Abstract
Increasing soil nutrients through litter manipulation, pollution, or fertilization can adversely affect ectomycorrhizal (EM) communities by inhibiting fungal growth. In this study, we used molecular genetic methods to determine the effects of litter addition on the EM community of a Pinus contorta stand in Yellowstone National Park that regenerated after a stand-replacing fire. Two controls were used; in unmodified control plots nothing was added to the soil, and in perlite plots perlite, a chemically neutral substance, was added to maintain soil moisture and temperature at levels similar to those under litter. We found that (i) species richness did not change significantly following perlite addition (2.6 ± 0.3 species/core in control plots, compared with 2.3 ± 0.3 species/core in perlite plots) but decreased significantly (P < 0.05) following litter addition (1.8 ± 0.3 species/core); (ii) EM infection was not affected by the addition of perlite but increased significantly (P < 0.001) in response to litter addition, and the increase occurred only in the upper soil layer, directly adjacent to the added litter; and (iii) Suillus granulatus, Wilcoxina mikolae, and agaricoid DD were the dominant organisms in controls, but the levels of W. mikolae and agaricoid DD decreased significantly in response to both perlite and litter addition. The relative levels of S. granulatus and a fourth fungus, Cortinariaceae species 2, increased significantly (P < 0.01 and P < 0.05, respectively) following litter addition. Thus, litter addition resulted in some negative effects that may be attributable to moisture-temperature relationships rather than to the increased nutrients associated with litter. Some species respond positively to litter addition, indicating that there are differences in their physiologies. Hence, changes in the EM community induced by litter accumulation also may affect ecosystem function.
Ectomycorrhizal (EM) fungi play a significant role in uptake and distribution of nutrients in temperate forest ecosystems, including the pine-dominated forests of the northern Rocky Mountains and Yellowstone National Park (9, 13, 14, 40, 42). Forest development often is fire driven and progresses from reestablishment of Pinus contorta (lodgepole pine) via serotinous cones through mixed P. contorta-Picea engelmannii (Engelmann spruce) or P. contorta-Abies lasiocarpa (subalpine fir) stands, depending on the soil conditions (41, 45). In the absence of fire, this progression to spruce-fir stands occurs in approximately 300 years. Litter accumulates and adds to the fire potential with the addition of the second tree species, and it continues to increase until fire reduces the fuel load.
Fungal fruiting body (mushroom) assessments of EM fungal diversity indicate that patterns of EM diversity change during forest development. For example, after seedling establishment in young birch stands, EM fungal species diversity is relatively high. As stands age, the EM fungus species composition changes, the EM fungal diversity decreases, and a different functional group of fungi becomes established, which is adapted to the changing conditions and may utilize litter as a nutrient source (33). A similar pattern of increasing fruiting body diversity up to canopy closure, followed by decreasing diversity as stands age, has been observed in P. contorta stands (23). Also, in Jack pine stands, EM fruiting body and morphotype diversity is relatively low at the beginning, increases rapidly until 65 years, and then levels off, a pattern attributed to adaptation to the fire disturbance cycle (47). While mixed stands may have a more diverse fungal flora (35), a decrease in fruiting body diversity also occurs in 250-year-old mixed Douglas fir-larch stands (28). The apparent decrease in EM diversity in later-stage stands is apparently due to growth inhibition of some species resulting from changing soil chemistry (particularly increased nitrogen contents) and increases in the amounts of phenolic compounds resulting from accumulated soil litter (6, 22, 33).
Litter manipulation experiments can induce similar changes in the EM community (21). Specifically, litter addition can cause a decrease in EM infection and morphotype diversity (5). Like the natural pattern of succession in the EM fungal community, these effects are thought to be due to increased amounts of nitrogen and phenolic compounds (4, 5, 22). The effects are most marked in nutrient-poor soils, and similar effects can be induced by soil amendment with nitrogen fertilizers (2, 10), supporting the assertion that EM infection responds negatively to increased nitrogen levels. Interestingly, although the overall pattern is negative in response to litter addition and increased nitrogen levels, some fungal species react positively (6), suggesting that there are functional differences among fungal species. Conversely, litter removal in late stands can induce increases in EM infection and morphotype diversity, raising both to levels more like those in younger stands (5, 6). The increase in diversity is due in large part to the release of growth inhibition and at least partially to recruitment of new fungal species via spore dispersal (4, 5).
