Abstract
Batch cultures of aquatic bacteria and dissolved organic matter were used to examine the impact of carbon source concentration on bacterial growth, biomass, growth efficiency, and community composition. An aged concentrate of dissolved organic matter from a humic lake was diluted with organic compound-free artificial lake water to obtain concentrations of dissolved organic carbon (DOC) ranging from 0.04 to 2.53 mM. The bacterial biomass produced in the cultures increased linearly with the DOC concentration, indicating that bacterial biomass production was limited by the supply of carbon. The bacterial growth rate in the exponential growth phase exhibited a hyperbolic response to the DOC concentration, suggesting that the maximum growth rate was constrained by the substrate concentration at low DOC concentrations. Likewise, the bacterial growth efficiency calculated from the production of biomass and CO2 increased asymptotically from 0.4 to 10.4% with increasing DOC concentration. The compositions of the microbial communities that emerged in the cultures were assessed by separation of PCR-amplified 16S rRNA fragments by denaturing gradient gel electrophoresis. Nonmetric multidimensional scaling of the gel profiles showed that there was a gradual change in the community composition along the DOC gradient; members of the β subclass of the class Proteobacteria and members of the Cytophaga-Flavobacterium group were well represented at all concentrations, whereas members of the α subclass of the Proteobacteria were found exclusively at the lowest carbon concentration. The shift in community composition along the DOC gradient was similar to the patterns of growth efficiency and growth rate. The results suggest that the bacterial growth efficiencies, the rates of bacterial growth, and the compositions of bacterial communities are not constrained by substrate concentrations in most natural waters, with the possible exception of the most oligotrophic environments.
Dissolved organic matter (DOM) is the dominant pool of reduced carbon in most aquatic ecosystems, and bacterial respiration during DOM utilization is the major component of total respiration in many environments (14). The amount of bacterial biomass carbon produced per unit of carbon substrate utilized (i.e., the bacterial growth efficiency [BGE]) determines to what extent bacterial metabolism results in biomass that can be utilized in the phagotrophic food chain and to what extent biomass is directly dissipated through the mineralization of organic matter.
Only a minor fraction of DOM is directly available for utilization by heterotrophic microorganisms (43), and intrinsic factors, such as the thermodynamic energy content of the bioavailable DOM, may influence BGE (52). Estimates of BGE for natural planktonic bacteria growing on surface water DOM range from <0.4% to as high as 80% (6, 8, 12, 25, 33, 51). BGE tends to be high in eutrophic environments and decreases with increasing oligotrophy. This has been hypothesized to be due to respiration of a higher proportion of the organic substrates utilized when the available energy resource is scarce compared to the respiration in environments rich in organic substrates (14). However, this conclusion was based on a cross-system comparison in which the variation in DOM quality between different systems was not taken into account. Possibly, the average substrate quality (e.g., thermodynamic energy content and bioavailability) of the DOM is higher in eutrophic systems than in oligotrophic systems, and this could be an alternative explanation for the observed differences in BGE.
Microbial communities may respond to a varying supply of substrates either by physiological adaptation or by changes in the community composition. Even small additions of organic substrates may trigger a shift in the composition of the microbial community and an accompanying change in the relative abundance of specific hydrolytic ectoenzymes (38). Furthermore, van Hannen et al. (55) showed that the quality of DOM influenced the bacterial community structure in dilution cultures receiving detritus from either Oscillatoria limnetica or Ankistrodesmus falicatus. These studies clearly showed that the quality and supply of organic substrates may affect the function as well as the composition of heterotrophic microbial communities.
To test whether the previously observed decrease in growth efficiency with decreasing productivity occurs irrespective of intrinsic differences in DOM quality, we studied the BGE, the bacterial growth rate (BGR), and the carrying capacity, as well as the bacterial community composition, of cultures containing different concentrations of a qualitatively identical source of recalcitrant lake water DOM. Our results suggest that the concentration of natural organic carbon supporting bacterial growth and respiration does not influence BGE, BGR, and bacterial community composition at the concentrations of bioavailable DOC typical of most freshwater and coastal marine water environments.
MATERIALS AND METHODS
Sampling and experimental design.
Bacterial biomass and CO2 levels were monitored over time in batch cultures with different initial concentrations of DOC. Surface water from a humic lake in southern Sweden (Lindhultsgölen [for a description see reference 5]) was concentrated in 1998 by using a RealSoft PROS/2S system, as described by Serkiz and Perdue (42). The DOC was subsequently aged for 2 years in the dark at 4°C, and this was followed by removal of inorganic constituents and DOC with nominal molecular masses less than 1,000 Da by tangential flow ultrafiltration (45). The pH was adjusted to 7.0 with NaOH, and the resulting high-molecular-weight DOM stock solution was filter sterilized through a 0.2-μm-pore-size Supor membrane filter (Gelman). The final DOC concentration was 16.7 mM. The rationale behind using an aged DOM stock was that refractory DOM is the most abundant organic material in aquatic systems. In addition, the use of recalcitrant organic matter with minimal concentrations of labile components should have minimized the risk of bias introduced by transformations that were not directly related to the experimental treatments.
The DOM stock solution was diluted with Milli-Q water (Millipore) to obtain final concentrations of aged DOC ranging from 0.15 to 2.53 mM. Inorganic micro- and macronutrients were added from a stock solution described by Lehman (26) to make carbon the sole limiting factor. Nitrogen and phosphorus were added at final concentrations of 70 μM NH4 N and 3.2 μM PO4 P. The highest bacterial C biomass measured in the experiment corresponded to utilization of at most 1.4 μM N and 0.07 μM P, assuming that the average molar C/N/P ratio is 34:9.2:1 for bacterial cells (56). A parallel control without addition of the aged DOM concentrate showed that extraneous carbon from the Milli-Q water and the various treatments involved in our experimental setup contributed 0.04 mM DOC to the total DOC in the incubation mixtures. This corresponded to 1.9 to 25.9% of the DOC that was attributable to extraneous carbon at the different dilutions of the aged DOM concentrate.
Acid-washed glass bottles (1.2 liters) were completely filled with no headspace and subsequently sealed with Teflon-coated butyl rubber stoppers. Each replicate consisted of two bottles connected by Teflon tubing. Three replicates were set up for each of the seven different DOC concentrations (0.04, 0.15, 0.28, 0.54, 0.83, 1.27, and 2.53 mM). The bacterial inoculum was composed of a mixture of two contrasting waters, one from the outlet of oligotrophic Lake Vättern in southern Sweden (58°28′0"N, 14°55′2"E) and the other from a humic matter-rich inlet to this lake, in order to ensure that there was a diverse bacterial inoculum which was capable of degrading both autochthonous and allochthonous DOM. The mixed sample was sequentially filtered through two glass fiber filters with a nominal pore size of 0.7 μm (Whatman GF/F) to remove large particles and eukaryotic organisms. Ten milliliters of this bacterial inoculum was injected into each bottle, and the resulting batch cultures were incubated in the dark at 20°C.
