Abstract
Survival of Mycobacterium bovis after ingestion by protozoa would provide an environmental reservoir for infection of cattle. We have shown that M. bovis survived ingestion by Acanthamoeba castellanii. In contrast, two strains of M. bovis BCG did not survive well within Acanthamoeba.
Bovine tuberculosis continues to pose an economic problem in Ireland and the United Kingdom. There is strong circumstantial evidence that badgers are a reservoir of the disease (14) and that ingestion of grass contaminated by badger waste is a likely route of infection. The length of survival of Mycobacterium bovis in the environment appears to vary considerably according to environmental conditions, ranging from only a few weeks (6, 24) to a year or more (18, 26). However, there is scant understanding of how M. bovis survives in an environment to which it is ill adapted. Ingestion of M. bovis by protozoa might provide a protected environmental niche, enabling extended survival in the soil (4).
Since the discovery that Legionella pneumophila can infect and replicate in amoebae (21), there has been increasing interest in the role of protozoa (especially Acanthamoeba spp.) in the environmental survival of pathogenic bacteria (4, 7, 8, 16, 17, 23, 25). Mycobacterium leprae and Mycobacterium avium have been shown to survive within protozoa (4, 5, 15, 22), providing protection against adverse conditions (19). However, there is no published evidence that M. bovis can survive ingestion by amoebae.
The purpose of this study was to test the survival of M. bovis in a model amoebal species, Acanthamoeba castellanii (Neff CCAP 1501/1A), which was maintained as axenic monolayers at 15°C in proteose-peptone-glucose medium (PPG). PPG is composed of 15 g of proteose-peptone, 18 g of d-glucose, and 1 liter of Page amoeba saline (PAS) (20). For bacterial infection, amoebal monolayers at approximately 90% confluency were resuspended, and viable counts of amoebae were determined by trypan blue exclusion in an improved Neubauer hemocytometer (11). Amoebae were added in 0.5 ml of PPG to each well of flat-bottom 24-well tissue culture plates (Nunc). For electron microscopy, monolayers were adhered to a piece of Melinex film in the tissue culture plate wells.
After 2 h of incubation at 15°C, the PPG broth was removed and replaced with 0.5 ml of a bacterial suspension of M. bovis NCTC 10772 and two strains of M. bovis BCG (Pasteur, ATCC 35734; and Japan, laboratory collection), grown at 37°C to the log phase in Middlebrook 7H9 broth supplemented with 5% (vol/vol) OADC (oleic acid, albumin, dextrose, catalase enrichment; Becton Dickinson), 0.05% (vol/vol) Tween 80, and 4.1 g of sodium pyruvate per liter (for M. bovis) or 0.2% (vol/vol) glycerol (for BCG). The identity of the BCG strains was checked with IS6110 (9). Numbers of bacteria were estimated by optical density at 600 nm, and the cultures were resuspended in PAS for infection of Acanthamoeba at a multiplicity of infection of approximately 10, which was shown in preliminary experiments to achieve effective infection of the amoebal monolayer. The plates were then reincubated for 24 h to allow ingestion of the mycobacteria, which was confirmed by transmission electron microscopy.
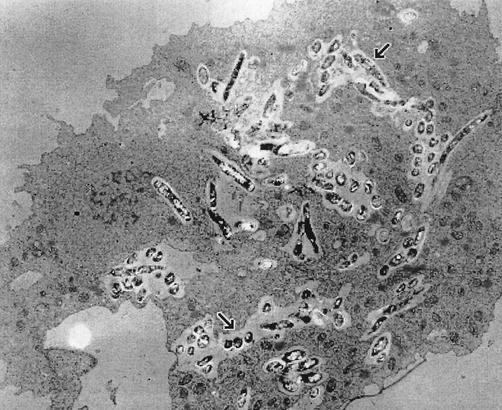
For electron microscopy, the monolayer was fixed by adding 25% glutaraldehyde to a concentration of 2.5% for 30 min and then washed in 0.1 M cacodylate buffer (pH 7.4). Postfixing was carried out in 2% osmium tetroxide in 0.1 M cacodylate buffer for 1 h. The samples were then dehydrated through increasing concentrations of ethanol (30, 50, 70, 90, and 100%), followed by ethanol-propylene oxide (1:1), propylene oxide, propylene oxide-Epon 812 (1:1), and finally Epon 812, with the Melinex film applied to the resin so that the cell monolayer was facing down. After polymerization of the resin, thin sections were cut, stained with lead citrate and uranyl acetate, mounted on copper grids, and viewed with a transmission electron microscope (Philips 400T). The results (Fig. 1) confirmed that mycobacteria had been ingested by the protozoa. Further results (not shown) obtained with a strain expressing green fluorescent protein, visualized by fluorescence microscopy, showed the presence of bacteria within vacuoles after as little as 30 min of coincubation
FIG. 1.