In many previous litter manipulation studies the workers focused on fruiting body and EM morphotype diversity (4, 5, 10, 22). In this study we used molecular methods to determine effects of litter addition on the EM fungal community of a P. contorta stand in nutrient-poor rhyolite soils. This stand experienced stand-replacing fire in 1988 and as a result has very little, if any, resident litter and organic matter. Litter addition affects not only the nutrient levels in soils but also the moisture content and temperature. As a control, we added perlite (a chemically neutral substance that can maintain moisture and temperature at levels similar to those measured under litter) to replicate plots so that the relative importance of changes associated with moisture and temperature and changes associated with soil nutrient levels on the EM fungal community could be determined.
MATERIALS AND METHODS
The study site was located within Yellowstone National Park, on the road from Canyon Junction to Madison Junction, approximately 1 mile east of the Virginia Cascade cutoff. The stand selected had undergone stand-replacing fire in 1988. Within this stand, three blocks that were 5 by 5 m for each treatment (control [no modification], perlite addition, and litter addition) were designated. The plot size was designed to ensure that the complete root systems of ≥20 individual P. contorta trees were included in each block. Litter was collected from neighboring old-growth stands, solarized for 3 months under black plastic, and spread to a uniform depth of 8 cm. This depth was similar to that in the surrounding forest. The perlite depth was determined empirically so that the moisture and temperature levels matched the levels under litter.
Three arbitrarily located, nested pairs of cores were taken from each of the three control, perlite, and litter addition blocks (Fig. 1). This sampling protocol provided six cores per block and thus a 18 total of cores for each treatment type. Each core was 8 cm in diameter and 24 cm deep. Cores were taken two growing seasons following litter addition. The coring depth was based on the depth of rooting in the soils and was standardized to provide equal volumes of soil for statistical analyses.
FIG. 1.
Schematic diagram of collection blocks. The large ellipses represent plots from which two cores (solid ellipses) were taken.
To ensure that changes in the fungal community were not due to transfer of species from the litter collection site, a molecular survey of nine cores from the old growth was performed. The results of this survey indicated that there was no overlap of species between the old-growth site and the litter addition site (16).
Cores were separated into three 8-cm-deep segments, and each layer was sifted to remove the soil. Mycorrhizae were separated by color morphology (1) and then stored dry at −20°C within 10 days of collection. EM tips of each morphotype in each core were pooled for quantification (17, 18, 29) and then freeze-dried for long-term storage. All morphotypes from all cores were archived for future analyses, but no living cultures were stored or deposited in international culture collections. To ensure genetic consistency within morphotypes, two individuals of each morphotype were selected for identification of fungi forming individual EM. This sampling scheme has been used successfully by workers in our laboratory in Yellowstone National Park (16, 17, 18) and also by other workers (29).
Fungi forming individual EM were identified by PCR-based methods. DNA was amplified from root tips and from fruiting bodies by using primers ITS1F and ITS4B specific for the internal transcribed spacer region of the nuclear rRNA repeat unit of basidiomycete fungi (25). To identify ascomycete EM fungi, the universal fungal primers ITS1F and ITS4 were used (25). We used the following parameters for PCR: initial denaturation at 95°C for 1 min 35 s, followed by 13 cycles of denaturation at 94°C, primer annealing for 55 s at 55°C, and polymerization for 45 s at 72°C and then nine additional cycles with the polymerization time extended to 2 min, nine cycles with 3 min of extension, and a final 10-min polymerization step at 72°C.
Amplified DNA was digested with restriction enzymes AluI and HinfI, and the band patterns of EM were compared to those of fruiting bodies. Restriction fragment length polymorphism (RFLP) patterns that were identical for EM and fruiting bodies were deemed a match. The utility and accuracy of this method have been demonstrated previously (26) and have used by workers in our laboratory in Yellowstone National Park (5, 12, 17, 18).
