Abstract
Although thermostable direct hemolysin (TDH)-producing Vibrio parahaemolyticus has caused many infections in Asian countries, the United States, and other countries, it has been difficult to detect the same pathogen in seafoods and other environmental samples. In this study, we detected and enumerated tdh gene-positive V. parahaemolyticus in Japanese seafoods with a tdh-specific PCR method, a chromogenic agar medium, and a most-probable-number method. The tdh gene was detected in 33 of 329 seafood samples (10.0%). The number of tdh-positive V. parahaemolyticus ranged from <3 to 93/10 g. The incidence of tdh-positive V. parahaemolyticus tended to be high in samples contaminated with relatively high levels of total V. parahaemolyticus. TDH-producing strains of V. parahaemolyticus were isolated from 11 of 33 tdh-positive samples (short-necked clam, hen clam, and rock oyster). TDH-producing strains of V. parahaemolyticus were also isolated from the sediments of rivers near the coast in Japan. Representative strains of the seafood and sediment isolates were examined for the O:K serovar and by the PCR method specific to the pandemic clone and arbitrarily primed PCR and pulsed-field gel electrophoresis techniques. The results indicated that most O3:K6 tdh-positive strains belonged to the pandemic O3:K6 clone and suggested that serovariation took place in the Japanese environment.
Vibrio parahaemolyticus inhabits the marine environment and is a major food-borne pathogen. Strains carrying the tdh gene, encoding the thermostable direct hemolysin (TDH), or the trh gene, encoding the TDH-related hemolysin (TRH), or both genes are considered virulent strains (18).
In recent years, outbreaks of V. parahaemolyticus infection have increased in Japan and Taiwan with the increase in the incidence of the O3:K6 serovar (8, 14). Outbreaks of O3:K6 infection also occurred in the United States, linked to ingestion of raw seafoods (6, 7). The O3:K6 strains were demonstrated to carry the tdh gene but not the trh gene and to be a new clone by an arbitrarily primed PCR (AP-PCR) technique (17, 20). AP-PCR and group-specific PCR (GS-PCR), a PCR method targeting the clone-specific bases in the toxRS operon (17), revealed that the new clone was responsible for pandemic spread to Taiwan, Laos, Japan, Thailand, Korea, and the United States since 1996 (4, 17, 24, 25). Furthermore, serovariants of the pandemic clone belonging to O4:K68, O1:K untypeable, O1:K25, O1:K41, and O4:12 were found (5, 17; M. Nishibuchi, unpublished data).
The new O3:K6 clone, in addition to other virulent strains of V. parahaemolyticus, has rarely been isolated from food and other environmental samples, although many infections with the O3:K6 clone have been epidemiologically linked to consumption of seafoods after 1997 in Japan (14). Virulent strains of V. parahaemolyticus are usually found together with much larger populations of avirulent strains in the environment. The relatively low population of virulent strains in environmental samples (3, 10, 21) and the similarity in growth kinetics of the virulent and avirulent strains have made it difficult to selectively detect and enumerate virulent strains in environmental samples. Therefore, undifferentiated total V. parahaemolyticus instead of virulent V. parahaemolyticus has long been used as an indicator for control of food contamination toward prevention of infection, but the appropriateness of this approach remains uncertain because of the lack of adequate information on the incidence of virulent strains in environmental samples.
To gain insights into the incidence and population of TDH-producing O3:K6 strains in environmental samples, we tried to isolate and enumerate these strains in seafoods marketed in Japan. The most-probable-number (MPN) method (2) and a PCR method for tdh gene detection were used for quantitative analysis, and the isolation method employing a chromogenic agar medium (12) combined with selective enrichment was used for qualitative analysis. We examined primarily molluscan shellfish, because mollusks are filter feeders that accumulate bacteria in aquatic environments and thus are likely be the source of V. parahaemolyticus infection. The sediments of rivers near the coast in Japan were also examined for the prevalence of TDH-producing strains. To investigate whether the strains isolated in this study belong to the pandemic clone, their characteristics were compared with those of the pandemic strains isolated previously from clinical sources in various countries.
MATERIALS AND METHODS
Samples for detection of V. parahaemolyticus.
Seafood samples were collected in the north region (four prefectures), the central region (four prefectures), and the south region (five prefectures) of Japan from June to October 2001. Unless otherwise specified, the seafoods were packed in polyethylene bags and kept in styrene foam boxes with ice. Short-necked clam (Tapes japonica), scallop (Patinopecten yessoenisis), Japanese abalone (Haliotis discus), sea urchin (Echinoidea sp.), rock oyster (Crassostrea nippona), spiny top shells (Turbo cornutus), Japanese razor shell (Solen strictus), underlined fig shell (Ficus subintermedius), pen shell (Atrina pectinata japonica), Tokobushi abalone (Sulculus diversicolor aquatilis), Japanese hard clam (Meretrix lusoria), Japanese oyster (Crassostrea gigas), and hen clam (Mactra sulcatari) samples were purchased from nurseries. The shells were removed aseptically, and the meat was subjected to examination. The meat of hen clams sold without shells at a fresh seafood market was also examined. Common freshwater clams (Corbicula leana) were purchased from retail shops and examined without removing the shells. Horse mackerel (Trachurus japonicus) purchased from retail shops and markets were cut into several portions, and the head with the gills and surface skin was used as the test sample.
Procedure for analysis of tdh-positive V. parahaemolyticus in seafood.