Sampling was performed by injecting filter-sterilized medium into one of the two connected bottles and simultaneously removing a sample from the other bottle (2). The resulting dilution factor during one sampling event was less than 0.3% (vol/vol) (final concentration). Samples were withdrawn over a 10-day period at 20- to 28-h intervals.
Analysis of DOC and carbon dioxide.
DOC samples were obtained at the start and at the end of the experiment. Water samples were filtered through acid-rinsed 0.2-μm-pore-size Supor membrane filters (Gelman) by using acid-rinsed syringes and filter holders (58). Filtered water (6 ml) was acidified with 100 μl of 1.2 M HCl and purged with CO2-free air to remove inorganic carbon. Samples were immediately analyzed by high-temperature catalytic oxidation and infrared detection with a Shimadzu TOC-5000 instrument (5).
The total inorganic carbon was analyzed by analyzing CO2 with a gas chromatograph (Shimadzu GC-14A) after acidification and headspace equilibration (34). The concentration of CO2 was calculated by linear regression of the calibration standards Na2CO3 and NaHCO3 (R2 = 0.98, n = 18).
Analysis of bacterial growth and growth efficiency.
Subsamples were preserved with borax-buffered, filtered (pore size, 0.2 μm) formaldehyde (final concentration, 2% [vol/vol]) for analysis of bacterial cell number by flow cytometry after Syto 13 nucleic acid staining (13) with minor modifications from the original protocol (15). Selected samples were also counted microscopically after staining with acridine orange (21). At least 12 fields or 200 cells were counted. The flow cytometric counts were slightly less than the microscopic counts (slope = 0.87, R2 = 0.94, n = 8).
During the stationary phase, cell size was determined by fluorescence microscopy and image analysis of acridine orange-stained cells with a Nikon microscope equipped with a digital camera (IDXM 1200 Nikon) and a ×100 PlanFluor objective. Acquired images were transmitted to a digital grabber program (ACT 1) and transferred to the Easy Image Analyzer 2000 software for image analysis. The length (L) and width (w) of 70 to 250 cells were recorded for each sample. Acridine orange-stained particles with areas less than 0.012 μm2 were excluded from the analysis. Cell volumes (V) (in cubic micrometers) were calculated with the formula V = 4Π(w/2)3/3 + (L − w)Π(w/2)2, based on the assumption that the approximate form of each cell is a cylinder with hemispherical end caps. Biovolumes were converted to bacterial biomass by using the volume-to-dry weight relationship mb = 435 × V0.86, where mb is the dry weight of a bacterial cell (in femtograms) (28). Carbon was assumed to comprise 50% of the bacterial dry weight. The accumulated bacterial biomass was determined in the stationary phase, which was typically reached between 7 and 9 days after inoculation of the cultures. For a few replicates, in which the bacterial abundance declined towards the end of the stationary growth phase, the maximum bacterial abundance was used to calculate the yield.
The bacterial exponential growth phase was identified visually as the linear part of a plot of the natural logarithm of bacterial abundance versus incubation time (30). The exponential growth rate was calculated from linear regression of the plot of the natural logarithm of bacterial abundance versus time by using at least three data points covering at least 40 h of incubation.
BGE was estimated from the change in bacterial biomass (Δbiomass) and the change in CO2 content (ΔCO2) by using the following equation: BGE = Δbiomass/(Δbiomass + ΔCO2).
DNA extraction and denaturing gradient gel electrophoresis (DGGE) analysis.
At the end of the experiment, cells from 0.4 to 0.8 liter of water from each replicate were collected on separate 0.22-μm-pore-size Durapore filters (Millipore). Cells were treated with lysozyme (1 mg/ml, 1 n, 37°C), and genomic DNA was extracted and purified by standard protocols (3). The extracted DNA from the mixed bacterial community was used as a template for PCR amplification of a ∼500-bp fragment of the 16S ribosomal DNA gene (Escherichia coli positions 907 to 1406) by using the universal primers GM5F-GC clamp and DS907R (48). PCR was performed in 50-μl mixtures by using a Minicycler (MJ Research) and the following program: 5 min at 95°C, followed by 30 cycles of 30 s at 95°C, 1 min of annealing at 55°C, and 1 min of primer extension at 72°C and then a final extension of 7 min at 72°C. Each tube contained 1 to 2 ng of target DNA, 25 pmol of each primer, 1× Gene Amp PCR buffer, 3 mM MgCl2, each deoxynucleoside triphosphate at a concentration of 200 μM, 0.01% bovine serum albumin, and 0.25 U of AmpliTaq DNA polymerase (Applied Biosystems).
DGGE was performed in a 6.5% (wt/vol) polyacrylamide gel with a 25 to 70% denaturing gradient of formamide and urea in 1× TAE buffer (20 mM Tris, 10 mM acetate, 0.5 mM Na2EDTA; pH 7.4). Equal amounts of PCR product (∼160 ng) were applied to individual lanes and separated on the gels at 61°C by applying 200 V for 6 h. The gels were stained with SYBR GOLD (Molecular Probes) used according to the manufacturer's instructions, and nucleic acids were detected by UV transillumination.
To investigate the degree of dissimilarity (relative distance) between bacterial communities that developed at different DOC dilutions, the DGGE patterns were converted to a binary (0/1) matrix (53). A distance matrix (Dxy) was calculated from the binary data with the equation Dxy = 1 − (2Nxy/Nx + Ny), where Nx and Ny represent the number of bands present either in sample x or y, respectively, and Nxy is the number of bands present in both samples (54). Semistrong hybrid nonmetric multidimensional scaling (NMDS) was performed by using Statistica for Windows (Statsoft) to reduce the multidimensional similarity matrix of the different treatments to one dimension. This procedure transforms the band pattern of every sample to one point in the Euclidean plane. Hence, samples with similar band patterns appear to be close together in the plot (54, 55), and relative differences in community structure for the different dilutions could be visualized. With the reduction to one dimension, it was possible to study the relationship between the degree of community dissimilarity and other parameters, such as DOC concentration, BGE, and BGR.
Clone library, restriction fragment length polymorphism, and sequence analysis.
DGGE bands were excised from a gel and placed in sterile water at 4°C overnight. Aliquots of the water containing DGGE fragments were used as templates in a second PCR amplification performed as described above. The resulting PCR product was purified by separation on a 2% agarose gel, followed by extraction with a QIAquick gel extraction kit (Qiagen, Hilden, Germany). The PCR products were cloned with a TOPO TA cloning kit for sequencing (Invitrogen) used according to the manufacturer's instructions. At least five clones from each band were lysed by heating at 98°C for 10 min, and this was followed by centrifugation at 10,000 × g for 5 min to remove the cell debris. The supernatants were used as templates for PCR amplification of the insert with external (vector) primers M13f and M13r by using the PCR conditions described above.
