Abstract
The industrial production of β-carotene with the zygomycete Blakeslea trispora involves the joint cultivation of mycelia of opposite sex in the presence of β-ionone and other chemical activators. We have obtained improved strains by mutation and heterokaryosis. We chose wild strains on the basis of their growth and carotene content in single and mated cultures. Following exposure of their spores to N-methyl-N′-nitro-N-nitrosoguanidine, we obtained high-carotene mutants, which were more productive than their parents but similar to them in having β-carotene as the main product. Further increases in carotene content were obtained after a new round of mutagenesis in one of the mutants. The production was shifted to lycopene in cultures incubated in the presence of nicotine and in lycopene-rich mutants derived from the wild strains. The highest production levels were achieved in intersexual heterokaryons, which contained mutant nuclei of opposite sex. These contained up to 39 mg of β-carotene or 15 mg of lycopene per g (dry mass) under standard laboratory conditions in which the original wild strains contained about 0.3 mg of β-carotene per g (dry mass). β-Ionone did not increase the carotene content of these strains. Not all wild strains lent themselves to these improvements, either because they produced few mutants or because they did not increase their carotene production in mated cultures.
Blakeslea trispora, a zygomycete mold, is an industrial source of β-carotene following the optimization of liquid fermentation processes (10, 29). The organism is a saprophyte that performs its vegetative cycle of spores, filamentous mycelia, fruiting bodies, and again spores on chemically defined media in the laboratory. The genetic analysis of this organism is limited to the isolation of mutants and inferences from their phenotypes (19). Mycelia belong to either the (+) or the (−) mating type or sex. Mycelia of opposite sex that grow together increase their accumulation of β-carotene. Hyphal tips of opposite sex that grow near one another on solid media undergo a series of morphological differentiations that culminates in the production of zygospores, i.e., cells that contain protoplasm from both parents. We have not been able to induce the zygospores to germinate, and we have not obtained recombinant progeny.
In Blakeslea, as in other organisms, β-carotene results from the formation of β-rings at both ends of a linear precursor, lycopene, as shown by genetic (19) and chemical (20) inhibition of this reaction. Mixtures of lycopene, γ-carotene (the intermediate with a single ring), and β-carotene are accumulated, depending on the extent of the inhibition.
The regulation of carotene biosynthesis in Blakeslea differs from the regulation in Phycomyces blakesleeanus, another fungus of the order Mucorales. The latter exhibits a strong inhibition by the final product, β-carotene (25), which is lacking in the former (19). Both organisms increase their carotenogenesis during sexual interaction and after exposure to β-ionone, but they respond very differently to blue light and many chemicals (2, 8, 18). In Phycomyces the carotene content is increased by mutations in the genes carS (25), carD (32), and carF (21). Sexual stimulation occurs in mixed cultures of (+) and (−) strains, called mated cultures; in intersexual heterokaryons, which carry both sexual determinants in different nuclei of the same mycelium (26); and in intersexual diploids or aneudiploids, which carry both sexual determinants in different chromosomes of the same nucleus (22). Intersexual strains are readily recognized by their bright yellow color and velvety appearance, which is due to the scarcity of vegetative fruiting bodies and the abundance of short and contorted aerial hyphae known as pseudophores. Phycomyces strains that carry the genetic determinants for both sexes and mutations for increased carotene production accumulate hundreds of times more β-carotene than the wild type.
Young zygospores of Phycomyces and possibly other surrounding structures with mixed protoplasm may be picked and transferred to fresh medium to produce intersexual heterokaryons that contain nuclei of both sexes (4, 12). Similar structures should allow the production of Blakeslea heterokaryons, but the lack of a productive sexual cycle hinders the construction of intersexual diploids. In order to develop Blakeslea strains with high contents of β-carotene or lycopene, we have obtained mutants and constructed intersexual heterokaryons.
MATERIALS AND METHODS
The wild strains of B. trispora Thaxter (class Zygomycetes, order Mucorales, family Choanophoraceae) were obtained from the Vsiesoyuznaya Kollektsiya Mikroorganizmov, Moscow, Russia. These wild-type strains, the mutants listed in Table 1, mated cultures, and heterokaryons were grown according to the recommendations for Phycomyces (7) from spores (104 per plate) for 4 days on minimal agar at room temperature, unless otherwise stated. Distinct colonies were observed on acid minimal agar (pH 3.3). For mated cultures, two strains of opposite sex were inoculated and incubated together (104 spores of each strain per plate). Spores require no special activation to germinate. Heterokaryons were usually grown from pieces of mycelium (about 1 mg [dry mass]); they rarely were grown from spores.
TABLE 1.