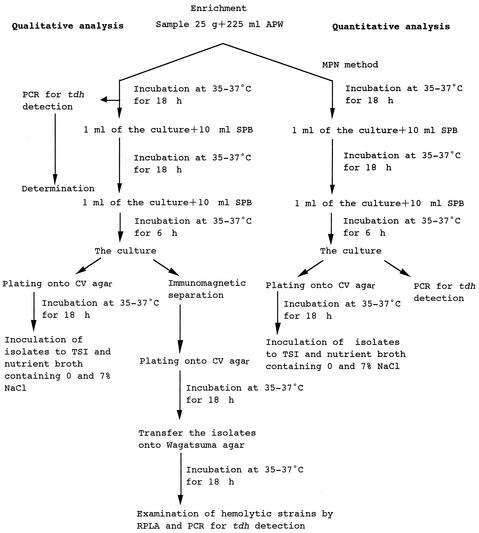
Qualitative and quantitative analyses of tdh-positive V. parahaemolyticus in seafood were carried out as shown schematically in Fig. 1. In the quantitative analysis with an MPN method, a 25-g portion of test sample was mixed with 225 ml of alkaline peptone water (APW; Nissui Co., Tokyo, Japan) in a stomacher bag, which was then gently homogenated with hands. Either 10 ml, 1 ml, or 0.1 ml of the homogenate and 1 ml or 0.1 ml of a 10−2 dilution of the homogenate were inoculated into 10 ml of APW in triplicate and incubated at 35 to 37°C for 18 h. One-milliliter portions of each APW culture were inoculated into 10 ml of salt polymyxin broth (SPB; Nissui Co.) and incubated for 18 h at 35 to 37°C, and then 1 ml of the SPB culture was inoculated into 10 ml of fresh SPB and incubated for 6 h at 35 to 37°C. A portion of the third enrichment culture in SPB was plated onto CHROMagar Vibrio (CV) agar (CHROMagar, Paris, France) (12) for detection of total V. parahaemolyticus by isolation and biochemical identification. Another 1-ml portion of the third enrichment culture served as the material for PCR analysis for detection of tdh-positive V. parahaemolyticus.
FIG. 1.
Schematic representation of qualitative and quantitative analyses of total and tdh-positive V. parahaemolyticus in seafood. APW, alkaline peptone water; SPB, salt polymyxin broth; CV, CHROMagar Vibrio; RPLA, reversed passive latex agglutination test; TSI, triple sugar iron.
For qualitative analysis, the remaining portion of the sample homogenate in APW was incubated at 35 to 37°C for 18 h, and then two further steps of enrichment culture were conducted in the same manner as for the quantitative analysis except that only one tube was used for each enrichment step. During the course of the three-step enrichment cultures, the first enrichment culture in APW was screened for the presence of tdh-positive V. parahaemolyticus by the PCR method. If the PCR analysis showed a positive reaction, portions of the third enrichment culture were plated directly and also after the immunomagnetic separation step (described below) onto CV agar for isolation and identification of tdh-positive V. parahaemolyticus.
In the previous study, we found that repeated enrichment with fresh medium improved detection of V. parahaemolyticus in seafood (12). Preliminary experiments showed that TDH-producing V. parahaemolyticus was isolated from all of five seawater samples by the three-step enrichment, whereas that was isolated from two out of five samples by single-step enrichment, indicating that the three-step enrichment procedure is more effective in detection of TDH-producing V. parahaemolyticus than the one-step enrichment procedure.
Procedure for isolation of tdh-positive V. parahaemolyticus from river sediments.
The following three-step enrichment procedure was employed for isolation of tdh-positive V. parahaemolyticus from river sediments. Each sample (10 g) was added to 100 ml of tryptic soy broth (TSB; Difco, Detroit, Mich.) containing 2% NaCl and cultured for 6 h at 36°C. Ten milliliters of the culture was further cultured with 100 ml of SPB for 18 h at 36°C. The SPB culture (0.5 ml) was again cultured with 10 ml of SPB for 6 h at 36°C. Preliminary experiments showed that the three-step enrichment procedure was superior to the one-step enrichment procedure with SPB; tdh was detected by PCR in four out of eight river water samples by the three-step enrichment, whereas tdh was detected in two out of eight samples by the one-step enrichment. After inoculation of the culture onto thiosulfate citrate bile salts sucrose (Nissui Co.) and incubation for 18 h at 36°C, colonies suspected as V. parahaemolyticus were confirmed for production of TDH on Wagatsuma agar, which is described below.
Identification of V. parahaemolyticus on CV agar.
After inoculation of the enrichment culture, the CV agar plate was incubated at 37°C for 18 h. Purple colonies on the CV agar plate were presumptively identified as V. parahaemolyticus and inoculated into triple sugar iron medium (Eiken Co, Tokyo, Japan), nutrient broth (Difco), and nutrient broth supplemented with 8% NaCl. Test strains showing alkaline slant and acid butt reactions in the triple sugar iron medium, and, in nutrient broth, no growth without and growth with 8% NaCl were identified as V. parahaemolyticus.
Immunomagnetic separation of K6 strains and detection of TDH production.
Since investigations of recent outbreaks have indicated that O3:K6 is a major serotype of TDH-producing V. parahaemolyticus, immunomagnetic separation of K6 strains was performed to improve isolation of TDH-producing organisms (23). A 1-ml portion of the enrichment culture was added to 25 μl of immunomagnetic beads coated with antibody against the V. parahaemolyticus K6 antigen (Denka Seiken, Tokyo, Japan). According to the manufacturer's instructions, the beads were incubated, separated, and then suspended in 0.1 ml of washing buffer. Then 20 ml of the suspension was streaked onto CV agar. After the agar was incubated at 37°C for 18 h, purple colonies presumptively identified as V. parahaemolyticus were examined for TDH production with Wagatsuma agar medium for the Kanagawa phenomenon.
To prepare Wagatsuma agar medium, melted basal medium [yeast extract, 5 g/liter; Bacto-peptone, 10 g/liter; sodium chloride, 70 g/liter; d-(−)-mannitol, 5 g/liter; agar, 15 g/liter; crystal violet, 1 mg/liter; dipotassium hydrogen phosphate, 5 g/liter (pH 7.5)] (Kyokuto Pharmaceutical Ind. Co., Tokyo, Japan) was mixed with a 20% solution of washed human blood (100 ml/liter). The test strains were inoculated onto the Wagatsuma agar plate. After incubation for 18 h at 35 to 37°C, the strain showing a clear zone around the colony was examined for TDH production by an immunological method with anti-TDH antibody. The agglutination test with a reversed passive latex agglutination assay kit (KAP-RPLA; Denka Seiken) was performed according to the manufacturer's specifications. Finally, the presence of the tdh gene in the TDH-producing strains was confirmed by the PCR method described below.
Serotyping.