Electron microscopy of M. bovis BCG within A. castellanii. Samples were prepared for transmission electron microscopy after 3 h of coincubation. The arrows show examples of mycobacteria within vacuoles.
To determine the survival of mycobacteria after ingestion by the amoebae, the monolayers were washed in PAS and treated with 100 μg of amikacin per ml for 2 h to remove any extracellular or adherent bacteria (2); the short period of amikacin treatment makes it unlikely to have any persistent effect on the amoebae. Amikacin was removed by washing with PAS, and the monolayers were then incubated in 0.5 ml of PAS at 15°C, a temperature that is characteristic of soils in temperate regions during the summer months, but does not permit growth of M. bovis. Preliminary experiments established that this combination of washing and treatment with amikacin reduced the levels of extracellular bacteria at least 105-fold. At intervals, a set of three wells was treated by removing the PAS and washing the monolayers twice with 0.5 ml of PAS. Intracellular mycobacteria were released by lysing the monolayers with 0.5 ml of 0.5% sodium dodecyl sulfate, followed by passage through 27-gauge needles twice to ensure breakage of the amoebae. Preliminary experiments, counting viable numbers of amoebae in a hemocytometer as described above, established that this procedure resulted in 100% lysis of trophozoites and 85.7% ± 7.1% lysis of encysted amoebae.
Viable counts of mycobacteria in the lysate were determined on Middlebrook 7H11 agar containing 5% (vol/vol) OADC and either 4.1 g of sodium pyruvate per liter (for M. bovis) or 1% (vol/vol) glycerol (for BCG).
Figure 2A shows that M. bovis survived ingestion by the amoebae and persisted for the duration of the experiment, with more than 90% being recovered at day 14 compared to the number recovered at day 0 (immediately after amikacin treatment). In contrast, viable numbers of both M. bovis BCG strains had already fallen by day 1; the proportion surviving was significantly less than that of M. bovis (P = 0.005). The numbers of viable BCG continued to decrease over the course of the experiment. By day 14, the recovery of viable M. bovis BCG Pasteur was <1 in 104, while the numbers of M. bovis BCG Japan were below the limit of detection. There was no statistically significant difference between the levels of survival of the two BCG strains. In a control experiment in PAS, in the absence of amoebae (Fig. 2B), there was no statistically significant difference between the levels of survival of M. bovis and BCG (P > 0.05) until day 8.
FIG. 2.
Survival of mycobacteria within A. castellanii at 15°C. Time zero represents 24 h after infection, immediately after treatment with amikacin. Each bar represents the mean of triplicate wells, and error bars show the standard error of the mean. Approximately 3 × 106 amoebae were added to each monolayer. (A) Survival in the presence of amoebae. (B) Survival in PAS.
These results were confirmed by a further experiment (results not shown) using a second strain of M. bovis (field isolate 2122/97, obtained from G. Hewinson, Veterinary Laboratories Agency, Weybridge, United Kingdom), which was indistinguishable from M. bovis NCTC 10772 in its survival within amoebae, while the recovery of BCG from amoebae again declined rapidly.
Observation of the monolayers with an inverted light microscope revealed that the amoebae started to form cysts after 48 h, and by 14 days, approximately 50% of the amoebae had encysted. Since the lysis method was only 85% effective for cysts, the numbers of viable intracellular bacteria after 48 h of incubation may be somewhat higher than indicated. Nevertheless, the recovery of viable M. bovis from amoebae in the late stages of the experiment, when most of the amoebae had encysted, indicates that M. bovis is able to survive encystment of the amoebae. Since amoebal cysts have been found to protect bacteria from a variety of physical and chemical stresses (1, 12, 13, 19), it seems likely that survival of M. bovis in cysts will confer similar protection.