Carotene contents of single and mated cultures of wild-type and mutant strains
| Strain | Origin | Genotype | Main carotene | Concn (mg/g [dry mass])a |
|---|---|---|---|---|
| F921 | Wild type | (−) | β-Carotene | 0.33 ± 0.07 |
| F986 | Wild type | (+) | β-Carotene | 0.26 ± 0.04 |
| F989 | Wild type | (+) | β-Carotene | 0.26 ± 0.05 |
| SB29 | From F921 | car-1 (−) | β-Carotene | 0.61 ± 0.12 |
| SB30 | From F921 | car-2 (−) | β-Carotene | 0.64 ± 0.20 |
| SB31 | From F921 | car-3 (−) | β-Carotene | 0.69 ± 0.14 |
| SB32 | From F921 | car-4 (−) | β-Carotene | 0.85 ± 0.27 |
| SB38 | From F986 | car-6 (+) | β-Carotene | 0.49 ± 0.03 |
| SB39 | From F986 | car-7 (+) | β-Carotene | 0.52 ± 0.05 |
| SB34 | From F921 | car-5 (−) | Lycopene | 0.25 ± 0.06 |
| SB40 | From F986 | car-8 (+) | Lycopene | 0.18 ± 0.05 |
| SB41 | From F986 | car-9 (+) | Lycopene | 0.20 ± 0.03 |
| SB51 | From F986 | car-19 (+) | Mixture | 0.46 ± 0.08 |
| SB52 | From F921 | car-20 (−) | β-Carotene | 1.68 ± 0.10 |
| SB53 | From F921 | car-21 (−) | β-Carotene | 0.67 ± 0.14 |
| SB63 | From SB52 | car-20 car-22 (−) | β-Carotene | 2.56 ± 0.34 |
| F986 + F921 | Mated wild-type strains | β-Carotene | 4.30 ± 1.05 | |
| F989 + F921 | Mated wild-type strains | β-Carotene | 3.94 ± 1.12 | |
| SB38 + SB32 | Mated high-carotene mutants | β-Carotene | 27.1 ± 7.1 | |
| SB39 + SB32 | Mated high-carotene mutants | β-Carotene | 28.2 ± 5.8 | |
| SB51 + SB52 | Mated high-carotene mutants | β-Carotene | 5.0 ± 0.4 | |
| SB51 + SB63 | Mated high-carotene mutants | β-Carotene | 22.7 ± 1.2 | |
| SB40 + SB34 | Mated lycopene mutants | Lycopene | 3.5 ± 0.28 | |
| SB41 + SB34 | Mated lycopene mutants | Lycopene | 4.2 ± 0.21 |
Mean and standard error for two to seven mycelia grown for 4 days on minimal agar.
To count the nuclei, spores were suspended in ethanol for 5 min, spread on a glass slide, allowed to dry, covered with 40 μl of a 5-mg/liter solution of 4′,6-diamidino-2-phenylindole (DAPI) in Vectashield mounting medium (Vector Laboratories, Burlingame, Calif.), and observed under a fluorescence microscope.
For mutagenesis (30), 106 spores were suspended in 1 ml of distilled water with 1 or 0.2 mg of N-methyl-N′-nitro-N-nitrosoguanidine and incubated at 30°C until only 1 to 5% of them remained viable (exposure of 20 or 60 min, respectively). Colonies with apparently deviant color were picked up and purified by repeated subculturing (two to five times) from spores of single colonies.
To construct heterokaryons of Blakeslea, we adapted the method described by Gauger et al. (12) for Phycomyces. Pieces of mycelium of two strains of opposite sex were inoculated on potato dextrose agar and incubated until bright yellow bands appeared along the mycelial meeting line. Fragments (about 1 mg [dry mass]) of mycelia from these bands were subcultured on minimal agar. The patches of intersexual mycelia that appeared were subcultured until they were relatively uniform. The intersexual mycelia sporulate rarely, but they could be purified by vigorously shaking pieces in 0.2 ml of 0.55 M sorbitol with a small amount of sterile sea sand in a vortex mixer for about 5 min, plating on acid minimal agar, and incubating for about 4 days to obtain individual colonies.
Nicotine (5 mmol/liter) was added to the cultures directly, and β-ionone (0.5 mmol/liter) was emulsified in ethanol and Tween 80 (polyoxyethylenesorbitan monooleate). The final concentrations of the solvents in the cultures were 3 and 1.5 ml/liter, respectively.
The carotene content in n-hexane extracts from lyophilized mycelia was estimated spectrophotometrically (14) from the specific absorption coefficients (A1cm1 g/liter = 250 at 450 nm for β-carotene and 320 at 502 nm for lycopene).