Serovars of the strains isolated from CV agar and identified as V. parahaemolyticus were determined with a serotyping kit (Denka Seiken) according to the manufacturer's instruction.
PCR.
Enrichment cultures at the first step in APW and third step in SPB and the SPB culture of the isolated strain grown for 18 h at 37°C served as the materials for the PCR assay for tdh detection. One milliliter of the test culture was centrifuged at 5,000 × g for 10 min. After the supernatant was removed, the pellet was resuspended in 0.1 ml of sterilized distilled water and heated at 100°C for 5 min. After centrifugation at 10,000 × g for 10 min, the supernatant was transferred to a new tube and stored at 4°C or −20°C until the PCR assay.
PCR for detection of the tdh gene was performed with primer 1 (5′-GGTACTAAATGGCTGACATC) and primer 2 (5′-CCACTACCACTCTCATATGC) as described by Tada et al. (22). Briefly, the PCR mixture (50 μl) consisted of 10 mM Tris-HCl (pH 8.3), 50 mM KCl, 1.5 mM MgCl2, 0.01 mM EDTA, 0.1 mM dithiothreitol, 0.05% Tween 20, 0.05% Nonidet P-40, 5% glycerol, 0.2 mM each of the four deoxynucleoside triphosphates (dNTP mixture; Takara, Ohtsu, Japan), and 0.5 U of Taq polymerase (Takara Ex Taq; Takara). The amplification conditions were set at one cycle of 96°C for 5 min, followed by 35 cycles of amplification consisting of denaturation at 94°C for 1 min, annealing at 55°C for 1 min, and extension at 72°C for 1 min, followed by one cycle of 72°C for 7 min.
For detection of the toxR gene and detection of the pandemic strains by GS-PCR, the test strains were grown in tryptic soy broth containing 2% NaCl at 37°C for 18 h. One milliliter of the culture was centrifuged at 5,000 × g for 10 min. After the supernatant was removed, the pellet was resuspended in 0.1 ml of sterile distilled water and heated at 100°C for 5 min. After centrifugation at 10,000 × g for 10 min, the supernatant was transferred to a new tube and stored at 4°C or −20°C. The PCRs for toxR detection and for GS-PCR were carried out following the methods described by Kim et al. (16) and by Matsumoto et al. (17), respectively.
AP-PCR.
AP-PCR was carried out according to the method described by Okuda et al. (20) with primer 2 (5′-GTTTCGCTCC) and primer 4 (5′-AAGAGCCCGT).
PFGE analysis.
For pulsed-field gel electrophoresis (PFGE) analysis, the test strain was grown to mid-log phase (optical density of 0.9 to 1.0 at 600 nm) in Luria-Bertani broth containing 1% NaCl. Bacterial cells were harvested by centrifugation (3,000 × g), washed, mixed with 2% agarose, and dispensed into a plug mold as described by Albert et al. (1) except that low-melting-point agarose (Bethesda Research Laboratories, Gaithersburg, Md.) was used. The agarose plug was treated with lysozyme solution, deproteinated, and digested with 50 U of NotI restriction enzyme as described by Chowdhury et al. (9). The digested DNA fragments were separated by the contour-clamped homogeneous electric field method on a CHEF-DRIII system (Bio-Rad Laboratories, Hercules, Calif.) in 1% agarose (pulse field certified agarose; Bio-Rad Laboratories) with 0.5× TBE buffer (44.5 mM Tris-HCl, 44.5 mM boric acid, 1.0 mM EDTA [pH 8.0]). The running conditions were 6 V/cm at 14°C for 24 h at a field angle of 120°, and switch times were 1 to 18 s for 12 h and 3 to 80 s for 12 h. Following electrophoresis, the gels were stained with ethidium bromide (10 μg/ml) for 30 min, destained in distilled water for 1 h, and photographed with a UV transilluminator.
The dendrogram was constructed from the PFGE profiles according to an unweighted pair-group method with arithmetic average (UPGMA) clustering analysis with Dendron software, version 3 (Solltech Inc., Oakdale, La.).
RESULTS
Qualitative analysis: incidence of total and TDH-producing V. parahaemolyticus in seafood.
Three hundred and twenty-nine seafood samples belonging to 13 species of molluscan shellfish (bivalves) and two other seafoods were purchased in various regions in Japan between June and October 2001. The presence of total V. parahaemolyticus was examined for 173 of 329 samples (Table 1). The unknown region means that the samples were purchased at the Central Market in Tokyo but their exact harvest locations could not be identified. Ninety-five percent (n = 165) of the 173 samples were positive. All 329 samples were examined for tdh-positive V. parahaemolyticus in the qualitative analysis (Table 1). PCR analysis of the first enrichment culture in APW of the seafoods revealed the presence of the tdh gene in 33 samples (10.0%): 8 of 137 samples from the north region, 15 of 141 samples from central region, 3 of 27 samples from the south region, and 7 of 24 samples from the unknown region. TDH-producing V. parahaemolyticus was isolated from 11 of 33 tdh-positive samples.
TABLE 1.