The PCR products were screened by restriction fragment length polymorphism analysis by simultaneous digestion with two tetrameric restriction enzymes (HhaI and HaeIII [Invitrogen]) for 16 h at 37°C. The fragments were separated on a 2% agarose gel and were subsequently detected by ethidium bromide staining and UV transillumination. All clones with unique restriction fragment length polymorphism patterns were sequenced bidirectionally by using an ABI 3700 DNA sequencer, primers M13f and M13r, and a BigDye terminator kit (v. 3.1; Applied Biosystems). The sequences recovered were aligned with known sequences in the GenBank database by using BLAST (basic local alignment search tool) (National Center for Biotechnology Information) (1). The sequences for the different clones were aligned by using the Pileup software of the Wisconsin Package (Genetics Computer Group).
Nucleotide sequence accession numbers.
All nucleotide sequence data have been deposited in the GenBank database under accession numbers AF550593 to AF550606.
RESULTS
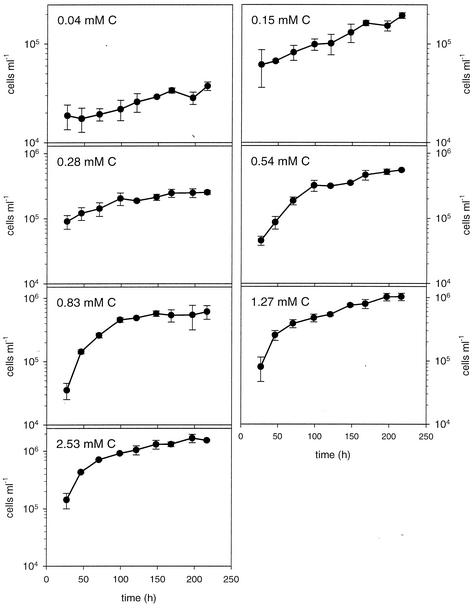
An exponential growth phase was identified for bacteria cultivated at all DOC concentrations and was over before the fifth day of incubation for all cultures except the controls (Fig. 1). In general, growth leveled off before the end of the experiment; the only exceptions were the controls without added DOM and the treatment that received the lowest DOC, in which cell abundance continued to increase at a low rate. Between 1.9 and 8.7% of the total DOC was utilized by bacteria in the cultures when aged DOM was added, whereas the fraction was 25.9% for the controls containing only extraneous DOC.
FIG. 1.
Changes in bacterial numbers in dilution cultures with various concentrations of organic carbon substrates. The data are means of triplicate cultures, and the error bars indicate standard deviations.
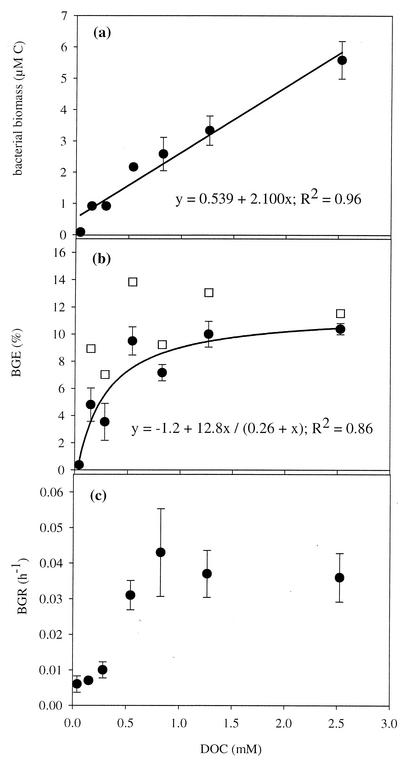
The average cell volume of the bacteria in the controls was significantly smaller (average volume, 0.088 ± 0.003 μm3; n = 3) than the average cell volume of cells analyzed in the various treatments (average volume, 0.168 ± 0.038 μm3; range of all individual treatments 0.114 to 0.272 μm3; n = 18) (P < 0.005, as determined by contrast analysis). When the controls were excluded from the analysis, the average cell volume of bacteria was not influenced by the DOC concentration (P = 0.12, as determined by one-way analysis of variance). The estimates of stationary-phase bacterial biomass contents ranged from 0.07 to 6.2 μM C and increased linearly with DOC concentration (Fig. 2a).
FIG. 2.
Impact of DOC concentration on bacterial carrying capacity (a), BGE (b), and maximal intrinsic growth rate (c). The solid circles indicate values that were not corrected for the control, whereas the open squares indicate values that were corrected for the influence of extraneous carbon. The corrected values were calculated by subtracting the biomass and the dissolved inorganic carbon produced in the controls from the values obtained for the various treatments (for further description see the text). All values are averages for triplicate cultures, and the error bars indicate the standard deviations, except for the corrected BGE values. The lines indicate the best-fit regression obtained by using Sigma Plot for Windows.
Bacterial mineralization of organic carbon was measured by measuring the increase in inorganic carbon content, which ranged from 10 to 47 μM C for the different incubations. Furthermore, the increase was linearly related to the initial DOC concentration according to the following equation: [produced CO2 C] = 0.013[DOC] + 0.13 (R2 = 0.78).
The BGE ranged from 0.4 to 10.4% and increased logarithmically with the DOC concentration (Fig. 2b). The maximum intrinsic growth rate was low (<0.01 h−1) in the cultures prepared with the lowest DOC concentrations (0.04 to 0.28 mM), whereas the corresponding growth rates in cultures prepared with higher DOC concentrations (0.54 to 2.53 mM) were all close to 0.04 h−1 (Fig. 2c). There was a highly significant correlation between BGE and BGR (R = 0.82; P < 0.001), and both variables were also significantly correlated with the biomass (R = 0.84 and R = 0.79, respectively; P < 0.001).
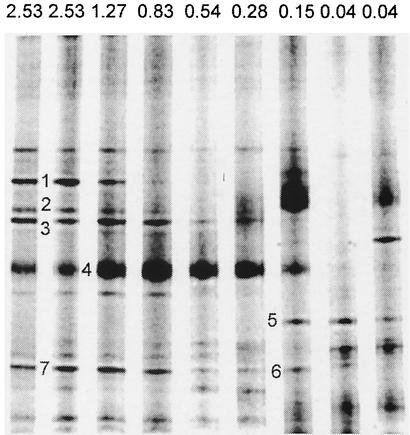
The DOC concentration had a major influence on the compositions of the bacterial communities that developed in the cultures. We compared the DGGE patterns of PCR-amplified 16S rRNA gene fragments from all replicates of the seven different treatments to determine differences in the emerging bacterial assemblages (Fig. 3). The DGGE band patterns for the triplicate microcosms analyzed for each treatment were identical except for the 0.15 mM DOC treatment (a single band did not appear in all replicates) and the controls to which DOC was not added. A total of 20 different bands were detected in the analysis.