Cultures for liquid chromatography were incubated for 5 days at 28°C in the dark. n-Hexane extracts were loaded into a 4.6- by 100-mm Hypersil octyldecyl silane analytical column (particle size, 5 μm) (Waters, Milford, Mass.) in a series 1100 liquid chromatograph (Hewlett-Packard, Palo Alto, Calif.), eluted at room temperature with methanol-acetonitrile-chloroform (47:47:6, vol/vol) at a flow rate of 1 ml/min, and monitored at 286, 450, 462, and 473 nm (the absorption maxima for phytoene, β-carotene, γ-carotene, and lycopene, respectively). Carotene amounts were obtained from the absorption maxima following calibration with pure samples.
RESULTS
Choice of starting wild strains.
We chose our standard (+) and (−) strains for the genetic analysis of carotenogenesis after careful observations of a collection of 25 wild strains carried out by I. N. Obraztsova in this laboratory. We paid particular attention to growth, sporulation, and β-carotene production. Strain F921 was a clear choice as the standard (−) strain, a decision that was reinforced by successive experiments. Most (+) strains that were attractive for the reasons given above had to be discarded because they lacked the sexual stimulation of carotenogenesis that is essential in the current industrial application of this fungus. Two (+) strains, F986 and F989, were retained; their mated cultures with F921 contained 10 to 15 times as much β-carotene as single cultures (Table 1).
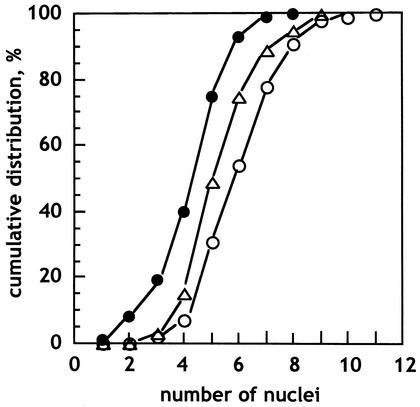
The spores of Blakeslea are multinucleate; our chosen wild-type strains are not among those with the highest numbers of nuclei per spore. Strains F986, F989, and F921 (Fig. 1) average 4.7, 5.6, and 6.4 nuclei per spore, respectively.
FIG. 1.
Cumulative distribution of the number of nuclei per spore in the wild-type strains F921 (○), F986 (•), and F989 (▵) as determined by fluorescence microscopy of DAPI-stained samples.
High-carotene mutants.
Over 200.000 colonies produced by mutagen-treated spores of the wild-type strains F986 and F921 were screened. Some were more yellow than the wild type, and others were more pink, but none were white. The mutants were isolated by picking up the more yellow colonies and purified by subculturing them repeatedly through spores. In most cases, the spores from the first rounds of purification produced colonies of various shades of yellow, as would be expected from dominant mutations in heterokaryosis. Rare mutations in multinucleate spores would appear first in heterokaryosis, but some of the spores produced by the heterokaryons would be homokaryotic. Prolonged attempts to derive stable color mutants from F989 failed.
The mutant strains exhibited significant increases in carotene content over the original wild type (Table 1). The increase was about fivefold in the best mutant, strain SB52, and about eightfold in a double mutant, SB63, obtained after a second mutagen exposure.
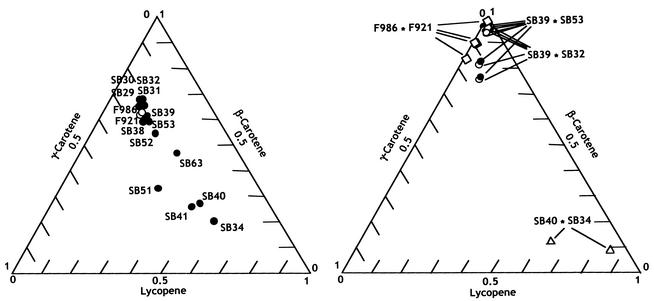
The mutants contained mixtures of colored carotenes similar to those of the wild-type strains (Fig. 2). β-Carotene represented about two-thirds of the total, and its immediate precursors, γ-carotene and lycopene, were present in sizeable amounts. In addition, all strains contained phytoene, a colorless carotene, in concentrations of one-sixth to one-third of that of the total colored carotenes (0.04 to 0.25 mg/g [dry mass]).
FIG. 2.
Colored carotenes in the wild-type strains, mutant strains, and intersexual heterokaryons. High-pressure liquid chromatography analyses of mycelia grown for 5 days on minimal agar were performed. The three homogeneous coordinates represent the proportions of lycopene, γ-carotene, and β-carotene in the sum of the three compounds. Values are the means of two to five determinations. The mean relative error in 45 determinations was 2.2%. (Left) Wild and mutant strains as indicated. (Right) Intersexual heterokaryons.