Detection of tdh-positive and total V. parahaemolyticus in seafood samples in Japan in 2001
| Region of Japan | Seafood | No. of samples
|
||||
|---|---|---|---|---|---|---|
|
tdh-positive V. parahaemolyticus
|
Total V. parahaemolyticus
|
|||||
| Total | tdh positivea | Positive for TDH-producing strainsb | Total | V. parahaemolyticus isolated | ||
| North | Rock oyster | 78 | 7 | 6 | 1 | 1 |
| Scallop | 29 | 0 | NTc | NT | NT | |
| Japanese abalone | 18 | 0 | NT | NT | NT | |
| Hen clam | 7 | 1 | 0 | 4 | 4 | |
| Sea urchin | 3 | 0 | NT | NT | NT | |
| Spiny top shell | 1 | 0 | NT | NT | NT | |
| Japanese oyster | 1 | 0 | NT | 1 | 0 | |
| Total | 137 | 8 | 6 | 6 | 5 | |
| Central | Hen clam | 97 | 8 | 2 | 76 | 72 |
| Short-neck clam | 32 | 7 | 2 | 30 | 30 | |
| Horse mackerel | 9 | 0 | NT | 8 | 7 | |
| Common freshwater clam | 3 | 0 | NT | 3 | 3 | |
| Total | 141 | 15 | 4 | 117 | 112 | |
| South | Horse mackerel | 8 | 0 | NT | 7 | 6 |
| Spiny top shell | 6 | 1 | 0 | 6 | 5 | |
| Short-neck clam | 4 | 1 | 0 | 4 | 4 | |
| Common freshwater clam | 2 | 0 | NT | 2 | 2 | |
| Japanese hard clam | 2 | 0 | NT | 2 | 2 | |
| Japanese razor shell | 1 | 0 | NT | 1 | 1 | |
| Underlined fig shell | 1 | 0 | NT | 1 | 1 | |
| Japanese oyster | 1 | 0 | NT | 1 | 1 | |
| Pen shell | 1 | 0 | NT | 1 | 1 | |
| Tokobushi abalone | 1 | 1 | 0 | 1 | 1 | |
| Total | 27 | 3 | 0 | 26 | 24 | |
| Unknown | Hen clam | 24 | 7 | 1 | 24 | 24 |
| Total | 329 | 33 | 11 | 173 | 165 | |
APW enrichment culture directly examined by the PCR method.
TDH-producing strains were actually isolated.
NT, not tested.
Quantitative analysis: MPN of total and tdh-positive V. parahaemolyticus in seafood samples.
In a preliminary study, we confirmed the effectiveness of the PCR for the MPN method. The MPN of tdh-positive V. parahaemolyticus was determined by PCR and plating methods with short-neck clam samples which had been inoculated with tdh-positive V. parahaemolyticus. The PCR method gave 2.5-fold lower MPN than the plating method in 1 out of 10 samples, but both methods gave the same MPN values for nine samples, indicating that the two methods have similar sensitivities. Furthermore, a tdh-negative (107 CFU/0.1 ml) and a tdh-positive (102, 103, or 104 CFU/0.1 ml) strain of V. parahaemolyticus were inoculated into the enrichment cultures of short-neck clams and scallops, which contained background bacteria at concentrations of 102 to 103 CFU/ml, and PCR for tdh detection was performed. tdh was detected in all the samples by the three-step enrichment procedure, demonstrating that tdh-positive V. parahaemolyticus can be detected in the presence of a large population of tdh-negative V. parahaemolyticus.
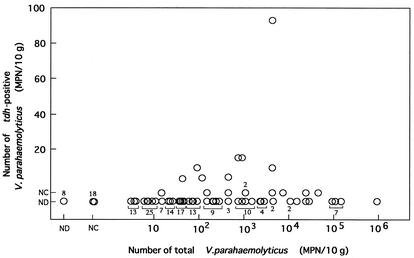
Accordingly, the number of V. parahaemolyticus was estimated by the MPN method described in the Materials and Methods section for 173 samples spanning more than 12 species of molluscan shellfish and one other seafood (details not shown). The total number of V. parahaemolyticus ranged from <3 to >110,000 MPN/10 g (Fig. 2). Sixteen of 173 samples were tdh positive in the qualitative analysis, and therefore these tdh-positive samples were analyzed for the MPN of tdh-positive V. parahaemolyticus (Fig. 2). The numbers of tdh-positive V. parahaemolyticus were <3 MPN/10 g for two hen clam, five short-neck clam, and one Tokobushi abalone sample, 4 to 15 MPN/10 g for four hen clam, one spiny top shell, and two short-neck clam samples, and 93 MPN/10 g for one short-neck clam sample.
FIG. 2.
Relationship between total number of V. parahaemolyticus organisms and number of tdh-positive V. parahaemolyticus organisms in seafood. Each circle indicates the number in each sample. ND, tdh gene not detected by qualitative analysis (<1 CFU/25 g), NC, tdh gene not detected by quantitative analysis (<3 MPN/10 g) but detected by qualitative analysis (>1 CFU/25 g).
The ratio of tdh-positive and total V. parahaemolyticus ranged widely (93 of 4,300 to <3 of 46,000) (Fig. 2). The number of tdh-positive V. parahaemolyticus was not exactly in parallel to that of total V. parahaemolyticus. However, the incidence of tdh-positive V. parahaemolyticus tended to be high in samples contaminated with relatively high levels of total V. parahaemolyticus. The tdh gene was detected in 14 of 55 samples (25.5%) contaminated with total V. parahaemolyticus at equal to or greater than 100 MPN/10 g, whereas only 3 (2.5%) of 118 samples contaminated with total V. parahaemolyticus of less than 100 MPN/10 g were tdh positive.
Effect of clam storage at 30°C for 3 h on detection of tdh gene.
Whether storage of clam samples at a high temperature before examination improves tdh detection was examined. Twenty-two batches of hen clam samples were purchased in the markets between August and October. Two portions were sampled from each of the 22 hen clam samples and subjected to qualitative and quantitative analyses. A portion was transferred immediately from an iced styrene foam box into APW enrichment broth warmed to 37°C, while the other portion of the clam sample was stored at 30°C for 3 h before the APW enrichment step. The tdh gene was detected in three samples by the routine procedure without storage at 30°C for 3 h and in seven samples by the procedure with storage at 30°C for 3 h (Table 2). In all samples except for sample 7, the MPN of total V. parahaemolyticus increased remarkably after storing the samples at 30°C for 3 h.
TABLE 2.
