatol. 32:768. [DOI] [PubMed]
- 6.Davies, R., M. Breslin, J. E. Correy, W. Hudson, and V. M. Allen. 2001. Observations on the distribution and control of Salmonella species in two integrated broiler companies. Vet. Rec. 149:227-232. [DOI] [PubMed] [Google Scholar]
- 7.Gordon, M. A., H. T. Banda, M. Gondwe, S. B. Gordon, M. J. Boree, A. L. Walsh, J. E. Corkill, C. A. Hart, C. F. Gilks, and M. E. Molyneux. 2002. Non-typhoidal salmonella bacteremia among HIV-infected Malawian adults: high mortality and frequent recrudescence. AIDS 16:1-9. [DOI] [PubMed] [Google Scholar]
- 8.Graham, S. M., E. M. Molyneux, A. L. Walsh, J. S. Cheesbrough, M. E. Molyneux, and C. A. Hart. 2000. Nontyphoidal salmonella infections of children in tropical Africa. Pediatr. Infect. Dis. J. 19:1189-1196. [DOI] [PubMed] [Google Scholar]
- 9.Humphrey, T. J., and A. Bygrave. 1988. Abortion in a cow associated with salmonella infection in badgers. Vet. Rec. 123:160. [DOI] [PubMed] [Google Scholar]
- 10.Jones, Y. E., S. Chappell, I. M. McLaren, R. H. Davies, and C. Wray. 2002. Antimicrobial resistance in Salmonella isolated from animals and their environment in England and Wales from 1988 to 1999. Vet. Rec. 150:649-654. [DOI] [PubMed] [Google Scholar]
- 11.Khakria, R., D. Woodward, W. M. Johnson, and C. Poppe. 1997. Salmonella isolated from humans, animals and other sources in Canada, 1983-92. Epidemiol. Infect. 119:15-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kariuki, S., G. Revathi, F. Gakuya, V. Yamo, J. Muyodi, and C. A. Hart. 2002. Lack of clonal relationship between non-typhi Salmonella strain types from humans and those isolated from animals living in close contact. FEMS Immunol. Med. Microbiol. 33:165-171. [DOI] [PubMed] [Google Scholar]
- 13.Kumar, A. A., and A. N. Sawhney. 1970. Salmonellae affecting poultry in India: isolation of S. lomita and S. wien. Indian Vet. J. 47:701-702. [PubMed] [Google Scholar]
- 14.Lax, A. J., P. A. Barrow, P. W. Jones, and T. S. Wallis. 1995. Current perspectives in salmonellosis. Br. Vet. J. 151:352-377. [DOI] [PubMed] [Google Scholar]
- 15.Liebana, E., L. Garcia-Migura, M. F. Breslin, R. H. Davies, and M. J. Woodward. 2001. Diversity of strains of Salmonella enterica serotype Enteritidis from English poultry farms assessed by multiple genetic fingerprinting. J. Clin. Microbiol. 39:154-161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mallon, D. J. P., J. E. Corkill, S. M. Hazel, J. S. Wilson, N. P. French, M. Bennett, and C. A. Hart. 2002. Excretion of vancomycin-resistant enterococci by wild mammals. Emerg. Infect. Dis. 8:636-638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mascher, F., F. F. Reinthaler, and W. Sixl. 1988. Serotyping of Salmonella strains in southwest Nigeria: epidemiological aspects. Geograph. Med. 18:171-174. [PubMed] [Google Scholar]
- 18.Mead, P. S., L. Slutsker, V. Dietz, L. F. McCaig, J. S. Bresee, C. Shapiro, P. M. Griffin, and R. V. Tauxe. 1999. Food-related illness and death in the United States. Emerg. Infect. Dis. 5:607-625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Moxley, R. A., and G. E. Duhamel. 1999. Comparative pathology of bacterial enteric diseases of swine. Adv. Exp. Med. Biol. 473:83-101. [DOI] [PubMed] [Google Scholar]
- 20.Nadelman, R. B., U. Mathur-Waugh, S. R. Yancovitz, and D. Mildvan. 1985. Salmonella bacteremia associated with the acquired immunodeficiency syndrome (AIDS). Arch. Intern. Med. 145:1968-1971. [PubMed] [Google Scholar]
- 21.Neal, E. 1990. The natural history of badgers. Christopher Helm, London, United Kingdom.
- 22.Oboegbulem, S. I., and I. Okoronkwo. 1990. Salmonellae in the African great cane rat (Thyonomys swinderianus). J. Wildl. Dis. 26:119-121. [DOI] [PubMed] [Google Scholar]
- 23.Pegues, D. A., and S. I. Miller. 1994. Salmonellosis including typhoid fever. Curr. Opin. Infect. Dis. 7:616-623. [Google Scholar]
- 24.Radke, B. R., M. McFall, and S. M. Radostits. 2002. Salmonella Muenster infection in a dairy herd. Can. Vet. J. 43:443-453. [PMC free article] [PubMed] [Google Scholar]
- 25.Ripley, B. D. 1976. The second-order analysis of stationary point processes. J. Appl. Probab. 13:255-266. [Google Scholar]
- 26.Schubert, H. W., and P. Scheiber. 1979. Investigation of the presence of salmonella in drinking water from water supplies and distribution systems in Togo. Zentralbl. Bakteriol. 168:356-360. [PubMed] [Google Scholar]
- 27.Smith, M. G. 1977. Transfer of R factors from Escherichia coli to salmonellas in the rumen of sheep. J. Med. Microbiol. 10:29-35. [DOI] [PubMed] [Google Scholar]
- 28.Spier, S. J. 1993. Salmonellosis. Vet. Clin. North Am. Equine Pract. 9:385-397. [DOI] [PubMed] [Google Scholar]
- 29.Taylor, J. 1968. Salmonella in wild animals. Symp. Zool. Soc. Lond. 24:51-73. [Google Scholar]
- 30.Tenover, F. C., R. D. Arbeit, R. V. Goering, P. A. Mickelsen, B. E. Murray, D. H. Persing, and B. Swaminathan. 1995. Interpreting chromosomal DNA restriction patterns produced by pulsed-field gel electrophoresis: criteria for bacterial strain typing. J. Clin. Microbiol. 33:2233-2239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Threlfall, E. J., L. R. Ward, J. A. Skinner, and A. Graham. 2000. Antimicrobial drug resistance in non-typhoidal salmonellas from humans in England and Wales in 1999: decrease in multiple resistance in Salmonella enterica serotypes Typhimurium, Virchow, and Hadar. Microb. Drug Resist. 6:319-325. [DOI] [PubMed] [Google Scholar]
- 32.Working Party on Antibiotic Sensitivity. Testing of the British Society for Antimicrobial Chemotherapy. 1998. Antimicrobial sensitivity guidelines. British Society of Antimicrobial Chemotherapy, Birmingham, United Kingdom.
- 33.Wray, C. 1995. Salmonellosis: a hundred years old and still going strong. Br. Vet. J. 151:351-352. [DOI] [PubMed] [Google Scholar]
- 34.Wray, C., K. Baker, J. Gallagher, and P. Naylor. 1977. Salmonella infection in badgers in the south west of England. Br. Vet. J. 133:526-529. [DOI] [PubMed] [Google Scholar]