Lycopene-rich mutants.
Mutant strains with a high lycopene content were obtained by picking up and purifying the pinkish yellow colonies found in the mutagen-exposed populations. These mutants, which were less frequent than the high-carotene mutants, differed not quantitatively but qualitatively from the wild-type strains (Table 1 and Fig. 2). Lycopene predominated in strains SB34, SB40, and SB41, and strain SB51 had about equal amounts of the three colored carotenes.
Mated cultures.
The mutants mentioned in this work exhibited sexual stimulation of carotenogenesis (Table 1). The lycopene-rich mutants were similar to the wild-type strains in that their mated cultures contained about 15 times more carotene than separate cultures. The results with the high-carotene strains were particularly striking. The mycelia of their mated cultures exhibited a strong orange-yellow color and contained 40 times more carotene than the average for the single cultures and 32 times more than the amount in the best single culture.
Intersexual heterokaryons.
Intersexual heterokaryons contain nuclei of opposite sex in the same cytoplasm. They are produced spontaneously early in the sexual cycle at the frontier of mycelia of opposite sex growing on the same agar plates. They can be purified by mycelial subcultures and recognized by their peculiar velvety appearance.
Intersexual heterokaryons sporulate very rarely. Heterokaryons with different proportions of the constituent nuclei can be obtained by plating on acid agar the rare spores or small pieces from vegetative mycelia obtained by mechanical breaking.
Intersexual heterokaryons prepared with wild-type strains and various mutants contained much more carotene than their homokaryotic constituent strains (Table 2). The intersexual heterokaryons of high-carotene mutants were deep orange, with various tints that can be attributed to variations in the proportions of the constitutive nuclei. Our best result, 39 mg of β-carotene per g (dry mass) in a mycelium of the heterokaryon SB39 ∗ SB32, represented more than a 100-fold increase over the average content of the wild-type strains, more than a 50-fold increase over the average content of the mutants, and a 7-fold increase over the content of the best wild-type intersexual heterokaryon. Most of this extraordinary overproduction was achieved with intersexual heterokaryons of a mutant, such as SB32, and a wild-type strain of the opposite sex. One could say that overproduction in heterokaryosis behaved as a dominant marker.
TABLE 2.
Carotene contents of heterokaryons of wild-type and mutant strains
| Heterokaryon | Main carotene | No. of mycelia | Carotene (mg/g [dry mass])a
|
|
|---|---|---|---|---|
| Mean ± SD | Best myceliumb | |||
| F989 ∗ F921 | β-Carotene | 6 | 3.6 ± 1.0 | 5.5 |
| F989 ∗ SB32 | β-Carotene | 11 | 23.3 ± 8.0 | 34.6 |
| F989 ∗ SB34 | Lycopene | 4 | 7.1 ± 1.1 | 8.4 |
| F986 ∗ F921 | β-Carotene | 8 | 4.2 ± 1.1 | 5.8 |
| F986 ∗ SB32 | β-Carotene | 2 | 25.5 ± 8.3 | 33.8 |
| F986 ∗ SB34 | Lycopene | 2 | 9.4 ± 3.1 | 12.4 |
| SB39 ∗ SB32 | β-Carotene | 7 | 27.1 ± 6.6 | 38.9 |
| SB40 ∗ SB34 | Lycopene | 7 | 10.3 ± 3.3 | 15.5 |
The samples had been grown for 3 days on minimal agar.
Highest result in analyses of mycelia with different nuclear proportions.
Intersexual heterokaryotic mycelia were not completely uniform. On agar they produced sectors that were less colorful than the original mycelium, down to the color of the constituent strains. The high production levels could be recovered by collecting the relatively few spores, plating them on acid agar, and choosing the most colorful colony. Another way to recover high-yield heterokaryons was by breaking the mycelia mechanically and plating the pieces on acid agar. The resulting colonies showed a broad range of colors and carotene contents, including deep-orange colonies that could be isolated and grown in pure culture. For example, all of the F989 ∗ SB32 intersexual mycelia that were used to obtain the values in Table 2 produced some spore progeny mycelia with more than 30 mg of β-carotene per g (dry mass).
Similar results were obtained with the high-lycopene mutants. Their intersexual heterokaryons contained more lycopene (Table 2) than the corresponding single and mated cultures. The best SB40 ∗ SB34 heterokaryons contained 15 mg of lycopene per g (dry mass), or about a 50-fold increase over that of their best homokaryotic component. Again, most of the increase could be obtained with heterokaryons of a mutant and a wild-type strain of opposite sex.