Effect of storage at 30°C for 3 h on detection of tdh gene and the MPN of total and tdh-positive V. parahaemolyticus in hen clam samples
| Sample no. | Sampling date in 2001 | Without storage at 30°C for 3 h
|
After storage at 30°C for 3 h
|
||||
|---|---|---|---|---|---|---|---|
| tdh gene | MPN/10 g
|
tdh gene | MPN/10 g
|
||||
| Total V. parahaemolyticus | tdh-positive V. parahaemolyticus | Total V. parahaemolyticus | tdh-positive V. parahaemolyticus | ||||
| 1 | August 13 | − | 43 | NTa | + | >110,000 | 15 |
| 2 | − | 43 | NT | + | 46,000 | <3 | |
| 3 | + | 1,100 | <3 | + | >110,000 | <3 | |
| 4 | − | 23 | NT | − | 9,300 | NT | |
| 5 | + | 4,300 | <3 | + | >110,000 | 240 | |
| 6 | August 20 | − | 93 | NT | − | 2,100 | NT |
| 7 | − | 750 | NT | + | 460 | 7 | |
| 8 | September 10 | − | 43 | NT | + | 46,000 | 20 |
| 9 | − | 150 | NT | − | 24,000 | NT | |
| 10 | − | 93 | NT | − | 29,000 | NT | |
| 11 | − | 93 | NT | − | 9,300 | NT | |
| 12 | September 17 | − | 21 | NT | − | 210 | NT |
| 13 | September 25 | − | 4 | NT | − | 23 | NT |
| 14 | − | <3 | NT | − | 24,000 | NT | |
| 15 | − | 21 | NT | − | 24,000 | NT | |
| 16 | + | 43 | 3 | + | 3,800 | NT | |
| 17 | October 1 | − | 4 | NT | − | 210 | NT |
| 18 | − | 9 | NT | − | 1,100 | NT | |
| 19 | − | <3 | NT | − | 210 | NT | |
| 20 | − | 7 | NT | − | 21,000 | NT | |
| 21 | − | <3 | NT | − | >1,500 | NT | |
| 22 | October 9 | − | 9 | NT | − | 240 | NT |
NT, not tested.
Characteristics of selected isolates.
The strains isolated from CV agar of the seafood samples and TDH-producing and -nonproducing strains isolated from the river sediment (see the Materials and Methods section for the latter) in Japan were examined for the O:K serovar. Seventeen O3:K6, one O4:K68, and one O1:K25 strain, each isolated from different samples, and the reference strains of the pandemic clone were characterized to examine their relatedness (Table 3). All strains were confirmed to be V. parahaemolyticus by detecting the species-specific toxR gene. Only one strain isolated from rock oyster was negative in the GS-PCR test. Of the strains isolated from Japanese seafood and the river sediment, four strains were atypical in that they lacked the ability to produce TDH but were GS-PCR positive.
TABLE 3.
Characteristics of selected strains of V. parahaemolyticus
| Class | Strain no. | Sample | Origina | Date of isolation | Serotype | toxR gene | tdh geneb | GS-PCR | AP-PCR patternc obtained with:
|
PFGE profiled | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Primer 2 | Primer 4 | ||||||||||
| Japanese environmental samples | VP1152 | Rock oyster | N | 24 July 2001 | O3:K6 | + | + | + | a | i | H |
| VP1153 | Rock oyster | N | 24 July 2001 | O3:K6 | + | + | + | a | i | F | |
| VP1219 | Rock oyster | N | 7 Aug. 2001 | O3:K6 | + | + | + | a | i | F | |
| VP1280 | Rock oyster | N | 30 Aug. 2001 | O3:K6 | + | + | + | a | i | E | |
| VP1281 | Rock oyster | N | 30 Aug. 2001 | O3:K6 | + | + | + | a | i | E | |
| VP1282 | Rock oyster | N | 30 Aug. 2001 | O3:K6 | + | + | − | c | ii | NDe | |
| 1001A44 | Hen clamf | N | 17 Sep. 2001 | O3:K6 | + | − | + | b | i | J | |
| 1001A46 | Hen clamf | N | 17 Sep. 2001 | O3:K6 | + | − | + | b | i | K | |
| VPF01-13 | Hen clam | C | 6 Aug. 2001 | O3:K6 | + | + | + | a | i | ND | |
| VP80 | Hen clam | C | 20 Aug. 2001 | O3:K6 | + | + | + | a | i | F | |
| VPF01-5 | Short-neck clam | C | 16 July 2001 | O3:K6 | + | + | + | a | i | ND | |
| 19-13 | Hen clam | U | 14 Aug. 2001 | O3:K6 | + | + | + | a | i | F | |
| VPF00-18 | Short-neck clam | C | 16 Oct. 2000 | O3:K6 | + | + | + | a | i | G | |
| VPF00-10 | Short-neck clam | C | 2 Oct. 2000 | O3:K6 | + | + | + | a | i | E | |
| APCC VP 00157 | Sediment of river N | N | July 2001 | O1:K25 | + | + | + | a | i | O | |
| APCC VP 00190 | Sediment of river N | N | Aug. 2001 | O4:K68 | + | + | + | a | i | P | |
| APCC VP 00030 | Sediment of river A | N | Dec. 2000 | O3:K6 | + | − | + | b | i | L | |
| APCC VP 00031 | Sediment of river K | N | Dec. 2000 | O3:K6 | + | − | + | b | i | M | |
| APCC VP 9810 | Sediment of river N | N | Aug. 1998 | O3:K6 | + | + | + | a | i | I | |
| Reference strainsg | FIHES98V1-32-4 | Clinical specimen | Japan | 1998 | O3:K6 | + | − | + | b | i | A |
| JKY-VP6 | Clinical specimen | Japan | 1998 | O3:K6 | + | + | + | a | i | B | |
| VP-2 | Clinical specimen | Korea | 1998 | O3:K6 | + | + | + | a | i | C | |
| BE98-2062 | Clinical specimen | USA | 1998 | O3:K6 | + | + | + | a | i | D | |
| KX-V225 | Clinical specimen | Int.travel. | 1996 | O3:K6 | + | + | + | a | i | E | |
| AN-8373 | Clinical specimen | Bangladesh | 1998 | O3:K6 | + | + | + | a | i | E | |
| VP-81 | Clinical specimen | India | 1996 | O3:K6 | + | + | + | a | i | E | |
| DOH958-15 | Clinical specimen | Taiwan | 1997 | O3:K6 | + | + | + | a | i | E | |
| 97LVP2 | Clinical specimen | Laos | 1997 | O3:K6 | + | + | + | a | i | E | |
| VP-47 | Clinical specimen | Thailand | 1998 | O3:K6 | + | + | + | a | i | E | |
| VP-67 | Clinical specimen | Thailand | 1999 | O3:K6 | + | + | + | a | i | E | |
| 4917 | Clinical specimen | Thailand | 1999 | O1:K25 | + | + | + | a | i | N | |
| 5095 | Clinical specimen | Thailand | 1999 | O4:K68 | + | + | + | a | i | P | |
N, northern Japan; C, central Japan; U, unknown; Int. travel., International traveler to Thailand.