When identification of fungal species comprising more than 8% of the total community could not be made by RFLP comparisons with fruiting bodies, identification to some taxonomic level was made by comparing the DNA sequences obtained from root tips to the DNA sequences available in the GenBank database. For identification to a family-level monophyletic group we used mitochondrial large rRNA gene sequences (11), and for placement in either the basidiomycetes or the ascomycetes we used the 5.8S rRNA gene (15).
The soil chemistry (pH and organic matter, total nitrogen, ammonium, nitrate, and phosphorous contents) in collection plots was analyzed by the DANR Analytical Laboratory, University of California at Davis. Soil samples were collected (six 300-g cores/plot), oven dried at 55 to 60°C, and sieved through a 2-mm-mesh screen. Soil was analyzed with a pH meter after aqueous extraction (46); soil was analyzed for organic matter content by potassium dichromate reduction of organic carbon and subsequent spectrophotometric measurement (modified Walkley-Black method [36]); soil was analyzed for P (Olsen) content by alkaline extraction in 0.5 N NaHCO3; soil was analyzed for P (Bray) content by extraction of acid soil (pH less than 7.0) by using a dilute acid-fluoride extractant (37); soil was analyzed for total nitrogen content by combustion (38, 39); and soil was analyzed for nitrate and ammonium contents by extraction with potassium chloride and subsequent determination with a diffusion-conductivity analyzer (30).
Differences in species richness (number of EM fungal species per core) and EM infection (number of individual EM per core) and the significance of differences in the relative levels of EM fungal species among treatments were analyzed by a Mann-Whitney U test. The significance of differences in soil chemistry were determined by the Student t test.
RESULTS
Perlite maintained the soil temperature and moisture at levels comparable to those under added litter (Table 1). However, the nitrate and phosphorous levels were significantly lower in the plots to which perlite was added than in either the control plots or the plots to which litter was added, while the ammonium level was slightly (although not significantly) higher. The changes in the perlite plots were consistent with expectations given the increased soil moisture without a new source of nutrients in a low-pH environment (3). The organic matter content, total N content, and pH were not changed significantly by either perlite or litter addition. The soil ammonium level was significantly higher in the plots to which litter was added than in the control plots and the plots to which perlite was added.
TABLE 1.
Mean effects, based on 18 cores for each treatment, of litter and perlite addition on soil conditions and EM fungal species richness
| Treatment | Species richness (species/core) | Temp (°C)
|
Moisture (% saturation)
|
NH4 concn (ppm) | NO3 concn (ppm) | Total N concn (%) | P concn (ppm) | Organic matter concn (%) | pH | ||
|---|---|---|---|---|---|---|---|---|---|---|---|
| July | Sept | July | Sept | ||||||||
| Control | 2.6 (0.3)a | 24.5 (1) | 23.9 (0.8) | 0.2 (0.2) | 5.0 (0.7) | 5.0 (1) | 1.2 (0.4) | 0.109 (0.01) | 29 (2) | 2.4 (0.4) | 5.1 (0.2) |
| Perlite | 2.3 (0.4) | 13.3 (0.4)b | 17.1 (0.3)b | 7.6 (0.3)b | 8.1 (1)b | 5.2 (1) | 0.4 (0.1) | 0.094 (0.07) | 23 (4) | 2.7 (0.1) | 4.9 (0.2) |
| Litter | 1.8 (0.3)b,c | 14.6 (0.4)b | 18.1 (0.5)b | 5.4 (0.6)b | 7.2 (1.2)b | 7.4 (1)d,e | 1.4 (0.7)e | 0.119 (0.02) | 29 (3)c | 3.0 (0.4) | 5.0 (0.4) |
The values in parentheses are standard errors.
Significantly different (P < 0.01) from the control value.
Significantly different (P < 0.001) from the perlite value.
Significantly different (P < 0.05) from the perlite value.
Significantly different (P < 0.05) from the control value.