FIG. 3.
Comparison of DGGE profiles of PCR-amplified bacterial 16S rRNA gene fragments derived from experimental cultures with different concentrations of natural DOM. The DOC concentrations (expressed as millimolar carbon) for the various treatments are indicated at the top. Bands that were excised cloned, and sequenced are indicated by the numbers to the left of bands (see Tables 1 and 2).
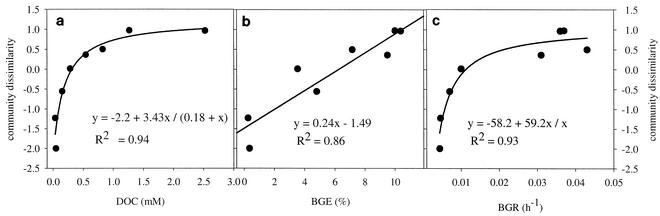
Since DNA-derived fingerprints of triplicate treatments were reproducible, the analysis of dissimilarity was carried out with only one replicate (except for the more variable control treatment). NMDS analysis of the absence or presence of the bands identified (stress value, 0.07; alienation, 0.11) showed that there was a gradual change in community structure from low DOC concentrations to high DOC concentrations. The correlation between DOC concentration and community dissimilarity followed an asymptotic function, with only minor changes in the community composition at higher DOC concentrations (Fig. 4a). In general, the number of bands did not seem to be influenced by the substrate supply (Fig. 3), and therefore the observed differences in the DGGE pattern along the DOM concentration gradient originated mainly from differences in the identities of the bands.
FIG. 4.
(a) Relationship between community dissimilarity and the concentration of DOC in the cultures. (b) Relationship between community dissimilarities and BGE. (c) Relationship between community dissimilarity and BGR. The degrees of dissimilarity in the DGGE patterns of the dilution cultures were converted to a binary (0/1) matrix (53). The multidimensional dissimilarity matrix was reduced to one dimension by using semistrong hybrid NMDS to visualize the relative differences in community structure between the cultures. Similar patterns are plotted close together (53, 54). Regressions were fitted by using Sigma Plot for Windows.
The observed changes in bacterial community structure were linearly correlated with BGE (Fig. 4b) and were significantly correlated with the accumulated biomass in the cultures (R = 0.81; P < 0.001). Furthermore, the correlation between community dissimilarity and BGR also followed an asymptote, with only minor changes at higher growth rates (Fig. 4c).
Seven bands representing different patterns of occurrence along the DOC concentration gradient were excised, cloned, and sequenced to identify bacterial populations (Fig. 3). Sequence analysis of the partial 16S rRNA fragments separated on the DGGE gel showed that single bands often contained more than one sequence. The multiple sequences obtained from bands 1 and 2 were all very similar to the sequence of an uncultured Cytophagales species (Tables 1 and 2). The three sequences obtained from band 7 and the single sequence in band 6 were also similar to each other and clustered with sequences of various uncultured members of the β subclass of the class Proteobacteria (β-proteobacteria) (Tables 1 and 2). However, band 3 contained sequences both from an uncultured member of the Cytophaga-Flavobacterium group and from a member of the β-proteobacteria, whereas the three sequences from band 5 included sequences related primarily to sequences of members of the α-proteobacteria (e.g., Caulobacter and Sphingomonadaceae) but also to sequences of β-proteobacteria (Tables 1 and 2). Sequences closely related to sequences of members of the Cytophaga-Flavobacterium group were typically found in the upper part of the gel and appeared to be more common at higher DOC concentrations. On the other hand, sequences from members of the β-proteobacteria, typically found in the lower portion of the gel, were present in most of the treatments. The band which contained sequences related to sequences of α-proteobacteria was obtained only at the two lowest DOC concentrations.
TABLE 1.
Similarity matrix for -500-bp 16s ribosomal DNA fragments from the DGGE analysis
| Banda | % Sequence similarity with band:
|
||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1b | 2a | 2b | 3a | 3b | 4 | 5a | 5b | 5c | 6 | 7a | 7b | 7c | |
| 1a | 95.2 | 99.6 | 93.3 | 91.7 | 75.4 | 82.7 | 75.7 | 76.9 | 72.5 | 74.1 | 73.2 | 73.0 | 73.2 |
| 1b | 94.8 | 96.9 | 92.9 | 74.7 | 77.5 | 78.1 | 81.0 | 77.0 | 77.0 | 77.0 | 77.3 | 77.2 | |
| 2a | 93.3 | 91.3 | 75.3 | 82.9 | 75.4 | 76.5 | 72.5 | 74.2 | 73.3 | 73.1 | 73.3 | ||
| 2b | 91.1 | 73.8 | 76.8 | 76.1 | 79.8 | 76.6 | 79.0 | 79.4 | 79.4 | 79.6 | |||
| 3a | 74.7 | 77.7 | 75.7 | 76.9 | 72.2 | 73.5 | 72.9 | 72.7 | 72.9 | ||||
| 3b | 76.0 | 81.0 | 78.8 | 86.6 | 86.8 | 84.0 | 85.7 | 85.9 | |||||
| 4 | 75.0 | 76.3 | 73.5 | 73.5 | 72.7 | 72.4 | 72.5 | ||||||
| 5a | 85.7 | 82.0 | 79.9 | 79.3 | 79.9 | 79.7 | |||||||
| 5b | 80.8 | 81.5 | 82.3 | 82.3 | 82.5 | ||||||||
| 5c | 89.4 | 87.2 | 90.1 | 90.3 | |||||||||
| 6 | 95.6 | 98.9 | 99.1 | ||||||||||
| 7a | 96.3 | 96.5 | |||||||||||
| 7b | 99.8 | ||||||||||||
The numbers 1 to 7 represent unique bands excised from the gel (Fig. 3). The letters a to c represent unique ribotypes obtained from the cloned bands.
TABLE 2.