Ability to produce TDH was confirmed by detection of TDH by reversed-phase passive latex agglutination test.
The patterns are shown in Fig. 3.
The profiles are shown in Fig. 4A.
ND; not determined because of DNA degradation.
From the same sample.
Matsumoto et al. (17) and M. Nishibuchi (unpublished data).
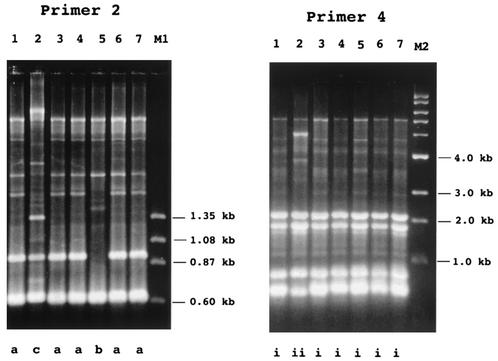
The test strains were compared by two genetic fingerprinting techniques. In the AP-PCR analysis with primer 2, the test strains were classified into three types, arbitrarily designated a, b, and c (Fig. 3; Table 3). The TDH-producing and GS-PCR-positive strains and TDH-nonproducing and GS-PCR-positive strains isolated from Japanese seafood and river sediment belonged to types a and b, respectively. The TDH-producing and GS-PCR-negative O3:K6 strain belonged to type c.
FIG. 3.
Representative AP-PCR patterns exhibited by selected strains. The results obtained with primer 2 and primer 4 are shown as indicated. Lanes: 1, VP1281; 2, VP1282; 3, VPFO1-5; 4, APCCVP9810; 5, 1001A46; 6, VPFOO-10; 7, VP-81; M1, φX174 DNA digested with HaeIII; M2, 1-kb DNA ladder. Arbitrarily designated AP-PCR patterns are indicated at the bottom. The test strains and their AP-PCR patterns are listed in Table 3.
In the AP-PCR analysis with primer 4, the test strains were classified into two types, arbitrarily designated i and ii (Fig. 3; Table 3). The TDH-producing and GS-PCR-negative O3:K6 strain belonged to type ii, but the other strains belonged to type i.
PFGE profiles of the NotI-digested DNA of the test strains were obtained except that those of three strains were not available because of DNA degradation. All available profiles were from GS-PCR-positive strains. These profiles were classified into 15 patterns, arbitrarily designated A to O (Fig. 4A; Table 3). The reference pandemic O3:K6 strains isolated in 1996 to 1999 exhibited one of the five (A to E) profiles. Four isolates from Japanese seafood showed profile E. However, the other seafood isolates and river sediment isolates belonging to the TDH-producing O3:K6 group showed PFGE profiles (F to I) that were different from those of the reference strains. The distribution of the PFGE profiles of the seafood and river sediment isolates did not correlate with their location of isolation.
FIG. 4.
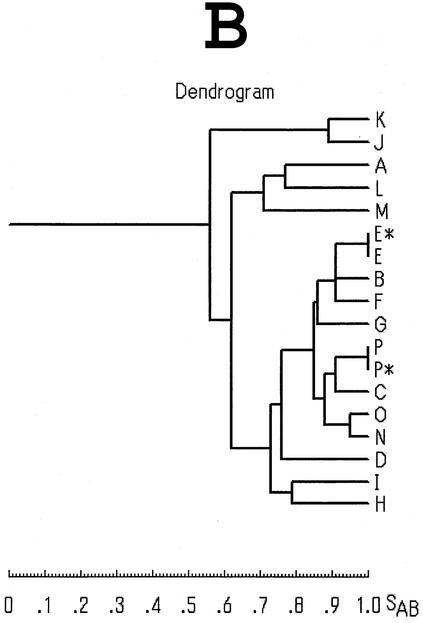
PFGE profiles of selected strains of V. parahaemolyticus and their relations. (A) PFGE profiles of NotI-digested genomic DNAs. The profiles were considered different if they differed by one or more DNA fragment. The profiles were classified into 15 profiles, arbitrarily designated A through P (indicated at the bottom). The asterisk denotes the profile obtained from reference strains. Lanes: M, molecular size markers (DNA size standard maker, lambda ladder; Bio-Rad); 1, FIHES98V1-32-4; 2, JKY-VP6; 3, VP-2; 4, BE98-2062; 5, VP1280; 6, KX-V225; 7, VP80; 8, VPF00-18; 9, VP1152; 10, APCC VP 9810; 11, 1001A44; 12, 1001A46; 13, APCC VP 00030; 14, APCC VP 00031; 15, 4917; 16, APCC VP 00157; 17, APCC VP 00190; 18, 5095. Test strains and their PFGE profiles are listed in Table 3. (B) Dendrogram constructed from the 15 PFGE profiles shown in panel A. The scale for the similarity coefficient is indicated as SAB.
TDH-nonproducing O3:K6 isolates exhibited PFGE profile A and J to M patterns. These profiles were different from those of TDH-positive isolates. The PFGE profiles of O4:K68 and O1:K25 strains isolated from the river sediment (O and P) were the same as or resembled those of the reference strains of the same serovars (N and P) (Table 3; Fig. 4A).
The dendrogram analysis of the PFGE profiles (Fig. 4B) revealed that TDH-negative isolates (profiles A, J, K, L, and M) were rather distant from TDH-positive isolates (all other profiles) and that the TDH-positive strains were closely related to each other regardless of the serovar.