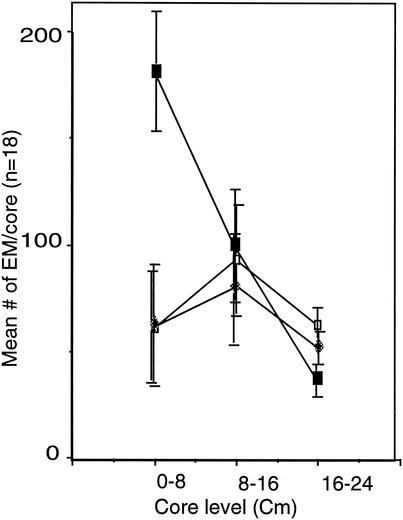
Litter addition significantly affected both EM fungal species richness (Table 1) and EM infection (P < 0.001) (Fig. 2), causing a decrease in the former and an increase in the latter, which was concentrated in the upper soil layer, closest to the added litter. Perlite addition had no significant effect on either EM fungal species richness or EM infection in any of the three soil layers
FIG. 2.
Changes in the distribution of individual EM roots with depth. Symbols: ▪, litter added; □, perlite added; ⧫, unmodified control.
Four EM fungal species comprised 60 to 80% of the total EM communities for all three treatments (Table 2). The remainder of the EM fungal communities was composed of relatively rare species (seven species per plot in controls and plots to which perlite was added and three species per plot in plots to which litter was added), each of which was detected in only a single core (Table 2). This pattern of relatively few dominant species along with many rare species has been detected previously (12, 16, 18). Since the latter species were rare and changes in their relative abundance in response to treatment could not be analyzed statistically, they are not included in Table 2. However, RFLP patterns for these species are available upon request from one of us (K.W.C.).
TABLE 2.
Abundance and RFLP patterns of dominant EM fungal species detected
| Species | Relative abundance (no. of tips/core)
|
% of total community
|
RFLP pattern (bp)
|
|||||
|---|---|---|---|---|---|---|---|---|
| Control | Perlite | Litter | Control | Perlite | Litter | AluI | HinfI | |
| Suillus granulatus | 56 (18)a | 77 (18) | 164 (15) | 53 | 59 | 60 | 680 | 220, 190, 150, 70, 40 |
| Cortinariaceae species 2 | 3 (3) | 0 | 84 (23) | 0.6 | 0 | 13 | 404, 320 | 242, 190, 130, 120, 70 |
| Agaricoid DD | 61 (25) | 7 (5) | 0 | 12 | 2 | 0 | 480, 130, 100 | 320, 250, 120 |
| Wilcoxina mikolae | 98 (43) | 0 | 0 | 15 | 0 | 0 | 692 | 300, 200, 180 |
| Otherb | 7c | 7 | 3 | 20 | 39 | 27 | NAd | NAd |
The numbers in parentheses are standard errors.
Species detected in only a single core.
Number of rare species per plot.
NA, not applicable.
Treatment affected each of the four dominant EM fungal species differently. The relative abundance (number of EM tips per core) of Suillus granulatus increased significantly (P < 0.01) in response to litter addition but not in response to addition of perlite (Table 2). Similarly, the level of Cortinariaceae species 2 (accession no. AY169695), an EM fungus that did not fruit (but was identified as a member of a family-level monophyletic group), increased significantly (P < 0.05) only in response to litter addition. Conversely, Wilcoxina mikolae, an ascomycete fungus, and agaricoid DD (accession no. AY169696; a basidiomycete identified to the ordinal level by 5.8S rRNA sequence analysis) responded negatively (P < 0.01) to both litter addition and perlite addition. We performed individual comparisons of each block combination (i.e., control plot 1 was compared to all litter blocks individually, control block 2 was compared to all litter blocks, etc.) and found no plot effect that detracted from our conclusions.
DISCUSSION
Conditions and chemistry associated with soil litter can decrease both EM infection and the number of EM morphotypes (5, 6). Previously, these effects were thought to be due to nitrogen inhibition of fungal growth (4, 5). The effects were most marked in nutrient-poor soils, and similar effects could be induced by soil amendment with nitrogen fertilizers (2, 8, 10), supporting the hypothesis that EM infection decreases with increased soil fertility.
Litter addition decreased species richness in our system by eliminating species that were dominant in control plots and also by reducing the number of rare species. In contrast, perlite addition had no effect on species richness, supporting the hypothesis that conditions associated with added litter can reduce the number of EM fungi in the community. Rare species accounted for roughly 20 to 40% of each EM fungal community, although the number of species contributing to this value was significantly lower following litter addition. This result indicates that the relatively few remaining species, species detected in only a single core each, were present in large numbers in their respective cores.