Relationship of ribotypes sequenced to other sequences in the GenBank databasea
| Band | % Similarity | Alignment | Closest relative (accession no.) | Accession no. | Taxonomic affiliation |
|---|---|---|---|---|---|
| 1a | 98.0 | 408/413 | Uncultured Cytophagales bacterium; freshwater (AF361185) | AF550605 | Cytophaga-Flavobacterium |
| 1b | 99.0 | 401/405 | Uncultured Cytophagales bacterium; freshwater (AF361185) | AF550606 | Cytophaga-Flavobacterium |
| 2a | 98.0 | 400/406 | Uncultured Cytophagales bacterium; freshwater (AF361185) | AF550603 | Cytophaga-Flavobacterium |
| 2b | 98.0 | 407/412 | Uncultured Cytophagales bacterium; freshwater (AF361185) | AF550604 | Cytophaga-Flavobacterium |
| 3a | 97.0 | 526/541 | Uncultured bacterium FukuN24, Lake Fuchskuhle (AJ289995) | AF550601 | Cytophaga-Flavobacterium |
| 3b | 99.0 | 547/548 | Uncultured β-proteobacterium clone PRD01b002B; Parker River (Massachusetts) (AF289162) | AF550602 | β-Proteobacteria |
| 4 | 99.0 | 539/540 | Uncultured Cytophagales bacterium clone 30; mesocutrophic reservoir (AF361200) | AF550593 | Cytophagaz Flavobacterium |
| 5a | 97.0 | 439/452 | Caulobacter henricii (AJ007805) | AF550594 | α-Proteobacteria, Caulobacter |
| 5b | 98.0 | 515/524 | Sphingomonas sp. strain B28161 (AJ001052) | AF550595 | α-Proteobacteria, Sphingomonadaceae |
| 5c | 97.0 | 534/548 | Uncultured β-proteobacterium clone CLH3; environmental samples (AF529324) | AF550596 | β-Proteobacteria |
| 6 | 98.0 | 541/547 | Uncultured bacterium FukuS35; Lake Fuchskuhle (AJ290013) | AF550597 | β-Proteobacteria |
| 7a | 96.0 | 527/547 | Uncultured bacterium FukuS35; Lake Fuchskuhle (AJ290013) | AF550598 | β-Proteobacteria |
| 7b | 99.0 | 546/547 | Uncultured β-proteobacterium clone PRD01a006B; Parker River (Massachusetts) (AF289154) | AF550599 | β-Proteobacteria |
| 7c | 99.0 | 546/547 | Uncultured bacterium FukuS35: Lake Fuchskuhle (AJ290013) | AF550600 | β-Proteobacteria |
Sequences were aligned with the closest relatives in the GenBank database by using BLAST, The numbers 1 to 7 represent unique bands excised from the gel (Fig. 3). The letters a to c represent unique ribotypes obtained from the cloned bands. The percentage of similarity was calculated without taking gaps into account. The part of the total sequence used is indicated by the alignment data (number of positions used/total number of positions). Nucleotide sequences can be accessed by using their accession numbers at http://www.ncbi.nim.nih.gov.
DISCUSSION
Impact of carbon supply on microbial growth and growth efficiency.
In natural systems, the relative mass transfer from DOM to higher trophic levels via the heterotrophic bacterial route is partly regulated by BGE. Hence, at a low BGE a high proportion of the DOM utilized is lost as CO2. There is little doubt that BGE can be constrained by the quality of the available substrate (52). The similar BGE at the four highest substrate concentrations used in the present study probably reflect such constraints (Fig. 2b). The highest BGE observed for our aged humic DOM was 10.4%, which is low compared to the values obtained in previous studies of bacterial utilization of DOC in humic matter-rich waters (50, 51). This is likely due to intrinsic differences in the quality of the DOC caused by the selective loss of labile moieties constituting higher-quality substrates during the aging process.
Substrate concentration seemed to influence BGE only at DOC concentrations lower than 0.54 mM (Fig. 2). The total concentration of bacteria that accumulated in these low-DOC cultures never exceeded about 0.5 × 109 cells liter−1, and this cell yield is substantially below values expected for a wide range of natural waters with considerably lower total DOC concentrations. Accordingly, the bacterial cell yield was lower than the yields in comparable regrowth studies performed with water from both oligotrophic humic and clearwater lakes (4, 51), eutrophic lakes (44), and coastal waters (12, 19). For the open ocean, including the North Atlantic Ocean (22) and the Southern Ocean (7, 11), similar batch culture experiments have sometimes resulted in accumulations of bacterial biomass comparable to those in the incubation preparations in which we found no effect of substrate concentration on BGE. The results of other experiments (e.g., experiments with nonamended Sargasso Seawater [8]) match the low end of our gradient data, in which BGE was linearly related to substrate concentration. Hence, we concluded that DOC concentration is probably not an important regulator of BGE at the concentrations found in most natural aquatic systems. Consequently, variation in BGE found in such environments may instead be attributed to the quality of the organic matter or other factors.
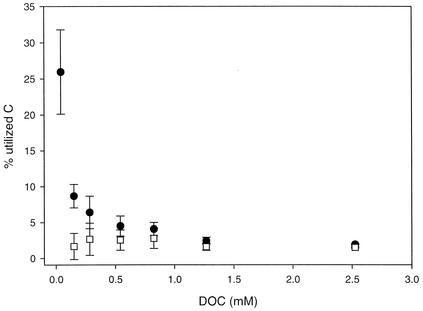
There was a gradual increase in the fraction of DOC that was utilized from the culture containing the largest concentration of aged DOC to the culture containing the lowest concentration of aged DOC (Fig. 5). Still, the observed range (1.9 to 8.7%) was far below the 25.9% of the total DOC that was utilized in the controls to which no aged DOC was added. The concentration of DOC in the control treatment was only 0.04 mM, and this low background level of bioavailable extraneous DOC originated mainly from the Milli-Q water used to prepare the cultures and the inoculum. The low BGE in the control incubation mixtures (0.37%) suggest that this extraneous DOC was energetically very poor (e.g., highly oxidized compounds). When utilization of the extraneous material was excluded from the analysis, the amount of DOC utilized was not significantly different for the various treatments (P = 0.73, as determined by one-way analysis of variance) (Fig. 5).
FIG. 5.
Fractions of total DOC utilized in the six treatments and the blank (0.04 mM DOC) (solid circles) and fractions of the aged DOC utilized (estimated by subtracting the DOC utilized in the control from the total DOC utilized in the treatments) (open squares). All values are averages for triplicate cultures, and the error bars indicate standard deviations.