DISCUSSION
Various non-V. parahaemolyticus bacteria distributed in the coastal environment can grow in enrichment media and on selective agar media commonly used for isolation of V. parahaemolyticus. In addition, both virulent and avirulent strains of V. parahaemolyticus inhabit the coastal environment. Previous studies showed that the proportion of virulent strains of V. parahaemolyticus in the coastal environment is very low (10, 15). These make it difficult to isolate virulent strains of V. parahaemolyticus from environmental samples such as seafood, coastal water, and sediments (13). To overcome such difficulties, we used a three-step enrichment procedure, a PCR method for detection of the tdh gene, and new chromogenic agar plates highly specific for V. parahaemolyticus (13). As a result, we detected the tdh gene in 33 (10.0%) of 329 seafood samples (mostly molluscan shellfish) and isolated TDH-producing strains of V. parahaemolyticus from 3.3% of the samples. The result that TDH-producing V. parahaemolyticus was isolated from only one third of tdh-positive samples suggests that the method for isolation of TDH-producing V. parahaemolyticus needs to be improved.
The difficulty in isolating TDH-producing colonies on agar plates may be due at least in part to the fact that the relative proportion of TDH-producing V. parahaemolyticus to total V. parahaemolyticus is extremely low in some samples. A study on the prevalence of V. parahaemolyticus in oysters in the United States suggested that the ratio of tdh-positive V. parahaemolyticus to total V. parahaemolyticus ranged from 1:5.6 to 1:64 (11), while the trial to detect TDH-producing V. parahaemolyticus in foods in Japan revealed only one TDH-producing colony in 1,369 V. parahaemolyticus-like colonies (19). In this study, the tdh gene was not detected even in the samples containing high levels of total V. parahaemolyticus (104 to 106 MPN/10 g; Fig. 2). Therefore, the number of total V. parahaemolyticus does not appear to correlate directly with the number or with the presence of TDH-producing V. parahaemolyticus.
Storing samples at 30°C for 3 h prior to the initial enrichment step resulted in an increase of the detection rate of tdh-positive V. parahaemolyticus with increases in the number of total V. parahaemolyticus (Table 2). This suggests that there may be some conditions in hen clam that are more favorable for the growth or resuscitation of tdh-positive V. parahaemolyticus than the enrichment condition used in this study. Therefore, there is some room to improve the enrichment conditions for isolation of tdh-positive V. parahaemolyticus. The increase in the detection rate of tdh-positive V. parahaemolyticus at a relatively high temperature may explain the increase in V. parahaemolyticus infection in the summer season in Japan (14). Avoiding consumption of raw shellfish with a large number of total V. parahaemolyticus may be effective in reducing the incidence of V. parahaemolyticus infection, although raw shellfish might not always contain adequate numbers of TDH-producing V. parahaemolyticus to cause food poisoning.
Characterization of the strains representing seafood and sediment isolates revealed for the first time that the pandemic strains are distributed throughout the Japanese coastal environment. Of 19 strains examined, 14 strains were tdh positive and GS-PCR positive and showed the same AP-PCR profiles as the reference strains of the pandemic clone. These 14 strains were thus judged to be typical pandemic strains. These strains belonged not only to the O3:K6 serovar but also to two other pandemic serovars (O4:K68 and O1:K25). High-resolution analysis by PFGE showed that three of the O3:K6 pandemic strains isolated from the Japanese environment were indistinguishable from the O3:K6 pandemic strains prevalent in various countries (Table 3, profile E). This result suggests that the O3:K6 strains with profile E may belong to the ancestral pandemic clone. Other O3:K6 pandemic strains isolated from the Japanese environment showed PFGE profiles F to I. These profiles and the PFGE profiles of other pandemic strains of O3:K6 serovar (profiles B to D), O4:K68 serovar (profile P), and O1:K25 serovar (profiles N and O) were closely related to profile E (Fig. 4B).
The present results suggest that the strains showing profiles other than E may have diverged from the ancestral clone (profile E). In contrast, tdh-negative and GS-PCR-positive O3:K6 strains showed profiles (A, J, K, L, and M) that were rather distant from the other profiles (Fig. 4B). The strains showing these profiles were also differentiated from tdh-positive pandemic strains by the AP-PCR pattern. These strains seem to be a variants that diverged from the ancestor of the current pandemic strains. They may have lost the tdh gene at some point during adaptation to the environment and are no longer virulent.
Acknowledgments
This work was supported by a Health Sciences Research Grant from the Ministry of Health, Labor and Welfare, Japan.
REFERENCES
- 1. Albert, M. J., N. A. Bhuiyan, K. A. Talukder, A. S. Faruque, S. Nahar, S. M. Faruque, M. Ansaruzzaman, and M. Rahman. 1997. Phenotypic and genotypic changes in Vibrio cholerae O139 Bengal. J. Clin. Microbiol. 35:2588-2592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. American Public Health Association. 1970. Recommended procedure for the examination of seawater and shellfish, 4th ed. American Public Health Association, Washington, D.C.