Along with the loss of several rare types that occurred in only a single soil core each, two of the four dominant organisms (W. mikolae and agaricoid DD) were eliminated by the addition of litter. W. mikolae is a species that forms ectendomycorrhizae, rapidly colonizes roots after disturbance (44), and prefers heavily disturbed soils with low organic contents (e.g., soils from burns, mine spoils [7, 24, 34], or clear-cuts [27]). However, these species were eliminated from both the perlite plots and the litter addition plots, so their disappearance may have been due to the temperature-moisture relationships rather than the nutritive properties of litter. Container experiments with Jack pine in association with W. mikolae demonstrated that increased fertilization does not inhibit infection at all levels except the highest nitrogen levels (19), supporting this notion.
Although species richness decreased, EM infection increased significantly in response to litter addition. This increase occurred in the upper soil layers, directly adjacent to the added litter, and was due largely to increases in both S. granulatus infection and Cortinariaceae species 2 infection (Table 2). This phenomenon maintained the overall percentage of the dominant organism in the system, S. granulatus, at 60% of the community, while the relative abundance of this species increased significantly following litter addition.
The increase in EM infection in response to litter addition might have been due to increased growth of some fungal species in response to the increased moisture and decreased temperature resulting from the insulating nature of litter. We consider this interpretation of the results unlikely since there was no significant effect on the relative abundance of the species or on total EM infection after addition of perlite, which maintained both the moisture and the temperature at levels similar to those found under litter. Rather, we think that these results were due to a positive growth response by some species to added nutrients. Another Suillus species, Suillus intermedius, has a strong positive growth response to pine needle extracts when it is grown in culture (32). Increasing the nitrogen content by urea amendment or fertilization also failed to alter the relative levels of several EM fungal species in a Tsuga heterophylla forest in British Columbia (31) and in Jack pine (20). This lack of change was attributed to the low levels of nitrogen amendment used (31) or to the ability of some species to grow even under high-nitrogen conditions (20).
Clearly, conditions associated with litter addition did not deter growth and mycorrhization by either S. granulatus or Cortinariaceae species 2, and the increases exhibited by these two fungi may reflect positive reactions to increased levels of ammonium. Alternatively, the increases could have been due to increased root growth in response to conditions associated with litter addition and subsequent infection by fungal species that could adapt to the changing conditions. Targeted experiments in which ammonium is added to perlite plots (in order to produce the temperature-moisture relationships in conjunction with increased levels of nutrients) and experiments in which the responses of S. granulatus to increasing ammonium levels in culture are examined are required to determine which of these possibilities is correct. Unfortunately, until fruiting bodies of Cortinariaceae species 2 are found, a culture experiment involving this species cannot be conducted.
Before treatment, our system was dominated by only four species, the ascomycete W. mikolae, S. granulatus, agaricoid DD, and a member of the Cortinariaceae. All of the other species were detected in only a single core, and each of these species comprised <8% of the EM fungal community (Table 2). This result is not consistent with the hypothesis (43) that the fungal communities in pinaceous tree-dominated systems less than 100 years old are universally comprised of thelephoroid and russuloid species. The results of previous studies in Yellowstone National Park also do not support this hypothesis (12, 16, 17, 18) since in no case were thelephoroids the dominant EM fungi in the system, although russuloids were abundant in old-growth, mixed-conifer forests that were >300 years old (16, 17). The high relative level of the ascomycete W. mikolae is consistent with previous results obtained with systems disturbed by fire (44) and probably was due to the ability of this species to form resistant propagules that persist following fire (47).