The carotene mixtures in the intersexual heterokaryons were enriched in the final product in comparison with those of the homokaryons (Fig. 3). β-Carotene constituted up to 97% and lycopene constituted up to 86% of the colored carotenes in the appropriate intersexual heterokaryons. These and the other intersexual heterokaryons contained colorless phytoene in amounts that represented 10 to 15% of the colored carotenes.
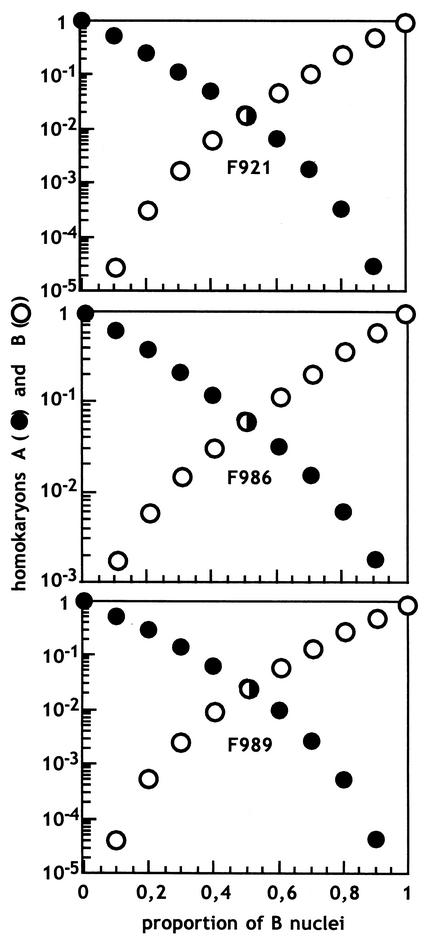
FIG. 3.
Predicted genotype distributions in the spores produced by heterokaryotic mycelia of the wild-type strains F921, F986, and F989. The two kinds of nuclei in the heterokaryons are called A and B, and the spores can be heterokaryons, homokaryons with A nuclei (○), or homokaryons with B nuclei (•).
Chemical modification of carotenogenesis.
Nicotine, an inhibitor of lycopene cyclase (6, 16, 20), caused the accumulation of lycopene in β-carotene-producing strains when present in the medium at 5 mmol/liter. The total carotene contents (for example, 0.30 ± 0.01 and 0.61 ± 0.20 mg/g [dry mass] in strains F921 and SB32, respectively) remained approximately unchanged. Nicotine had little or no effect on the contents of the lycopene-rich mutant strains SB34, SB40, and SB41.
Intersexual heterokaryons produced mostly lycopene in the presence of nicotine, but the concentrations were erratic. Small activations were seen in F986 ∗ F921 (1.5 times the control value in Table 2) and SB34 ∗ SB40 (1.3 times the control value), and a considerable loss was seen in SB32 ∗ SB39 (0.4 times the control value).
A strong activator of carotenogenesis, β-ionone, used at 0.5 mmol/liter, had no beneficial effects on intersexual heterokaryons. Production was much worse than in the controls (the carotene contents in the heterokaryons F986 ∗ F921, SB34 ∗ SB40, and SB32 ∗ SB39 were 0.15, 0.07, and 0.4 times the control values in Table 2, respectively), but much of the reduction was caused by the solvents added to the cultures with the drug.
DISCUSSION
Isolation of mutants.
Not all Blakeslea wild-type strains were equally appropriate for mutational modifications. The mutants that we isolated from the (−) strain F921 occurred more frequently, produced more carotene, and were more stable than those from the (+) strain F986. Another (+) strain, F989, was even less convenient. We do not know the differences in their genetic makeup that explain these results, but the distribution of nuclei per spore does not: the best wild-type strain, F921, had more nuclei per spore, on the average, than the other strains. One can hardly overstress the need to devote considerable effort to choosing an appropriate wild-type strain before launching a program of genetic improvement.
The multiplicity of nuclei in Blakeslea spores is a drawback for the isolation of recessive mutants and for the purification of dominant mutations to homokaryosis. The problem is more severe in Blakeslea than in the standard Phycomyces strain, because the average number of nuclei per spore is larger, but can be addressed with the same general solutions. Many agents that damage DNA inactivate nuclei to the extent that they cannot reproduce further. Some surviving spores become functionally uninucleate, having lost all of their nuclei except one. The proportion of functionally uninucleate survivors in Phycomyces can be calculated and determined experimentally (9, 30). Application of the same formula that fit the Phycomyces results (formula on p. 382 of reference 9 with parameters m = 0.6 and r = 0.4) suggests that about 12% of the spores of Blakeslea strain F921 that survived the exposure to N-methyl-N′-nitro-N-nitrosoguanidine were functionally uninucleate. This minority subpopulation should allow the isolation of all kinds of viable mutants, including those with recessive mutations.