- 3.Ayres, P. A., and G. I. Barrow. 1977. The distribution of Vibrio parahaemolyticus in British coastal waters: report of a collaborative study 1975-6. J. Hyg. Camb. 80:281-294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bag, P. K., S. Nandi, R. K. Bhadra, T. Ramamurthy, S. K. Bhattacharya, M. Nishibuchi, T. Hamabata, S. Yamasaki, Y. Takeda, and G. B. Nair. 1999. Clonal diversity among recently emerged strains of Vibrio parahaemolyticus O3:K6 associated with pandemic spread. J. Clin. Microbiol. 37:2354-2357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bhuiyan, N. A., M. Ansaruzzaman, M. Kamruzzaman, K. Alam, N. R. Chouwdhury, M. Nishibuchi, S. M. Faruque, D. A. Sack, Y. Takeda, and G. B. Nair. 2002. Prevalence of the pandemic genotype of Vibrio parahaemolyticus in Dhaka, Bangladesh, and significance of its distribution across different serotypes. J. Clin. Microbiol. 40:284-286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Centers for Disease Control and Prevention. 1998. Outbreak of Vibrio parahaemolyticus infections association with eating raw oysters — Pacific Northwest, 1997. Morb. Mortal. Wkly. Rep. 47:457-462. [PubMed] [Google Scholar]
- 7.Centers for Disease Control and Prevention. 1999. Outbreak of Vibrio parahaemolyticus infection association with eating raw oysters and clams harvested from Long Island Sound—Connecticut, New Jersey, and New York, 1998. Morb. Mortal. Wkly. Rep. 48:48-51. [PubMed] [Google Scholar]
- 8.Chiou, C.-S., S.-Y. Hsu, S.-I. Chiu, T.-K. Wang, and C.-S. Chao. 2000. Vibrio parahaemolyticus serovar O3:K6 as cause of unusually high incidence of food-borne disease outbreaks in Taiwan from 1996 to 1999. J. Clin. Microbiol. 38:4621-4625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chowdhury, N. R., S. Chakraborty, T. Ramamurthy, M. Nishibuchi, S. Yamasaki, Y. Takeda, and G. B. Nair. 2000. Molecular evidence of clonal Vibrio parahaemolyticus pandemic strains. Emerg. Infect. Dis. 6:631-636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.DePaola, A., L. H. Hopkins, J. T. Peeler, B. Wentz, and R. M. McPhearson. 1990. Incidence of Vibrio parahaemolyticus in U.S. coastal water and oysters. Appl. Environ. Microbiol. 56:2299-2302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.DePaola, A., C. A. Kaysner, J. Bowers, and D. W. Cook. 2000. Environmental investigations of Vibrio parahaemolyticus in oyster after outbreaks in Washington, Texas, and New York (1997 and 1998). Appl. Environ. Microbiol. 66:4649-4654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hara-Kudo, Y., T. Nishina, H. Nakagawa, H. Konuma, J. Hasegawa, and S. Kumagai. 2001. An improved detection method for Vibrio parahaemolyticus in seafood. Appl. Environ. Microbiol. 67:5819-5823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hara-Kudo, Y., T. Nishina, K. Sugiyama, A. Saitoh, H. Nakagawa, T. Ichihara, H. Konuma, J. Hasegawa, and S. Kumagai. 2001. Detection of TDH-producing Vibrio Parahaemolyticus O3:K6 from naturally contaminated shellfish with a immunomagnetic separation method and a chromogenic agar medium. Kansenshogaku Zasshi 75:955-960. (In Japanese.) [DOI] [PubMed]
- 14.Infectious Diseases Surveillance Center, National Institute of Infectious Diseases. 1999. Vibrio parahaemolyticus, Japan, 1996-1998. Infect. Agents Surv. Rep. 20:159-160. [Google Scholar]
- 15.Kaysner, C. A., C. Abeyta, R. F. Stott, J. L. Lilja, and M. M. Wekkell. 1990. Incidence of urea-hydrolyzing Vibrio parahaemolyticus in Willapa Bay, Washington. Appl. Environ. Microbiol. 56:904-907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kim, Y. B., J. Okuda, C. Matsumoto, N. Takahashi, S. Hashimoto, and M. Nishibuchi. 1999. Identification of Vibrio parahaemolyticus at the species level by PCR targeted to the toxR gene. J. Clin. Microbiol. 37:1173-1177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Matsumoto, C., J. Okuda, M. Ishibashi, M. Iwanaga, P. Garg, T. Rammamurthy, H. C. Wong, A. Depaola, Y. B. Kim, M. J. Albert, and M. Nishibuchi. 2000. Pandemic spread of an O3:K6 clone of Vibrio parahaemolyticus and emergence of related strains evidenced by arbitrarily primed PCR and toxRS sequence analyses. J. Clin. Microbiol. 38:578-585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nishibuchi, M., and J. B. Kaper. 1995. Thermostable direct hemolysin gene of Vibrio parahaemolyticus: a virulence gene acquired by a marine bacterium. Infect. Immun. 63:2093-2099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Numata, N., M. Ushimizu, M. Ohtomo, K. Chida, H. Fujita, T. Saito, D. Suto, M. Oguro, Y. Hayakawa, K. Sasaki, E. Arakawa, T. Shimada, and H. Watanabe. 2000. The use of colony hybridization in the isolation of thermostable direct hemolysin-producing Vibrio parahaemolyticus from foods implicated in an incidence of food poisoning. Jpn. J. Infect. Dis. 53:75-77. [PubMed] [Google Scholar]
- 20.Okuda, J., M. Ishibashi, S. L. Abbott, J. M. Janda, and M. Nishibuchi. 1997. Analysis of the thermostable direct hemolysin (tdh) gene and the tdh-related hemolysin (tdh) genes in urease-positive strains of Vibrio parahaemolyticus isolated on the west coast of the United States. J. Clin. Microbiol. 35:1965-1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sakazaki, R., K. Tamura, T. Kato, Y. Obara, S. Yamai, and K. Hobo. 1968. Studies on the enteropathogenic, facultatively halophilic bacteria Vibrio parahaemolyticus. III. Enteropathogenicity. Jpn. J. Med. Sci. Biol. 21:325-331. [DOI] [PubMed] [Google Scholar]
- 22.Tada, J., T. Ohashi, N. Nishimura, Y. Shirasaki, H. Ozaki, S. Fukushima, J. Takano, M. Nishibuchi, and Y. Takeda. 1992. Detection of the thermostable direct hemolysin gene (tdh) and the thermostable direct hemolysin-related hemolysin gene (trh) of Vibrio parahaemolyticus by polymerase chain reaction. Mol. Cell. Probes 6:477-487. [DOI] [PubMed] [Google Scholar]
- 23.Tomoyasu, T. 1992. Development of the immunomagnetic enrichment method selective for Vibrio parahaemolyticus serotype K and its application to food poisoning study. Appl. Environ. Microbiol. 58:2679-2682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Vuddhakul, V., A. Chowdhury, V. Laohaprertthisan, P. Pungrasamee, N. Patararungrong, P. Thianmontri, M. Ishibashi, C. Matsumoto, and M. Nishibuchi. 2000. Isolation of a pandemic O3:K6 clone of a Vibrio parahaemolyticus strain from environmental and clinical sources in Thailand. Appl. Environ. Microbiol. 66:2685-2689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wong, H. C., S.-H. Liu, T.-K. Wang, C. L. Lee, C. S. Chiou, D. P. Liu, M. Nishibuchi, and B.-K. Lee. 2000. Characterization of Vibrio parahaemolyticus O3:K6 from Asia. Appl. Environ. Microbiol. 66:3981-3986. [DOI] [PMC free article] [PubMed] [Google Scholar]