We were concerned that the positive correlation between substrate concentration and BGE at DOC concentrations below 0.54 mM was caused by differences in substrate quality due to the extraneous DOC. This material may have constituted a significant fraction of the available DOC at the lowest concentrations. To evaluate this possibility, we assumed that 25.9% of the extraneous carbon was utilized in all treatments and that the BGE on the extraneous DOC was 0.37%, as observed for the extraneous DOC in the control cultures. Based on these assumptions, the BGE varied only from 7 to 14% for the different DOM concentrations, and the effects of the extraneous DOC on the BGE decreased with increasing total DOC concentration (Fig. 2b and 5). Our data indicate that the fraction of the total acquired energy allocated to support nongrowth processes decreased with increasing total substrate concentration within this concentration interval. The reasons that there are higher energy requirements per unit of biomass produced at lower substrate concentrations may include a higher absolute energy expenditure for active transport of the substrate into the cell at lower substrate concentrations (29) and a higher relative cost for maintaining essential functions, such as catabolic enzymes and functional membrane systems, at lower levels of growth (35). Previous work has shown that at very low substrate concentrations, the substrate is used primarily in catabolic reactions and the resulting BGE is low (20, 39, 46). Additionally, in natural aquatic environments synthesis of extracellular enzymes necessary for hydrolysis and subsequent utilization of polymeric substrates represent a major cost of energy for bacteria (33), and this may result in lower growth efficiency under energy-limited conditions in oligotrophic systems.
Coupling of bacterial growth and growth efficiency.
The bacterial carrying capacity and BGE observed in our experiments reflect the potential values when the organic carbon is the limiting factor, since the cultures received excess inorganic nutrients. We calculated the BGR from the increase in abundance during the log phase and found that there was a positive correlation with BGE. In previous studies workers have suggested that BGE is positively correlated with BGR, independent of temperature, the organic substrate, and other constraining factors (24, 32). In other studies, no such relationship was observed between BGR and BGE (14, 40), and Bjørnsen (6) even observed a negative correlation between BGE and BGR. Our results support the hypothesis that there is covariation of BGE and BGR (Fig. 2b and c) when the same DOC source is supplied at different concentrations, since the BGE were typically much lower in cultures with low growth rates (ca. 0.01 h−1) than in cultures with higher growth rates (>0.04 h−1). The highest BGE was observed at a high BGR, and this is in agreement with the results of a comparison of previously published data from a large number of systems (14).
Changes in bacterial community composition along a DOM gradient.
A recent study demonstrated that addition of equal amounts of two qualitatively different organic substrates to lake water resulted in emergence of significantly different bacterial communities (55). In our study, the composition of the bacterial community was influenced by the quantitative supply of carbon, and there was a gradual shift along a DOC gradient. The effect was most dramatic in the more dilute systems, as visualized by the NMDS data and the DGGE gel (Fig. 3 and 4a). At the lowest DOC concentrations, different band patterns were even observed for the replicates, while all band patterns that were obtained at DOC concentrations above 0.54 mM were similar. This indicates that even a small change in the DOC concentration could have an effect on the community composition in the most oligotrophic systems, while the band patterns in the richer systems were more resistant to a change in the substrate supply.
DGGE analysis of PCR-amplified 16S rRNA genes is a useful method for detecting major changes in the microbial community (9, 10, 36, 49). However, the results should not be interpreted as a quantitative representation of the bacterial community without extensive control experiments, since a mixed-template PCR may alter the template-to-product ratio for different genotypes (47) and since the 16S ribosomal DNA copy number may be different in different bacteria (15, 27, 37). Thus, we only analyzed the presence or absence of the different DGGE bands.
We are aware that DGGE analysis of PCR-amplified 16S rRNA fragments does not adequately detect less abundant populations in a community. The method rarely detects organisms that account for <1% of the total bacterial community (46). Hence, organisms that produce bands that disappear along the DOC gradient could still be present at low levels in the cultures in which these bands were not detected. Furthermore, individual bands often contain several sequences representing different bacterial populations (Table 1) (41). As a consequence, the microbial communities in different cultures contain bacterial populations far greater than the numbers of bands detected in the DGGE analysis.
The sequence analysis corroborated several recent studies suggesting that freshwater bacteria form distinct phylogenetic clusters separate from bacteria retrieved from marine and terrestrial ecosystems (18, 57). Most sequences were most closely related to strains recovered from Lake Fuchskuhle, a small humic lake in northern Germany (18). Furthermore, β-proteobacteria were well represented, and this group of bacteria is abundant in lakes and other freshwater ecosystems (17, 31). Members of the Cytophaga-Flavobacterium group are found in nearly every habitat of the biosphere and are known to play an important role in the degradation of complex biopolymers in marine and freshwater environments (23). In freshwater, this key role in the degradation of macromolecules is likely shared with the β-proteobacteria (23). Hence, the presence of several representatives of the Cytophaga-Flavobacterium group and β-proteobacteria is in accordance with the predominance of recalcitrant aged, high-molecular-weight humic substances in the DOC used in our experiment.
So far, strain RB2256 is the only isolated representative of the phylogenetic heterogeneous genus Sphingomonas. This organism was characterized as an oligotrophic “ultramicrobium” (16). Its physiological characteristics suggest that it should be adapted to very low substrate concentrations. This is in agreement with the phylogenetic data from our DGGE analysis of the bacterial communities in the dilution cultures, since α-proteobacterial sequences, including the sequence of a representative that clustered with the genus Sphingomonas, were found exclusively at the lowest carbon concentrations (Fig. 3 and Table 2).
In conclusion, we found that BGE, BGR, and bacterial community composition covaried along a concentration gradient of DOC. The lower BGE at low DOC concentrations could have been due either to a shift in the active bacterial populations to species with intrinsically low growth efficiency or to a simultaneous shift in species composition and the physiology of the active populations. However, the impact of substrate concentration on BGE, BGR, and bacterial community structure was apparent only at the lowest DOC concentrations, at which the ability of the DOC to support bacterial growth was lower than that the ability in most previous studies of eutrophic and oligotrophic aquatic systems, including many oceanic surface waters.
Acknowledgments
We thank J. Johansson for technical assistance and E. von Wachenfeldt for his invaluable help during preparation for the experiments. D. Bastviken assisted in preparation of the concentrated lake water DOM.
This study was funded by the Swedish Research Council (a grant to L.J.T.), the Swedish Research Council for Environment, Agricultural Sciences and Spatial Planning (a grant to S.B.), and ERASMUS (a scholarship to A.E.).
REFERENCES
- 1.Altschul, S. F., W. Gish, W. Miller, E. W. Myers, and D. J. Lipman. 1990. Basic local alignment search tool. J. Mol. Biol. 215:403-410. [DOI] [PubMed] [Google Scholar]
- 2.Amon, R. M. W., and R. Benner. 1996. Bacterial utilization of different size classes of dissolved organic matter. Limnol. Oceanogr. 41:41-51. [Google Scholar]
- 3.Ausubel, F. M., R. Brent, R. E. Kingston, D. D. Moore, J. G. Seidman, J. A. Smith, and K. Struhl. 1998. Current protocols in molecular biology, vol. 4, unit 2.5. John and Sons, Wiley, New York, N.Y.