The demonstration that M. bovis can survive ingestion by amoebae suggests that protozoa could significantly enhance the survival of M. bovis in the soil and hence may be instrumental in the transmission of bovine tuberculosis. A full understanding of the potential role of amoebae in this respect will require investigation of the uptake and survival of M. bovis in soil microcosms in the presence of mixed populations of bacteria.
An unexpected aspect of the data is the apparent disparity in the abilities of M. bovis itself and the BCG vaccine strains to survive within A. castellanii. Although a number of differences in genome structure between M. bovis and M. bovis BCG have been identified (3, 10), the reasons for the attenuation of BCG are still not understood. Further studies are needed to confirm the association between the attenuation of BCG and its inability to survive within amoebae and to understand the reasons for this effect, which would be invaluable information for attempts to develop an improved vaccine against tuberculosis.
Acknowledgments
S.J.T. was supported by a studentship from the Department for Environment, Food and Rural Affairs (DEFRA), formerly the Ministry of Agriculture, Fisheries and Food (MAFF).
We thank Glyn Hewinson (Veterinary Laboratories Agency, Weybridge, United Kingdom) for the kind provision of M. bovis 2122/97. We are grateful to Jenny Mullerwy, Miloslav Dobrota, and Tony Chamberlain for assistance with electron microscopy.
REFERENCES
- 1.Barker, J., M. R. W. Brown, P. J. Collier, I. Farrell, and P. Gilbert. 1992. Relationship between Legionella pneumophila and Acanthamoeba polyphaga: physiological status and susceptibility to chemical inactivation. Appl. Environ. Microbiol. 58:2420-2425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bermudez, L. E., and L. S. Young. 1994. Factors affecting invasion of HT-29 and HEp-2 epithelial cells by organisms of the Mycobacterium avium complex. Infect. Immun. 62:2021-2026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brosch, R., S. V. Gordon, K. Eiglmeier, T. Garnier, F. Tekaia, E. Yeramian, and S. T. Cole. 2000. Genomics, biology, and evolution of the Mycobacterium tuberculosis complex, p. 19-36. In G. F. Hatfull and W. R. Jacobs, Jr. (ed.), Molecular genetics of mycobacteria. ASM Press, Washington, D.C.
- 4.Brown, M. R. W., and J. Barker. 1999. Unexplored reservoirs of pathogenic bacteria: protozoa and biofilms. Trends Microbiol. 7:46-50. [DOI] [PubMed] [Google Scholar]
- 5.Cirillo, J. D., S. Falkow, L. S. Tompkins, and L. E. Bermudez. 1997. Interaction of Mycobacterium avium with environmental amoebae enhances virulence. Infect. Immun. 65:3759-3767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Duffield, B. J., and D. A. Young. 1985. Survival of Mycobacterium bovis in defined environmental conditions. Vet. Microbiol. 10:193-197. [DOI] [PubMed] [Google Scholar]
- 7.Essig, A., M. Heinemann, U. Simnacher, and R. Marre. 1997. Infection of Acanthamoeba castellanii by Chlamydia pneumoniae. Appl. Environ. Microbiol. 63:1396-1399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fields, B. S. 1996. The molecular ecology of legionellae. Trends Microbiol. 4:286-290. [DOI] [PubMed] [Google Scholar]
- 9.Fomukong, N. G., J. W. Dale, T. W. Osborn, and J. M. Grange. 1992. Use of gene probes based on the insertion sequence IS986 to differentiate between BCG vaccine strains. J. Appl. Bacteriol. 72:126-133. [DOI] [PubMed] [Google Scholar]
- 10.Gordon, S. V., K. Eiglmeier, R. Brosch, T. Garnier, N. Honore, B. G. Barrell, and S. T. Cole. 1999. Genomics of Mycobacterium tuberculosis and Mycobacterium leprae, p. 93-109. In C. Ratledge and J. W. Dale (ed.), Mycobacteria: molecular biology and virulence. Blackwell Science, Oxford, United Kingdom.