In summary, the results of this study support the hypothesis that EM species richness can decrease in response to litter addition. However, measurements of the relative abundance of individual fungal species indicate that some organisms responded positively to litter addition, while others responded negatively to perlite addition as well as to litter addition, which altered only the soil moisture and temperature and not the soil nutrient levels. The hypothesis that EM infection decreases in response to litter addition was not supported. In fact, EM infection increased in the soil layers directly adjacent to the added litter. These results suggest that the negative responses observed in some litter addition experiments (and hence some changes that occur through forest succession) are not due solely to nutrient effects. Rather, changes in soil temperature and moisture may be more important than changes in nitrogen levels. Our results also indicate that there are significant physiological differences among EM fungal species in their responses to changes in nutrients. Thus, the loss, gain, or changes in relative abundance of an EM fungal species that occur as a result of changes in soil fertility also could result in significant changes in the ecosystem.
Acknowledgments
This work was supported by NSF grant DEB/RUI 9420141 to K. W. Cullings and V. T. Parker and by a NASA-DDF grant to K. W. Cullings.
We thank J. R. Blair for mushroom identification and Bob Lindstrom and Ann Deutch of the Yellowstone Center for Resources and Ann Rodman of the Yellowstone Soil Survey for logistical support.
REFERENCES
- 1.Agerer, R. 1987. Colour atlas of ectomycorrhizae. Einhorn-Verlag, Eduard Dietenberger, Schwäbisch Gmünd, Germany.
- 2.Arnebrant, K. 1994. Nitrogen amendments reduce the growth of extramatrical ectomycorrhizal mycelium. Mycorrhiza 5:7-15. [Google Scholar]
- 3.Atlas, R. M., and R. Bartha. 1993. Microbial ecology: fundamentals and applications, 3rd ed., p. 230 and 314-333. The Benjamin/Cummings Publishing Company, Inc., Redwood City, Calif.
- 4.Baar, J., W. A. Ozinga, I. L. Sweers, and T. W. Kuyper. 1994. Stimulatory and inhibitory effects of needle litter and grass extracts on the growth of some ectomycorrhizal fungi. Soil Biol. Biochem. 26:1073-1079. [Google Scholar]
- 5.Baar, J., and F. W. de Vries. 1995. Effects of manipulation of litter and humus layers on ectomycorrhizal colonization potential in Scots pine stands of different age. Mycorrhiza 5:267-272. [Google Scholar]
- 6.Baar, J. 1996. The ectomycorrhizal flora of primary and secondary stands of Pinus sylvestris in relation to soil conditions and ectomycorrhizal succession. J. Veg. Sci. 7:497-504. [Google Scholar]
- 7.Baar, J., T. R. Horton, A. M. Kretzer, and T. D. Bruns. 1999. Mycorrhizal colonization of Pinus muricata from resistant propogules after a stand-replacing wildfire. New Phytol. 143:409-418. [Google Scholar]
- 8.Baum, C., and F. Makeschin. 2000. Effects of nitrogen and phosphorous fertilization on mycorrhizal formation of two poplar clones (Populus trichocarpa and P. tremula × tremuloides). J. Plant Nutr. Soil Sci. 163:491-497. [Google Scholar]
- 9.Bendig, G. D., and D. J. Read. 1995. The structure and function of the vegetative mycelium of ectomycorrhizal plants. New Phytol. 130:401-409. [DOI] [PubMed] [Google Scholar]
- 10.Brandrud, T. E. 1995. The effects of experimental nitrogen addition on the ectomycorrhizal fungus flora in an oligotrophic spruce forest at Gardsjon, Sweden. For. Ecol. Manage. 71:111-122. [Google Scholar]
- 11.Bruns, T. D., T. M. Szaro, M. Gardes, K. W. Cullings, J. J. Pan, D. L. Taylor, T. R. Horton, A. Kretzer, M. Garbelotto, and Y. U. Li. 1998. A sequence database for the identification of ectomycorrhizal basidiomycetes by phylogenetic analysis. Mol. Ecol. 7:257-272. [Google Scholar]
- 12.Byrd, K. B., V. T. Parker, D. R. Vogler, and K. W. Cullings. 2000. The influence of clear-cutting on ectomycorrhizal fungus diversity in a lodgepole pine (Pinus contorta) stand, Yellowstone National Park, Wyoming, and Gallatin National Forest, Montana. Can. J. Bot. 78:149-156. [Google Scholar]