Most dominant mutations would have been induced in the majority subpopulation of functionally multinucleate survivors, leading to the appearance of heterokaryons. For most practical purposes these are less attractive than the corresponding homokaryons with mutant nuclei only.
It was proven for Phycomyces that the relative proportions of different kinds of nuclei remain constant during the vegetative growth of heterokaryotic mycelia and during subcultivation of pieces of young mycelia in fresh media. Additionally, it was shown that the nuclei in the spores are random samples of nuclei from the mycelium. This allowed the calculation of the proportion of nuclei in a heterokaryon from the distribution of phenotypes in the colonies grown from its spores (15). Under the assumption that these conclusions apply to Blakeslea, the genotype distributions in the spores produced by heterokaryons can be predicted (Fig. 3). These considerations explain our qualitative observations of Blakeslea heterokaryons. It is worth pointing out that the spores from a heterokaryon are very rarely homokaryotic for minority nuclei. In the absence of additional genetic markers, it is very difficult to distinguish a homokaryon for a dominant mutation from a heterokaryon where the majority of the nuclei carry that mutation.
The mutants.
Our high-carotene mutants are attractive as starting steps in the improvement of β-carotene production, although their carotene contents are modest, particularly compared to those found in other organisms. The Blakeslea wild-type strains used in this work produced about six times more β-carotene than the standard Phycomyces wild-type strain, but the Blakeslea mutants known to us produce far less β-carotene than the Phycomyces carF and carS mutants (21), which accumulate up to 5 mg of β-carotene per g (dry mass) separately and twice as much when combined.
In lycopene-rich mutants, noted for their pinkish color, most of the β-carotene was replaced by its precursors lycopene and γ-carotene. This indicated an incomplete loss of the ability to cyclicize the straight ends of lycopene and γ-carotene. The total carotene content of the lycopene-rich mutants did not exceed that of the wild type. This indicates a lack of the end product regulation that operates in Phycomyces, by which scarcity of the end product, β-carotene, leads to vast increases in the activity of the pathway and accumulation of precursors (25). The lycopene content might be increased by separate mutations, as suggested by the fact that our high-carotene mutants grown in the presence of nicotine contained more lycopene than our lycopene-rich mutants.
Blakeslea color mutants with various modifications in carotene biosynthesis were isolated previously (19) from wild-type strains other than the ones used here, but those mutants were abandoned because of their inability to sporulate. White mutants that were unable either to produce phytoene or to metabolize it to colored carotenes were described. The same two kinds of white mutants were found in Phycomyces (15, 23), in Mucor circinelloides (28, 31), and in organisms outside the class Zygomycetes but were not found in this study. We suspect that our strains may have structural features, such as gene duplications, that hinder the isolation of certain kinds of mutants. A duplication of the gene for lycopene cyclase would explain the 2:1:1 distribution of the colored carotenes in several mutants, because this is expected for strains with an equal mixture of wild-type lycopene cyclase and inactive mutant protein under some simple assumptions (11).
The heterokaryons.
Blakeslea was chosen for the development of industrial carotene production because its mated cultures contain much more β-carotene than single cultures, whether grown on agar or in liquid medium (1). Sexual stimulation can be mimicked by the addition of trisporic acids to single cultures (5), but industry finds the costs too high.
There are two additional ways to achieve the sexual stimulation of carotene biosynthesis in Phycomyces: heterokaryosis (25) and heterozygosis (22). We have isolated for the first time heterokaryons of Blakeslea and have found them to contain more carotene than the homokaryons with the same nuclei, even when these were grown in mated cultures. In addition, we observed a synergy between sexual stimulation and the action of mutations that caused modest increases in the carotene content of the homokaryons; the cooperation resulted in very high carotene contents, with a prevalence of β-carotene. Even less expected was the synergy between sexual stimulation and mutations that made lycopene the most abundant carotene, resulting in very high lycopene contents. Our β-carotene and lycopene contents are the highest reported for any strain of any fungus.
A drawback of the heterokaryons was their tendency to produce less-pigmented sectors during vegetative growth. The segregants were strains of opposite sex, and therefore interact to maintain a high carotene production if well mixed. The instability varied for different heterokaryons. In all cases, high-producing heterokaryons could be recovered and repurified by use of simple procedures. A more permanent remedy for the instability would be to introduce recessive lethal mutations in both components of the heterokaryon, as was done with Phycomyces (26).