- 11.Holden, E. P., H. H. Winkler, D. O. Wood, and E. D. Leinbach. 1984. Intracellular growth of Legionella pneumophila within Acanthamoeba castellanii Neff. Infect. Immun. 45:18-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kilvington, S., and J. Price. 1990. Survival of Legionella pneumophila within cysts of Acanthamoeba polyphaga following chlorine exposure. J. Appl. Bacteriol. 68:519-525. [DOI] [PubMed] [Google Scholar]
- 13.King, C. H., E. B. Shotts, Jr., R. E. Wooley, and K. G. Porter. 1988. Survival of coliforms and bacterial pathogens within protozoa during chlorination. Appl. Environ. Microbiol. 54:3023-3033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Krebs, J. R., R. M. Anderson, T. Clutton-Brock, C. A. Donnelly, S. Frost, W. I. Morison, R. Woodroffe, and D. Young. 1998. Badgers and bovine TB: conflicts between conservation and health. Science 279:817-818. [DOI] [PubMed] [Google Scholar]
- 15.Krishna-Prasad, B. N., and S. K. Gupta. 1978. Preliminary report on the engulfment and retention of mycobacteria by trophozoites of axenically grown Acanthamoeba castellanii Douglas, 1930. Curr. Sci. India 47:245-247. [Google Scholar]
- 16.Landers, P., K. G. Kerr, T. J. Rowbowtham, J. L. Tipper, P. M. Keig, E. Ingham, and M. Denton. 2000. Survival and growth of Burkholderia cepacia within the free-living amoeba Acanthamoeba polyphaga. Eur. J. Clin. Microbiol. 19:121-123. [DOI] [PubMed] [Google Scholar]
- 17.Ly, T. M. C., and H. E. Muller. 1990. Ingested Listeria monocytogenes survive and multiply in protozoa. J. Med. Microbiol. 33:51-54. [DOI] [PubMed] [Google Scholar]
- 18.Maddock, E. C. G. 1933. Studies on the survival time of the bovine tubercle bacillus in soil, soil and dung, in dung and on grass, with experiments on the preliminary treatment of infected organic matter and the cultivation of the organism. J. Hyg. 33:103-117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Miltner, E. C., and L. E. Bermudez. 2000. Mycobacterium avium grown in Acanthamoeba castellanii is protected from the effects of antimicrobials. Antimicrob. Agents Chemother. 44:1990-1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Page, F. C. 1967. Taxonomic criteria for limax amoebae, with description of 3 new species of Hartmannella and 3 of Vahlkamfia. J. Protozool. 14:499-521. [DOI] [PubMed] [Google Scholar]
- 21.Rowbowtham, T. J. 1980. Preliminary report on the pathogenicity of Legionella pneumophila for freshwater and soil amoebae. J. Clin. Pathol. 33:1179-1183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Steinert, M., K. Birkness, E. White, B. Fields, and F. Quinn. 1998. Mycobacterium avium bacilli grow saprozoically in coculture with Acanthamoeba polyphaga and survive within cyst walls. Appl. Environ. Microbiol. 64:2256-2261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Strahl, E. D., G. E. Gillaspy, and J. O. Falkinham III. 2001. Fluorescent acid-fast microscopy for measuring phagocytosis of Mycobacterium avium, Mycobacterium intracellulare, and Mycobacterium scrofulaceum by Tetrahymena pyriformis and their intracellular growth. Appl. Environ. Microbiol. 67:4432-4439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tanner, M., and A. L. Michel. 1999. Investigation of the viability of M. bovis under different environmental conditions in the Kruger National Park. Onderstepoort J. Vet. Res. 66:185-190. [PubMed] [Google Scholar]
- 25.Thom, S., D. Warhurst, and B. S. Drasar. 1992. Association of Vibrio cholerae with fresh water amoebae. J. Med. Microbiol. 36:303-306. [DOI] [PubMed] [Google Scholar]
- 26.Williams, R. S., and W. A. Hoy. 1930. The viability of B. tuberculosis (bovinus) in pasture land, in stored faeces and in liquid manure. J. Hyg. 30:413-419. [DOI] [PMC free article] [PubMed] [Google Scholar]