- 13.Colpaert, J. W., and A. Van Laere. 1996. A comparison of the extracellular enzyme activities of two ectomycorrhizal and a leaf-saprotrophic basidiomycete colonizing beech leaf litter. New Phytol. 133:133-141. [Google Scholar]
- 14.Colpaert, J. W., and K. K. Van Tichelen. 1996. Decomposition, nitrogen and phosphorous mineralization from beech leaf litter colonized by ectomycorrhizal or litter-decomposing basidiomycetes. New Phytol. 134:123-132. [Google Scholar]
- 15.Cullings, K. W., and D. R. Vogler. 1998. A 5.8S nuclear ribosomal RNA gene sequence database: applications to ecology and evolution. Mol. Ecol. 7:919-923. [DOI] [PubMed] [Google Scholar]
- 16.Cullings, K. W., V. T. Parker, S. K. Finley, and D. R. Vogler. 2000. Ectomycorrhizal specificity patterns in a mixed Pinus contorta and Picea engelmannii stand in Yellowstone National Park. Appl. Environ. Microbiol. 66:4988-4991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cullings, K. W., D. R. Vogler, V. T. Parker, and S. Makhija. 2001. Effects of artificial defoliation on the ectomycorrhizal community of a mixed Pinus contorta/Picea engelmannii stand in Yellowstone Park. Oecologia 127:533-539. [DOI] [PubMed] [Google Scholar]
- 18.Cullings, K. W., and S. Makhija. 2002. Ectomycorrhizal associates of Pinus contorta in soils associated with a hot spring in Norris Geyser Basin, Yellowstone National Park, Wyoming. Appl. Environ. Microbiol. 67:5538-5543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Danielson, R. M. 1984. Ectomycorrhiza formation by the operculate discomycete Sphaerosporella brunnes (Pezizales). Mycologia 76:454-461. [Google Scholar]
- 20.Danielson, R. M., C. L. Griffiths, and D. Parkinson. 1984. Effects of fertilization on the growth and mycorrhizal development of container-grown Jack pine seedlings. For. Sci. 30:828-835. [Google Scholar]
- 21.De Vries, B. W. L., E. Jansen, H. F. Van Dobben, and T. W. Kuyper. 1995. Partial restoration of fungal and plant species diversity by removal of litter and humus layers in stands of Scots pine in the Netherlands. Biodiv. Conserv. 4:156-164. [Google Scholar]
- 22.Dighton, J., and P. A. Mason. 1985. Mycorrhizal dynamics during forest tree development, p. 117-139. In D. Moore, L. A. Casselton, D. Wood, and J. C. Frankland (ed.) Developmental biology of higher fungi. Cambridge University Press, Cambridge, United Kingdom.
- 23.Dighton, J., J. M. Poskitt, and D. M. Howard. 1986. Changes in occurrence of basidiomycete fruit bodies during forest stand development: with specific reference to mycorrhizal species. Trans. Br. Mycol. Soc. 87:163-171. [Google Scholar]
- 24.Egger, K. N., R. M. Danielson, and J. A. Fortin. 1991. Taxonomy and population structure of e-strain mycorrhizal fungi inferred from ribosomal and mitochondrial DNA polymorphisms. Mycol. Res. 95:866-872. [Google Scholar]
- 25.Gardes, M., and T. D. Bruns. 1993. ITS primers with enhanced specificity for higher fungi and basidiomycetes: application to identification of mycorrhizae and rusts. Mol. Ecol. 2:113-118. [DOI] [PubMed] [Google Scholar]
- 26.Gardes, M., and T. D. Bruns. 1996. Community structure of ectomycorrhizal fungi in a Pinus muricata forest: above- and below-ground views. Can. J. Bot. 74:1572-1583. [Google Scholar]
- 27.Hagerman, S. M., M. D. Jones, G. E. Bradfield, M. Gillespie, and D. M. Durall. 1999. Effects of clear-cut logging on the diversity and persistence of ectomycorrhizae at a subalpine forest. Can. J. For. Res 29:124-134. [Google Scholar]
- 28.Harvey, A. E., M. J. Larsen, and M. F. Jugensen. 1976. Distribution of ectomycorrhizae in a mature Douglas-fir/larch forest soil in western Montana. For. Sci. 22:393-398. [Google Scholar]
- 29.Horton, T. R., and T. D. Bruns. 1998. Multiple-host fungi are the most frequent and abundant ectomycorrhizal types in a mixed stand of Douglas-fir (Pseudotsuga menziesii) and bishop pine (Pinus muricata). New Phytol. 139:331-339. [Google Scholar]
- 30.Keeney, D. R., and D. W. Nelson. 1982. Nitrogen—inorganic forms, p. 643-698. In A. L. Page (ed.), Methods of soil analysis. Part 2. Chemical and microbiological properties, 2nd ed. Monograph 9. ASA, Madison, Wis.