The industrial procedures described previously (10, 29) were aimed at the production of β-carotene, an attractive pigment with provitamin A activity and various positive health effects. Lycopene, the red tomato pigment, was not in much demand, but the situation has changed recently. Lycopene is not a source of vitamin A, but it is the strongest antioxidant among the usual carotenoids (24) and has been the subject of a wave of reports on protective effects against infarction, senile degeneration of the eye macula, cancers, spontaneous and induced mutations, radiation, and toxic chemicals (3, 13, 17, 27, 33).
Acknowledgments
We thank Carmen Vallejo and Javier Avalos for their help.
We thank Antibióticos S. A., the European Union (grant QLK-CT-2001-00780), the Spanish Government (grants PB96-1336, 1FD97-1476, and AGL2002-01799), and Junta de Andalucía (grupo CVI-119) for financial support.
REFERENCES
- 1.Anderson, R. F., M. Arnold, G. E. N. Nelson, and A. Ciegler. 1958. Microbiological production of β-carotene in shaken flasks. Agric. Food Chem. 6:543-545. [Google Scholar]
- 2.Bejarano, E. R., F. Parra, F. J. Murillo, and E. Cerdá-Olmedo. 1988. End product regulation of carotenogenesis in Phycomyces. Arch. Microbiol. 150:209-214. [Google Scholar]
- 3.Bohm, F., R. Edge, M. Burke, and T. G. Truscott. 2001. Dietary uptake of lycopene protects human cells from singlet oxygen and nitrogen dioxide-ROS components from cigarette smoke. J. Photochem. Photobiol. B 64:176-178. [DOI] [PubMed] [Google Scholar]
- 4.Burgeff, H. 1915. Untersuchungen über Variabilität, Sexualität und Erblichkeit bei Phycomyces nitens Kuntze. II. Flora 108:353-448. [Google Scholar]
- 5.Caglioti, L., G. Cainelli, B. Camerino, R. Mondelli, A. Prieto, A. Quilico, T. Salvatori, and A. Selva. 1966. The structure of trisporic-C acid. Tetrahedron Suppl. 7:175-187. [Google Scholar]
- 6.Candau, R., E. R. Bejarano, and E. Cerdá-Olmedo. 1991. In vivo channeling of substrates in an enzyme aggregate for β-carotene biosynthesis. Proc. Natl. Acad. Sci. USA 88:4936-4940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cerdá-Olmedo, E. 1987. Standard growth conditions and variations. p. 337-339. In E. Cerdá-Olmedo and E. D. Lipson (ed.), Phycomyces. Cold Spring Harbor Laboratory, Cold Spring Harbor, N.Y.
- 8.Cerdá-Olmedo, E. 1989. Production of carotenoids with fungi, p. 27-42. In E. J. Vandamme (ed.), Biotechnology of vitamins, pigments, and growth factors. Elsevier Applied Science, Amsterdam, The Netherlands.
- 9.Cerdá-Olmedo, E., and P. Reau. 1970. Genetic classification of the lethal effects of various agents on heterokaryotic spores of Phycomyces. Mutat. Res. 9:369-384. [DOI] [PubMed] [Google Scholar]
- 10.Ciegler, A. 1965. Microbial carotenogenesis. Adv. Appl. Microbiol. 7:1-34. [DOI] [PubMed] [Google Scholar]
- 11.de la Guardia, M. D., C. M. G. Aragón, F. J. Murillo, and E. Cerdá-Olmedo. 1971. A carotenogenic enzyme aggregate in Phycomyces: evidence from quantitative complementation. Proc. Natl. Acad. Sci. USA 68:2012-2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gauger, W., M. I. Peláez, M. I. Alvarez, and A. P. Eslava. 1980. Mating type heterokaryons in Phycomyces blakesleeanus. Exp. Mycol. 4:56-64. [Google Scholar]
- 13.Giovannucci, E., E. B. Rimm, Y. Liu, M. J. Stampfer, and W. C. Willett. 2002. A prospective study of tomato products, lycopene and prostate cancer risk. J. Natl. Cancer Inst. 94:391-398. [DOI] [PubMed] [Google Scholar]
- 14.Govind, N. S., and E. Cerdá-Olmedo. 1986. Sexual activation of carotenogenesis in Phycomyces blakesleeanus. J. Gen. Microbiol. 132:2775-2780. [Google Scholar]