- 4.Bano, N., M. A. Moran, and R. E. Hodson. 1997. Bacterial utilization of dissolved humic substances from a freshwater swamp. Aquat. Microb. Ecol. 12:233-238. [Google Scholar]
- 5.Bertilsson, S., and L. J. Tranvik. 2000. Photochemical transformation of dissolved organic matter in lakes. Limnol. Oceanogr. 45:753-762. [Google Scholar]
- 6.Bjørnsen, P. K. 1986. Bacterioplankton growth yield in continuous seawater cultures. Mar. Ecol. Prog. Ser. 30:191-196. [Google Scholar]
- 7.Bjørnsen, P. K., and J. Kuparinen. 1991. Determination of bacterioplankton biomass, net production and growth efficiency in the Southern Ocean. Mar. Ecol. Prog. Ser. 71:185-194. [Google Scholar]
- 8.Carlson, C. A., and H. W. Ducklow. 1996. Growth of bacterioplankton and consumption of dissolved organic carbon in the Sargossa Sea. Aquat. Microb. Ecol. 10:69-85. [Google Scholar]
- 9.Casamayor, E. O., H. Schäfer, L. Bañeras, C. Pedrós-Alio, and G. Muyzer. 2000. Identification of and spatio-temporal differences between microbial assemblages from two neighboring sulfurous lakes: comparison by microscopy and denaturing gradient gel electrophoresis. Appl. Environ. Microbiol. 66:499-508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Casamayor, E. O., R. Massana, S. Benlloch, L. Øvreås, B. Diez, V. J. Goddard, J. M. Gasol, I. Joint, F. Rodríguez-Valera, and C. Pedrós-Alió. 2002. Changes in archaeal bacterial and eukaryal assemblages along a salinity gradient by comparison of genetic fingerprinting methods in a multipond solar saltern. Environ. Microbiol. 4:338-348. [DOI] [PubMed] [Google Scholar]
- 11.Church, M. J., D. A. Hutchins, and H. W. Ducklow. 2000. Limitation of bacterial growth by dissolved organic matter and iron in the Southern Ocean. Appl. Environ. Microbiol. 66:455-466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Coffin, R. B., J. P. Connely, and P. S. Harris. 1993. Availability of dissolved organic carbon to bacterioplankton examined by oxygen utilization. Mar. Ecol. Prog. Ser. 101:9-22. [Google Scholar]
- 13.Del Giorgio, P. A., A. F. Bird, Y. T. Prairie, and D. Planas. 1996. The flow cytrometric determination of bacterial abundance in lake plankton with the green acid stain SYTO 13. Limnol. Oceanogr. 41:783-789. [Google Scholar]
- 14.Del Giorgio, P. A., and J. J. Cole. 1998. Bacterial growth efficiency in natural systems. Annu. Rev. Ecol. Syst. 29:503-541. [Google Scholar]
- 15.Fogel, G. B., C. R. Collins, J. Li, and C. F. Brunk. 1999. Prokaryotic genome size and SSU rDNA copy number: estimation of microbial relative abundance from a mixed population. Microb. Ecol. 38:93-113. [DOI] [PubMed] [Google Scholar]
- 16.Giovanni, S., and M. Ráppe. 2000. Evolution, diversity, and molecular ecology of marine prokaryotes, p. 47-84. In D. L Kirchman (ed.), Microbial ecology of the oceans. Wiley-Liss, New York, N.Y.
- 17.Glöckner, F. O., B. M. Fuchs, and R. Amann. 1999. Bacterioplankton composition of lakes and oceans: a first comparison based on fluorescence in situ hybridization. Appl. Environ. Microbiol. 65:3721-3726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Glöckner, F. O., E. Zaichikov, N. Belkova, L. Denissova, J. Pernthaler, A. Pernthaler, and R. Amann. 2000. Comparative 16S rRNA analysis of lake bacterioplankton reveals globally distributed phylogenetic clusters, including an abundant group of actinobacteria. Appl. Environ. Microbiol. 66:5053-5065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Goldman, J. C., and M. R. Dennett. 2000. Growth of marine bacteria in batch and continuous cultures under carbon and nitrogen limitation. Limnol. Oceanogr. 45:789-800. [Google Scholar]
- 20.Harder, J. 1997. Species-independent maintenance energy and natural population sizes. FEMS Microbiol. Ecol. 23:39-44. [Google Scholar]
- 21.Hobbie, J. E., R. J. Daley, and S. Jasper. 1977. Use of Nuclepore filters for counting bacteria by fluorescence microscopy. Appl. Environ. Microbiol. 33:1225-1228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kirchman, D. L., Y. Suzuki, C. Garside, and H. W. Ducklow. 1991. High turnover rates of dissolved organic-carbon during a spring phytoplankton bloom. Nature 352:612-614. [Google Scholar]
- 23.Kirchman, D. L. 2002. The ecology of Cytophaga-Flavobacteria in aquatic environments. FEMS Microb. Ecol. 39:91-100. [DOI] [PubMed] [Google Scholar]
- 24.Kristiansen, K., H. Nielsen, B. Riemann, and J. A. Fuhrman. 1992. Growth efficiencies of freshwater bacterioplankton. Microb. Ecol. 24:145-160. [DOI] [PubMed] [Google Scholar]
- 25.Kroer, N. 1993. Bacterial growth efficiency on natural dissolved organic matter. Limnol. Oceanogr. 38:1282-1290. [Google Scholar]
- 26.Lehman, J. T. 1980. Release and cycling of nutrients between planktonic algae and herbivores. Limnol. Oceanogr. 25:620-632. [Google Scholar]
- 27.Lionel, R., P. Franck, and N. Sylvie. 2000. Monitoring complex bacterial communities using culture-independent molecular techniques: application to soil environment. Res. Microbiol. 1551:167-177. [DOI] [PubMed] [Google Scholar]
- 28.Loferer-Krössbacher, M., J. Klima, and R. Psenner. 1998. Determination of bacterial cell dry mass by transmission electron microscopy and densitometric image analysis. Appl. Environ. Microbiol. 64:668-694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Marden, P., T. Nystrom, and S. Kjelleberg. 1987. Uptake of leucine by a marine gram-negative heterotrophic bacterium during exposure to starvation conditions. FEMS Microbiol. Ecol. 45:233-241. [Google Scholar]
- 30.McMannus, G. B. 1993. Growth rates of natural populations of heterotrophic nanoplankton, p. 557-562. In P. F. Kemp, E. B. Sherr, and J. J. Cole (ed.), Current methods in aquatic microbial ecology. Lewis Publishers, Boca Raton, Fla.