- 31.Kernaghan, G., S. Berch, and R. Carter. 1995. Effect of urea fertilization on ectomycorrhizae of 20-year-old Tsuga heterophyla. Can. J. For. Res. 25:891-901. [Google Scholar]
- 32.Koide, R. T., L. Suome, C. M. Stevens, and L. McCormick. 1998. Interactions between needles of Pinus resinosa and ectomycorrhizal fungi. New Phytol. 140:539-547. [DOI] [PubMed] [Google Scholar]
- 33.Last, F. T., J. Dighton, and P. A. Mason. 1985. Successions of sheathing mycorrhizal fungi. Trends Ecol. E. 2:157-161. [DOI] [PubMed] [Google Scholar]
- 34.Mah, K., L. E. Tackaberry, K. N. Egger, and H. B. Massicotte. 2001. The impacts of broadcast burning after clear-cutting on the diversity of ectomycorrhizal fungi associated with hybrid spruce seedlings in central British Columbia. Can. J. For. Res. 31:224-235. [Google Scholar]
- 35.Mosse, B., D. O. Stribley, and F. Le Tacon. 1981. Ecology of mycorrhizae and mycorrhizal fungi. Adv. Microb. Ecol. 5:137-210. [Google Scholar]
- 36.Nelson, D. W., and L. E. Sommers. 1982. Total carbon, organic carbon, and organic matter, p. 539-579. In A. L. Page (ed.), Methods of soil analysis. Part 2. Chemical and microbiological properties, 2nd ed. Monograph 9. ASA, Madison, Wis.
- 37.Olsen, S. R., C. V. Cole, F. S. Watanabe, and L. A. Dean. 1954. Estimation of available phosphorus in soils by extraction with sodium bicarbonate. U. S. Dep. Agric. Circ. 939:1-19. [Google Scholar]
- 38.Pella, E. 1990. Elemental organic analysis, part 1: historical developments. Am. Lab. 22:116. [Google Scholar]
- 39.Pella, E. 1990. Elemental organic analysis, part 2: state of the art. Am. Lab. 22:28. [Google Scholar]
- 40.Read, D. J. 1991. Mycorrhizas in ecosystems. Experientia 47:376-391. [Google Scholar]
- 41.Romme, W. H., and D. G. Despain. 1989. The Yellowstone fires. Sci. Am. 261:37-43. [Google Scholar]
- 42.Smith, S. E., and D. J. Read. 1997. Mycorrhizal symbiosis. Academic Press, London, United Kingdom.
- 43.Stendell, E., T. R. Horton, and T. D. Bruns. 1999. Early effects of prescribed fire on the structure of the ectomycorrhizal fungus community in a Sierra Nevada ponderosa pine forest. Mycol. Res. 103:1353-1359. [Google Scholar]
- 44.Torres, P., and M. Honrubia. 1997. Changes and effects of a natural fire on ectomycorrhizal inoculum potential of soil in a Pinus halepensis forest. For. Ecol. Manage. 96:189-196. [Google Scholar]
- 45.Turner, M. G., W. H. Romme, R. H. Gardner, and W. W. Hargrove. 1997. Effects of fire size and pattern on early succession in Yellowstone National Park. Ecol. Monogr. 67:411-433. [Google Scholar]
- 46.U.S. Salinity Laboratory Staff. 1954. Diagnosis and improvement of saline and alkali soils. Agricultural handbook no. 60. U.S. Department of Agriculture, Washington, D.C.
- 47.Visser, S. 1995. Ectomycorrhizal fungal succession in jack pine stands following wildfire. New Phytol. 129:389-401. [Google Scholar]