- 15.Heisenberg, M., and E. Cerdá-Olmedo. 1968. Segregation of heterokaryons in the asexual cycle of Phycomyces. Mol. Gen. Genet. 102:187-195. [DOI] [PubMed] [Google Scholar]
- 16.Howes, C. D., and P. P. Batra. 1970. Accumulation of lycopene and inhibition of cyclic carotenoids in Mycobacterium in the presence of nicotine. Biochim. Biophys. Acta 222:174-179. [DOI] [PubMed] [Google Scholar]
- 17.Kohlmeier, L., J. D. Kark, E. Gomez-Gracia, B. C. Martin, S. E. Steck, A. F. Kardinaal, J. Ringstad, M. Thamm, V. Masaev, R. Riemersma, J. M. Martin-Moreno, J. K. Huttunen, and F. J. Kok. 1997. Lycopene and myocardial infarction risk in the EURAMIC study. Am. J. Epidemiol. 146:618-626. [DOI] [PubMed] [Google Scholar]
- 18.Lampila, L. E., S. E. Wallen, and L. B. Bullerman. 1985. A review of factors affecting biosynthesis of carotenoids by the order Mucorales. Mycopathologia 90:65-80. [DOI] [PubMed] [Google Scholar]
- 19.Mehta, B. J., and E. Cerdá-Olmedo. 1995. Mutants of carotene production in Blakeslea trispora. Appl. Microbiol. Biotechnol. 42:836-838. [Google Scholar]
- 20.Mehta, B. J., and E. Cerdá-Olmedo. 1999. Lycopene cyclization in Blakeslea trispora. Mycoscience 40:307-310. [Google Scholar]
- 21.Mehta, B. J., L. M. Salgado, E. R. Bejarano, and E. Cerdá-Olmedo. 1997. New mutants of Phycomyces blakesleeanus for β-carotene production. Appl. Environ. Microbiol. 63:3657-3661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mehta, B. J., and E. Cerdá-Olmedo. 2001. Intersexual partial diploids of Phycomyces. Genetics 158:635-641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Meissner, G., and M. Delbrück. 1968. Carotenes and retinal in Phycomyces mutants. Plant Physiol. 43:1279-1283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mortensen, A., L. H. Skibsted, and T. G. Truscott. 2001. The interaction of dietary carotenoids with radical species. Arch. Biochem. Biophys. 385:13-19. [DOI] [PubMed] [Google Scholar]
- 25.Murillo, F. J., and E. Cerdá-Olmedo. 1976. Regulation of carotene synthesis in Phycomyces. Mol. Gen. Genet. 148:19-24. [DOI] [PubMed] [Google Scholar]
- 26.Murillo, F. J., I. L. Calderón, I. López-Díaz, and E. Cerdá-Olmedo. 1978. Carotene-superproducing strains of Phycomyces. Appl. Environ. Microbiol. 36:639-642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nara, E., H. Hayashi, M. Kotake, K. Miyashita, and A. Nagao. 2001. Acyclic carotenoids and their oxidation mixtures inhibit the growth of HL-60 human promyelocytic leukemia cells. Nutr. Cancer 39:273-383. [DOI] [PubMed] [Google Scholar]
- 28.Navarro, E., G. Sandmann, and S. Torres-Martínez. 1995. Mutants of the carotenoid biosynthetic pathway of Mucor circinelloides. Exp. Mycol. 19:186-190. [Google Scholar]
- 29.Ninet, L., and J. Renaut. 1979. Carotenoids, p. 529-544. In H. J. Peppler and D. Perlman (ed.), Microbial technology, vol. 1. Academic Press, New York, N.Y.
- 30.Roncero, M. I. G., C. Zabala, and E. Cerdá-Olmedo. 1984. Mutagenesis in multinucleate cells: the effects of N-methyl-N′-nitro-N-nitrosoguanidine on Phycomyces spores. Mutat. Res. 125:195-204. [DOI] [PubMed] [Google Scholar]
- 31.Ruiz-Hidalgo, M. J., M. A. López-Matas, A. Velayos, P. D. Fraser, P. M. Bramley, and A. P. Eslava. 1995. Carotenoid mutants of Mucor circinelloides. Bot. Acta 108:396-400. [Google Scholar]
- 32.Salgado, L. M., E. R. Bejarano, and E. Cerdá-Olmedo. 1989. Carotene-superproducing mutants of Phycomyces blakesleeanus. Exp. Mycol. 13:332-336. [Google Scholar]
- 33.Stahl, W., U. Heinrich, H. Jungmann, H. Sies, and H. Tronnier. 2000. Carotenoids and carotenoids plus vitamin E protect against ultraviolet light-induced erythema in humans. Am. J. Clin. Nutr. 71:795-798. [DOI] [PubMed] [Google Scholar]