- 31.Méthe, B. A., W. D. Hiorns, and J. P. Zehr. 1998. Contrasts between marine and freshwater bacterial community composition: analyses of communities in Lake George and six other Adirondack lakes. Limnol. Oceanogr. 43:368-374. [Google Scholar]
- 32.Middelboe, M., B. Nielsen, and M. Søndergaard. 1992. Bacterial utilization of dissolved organic carbon (DOC) in coastal waters—determination of growth yield. Arch. Hydrobiol. Erg. Limnol. 37:51-61. [Google Scholar]
- 33.Middelboe, M., and M. Søndergaard. 1993. Bacterioplankton growth yield: seasonal variations and coupling to substrate lability and β-glucosidase activity. Appl. Environ. Microbiol. 59:3916-3921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mille-Lindblom, C., and L. J. Tranvik. 2003. Antagonism between bacteria and fungi on decomposing aquatic plant litter. Microb. Ecol. 45:173-182. [DOI] [PubMed] [Google Scholar]
- 35.Morita, R. Y. 1997. Bacteria in oligotrophic environments. Chapman & Hall, New York, N.Y.
- 36.Muyzer, G., E. C. de Waal, and A. G. Uitterlinden. 1993. Profiling of complex microbial populations by denaturing gradient gel electrophoresis analysis of polymerase chain reaction-amplified genes coding for 16S rRNA. Appl. Environ. Microbiol. 59:695-700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Muyzer, G. 1998. Structure, function and dynamics of microbial communities: the molecular biological approach, p. 157-187. In G. R. Carvalho (ed.), Advances in molecular ecology. IOS Press, Amsterdam, The Netherlands.
- 38.Pinhassi, J., F. Azam, J. Hemphälä, R. A. Long, J. Martinez, U. L. Zweifel, and Å. Hagström. 1999. Coupling between bacterioplankton species composition, population dynamics, and organic matter degradation. Aquat. Microb. Ecol. 17:13-26. [Google Scholar]
- 39.Russell, J. B., and G. M. Cook. 1995. Energetics of bacterial growth: balance of anabolic and catabolic reactions. Microbiol. Rev. 59:48-62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Schweitzer, B., and M. Simon. 1995. Growth limitation of planktonic bacteria in a large mesotrophic lake. Microb. Ecol. 30:89-104. [DOI] [PubMed] [Google Scholar]
- 41.Sekiguchi, H., N. Tomioka, T. Nakahara, and H. Uchiyama. 2001. A single band does not always represent single bacterial strains in denaturing gradient gel electrophoresis analysis. Biotechnol. Lett. 23:1205-1208. [Google Scholar]
- 42.Serkiz, S. M., and E. M. Perdue. 1990. Isolation of dissolved organic-matter from the Suwannee River using reverse-osmosis. Water Res. 24:911-916. [Google Scholar]
- 43.Søndergaard, M., and M. Middelboe. 1995. A cross-system analysis of labile dissolved organic carbon. Mar. Ecol. Prog. Ser. 118:283-294. [Google Scholar]
- 44.Søndergaard, M., and J. Theil-Nielsen. 1997. Bacterial growth efficiency in lakewater cultures. Aquat. Microb. Ecol. 12:115-122. [Google Scholar]
- 45.Stepanauskas, R., L. Leonardson, and L. J. Tranvik. 1999. Bioavailability of wetland-derived DON to freshwater and marine bacterioplankton. Limnol. Oceanogr. 44:1477-1485. [Google Scholar]
- 46.Stouthamer, A. H., and C. Bettenhaussen. 1973. Utilization of energy for growth and maintenance in continuous and batch cultures of microorganisms. Biochem. Biophs. Acta 301:53-70. [DOI] [PubMed] [Google Scholar]
- 47.Suzuki, M. T., and S. J. Giovannoni. 1996. Bias caused by template annealing in the amplification of mixtures of 16S rRNA genes by PCR. Appl. Environ. Microbiol. 62:625-630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Teske, A., C. Wawer, G. Muyzer, and N. B. Ramsing. 1996. Distribution of sulfate-reducing bacteria in a stratified fjord (Mariager Fjord, Denmark) as evaluated by most-probable-number counts and denaturing gradient gel electrophoresis of PCR-amplified ribosomal DNA fragments. Appl. Environ. Microbiol. 62:1405-1415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Torsvik, V., F. Daae, R.-A. Sandaa, and L. Øvreås. 1998. Novel techniques for analysing microbial diversity in natural and perturbed environments. J. Biotechnol. 64:53-62. [DOI] [PubMed] [Google Scholar]
- 50.Tranvik, L. J., and M. G. Höfle. 1987. Bacterial growth in mixed cultures on dissolved organic carbon from humic and clear waters. Appl. Environ. Microbiol. 53:482-488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Tranvik, L. J. 1988. Availability of dissolved organic carbon for planktonic bacteria in oligotrophic lakes of differing humic content. Microb. Ecol. 16:311-322. [DOI] [PubMed] [Google Scholar]
- 52.Vallino, J. J., C. S. Hoppkinson, and J. E. Hobbie. 1996. Modeling bacterial utilization of dissolved organic matter: optimization replaces Monod growth kinetics. Limnol. Oceanogr. 41:1591-1609. [Google Scholar]
- 53.Van Hannen, E. J., M. P. Van Agterveld, H. J. Gons, and H. J. Laanbroek. 1998. Revealing genetic diversity of eukaryotic microorganisms in aquatic environments by denaturing gradient gel electrophoresis. J. Phycol. 34:206-213. [Google Scholar]
- 54.Van Hannen, E. J., G. Zwart, M. P. Van Agterveld, H. J. Gons, J. Ebert, and H. J. Laanbroek. 1999. Changes in bacterial and eukaryotic community structure after mass lysis of filamentous cyanobacteria associated with viruses. Appl. Environ. Microbiol. 65:795-801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Van Hannen, E. J., W. Mooij, M. P. Van Agterveld, H. J. Gons, and H. J. Laanbroek. 1999. Detritus-dependent development of microbial community in an experimental system: qualitative analysis by denaturing gradient gel electrophoresis. Appl. Environ. Microbiol. 65:2478-2484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Vrede, K., M. Heldal, S. Norland, and G. Bratbak. 2002. Elemental composition (C, N, P) and cell volume of exponentially growing and nutrient-limited bacterioplankton. Appl. Environ. Microbiol. 68:2965-2971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Zwart, G., B. C. Crump, M. P. K. van Agterveld, F. Hagen, and S.-K. Han. 2002. Typical freshwater bacteria: an analysis of available 16S rRNA gene sequences from plankton in lakes and rivers. Aquat. Microb. Ecol. 28:141-155. [Google Scholar]
- 58.Zweifel, U. L., B. Norrman, and Å. Hagström. 1993. Consumption of dissolved organic carbon by marine bacteria and demand for inorganic nutrients. Mar. Ecol. Prog. Ser. 101:23-32. [Google Scholar]